Abstract

As the most studied two-dimensional (2D) material from the MXene family, Ti3C2Tx has constantly gained interest from academia and industry. Ti3C2Tx MXene has the highest electrical conductivity (up to 24,000 S cm–1) and one of the highest stiffness values with a Young’s modulus of ∼ 334 GPa among water-dispersible conductive 2D materials. The negative surface charge of MXene helps to disperse it well in aqueous and other polar solvents. This solubility across a wide range of solvents, excellent interface interaction, tunable surface functionality, and stability with other organic/polymeric materials combined with the layered structure of Ti3C2Tx MXene make it a promising material for anticorrosion coatings. While there are many reviews on Ti3C2Tx MXene polymer composites for catalysis, flexible electronics, and energy storage, to our knowledge, no review has been published yet on MXenes’ anticorrosion applications. In this brief report, we summarize the current progress and the development of Ti3C2Tx polymer composites for anticorrosion. We also provide an outlook and discussion on possible ways to improve the exploitation of Ti3C2Tx polymer composites as anticorrosive materials. Finally, we provide a perspective beyond Ti3C2Tx MXene composition for the development of future anticorrosion coatings.
Keywords: 2D materials, MXene, Ti3C2Tx, anticorrosion, polymer composites, coatings, MAX phase
1. Introduction
Ti3C2Tx, the most studied two-dimensional (2D) material in the MXene family, has gained great attention since its first synthesis in 2011.1 The chemical formula of MXene indicates the number of atomic layers of the elements present in a sandwich-like layered morphology. For example, Ti3C2Tx consists of three layers of Ti atoms and two layers of C atoms arranged in layers of Ti–C–Ti–C–Ti. The Tx component in the formula represents the surface terminations (typically −OH, −F, −O, −Cl) existing on the outer planes of Ti as an outcome of the synthesis method.1−3 Thus, it is easily well-dispersed in water or other solvents, with the highest electrical conductivity (up to 24,000 S cm–1) and Young’s modulus (∼334 GPa) among all solution-processed 2D materials.4−6 In addition, the top-down synthesis method via wet chemical selective etching from its precursor, the Ti3AlC2 MAX phase,7,8 makes it quite scalable for industrial synthesis. Owing to these superior properties and its feasibility for solution processability, scalability, and surface functionality, various applications of Ti3C2Tx such as in catalysis,9,10 electromagnetic interference shielding,11 energy-storage applications,12 flexible electronics, and biosensors13 have been reported.
Corrosion is a tendency of a metal to convert to its oxide form. It has a significant environmental and economic impact on society. Unlike graphene, Ti3C2Tx-based coatings for anticorrosion are not widely explored. For instance, Ti3C2Tx MXene was projected as a robust current collector for water desalination applications14 and lithium-ion batteries.15 Chloride anions present in saline water could corrode the current collectors beyond threshold potentials, impacting the efficiencies. The use of Ti3C2Tx MXene as a current collector is due to its high specific surface area, suitable pore structure, high redox activity, high electrical conductivity, and stability in aqueous electrolytes. These properties enable Ti3C2Tx MXene electrode operation at a high salt adsorption capacity within a large voltage window of electrochemical stability, exhibiting high reversibility without corrosion.14 However, despite these advantages, no works on grafting polymeric materials on Ti3C2Tx for anticorrosion protection are reported to the best of our knowledge. For anticorrosive coating applications, some reports have investigated Ti3C2Tx-based polymer composites by either noncovalent or covalent functionalization16,17 with noncovalent functionalization, employing physical mixing of Ti3C2Tx with polymeric materials. In the first part of this review, we discuss recent research on Ti3C2Tx–polymer composites for anticorrosion. Next, we present how to improve Ti3C2Tx MXene integration into polymer matrices to further enhance the anticorrosion properties of these materials. Finally, we provide a perspective beyond Ti3C2Tx, which can be useful for the future development of anticorrosion coatings.
2. Ti3C2Tx MXene Polymer Composites for Anticorrosion
Ti3C2Tx MXene polymer composites prepared via surface functionalization will be discussed in detail in the subsequent sections. The discussion is organized as follows: (a) first, the efficacy of pristine MXenes/polymer matrix composites, followed by (b) MXene coatings derived via surface functionalization methods.
2.1. Pristine Ti3C2Tx
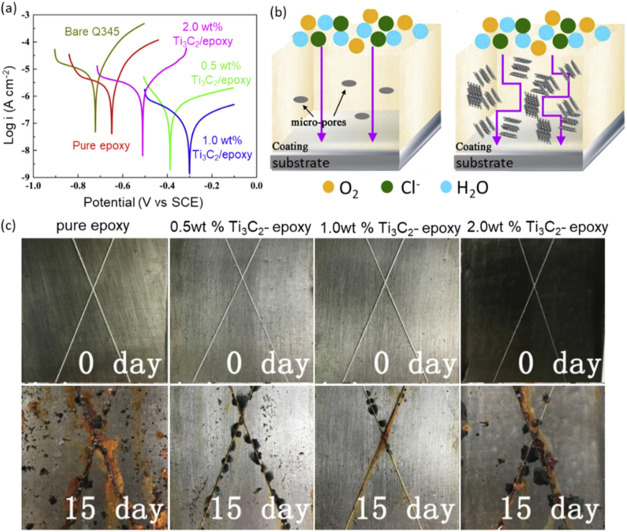
To exploit the anticorrosion properties of pristine Ti3C2Tx nanosheets, single- to few-layer Ti3C2Tx nanosheets were physically mixed via magnetic stirring in a waterborne epoxy coating (WEC)18,19 or waterborne polyurethane (WPU).20 The anticorrosion properties of pristine Ti3C2Tx were first reported by Yan et al.,18 where they incorporated Ti3C2Tx nanosheets in epoxy resin with an amine curing agent. Ti3C2Tx exhibited stable dispersions in the epoxy matrix due to its hydrophilic nature, which is vital to create a perfect physical barrier for anticorrosion. Figure 1a shows the Tafel plots of the uncoated Q345 sample, pure epoxy, and Ti3C2Tx/epoxy composites with different ratios of Ti3C2Tx (0.5, 1, and 2 wt % Ti3C2Tx/epoxy). After immersion in 3.5% NaCl solution for 96 h, the MXene showed enhanced corrosion protection on the steel substrates compared to pure epoxy coatings. The improvement in anticorrosion properties was attributed to the presence of MXene flakes as thin film barriers for the diffusion of electrolyte and providing corrosion protection to the substrate (Figure 1b).18
Figure 1.
(a) Tafel plots of the anticorrosion properties of uncoated and coated samples after immersion in 3.5% NaCl for 96 h. The 1 wt %-coated sample shows the highest protection, indicated by the most positive shifting of potential value Ecorr and the lowest corrosion current, Icorr. Here, potential (V vs SCE) refers to potential versus saturated calomel electrode, which serves as the reference electrode. (b) Schematic illustration of corrosion process without and with a Ti3C2Tx-contained epoxy coating. (c) Photographs of the samples before and after the salt spray test, where 1.0 wt % Ti3C2 offers the highest protection, in agreement with results in a. Reprinted with permission from ref (18) Copyright 2019 Elsevier.
Moreover, the 1 wt % Ti3C2Tx-coated sample showed the highest protection, indicated by the shift of the potential (Ecorr) to the most positive value and the lowest corrosion current (Icorr) as shown in Figure 1a. Table 1 shows the anticorrosion parameters of each sample, as derived from the Tafel plot in Figure 1a.
Table 1. Corrosion Properties for Pristine and Coated Q345 Steel Substrates after Immersion for 96 h in a 3.5% NaCl Solutionab.
| samples | Ecorr (V) | icorr (A·cm–2) | ba (mV·dec–1) | bc (mV·dec–1) | Rcorr (MΩ·cm2) | CR (mm y–1) | PE (%) |
|---|---|---|---|---|---|---|---|
| bare Q345 | –0.75 | 7.51 × 10–5 | 0.120 | –0.364 | 0.09 | 87.07 × 10–3 | |
| pure epoxy | –0.71 | 1.00 × 10–6 | 0.154 | –0.369 | 0.71 | 11.62 × 10–3 | 98.67 |
| 0.5 wt % Ti3C2/epoxy | –0.41 | 1.28 × 10–7 | 0.423 | –0.094 | 3.20 | 1.48 × 10–3 | 99.83 |
| 1.0 wt % Ti3C2/epoxy | –0.29 | 3.39 × 10–8 | 0.257 | –0.218 | 8.55 | 0.39 × 10–3 | 99.95 |
| 2.0 wt % Ti3C2/epoxy | –0.53 | 7.05 × 10–7 | 0.191 | –0.473 | 0.75 | 8.17 × 10–3 | 99.06 |
Adapted from ref (18).
Note: Ecorr: corrosion potential; Icorr: corrosion current density; ba: anodic Tafel slope; bc: cathodic Tafel slope; corrosion resistance, Rcorr was calculated from Ecorr/Icorr. CR: corrosion rate; PE: protection efficiency.
Figure 1c shows the optical photographs of pure epoxy and Ti3C2Tx–epoxy coatings before (0 days) and after 15 days of exposure time in a salt spray chamber. In agreement with Figure 1a, 1.0 wt % Ti3C2Tx–epoxy shows the highest corrosion protection, outperforming 2.0 wt % Ti3C2Tx–epoxy. These results indicate that adding more Ti3C2Tx does not necessarily result in higher protection. Rather, the optimized value for the mixing of Ti3C2Tx and the epoxy where the best dispersibility can be obtained is more structurally important. The higher content of Ti3C2Tx leads to the stacking of flakes, creating higher volume densities and resulting in phase aggregation and separation between Ti3C2 and the epoxy matrix, further impeding the effectiveness of Ti3C2Tx 2D flakes as a physical barrier for anticorrosion. These results corroborate the work on graphene-based coatings, where the optimized ratio of graphene and the polymer matrix is found to be important to achieve the highest degree of protection against corrosion.21
Another aspect that reflects the anticorrosion properties is the impedance modulus at the lowest frequencies, |z|f = 0.01 Hz, where a higher impedance modulus results in better corrosion protection. In the same study, the authors observed that for the first 2 h, the impedance moduli were 9.42 × 107, 3.55 × 108, 6.23 × 108, and 3.95 × 108 Ω cm2 for pure epoxy, 0.5, 1, and 2 wt % Ti3C2Tx, respectively. However, after 96 h immersion in saline environments (3.5% NaCl), the impedance moduli significantly decreased to 2.27 × 106, 7.6 × 106, 2.96 × 107, and 6.11 × 106 Ω cm2, respectively. The decrease of the impedance modulus at longer exposures to saline environments is attributed to the instability of Ti3C2Tx due to oxidation and hydrolysis.22−24 It is known that despite its superior intrinsic properties, Ti3C2Tx flakes are prone to hydrolysis and oxidation in hydrated environments and potential transformation to TiOx and TiO2.17,23−25 The degradation is usually initiated at the defect and edge sites of MXenes and is a multistep process where the defect sites undergo systematic hydrolysis with the formation of TiO2 as the final degraded product.26 This chemical degradation may impede the anticorrosion behavior of MXene in the long term, therefore requiring strategies to mitigate oxidation.
The oxidation of MXenes due to the reaction with dissolved oxygen can be limited by storing them in solutions saturated with inert gas.24 Another way to slow the oxidation rates of MXenes is by freezing at ultralow temperatures (−20 or −80 °C), extending the shelf life to two years.27 Stability of MXene can be effectively improved by hydrogen annealing but causes loss of dispersibility in solvents and surface reactivity.28 When excess aluminum was used in the synthesis mixture of Ti3AlC2 resulting Ti3C2Tx MXene had lower number of Ti and C vacancies and exhibited a better oxidation resistance.29 A long-term storage of MXenes in an aqueous solution utilizing hydration chemistry with nontoxic inorganic salts inhibits the attack of MXene by free water and oxygen molecules.30−32 As a result, oxidation can be largely inhibited, prolonging the shelf life to up to 400 days with negligible loss of surface chemistry. Other methods such as surface functionalization33,34 with organic ligands can maintain MXene chemical stability for long-term applications such as anticorrosion additives.
2.2. Surface-Functionalized Ti3C2Tx MXene
Aminopropyl triethoxysilane (APTES) is the most used silane for surface functionalization. The primary amino functional groups in APTES offer several possibilities for postfunctionalization from bioconjugation to nanoparticle impregnation. Ji et al.35 reported that functionalized Ti3C2Tx with APTES demonstrates improved stability against oxidation with adjustable hydrophilicity in comparison to pristine Ti3C2Tx MXene.
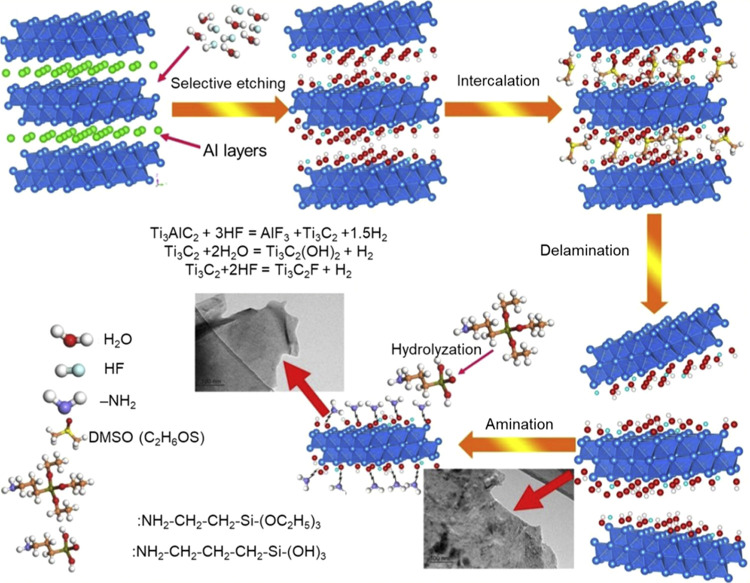
Yan et al.36 were the first to investigate the anticorrosion properties of amino-functionalized Ti3C2Tx MXene. In their work, APTES was first attached to Ti3C2Tx via a simple wet deposition, using the abundant hydroxyl functional groups on Ti3C2Tx in the colloidal solution form. Figure 2 shows the schematic illustration of the preparation of Ti3C2Tx MXene and its surface functionalization with APTES. The amino-functionalized Ti3C2Tx exhibited higher mechanical properties and showed better dispersibility in water, in comparison to pristine Ti3C2Tx.36 Note that the synthesis of Ti3C2Tx MXene has been reported in numerous studies and reviews elsewhere3,37−40 and is not in the scope of this review.
Figure 2.
Schematic illustration of synthesis and surface functionalization of Ti3C2Tx with APTES. Reprinted with permission from ref (36) Copyright 2020 Elsevier.
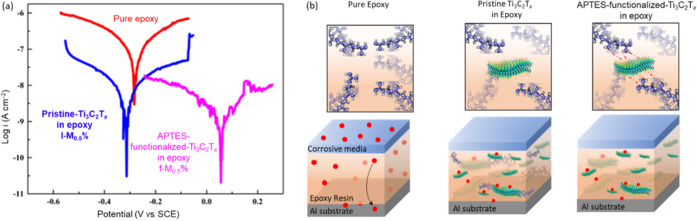
Composites with 0.25 and 0.5 wt % of pristine Ti3C2Tx (0.25 l-M and 0.5 l-M, respectively) and 0.25 and 0.5 wt % APTES-functionalized Ti3C2Tx (0.25 and 0.5 f-M, respectively) in the waterborne epoxy polymer were prepared by physical mixing. The addition of pristine Ti3C2Tx and APTES-functionalized Ti3C2Tx has enhanced the anticorrosion properties of the epoxy coatings (Figure 3a) where MXene 2D flakes acted as a physical barrier to the corrosion agents (Figure 3b). The corrosion resistance was 2.34 × 108 Ω cm2 for pure epoxy. The functionalized Ti3C2Tx exhibits higher corrosion resistance in comparison to pristine Ti3C2Tx. The 0.5 wt % APTES-functionalized Ti3C2Tx/epoxy coatings (f-M0.5%) demonstrated the best anticorrosion protection, as indicated by the most positive value in the Tafel plot (Figure 3a and Table 2). f-M0.5% showed the highest corrosion protection of 3.09 × 109 Ω cm2. However, after 4 weeks of immersion in 3.5% NaCl, a significant decrease in corrosion resistance was observed for all samples. The pure epoxy exhibited the highest degradation to 3.45 × 105 Ω cm2, whereas the 0.5 wt % APTES-functionalized Ti3C2Tx/epoxy coatings exhibited the lowest degradation (from 3.09 × 109 to 1.02 × 107 Ω cm2). The study shows the importance of the ligand functionalization of Ti3C2Tx in improving its dispersibility as well as maintaining its chemical stability while slowing down degradation due to oxidation in the polymer matrix.36
Figure 3.
(a) Tafel plot of pure epoxy, l-M0.5%, and f-M0.5% after 4-week immersion in a 3.5% NaCl solution. f-M0.5% shows the best anticorrosion performance, indicated by the most positive value. The potential (V vs SCE) refers to potential versus saturated calomel electrode, which serves as the reference electrode. (b) Schematic illustration of the corrosion protection process in pure epoxy, pristine Ti3C2Tx/epoxy, and APTES-functionalized/epoxy coatings. Reprinted with permission from ref (36) Copyright 2020 Elsevier.
Table 2. Anticorrosion Resistance of the Pure Epoxy, Pristine Ti3C2Tx/Epoxy (l-M), and APTES-Functionalized Ti3C2Tx/Epoxy (f-M)-Coated Samples, after 1-Day and 4-Week Immersion in 3.5% NaCla.
| sample | Rcorr (1 day) (Ω cm2) | Rcorr (4 weeks) (Ω cm2) |
|---|---|---|
| pure | 2.34 × 108 | 3.46 × 105 |
| 0.25 l-M | 6.45 × 108 | 4.68 × 106 |
| 0.5 l-M | 7.94 × 108 | 5.89 × 106 |
| 0.25 f-M | 2.04 × 109 | 8.91 × 106 |
| 0.5 f-M | 3.09 × 109 | 1.02 × 107 |
Adapted from ref (36).
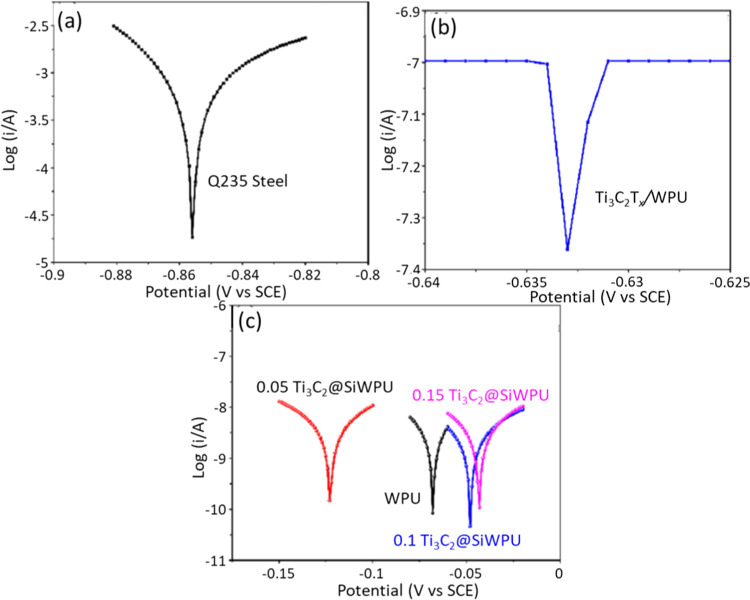
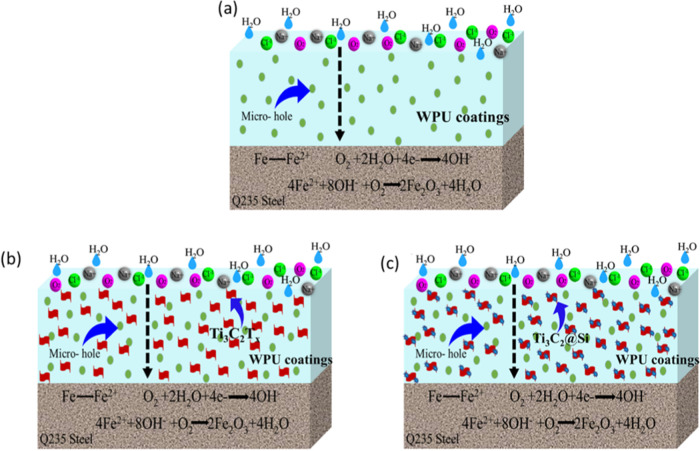
Similarly, Zhang et al.,41 reported surface functionalization of [3-(2-aminoethyl) aminopropyl] trimethoxysilane (AEAPTES) on Ti3C2Tx MXene. The AEAPTES-functionalized Ti3C2Tx (named Ti3C2@Si) was then mixed with waterborne polyurethane (WPU) with Ti3C2Tx ratios of 0.05, 0.1, and 0.15 wt %. Figure 4 shows the Tafel plots for the uncoated steel substrate, pristine Ti3C2Tx/WPU, and WPU and Ti3C2@Si. Ti3C2@Si exhibited a positive shift in the Tafel plot (Figure 4c). The 0.1 wt % Ti3C2Tx sample showed the lowest current density, indicating the best anticorrosion performance, outperforming 0.15, 0.05 wt %, WPU, Ti3C2Tx /WPU, and bare Q235 steel substrate.
Figure 4.
Tafel plots of (a) uncoated Q235 steel, (b) pristine Ti3C2/WPU, and (c) WPU and functionalized Ti3C2@Si/WPU. The functionalized coated samples exhibit a positive shift value, indicating the highest corrosion protection. The potential (V vs SCE) refers to potential versus saturated calomel electrode, which serves as the reference electrode. Reprinted with permission from ref (41) Copyright 2021 Elsevier.
The parameters extracted from the Tafel plots (Table 3) confirm better corrosion protection of the functionalized-Ti3C2Tx coated samples in comparison to pristine Ti3C2Tx MXene. The results indicated that pristine Ti3C2Tx MXene in WPU was less effective in anticorrosion compared with pure WPU (Table 3). The poor performance of pristine Ti3C2Tx MXene in WPU may be attributed to the intermittent and noncontinuous interfacial adhesion between pristine Ti3C2Tx and WPU. This may create spaces or micropores that facilitate the corrosive ions to permeate and subsequently propagate the degradation beneath the coating. All functionalized Ti3C2Tx MXene–WPU coatings showed higher corrosion protection with 0.1 wt % exhibiting the lowest current density of 2.67 × 10–9 A cm–2 and the highest contact resistance of 3.05 × 106 Ω·cm2. Impressively, after 42 days of immersion in 3.5% NaCl, no degradation was observed. This finding corroborates with the work of Yan et al.,36 where the surface functionalization of Ti3C2Tx with APTES was found to enhance the chemical enhance and stabilize the corrosion resistance.
Table 3. Resistance Parameters as Derived from the Tafel Plot in Figure 4, Adapted from ref (41).
| sample | Ecorr (V) | Icorr (A cm–2) | Rp (Ω) | Ba | Bc | CR (mm year–1) |
|---|---|---|---|---|---|---|
| Q235 steel | –0.86 | 3.23 × 10–4 | 11 × 100 | 2.26 | 1.58 | 3.76 × 100 |
| WPU | –0.07 | 2.32 × 10–8 | 1.9 × 106 | 4.90 | 4.98 | 2.74 × 10–4 |
| Ti3C2/WPU | –0.633 | 2.8 × 10–5 | 6.63 × 104 | 0.08 | 0.15 | 3.27 × 10–1 |
| 0.05% Ti3C2@Si/WPU | –0.12 | 9.94 × 10–9 | 2.09 × 106 | 10.78 | 10.18 | 1.16 × 10–4 |
| 0.1% Ti3C2@Si/WPU | –0.05 | 2.67 × 10–9 | 3.05 × 106 | 26.94 | 26.46 | 3.11 × 10–5 |
| 0.15% Ti3C2@Si/WPU | –0.04 | 3.48 × 10–9 | 2.19 × 106 | 30.02 | 27.11 | 4.06 × 10–5 |
Figure 5 shows the mechanism of the corrosion protection of pure WPU, Ti3C2Tx/WPU, and functionalized Ti3C2@Si/WPU. Steels coated with pure WPU corrode easily, probably due to the defects and micron-sized holes through which the ions can permeate with relative ease. Contrary to expectations, Ti3C2/WPU coatings were less efficient than pristine WPU. This may be due to abundant oxygen functional groups on Ti3C2Tx surfaces, attracting water molecules and other corrosive media, acting as initiator sites for oxidation. The introduction of amino functional groups on Ti3C2Tx facilitates intercalation with isocyanate groups in WPU, giving a strong and compact structure and leading to stable dispersions of Ti3C2@Si in WPU. Therefore, a network of an effective barrier was formed by good compatibility and dispersibility while creating complex diffusion paths, thereby slowing the diffusion rates. Importantly, the functionalized Ti3C2@Si increased the hydrophobicity of WPU, which decreased the absorption of water and enhanced the corrosion performance of Ti3C2/WPU composite coatings. These findings are similar to those of graphene-based materials,21,42,43 where covalently functionalized graphene demonstrated better corrosion protection compared to pristine graphene.
Figure 5.
Mechanism of the corrosion protection for (a) pristine WPU, (b) Ti3C2Tx/WPU, and (c) functionalized Ti3C2Tx@Si/WPU. Reprinted with permission from ref (41) copyright 2021 Elsevier.
2.3. Ti3C2Tx/PANI Composites
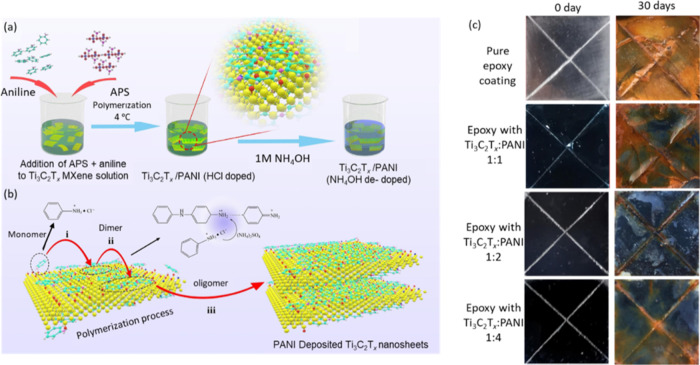
Conductive polymers (CPs) such as polythiophene (PT),44 polypyrrole (PPy),45,46 and polyaniline (PANI)47−49 exhibit anticorrosion properties. Among these, PANI has attracted more attention for anticorrosion owing to its ease of synthesis, thermal stability, and reversible acid/base doping/dedoping.50 Cai et al.51 combined PANI and the 2D Ti3C2Tx to enhance the anticorrosion properties of waterborne epoxy (WEP) resins. Ti3C2Tx/PANI composites were prepared via in situ polymerization (Figure 6a,b) in Ti3C2Tx/PANI mass ratios of 1:1, 1:2, and 1:4, followed by sandwiching the Ti3C2Tx/PANI composite between two WEP layers to create multilayer coatings on mild steel substrates.
Figure 6.
Schematic Illustration of the preparation of Ti3C2/PANI composites. (a) Preparation of Ti3C2 nanosheets. (b) Synthesis of Ti3C2/PANI composites (TPCs) with the mechanism of oxidative polymerization of aniline on Ti3C2. (c) Photograph of the samples before and after the salt spray test for 30 days. Reprinted with permission from ref (51) copyright 2021 Elsevier.
Among all of the Ti3C2Tx/PANI composite coatings, Ti3C2Tx/PANI with a 1:2 ratio exhibited the best corrosion protection (Figure 6c). The initial impedance modulus after 1-day immersion in 3.5% NaCl was 7 × 108 Ω·cm2 and decreased to 1 order of magnitude to 1.05 × 107 Ω·cm2 after 5 weeks of immersion. Furthermore, Ti3C2Tx/PANI (1:2) sprayed with salts for 45 days demonstrated minimum contents of oxygen and chlorine. The excellent corrosion protection was attributed to the 3D structures of Ti3C2Tx/PANI composites, which served as a reservoir and as a trap for corrosive ions.
2.4. Ti3C2Tx/Graphene Hybrid Composites
Graphene has excellent impermeable and physical barrier properties. However, its ability to be dispersed in the polymer matrix is poor. To improve graphene dispersion, the covalent functionalization of graphene with polymeric materials is an option. Another novel strategy is to combine graphene and Ti3C2Tx to achieve graphene/Ti3C2Tx heterostructures. Since Ti3C2Tx is hydrophilic, the resulting graphene/Ti3C2Tx heterostructures are also hydrophilic and may show beneficial synergistic effects for anticorrosion.
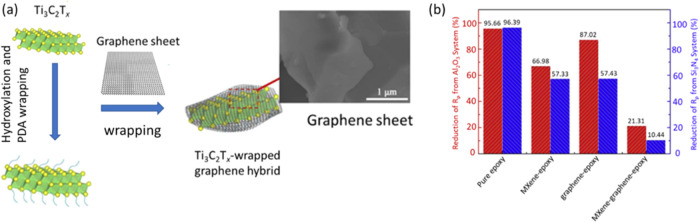
Recently, Yan et al.,52 have demonstrated the anticorrosion properties of Ti3C2Tx/graphene heterostructures in which Ti3C2Tx is wrapped by graphene. First, they prepared the Ti3C2Tx/graphene heterostructures by making a graphene-wrapped Ti3C2Tx via polydopamine interfacial chemistry (Figure 7a). Ti3C2Tx/graphene was then mixed with the epoxy to make Ti3C2Tx/graphene–epoxy coating composites. The corrosion properties of the Ti3C2Tx/graphene–epoxy coating composites in 3.5% NaCl were studied.
Figure 7.
(a) Schematic illustration of the synthesis of Ti3C2Tx/graphene heterostructures with wrapping structures where MXene sheets are wrapped by graphene sheets. (b) Reduction of the corrosion polarization resistance Rp for all coatings. MG-EP shows the lowest reduction, while pure EP exhibits the highest loss of corrosion resistance. Reprinted with permission from ref (52) copyright 2020 Elsevier.
The Ti3C2Tx/graphene–epoxy coating exhibited a corrosion resistance modulus of 2.14 × 109 Ω·cm2, higher than those of pure epoxy (1.06 × 108 Ω·cm2), Ti3C2Tx MXene–epoxy (1.51 × 109 Ω·cm2), and graphene–epoxy (1.53 × 109 Ω·cm2). The Ti3C2Tx/graphene–epoxy coating exhibited a significant decrease in corrosion impedance modulus of 21.3 and 10.4% after the wear test with Al2O3 and Si3N4 balls, respectively. For pure epoxy, the corrosion impedance modulus was decreased by 95.7 and 96.4% after the wear test with Al2O3 and Si3N4, respectively (Figure 7b). The enhanced performance of the Ti3C2Tx/graphene–epoxy composite was attributed to the (i) thermal conductivity and excellent lubricant properties of Ti3C2Tx and graphene, (ii) dual hybrid surfaces that form protective films, and (iii) the synergistic effects of Ti3C2Tx/graphene-interweaved structures that greatly improved the anticorrosion properties of organic coatings.
3. Conclusions and Future Perspectives
We have discussed the current progress on the Ti3C2Tx MXene for anticorrosion applications. Both pristine and functionalized Ti3C2Tx may be used for making anticorrosive coatings. Physical mixing of Ti3C2Tx with waterborne epoxy (WEP) or polyurethane (PU) is the most popular route for preparing anticorrosion coatings. In general, studies on the behavior of the Ti3C2Tx MXene in corrosive environments indicate that its hydrophilicity has both advantages and disadvantages. Ti3C2Tx hydrophilicity improves its dispersibility in WEP and PU matrices. However, the abundant oxygen functional groups on the Ti3C2Tx surface may trigger more corrosion. Ti3C2Tx MXene is prone to chemical degradation due to active hydrolysis and surface oxidation, leading to the formation of titanium oxide,24 which may impede the long-term corrosion protection.
There are at least three main approaches to slow down or prevent the oxidation and chemical degradation of MXenes and to improve their shelf life. The first route is to improve the purity and stoichiometry of the precursor MAX phase to enhance the quality of the resulting MXene flakes by lowering the number of defects in the resulting MXene 2D flakes.29 The second approach is to increase the flake size and decrease the concentrations of the defects during the selective etching and delamination processes.53 The third method is to improve MXenes’ oxidation resistance by decorating their surface with organic/inorganic moieties.31,33,54 The presence of multiple species of ions in-between the MXene sheets can also influence the rate of oxidation.
Surface functionalization of Ti3C2Tx is important to maintain its chemical stability for long-term application in anticorrosion coatings.36,41 The attachment of organic functional groups was used to functionalize Ti3C2Tx to maintain its chemical stability.35 A strategy that may further improve the chemical stability of Ti3C2Tx is the covalent functionalization with polymer brushes.55 However, the grafting of polymer brushes on Ti3C2Tx for its application in anticorrosion is not explored yet. This can be an easier task in comparison to the assembly of MXene with other 2D materials, such as graphene or hexagonal boron nitride. The abundant functional groups on the surface of Ti3C2Tx can be functionalized with initiators to perform surface-initiated atom transfer radical polymerization. Furthermore, direct photografting, known as self-initiated photografting and photopolymerization (SIPGP), can also be used, which is a one-step polymerization process where no initiator attachment is needed. Polymer brush grafting methods via SIPGP have been utilized on other 2D materials such as graphene,56,57 graphitic carbon nitride,58 and hexagonal boron nitride.59 Unlike graphene,60 Ti3C2Tx-based coatings for anticorrosion are not widely explored and the research area of MXenes for anticorrosion coatings is at its initial stage. Ti3C2Tx is only one composition in the large compositional space of MXenes, which suggests the potential of this field due to the tailorable compositions and properties of MXenes.
4. Beyond Ti3C2Tx MXene
Research on the anticorrosive behavior of this large family of 2D layered carbides/nitrides and carbonitrides is very limited and is yet to expand beyond Ti3C2Tx MXene. MXenes consisting of transition metals with high electronegativities may be more effective for inhibiting the propagation of corrosion. The introduction of two different MXene compositions in aqueous or other solvent media together can improve their combined efficacy for inhibiting corrosion. For example, the use of different MXene types mixed together has shown improved electrochemical activity.61 Preparation of MXenes in nonaqueous, polar solvents can further eliminate the potential of oxidation due to hydrolysis. In addition, a more in-depth understanding of surface terminations' effect toward high impedance behavior and stability in saline environments must be developed, which leads to lower protection/inhibition of corrosion.
Since corrosion is a surface phenomenon, tailoring of surface functional groups is vital to develop coatings that are resistant to oxide growth, therefore requiring more oxidation-resistant species on the basal (outer) planes of MXenes. MXenes with two transition metals, known as double transition metal MXenes, as either random solid solutions or in-plane ordered or out-of-plane ordered MXenes62−64 can potentially inhibit corrosion via the contribution from the transition metals order/disorder, as well as via providing better control on their charge transport properties.65
Extending the concept of multiple M elements in MXenes, the newest addition of entropy-stabilized MAX phases and the isolation of high-entropy MXenes with multiple principal metals have further expanded the scope of MXenes for exploration toward anticorrosion materials.66−69 These systems, where the metal elements have random occupancies, exhibit a high rate of disorder with diverse electronegativities. This can potentially contribute to lower rates of oxidation and extend the service life of coatings due to the availability of nonhomogeneous active sites for corrosion initiation. The diversity of MXenes in terms of constituent elements, layer thicknesses with two to five layers of transition metals, and different surface terminations70 provide a platform to further expand the available tools in corrosion science and engineering.
Acknowledgments
I.A. and N.R.S. thank the Dutch Research Council (NWO) for the LIFT Grant (731.017.413). S.K.N. and B.A. acknowledge the support from Indiana University Research Support Funds Grant (RSFG) and the National Science Foundation (NSF) grant Future Manufacturing 2134607.
The authors declare no competing financial interest.
References
- Naguib M.; Kurtoglu M.; Presser V.; Lu J.; Niu J.; Heon M.; Hultman L.; Gogotsi Y.; Barsoum M. W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. 10.1002/adma.201102306. [DOI] [PubMed] [Google Scholar]
- Slot T. K.; Riley N.; Shiju N. R.; Medlin J. W.; Rothenberg G. An Experimental Approach for Controlling Confinement Effects at Catalyst Interfaces. Chem. Sci. 2020, 11, 11024–11029. 10.1039/D0SC04118A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.; Wang N.; Legut D.; Si C.; Zhang Q.; Du S.; Germann T. C.; Francisco J. S.; Zhang R. Rational Design of Flexible Two-Dimensional MXenes with Multiple Functionalities. Chem. Rev. 2019, 119, 11980–12031. 10.1021/acs.chemrev.9b00348. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Kong N.; Uzun S.; Levitt A.; Seyedin S.; Lynch P. A.; Qin S.; Han M.; Yang W.; Liu J.; Wang X.; Gogotsi Y.; Razal J. M. Scalable Manufacturing of Free-Standing, Strong Ti3C2Tx MXene Films with Outstanding Conductivity. Adv. Mater. 2020, 32, 2001093 10.1002/adma.202070393. [DOI] [PubMed] [Google Scholar]
- Lipatov A.; Lu H.; Alhabeb M.; Anasori B.; Gruverman A.; Gogotsi Y.; Sinitskii A. Elastic Properties of 2D Ti3C2Tx MXene Monolayers and Bilayers. Sci. Adv. 2018, 4, eaat0491 10.1126/sciadv.aat0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayesteh Zeraati A.; Mirkhani S. A.; Sun P.; Naguib M.; Braun P. V.; Sundararaj U. Improved Synthesis of Ti3C2Tx MXenes Resulting in Exceptional Electrical Conductivity, High Synthesis Yield, and Enhanced Capacitance. Nanoscale 2021, 13, 3572–3580. 10.1039/D0NR06671K. [DOI] [PubMed] [Google Scholar]
- Barsoum M. W. The MN+1AXN phases: A New Class of Solids: Thermodynamically Stable Nanolaminates. Prog. Solid State Chem. 2000, 28, 201–281. 10.1016/S0079-6786(00)00006-6. [DOI] [Google Scholar]
- Tzenov N. V.; Barsoum M. W. Synthesis and Characterization of Ti3AlC2. J. Am. Ceram. Soc. 2004, 83, 825–832. 10.1111/j.1151-2916.2000.tb01281.x. [DOI] [Google Scholar]
- Slot T. K.; Natu V.; Ramos-Fernandez E. V.; Sepúlveda-Escribano A.; Barsoum M.; Rothenberg G.; Shiju N. R. Enhancing Catalytic Epoxide Ring-Opening Selectivity Using Surface-Modified Ti3C2Tx MXenes. 2D Mater. 2021, 8, 035003 10.1088/2053-1583/abe951. [DOI] [Google Scholar]
- Slot T. K.; Yue F.; Xu H.; Ramos-Fernandez E. V.; Sepúlveda-Escribano A.; Sofer Z.; Rothenberg G.; Shiju N. R. Surface Oxidation of Ti3C2Tx Enhances the Catalytic Activity of Supported Platinum Nanoparticles in Ammonia Borane Hydrolysis. 2D Mater. 2021, 8, 015001 10.1088/2053-1583/ababef. [DOI] [Google Scholar]
- Han M.; Shuck C. E.; Rakhmanov R.; Parchment D.; Anasori B.; Koo C. M.; Friedman G.; Gogotsi Y. Beyond Ti3C2Tx: MXenes for Electromagnetic Interference Shielding. ACS Nano 2020, 14, 5008–5016. 10.1021/acsnano.0c01312. [DOI] [PubMed] [Google Scholar]
- Xu X. D.; Zhang Y. L.; Sun H. Y.; Zhou J. W.; Yang F.; Li H.; Chen H.; Chen Y. C.; Liu Z.; Qiu Z. P.; Wang D.; Ma L. P.; Wang J. W.; Zeng Q. G.; Peng Z. Q. Progress and Perspective: MXene and MXene-Based Nanomaterials for High-Performance Energy Storage Devices. Adv. Electron. Mater. 2021, 7, 2000967 10.1002/aelm.202000967. [DOI] [Google Scholar]
- Huang W.; Hu L.; Tang Y.; Xie Z.; Zhang H. Recent Advances in Functional 2D MXene-Based Nanostructures for Next-Generation Devices. Adv. Funct. Mater. 2020, 30, 2005223 10.1002/adfm.202005223. [DOI] [Google Scholar]
- Buczek S.; Barsoum M. L.; Uzun S.; Kurra N.; Andris R.; Pomerantseva E.; Mahmoud K. A.; Gogotsi Y. Rational Design of Titanium Carbide MXene Electrode Architectures for Hybrid Capacitive Deionization. Energy Environ. Mater. 2020, 3, 398–404. 10.1002/eem2.12110. [DOI] [Google Scholar]
- Wang C.-H.; Kurra N.; Alhabeb M.; Chang J.-K.; Alshareef H. N.; Gogotsi Y. Titanium Carbide (MXene) as a Current Collector for Lithium-Ion Batteries. ACS Omega 2018, 3, 12489–12494. 10.1021/acsomega.8b02032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M.; Barsoum M. W. MXene Polymer Nanocomposites: a Review. Mater. Today Adv. 2021, 9, 100120 10.1016/j.mtadv.2020.100120. [DOI] [Google Scholar]
- Riazi H.; Nemani S. K.; Grady M. C.; Anasori B.; Soroush M. Ti3C2 MXene–Polymer Nanocomposites and their Applications. J. Mater. Chem. A 2021, 9, 8051–8098. 10.1039/D0TA08023C. [DOI] [Google Scholar]
- Yan H.; Li W.; Li H.; Fan X.; Zhu M. Ti3C2 MXene Nanosheets Toward High-Performance Corrosion Inhibitor for Epoxy Coating. Prog. Org. Coat. 2019, 135, 156–167. 10.1016/j.porgcoat.2019.06.013. [DOI] [Google Scholar]
- Yan H.; Cai M.; Wang J.; Zhang L.; Li H.; Li W.; Fan X.; Zhu M. Insight into Anticorrosion/Antiwear Behavior of Inorganic-Organic Multilayer Protection System Composed of Nitriding Layer and Epoxy Coating with Ti3C2Tx MXene. Appl. Surf. Sci. 2021, 536, 147974 10.1016/j.apsusc.2020.147974. [DOI] [Google Scholar]
- Sheng X.; Li S.; Huang H.; Zhao Y.; Chen Y.; Zhang L.; Xie D. Anticorrosive and UV-Blocking Waterborne Polyurethane Composite Coating Containing Novel Two-Dimensional Ti3C2 MXene Nanosheets. J. Mater. Sci. 2021, 56, 4212–4224. 10.1007/s10853-020-05525-2. [DOI] [Google Scholar]
- Zhang F.; Liu W.; Liang L.; Wang S.; Shi H.; Xie Y.; Yang M.; Pi K. The Effect of Functional Graphene Oxide Nanoparticles on Corrosion Resistance of Waterborne Polyurethane. Colloids Surf., A 2020, 591, 124565 10.1016/j.colsurfa.2020.124565. [DOI] [Google Scholar]
- Iqbal A.; Hong J.; Ko T. Y.; Koo C. M. Improving Oxidation Stability of 2D MXenes: Synthesis, Storage Media, and Conditions. Nano Convergence 2021, 8, 9 10.1186/s40580-021-00259-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib T.; Zhao X.; Shah S. A.; Chen Y.; Sun W.; An H.; Lutkenhaus J. L.; Radovic M.; Green M. J. Oxidation Stability of Ti3C2Tx MXene Nanosheets in Solvents and Composite Films. npj 2D Mater. Appl. 2019, 3, 8 10.1038/s41699-019-0089-3. [DOI] [Google Scholar]
- Zhang C. J.; Pinilla S.; McEvoy N.; Cullen C. P.; Anasori B.; Long E.; Park S.-H.; Seral-Ascaso A.; Shmeliov A.; Krishnan D.; Morant C.; Liu X.; Duesberg G. S.; Gogotsi Y.; Nicolosi V. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. 10.1021/acs.chemmater.7b00745. [DOI] [Google Scholar]
- Cao F.; Zhang Y.; Wang H.; Khan K.; Tareen A. K.; Qian W.; Zhang H.; Ågren H. Recent Advances in Oxidation Stable Chemistry of 2D MXenes. Adv. Mater. 2022, 34, 2107554 10.1002/adma.202107554. [DOI] [PubMed] [Google Scholar]
- Huang S.; Mochalin V. N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. 10.1021/acs.inorgchem.8b02890. [DOI] [PubMed] [Google Scholar]
- Zhang J.; Kong N.; Hegh D.; Usman K. A. S.; Guan G.; Qin S.; Jurewicz I.; Yang W.; Razal J. M. Freezing Titanium Carbide Aqueous Dispersions for Ultra-long-term Storage. ACS Appl. Mater. Interfaces 2020, 12, 34032–34040. 10.1021/acsami.0c06728. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Kim S. J.; Kim Y.-J.; Lim Y.; Chae Y.; Lee B.-J.; Kim Y.-T.; Han H.; Gogotsi Y.; Ahn C. W. Oxidation-Resistant Titanium Carbide MXene Films. J. Mater. Chem. A 2020, 8, 573–581. 10.1039/C9TA07036B. [DOI] [Google Scholar]
- Mathis T. S.; Maleski K.; Goad A.; Sarycheva A.; Anayee M.; Foucher A. C.; Hantanasirisakul K.; Shuck C. E.; Stach E. A.; Gogotsi Y. Modified MAX Phase Synthesis for Environmentally Stable and Highly Conductive Ti3C2 MXene. ACS Nano 2021, 15, 6420–6429. 10.1021/acsnano.0c08357. [DOI] [PubMed] [Google Scholar]
- Wang X.; Wang Z.; Qiu J. Stabilizing MXene by Hydration Chemistry in Aqueous Solution. Angew. Chem., Int. Ed. 2021, 60, 26587–26591. 10.1002/anie.202113981. [DOI] [PubMed] [Google Scholar]
- VahidMohammadi A.; Mojtabavi M.; Caffrey N. M.; Wanunu M.; Beidaghi M. Assembling 2D MXenes into Highly Stable Pseudocapacitive Electrodes with High Power and Energy Densities. Adv. Mater. 2019, 31, 1806931 10.1002/adma.201806931. [DOI] [PubMed] [Google Scholar]
- Matthews K.; Zhang T.; Shuck C. E.; VahidMohammadi A.; Gogotsi Y. Guidelines for Synthesis and Processing of Chemically Stable Two-Dimensional V2CTx MXene. Chem. Mater. 2022, 34, 499–509. 10.1021/acs.chemmater.1c03508. [DOI] [Google Scholar]
- Natu V.; Hart J. L.; Sokol M.; Chiang H.; Taheri M. L.; Barsoum M. W. Edge Capping of 2D-MXene Sheets with Polyanionic Salts To Mitigate Oxidation in Aqueous Colloidal Suspensions. Angew. Chem., Int. Ed. 2019, 58, 12655–12660. 10.1002/anie.201906138. [DOI] [PubMed] [Google Scholar]
- Choi E.; Lee J.; Kim Y.-J.; Kim H.; Kim M.; Hong J.; Kang Y. C.; Koo C. M.; Kim D. W.; Kim S. J. Enhanced Stability of Ti3C2Tx MXene Enabled by Continuous ZIF-8 Coating. Carbon 2022, 191, 593–599. 10.1016/j.carbon.2022.02.036. [DOI] [Google Scholar]
- Ji J.; Zhao L.; Shen Y.; Liu S.; Zhang Y. Covalent Stabilization and Functionalization of MXene via Silylation Reactions with Improved Surface Properties. FlatChem 2019, 17, 100128 10.1016/j.flatc.2019.100128. [DOI] [Google Scholar]
- Yan H.; Cai M.; Li W.; Fan X.; Zhu M. Amino-functionalized Ti3C2Tx with Anti-Corrosive/Wear Function for Waterborne Epoxy Coating. J. Mater. Sci. Technol. 2020, 54, 144–159. 10.1016/j.jmst.2020.05.002. [DOI] [Google Scholar]
- Gao L.; Li C.; Huang W.; Mei S.; Lin H.; Ou Q.; Zhang Y.; Guo J.; Zhang F.; Xu S.; Zhang H. MXene/Polymer Membranes: Synthesis, Properties, and Emerging Applications. Chem. Mater. 2020, 32, 1703–1747. 10.1021/acs.chemmater.9b04408. [DOI] [Google Scholar]
- Jimmy J.; Kandasubramanian B. Mxene Functionalized Polymer Composites: Synthesis and Applications. Eur. Polym. J. 2020, 122, 109367 10.1016/j.eurpolymj.2019.109367. [DOI] [Google Scholar]
- Chen X.; Zhao Y.; Li L.; Wang Y.; Wang J.; Xiong J.; Du S.; Zhang P.; Shi X.; Yu J. MXene/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2021, 61, 80–115. 10.1080/15583724.2020.1729179. [DOI] [Google Scholar]
- Alhabeb M.; Maleski K.; Anasori B.; Lelyukh P.; Clark L.; Sin S.; Gogotsi Y. Guidelines for Synthesis and Processing of Two-Dimensional Titanium Carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. 10.1021/acs.chemmater.7b02847. [DOI] [Google Scholar]
- Zhang F.; Liu W.; Wang S.; Liu C.; Shi H.; Liang L.; Pi K. Surface Functionalization of Ti3C2Tx and its Application in Aqueous Polymer Nanocomposites for Reinforcing Corrosion Protection. Composites, Part B 2021, 217, 108900 10.1016/j.compositesb.2021.108900. [DOI] [Google Scholar]
- Wang H.; Qin S.; Yang X.; Fei G.; Tian M.; Shao Y.; Zhu K.; Waterborne A. Uniform Graphene-Poly(urethane-acrylate) Complex with Enhanced Anticorrosive Properties Enabled by Ionic Interaction. Chem. Eng. J. 2018, 351, 939–951. 10.1016/j.cej.2018.06.151. [DOI] [Google Scholar]
- Wen J.-G.; Geng W.; Geng H.-Z.; Zhao H.; Jing L.-C.; Yuan X.-T.; Tian Y.; Wang T.; Ning Y.-J.; Wu L. Improvement of Corrosion Resistance of Waterborne Polyurethane Coatings by Covalent and Noncovalent Grafted Graphene Oxide Nanosheets. ACS Omega 2019, 4, 20265–20274. 10.1021/acsomega.9b02687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrani M.; Sharif M.; Amirazodi K. Preparation and Characterization of Polythiophene/Graphene Oxide/Epoxy Nanocomposite Coatings with Advanced Properties. Polym. Bull. 2022, 79, 263–284. 10.1007/s00289-020-03529-1. [DOI] [Google Scholar]
- Contri G.; Zimmermann C. A.; Ramoa S. D. A. D. S.; Schmitz D. P.; Ecco L. G.; Barra G. M. O.; Fedel M. Polypyrrole Modified E-Coat Paint for Corrosion Protection of Aluminum AA1200. Front. Mater. 2020, 7, 1–9. 10.3389/fmats.2020.00045. [DOI] [Google Scholar]
- Lu F.; Liu C.; Chen Z.; Veerabagu U.; Chen Z.; Liu M.; Hu L.; Xia H.; Cha L.; Zhang W. Polypyrrole-Functionalized Boron Nitride Nanosheets for High-Performance Anti-Corrosion Composite Coating. Surf. Coat. Technol. 2021, 420, 127273 10.1016/j.surfcoat.2021.127273. [DOI] [Google Scholar]
- Li A.; Chen S.; Ma Z.; Sun M.; Zhu G.; Zhang Y.; Wang W. Corrosion Protection Properties of Polyvinyl Butyral/Polyaniline-Graphene Oxide/Poly(methylhydrosiloxane) Composite Coating for AA2024 aluminum alloy. Diamond Relat. Mater. 2021, 116, 108397 10.1016/j.diamond.2021.108397. [DOI] [Google Scholar]
- Kadri Y.; Srasra E.; Bekri-Abbes I.; Herrasti P. Facile and Eco-friendly Synthesis of Polyaniline/ZnO Composites for Corrosion Protection of AA-2024 Aluminium Alloy. J. Electroanal. Chem. 2021, 893, 115335 10.1016/j.jelechem.2021.115335. [DOI] [Google Scholar]
- Gao M.; Quan X.; Wang J.; Wang Z. Preparation and Characterization of Coatings Incorporated with Poly(aniline-co-nitroaniline) Nanoparticles Having Antifouling and Anticorrosion Behavior. Ind. Eng. Chem. Res. 2020, 59, 22173–22186. 10.1021/acs.iecr.0c05329. [DOI] [Google Scholar]
- Gao F.; Mu J.; Bi Z.; Wang S.; Li Z. Recent Advances of Polyaniline Composites in Anticorrosive Coatings: A Review. Prog. Org. Coat. 2021, 151, 106071 10.1016/j.porgcoat.2020.106071. [DOI] [Google Scholar]
- Cai M.; Yan H.; Li Y.; Li W.; Li H.; Fan X.; Zhu M. Ti3C2Tx/PANI Composites with Tunable Conductivity Towards Anticorrosion Application. Chem. Eng. J. 2021, 410, 128310 10.1016/j.cej.2020.128310. [DOI] [Google Scholar]
- Yan H.; Zhang L.; Li H.; Fan X.; Zhu M. Towards High-Performance Additive of Ti3C2/Graphene Hybrid with a Novel Wrapping Structure in Epoxy Coating. Carbon 2020, 157, 217–233. 10.1016/j.carbon.2019.10.034. [DOI] [Google Scholar]
- Maleski K.; Ren C. E.; Zhao M.-Q.; Anasori B.; Gogotsi Y. Size-Dependent Physical and Electrochemical Properties of Two-Dimensional MXene Flakes. ACS Appl. Mater. Interfaces 2018, 10, 24491–24498. 10.1021/acsami.8b04662. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Vashisth A.; Prehn E.; Sun W.; Shah S. A.; Habib T.; Chen Y.; Tan Z.; Lutkenhaus J. L.; Radovic M.; Green M. J. Antioxidants Unlock Shelf-Stable Ti3C2Tx (MXene) Nanosheet Dispersions. Matter 2019, 1, 513–526. 10.1016/j.matt.2019.05.020. [DOI] [Google Scholar]
- Lee J. T.; Wyatt B. C.; Davis G. A.; Masterson A. N.; Pagan A. L.; Shah A.; Anasori B.; Sardar R. Covalent Surface Modification of Ti3C2Tx MXene with Chemically Active Polymeric Ligands Producing Highly Conductive and Ordered Microstructure Films. ACS Nano 2021, 15, 19600–19612. 10.1021/acsnano.1c06670. [DOI] [PubMed] [Google Scholar]
- Steenackers M.; Gigler A. M.; Zhang N.; Deubel F.; Seifert M.; Hess L. H.; Lim C. H. Y. X.; Loh K. P.; Garrido J. A.; Jordan R.; Stutzmann M.; Sharp I. D. Polymer Brushes on Graphene. J. Am. Chem. Soc. 2011, 133, 10490–10498. 10.1021/ja201052q. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Rodriguez R. D.; Amin I.; Gasiorowski J.; Rahaman M.; Sheng W.; Kalbacova J.; Sheremet E.; Zahn D. R. T.; Jordan R. Bottom-Up Fabrication of Graphene-Based Conductive Polymer Carpets for Optoelectronics. J. Mater. Chem. C 2018, 6, 4919–4927. 10.1039/C8TC00554K. [DOI] [Google Scholar]
- Sheng W.; Li W.; Tan D.; Zhang P.; Zhang E.; Sheremet E.; Schmidt B. V. K. J.; Feng X.; Rodriguez R. D.; Jordan R.; Amin I. Polymer Brushes on Graphitic Carbon Nitride for Patterning and as a SERS Active Sensing Layer via Incorporated Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 9797–9805. 10.1021/acsami.9b21984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W.; Amin I.; Neumann C.; Dong R.; Zhang T.; Wegener E.; Chen W.-L.; Förster P.; Tran H. Q.; Löffler M.; Winter A.; Rodriguez R. D.; Zschech E.; Ober C. K.; Feng X.; Turchanin A.; Jordan R. Polymer Brushes on Hexagonal Boron Nitride. Small 2019, 15, 1805228 10.1002/smll.201805228. [DOI] [PubMed] [Google Scholar]
- Amin I.; Batyrev E.; de Vooys A.; van der Weijde H.; Shiju N. R. Covalent Polymer Functionalization of Graphene/Graphene Oxide and its Application as Anticorrosion Materials. 2D Mater. 2022, 9, 032002 10.1088/2053-1583/ac54ee. [DOI] [Google Scholar]
- VahidMohammadi A.; Liang W.; Mojtabavi M.; Wanunu M.; Beidaghi M. 2D Titanium and Vanadium Carbide MXene Heterostructures for Electrochemical Energy Storage. Energy Storage Mater. 2021, 41, 554–562. 10.1016/j.ensm.2021.06.014. [DOI] [Google Scholar]
- Anasori B.; Xie Y.; Beidaghi M.; Lu J.; Hosler B. C.; Hultman L.; Kent P. R. C.; Gogotsi Y.; Barsoum M. W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. 10.1021/acsnano.5b03591. [DOI] [PubMed] [Google Scholar]
- Hong W.; Wyatt B. C.; Nemani S. K.; Anasori B. Double Transition-Metal MXenes: Atomistic Design of Two-Dimensional Carbides and Nitrides. MRS Bull. 2020, 45, 850–861. 10.1557/mrs.2020.251. [DOI] [Google Scholar]
- Rosen J.; Dahlqvist M.; Tao Q.; Hultman L.. In- and Out-of-Plane Ordered MAX Phases and Their MXene Derivatives. In 2D Metal Carbides and Nitrides (MXenes): Structure, Properties and Applications, Anasori B.; Gogotsi Y., Eds. Springer: Cham, 2019; Vol. 1, pp 37–52. [Google Scholar]
- Anasori B.; Shi C.; Moon E. J.; Xie Y.; Voigt C. A.; Kent P. R. C.; May S. J.; Billinge S. J. L.; Barsoum M. W.; Gogotsi Y. Control of Electronic Properties of 2D Carbides (MXenes) by Manipulating their Transition Metal Layers. Nanoscale Horiz. 2016, 1, 227–234. 10.1039/C5NH00125K. [DOI] [PubMed] [Google Scholar]
- Nemani S. K.; Zhang B.; Wyatt B. C.; Hood Z. D.; Manna S.; Khaledialidusti R.; Hong W.; Sternberg M. G.; Sankaranarayanan S. K. R. S.; Anasori B. High-Entropy 2D Carbide MXenes: TiVNbMoC3 and TiVCrMoC3. ACS Nano 2021, 15, 12815–12825. 10.1021/acsnano.1c02775. [DOI] [PubMed] [Google Scholar]
- Du Z.; Wu C.; Chen Y.; Cao Z.; Hu R.; Zhang Y.; Gu J.; Cui Y.; Chen H.; Shi Y.; Shang J.; Li B.; Yang S. High-Entropy Atomic Layers of Transition-Metal Carbides (MXenes). Adv. Mater. 2021, 33, 2101473 10.1002/adma.202101473. [DOI] [PubMed] [Google Scholar]
- Du Z.; Wu C.; Chen Y.; Zhu Q.; Cui Y.; Wang H.; Zhang Y.; Chen X.; Shang J.; Li B.; Chen W.; Liu C.; Yang S.; High-Entropy Carbonitride M. A. X. Phases and Their Derivative MXenes. Adv. Energy Mater. 2022, 12, 2103228 10.1002/aenm.202103228. [DOI] [Google Scholar]
- Etman A. S.; Zhou J.; Rosen J. Ti1.1V0.7CrxNb1.0Ta0.6C3Tz high-entropy MXene freestanding films for charge storage applications. Electrochem. Commun. 2022, 137, 107264 10.1016/j.elecom.2022.107264. [DOI] [Google Scholar]
- VahidMohammadi A.; Rosen J.; Gogotsi Y. The world of Two-Dimensional Carbides and Nitrides (MXenes). Science 2021, 372, eabf1581 10.1126/science.abf1581. [DOI] [PubMed] [Google Scholar]