Abstract
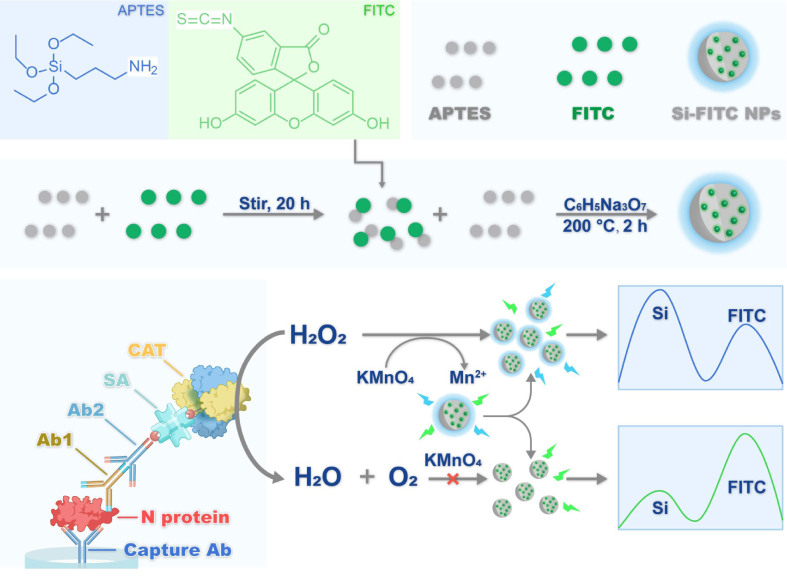
Coronavirus disease 2019 (COVID-19) highlights the importance of rapid and reliable diagnostic assays for the management of virus transmission. Here, we developed a one-pot hydrothermal method to prepare Si-FITC nanoparticles (NPs) for the fluorescent immunoassay of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein (N protein). The synthesis of Si-FITC NPs did not need post-modification, which addressed the issue of quantum yield reduction during the coupling reaction. Si-FITC NPs showed two distinct peaks, Si fluorescence at λem = 385 nm and FITC fluorescence at λem = 490 nm. In the presence of KMnO4, Si fluorescence was decreased and FITC fluorescence was enhanced. Briefly, in the presence of N protein, catalase (CAT)-linked secondary antibody/reporter antibody/N protein/capture antibody immunocomplexes were formed on microplates. Subsequently, hydrogen peroxide (H2O2) and Si-FITC NPs/KMnO4 were injected into the microplate together. The decomposition of H2O2 by CAT resulted in remaining of KMnO4, which changed the fluorescence intensity ratio of Si-FITC NPs. The fluorescence intensity ratio correlated significantly with the N protein concentration ranging from 0.02 to 50.00 ng/mL, and the detection limit was 0.003 ng/mL, which was more sensitive than the commercial ELISA kit with a detection limit of 0.057 ng/mL. The N protein concentration can be accurately determined in human serum. Furthermore, the COVID-19 and non-COVID-19 patients were distinguishable by this method. Therefore, the ratiometric fluorescent immunoassay can be used for SARS-CoV-2 infection diagnosis with a high sensitivity and selectivity.
Electronic Supplementary Material
Supplementary material (characterization of Si-FITC NPs (FTIR, HRXPS); stability investigation of Si-FITC NPs (photostability, pH stability, anti-interference ability); stability investigation of free FITC (pH value, KMnO4); quenching mechanism of KMnO4 (UV-vis absorption spectra, fluorescence lifetime decay curves); reaction condition optimization of biotin-CAT with H2O2 (pH value, temperature, time); detection of N protein using commercial ELISA Kit; selectivity investigation of assays for SARS-CoV-2 N protein detection; determination results of SARS-CoV-2 N protein in human serum) is available in the online version of this article at 10.1007/s12274-022-5005-z.
Keywords: Si-FITC nanoparticles, ratiometric fluorescent probe, SARS-CoV-2, ELISA
Electronic Supplementary Material
Ratiometric fluorescent Si-FITC nanoprobe for immunoassay of SARS-CoV-2 nucleocapsid protein
Acknowledgements
This work was supported by National Key Research and Development Program of China (No. 2021YFA0910900), the Sino-German rapid response funding call for COVID-19 related research (No. C-0008), the National Natural Science Foundation of China (Nos. 32222044, 22104147), Shenzhen Municipal Science and Technology Innovation Council (No. RCYX20210609103823046), Youth Innovation Promotion Association CAS (No. 2021359), Natural Science Foundation of Guangdong (Nos. 2018B030306046, 2020A1515111130), Guangdong Provincial Key Laboratory of Synthetic Genomics (No. 2019B030301006), Shenzhen Science and Technology Program (No. KQTD20180413181837372), and Shenzhen Outstanding Talents Training Fund.
Ethics declarations
The study protocol was approved by the Ethics Review Committee of Shenzhen Third People’s Hospital (No. 2020-010). Written informed consent was obtained from all patients. The study was conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice, the Declaration of Helsinki and institutional ethics guidelines.
Footnotes
Guobin Mao and Silu Ye contributed equally to this work.
Contributor Information
Zhike He, Email: zhkhe@whu.edu.cn.
Yingxin Ma, Email: yx.ma1@siat.ac.cn.
References
- [1].Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al. A novel coronavirus from patients with pneumonia in China, 2019. New. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou P, Yang X L, Wang X G, Hu B, Zhang L, Zhang W, Si H R, Zhu Y, Li B, Huang C L, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rosenthal P J. The importance of diagnostic testing during a viral pandemic: early lessons from novel coronavirus disease (COVID-19) Am. J. Trop. Med. Hyg. 2020;102:915–916. doi: 10.4269/ajtmh.20-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang Y, Yang M, Yuan J, Wang F, Wang Z, Li J, Zhang M, Xing L, Wei J, Peng L, et al. Laboratory diagnosis and monitoring the viral shedding of SARS-CoV-2 infection. Innovation (N Y) 2020;1:100061. doi: 10.1016/j.xinn.2020.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiersinga W J, Rhodes A, Cheng A C, Peacock S J, Prescott H C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- [6].Vogels C B F, Brito A F, Wyllie A L, Fauver J R, Ott I M, Kalinich C C, Petrone M E, Casanovas-Massana A, Muenker M C, Moore A J, et al. Analytical sensitivity and efficiency comparisons of SARS-CoV-2 RT-qPCR primer-probe sets. Nat. Microbiol. 2020;5:1299–1305. doi: 10.1038/s41564-020-0761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang Y, Kang H, Liu X, Tong Z. Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak. J. Med. Virol. 2020;92:538–539. doi: 10.1002/jmv.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller S, Chiu C, Rodino K G, Miller M B. Point-counterpoint: should we be performing metagenomic next-generation sequencing for infectious disease diagnosis in the clinical laboratory? J. Clin. Microbiol. 2020;58:e01739–19. doi: 10.1128/JCM.01739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Augustine R, Hasan A, Das S, Ahmed R, Mori Y, Notomi T, Kevadiya B D, Thakor A S. Loop-mediated isothermal amplification (LAMP): A rapid, sensitive, specific, and cost-effective point-of-care test for coronaviruses in the context of COVID-19 pandemic. Biology (Basel). 2020;9:182. doi: 10.3390/biology9080182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lalli M A, Langmade S J, Chen X, Fronick C C, Sawyer C S, Burcea L C, Wilkinson M N, Fulton R S, Heinz M, Buchser W J, et al. Rapid and extraction-free detection of SARS-CoV-2 from saliva by colorimetric reverse-transcription loopmediated isothermal amplification. Clin. Chem. 2021;67:415–424. doi: 10.1093/clinchem/hvaa267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fozouni P, Son S, Derby M D D L, Knott G J, Gray C N, D’Ambrosio M V, Zhao C, Switz N A, Kumar G R, Stephens S I, et al. Amplification-free detection of SARS-CoV-2 with CRISPR-Cas13a and mobile phone microscopy. Cell. 2021;184:323–333.e9. doi: 10.1016/j.cell.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Broughton J P, Deng X, Yu G, Fasching C L, Servellita V, Singh J, Miao X, Streithorst J A, Granados A, Sotomayor-Gonzalez A. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. biotechnol. 2020;38:870–874. doi: 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ding X, Yin K, Li Z, Lalla R V, Ballesteros E, Sfeir M M, Liu C. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020;11:4711. doi: 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shen M, Zhou Y, Ye J, Al-Maskri A A A, Kang Y, Zeng S, Cai S. Recent advances and perspectives of nucleic acid detection for coronavirus. J. Pharm. Anal. 2020;10:97–101. doi: 10.1016/j.jpha.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen M, Cui D, Zhao Z, Kang D, Li Z, Albawardi S, Alsageer S, Alamri F, Alhazmi A, Amer M R, et al. Highly sensitive, scalable, and rapid SARS-CoV-2 biosensor based on In2O3 nanoribbon transistors and phosphatase. Nano. Res. 2022;15:5510–5516. doi: 10.1007/s12274-022-4190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hu R, Liao T, Ren Y, Liu W, Ma R, Wang X, Lin Q, Wang G, Liang Y. Sensitively detecting antigen of SARS-CoV-2 by NIR-II fluorescent nanoparticles. Nano Res. 2022;15:7313–7319. doi: 10.1007/s12274-022-4351-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Deeks J J, Dinnes J, Takwoingil Y, Davenport C, Spijker R, Taylor-Phillips S, Adrianol A, Beesel S, Dretzkel J, di Ruffanol L F, et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Db. Syst. Rev. 2020;6:CD013652. doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, Wang Q, Tan L, Wu W, Tang S, et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020;58:e00461–20. doi: 10.1128/JCM.00461-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Scohy A, Anantharajah A, Bodeus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020;129:104455. doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dinnes J, Deeks J J, Adriano A, Berhane S, Davenport C, Dittrich S, Emperador D, Takwoingi Y, Cunningham J, Beese S, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection (Review) Cochrane Db. Syst. Rev. 2020;8:CD013705. doi: 10.1002/14651858.CD013705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Reen D J. Enzyme-linked immunosorbent assay (ELISA) Methods. Mol. Bioll. 1994;32:461–166. doi: 10.1385/0-89603-268-X:461. [DOI] [PubMed] [Google Scholar]
- [22].Zhang D, Li W, Ma Z, Han H. Improved ELISA for tumor marker detection using electro-readout-mode based on label triggered degradation of methylene blue. Biosens. Bioelectron. 2019;126:800–805. doi: 10.1016/j.bios.2018.11.038. [DOI] [PubMed] [Google Scholar]
- [23].Wu L, Li G, Xu X, Zhu L, Huang R, Chen X. Application of nano-ELISA in food analysis: Recent advances and challenges. Trac-Trend Anal. Chem. 2019;113:140–156. doi: 10.1016/j.trac.2019.02.002. [DOI] [Google Scholar]
- [24].de la Rica R, Stevens M M. Plasmonic ELISA for the ultrasensitive detection of disease biomarkers with the naked eye. Nat. Nanotechnol. 2012;7:821–824. doi: 10.1038/nnano.2012.186. [DOI] [PubMed] [Google Scholar]
- [25].Luo Q, Wang H, Yin X, Wang L. Hydrophilic perovskite microdisks with excellent stability and strong fluorescence for recyclable temperature sensing. Sci. China. Mater. 2019;62:1065–1070. doi: 10.1007/s40843-018-9396-9. [DOI] [Google Scholar]
- [26].Liang Y, Huang X, Yu R, Zhou Y, Xiong Y. Fluorescence ELISA for sensitive detection of ochratoxin A based on glucose oxidase-mediated fluorescence quenching of CdTe QDs. Anal. Chim. Acta. 2016;936:195–201. doi: 10.1016/j.aca.2016.06.018. [DOI] [PubMed] [Google Scholar]
- [27].Chu B, Song B, Ji X, Su Y, Wang H, He Y. Fluorescent silicon nanorods-based ratiometric sensors for long term and realtime measurements of intracellular pH in live cells. Anal. Chem. 2017;89:12152–12159. doi: 10.1021/acs.analchem.7b02791. [DOI] [PubMed] [Google Scholar]
- [28].Zhai X, Song B, Chu B, Su Y, Wang H, He Y. Highly fluorescent, photostable, and biocompatible silicon theranostic nanoprobes against Staphylococcus aureus infections. Nano Res. 2018;11:6417–6427. doi: 10.1007/s12274-018-2166-x. [DOI] [Google Scholar]
- [29].Guo D, Ji X, Peng F, Zhong Y, Chu B, Su Y, He Y. Photostable and biocompatible fluorescent silicon nanoparticles for imaging-guided co-delivery of siRNA and doxorubicin to drug-resistant cancer cells. Nano-micro. Lett. 2019;11:27. doi: 10.1007/s40820-019-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun J, Hu T, Chen C, Zhao D, Yang F, Yang X. Fluorescence immunoassay system via enzyme-enabled in situ synthesis of fluorescent silicon nanoparticles. Anal. Chem. 2016;88:9789–9795. doi: 10.1021/acs.analchem.6b02847. [DOI] [PubMed] [Google Scholar]
- [31].Chen C, Zhao D, Wang B, Ni P, Jiang Y, Zhang C, Yang F, Lu Y, Sun J. Alkaline phosphatase-triggered in situ formation of silicon-containing nanoparticles for a fluorometric and colorimetric dual-channel immunoassay. Anal. Chem. 2020;92:4639–4646. doi: 10.1021/acs.analchem.0c00224. [DOI] [PubMed] [Google Scholar]
- [32].Wang J, Liu X, Huang L, Jin J, Jiang C, Li D, Wen H, Hu J. Controllable and robust dual-emissive quantum dot nanohybrids as inner filter-based ratiometric probes for visualizable melamine detection. Nanoscale. 2020;12:4562–4572. doi: 10.1039/C9NR08849K. [DOI] [PubMed] [Google Scholar]
- [33].Zhang J, Qian J, Mei Q, Yang L, He L, Liu S, Zhang C, Zhang K. Imaging-based fluorescent sensing platform for quantitative monitoring and visualizing of fluoride ions with dualemission quantum dots hybrid. Biosens. Bioelectron. 2019;128:61–67. doi: 10.1016/j.bios.2018.12.044. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Guo S, Jiang Z, Mao G, Ji X, He Z. Rox-DNA functionalized silicon nanodots for ratiometric detection of mercury ions in live cells. Anal. Chem. 2018;90:9796–9804. doi: 10.1021/acs.analchem.8b01574. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ratiometric fluorescent Si-FITC nanoprobe for immunoassay of SARS-CoV-2 nucleocapsid protein