Janus nanoarchitectures, an emerging class of nanostructures, named after the Roman god with two faces, are a fascinating class of nanomaterials with promising applications in various areas, including catalysis, optical imaging, and so on. Although matter with structural and chemical homogeneity tends to display high stability owing to the low entropy, its heterogeneous counterpart is also attractive because of the high reactivity arising therefrom. To date, several bimetallic Janus nanocrystals have been fabricated; however, the atomic-level investigation of their structure–property correlations remains highly challenging due to two intrinsic characteristics: ununiform sizes and imprecise surface chemistry. In this issue of ACS Central Science, Shuang-Quan Zang and co-workers have structurally resolved Janus nanoarchitectures at the atomic level and mapped out their interfacial linkages and the synergistic effect.1
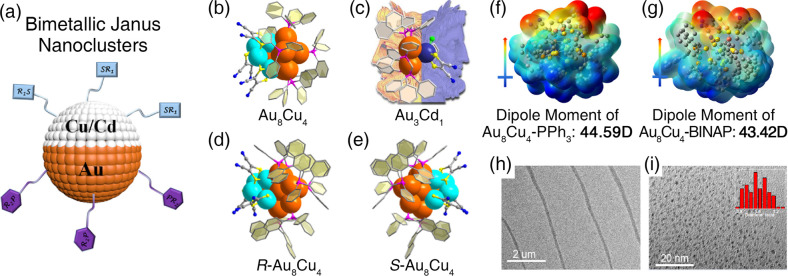
In this work, four Janus nanoclusters, including racemate Au8Cu4, R/S-Au8Cu4 enantiomers, and racemate Au3Cd1, costabilized by thiol and phosphine ligands, were controllably synthesized and structurally determined, serving as research templates to resolve fundamental issues of asymmetric bimetallic Janus nanocrystals. Structurally, these four alloy nanoclusters perfectly reflected the two-face characterization of Janus architectures (Figure 1a–e): the gold sides are stabilized by phosphine ligands (i.e., PPh3, BINAP, and DPPM), whereas the transition metal copper/cadmium sides are anchored by thiol ligands (i.e., MNT). In addition, the six achiral PPh3 ligands in racemate Au8Cu4 could be substituted by diphosphine BINAP ligands, and the postmodified synthesized R/S-Au8Cu4 nanocluster enantiomers exhibited obvious chiroptical properties. By analyzing the density functional theory calculation results of the Au8Cu4 nanocluster, the authors demonstrated that the Au–Cu interaction may play a critical role in forming the Janus nanocluster, and the lattice adaptability from the protecting thiol/phosphine ligands induced asymmetric growth. In addition, the dipolar distribution of Au/Cu bicomponents in Au8Cu4 led to a maximum dipole moment up to 45 D (Figure 1f,g), which further drove the self-assembly of Au8Cu4 nanocluster molecules into one-dimensional nanowires (Figure 1h,i).
Figure 1.
(a) Scheme illustration of the bimetallic Janus structures of the obtained nanoclusters. (b, c) Structures of the racemate Au8Cu4 and Au3Cd1 nanoclusters. (d, e) Structures of R/S-Au8Cu4 enantiomers. Color legends: orange sphere, Au; turquoise sphere, Cu; indigo sphere, Cd; pink sphere, P; yellow sphere, S; blue sphere, N; green sphere, Cl; gray sphere, C. Hydrogen atoms are omitted for clarity. (f, g) Directions and values of the dipole moment of Au8Cu4 nanoclusters. (h, i) TEM images of the one-dimensional nanowires made from Au8Cu4 nanocluster molecules. Reproduced with permission from ref (1). Copyright 2022 The Authors. Published by American Chemical Society.
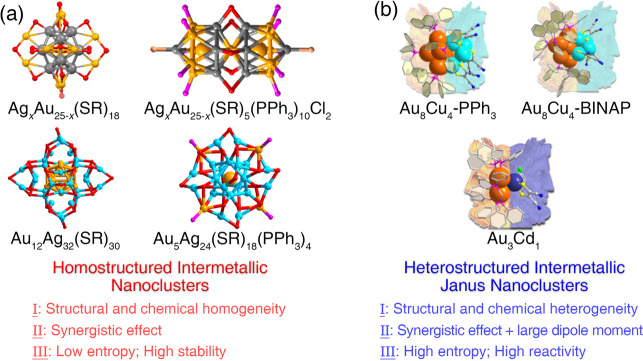
Through the continuous accumulation of synthetic experience and advances in analytical methods, metal nanoclusters can now easily be tailored to desired composition and morphology. The concept of alloying has been extensively exploited in dictating the geometric/electronic structures and customizing the chemical/physical properties of metal nanoclusters.2 In most alloying cases, the active alloying sites tend to have more equal distributions in metallic skeletons of metal nanoclusters (Figure 2a). For example, the incorporated Ag heteroatoms prefer to occupy the icosahedral kernel surface with a uniform pattern in AgxAu25–x(SR)18 nanoclusters;3 the architecture of the rodlike AgxAu25–x(SR)5(PPh3)10Cl2 nanocluster perfectly follows a symmetrical alloying mode along the central plane;4 the alloyed Au12Ag32(SR)30 nanocluster follows a Au12 core@Ag32 shell architecture of the structural aesthetic;5 the five Au heteroatoms in the tetrametallic Au5Ag24(SR)18(PPh3)4 nanocluster are evenly arranged into the innermost kernel or onto the outermost vertexes of the cluster skeleton.6 The equal distribution of heterometals in these commonly researched alloy nanoclusters is reasonable because the structural and chemical homogeneity should reduce the entropy value of a cluster system and produce an alloy nanocluster with high stability. Such a tendency is in line with the expressions of “life feeds on negative entropy” and “survival of the fittest”.7,8
Figure 2.
(a) Structural and chemical homogeneity in several alloy nanoclusters with high stability, such as AgxAu25–x(SR)18, AgxAu25–x(SR)5(PPh3)10Cl2, Au12Ag32(SR)30, and Au5Ag24(SR)18(PPh3)4 nanoclusters. (b) Structural and chemical heterogeneity in several Janus nanoclusters with high reactivity, such as Au8Cu4 and Au3Cd1 nanoclusters. Reproduced with permission from ref (1). Copyright 2022 The Authors. Published by American Chemical Society.
Janus nanoclusters, on the other hand, exhibit structural and chemical heterogeneity. The asymmetrical structures endow these Janus nanoclusters with distinctively chemical–physical properties owing to the emerging large dipole moments, in addition to the synergistic effect between different metal components. In addition, the high reactivity of Janus nanomaterials originating from their dynamically unfavorable characterization makes them well-suited as optical devices or nanocatalysts.9 In this work, the Janus Au8Cu4 nanoclusters displayed high surface energy and were self-assembled into cluster-based nanowires with the driving force of their inherent bipolar phase and intercluster dipole interactions. In addition, the photocurrent response properties of such Janus nanoclusters were excellent, manifesting good photogenerated electron/hole pair generation and separation efficiencies.1
However, the bottom-up synthesis of Janus nanoclusters remains highly challenging since they are dynamically unfavorable and require a delicate interplay balance between entropy and enthalpy.10 Shuang-Quan Zang and co-workers prepared such Janus nanoclusters by using mixed ligands with different metallic affinities and electronegativities of the substituents.1 We envision that such a controllable method (i.e., mixed ligands stabilizing noble/transition metals) will allow for the efficient fabrication of more atomically precise Janus nanostructures in the near future.
In summary, the four reported Au–Cu or Au–Cd Janus nanoclusters reported by Shuang-Quan Zang and co-workers resolved several fundamental issues at the atomic level, including the interfacial linkages and the synergistic effects in Janus nanoarchitectures. The new findings provide atomic-level clues for understanding the heterogeneous architectures of Janus nanomaterials. In addition, such new findings will hopefully pave the way for the future fabrication of heterostructured intermetallic Janus systems for several downstream applications in optics and catalysis.
References
- Li Y.; Zang Q.-X.; Dong X.-Y.; Wang Z.-Y.; Luo P.; Luo X.-M.; Zang S.-Q.. Atomically Precise Enantiopure Bimetallic Janus clusters. ACS Cent. Sci. 2022, in press. 10.1021/acscentsci.2c00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X.; Li Y.; Zhu M.; Jin R. Atomically Precise Alloy Nanoclusters: Syntheses, Structures, and Properties. Chem. Soc. Rev. 2020, 49, 6443–6514. 10.1039/C9CS00633H. [DOI] [PubMed] [Google Scholar]
- Kumara C.; Aikens C. M.; Dass A. X-ray Crystal Structure and Theoretical Analysis of Au25-xAgx(SCH2CH2Ph)18– Alloy. J. Phys. Chem. Lett. 2014, 5, 461–466. 10.1021/jz402441d. [DOI] [PubMed] [Google Scholar]
- Wang S.; Meng X.; Das A.; Li T.; Song Y.; Cao T.; Zhu X.; Zhu M.; Jin R. A 200-Fold Quantum Yield Boost in the Photoluminescence of Silver-Doped AgxAu25-x Nanoclusters: The 13th Silver Atom Matters. Angew. Chem., Int. Ed. 2014, 53, 2376–2380. 10.1002/anie.201307480. [DOI] [PubMed] [Google Scholar]
- Yang H.; Wang Y.; Huang H.; Gell L.; Lehtovaara L.; Malola S.; Häkkinen H.; Zheng N. All-Thiol-Stabilized Ag44 and Au12Ag32 Nanoparticles with Single-Crystal Structures. Nat. Commun. 2013, 4, 2422. 10.1038/ncomms3422. [DOI] [PubMed] [Google Scholar]
- Kang X.; Wei X.; Jin S.; Yuan Q.; Luan X.; Pei Y.; Wang S.; Zhu M.; Jin R. Rational Construction of a Library of M29 Nanoclusters from Monometallic to Tetrametallic. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 18834–18840. 10.1073/pnas.1912719116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrödinger E.What is Life? The Physical Aspects of a Living Cell; Cambridge University Press: Cambridge, 1944. [Google Scholar]
- Darwin C.The origin of species; Oxford World’s Classics; Oxford University Press: Oxford, 1996. [Google Scholar]
- Chen P. C.; Liu M.; Du J. S.; Meckes B.; Wang S.; Lin H.; Dravid V. P.; Wolverton C.; Mirkin C. A. Interface and Heterostructure Design in Polyelemental Nanoparticles. Science 2019, 363, 959–964. 10.1126/science.aav4302. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhang J. Nanointerface Chemistry: Lattice-Mismatch Directed Synthesis and Application of Hybrid Nanocrystals. Chem. Rev. 2020, 120, 2123–2170. 10.1021/acs.chemrev.9b00443. [DOI] [PubMed] [Google Scholar]