Melatonin is an antioxidant with signaling properties that is especially relevant to fruit ripening. Melatonin crosstalk with NO and H2O2broadens the regulatory networks of these molecules.
Keywords: Hydrogen peroxide, nitric oxide, nitrosomelatonin, melatonin, postharvest, ripening
Abstract
Fruit ripening is a physiological process that involves a complex network of signaling molecules that act as switches to activate or deactivate certain metabolic pathways at different levels, not only by regulating gene and protein expression but also through post-translational modifications of the involved proteins. Ethylene is the distinctive molecule that regulates the ripening of fruits, which can be classified as climacteric or non-climacteric according to whether or not, respectively, they are dependent on this phytohormone. However, in recent years it has been found that other molecules with signaling potential also exert regulatory roles, not only individually but also as a result of interactions among them. These observations imply the existence of mutual and hierarchical regulations that sometimes make it difficult to identify the initial triggering event. Among these ‘new’ molecules, hydrogen peroxide, nitric oxide, and melatonin have been highlighted as prominent. This review provides a comprehensive outline of the relevance of these molecules in the fruit ripening process and the complex network of the known interactions among them.
Introduction
Fruits are specialized organs whose function is to provide an appropriate environment for the formation and maturation of seeds that will be propagated by various procedures to preserve the species (Dardick and Callahan, 2014). Regardless of the different classifications of fruits, their ripening involves complex physiological processes that are associated with multiple changes at the genetic, proteomic, biochemical, and metabolic levels, which are highly coordinated (Klee and Giovannoni, 2011; Palma et al., 2011; Karlova et al., 2014; Lü et al., 2018; Palma et al., 2019; Aghdam et al., 2020b). Fleshy fruits are a good example in which endogenous metabolic fluctuations can be translated into external changes that are easily observed at the phenotypic level, in many cases consisting of drastic changes in organoleptic features (e.g. color, emission of volatiles, and flavor) (Lalel et al., 2003; Wu et al., 2018).
Plants contain a wide variety of molecules that exert regulatory functions either independently or through their interactions with other molecules, acting as plant hormones. Among the classical phytohormones are auxins, cytokinins, gibberellins (GA), abscisic acid (ABA), and ethylene, but there are also other groups, including brassinosteroids (BR), salicylates, jasmonates, and strigolactones (Vanstraelen and Benková, 2012; Asgher et al., 2017). Recently, different types of molecules previously considered toxic to cells have been found to exert signaling functions either directly or indirectly. Accordingly, molecules such as hydrogen peroxide (H2O2) and nitric oxide (NO), which are part of the metabolism of reactive oxygen species (ROS) and reactive nitrogen species (RNS), have also been shown to be regulators of plant cellular metabolism, participating in all stages of plant development including seed germination, root and plant development, stomatal movement, senescence, flowering, and fruit ripening, as well as in the mechanisms of response to adverse environmental conditions (Smirnoff and Arnaud, 2019; Liu et al., 2020; Rodrigues and Shan, 2021; Corpas et al., 2022; Gupta et al., 2022). Other molecules could also be placed in the same category, such as hydrogen sulfide (H2S), which has recently been shown to exert regulatory functions in numerous processes, including fruit ripening (Gotor et al., 2019; Corpas, 2019; Corpas et al., 2021; Mishra et al., 2021).
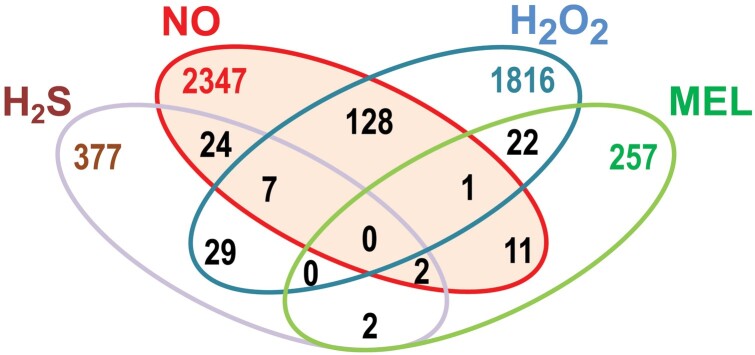
The hormone melatonin, which was discovered in higher plants in 1995 (Dubbels et al., 1995; Hattori et al., 1995) ,is a well-known regulatory molecule in mammals; in humans, for example, it influences numerous physiological and pathological processes such as circadian rhythms (Dominguez-Rodriguez et al., 2010), metabolism (Korkmaz et al., 2009), aging (Majidinia et al., 2018), neurodegenerative diseases (Shukla et al., 2019), and a wide range of cancers (González et al., 2021; Moloudizargari et al., 2021). Melatonin also has a broad spectrum of functions in higher plants (Zhao et al., 2021; Arnao et al., 2022; Hernández-Ruiz et al., 2022). Consequently, melatonin has been defined as a ‘master regulator’ in animal and plant cells (Reiter et al., 2010; Wang et al., 2018; Arnao and Hernández-Ruiz, 2019, 2021a; C. Sun et al., 2021), although the available information on melatonin in plants is still limited in comparison to that in animals. Figure 1 shows a Venn diagram analysis of the number of publications from the 1980s to date covering the different signaling molecules (NO, H2O2, H2S, and melatonin) as they relate to fruit physiology, with NO having the greatest number of publications, followed by H2O2, H2S, and melatonin. A small number of publications have simultaneously analyzed melatonin in combination with other signaling molecules, indicating that this is an emerging area that should be addressed.
Fig. 1.
Venn diagram analysis of the number of publications on the different signal molecules, namely nitric oxide (NO), hydrogen peroxide (H2O2), hydrogen sulfide (H2S), and melatonin (MEL), related to fruits, found in the PubMed database in the period 1980–2022.
The present review provides a framework of the relevance of melatonin in fruit ripening from the perspective of H2O2 and NO metabolism. Thus, melatonin, as a free radical scavenger, exerts regulatory actions over some ROS and RNS. The biochemical interactions among melatonin and these reactive species provide a promising new area of research due to the potential signaling functions of these interactions. Furthermore, the biotechnological significance of these molecules during fruit postharvest storage is discussed, as well as the effects of their exogenous application.
Fleshy fruit ripening
Fleshy fruits are classically divided into two main categories according to their dependence on the ethylene profile and the respiratory burst: climacteric (e.g. apple, apricot, avocado, banana, melon, pear, persimmon, and tomato), which are dependent on ethylene and the respiratory burst, and non-climacteric (e.g. cherry, grape, orange, lemon, other citrus, olive, pepper, raspberry, and strawberry), which are not dependent on these factors (Cherian et al., 2014; Chen et al., 2018). However, fruit ripening also involves the integration of other molecules, including abscisic acid, auxin, jasmonic acid, and salicylic acid, which exert regulatory functions (Symons et al., 2012; Kumar et al., 2014; Hou et al., 2018; Kou et al., 2021; P. Li et al., 2021; Alferez et al., 2021). Recently, it was found that fruit ripening involves a physiological nitro-oxidative stress, which influences many subcellular processes such that some metabolic pathways are down-regulated whereas others are up-regulated (Chaki et al., 2015; Rodríguez-Ruiz et al., 2017; Corpas et al., 2018a; Chu-Puga et al., 2019; Palma et al., 2019; González-Gordo et al., 2019; Zuccarelli et al., 2021). Furthermore, accumulating data indicate that the exogenous application of some key molecules, such as NO, H2O2, or H2S, and most recently melatonin, has beneficial effects at different levels, including delay of fruit senescence, palliating chilling injury, ameliorating fungal decay, and improving nutritional quality. In many cases, all these molecules participate in complex signaling cascades that, in general, stimulate the enzymatic and non-enzymatic antioxidant systems.
Melatonin
Melatonin (N-acetyl-5-methoxytryptamine) is generated from the amino acid tryptophan. In vertebrates, melatonin is the main secretory product of the pineal gland located in the brain and is probably best known for its influence on sleep. However, melatonin has multiple regulatory functions in physiological and pathological conditions (Shukla et al., 2019; Ma et al., 2020; Back, 2021). In plant cells, this molecule is referred to as phytomelatonin and it has phytohormonal actions in higher plants, which include its antioxidant properties. A putative melatonin receptor in the plasma membrane designated CAND2/PMTR1 (Candidate G-protein coupled receptor 2/Phytomelatonin receptor 1), which participates in the signaling mechanisms related to stomatal closure of Arabidopsis thaliana, has been identified. This signaling involves a cascade of signals, including H2O2, Ca2+ influx, and K+ efflux, in stomatal guard cells (Wei et al., 2018). More recently, new data obtained using confocal microscopy and Cand2-defective Arabidopsis mutants indicate that the Cand2 protein is actually localized in the cytosol and may not be a G protein that mediates melatonin-induced defense (Back and Lee, 2020). Therefore, the presence of a melatonin receptor on the plasma membrane in higher plant cells obviously remains an open question. It is, however, well recognized in higher plants that melatonin participates in regulatory functions at different levels, such as promoting lateral root growth, delaying senescence, flowering, and fruit ripening, ameliorating iron deficiency, and mediating the response to environmental stresses (Korkmaz et al., 2014; Zhang et al., 2015; Zhu et al., 2019; Zhao et al., 2019; Arnao and Hernández-Ruiz, 2020; Siddiqui et al., 2020), and that these regulatory processes involve molecules such as H2O2, NO, or H2S that have signaling properties, although the existence of a receptor is still under analysis (S. Li et al., 2021b; Pardo-Hernández et al., 2021; Singh et al., 2022).
Reactive oxygen species: H2O2 as a signal molecule
ROS are a family of molecules generated during the reduction of molecular oxygen. They include hydrogen peroxide (H2O2), superoxide anion (O2•–), hydroxyl radical (•OH), and other species that do not involve electron gains, such as singlet oxygen (1O2). In higher plants, the main sources of ROS are the electron transport chains of chloroplasts and mitochondria, as well as peroxisomes, which are a particularly important source of H2O2 due to the β-oxidation and photorespiration pathways (Corpas et al., 2020). Additionally, there are other minor cellular sites of ROS production including the cytosol, plasma membrane, and cell wall (Corpas et al., 2015; Podgórska et al., 2017; Kámán-Tóth et al., 2019). Furthermore, the uncontrolled overproduction of ROS, such as occurs as a consequence of adverse environmental conditions, can trigger oxidative damage to the various cellular macromolecules, causing their dysfunction (Møller et al., 2007). However, some ROS have signaling properties, in particular H2O2, which has been extensively studied (Exposito-Rodriguez et al., 2017; Foyer, 2018, 2020; Smirnoff and Arnaud, 2019; Nazir et al., 2020; Liu et al., 2021; Zhang et al., 2021; Zentgraf et al., 2022). Recent reports have identified two H2O2 plasma membrane receptors, designated leucine-rich repeat (LRR) receptor protein kinase HPCA1 (Wu et al., 2020) and LRR-receptor-like kinase (RLK) protein HSL3 (Liu et al., 2020), which sense the apoplastic content of H2O2 and initiate a cascade of signals as a response mechanism to different exogenous stimuli (Foyer et al., 2020; Singh et al., 2022). Likewise, during the plant immune response, O2•– generation is controlled by a receptor-like cytoplasmic kinase (RLCK)-mediated phosphorylation of respiratory burst oxidase homolog D (RBOHD) (P. Li et al, 2021a; Singh et al., 2022).
Reactive nitrogen species: nitric oxide as a signal molecule
The discovery that plant cells have the capacity to generate the free radical nitric oxide (•NO) opened a new area of research (Kolbert et al., 2019). Unlike animals, in which the enzymatic NO source from the amino acid l-arginine involves a group of well-characterized enzymes named nitric oxide synthases (NOSs), in higher plants, the enzymatic source remains undefined, although it is generally accepted that there are two main routes: (i) a reductive pathway from nitrate and nitrite that is mediated by nitrate reductase (NR), and (ii) an oxidative pathway from l-arginine through a NOS-like activity, designated thus because it has the same biochemical requirements as animal NOS (Astier et al., 2018; Corpas et al., 2022). In higher plants, the main NO sources are the cytosol, peroxisomes, chloroplasts, and mitochondria.
Metabolism of NO leads to the formation of derived molecules, called RNS, which include nitrogen dioxide (•NO2), peroxynitrite (ONOO–), and S-nitrosoglutathione (GSNO), among others (Corpas, 2017). ONOO– is a highly reactive molecule and also a strong oxidizing and nitrating agent (Ferrer-Sueta et al., 2018) that is formed by the chemical reaction between two radicals, •NO and O2•–, with a very high rate constant (~1010 M–1 s–1), even higher than the rate constant for O2•– dismutation by the CuZn superoxide dismutase (SOD) enzyme, which is 2 × 109 M–1 s–1 (Gray and Carmichael, 1992). This characteristic guarantees that when both radicals are simultaneously present in any plant subcellular location, ONOO– will be generated. On the other hand, GSNO is also generated by the binding of NO to the thiol group of the reduced form of glutathione (GSH, γ-l-glutamyl-l-cysteinylglycine), which is considered to be the main S-nitrosothiol in plant cells and whose content is regulated by the enzyme GSNO reductase (Lee et al., 2008; Corpas et al., 2013). These two examples, ONOO– and GSNO, demonstrate the close relationship between ROS and RNS metabolism.
In plants, RNS regulate protein functions by post-translational modifications (PTMs). Tyrosine nitration is an irreversible process that usually causes inhibition of the target proteins (Radi, 2013; Mata-Pérez et al., 2016), whereas S-nitrosation in the thiol group of key cysteine residues, which is reversible, can either up-regulate or down-regulate enzyme functions (Corpas et al., 2008; Gupta et al., 2020). To date, it has been demonstrated that the main antioxidant enzymes in plant cells, such as catalase, ascorbate peroxidase (APX), monodehydroascorbate reductase, and SODs, can undergo either nitration, S-nitrosation, or both (Begara-Morales et al., 2014a, 2015, 2016; Palma et al., 2020). Under adverse environmental conditions, RNS are also overproduced in an uncontrolled way and trigger dysfunction in macromolecules, causing nitrosative stress; ONOO– has a particular relevance for protein nitration (Corpas and Barroso, 2014). In a mirrored manner to H2O2, which is the most studied ROS participating in signaling functions in plant cells, NO is the most highly investigated RNS (Corpas et al., 2018b; Kohli et al., 2019; Gupta et al., 2022).
Chemical and biochemical interactions of melatonin with ROS and RNS
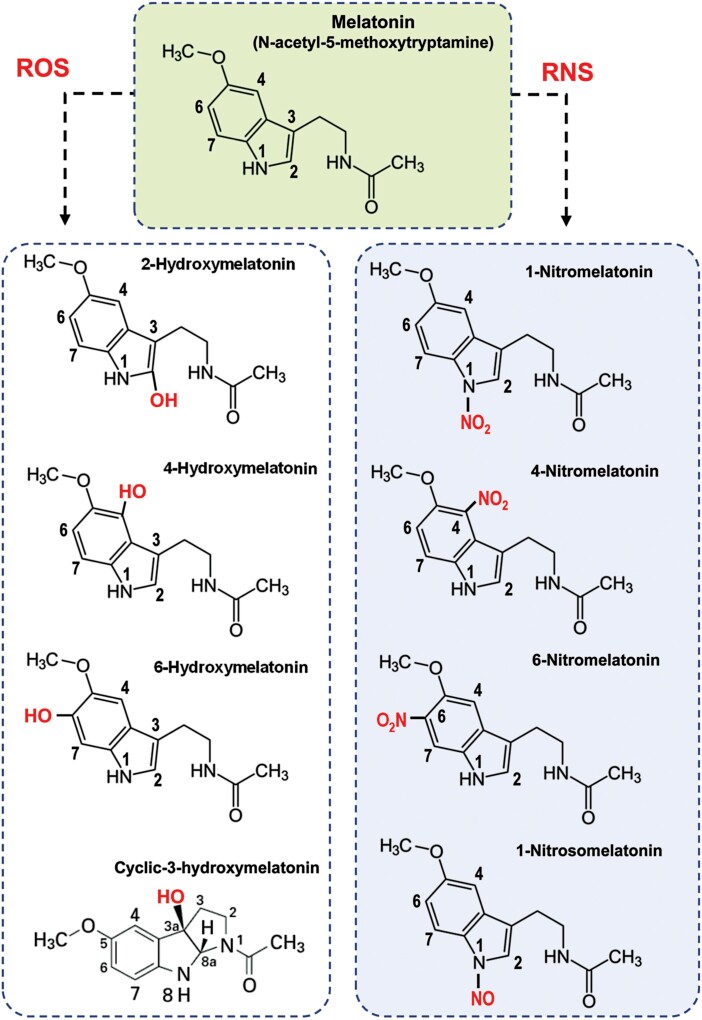
It is well recognized that melatonin is a potent free radical scavenger (Reiter et al., 2001; Sofic et al. 2005; Tan et al., 2007; Galano et al., 2013; Zhang and Zhang, 2014) that can diffuse through biological membranes, exerting its antioxidant capacity in the different subcellular compartments. Melatonin reacts with different ROS or RNS to form a family of molecules that involve either the addition of a hydroxyl group (-OH) in position 1, 4, or 6 to generate hydroxymelatonin; the addition of a nitro group (-NO2) in position 1, 4, or 6 to generate N-nitromelatonin (Kirsch and de Groot, 2009); or the addition of NO in position 1 to generate 1-nitrosomelatonin (NOMel) (Blanchard et al., 2000) (Fig. 2). Among the different hydroxymelatonin metabolites, 4-hydroxymelatonin (4-OHM) is an excellent peroxyl radical (ROO•) scavenger, whereas 2-hydroxymelatonin (2-OHM) is predicted to have low antioxidant protection. Under in vitro conditions at physiological pH, it was shown that 4-OHM reacted with ROO• ~200 times faster than trolox (an analog of vitamin E used to measure antioxidant capacity). Furthermore, 4-OHM was predicted to have a higher antioxidant capacity than natural antioxidants present in different fruits, such as gallic and ellagic acids in blueberry, blackberry, strawberry, plum, or grape (Pérez-González et al., 2017). In humans, mice, and rats, another metabolite of melatonin has been described, namely cyclic 3-hydroxymelatonin, which was found to have a higher hydroxyl radical (•OH) scavenging capacity than either melatonin or vitamin C (Tan et al., 2014). To corroborate the formation of these compounds, an in vitro assay using 5 mM melatonin in the presence of 5 mM 3-morpholinosydnonimine (SIN-1, a peroxynitrite donor that simultaneously generates equimolar amounts of O2•– and •NO) was carried out at 37 °C for 60 min in the dark. Subsequently, the profile of compounds was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and all the hydroxy-, nitro-, and nitrosomelatonin derivatives were found.
Fig. 2.
Melatonin-derived metabolites resulting from the interaction of melatonin with ROS and RNS. The reactions involve the addition of a hydroxyl group (-OH) in position 2, 4, or 6; the addition of NO (1-nitrosomelatonin); or the addition of a nitro group (NO2) in position 1, 4, or 6.
Byeon et al. (2015) performed a systematic analysis using a LC-MS/MS approach to evaluate the content of melatonin and some of its hydroxy-derived molecules in 24 plant species. This study found that in most plant species the melatonin concentration is ~1 ng g–1 fresh weight (FW). In contrast, the content of 2-OHM is ~6 ng g–1 FW. A deeper analysis of the hydroxyl forms of melatonin in the selected plants indicated that the predominant form was 2-OHM (99%), followed by 4-OHM (~0.5%), with 6-hydroxymelatonin being undetected. Unfortunately, any melatonin molecule related to NO was not analyzed in this study.
Melatonin is known to react with peroxynitrite, and it could be expected that in a cellular environment, this would be a mechanism of protection against protein nitration processes that are usually associated with a down-regulation in the function of the affected protein (Begara-Morales et al., 2013; Radi, 2013; Ferrer-Sueta et al., 2018; Muñoz-Vargas et al., 2018; Corpas et al., 2021). It is important to consider that the formation of peroxynitrite is usually associated with the overproduction of both •NO and O2•–, whose coupling activity is very high; as a result, the product, peroxynitrite, has major negative effects where it is generated. Thus, the interaction of peroxynitrite with melatonin is an additional mechanism of protection of proteins against nitration. particularly under stress conditions; this deserves to be further investigated.
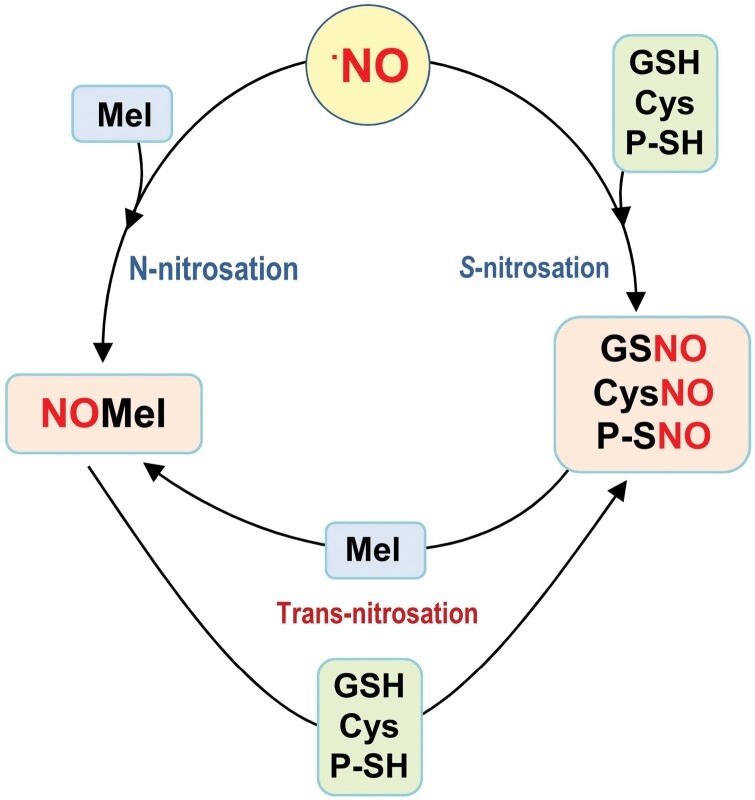
Interaction among N-nitrosomelatonin, NO, and S-nitrosothiols
Early in vitro studies evaluated the capacity of NOMel to release NO. Subsequently, these assays were completed under physiological conditions where NOMel, in the presence of reducing compounds such as ascorbate, released NO and melatonin (De Biase et al., 2005). The physiological relevance of this process is similar to that exerted by S-nitrosothiols of low and high molecular weight, including GSNO, nitrosocysteine, and S-nitrosated proteins, which are also NO-releasing compounds. For example, in the presence of reductants (ascorbate and GSH, and Cu2+), GSNO decomposes to produce •NO and oxidized glutathione (Gorren et al. 1996; Noble et al., 1999; Holmes and Williams 2000; Smith and Dasgupta 2000). In 30-day-old Arabidopsis plants, exogenous GSNO applied to the root system was available to release NO and modulate up to 1945 genes that were expressed differently in leaves and roots, with 114 genes being exclusive to one of these organs, indicating the capacity of the GSNO to move long distances through the vascular system (Begara-Morales et al., 2014b). Based on this property, Fig. 3 shows a working model in which NOMel could release NO and mediate a process of S-nitrosation of GSH, free cysteine, and thiol groups of proteins. At the same time, these S-nitrosothiols release NO and, by a trans-nitrosation process, mediate the formation of NOMel (Peyrot et al., 2006; Berchner-Pfannschmidt et al., 2008; Hickok et al., 2012; Mukherjee, 2019; Hardeland, 2021). These mechanisms could be considered a cellular strategy to extend the functional actions of the involved molecules in the different subcellular compartments in which they are generated. Consequently, a close relationship among all these molecules with the capacity to carry and release NO should be anticipated, with this being a long-distance signaling mechanism (Singh et al., 2016).
Fig. 3.
Simple model of melatonin (Mel) nitrosation, S-nitrosation of glutathione (GSH), cysteine (Cys), or protein thiol (P-SH), and trans-nitrosation. Nitric oxide (NO) interacts with Mel, GSH, Cys, and P-SH to generate nitrosomelatonin (NOMel), S-nitrosoglutathione (GSNO), S-nitrosocysteine (CysNO), or nitrosated protein (P-SNO), respectively, which can undergo trans-nitrosation processes.
In higher plants, the information about the formation of hydroxy- and nitromelatonin metabolites is, to the best of our knowledge, limited, and it is mostly based on in vitro and in vivo studies of animal cells (Hardeland, 2021). However, it could be expected that these compounds should have analogous functions in plant cells. In addition to the direct interactions between melatonin and the different ROS and RNS, there are also some mechanisms in plants that mediate the conversion of melatonin into 2-OHM and cyclic 3-hydroxymelatonin through the enzymatic action of melatonin 2-hydroxylase (M2H) and melatonin 3-hydroxylase (M3H), respectively (Lee and Back, 2016; Lee et al., 2016). Thus, some genetic studies in rice plants using RNA interference approaches to down-regulate the expression of M2H caused an increase the content of melatonin, conferring a higher tolerance to diverse stresses including cadmium, senescence, salt, and tunicamycin (Choi and Back, 2019). Similarly, treatment with 2-OHM induced plant defense genes in Arabidopsis, although to a smaller extent than melatonin (Byeon et al., 2015), and in rice (Oryza sativa) 2-OHM triggered resistance against cold and drought stress (Lee and Back, 2016, 2019).
In cassava (Manihot esculenta) plants, there are obvious protein interactions among cytosolic ascorbate peroxidase (MeAPX2) and two cytosolic isozymes of the melatonin biosynthesis, tryptophan decarboxylase (MeTDC2) and N-aceylserotonin O-methyltransferase (MeASMT2), which provide a higher antioxidant capacity against H2O2 (Bai et al., 2020). This observation prompts several questions focused on the molecular mechanism underlying how these protein interactions (MeAPX2–MeTDC2 and MeAPX2–MeASMT2) occur, where they take place, and whether they increase the APX activity and therefore provide greater protection against high concentrations of H2O2.
In higher plants, some experimental studies have reported an interaction between melatonin and NO (Y. Sun et al., 2021). In sunflower (Helianthus annuus L.) seedlings subjected to salinity stress, the exogenous application of 15 µM melatonin altered the content of NO, O2•–, and ONOO–, and consequently the modulation of CuZn-SOD and Mn-SOD as well as protein tyrosine nitration (Arora and Bhatla, 2017). Recently, using 3-day-old Arabidopsis seedlings as a model, Singh et al. (2021) estimated the NO release capacity of two compounds, 250 μM GSNO and NOMel, applied through the root system, and evaluated the NO content in green cotyledons by confocal laser scanning microscopy. The results showed that NOMel is more efficient than GSNO in releasing NO. Consequently, these data indicate that both NOMel and GSNO have the capacity to travel through the vascular system and release NO in other parts of the plant, as was previously proposed (Airaki et al., 2011; Begara-Morales et al., 2014b; Singh et al., 2016).
Crosstalk among melatonin, H2O2, and NO in fruit ripening and postharvest storage
Knowledge of the mechanism of regulation among melatonin, NO and H2O2 during fruit ripening is in a nascent phase, especially due to the fact that there are earlier unresolved issues such as the identity of the genes involved in the melatonin biosynthesis pathway, as well as how the NO is generated. Since the first descriptions of the presence of melatonin in plants (Dubbels et al.,1995; Hattori et al., 1995), interest in this molecule in the field of plant physiology has grown exponentially. This is especially due to its antioxidant properties, as well as its regulatory functions affecting both gene and protein expression, enzyme activities, and their crosstalk with different phytohormones (Arnao and Hernández-Ruiz, 2021b; Arnao et al., 2022). Likewise, the study of the interactions of endogenous melatonin with ROS and RNS in higher plants has also been increasing, mainly based on the biochemical information established from animal studies that have provided basic knowledge in this field. However, research studies of the interactions between melatonin with both ROS and RNS during fruit ripening are still scarce (Aghdam et al., 2022), possibly due to the low levels of endogenous melatonin in fruits, which make it difficult to identify the related melatonin metabolites. For example, a comparative analysis of the melatonin content of the most consumed horticultural fruits worldwide, pepper (Capsicum annuum L.) and tomato (Solanum lycopersicum L.), which are representative examples of non-climacteric and climacteric fruits, respectively, indicated that the melatonin concentration in red pepper fruits of six cultivars ranged from 5 ng g–1 to 12 ng g–1 FW, whereas in red tomato fruits of seven cultivars, the melatonin concentration ranged from 0.6 ng g–1 to 15 ng g–1 FW (Riga et al., 2014). A similar situation is apparent concerning studies on the metabolism of endogenous NO in fruits, about which information is also very limited (Corpas et al., 2018a), although in the case of ROS metabolism there is more information available.
Other challenges in delving into the regulatory mechanisms at the genetic level are to identify all the genes/enzymes involved in melatonin and NO biosynthesis. In the case of melatonin, its synthesis from the amino acid tryptophan in higher plants seems to involve six enzymes that are present in different subcellular compartments, namely tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), tryptophan hydroxylase (TPH), serotonin N-acetyltransferase (SNAT), N-acetylserotonin methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT). However, not all genes/enzymes have been identified in all plants (Aghdam et al., 2022 and references therein) suggesting the existence of diverse biosynthesis pathways. In the case of NO, its biosynthetic pathway is even more disputed (Corpas et al., 2022 and references therein). It would be of great interest if any of the enzymes involved in melatonin biosynthesis were found to be targets of NO-derived PTMs such as S-nitrosation and nitration, although, to our knowledge, this information has not yet been uncovered.
Consequently, the majority of the studies on fruits have been carried out after the exogenous application of either melatonin, NO, or H2O2. The few results reported indicate that these molecules regulate the ripening process, either slowing or accelerating it, or provide beneficial effects during postharvest storage; as a result, these compounds could be used as tools for biotechnological approaches to maintain the quality of the fruits as well as protecting them against infections by pathogens or chilling damage associated with postharvest storage. It should be pointed out, however, that the effects of these molecules on fruit ripening depend on the type of fruit (climacteric or non-climacteric) and the dose and duration of the treatment, among other parameters that must be optimized. Table 1 summarizes representative examples of climacteric and non-climacteric fruits treated with melatonin, H2O2, or NO and the beneficial effects of these treatments, such as extending postharvest storage life or preserving nutritional quality. However, it seems evident that the metabolic triangle constituted by melatonin, NO, and H2O2 has a common characteristic that implies the activation of both enzymatic and non-enzymatic antioxidant systems (Tan et al., 2015; Chumyam et al., 2019; Zuccarelli et al., 2021). This may serve to control the overproduction of ROS and RNS that could trigger uncontrolled nitro-oxidative stress resulting in an alteration in the quality of the fruits, in terms of both their external appearance and their organoleptic qualities (aroma, flavor, acidity, sweetness, etc.).
Table 1.
Representative examples of the beneficial effects triggered by exogenous molecules with signaling properties (melatonin, H2O2, and NO) in fruits to extend postharvest life or to preserve nutritional quality
| Fruit | Concentration | Main effects | Reference |
|---|---|---|---|
| Melatonin | |||
| Peach (Prunus persica L.) |
0.1 mM | Delays postharvest senescence by lowering O2•– and H2O2 accumulation. Higher AA accumulation and increased activity of catalase, SOD, and APX | Gao et al., (2016) |
| Grapevine (Vitis vinifera × labrusca). |
0.2 mM | Stimulates ripening by increasing the levels of ABA, H2O2, and ethylene | Xu et al., (2018) |
| Pear (Pyrus communis L.) |
0.1 mM | Delays postharvest senescence and induces NO accumulation. Higher NOS-like gene expression and enzyme activity. Lower ACS, ACO, PG, and Cel genes expression | Liu et al., (2019) |
| Pear (Pyrus communis L.) |
0.1 mM | Induces anthocyanin accumulation through the H2O2 generated by RBOHF | H. Sun et al., (2021) |
| Sweet cherry (Prunus avium L.) |
0.1 mM | Higher endogenous melatonin accumulation. Higher SOD, CAT, APX, and GR enzyme activity. Higher ascorbate and GSH accumulation. Higher membrane integrity. Lower electrolyte leakage and MDA accumulation. Lower O2•– and H2O2 accumulation | Wang et al., (2019) |
| Sweet cherry (Prunus avium L. var Prime Giant) |
0.01 and 0.1 mM | Delays ripening by modulating the contents of endogenous hormones, mainly ABA and auxin | Tijero et al., (2019) |
| Sweet cherry (Prunus avium L.) |
0.50 and 0.1 mM | Treatment of leaves treated with melatonin improved the antioxidant content of sweet cherry fruit | Xia et al., (2020) |
| Jujube (Ziziphus jujuba Mill.) |
25 µM | Higher APX and GR enzyme activity. Higher ascorbate and GSH accumulation. Lower PG and PME enzymes activity, maintaining firmness | Tang et al., (2020) |
| Pomegranate (Punica granatum L.) |
0.1 mM | Higher NADPH accumulation. Higher APX, GR, G6PDH, 6PGDH, and PAL enzyme activity. Higher AOX gene expression. Higher phenol and anthocyanin accumulation and DPPH-scavenging capacity. Higher AA and GSH accumulation. Lower AAO enzyme activity. | Aghdam et al., (2020a) |
| Mango (Mangifera indica L.) |
0.2 mM | Delays the ripening process. Decreases the contents of H2O2 and MDA in the exocarp of the fruit | Dong et al., (2021) |
| Apple (Malus domestica L. Borkh) |
1 mM | Reduces ethylene production. Increase the activity of catalase, SOD and peroxidase and keeps apple quality during postharvest storage | Onik et al., (2021) |
| Blueberry (Vaccinium corymbosum L.) |
1 mM | Reduces qualitative decay and improves antioxidant system (catalase, SOD, APX, ascorbate, polyphenols, anthocyanins, and flavonoids) during cold storage | Magri and Petriccione, (2022) |
| Kiwifruit (Actinidia chinensis) |
0.1 mM | Palliates chilling injury during cold postharvest storage by inhibition of lignin metabolism and increasing the activity of antioxidant enzymes and the content of soluble antioxidants (ascorbate and GSH) | Jiao et al., (2022) |
| Tomato (Solanum lycopersicum) |
0.5 mM | Promotes ripening of postharvest fruit through DNA methylation of ethylene-signalling genes | Shan et al., (2022) |
| H 2 O 2 | |||
| Melon (Cucumis melo L.) |
20 mM | Treatment of melon plants increases the soluble sugar content in leaves and fruits, thus improving the fruit quality. Increases photosynthetic activity and the activities of chloroplastic and cytosolic fructose-1,6-bisphosphatase, sucrose phosphate synthase, and invertases | Ozaki et al., (2009) |
| Longan (Dimocarpus longan Lour) |
1.96 mM | Increases the activities of pulp PLD, lipase, and LOX. Destroys longan pulp membrane structure and increases cell membrane permeability | Lin et al., (2019) |
| Guava (Psidium guajava L.) |
250 mM | Reduces enzymatic browning of freshly cut fruit by reducing PPO and POD activities. Stimulates the peroxiredoxin/thioredoxin system | Chumyam et al., (2019) |
| Kyoho grape (Vitis vinifera × Vitis labrusca) |
300 mM | Promotes early ripening. Affects the gene expression of HSP, GDSL, XTH, and CAB1, involved in oxidative stress, cell wall deacetylation, cell wall degradation, and photosynthesis, respectively. | Guo et al., (2020) |
| Mango (Mangifera indica L.) |
20 mM | Treated mango plants have fruits with a higher content of total sugar, phenol, and carotenoids | Mostafa, (2021) |
| Tomato (Solanum lycopersicum L. cv. Verty F1) |
100 mM | Increases tomato fruit firmness, decreases water-soluble pectin and expression of cell-wall-related genes, polygalacturonase, and pectate lyase. Maintains morphological and biochemical quality of tomato fruits during postharvest storage | Torun and Uluisik, (2022) |
| NO | |||
| Strawberry (Fragaria × ananassa Duch.) |
5 µM sodium nitroprusside solution | Extends postharvest life | Wills et al., (2007); Zhu and Zhou, (2007) |
| Peach fruit (Prunus persica L. cv. Xiahui 6) |
10 ppm NO gas | Delays the ripening process. Affects sucrose metabolism by changing the expression of related genes | Han et al., (2018) |
| Jujube (Ziziphus jujuba Mill.) |
20 ppm NO gas | Retards cell wall degradation | Zhao et al., (2019) |
| Sweet pepper (Capsicum annuum L. cv. Melchor) |
5 ppm NO gas | Delays fruit ripening. Increases ascorbate content, protein nitration, and S-nitrosation. Decreases catalase and APX activities | Rodríguez-Ruiz et al., (2017); González-Gordo et al., (2019) |
| Tomato (Solanum lycopersicum L. cv. ‘Micro-Tom’) |
300 ppm NO gas | Promotes ascorbate biosynthesis and intensifies protein S-nitrosation and nitration. Affects carotenoid, tocopherol, and flavonoid metabolism | Zuccarelli et al., (2021) |
| Melon (Cucumis melo L.) |
100 ppm NO gas | Enhances postharvest disease resistance to the fungus Alternaria alternata by postponing ethylene biosynthesis | Wei et al., (2021) |
AA, ascorbic acid; AAO, ascorbic acid oxidase; AOX, alternative oxidase; ABA, abscisic acid; ACS, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase; ACO, ACC oxidase; APX, ascorbate peroxidase; CAB1, chlorophyll a-b binding protein; CAT, catalase; Cel, cellulose; DPPH, 2,2-diphenyl-1-picrylhydrazyl; GDSL, GDSL-motif esterase/lipase; G6PDH, glucose-6-phosphate dehydrogenase; GR, glutathione reductase; GSH, reduced glutathione; HSP, heat shock protein; LOX, lipoxygenase; MDA, malondialdehyde; NOS, NO synthase; PG, polygalacturonase; 6PGDH, 6-phosphogluconate dehydrogenase; PLD, phospholipase D; POD, peroxidase; PPO, polyphenol oxidase; RBOHF, respiratory burst oxidase homolog F; SOD, superoxide dismutase; XTH, xyloglucan endotransglucosylase/hydrolase.
In pepper fruits, treatment with NO gas causes delayed ripening, which is accompanied by a modulation of the ROS metabolism characterized by an elevation in ascorbate content as a consequence of an increase in the expression and activity of the last enzyme of its biosynthesis pathway, the mitochondrial enzyme l-galactono-1,4-lactone dehydrogenase (GalLDH) (Rodriguez-Ruiz et al., 2017). Likewise, the NO-treated fruits had a higher GSH content, higher APX and lipoxygenase activities, lower lipid peroxidation, and lower O2•–-generating NADPH oxidase activity (González-Gordo et al., 2019, 2020). Interestingly, a higher content of nitrated proteins was apparent, particularly the peroxisomal enzyme catalase, whose activity decreased (Chaki et al., 2015). These observations related to APX and catalase activity are in good agreement with the previously reported effect of NO-derived PTMs, S-nitrosation, and nitration on these enzymes in other plant species (Begara-Morales et al., 2014a; Palma et al. 2020). Similarly, the exogenous application of NO to tomato at the pre-climacteric stage suppressed the activity of antioxidant enzymes, increased protein S-nitrosation and nitration, and favored the accumulation of ascorbate and flavonoids (Zuccarelli et al., 2021). Recently, it has been shown that melatonin exerts an epigenetic regulation through DNA methylation of ethylene signaling genes, which promotes the ripening of tomato fruit during postharvest storage (Shan et al., 2022). This observation suggests a scenario to be addressed in future investigations.
The cascade of events that takes place when any of these molecules is applied exogenously has been the subject of many studies because there are other elements involved, such as the type of fruit, the involvement of phytohormones such as ethylene, or the state of preservation of the fruit, for example, at low temperature. For example, in pear fruits, the exogenous application of melatonin inhibits the synthesis of ethylene, which seems to be mediated by NO (Liu et al., 2019), since this molecule can inhibit key enzymes in the ethylene biosynthesis pathway, such as S-adenosyl methionine synthetase, 1-aminocyclopropane-1-carboxylic acid (ACC) synthase, and ACC oxidase (Palma et al., 2019). In the case of non-climacteric fruits, the ripening process is essentially modulated by ABA, which mediates the accumulation of anthocyanins and sugars. For example, in sweet cherry, exogenous melatonin delays fruit ripening, counteracting the effect of ABA, since it affects the balance of other involved phytohormones such as cytokinins, jasmonic acid, and salicylic acid (Tijero et al., 2019; Michailidis et al., 2021).
Conclusions and future perspectives
As in mammals, melatonin is a multifunctional molecule in higher plants and specifically in fruits, where it exerts numerous beneficial functions as a protectant against biotic and abiotic stresses when it is exogenously applied. Melatonin has antioxidant properties, since it reacts with both ROS and RNS, although the information available on the derived molecules is scarce in higher plants and even non-existent in relation to the ripening of fruits, a process that is characterized by an important nitro-oxidative metabolism.
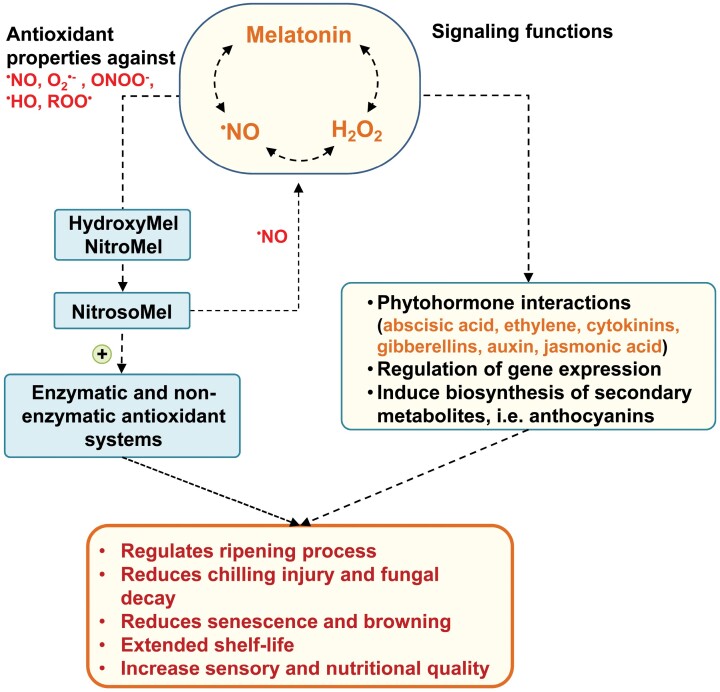
Future research should focus on the interactions and functions of these molecules, although a major technical challenge is their identification and specific localization, considering that they are endogenously generated at very low concentrations. Unquestionably, the exogenous application of melatonin has been shown to be a powerful biotechnological tool, since it exerts beneficial effects either directly as an antioxidant molecule, or by acting as a signaling molecule that acts upstream of H2O2 and NO. Furthermore, the interaction of melatonin with NO to generate nitrosomelatonin, a molecule that can release NO in the presence of reductants such as ascorbate, opens new research lines related to the complex crosstalk between these molecules. On the other hand, the use of exogenous melatonin to provide beneficial effects during postharvest storage could be considered as a novel biotechnological tool for application in the horticultural industry. However, it is important to note that the melatonin concentration, the time of exposure, and the means of application (by immersion, spraying, or other methods) should be optimized for each type of fruit. Figure 4 illustrates the cascade of signals mediated by the crosstalk among melatonin, NO, and H2O2 during fruit ripening, which, in general, stimulates antioxidant capacity through the activation of enzymatic and non-enzymatic systems as well as triggering regulatory functions in gene regulation by their interactions with the different phytohormones. Consequently, we conclude that melatonin, besides being an antioxidant molecule, it is also a key molecule with signaling properties. Beyond scientific interest in the basic research on the complex regulatory function of melatonin and its crosstalk with NO and H2O2 during the ripening of fruits or their subsequent storage, from an anthropological point of view, one of the stimuli that may promote its use is the nutraceutical benefits fruits enriched in melatonin can provide for human health.
Fig. 4.
Overview of the cascade of signals triggered by the application of exogenous melatonin (Mel), NO, or H2O2 to modulate fruit ripening and quality.
Contributor Information
Francisco J Corpas, Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín (Spanish National Research Council, CSIC), C/ Profesor Albareda, 1, 18008 Granada, Spain.
Marta Rodríguez-Ruiz, Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín (Spanish National Research Council, CSIC), C/ Profesor Albareda, 1, 18008 Granada, Spain.
María A Muñoz-Vargas, Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín (Spanish National Research Council, CSIC), C/ Profesor Albareda, 1, 18008 Granada, Spain.
Salvador González-Gordo, Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín (Spanish National Research Council, CSIC), C/ Profesor Albareda, 1, 18008 Granada, Spain.
Russel J Reiter, Department of Cell Systems and Anatomy, Joe R. and Teresa Lozano Long School of Medicine, UT Health San Antonio, San Antonio, TX 78229, USA.
José M Palma, Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín (Spanish National Research Council, CSIC), C/ Profesor Albareda, 1, 18008 Granada, Spain.
Marino Arnao, University of Murcia, Spain.
Conflict of interest
The authors declare no conflict of interest.
Funding
Our research work is supported by a European Regional Development Fund-co-financed grant from the Ministry of Science and Innovation (PID2019-103924GB-I00) and the Plan Andaluz de Investigación, Desarrollo e Innovación (PAIDI 2020; P18-FR-1359), Spain. MAMV acknowledges a Formación de Personal Investigador (FPI) pre-doctoral contract (PRE2020-093882) from the Spanish Ministry of Science, Innovation and Universities. MRR acknowledges a postdoctoral contract associated with the grant P18-FR-1359.
References
- Aghdam MS, Luo Z, Li L, Jannatizadeh A, Fard JR, Pirzad F.. 2020a. Melatonin treatment maintains nutraceutical properties of pomegranate fruits during cold storage. Food Chemistry 303, 125385. [DOI] [PubMed] [Google Scholar]
- Aghdam MS, Mukherjee S, Borja-Flores F, Arnao MB, Luo Z, Corpas FJ.. 2022. Functions of melatonin during postharvest of horticultural crops. Plant & Cell Physiology doi: 10.1093/pcp/pcab175 [DOI] [PubMed] [Google Scholar]
- Aghdam MS, Palma JM, Corpas FJ.. 2020b. NADPH as a quality footprinting in horticultural crops marketability. Trends in Food Science & Technology 103, 152–161. [Google Scholar]
- Airaki M, Sánchez-Moreno L, Leterrier M, Barroso JB, Palma JM, Corpas FJ.. 2011. Detection and quantification of S-nitrosoglutathione (GSNO) in pepper (Capsicum annuum L.) plant organs by LC-ES/MS. Plant & Cell Physiology 52, 2006–2015. [DOI] [PubMed] [Google Scholar]
- Alferez F, de Carvalho DU, Boakye D.. 2021. Interplay between abscisic acid and gibberellins, as related to ethylene and sugars, in regulating maturation of non-climacteric fruit. International Journal of Molecular Sciences 22, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J.. 2019. Melatonin: a new plant hormone and/or a plant master regulator? Trends in Plant Science 24, 38–48. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J.. 2020. Melatonin in flowering, fruit set and fruit ripening. Plant Reproduction 33, 77–87. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J.. 2021a. Melatonin as a plant biostimulant in crops and during post-harvest: a new approach is needed. Journal of the Science of Food and Agriculture 101, 5297–5304. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Hernández-Ruiz J.. 2021b. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biology 23, 7–19. [DOI] [PubMed] [Google Scholar]
- Arnao MB, Cano A, Hernández-Ruiz J.. 2022. Phytomelatonin: an unexpected molecule with amazing performances in plants. Journal of Experimental Botany 73, 5779–5800. doi: 10.1093/jxb/erac009 [DOI] [PubMed] [Google Scholar]
- Arora D, Bhatla S.. 2017. Melatonin and nitric oxide regulate sunflower seedling growth accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radical Biology and Medicine 106, 315–328. [DOI] [PubMed] [Google Scholar]
- Asgher M, Per TS, Masood A, Fatma M, Freschi L, Corpas FJ, Khan NA.. 2017. Nitric oxide signaling and its crosstalk with other plant growth regulators in plant responses to abiotic stress. Environmental Science and Pollution Research 24, 2273–2285. [DOI] [PubMed] [Google Scholar]
- Astier J, Gross I, Durner J.. 2018. Nitric oxide production in plants: an update. Journal of Experimental Botany 69, 3401–3411. [DOI] [PubMed] [Google Scholar]
- Back K. 2021. Melatonin metabolism, signaling and possible roles in plants. Plant Journal 105, 376–391. [DOI] [PubMed] [Google Scholar]
- Back K, Lee HY.. 2020. The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein–coupled melatonin receptor. Melatonin Research 3, 177–186. [Google Scholar]
- Bai Y, Guo J, Reiter RJ, Wei Y, Shi H.. 2020. Melatonin synthesis enzymes interact with ascorbate peroxidase to protect against oxidative stress in cassava. Journal of Experimental Botany 71, 5645–5655. [DOI] [PubMed] [Google Scholar]
- Begara-Morales JC, Chaki M, Sánchez-Calvo B, Mata-Pérez C, Leterrier M, Palma JM, Barroso JB, Corpas FJ.. 2013. Protein tyrosine nitration in pea roots during development and senescence. Journal of Experimental Botany 64, 1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, Sánchez-Calvo B, Chaki M, et al. 2014a. Dual regulation of cytosolic ascorbate peroxidase (APX) by tyrosine nitration and S-nitrosylation. Journal of Experimental Botany 65, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, Sánchez-Calvo B, Chaki M, et al. 2015. Differential molecular response of monodehydroascorbate reductase and glutathione reductase by nitration and S-nitrosylation. Journal of Experimental Botany 66, 5983–5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, Sánchez-Calvo B, Chaki M, Valderrama R, Mata-Pérez C, Padilla MN, Corpas FJ, Barroso JB.. 2016. Antioxidant systems are regulated by nitric oxide-mediated post-translational modifications (NO-PTMs). Frontiers in Plant Science 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begara-Morales JC, Sánchez-Calvo B, Luque F, Leyva-Pérez MO, Leterrier M, Corpas FJ, Barroso JB.. 2014b. Differential transcriptomic analysis by RNA-seq of GSNO-responsive genes between Arabidopsis roots and leaves. Plant & Cell Physiology 55, 1080–1095. [DOI] [PubMed] [Google Scholar]
- Berchner-Pfannschmidt U, Tug S, Trinidad B, Becker M, Oehme F, Flamme I, Fandrey J, Kirsch M.. 2008. The impact of N-nitrosomelatonin as nitric oxide donor in cell culture experiments. Journal of Pineal Research 45, 489–496. [DOI] [PubMed] [Google Scholar]
- Blanchard B, Pompon D, Ducrocq C.. 2000. Nitrosation of melatonin by nitric oxide and peroxynitrite. Journal of Pineal Research 29, 184–192. [DOI] [PubMed] [Google Scholar]
- Byeon Y, Tan DX, Reiter RJ, Back K.. 2015. Predominance of 2-hydroxymelatonin over melatonin in plants. Journal of Pineal Research 59, 448–454. [DOI] [PubMed] [Google Scholar]
- Chaki M, Álvarez de Morales P, Ruiz C, Begara-Morales JC, Barroso JB, Corpas FJ, Palma JM.. 2015. Ripening of pepper (Capsicum annuum) fruit is characterized by an enhancement of protein tyrosine nitration. Annals of Botany 116, 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Grimplet J, David K, et al. 2018. Ethylene receptors and related proteins in climacteric and non-climacteric fruits. Plant Science 276, 63–72. [DOI] [PubMed] [Google Scholar]
- Cherian S, Figueroa CR, Nair H.. 2014. ‘Movers and shakers’ in the regulation of fruit ripening: a cross-dissection of climacteric versus non-climacteric fruit. Journal of Experimental Botany 65, 4705–4722. [DOI] [PubMed] [Google Scholar]
- Choi GH, Back K.. 2019. Suppression of melatonin 2-hydroxylase increases melatonin production leading to the enhanced abiotic stress tolerance against cadmium, senescence, salt, and tunicamycin in rice plants. Biomolecules 9, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumyam A, Faiyue B, Saengnil K.. 2019. Reduction of enzymatic browning of fresh-cut guava fruit by exogenous hydrogen peroxide-activated peroxiredoxin/thioredoxin system. Scientia Horticulturae 255, 260–268. [Google Scholar]
- Chu-Puga A, González-Gordo S, Rodríguez-Ruiz M, Palma JM, Corpas FJ.. 2019. NADPH oxidase (Rboh) activity is up regulated during sweet pepper (Capsicum annuum L.) fruit ripening. Antioxidants 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ. 2017. Reactive nitrogen species (RNS) in plants under physiological and adverse environmental conditions: current view. Progress in Botany 78, 97–120. [Google Scholar]
- Corpas FJ. 2019. Hydrogen sulfide: a new warrior against abiotic stress. Trends in Plant Science 24, 983–988. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, Barroso JB.. 2014. Peroxynitrite (ONOO–) is endogenously produced in arabidopsis peroxisomes and is overproduced under cadmium stress. Annals of Botany 113, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, del Río LA, Barroso JB.. 2008. Post-translational modifications mediated by reactive nitrogen species: nitrosative stress responses or components of signal transduction pathways? Plant Signaling & Behavior 3, 301–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Alché JD, Barroso JB.. 2013. Current overview of S-nitrosoglutathione (GSNO) in higher plants. Frontiers in Plant Science 4, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, Gupta DK, Palma JM.. 2015. Production sites of reactive oxygen species (ROS) in organelles from plant cells. In: Gupta DK, Palma JM, Corpas FJ eds. Reactive oxygen species and oxidative damage in plants under stress. Cham: Springer International Publishing, 1–22. [Google Scholar]
- Corpas FJ, Freschi L, Rodríguez-Ruiz M, Mioto PT, González-Gordo S, Palma JM.. 2018a. Nitro-oxidative metabolism during fruit ripening. Journal of Experimental Botany 69, 3449–3463. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, del Río LA, Palma JM.. 2018b. A role for RNS in the communication of plant peroxisomes with other cell organelles? Subcellular Biochemistry 89, 473–493. [DOI] [PubMed] [Google Scholar]
- Corpas FJ, González-Gordo S, Palma JM.. 2020. Plant peroxisomes: a factory of reactive species. Frontiers in Plant Science 11, 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, González-Gordo S, Muñoz-Vargas MA, Rodríguez-Ruiz M, Palma JM.. 2021. The modus operandi of hydrogen sulfide (H2S)-dependent protein persulfidation in higher plants. Antioxidants 10, 1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas FJ, González-Gordo S, Palma JM.. 2022. NO source in higher plants: present and future of an unresolved question. Trends in Plant Science 27, 116–119. [DOI] [PubMed] [Google Scholar]
- Dardick C, Callahan AM.. 2014. Evolution of the fruit endocarp: molecular mechanisms underlying adaptations in seed protection and dispersal strategies. Frontiers in Plant Science 5, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biase PM, Turjanski AG, Estrin DA, Doctorovich F.. 2005. Mechanisms of NO release by N1-nitrosomelatonin: nucleophilic attack versus reducing pathways. Journal of Organic Chemistry 70, 5790–5798. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Sanchez-Sanchez JJ, Kaski JC, Reiter RJ.. 2010. Melatonin and circadian biology in human cardiovascular disease. Journal of Pineal Research 49, 14–22. [DOI] [PubMed] [Google Scholar]
- Dong J, Kebbeh M, Yan R, Huan C, Jiang T, Zheng X.. 2021. Melatonin treatment delays ripening in mangoes associated with maintaining the membrane integrity of fruit exocarp during postharvest. Plant Physiology and Biochemistry 169, 22–28. [DOI] [PubMed] [Google Scholar]
- Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W.. 1995. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. Journal of Pineal Research 18, 28–31. [DOI] [PubMed] [Google Scholar]
- Exposito-Rodriguez M, Laissue PP, Yvon-Durocher G, Smirnoff N, Mullineaux PM.. 2017. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nature Communications 8, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Campolo N, Trujillo M, Bartesaghi S, Carballal S, Romero N, Alvarez B, Radi R.. 2018. Biochemistry of peroxynitrite and protein tyrosine nitration. Chemical Reviews 118, 1338–1408. [DOI] [PubMed] [Google Scholar]
- Foyer CH. 2018. Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environmental and Experimental Botany 154, 134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH. 2020. How plant cells sense the outside world through hydrogen peroxide. Nature 578, 518–519. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Kyndt T, Hancock RD.. 2020. Vitamin C in plants: novel concepts, new perspectives, and outstanding issues. Antioxidants & Redox Signaling 32, 463–485. [DOI] [PubMed] [Google Scholar]
- Galano A, Tan DX, Reiter RJ.. 2013. On the free radical scavenging activities of melatonin’s metabolites, AFMK and AMK. Journal of Pineal Research 54, 245–257. [DOI] [PubMed] [Google Scholar]
- Gao H, Zhang ZK, Chai HK, Cheng N, Yang Y, Wang DN, et al. 2016. Melatonin treatment delays postharvest senescence and regulates reactive oxygen species metabolism in peach fruit. Postharvest Biology and Technology 118, 103–110. [Google Scholar]
- González A, Alonso-González C, González-González A, Menéndez-Menéndez J, Cos S, Martínez-Campa C.. 2021. Melatonin as an adjuvant to antiangiogenic cancer treatments. Cancers 13, 3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gordo S, Bautista R, Claros MG, Cañas A, Palma JM, Corpas FJ.. 2019. Nitric oxide-dependent regulation of sweet pepper fruit ripening. Journal of Experimental Botany 70, 4557–4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Gordo S, Rodríguez-Ruiz M, Palma JM, Corpas FJ.. 2020. Superoxide radical metabolism in sweet pepper (Capsicum annuum L.) fruits is regulated by ripening and by a NO-enriched environment. Frontiers in Plant Science 11, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorren ACF, Schrammel A, Schmidt K, Mayer B.. 1996. Decomposition of S-nitrosoglutathione in the presence of copper ions and glutathione. Archives of Biochemistry and Biophysics 330, 219–228. [DOI] [PubMed] [Google Scholar]
- Gotor C, García I, Aroca A, Laureano-Marín AM, Arenas-Alfonseca L, Jurado-Flores A, Moreno I, Romero LC.. 2019. Signaling by hydrogen sulfide and cyanide through post-translational modification. Journal of Experimental Botany 70, 4251–4265. [DOI] [PubMed] [Google Scholar]
- Gray B, Carmichael AJ.. 1992. Kinetics of superoxide scavenging by dismutase enzymes and manganese mimics determined by electron spin resonance. Biochemical Journal 281, 795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DL, Wang ZG, Pei MS, Guo LL, Yu YH.. 2020. Transcriptome analysis reveals mechanism of early ripening in Kyoho grape with hydrogen peroxide treatment. BMC Genomics 21, 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta KJ, Kolbert Z, Durner J, et al. 2020. Regulating the regulator: nitric oxide control of post-translational modifications. New Phytologist 227, 1319–1325. [DOI] [PubMed] [Google Scholar]
- Gupta KJ, Kaladhar VC, Fitzpatrick TB, Fernie AR, Møller IM, Loake GJ.. 2022. Nitric oxide regulation of plant metabolism. Molecular Plant 15, 228–242. [DOI] [PubMed] [Google Scholar]
- Han S, Cai H, An X, Huan C, Wu X, Jiang L, Yu M, Ma R, Yu Z.. 2018. Effect of nitric oxide on sugar metabolism in peach fruit (cv. Xiahui 6) during cold storage. Postharvest Biology and Technology 142, 72–80. [Google Scholar]
- Hardeland R. 2021. Melatonin, its metabolites and their interference with reactive nitrogen compounds. Molecules 26, 4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtani-Kaneko R, Hara M, Suzuki T, Reiter RJ.. 1995. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochemistry and Molecular Biology International 35, 627–634. [PubMed] [Google Scholar]
- Hernández-Ruiz J, Ruiz-Cano D, Giraldo-Acosta M, Cano A, Arnao MB.. 2022. Melatonin in Brassicaceae: role in postharvest and interesting phytochemicals. Molecules 27, 1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok JR, Vasudevan D, Thatcher GR, Thomas DD.. 2012. Is S-nitrosocysteine a true surrogate for nitric oxide? Antioxidants & Redox Signaling 17, 962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Williams DLH.. 2000. Reaction of ascorbic acid with S-nitrosothiols: clear evidence for two distinct reaction pathways. Journal of the Chemical Society, Perkin Transactions 2, 1639–1644. [Google Scholar]
- Hou BZ, Li CL, Han YY, Shen YY.. 2018. Characterization of the hot pepper (Capsicum frutescens) fruit ripening regulated by ethylene and ABA. BMC Plant Biology 18, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Jin M, Liu H, Suo J, Yin X, Zhu Q, Rao J.. 2022. Application of melatonin in kiwifruit (Actinidia chinensis) alleviated chilling injury during cold storage. Scientia Horticulturae 296, 110876. [Google Scholar]
- Kámán-Tóth E, Dankó T, Gullner G, Bozsó Z, Palkovics L, Pogány M.. 2019. Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Molecular Plant Pathology 20, 485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlova R, Chapman N, David K, Angenent GC, Seymour GB, de Maagd RA.. 2014. Transcriptional control of fleshy fruit development and ripening. Journal of Experimental Botany 65, 4527–4541. [DOI] [PubMed] [Google Scholar]
- Kirsch M, de Groot H.. 2009. N-nitrosomelatonin: synthesis, chemical properties, potential prodrug. Journal of Pineal Research 46, 121–127. [DOI] [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ.. 2011. Genetics and control of tomato fruit ripening and quality attributes. Annual Review of Genetics 45, 41–59. [DOI] [PubMed] [Google Scholar]
- Kohli SK, Khanna K, Bhardwaj R, Abd Allah EF, Ahmad P, Corpas FJ.. 2019. Assessment of subcellular ROS and NO metabolism in higher plants: multifunctional signaling molecules. Antioxidants 8, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbert Z, Barroso JB, Brouquisse R, et al. 2019. A forty year journey: the generation and roles of NO in plants. Nitric Oxide 93, 53–70. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Topal T, Tan DX, Reiter RJ.. 2009. Role of melatonin in metabolic regulation. Reviews in Endocrine and Metabolic Disorders 10, 261–270. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Değer O, Cuci Y.. 2014. Profiling the melatonin content in organs of the pepper plant during different growth stages. Scientia Horticulturae 172, 242–247. [Google Scholar]
- Kou X, Feng Y, Yuan S, Zhao X, Wu C, Wang C, Xue Z.. 2021. Different regulatory mechanisms of plant hormones in the ripening of climacteric and non-climacteric fruits: a review. Plant Molecular Biology 107, 477–497. [DOI] [PubMed] [Google Scholar]
- Kumar R, Khurana A, Sharma AK.. 2014. Role of plant hormones and their interplay in development and ripening of fleshy fruits. Journal of Experimental Botany 65, 4561–4575. [DOI] [PubMed] [Google Scholar]
- Lalel HJD, Singh Z, Tan SC.. 2003. Aroma volatiles production during fruit ripening of ‘Kensington Pride’ mango. Postharvest Biology and Technology 27, 323–336. [Google Scholar]
- Lee HJ, Back K.. 2016. 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). Journal of Pineal Research 61, 303–316. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Back K.. 2019. 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Research 2, 35–46. [Google Scholar]
- Lee U, Wie C, Fernandez BO, Feelisch M, Vierling E.. 2008. Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. The Plant Cell 20, 786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Zawadzka A, Czarnocki Z, Reiter RJ, Back K.. 2016. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). Journal of Pineal Research 61, 470–478. [DOI] [PubMed] [Google Scholar]
- Li P, Zhao L, Qi F, Htwe NMPS, Li Q, Zhang D, Lin F, Shang-Guan K, Liang Y.. 2021. The receptor-like cytoplasmic kinase RIPK regulates broad-spectrum ROS signaling in multiple layers of plant immune system. Molecular Plant 14, 1652–1667. [DOI] [PubMed] [Google Scholar]
- Li S, Chen K, Grierson D.. 2021. Molecular and hormonal mechanisms regulating fleshy fruit ripening. Cells 10, 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Lin H, Chen Y, Wang H, Ritenour MA, Lin Y.. 2019. Hydrogen peroxide-induced changes in activities of membrane lipids-degrading enzymes and contents of membrane lipids composition in relation to pulp breakdown of longan fruit during storage. Food Chemistry 297, 124955. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang J, Zhang H, Cong L, Zhai R, Yang C, et al. 2019. Melatonin inhibits ethylene synthesis via nitric oxide regulation to delay postharvest senescence in pears. Journal of Agricultural and Food Chemistry 67, 2279–2288. [DOI] [PubMed] [Google Scholar]
- Liu XS, Liang CC, Hou SG, Wang X, Chen DH, Shen JL, Zhang W, Wang M.. 2020. The LRR-RLK protein HSL3 regulates stomatal closure and the drought stress response by modulating hydrogen peroxide homeostasis. Frontiers in Plant Science 11, 548034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Huang L, Sun C, Wang L, Jin C, Lin X.. 2021. Cross-talk between hydrogen peroxide and nitric oxide during plant development and responses to stress. Journal of Agricultural and Food Chemistry 69, 9485–9497. [DOI] [PubMed] [Google Scholar]
- Lü P, Yu S, Zhu N, et al. 2018. Genome encode analyses reveal the basis of convergent evolution of fleshy fruit ripening. Nature Plants. 4, 784–791. [DOI] [PubMed] [Google Scholar]
- Ma Q, Reiter RJ, Chen Y.. 2020. Role of melatonin in controlling angiogenesis under physiological and pathological conditions. Angiogenesis 23, 91–104. [DOI] [PubMed] [Google Scholar]
- Magri A, Petriccione M.. 2022. Melatonin treatment reduces qualitative decay and improves antioxidant system in highbush blueberry fruit during cold storage. Journal of the Science of Food and Agriculture doi: 10.1002/jsfa.11774 [DOI] [PubMed] [Google Scholar]
- Majidinia M, Reiter RJ, Shakouri SK, Yousefi B.. 2018. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Research Reviews 47, 198–213. [DOI] [PubMed] [Google Scholar]
- Mata-Pérez C, Begara-Morales JC, Chaki M, Sánchez-Calvo B, Valderrama R, Padilla MN, Corpas FJ, Barroso JB.. 2016. Protein tyrosine nitration during development and abiotic stress response in plants. Frontiers in Plant Science 7, 1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michailidis M, Tanou G, Sarrou E, Karagiannis E, Ganopoulos I, Martens S, Molassiotis A.. 2021. Pre- and post-harvest melatonin application boosted phenolic compounds accumulation and altered respiratory characters in sweet cherry fruit. Frontiers in Nutrition 8, 695061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V, Singh P, Tripathi DK, Corpas FJ, Singh VP.. 2021. Nitric oxide and hydrogen sulfide: an indispensable combination for plant functioning. Trends in Plant Science 26, 1270–1285. [DOI] [PubMed] [Google Scholar]
- Møller IM, Jensen PE, Hansson A.. 2007. Oxidative modifications to cellular components in plants. Annual Review of Plant Biology 58, 459–481. [DOI] [PubMed] [Google Scholar]
- Moloudizargari M, Moradkhani F, Hekmatirad S, Fallah M, Asghari MH, Reiter RJ.. 2021. Therapeutic targets of cancer drugs: Modulation by melatonin. Life Sciences 267, 118934. [DOI] [PubMed] [Google Scholar]
- Mostafa L. 2021. Effect of hydrogen peroxide on the growth, fruit set, yield and quality of Ewais mango trees. Egyptian Journal of Horticulture 48, 71–81. [Google Scholar]
- Mukherjee S. 2019. Insights into nitric oxide–melatonin crosstalk and N-nitrosomelatonin functioning in plants. Journal of Experimental Botany 70, 6035–6047. [DOI] [PubMed] [Google Scholar]
- Muñoz-Vargas MA, González-Gordo S, Cañas A, López-Jaramillo J, Palma JM, Corpas FJ.. 2018. Endogenous hydrogen sulfide (H2S) is up-regulated during sweet pepper (Capsicum annuum L.) fruit ripening. In vitro analysis shows that NADP-dependent isocitrate dehydrogenase (ICDH) activity is inhibited by H2S and NO. Nitric Oxide 81, 36–45. [DOI] [PubMed] [Google Scholar]
- Nazir F, Fariduddin Q, Khan TA.. 2020. Hydrogen peroxide as a signalling molecule in plants and its crosstalk with other plant growth regulators under heavy metal stress. Chemosphere 252, 126486. [DOI] [PubMed] [Google Scholar]
- Noble DR, Swift HR, Williams DLH.. 1999. Nitric oxide release from S-nitrosoglutathione (GSNO). Chemical Communications 1999, 2317–2318. [Google Scholar]
- Onik JC, Wai SC, Li A, Lin Q, Sun Q, Wang Z, Duan Y.. 2021. Melatonin treatment reduces ethylene production and maintains fruit quality in apple during postharvest storage. Food Chemistry 337, 127753. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Uchida A, Takabe T, Shinagawa F, Tanaka Y, Takabe T, Hayashi T, Hattori T, Rai AK, Takabe T.. 2009. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. Journal of Plant Physiology 166, 569–578. [DOI] [PubMed] [Google Scholar]
- Palma JM, Corpas FJ, del Río LA.. 2011. Proteomics as an approach to the understanding of the molecular physiology of fruit development and ripening. Journal of Proteomics 74, 1230–1243. [DOI] [PubMed] [Google Scholar]
- Palma JM, Freschi L, Rodríguez-Ruiz M, González-Gordo S, Corpas FJ.. 2019. Nitric oxide in the physiology and quality of fleshy fruits. Journal of Experimental Botany 70, 4405–4417. [DOI] [PubMed] [Google Scholar]
- Palma JM, Mateos RM, López-Jaramillo J, Rodríguez-Ruiz M, González-Gordo S, Lechuga-Sancho AM, Corpas FJ.. 2020. Plant catalases as NO and H2S targets. Redox Biology 34, 101525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo-Hernández M, López-Delacalle M, Martí-Guillen JM, Martínez-Lorente SE, Rivero RM.. 2021. ROS and NO phytomelatonin-induced signaling mechanisms under metal toxicity in plants: a review. Antioxidants 10, 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-González A, Galano A, Alvarez-Idaboy JR, Tan DX, Reiter RJ.. 2017. Radical-trapping and preventive antioxidant effects of 2-hydroxymelatonin and 4-hydroxymelatonin: contributions to the melatonin protection against oxidative stress. Biochimica et Biophysica Acta, General Subjects 1861, 2206–2217. [DOI] [PubMed] [Google Scholar]
- Peyrot F, Houée-Levin C, Ducrocq C.. 2006. Melatonin nitrosation promoted by NO2; comparison with the peroxynitrite reaction. Free Radical Research 40, 910–920. [DOI] [PubMed] [Google Scholar]
- Podgórska A, Burian M, Szal B.. 2017. Extra-cellular but extra-ordinarily important for cells: apoplastic reactive oxygen species metabolism. Frontiers in Plant Science 8, 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi R. 2013. Protein tyrosine nitration: biochemical mechanisms and structural basis of functional effects. Accounts of Chemical Research 46, 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Manchester LC, Qi W.. 2001. Biochemical reactivity of melatonin with reactive oxygen and nitrogen species: a review of the evidence. Cell Biochemistry and Biophysics 34, 237–256. [DOI] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Fuentes-Broto L.. 2010. Melatonin: a multitasking molecule. Progress in Brain Research 181, 127–151. [DOI] [PubMed] [Google Scholar]
- Riga P, Medina S, García-Flores LA, Gil-Izquierdo A.. 2014. Melatonin content of pepper and tomato fruits: effects of cultivar and solar radiation. Food Chemistry 156, 347–352. [DOI] [PubMed] [Google Scholar]
- Rodrigues O, Shan L.. 2021. Stomata in a state of emergency: H2O2 is the target locked. Trends in Plant Science 27, 274–286. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ruiz M, Mateos RM, Codesido V, Corpas FJ, Palma JM.. 2017. Characterization of the galactono-1,4-lactone dehydrogenase from pepper fruits and its modulation in the ascorbate biosynthesis. Role of nitric oxide. Redox Biology 12, 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan S, Wang Z, Pu H, Duan W, Song H, Li J, Zhang Z, Xu X.. 2022. DNA methylation mediated by melatonin was involved in ethylene signal transmission and ripening of tomato fruit. Scientia Horticulturae 291, 110566. [Google Scholar]
- Shukla M, Chinchalongporn V, Govitrapong P, Reiter RJ.. 2019. The role of melatonin in targeting cell signaling pathways in neurodegeneration. Annals of the New York Academy of Sciences 1443, 75–96. [DOI] [PubMed] [Google Scholar]
- Siddiqui MH, Alamri S, Nasir Khan M, Corpas FJ, Al-Amri AA, Alsubaie QD, Ali HM, Kalaji HM, Ahmad P.. 2020. Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. Journal of Hazardous Materials 398, 122882. [DOI] [PubMed] [Google Scholar]
- Singh N, Kaur H, Yadav S, Bhatla SC.. 2016. Does N-nitrosomelatonin compete with S-nitrosothiols as a long distance nitric oxide carrier in plants? Biochemistry & Analytical Biochemistry 5, 262. [Google Scholar]
- Singh N, Jain P, Gupta S, Khurana JM, Bhatla SC.. 2021. N-Nitrosomelatonin, an efficient nitric oxide donor and transporter in Arabidopsis seedlings. Nitric Oxide 113-114, 50–56. [DOI] [PubMed] [Google Scholar]
- Singh P, Mishra V, Tripathi DK, Corpas FJ, Singh VP.. 2022. RIPK: a crucial ROS signaling component in plants. Trends in Plant Science 27, 214–216. [DOI] [PubMed] [Google Scholar]
- Smirnoff N, Arnaud D.. 2019. Hydrogen peroxide metabolism and functions in plants. New Phytologist 221, 1197–1214. [DOI] [PubMed] [Google Scholar]
- Smith JN, Dasgupta TP.. 2000. Kinetics and mechanism of the decomposition of S-nitrosoglutathione by l-ascorbic acid and copper ions in aqueous solution to produce nitric oxide. Nitric Oxide 4, 57–66. [DOI] [PubMed] [Google Scholar]
- Sofic E, Rimpapa Z, Kundurovic Z, Sapcanin A, Tahirovic I, Rustembegovic A, Cao G.. 2005. Antioxidant capacity of the neurohormone melatonin. Journal of Neural Transmission 112, 349–358. [DOI] [PubMed] [Google Scholar]
- Sun C, Liu L, Wang L, Li B, Jin C, Lin X.. 2021. Melatonin: a master regulator of plant development and stress responses. Journal of Integrative Plant Biology 63, 126–145. [DOI] [PubMed] [Google Scholar]
- Sun H, Cao X, Wang X, Zhang W, Li W, Wang X, Liu S, Lyu D.. 2021. RBOH-dependent hydrogen peroxide signaling mediates melatonin-induced anthocyanin biosynthesis in red pear fruit. Plant Science 313, 111093. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ma C, Kang X, Zhang L, Wang J, Zheng S, Zhang T.. 2021. Hydrogen sulfide and nitric oxide are involved in melatonin-induced salt tolerance in cucumber. Plant Physiology and Biochemistry 167, 101–112. [DOI] [PubMed] [Google Scholar]
- Symons GM, Chua YJ, Ross JJ, Quittenden LJ, Davies NW, Reid JB.. 2012. Hormonal changes during non-climacteric ripening in strawberry. Journal of Experimental Botany 63, 4741–4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Manchester LC, Galano A, Reiter RJ.. 2014. Cyclic-3-hydroxymelatonin (C3HOM), a potent antioxidant, scavenges free radicals and suppresses oxidative reactions. Current Medicinal Chemistry 21, 1557–1565. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ.. 2007. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? Journal of Pineal Research 42, 28–42. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Esteban-Zubero E, Zhou Z, Reiter RJ.. 2015. Melatonin as a potent and inducible endogenous antioxidant: synthesis and metabolism. Molecules 20, 18886–18906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q, Li C, Ge Y, Li X, Cheng Y, Hou J, et al. 2020. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT – Food Science and Technology 127, 109431. [Google Scholar]
- Tijero V, Muñoz P, Munné-Bosch S.. 2019. Melatonin as an inhibitor of sweet cherries ripening in orchard trees. Plant Physiology and Biochemistry 140, 88–95. [DOI] [PubMed] [Google Scholar]
- Torun H, Uluisik S.. 2022. Postharvest application of hydrogen peroxide affects physicochemical characteristics of tomato fruits during storage. Horticulture, Environment and Biotechnology doi: 10.1007/s13580-021-00403-5 [DOI] [Google Scholar]
- Vanstraelen M, Benková E.. 2012. Hormonal interactions in the regulation of plant development. Annual Review of Cell and Developmental Biology 28, 463–487. [DOI] [PubMed] [Google Scholar]
- Wang F, Zhang X, Yang Q, Zhao Q.. 2019. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chemistry 301, 125311. [DOI] [PubMed] [Google Scholar]
- Wang Y, Reiter RJ, Chan Z.. 2018. Phytomelatonin: a universal abiotic stress regulator. Journal of Experimental Botany 69, 963–974. [DOI] [PubMed] [Google Scholar]
- Wei J, Li DX, Zhang JR, Shan C, Rengel Z, Song ZB, et al. 2018. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. Journal of Pineal Research 65, e12500. [DOI] [PubMed] [Google Scholar]
- Wei J, Zhang Z, Zhang P, Wu B.. 2021. Regulation of ethylene biosynthesis and signal transduction by nitric oxide leading to resistance against Alternaria alternata in Hami melon. Journal of the Science of Food and Agriculture doi: 10.1002/jsfa.11697 [DOI] [PubMed] [Google Scholar]
- Wills RB, Soegiarto L, Bowyer MC.. 2007. Use of a solid mixture containing diethylenetriamine/nitric oxide (DETANO) to liberate nitric oxide gas in the presence of horticultural produce to extend postharvest life. Nitric Oxide 17, 44–49. [DOI] [PubMed] [Google Scholar]
- Wu F, Chi Y, Jiang Z, et al. 2020. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 578, 577–581. [DOI] [PubMed] [Google Scholar]
- Wu Q, Tao X, Ai X, Luo Z, Mao L, Ying T, Li L.. 2018. Contribution of abscisic acid to aromatic volatiles in cherry tomato (Solanum lycopersicum L.) fruit during postharvest ripening. Plant Physiology and Biochemistry 130, 205–214. [DOI] [PubMed] [Google Scholar]
- Xia H, Shen Y, Shen T, et al. 2020. Melatonin accumulation in sweet cherry and its influence on fruit quality and antioxidant properties. Molecules 25, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yue Q, Xiang G, Bian F, Yao Y.. 2018. Melatonin promotes ripening of grape berry via increasing the levels of ABA, H2O2, and particularly ethylene. Horticulture Research 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentgraf U, Andrade-Galan AG, Bieker S.. 2022. Specificity of H2O2 signaling in leaf senescence: is the ratio of H2O2 contents in different cellular compartments sensed in Arabidopsis plants? Cellular & Molecular Biology Letters 27, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Zhang Y.. 2014. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. Journal of Pineal Research 57, 131–146. [DOI] [PubMed] [Google Scholar]
- Zhang N, Sun Q, Zhang H, Cao Y, Weeda S, Ren S, Guo YD.. 2015. Roles of melatonin in abiotic stress resistance in plants. Journal of Experimental Botany 66, 647–656. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang Y, Xu C, Liu K, Bi H, Ai X.. 2021. H2O2 functions as a downstream signal of IAA to mediate H2S-induced chilling tolerance in cucumber. International Journal of Molecular Science 22, 12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Wang H, Chen S, Yu D, Reiter RJ.. 2021. Phytomelatonin: an emerging regulator of plant biotic stress resistance. Trends in Plant Science 26, 70–82. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Zhu X, Hou Y, Wang X, Li X.. 2019. Effects of nitric oxide fumigation treatment on retarding cell wall degradation and delaying softening of winter jujube (Ziziphus jujuba Mill. cv. Dongzao) fruit during storage. Postharvest Biology and Technology 156, 110954. [Google Scholar]
- Zhu S, Zhou J.. 2007. Effect of nitric oxide on ethylene production in strawberry fruit during storage. Food Chemistry 100, 1517–1522. [Google Scholar]
- Zhu Y, Gao H, Lu M, Hao C, Pu Z, Guo M, Hou D, Chen LY, Huang X.. 2019. Melatonin-nitric oxide crosstalk and their roles in the redox network in plants. International Journal of Molecular Sciences 20, 6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccarelli R, Rodríguez-Ruiz M, Lopes-Oliveira PJ, et al. 2021. Multifaceted roles of nitric oxide in tomato fruit ripening: NO-induced metabolic rewiring and consequences for fruit quality traits. Journal of Experimental Botany 72, 941–958. [DOI] [PubMed] [Google Scholar]