Abstract
The current pandemic of the acute severe respiratory syndrome coronavirus 2 (SARS-CoV-2) killed about 6.4 million and infected more than 600 million individuals by august of 2022, and researchers worldwide are searching for fast and selective approaches for this virus detection. Colorimetric biosensors are an excellent alternative because they are sensitive, simple, fast, and low-cost for rapid detection of SARS-CoV-2 compared to standard Enzyme-linked immunosorbent assay (ELISA) and Polymerase Chain Reaction (PCR) techniques. This study systematically searched and reviewed literature data related to colorimetric biosensors in detecting SARS-CoV-2 viruses, recovered from the Scopus (n = 16), Web of Science (n = 19), PubMed (n = 19), and Science Direct (n = 17) databases totalizing n = 71 articles. Data were analyzed for the type of nanomaterial, biorecognition material at the detection limit (LOD), and devices designed for diagnostics. The most applied nanomaterial were gold nanoparticles, in their original form and hybrid in quantum dots and core-shell. In addition, we show high specificity in point-of-care (POC) diagnostic devices as a faster and cheaper alternative for clinical diagnosis. Finally, the highlights of the colorimetric biosensor developed for diagnostic devices applied in swabs, surgical masks, and lateral flow immunoassays were presented.
Keywords: COVID-19, Coronavirus, POC, Diagnosis devices, Nanosensors, Biosensors
Graphical Abstract
1. Introduction
The pandemic caused by severe acute respiratory coronavirus 2 (SARS-CoV-2) infection resulted in the COVID-19 disease, killing about 6.4 million and infecting more than 600 million individuals by august 2022 [1]. Many deaths are due to cases of pneumonia and other complications, and the virus spreads from person to person, most commonly in hospitals and among families [2].
The virus’s widespread transmission raises significant concerns about rapid detection methods. Among the techniques for detecting SARS-CoV-2 (diagnostic tests), Enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) are the most indicated because they have high sensitivity and precision. However, they are high cost and depend on complex equipment and trained technicians only viable in centralized laboratories. In addition, PCR may take a few days to obtain the results, and immunological assays are a complex reaction of antibodies with recombinant proteins. The need to produce faster devices with lower cost and reliability for detecting SARS-CoV-2 was observed [3].
Biosensors are simple, fast, low cost, sensitive, and specific, with good portability and miniaturization potential. Miniature devices with different sensing platforms that can detect SARS-CoV-2, for example, through colorimetric detections, are popular due to their ease of use and ability to capture images through electronic benchtops and portable devices [4], [5], [6]. However, when working with analyses in such low concentrations, these devices have limitations because it is often difficult to convert detectable signals into color readings, resulting in low sensitivity [5].
Nanomaterials can improve the sensitivity of biosensors based on the colorimetry detection method [7]. Compounds such as noble metal nanoparticles, including gold nanoparticles (AuNPs) [8], [9], [10], silver nanoparticles (AgNPs) [11], [12], magnetic nanoparticles (MNPs) [13], [14], carbon-based nanostructures [15], [16], [17], nanohybrids corel shell [18], [19], quantum dots [20], [21] and others can be used for this purpose. Because nanomaterials have different proprieties as the large surface area of contact, they can increase the number of conjugated receptors on their surface, increasing recognition events and improving detection performance [5]. Moreover, DNA, proteins, peptides [22], and enzymes responsible for detection can be attached to the surfaces of nanoparticles [23]. The use of nanomaterials for the fabrication technology of biosensor devices creates a new set of biosensors called nanobiosensors.
Prior amplification of genetic material also is an efficient approach that significantly improves the sensitivity of biosensors. Colorimetric biosensors associated with molecular biology techniques increased sensitivity and specificity to SARS-CoV-2 RNA [23], [24]. Transcription Mediated Amplification (TMA), Rolling Circle Amplification (RCA), Clustered Regularly Spaced Short Palindromic Repeats (CRISPR), and Mediated Isothermal Amplification Loop (LAMP) are Isothermal amplification techniques that have gained popularity for their simplicity of execution and speed [23], [24].
Given the current pandemic scenario, mass screening is critical to address the need for rapid diagnosis. The purpose of this study was to conduct a systematic search of four scientific databases relevant to the topic at hand, analyzing all articles published in the literature that experimentally developed a colorimetric biosensor for detecting SARS-CoV-2. The databases used were Scopus (n = 16), Web of Science (n = 19), PubMed (n = 19) and Science Direct (n = 17), totaling n = 71 articles that reviewed and discussed the most recent advances in colorimetric biosensors in the detection of SARS-CoV-2. The benefits of using nanomaterials in colorimetric biosensors to improve sensitivity, the use of gold nanomaterials, the innovation of ACE2 as a biorecognition element, and finally the biosensors that were applied to POC devices were discussed throughout the text. In addition, the devices that stood out for their innovations were discussed, such as application in surgical masks, Nano-Amplified Colorimetric Test (NACT), optodiagnostic and lateral flow immunoassay (LFIA).
2. Systematic methodology
This systematic review searched over four scientific databases (Web of Science, SCOPUS, PubMed, and Science Direct) about biosensors to SARS-CoV-2 detection with the colorimetric transducer. The search followed a four-phase flow diagram and guidelines for systematic review and meta-analyses (PRISMA) [24].
2.1. Focus questions
The focus questions agreed with the problem, intervention, comparison, and outcome method by PICO. The research questions were: What types of colorimetric biosensors can be used to improve the detection of SARS-CoV-2? Which nanomaterial is most used to detect SARS-CoV-2 using a colorimetric biosensor? Which nanomaterial has presented the best sensitivity? What are the types of samples analyzed by these biosensors?
2.2. Information sources
This systematic review was conducted on January 18, 2022, through research in the four previously cited databases. The study was realized with the search components SC1 (Biosensor) AND SC2 (Biosensor) AND SC3 (SARS-CoV-2 OR COVID-19 OR coronavirus).
2.3. Selection criteria
This review considered original research articles and excluded revisions, thesis, and short communications. We first conducted the preliminary selection of abstracts, keywords, and titles identified independently. Articles were removed in this initial screening if the study did not investigate biosensors with colorimetric detection for SARS-CoV-2. Articles non-written in the English language were also excluded. After full-text reading, articles that did not meet any focus questions were rejected.
3. Dataset results
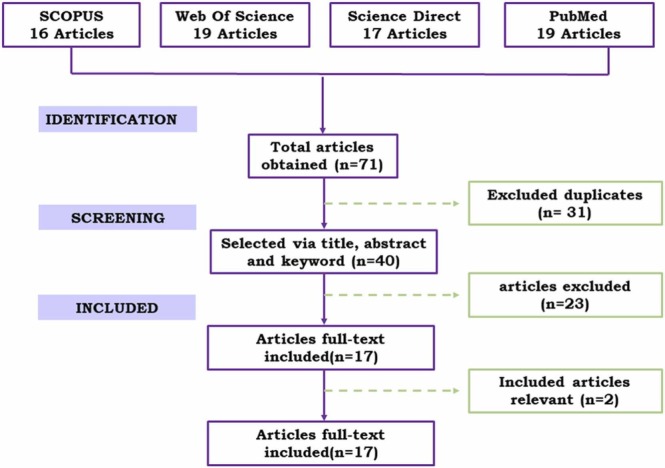
Data extraction and quality assessment were performed independently by three revisions. We only excluded studies after full-text reading discarded doubts about the study's eligibility. A total of 71 studies from the Scopus (n = 16), Web of Science (n = 19), PubMed (n = 19), and Science Direct (n = 17) databases were elected. The results of the systematic search are presented on the PRISMA flow chart, Fig. 1. After full-text reading of the articles, 17 were selected for quantitative analysis. All useful information obtained by each study were extracted and compiled in Table 1, Table 2, as presented in the following sections.
Fig. 1.
The schematic diagram for the selection of articles included based on PRISMA methodology.
Table 1.
Description of colorimetric biosensors for detection SARS-CoV-2.
| Nanomaterial | Size | Target | Biological recognition element | LOD | Reference |
|---|---|---|---|---|---|
| Polymer mPEG-PCL- AuNP | 222,1 nm | protein Spike | antibody | 0,11 ng/mL | [31] |
| AuNPs-N | 30 nm | IgG, IgM e IgA | protein N-antigen | [32] | |
| MNP (γ Fe2O3) | protein Spike | ACE2 receptor | 4,98 ng/mL | [33] | |
| AuNPs-ACE2 | 7 nm | Protein Spike | ACE2 receptor | 1.54×10-4 ng/mL | [34] |
| SiO2 @Au@QD NBs | ∼ 240 nm | Protein Spike | IgG and IgM | 1:106 dilution | [35] |
| f-AuNPs | 20 nm | spike, envelope, and membrane | antibody | Ct 7 | [36] |
| NPs core–shell Au@Pt | 25 nm | Protein spike S1 | polyclonal antibodies | 11 ng/mL | [37] |
| AuNPs | 40 nm | Protein N | antibody rabbit IgG | 3 ng/mL | [38] |
| AuNPs | – | Protein N | Antigen | 150 ng/mL | [39] |
| SiO2 @Au/QD | ∼200 nm | Protein spike S1 | antibody | 1 ng/mL | [40] |
| AuNPs | 17,7 nm | ORF1, ORF2, E1, and E2 | cDNA | 580 nM | [41] |
| AuNPs | 16 nm | Protein Spike | antibody monoclonal (mAb) | 48 ng/mL | [42] |
| AuNPs | – | Gene N | DNA+BoNT / A LC | 1 copie/μL | [43] |
| Fe3O4/Au core-shell | 30–80 nm | Protein Spike | anti-spike protein antibodies | 1200 PFU/mL | [44] |
| AuNPs | 80–120 nm | Gene N | ssDNA | 10 copies/μL | [45] |
| Not applicated | – | DNA biotinilados | ∼1 nM | [46] | |
| Not applicated | Gene N and E | ssDNA | 10 copies | [47] |
Table 2.
Description of biosensors in terms of POCs developed, AFOM, analyze time, sample type, and confirmation technique.
| Applicated in POC | AFOM |
Time | Samples | Confirmation thecnique | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Linear range | LOD | Sensitivity (%) | Recovery (%) | RSD (%) | Specificity (%) | |||||
| Colorimetric paper-based diagnosis | 0.17–3 ng/mL | 0,11 ng/mL | 4.59 * | 30 min | Serum human | RT-PCR and ELISA | [31] | |||
| Lateral flow immunoassay | 89 | 100 | 20 min | Serum human | ELISA | [32] | ||||
| Not applicated | 4.98 − 1 13 ng/mL | 498 ng/mL | 97.56 | 3.16 * | 90.24 | 30 min | Oropharyngeal and nasopharyngeal | RT-PCR | [78] | |
| Optodiagnostic | 10−3 − 103 ng/mL | 154 ng/mL | 96 | 5.7%* | 80 | 5 min | Nasopharyngeal and oropharyngeal | RT-PCR | [88] | |
| Lateral flow immunoassay | 1:106 dilution | 100 | 100 | 15 min | Serum human | RT-PCR | [35] | |||
| Not applicated | Ct 7 | 96 | 98 | 3 min | Nasal | RT-PCR | [36] | |||
| Not applicated | 10 – 100 ng/mL | 11 ng/mL | high | high | 20 min | Not applicated | Elisa | [37] | ||
| Surgical face masks | 0,3–102 ng/mL | 3 ng/mL | 96,20 | 100 | < 10 min | Face masks | RT-PCR | [38] | ||
| Not applicated | 150–550 ng/mL | 150 ng/mL | high | 5 min | Not applicated | not applicated | [39] | |||
| Lateral flow immunoassay | 0.1–100 ng/mL | 1 ng/mL | > 92.98 | 3.92–5.19 | high | 30 min | Throat and nose swab | RT-qPCR | [40] | |
| Not applicated | 10–10-5 nM | 580 nM | 40 min | Simulated samples | RT-qPCR | [41] | ||||
| Not applicated | 250–1000 ng/mL | 48 ng/mL | 94.1 ± 2.1 | 2.2 | high | 10 min | Saliva | RT-qPCR | [42] | |
| Not applicated | 1 copies/μL | 1–2 h | – | [43] | ||||||
| Lateral flow | 10 copies | inconsistent | 1 h | Swabs orofaríngeos e nasofaríngeos | RT-qPCR | [47] | ||||
| ‘Nano-amplified colorimetric test’ | 10 copies/μL | > 96.6 | 100 | < 1 h | Swabs orofaríngeos e nasofaríngeos | RT-qPCR | [45] | |||
| Not applicated | 0–500 nM | ∼ 1 nM | high | 2 h | Not applicated | [46] | ||||
| Magnetic-focus-enhanced lateral flow assay | – | 1200 PFU/mL | 66.7 | 100 | 50 min | Saliva | [44] | |||
Legend: (*) Relative standard deviation in relative to reproducibility; RSD (Relative standard deviation); LOD (Limite Of Detection); RT-PCR (Reverse.transcription polymerase chain reaction); RT-qPCR (Reverse transcription polymerase chain reaction in real-time quantitative)
3.1. Colorimetric biosensors
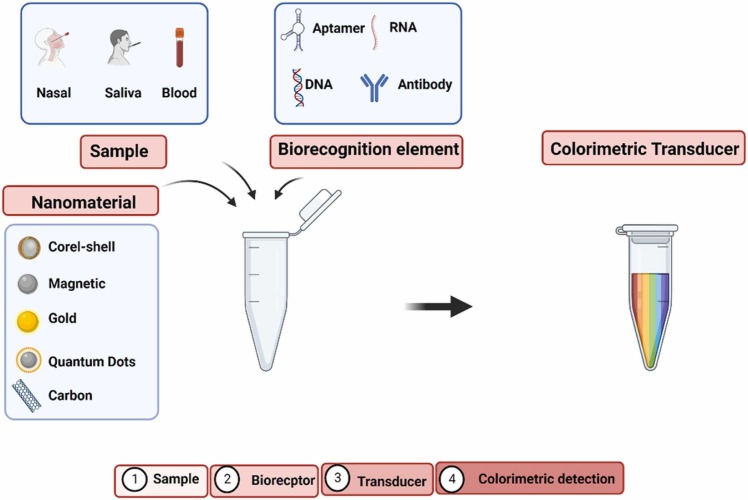
Colorimetric biosensors are a promising diagnostic device, particularly in areas with limited resources, emergencies, and home care, where external devices and reagents are not required [5], [25]. The advantage of this tool is its ability to provide a visible result to the naked eye, which overcomes the limitations of the gold standard, PCR, and ELISA techniques, regarding the time, cost, and techniques required for diagnosis [26]. Same with other biosensors that need expensive instruments to verify detection, such as electrochemical [17], SERS [27], and fluorescence [28], among others. Fast and easy detection systems are very desirable in the current pandemic scenario, where it is crucial to effectively detect the presence of viruses to combat and control the transmission of the SARS-CoV-2 that happens very quickly [29], [30]. Fig. 2 shows a diagram of colorimetric biosensors.
Fig. 2.
Diagram of main elements of a colorimetric biosensor and the steps in the detection.
Legend: (-) not reported; AuNPs (Gold nanoparticles); MNP (Magnetic nanoparticles); AuNPs-ACE2 (Gold nanoparticles with ACE2 protein acoplated); NBs (SiO2 @Au@QD nanobeads); mPEG-PCL (copolímeros dibloco de etileno glicol-caprolactona); AuNPs-N (Gold nanoparticles whit adsorbed nucleocapsid protein (N)); f-AuNPs (AuNPs functionalized with anti-SARS-CoV-2); SiO2 @Au/QD ( gold nanoparticles and quantum dots on SiO2); mPEG–PCL (methoxy poly(ethylene glycol-block-caprolactone)diblock copolymers); ssDNA (DNA de fita simples); DNA+BoNT / A LC (botulinum neurotoxin type A light chain);.
3.2. The role of nanomaterials in colorimetric biosensor
Nanomaterials have been used in biosensors (nanobiosensor) to improve sensitivity, especially colorimetric detection. Nanomaterials offer various physical and chemical properties and are easily functionalized with biomolecules [48]. Functionalization can happen with several biological structures, facilitating the capture of targets and amplifying the detection signal. Its works due to the greater contact area of the nanomaterials near the receptors. The more receptors in their surface area, the greater the chance of binding the target molecules and improving the sensitivity of the biosensor [14], [49], [50]. Among the selected studies that developed a colorimetric biosensor for detecting SARS-CoV-2, only two did not use nanomaterials (Table 1). This result shows that nanoparticles are already widely used as their benefits in detecting analytes with high sensitivity.
Nanomaterials are of interest in biosensing research because they can be exploited directly as signal reporters due to their inherent physical and optical properties. These characteristics include a high surface-to-volume ratio that enables suitable surface modification with bioactive compounds, an excellent capacity for reaction catalysis, electrical conductivity, excellent biocompatibility, a particularly high characteristic extinction coefficient in visible light, and a visual color transition resulting from the shift of surface plasma absorption as a result of varying size and shape. Using nanomaterials in colorimetric biosensors can dramatically magnify signal strength and increase the sensitivity of target biological molecules, including pathogenic bacteria and viruses, DNA, and proteins. Gold and silver nanoparticles are particularly intriguing among many nanomaterials due to their simplicity and sensitivity in producing a color response. Gold nanoparticles (AuNPs) are widely utilized in colorimetric biomedical assays because they are simple to produce, chemically and physically stable, biocompatible, exhibit a unique optoelectronic characteristic, and can be modified with bioactive and organic molecules [47]. Moreover, functionalization can occur with various biological configurations, facilitating target capture and amplifying detection signals due to the increased contact area of the nanomaterials near the receptors. The more receptors on their surface area, the better their chances of binding target molecules and improving biosensor sensitivity [14], [48], [49]. Due to these combined benefits, colorimetric biosensors have been used to detect SARS-CoV-2 antigens, antibodies, and/or their fragments at the molecular level. Table-1 demonstrates the application of these nanomaterials for the highly sensitive detection of SARS-CoV-2 spike protein [51]. Apart from the advantages, some technical issues need to be addressed right away with these nanomaterials for colorimetric detection of SARS-CoV-2, such as reliability and reproducibility in high ionic strength samples, such as in serum and urine, as well as lack of sensitivity is a concern in some applications because perceptible color change is difficult to measure, limiting their application to biological sample analysis in comparison to other analytical methods such as fluorescence and chemiluminescence [5].
SARS-CoV-2 detection can entail isolating and detecting nucleic acids in biological samples such as blood, feces, oral or nasal swab, tracheal, lung tissue, and sputum, which are used to investigate the level of infection during the pandemic. COVID-19 diagnostic methods must be effective, quick, and low-cost. Several biosensor improvements are being optimized and validated by researchers all over the world in order to meet the needs of the COVID-19 pandemic with faster detection and a low chance of false positive or false negative results [52], [53].
Colorimetric reactions can be performed on various platforms, such as paper sheets, for a simple and quick approach to colorimetric detection at a low cost. Colorimetric detections generally use simple equipment or can be seen with the naked eye, such as when a color change is involved or by fluorescence and luminescence [54]. Thus, in asymptomatic cases or individuals at the beginning of the infection, rapid and sensitive tests for the diagnosis of COVID-19 are required; this allows health professionals to diagnose more accurately. Aside from the possibility of early detection, this is an effective strategy for reducing the spread of the SARS-CoV-2 virus, with several advantages over the standard gold test, RT-qPCR.
Colorimetric detection with gold nanoparticles is one example, in which the chromogenic effect (color appearance) is caused by the aggregation of the gold nanoparticles. Colorimetric detections based on gold nanoparticles have a number of advantages, including stability and ease of fabrication. Gold nanoparticles can be linked to biorecognition components like antibodies or antigens. This device produces results that can be evaluated without the use of instruments. Several brands of test kits are currently available on the market that is based on the reactions between antigens or human antibodies anti-SARS-CoV-2 detected by immunochromatography in the presence of gold nanoparticles [52].
3.2.1. Gold-based nanomaterials
Gold metal (Au) has unique optical nanobiosensors and electronic properties responsible for the high popularity of this metal used in nanomaterials in the biomedical field. The high extinction coefficient of gold nanoparticles in 1010 M −1 cm −1 indicates that the metal strongly absorbs radiation [55], [56]. This strong absorption generates intense bands of localized surface plasmonic resonance (LSPR), which is caused by the oscillation of free electrons on the surface of the metal under light stimulation [57]. Metals with intense SPR bands and absorption in the visible region are called noble metals, and gold is among them [58], [59], [60], [61]. These optical characteristics and the fact that it is biocompatible and inert make gold the most used metal in biological sensors. The biocompatibility of gold nanomaterials (AuNMs) was explored with functionalization for DNA detection as an alternative to replace radioactive markers that were the main ones used at the time [62].
The main advantage of AuNMs in optical biosensors is the possibility of visualizing the detection result with the naked eye [58]. An example is spherical AuNPs (Gold nanoparticles) which are the most used for displaying the SPR present in the visible region of 400–700 nm without enlargements. The solution color, blue or red, will depend on the interparticle distances when functionalized. The solution will be red if the distances are more significant than the average particle diameter; otherwise, it will be blue [63]. Confirmation of the AuNPs functionalization can also be observed by the displacement of the SPR absorption band. After detecting the target by the AuNPs, the solution will change color, being possible to visualize the positive result with the naked eye without the need for optical instruments [58]. In addition to the color change, the detection can be observed by the difference in the intensity of the SPR bands.
The physicochemical properties of AuNPs allow adequate control of their size and shape in their synthesis and can generate different forms of AuNMs. Some forms most commonly used in sensors are nanorods [64], [65], nanostars [66], [67], nanoflowers [68], [69], core-shell [70], [71] and nanospheres [72], [73]. However, gold nanospheres are the most commonly used form due to their isotropic structure [74]. A study by Mustafa et al. (2010) compared the SPR bands of AuNPs of spherical forms, nanorods, and nano octahedrons. The different forms investigated their SPR effects in different sizes in a fixed excitation wavelength of 670 nm with a fixed concentration of NPs. It was noted that nanospheres and octahedrons had the SPR signal affected by mass changes based on the refraction index. The mass effect of the sharpness of the corners and edges plays an important role in the field and, consequently, in the displacement response of the SPR angle. In this case, the gold nanorods produce an SPR signal almost twice the displacement of the SPR angle as the gold nanospheres. Conclusion affirmed that gold nanospheres should be preferred for marking sensors because there is no strong dielectric effect. Moreover, nanorods are very considerable because a double increase can be obtained compared to gold nanospheres [75].
For the same material, the optical properties of nanoparticles are directly influenced by their shape. Due to the atoms being in different faces and angles, metal nanoparticles of the same metal but with different shapes have different properties, allowing multiple surface plasmonic resonances [74], [76]. Wiley et al. (2006) demonstrated how shape control can be used to adjust the optical properties of silver nanostructures using Mie's theory and Maxwell's equations calculations. Variations in the parameters: size, shape, or dielectric environment of the particles will result in the polarization change of its surface, affecting the resonance peak [59]. The spectrum of UV–vis extinction, absorption, and scattering obtained through theoretical calculations for different nanostructures: spherical, cube, tetrahedron, octahedron, and shell showed different absorption peaks [59]. The structures with sharp corners presented more peaks than the spherical shape because they had several distinct symmetries for dipolar resonance, and the loads accumulated in the corners of the structure. The hollow, spherical, or shells also present the plasmonic resonance peak diverted to red concerning a sphere because the loads on the inner and outer surface show the same signal for a given pole [59]. It concludes that the size and shape of the AuNPs and the dielectric constant of the surrounding environment have a high impact on the LOD [8]. All these factors make the spherical shape the most used.
Given the evidence discussed above, the data obtained by the articles selected in this systematic review did not let us establish any pattern between the nanomaterial size and the LOD obtained. The data showed the most varied sizes from 7 to 21 nm and LODs from 1 to 154 ng/L for studies that used nanomaterials. In 14 out of 17 studies (Table 1), gold was the most applied material/metal among the nanomaterials used to improve biosensor sensitivity.
The chromogenic effect (or chromatic alteration) produced by AuNPs explains their dominance in colorimetric biosensors. This effect is the aggregation of AuNPs, which results in a color change in virus detection. SARS-CoV-2 [77]. The interparticle distance is a critical parameter in nanoparticle aggregation and is responsible for the color displayed. Another important factor is particle diameter; NPs larger than 80 nm tend to shift their emission to the infrared region, making them impossible to detect with the naked eye. The fact that the gold nanoparticles present this effect offers an advantage compared to other nanomaterials. Of the published studies of colorimetric biosensors, only one study uses magnetic iron oxide nanoparticles (γ-Fe3O4), all other works use gold. In this single study, the visualization of color change is related to the oxidation of 3,3',5,5'-tetramethylbenzidine (TMB) in the presence of hydrogen peroxide (H2O2) and NPs of γ-Fe3O4 [78]. Unlike gold or nanoparticles of γ-Fe 3O4 cannot cause color change with the detection of the target. This can be explained by the iron (Fe) not absorbing in the region of the visible, ∼300 nm, causing the metal to have no SPR band [79]. Although iron nanoparticles are less expensive to synthesize, they do not detect the direct target, necessitating complementary reactions, making them less suitable for colorimetric biosensors. The sensitivity generated by the nanomaterial compared to the AuNPs is also an important issue. Büyüksünetçi et al., (2021) with γ-Fe3O4 nanoparticles present LOD 4.98 ng/mL, while Ferreira et al. [34] with AuNPs present LOD 1.54 × 10-4, a detection of about 104 more sensitives, in which the two studies used ACE2 as a biorecognition element. These presented facts provided solid justifications for the popularity of gold-based nanomaterials for biosensors with colorimetric detection.
3.3. The innovation of ACE2 as a biorecognition element in colorimetric biosensors
The biosensor's precision and accuracy are directly linked to the biorecognition element and the detection region within SARS-CoV-2 DNA. Biorecognition elements also should be very specific. For SARS-CoV-2, biosensors, antibodies, and nucleic acid probes are the biorecognition elements most used [80]. According to reported publications (Table 1), most contained antibodies as biorecognition material, followed by nucleic acid, the human angiotensin-converting enzyme 2 (ACE2), antigen, and Protein N. They used monoclonal or polyclonal antibodies in biosensors and presented sensitive results with LOD 0.11 ng/L for detecting SARS-CoV-2 [31] in 150 ng/L [39] . Because of their high specificity and affinity binding properties, antibodies or immunoglobulins (Ig) are very popular in immunodiagnostic assays. Immunoglobulin A (IgA), Immunoglobulin D (IgD), Immunoglobulin E (IgE), Immunoglobulin G (IgG), and Immunoglobulin M are the five isotypes of immunoglobulins (IgM). The most common types are IgG and IgM, which work together to provide immediate and long-term protection against infections, while IgE is linked to allergies. [81], [82]. Among the selected articles, we discovered that using aptamers in colorimetric detection was not observed among the biorecognition elements. cDNA or aptamers are gold standard diagnostic tools that use nucleic acids such as DNA (deoxyribonucleic acid) or RNA (ribonucleic acid). Nucleic acid-based biosensors are highly selective because they allow direct detection of a specific genetic fragment whose complementary sequence can be synthesized with high purity [83]. Because of this conjugation, they are highly specific for detecting SARS-CoV-2, for example. It can be tailored to a specific conserved genome region, avoiding regions where variants could result in false-negative detection [84]. Mutations occur naturally during virus replication within the host cell, and many mutations result in new variants. As a result, due to a high mutation rates of SARS-CoV-2, the correct selection of the detection region in the virus genome is critical for its identification [84]. Interestingly, the most significant number of mutations in SARS-CoV-2, about 4000, are encoded in the S gene, which is associated with viral entry into cells [85]. Despite the reported numerous mutations, more than half of the included studies chose this gene as a detection region.
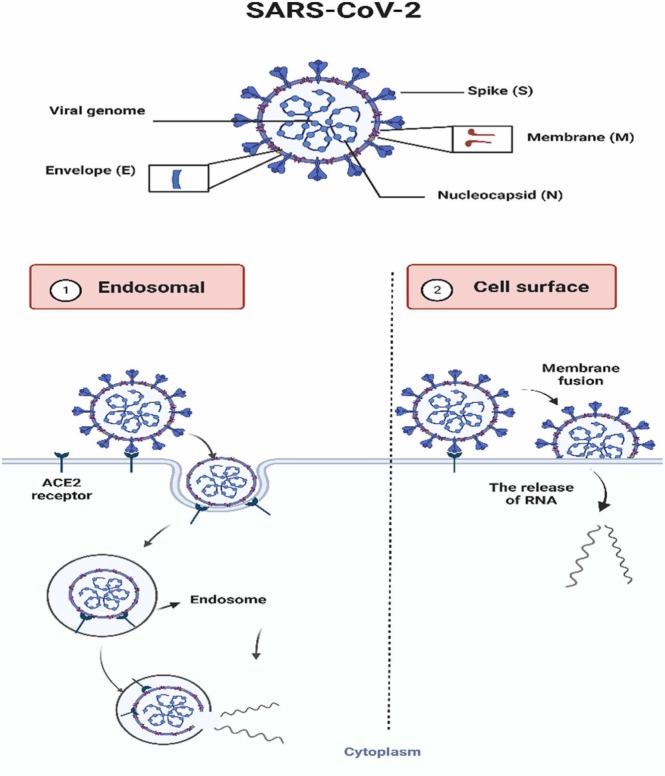
The ACE2 enzyme also can be used as a biorecognition element, and this use is highlighted as an innovation in colorimetric biosensors for diagnosing COVID-19. The diagnosis is possible because ACE2 is found in the membranes of the lungs, arteries, heart, kidney, and bowel cells and acts as an entry point for some coronaviruses, such as SARS-CoV and SARS-CoV-2 [78], [86], [87]. SARS-CoV-2 is an enveloped RNA virus containing crown-shaped tips on the outer surface called protein (S) Spike. The infection in human cells happens by the interaction of protein S with the ACE2 cell receptor [78], [86], [87]. After this contact with the cell receptor, the virus can release its genetic material into the cell, infecting it. Based on this interaction, recent studies use ACE2 as a biorecognition element in colorimetric biosensors to detect SARS-CoV-2 [78], [88]. In addition to protein S, there are three other main proteins: membrane (M), envelope (E), and nucleocapsid (N) [87], as represented in Fig. 3.
Fig. 3.
Description of the proteins mainly found in SARS-CoV-2 and the two routes of entry into human host cells. 1) The SARS-CoV-2-ACE2 complex can be internalized in lysosomes by endocytosis. 2) SARS-CoV-2 membrane fusion by action of host cell proteins and releasing RNA.
Ferreira et al. (2021) developed optodiagnosis for COVID-19, a colorimetric biosensor in the form of swabs made of gold nanoparticles (7 nm) modified with ACE2. The test had a low production cost of about 15 cents and a 5-minute detection time. The excellent sensitivity of this biosensor, capable of detecting very low viral particle loads, LOD 1.54 × 10-4 ng/mL, makes it an excellent choice. The optadiagonotic was applied to 100 nasopharyngeal and oropharyngeal clinical samples obtaining sensitivity, specificity, and accuracy values of 96%, 84%, and 90%, respectively [34]. Büyüksünetçi et al. (2021) also used ACE2 as a biorecognition element in its colorimetric biosensor. The enzyme was functionalized to a solution containing magnetic nanoparticles γ Fe2O3 and 3,3',5,5'-tetramethyl benzidine (TBM) oxidized, presenting a blue color. The change in coloration to colorless/transparent indicated the presence of spike protein (S), consequently detecting SARS-CoV-2. This method was applied to 40 real clinical samples and presented 90.24% specificity when tested with H1N1 and H3N2 influenza viruses and excellent sensitivity (LOD 4,98 ng/mL) [78]. The novelty of the optodiagnosis method is that ACE2 is the specific detection target rather than SARS-CoV, so virus mutations or antigen changes have little or no effect on detection efficiency. Furthermore, given the virus mutations reported thus far, this system may not require updates in other reagents such as primer and aptamers.
According to the literature, the ability to interact with all virus variants is the main advantage of using ACE2 as a biological element of recognition. The emergence of new variants in various genes may have an impact on biosensors that use RNA or antibodies. Wei et al., (2023) published a critical review in which its advantages over other elements were well established using various types of transduction [52]. Our main concern is the specificity of ACE2 to other viruses. Because the genome of the SARS-CoV-2 virus resembles SARS-CoV by 80%, an interaction with other viruses from the same family is very likely, resulting in false positives. Furthermore, we believe that additional research with other viruses, such as influenza, norovirus, and others, is required to confirm the specificity of ACE2.
3.4. Biosensors applied to point-of-care (POC) diagnostic devices
Point-of-care (POC) diagnostic devices have emerged as an excellent option for diagnosing various infectious diseases, particularly in the current pandemic scenario. This testing entails gathering detailed clinical data and parameters about the patient. POCs are very popular because they allow patients to be treated in the right direction as soon as possible [89] due to their detection speed, simplicity, and robustness [23], [90]. Lateral flow immunoassay (LFIA), colorimetric paper-based diagnosis, optodiagnostic, applicated surgical face masks, Nano-Amplified Colorimetric Test (NACT), and origami were the biosensors developed as POCs in the included studies. Table 2 shows their Analytical Figures of Merit (AFOM) in terms of LOD, sensitivity, recovery, RSD, and device specificity.
The lateral flow immunoassay (LFIA) was the most commonly used device with colorimetric biosensors to detect SARS-CoV-2 in the studies included in our systematic review (Table 2). This assay detects antigens in the early stages of infection, making it ideal for field use. Following that, we summarized the general aspects of three studies that focused on the most efficient application of LFIA to diagnostic POC devices.
Han et al. [40] created an LFIA based on rapid antigen detection of S protein, employing SiO2 @Au/QD fluorescent, colorimetric NPs as a quantum dots probe that can be viewed in two modes: naked eye and fluorescence. The sample is not pre-treated and can be directly placed on the device. The LOD showed 33 pg mL-1 and 1 ng mL-1 in fluorescence and naked eye detection, respectively. It was shown to be selective/specific when placed with interfering in a high concentration of SARS-CoV (100 ng/mL), MERS-CoV (100 ng mL-1), Influenza H1N1 (5 × 106 copies mL-1), Influenza-B (5 × 104 copies mL-1), Respiratory syncytial virus ( 5 × 105 PFU mL-1) and Adenovirus (5 μg mL-1). The recovery assay proved accuracy > 92.98%, and the device’s RSD of 3.92–5.19%. This study confirmed that the new biosensor has excellent accuracy and applicability for detecting SARS-CoV-2 in real samples [39].
Cavalera et al. [32] developed a device that detect the total antibodies produced by COVID-19. It is a dual-line LFIA in which both test lines could connect to various immunoglobulin classes (IgG, IgM, and IgA). The biosensor detects specific anti-SARS immunoglobulins with 100% specificity (95.75–100, 95% IC; n = 85 healthy individuals with other infections) and 94.6% sensitivity (84.9–98.9, 95% IC; n = 62 SARS CoV −2 infected individuals). The method also succeeded as a standard serological ELISA reference technique [31].
Ren and Irudayaraj's [44] study reported an improved lateral flow assay with magnetic focus (mLFA) detecting SARS-CoV-2 in non-inoculated saliva. Magnetic nanoparticles (MNPs) (Fe3O4/Au core-shell) conjugated with anti-spike protein antibodies were used to identify the SARS-CoV-2 virus in saliva samples during target detection. The higher concentration of the virus in the capture antibody region is due to the use of the magnet, and also the longer insertion time significantly improves the capture efficiency, resulting in high sensitivity capable of detecting 400 PFU mL-1 in buffer PBS and 1200 PFU mL -1 in saliva samples with sensitivity of 66.7% and 100% specificity without the prior need for amplification of genetic material [43].
Despite its broad applicability and sensitivity, LFIA detection of host immune response cells may be detrimental. This occurs because the time required for the host to produce antibodies can result in false negative results. Methods that detect the virus directly are alternatives for reducing this bias. In this context, we will discuss two of the most innovative studies in which researchers developed point-of-care (POC) devices capable of detecting SARS-CoV-2 directly in host samples.
Vaquer et al. (2021) pioneered the development of an antibody-decorated gold nanoparticle transfer biosensor for the non-invasive detection of SARS-CoV-2 antigens trapped in surgical masks of patients. Direct contact transfers AuNPs from the biosensor to the mask, emitting colorimetric signals that are later quantified using a mobile app. This requires the surgical mask be used for less than 10 min and be applied to 27 patients with mild or no symptoms. The analytical parameters presented by the authors were excellent: LOD 3 ng mL-1, sensitivity 96,2%, and specificity 100%. The high sensitivity, even for samples of asymptomatic patients, mobile detection, and non-invasive sample collection procedure, makes this biosensor ideal for mass triage [37].
Alafeef et al. (2021) created a test that combines two steps: amplifying viral RNA using the LAMP method and detecting SARS-CoV-2 in the same device. The nano-amplified colorimetric test does not require RNA extraction, and the sample can be placed crudely on the device before amplification and detection. AuNPs were coated with antisense oligonucleotides (ASOs), which served as a colorimetric reporter for detecting virus RNA. ASOs are specific to the N gene, which allowed a high specificity of 100%, accuracy > 98.4%, and sensitivity > 96.6% and with a detection limit of 10 copies μL-1 showing the optimal analytic results of the device and with response time < 1 h. The test is effective in viral detection and can be applied to other targets, changing the sequence of primers used in the LAMP amplification technique [44].
The application of colorimetric biosensors as POCs devices has become an excellent tool for detecting SARS-CoV-2 due to its speed in diagnosis, practicality to be performed in the field, and low production cost. They are easily handled without requiring highly qualified personnel, as demonstrated by the LFIA-based methods and surgical masks developed by Vaquer et al. (2021). The main advantage of these devices is that they do not require prior RNA amplification, as Alafeef et al. (2021). Fig. 4 depicts the diagnostic devices discussed above. In summary, they proved to be inexpensive, robust, and portable for diagnosing COVID-19 and assisting in virus control strategies.
Fig. 4.
Major diagnostic devices include lateral flow immunoassay (LFIA), colorimetric paper-based diagnosis, optodiagnostic, applicated surgical face masks, and Nano-Amplified Colorimetric Test (NACT) based on different nanomaterials.
(a) Reproduced with permission from Palmieri et al., [91] Copyright 2021 Elsevier. (b) Reproduced with permission from Bahadır and Sezgintürk [92] Copyright 2022 Elsevier. (c) Reproduced with permission from Brazaca [93] Copyright 2019, ACS. (d) Reproduced with permission from Ng Copyright 2016, ACS. (e) Reproduced with permission from Roh et al., [94] Copyright 2017, Asian Chemical Editorial Society. (f) Reproduced with permission from Loiseau et al.,[96] Copyright 2014, ACS. (g) Reproduced with permission from Yang et al., [95] Copyright 2019, ACS.
4. Final remarks
This systematic review selected remarkable studies of colorimetric biosensors developed to detect SARS-CoV-2. Most of them used nanomaterials in their construction to improve sensitivity. The predominant use of AuNPs, due to their unique optical properties, showed excellent sensitivity LOD (1–154 ng/mL) without RNA amplification, even placing the sample raw. Nanohybrids were also used in the core-shell (Au@Pt), and quantum dots (SiO2 @Au/QD) outs contained gold and the polymer mPEG-PCL. The sensitivity of the biosensor is directly linked to the type of nanomaterial employed and the type of transducer. The reported biosensors were applied to POCs of different configurations as an alternative to diagnostic devices. The most used model was the LFIA which has popularity due to its use in pregnancy tests. A highlighted model was manufactured by Ferreira et al. (2021) with swabs functionalized with ACE2 enzymes, which after nasopharyngeal, are placed in a solution containing AuNPs for visualization of results. However, the use of aptamer biorecognition element was not observed within the articles found during the investigation. The use of MNPs by Ren and Irudayaraj [44] was also highlighted as it can be applied directly to raw samples. Here we presented the latest advances in colorimetric biosensors used to develop devices capable of clinical diagnoses and provide information on exposure to the SARS-CoV-2, making them useful for a pandemic scenario. Biosensors proved themselves towards the alternative of fast, robust, and inexpensive detection methods, particularly for environments with limited resources, in controlling the spread of the virus in a pandemic state, such as COVID-19.
CRediT authorship contribution statement
Leticia Tessaro: Conceptualization, Methodology, Writing – review and editing. Adriano Aquino: Conceptualization, Writing – review and editing. Pedro Panzenhagen: Conceptualization, Writing – review and editing. Nirav Joshi: Conceptualization, Writing – review and editing. Carlos Adam Conte-Junior: Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are thankful for the financial support provided by the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) Brazil — grant number [E26/200.891/2021]; and [E-26/2002.227./2018]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) — grant number [313119/2020-1]; [163480/2020-6] and [152275/2022-3] and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Brazil — grant number [88887.518753/2020-00] and Finance Code 001.
Data Availability
Data will be made available on request.
References
- 1.WHO. WHO Coronavirus (COVID-19) Dashboard. World Health Organization (WHO) 2022. https://covid19.who.int/ (accessed August 30, 2022).
- 2.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikhzadeh E., Eissa S., Ismail A., Zourob M. Diagnostic techniques for COVID-19 and new developments. Talanta. 2020;220 doi: 10.1016/j.talanta.2020.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adrover-Jaume C., Alba-Patiño A., Clemente A., Santopolo G., Vaquer A., Russell S.M., et al. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B Chem. 2021;330 doi: 10.1016/j.snb.2020.129333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen Q.H., Kim M.Il. Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 2020;132 doi: 10.1016/j.trac.2020.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Y., Qiu Z., Lin X., Zeng W., Cao Y., Liu W., et al. Self-assembled nanomaterials based on aggregation-induced emission of AuNCs: Fluorescence and colorimetric dual-mode biosensing of organophosphorus pesticides. Sens Actuators B Chem. 2020:321. doi: 10.1016/j.snb.2020.128481. [DOI] [Google Scholar]
- 7.Ronkainen N.J., Okon S.L. Nanomaterial-based electrochemical immunosensors for clinically significant biomarkers. Materials. 2014;7:4669–4709. doi: 10.3390/ma7064669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holzinger M., Goff A.Le, Cosnier S. Nanomaterials for biosensing applications: a review. Front. Chem. 2014;2:1–10. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying N., Ju C., Li Z., Liu W., Wan J. Visual detection of nucleic acids based on lateral flow biosensor and hybridization chain reaction amplification. Talanta. 2017;164:432–438. doi: 10.1016/j.talanta.2016.10.098. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H., Ma X., Liu Y., Duan N., Wu S., Wang Z., et al. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015;74:872–877. doi: 10.1016/j.bios.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 11.Lu Y., Tan Y., Xiao Y., Li Z., Sheng E., Dai Z. A silver @ gold nanoparticle tetrahedron biosensor for multiple pesticides detection based on surface-enhanced Raman scattering. Talanta. 2021 doi: 10.1016/j.talanta.2021.122585. [DOI] [PubMed] [Google Scholar]
- 12.Wang T., Liu Y. A lanthanide-based ratiometric fluorescent biosensor for the enzyme-free detection of organophosphorus pesticides. Anal. Methods. 2021;13:2005–2010. doi: 10.1039/d1ay00345c. [DOI] [PubMed] [Google Scholar]
- 13.Vetrone S.A., Huarng M.C., Alocilja E.C. Detection of Non-PCR amplified S. enteritidis genomic DNA from food matrices using a gold-nanoparticle DNA biosensor: a proof-of-concept study. Sensors. 2012;12:10487–10499. doi: 10.3390/s120810487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheon H.J., Adhikari M.D., Chung M., Tran T.D., Kim J., Kim M. Il. Magnetic nanoparticles-embedded enzyme-inorganic hybrid nanoflowers with enhanced peroxidase-like activity and substrate channeling for glucose biosensing. Adv. Healthc. Mater. 2019;8:1–8. doi: 10.1002/adhm.201801507. [DOI] [PubMed] [Google Scholar]
- 15.Boron I., Juarez A., Battaglini F. Portable microalgal biosensor for herbicide monitoring. Chemelectrochem. 2020;7:1623–1630. doi: 10.1002/celc.202000210. [DOI] [Google Scholar]
- 16.Kumar T.H.V., Sundramoorthy A.K. Electrochemical biosensor for methyl parathion based on single-walled carbon nanotube/glutaraldehyde crosslinked acetylcholinesterase-wrapped bovine serum albumin nanocomposites. Anal. Chim. Acta. 2019;1074:131–141. doi: 10.1016/j.aca.2019.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Tharini J., Chen T.-W., Chen S.-M., Saraswathi R., Elshikh M.S., Darwish N.M., et al. A mexacarbate electrochemical biosensor on carbon materials based on a functionalized multiwalled carbon nanotube modified glassy carbon electrode. Int. J. Electrochem. Sci. 2019;14:8311–8325. doi: 10.20964/2019.08.103. [DOI] [Google Scholar]
- 18.Mahmoudpour M., Saadati A., Hasanzadeh M., Kholafazad-kordasht H. A stretchable glove sensor toward rapid monitoring of trifluralin: a new platform for the on-site recognition of herbicides based on wearable flexible sensor technology using lab-on-glove. J. Mol. Recognit. 2021 doi: 10.1002/jmr.2923. [DOI] [PubMed] [Google Scholar]
- 19.Dzudzevic Cancar H., Soylemez S., Akpinar Y., Kesik M., Göker S., Gunbas G., et al. A novel acetylcholinesterase biosensor: core-shell magnetic nanoparticles incorporating a conjugated polymer for the detection of organophosphorus pesticides. ACS Appl. Mater. Interfaces. 2016;8:8058–8067. doi: 10.1021/acsami.5b12383. [DOI] [PubMed] [Google Scholar]
- 20.Khalid K., Tan X., Mohd Zaid H.F., Tao Y., Lye Chew C., Chu D.T., et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11:328–355. doi: 10.1080/21655979.2020.1736240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiang L., Tang J. QD-aptamer as a donor for a FRET-based chemosensor and evaluation of affinity between acetamiprid and its aptamer. RSC Adv. 2017;7:8332–8337. doi: 10.1039/c6ra26118c. [DOI] [Google Scholar]
- 22.Verma N., Badhe Y., Gupta R., Maparu A., Rai B. Interactions of peptide coated gold nanoparticles with spike protein of the SARS-CoV-2: a basis for design of a simple and rapid detection tool. ChemRxiv. 2020 doi: 10.26434/chemrxiv.13341449.v1. [DOI] [Google Scholar]
- 23.Verma M.S., Rogowski J.L., Jones L., Gu F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015;33:666–680. doi: 10.1016/j.biotechadv.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G., Altman D., Antes G., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009:6. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharifi S., Vahed S.Z., Ahmadian E., Dizaj S.M., Eftekhari A., Khalilov R., et al. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020;150 doi: 10.1016/j.bios.2019.111933. [DOI] [PubMed] [Google Scholar]
- 26.Choi Y., Hwang J.H., Lee S.Y. Recent trends in nanomaterials-based colorimetric detection of pathogenic bacteria and viruses. Small Methods. 2018;2:1700351. doi: 10.1002/smtd.201700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karn-Orachai K., Sakamoto K., Laocharoensuk R., Bamrungsap S., Songsivilai S., Dharakul T., et al. Extrinsic surface-enhanced Raman scattering detection of influenza A virus enhanced by two-dimensional gold@silver core-shell nanoparticle arrays. RSC Adv. 2016;6:97791–97799. doi: 10.1039/c6ra17143e. [DOI] [Google Scholar]
- 28.Zhang D., Carr D.J., Alocilja E.C. Fluorescent bio-barcode DNA assay for the detection of Salmonella enterica serovar Enteritidis. Biosens. Bioelectron. 2009;24:1377–1381. doi: 10.1016/j.bios.2008.07.081. [DOI] [PubMed] [Google Scholar]
- 29.Zhao V.X.T., Wong T.I., Zheng X.T., Tan Y.N., Zhou X. Colorimetric biosensors for point-of-care virus detections. Mater. Sci. Energy Technol. 2020;3:237–249. doi: 10.1016/j.mset.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddali H., Miles C.E., Kohn J., O’Carroll D.M. Optical biosensors for virus detection: prospects for SARS-CoV-2/COVID-19. ChemBioChem. 2021;22:1176–1189. doi: 10.1002/cbic.202000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghorbanizamani F., Moulahoum H., Zihnioglu F., Evran S., Cicek C., Sertoz R., et al. Quantitative paper-based dot blot assay for spike protein detection using fuchsine dye-loaded polymersomes. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113484. [DOI] [PubMed] [Google Scholar]
- 32.Cavalera S., Colitti B., Rosati S., Ferrara G., Bertolotti L., Nogarol C., et al. A multi-target lateral flow immunoassay enabling the specific and sensitive detection of total antibodies to SARS COV-2. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Büyüksünetçi Y.T., Çitil B.E., Tapan U., Anık Ü. Development and application of a SARS-CoV-2 colorimetric biosensor based on the peroxidase-mimic activity of γ-Fe(2)O(3) nanoparticles. Mikrochim. Acta. 2021;188:335. doi: 10.1007/s00604-021-04989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A.L. Ferreira L.F. De Lima M.D.T. Torres W.R. De Araujo C. Low-Cost Optodiagnostic for Minute-Time Scale Detection of SARS-CoV-2 2021. [DOI] [PMC free article] [PubMed]
- 35.Wang C., Yang X., Gu B., Liu H., Zhou Z., Shi L., et al. Sensitive and simultaneous detection of SARS-CoV-2-Specific IgM/IgG using lateral flow immunoassay based on dual-mode quantum dot nanobeads. Anal. Chem. 2020;92:15542–15549. doi: 10.1021/acs.analchem.0c03484. [DOI] [PubMed] [Google Scholar]
- 36.Della Ventura B., Cennamo M., Minopoli A., Campanile R., Censi S.B., Terracciano D., et al. Colorimetric test for fast detection of SARS-CoV-2 in nasal and throat swabs. ACS Sens. 2020;5:3043–3048. doi: 10.1021/acssensors.0c01742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Z., Zeng W., Cai S., Li H., Ding J., Wang C., et al. Porous Au@Pt nanoparticles with superior peroxidase-like activity for colorimetric detection of spike protein of SARS-CoV-2. J. Colloid Interface Sci. 2021;604:113–121. doi: 10.1016/j.jcis.2021.06.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaquer A., Alba-Patiño A., Adrover-Jaume C., Russell S.M., Aranda M., Borges M., et al. Nanoparticle transfer biosensors for the non-invasive detection of SARS-CoV-2 antigens trapped in surgical face masks. Sens. Actuators B Chem. 2021;345 doi: 10.1016/j.snb.2021.130347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Behrouzi K., Lin L. Gold nanoparticle based plasmonic sensing for the detection of SARS-CoV-2 nucleocapsid proteins. Biosens. Bioelectron. 2022;195 doi: 10.1016/j.bios.2021.113669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han H., Wang C., Yang X., Zheng S., Cheng X., Liu Z., et al. Rapid field determination of SARS-CoV-2 by a colorimetric and fluorescent dual-functional lateral flow immunoassay biosensor. Sens. Actuators B Chem. 2022;351 doi: 10.1016/j.snb.2021.130897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao Y., Han Y., Wang C., Qiang L., Gao J., Wang Y., et al. Rapid and sensitive triple-mode detection of causative SARS-CoV-2 virus speci fi c genes through interaction between genes and nanoparticles. Anal. Chim. Acta. 2021:1154. doi: 10.1016/j.aca.2021.338330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karakus E., Erdemir E., Demirbilek N., Liv L. Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal. Chim. Acta. 2021:1182. doi: 10.1016/j.aca.2021.338939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song F., Shen Y., Wei Y., Yang C., Ge X., Wang A., et al. Botulinum toxin as an ultrasensitive reporter for bacterial and SARS-CoV-2 nucleic acid diagnostics. Biosens. Bioelectron. 2021;176 doi: 10.1016/j.bios.2020.112953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ren W., Irudayaraj J. Paper-based test for rapid on-site screening of SARS-CoV-2 in clinical samples. Biosensors. 2021:11. doi: 10.3390/bios11120488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alafeef M., Moitra P., Dighe K., Pan D. RNA-extraction-free nano-amplified colorimetric test for point-of-care clinical diagnosis of COVID-19. Nat. Protocols. 2021;16:3141. doi: 10.1038/s41596-021-00546-w. [DOI] [PubMed] [Google Scholar]
- 46.Do J.Y., Jeong J.Y., Hong C.A. Catalytic hairpin DNA assembly-based chemiluminescent assay for the detection of short SARS-CoV-2 target cDNA. Talanta. 2021:233. doi: 10.1016/j.talanta.2021.122505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ali Z., Aman R., Mahas A., Rao G.S., Tehseen M., Marsic T., et al. iSCAN: An RT-LAMP-coupled CRISPR-Cas12 module for rapid, sensitive detection of SARS-CoV-2. Virus Res. 2020;288 doi: 10.1016/j.virusres.2020.198129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y., Ren J., Qu X. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Acc. Chem. Res. 2014;47:1097–1105. doi: 10.1021/ar400250z. [DOI] [PubMed] [Google Scholar]
- 49.Cho S., Lee S.M., Shin H.Y., Kim M.S., Seo Y.H., Cho Y.K., et al. Highly sensitive colorimetric detection of allergies based on an immunoassay using peroxidase-mimicking nanozymes. Analyst. 2018;143:1182–1187. doi: 10.1039/c7an01866e. [DOI] [PubMed] [Google Scholar]
- 50.Malekzad H., Sahandi Zangabad P., Mirshekari H., Karimi M., Hamblin M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017;6:301–329. doi: 10.1515/ntrev-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karakuş E., Erdemir E., Demirbilek N., Liv L., Karakus E., Erdemir E., et al. Colorimetric and electrochemical detection of SARS-CoV-2 spike antigen with a gold nanoparticle-based biosensor. Anal. Chim. Acta. 2021;1182 doi: 10.1016/j.aca.2021.338939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MubarakAli D. Comprehensive review on rapid diagnosis of new infection COVID-19. Appl. Biochem. Biotechnol. 2022;194:1390–1400. doi: 10.1007/s12010-021-03728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G., Wang L., Meng Z., Su X., Jia C., Qiao X., et al. Visual detection of COVID ‑ 19 from materials aspect. Adv. Fiber Mater. 2022 doi: 10.1007/s42765-022-00179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moabelo K.L., Martin D.R., Fadaka A.O., Sibuyi N.R.S., Meyer M., Madiehe A.M. Nanotechnology-based strategies for effective and rapid detection of sars-cov-2. Materials. 2021:14. doi: 10.3390/ma14247851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008;37:2028–2045. doi: 10.1039/b712179m. [DOI] [PubMed] [Google Scholar]
- 56.Nie L., Liu F., Ma P., Xiao X. Applications of gold nanoparticles in optical biosensors. J. Biomed. Nanotechnol. 2014;10:2700–2721. doi: 10.1166/jbn.2014.1987. [DOI] [PubMed] [Google Scholar]
- 57.Yu L., Song Z., Peng J., Yang M., Zhi H., He H. Progress of gold nanomaterials for colorimetric sensing based on different strategies. TrAC Trends Anal. Chem. 2020;127 doi: 10.1016/j.trac.2020.115880. [DOI] [Google Scholar]
- 58.Tessaro L., Aquino A., de Carvalho A.P.A., Conte-Junior C.A. A systematic review on gold nanoparticles based-optical biosensors for Influenza virus detection. Sens. Actuators Rep. 2021;3 doi: 10.1016/j.snr.2021.100060. [DOI] [Google Scholar]
- 59.Wiley B.J., Im S.H., Li Z.Y., McLellan J., Siekkinen A., Xia Y. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J. Phys. Chem. B. 2006;110:15666–15675. doi: 10.1021/jp0608628. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Li Z., Li S., Zhang Y., Sun B., Yu Y., et al. LSPR optical fiber biosensor based on a 3D composite structure of gold nanoparticles and multilayer graphene films. Opt. Express. 2020;28:6071. doi: 10.1364/oe.385128. [DOI] [PubMed] [Google Scholar]
- 61.Kunwar S., Sui M., Pandey P., Gu Z., Pandit S., Lee J. Improved configuration and lspr response of platinum nanoparticles via enhanced solid state dewetting of In-Pt bilayers. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-018-37849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cao X., Ye Y., Liu S. Gold nanoparticle-based signal amplification for biosensing. Anal. Biochem. 2011;417:1–16. doi: 10.1016/j.ab.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 63.Elghanian R., Storhoff J.J., Mucic R.C., Letsinger R.L., Mirkin C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 64.Rao H., Xue X., Wang H., Xue Z. Gold nanorod etching-based multicolorimetric sensors: strategies and applications. J. Mater. Chem. C. 2019;7:4610–4621. doi: 10.1039/c9tc00757a. [DOI] [Google Scholar]
- 65.Cao J., Sun T., Grattan K.T.V. Gold nanorod-based localized surface plasmon resonance biosensors: a review. Sens. Actuators B Chem. 2014;195:332–351. doi: 10.1016/j.snb.2014.01.056. [DOI] [Google Scholar]
- 66.Indrasekara ASDS Meyers S., Shubeita S., Feldman L.C., Gustafsson T., Fabris L. Gold nanostar substrates for SERS-based chemical sensing in the femtomolar regime. Nanoscale. 2014;6:8891–8899. doi: 10.1039/c4nr02513j. [DOI] [PubMed] [Google Scholar]
- 67.Park S., Lee J., Ko H. Transparent and flexible surface-enhanced raman scattering (SERS) sensors based on gold nanostar arrays embedded in silicon rubber film. ACS Appl. Mater. Interfaces. 2017;9:44088–44095. doi: 10.1021/acsami.7b14022. [DOI] [PubMed] [Google Scholar]
- 68.Wang W., Cui H. Chitosan-luminol reduced gold nanoflowers: from one-pot synthesis to morphology-dependent SPR and chemiluminescence sensing. J. Phys. Chem. C. 2008;112:10759–10766. doi: 10.1021/jp802028r. [DOI] [Google Scholar]
- 69.Patel A.S., Juneja S., Kanaujia P.K., Maurya V., Prakash G.V., Chakraborti A., et al. Gold nanoflowers as efficient hosts for SERS based sensing and bio-imaging. Nano-Struct. Nano Objects. 2018;16:329–336. doi: 10.1016/j.nanoso.2018.09.001. [DOI] [Google Scholar]
- 70.Kawasaki D., Yamada H., Maeno K., Sueyoshi K., Hisamoto H., Endo T. Core-shell-structured gold nanocone array for label-free DNA sensing. ACS Appl. Nano Mater. 2019;2:4983–4990. doi: 10.1021/acsanm.9b00930. [DOI] [Google Scholar]
- 71.Chen Y., Lian Y., Huang M., Wei L., Xiao L. A dual-mode fluorometric/colorimetric sensor for Cu2+ detection based on hybridized carbon dots and gold-silver core-shell nanoparticles. Analyst. 2019;144:4250–4257. doi: 10.1039/c9an00850k. [DOI] [PubMed] [Google Scholar]
- 72.Arroquia A., Acosta I., Armada M.P.G. Self-assembled gold decorated polydopamine nanospheres as electrochemical sensor for simultaneous determination of ascorbic acid, dopamine, uric acid and tryptophan. Mater. Sci. Eng. C. 2020;109 doi: 10.1016/j.msec.2019.110602. [DOI] [PubMed] [Google Scholar]
- 73.Nhat H.N.T., Le N.T.T., Phong N.T.P., Nguyen D.H., Nguyen-Le M.T. Potential application of gold nanospheres as a surface plasmon resonance based sensor for in-situ detection of residual fungicides. Sensors. 2020:20. doi: 10.3390/s20082229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alex S., Tiwari A. Functionalized gold nanoparticles: synthesis, properties and applications-A review. J. Nanosci. Nanotechnol. 2015;15:1869–1894. doi: 10.1166/jnn.2015.9718. [DOI] [PubMed] [Google Scholar]
- 75.Mustafa D.E., Yang T., Xuan Z., Chen S., Tu H., Zhang A. Surface plasmon coupling effect of gold nanoparticles with different shape and size on conventional surface plasmon resonance signal. Plasmonics. 2010;5:221–231. doi: 10.1007/s11468-010-9141-z. [DOI] [Google Scholar]
- 76.Kelly, et al. Vol. 107. American Chemical Society; 2003. p. 668. (The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment). [DOI] [Google Scholar]
- 77.Martín Várguez P.E., Raimundo J.-M. Naked-eye chromogenic test strip for cyanide sensing based on novel phenothiazine push–pull derivatives. Biosensors. 2022;12:407. doi: 10.3390/bios12060407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Çitil B.E. Büyüksünetçi U. Tapan Ü. Anık Development and application of a SARS-CoV-2 colorimetric biosensor based on the peroxidase-mimic activity of γ-Fe2O3 nanoparticles 2021;188. [DOI] [PMC free article] [PubMed]
- 79.Anjum S., Saleem H., Rasheed K., Zia R., Riaz S., Usman A. Role of Ni2+ ions in magnetite nano-particles synthesized by co-precipitation method. J. Supercond. Nov. Magn. 2017;30:1177–1186. doi: 10.1007/s10948-016-3832-4. [DOI] [Google Scholar]
- 80.W. yin Lim Emerging Biosensors to Detect Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): A Review 2021;2. [DOI] [PMC free article] [PubMed]
- 81.M.L. Molecular Mechanisms of Multimeric Assembly of IgM and IgA 2022:221–247. [DOI] [PubMed]
- 82.Rezaei M.S. and N. Introduction on Monoclonal Antibodies. In Monoclonal Antibodies 2021;40:1–21. https://doi.org/10.5772/intechopen.98378.
- 83.Song S., Wang L., Li J., Fan C., Zhao J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008;27:108–117. doi: 10.1016/j.trac.2007.12.004. [DOI] [Google Scholar]
- 84.FDA. SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests 2021. https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests (accessed May 5, 2022).
- 85.Quaglia F., Salladini E., Carraro M., Minervini G., Tosatto S.C.E., Le Mercier P. SARS-CoV-2 variants preferentially emerge at intrinsically disordered protein sites helping immune evasion. FEBS J. 2022:1–11. doi: 10.1111/febs.16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. Ultrarapid communication a novel angiotensin-converting enzyme – related to angiotensin 1-9. Circ. Res. 2000;87:e1–e9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 87.Antiochia R. Nanobiosensors as new diagnostic tools for SARS, MERS and COVID-19: from past to perspectives. Microchim. Acta. 2020:187. doi: 10.1007/s00604-020-04615-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferreira De. Lima L.F., Torres M.D.T., de Araujo W.R., De La Fuente-Nunez C. Low-cost optodiagnostic for minute-time scale detection of SARS-CoV-2. ACS Nano. 2021 doi: 10.1021/acsnano.1c03236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Konwar A.N., Borse V. Current status of point-of-care diagnostic devices in the Indian healthcare system with an update on COVID-19 pandemic. Sens. Int. 2020;1 doi: 10.1016/j.sintl.2020.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luong A.-D., Roy I., Malhotra B.D., Luong J.H.T. Analytical and biosensing platforms for insulin: a review. Sens. Actuators Rep. 2021;3 doi: 10.1016/j.snr.2021.100028. [DOI] [Google Scholar]
- 91.Palmieri V., De Maio F., De Spirito M., Papi M. Face masks and nanotechnology: keep the blue side up. Nano Today. 2021;37 doi: 10.1016/j.nantod.2021.101077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bahadır E.B., Sezgintürk M.K. Lateral flow assays: principles, designs and labels. TrAC Trends Anal. Chem. 2016;82:286–306. doi: 10.1016/j.trac.2016.06.006. [DOI] [Google Scholar]
- 93.Brazaca L.C., dos Santos P.L., de Oliveira P.R., Rocha D.P., Stefano J.S., Kalinke C., et al. Biosensing strategies for the electrochemical detection of viruses and viral diseases – a review. Anal. Chim. Acta. 2021:1159. doi: 10.1016/j.aca.2021.338384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roh J., Lee S.Y., Park S., Ahn D.J. Polydiacetylene/Anti-HBs complexes for visible and fluorescent detection of hepatitis B surface antigen on a nitrocellulose membrane. Chem. Asian J. 2017;12:2033–2037. doi: 10.1002/asia.201700769. [DOI] [PubMed] [Google Scholar]
- 95.Yang X., Li J., Pei H., Zhao Y., Zuo X., Fan C., et al. DNA − Gold Nanoparticle Conjugates-Based Nanoplasmonic Probe for Speci fi c Di ff erentiation of Cell Types. Analytical Chemistry 2014. [DOI] [PubMed]
- 96.Loiseau A., Zhang L., Hu D., Salmain M., Mazouzi Y., Flack R., et al. Core-shell gold/silver nanoparticles for localized surface plasmon resonance-based naked-eye toxin biosensing. ACS Appl. Mater. Interfaces. 2019;11:46462–46471. doi: 10.1021/acsami.9b14980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.