Abstract
Objectives
Persistent post-acute coronavirus disease 2019 (COVID-19) symptoms (PACSs) have been reported up to 6 months after hospital discharge. Herein we assessed the symptoms that persisted 12 months (M12) after admission for COVID-19 in the longitudinal prospective national French coronavirus disease cohort.
Methods
Hospitalized patients with a confirmed virological diagnosis of COVID-19 were enrolled. Follow-up was planned until M12 after admission. Associations between persistence of ≥3 PACSs at M12 and clinical characteristics at admission were assessed through logistic regression according to gender.
Results
We focused on participants enrolled between 24 January 2020 and 15 July 2020, to allow M12 follow-up. The M12 data were available for 737 participants. Median age was 61 years, 475 (64%) were men and 242/647 (37%) were admitted to intensive care units during the acute phase. At M12, 27% (194/710) of the participants had ≥3 persistent PACS, mostly fatigue, dyspnoea and joint pain. Among those who had a professional occupation before the acute phase, 91 out of 339 (27%) were still on sick leave at M12. Presence of ≥3 persistent PACS was associated with female gender, both anxiety and depression, impaired health-related quality of life and Medical Muscle Research Council Scale <57. Compared with men, women more often reported presence of ≥3 persistent PACSs (98/253, 39% vs. 96/457, 21%), depression and anxiety (18/152, 12% vs. 17/268, 6% and 33/156, 21% vs. 26/264, 10%, respectively), impaired physical health-related quality of life (76/141, 54% vs. 120/261, 46%). Women had less often returned to work than men (77/116, 66% vs. 171/223, 77%).
Conclusions
One fourth of the individuals admitted to hospital for COVID-19 still had ≥3 persistent PACSs at M12 post-discharge. Women reported more often ≥3 persistent PACSs, suffered more from anxiety and depression and had less often returned to work than men.
Keywords: Cohort, Emerging infectious diseases, Moderate to severe COVID-19, Post-acute COVID-19 symptoms, SARS-CoV-2
Introduction
Clinical presentation of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection ranges from asymptomatic cases to severe distress respiratory syndrome. When symptomatic, the acute phase commonly features cough, dyspnoea, flu-like symptoms, myalgia, joint pain, gastrointestinal symptoms and anosmia/ageusia [1]. Several studies have reported the persistence of coronavirus disease 2019 (COVID-19)-related symptoms after the acute phase. In 2021, WHO developed a clinical definition of post-COVID condition [2]. According this definition, the proportion of patients experiencing at least one persistent post-acute COVID-19 symptom (PACS) reaches 66% at 2 months, 53% at 4 months and 32% at 7 months post-infection in outpatients [[3], [4], [5]], and rises to 62% to 68% at 6 months post-infection in patients hospitalized during the acute phase [6,7]. It was shown that intensive care unit (ICU) stay (with or without COVID-19) was associated with worse long-term outcomes [8].
Few data are available after 12 months post-infection with design heterogeneity [7,[9], [10], [11]]. In the Chinese cohort with a 12-month follow-up as well as in the study performed in France with a 6-month follow-up [6,7], female gender was associated with the persistence of PACSs. Furthermore, women of the same age report poorer health than men based on subjective health assessments, in general and in COVID-19 specific settings [[12], [13], [14], [15]].Therefore, to add relevant evidence to the current literature we report results stratified by gender from a large national multicentre cohort in which patients with COVID-19 were observed prospectively from hospital admission up to 12 months regardless development of PACS or not.
Patients and methods
Study oversight and data collection
The design of this national multicentre prospective cohort (French COVID cohort) has been described elsewhere [16]. Briefly, hospitalized patients with a confirmed virological diagnosis of COVID-19 were enrolled in the cohort (registered in clinicaltrials.gov NCT04262921); ethics approval was obtained from the Comité de Protection des Personnes (CPP) Ile-de-France-VI (ID-RCB: 2020-A00256-33). Patients were co-included in the European H2020 ORCHESTRA project.
Follow-up was planned with a physician's visit at month 3 (M3), M6 and M12 after hospital admission. Comorbidities were assessed according to the 4C Mortality Score [17].
We asked every centre to check the French register of deceased persons (https://arbre.app/en/insee) to record the vital status (causes of death was not available) of those who did not attend the follow-up visits.
Study definitions and outcomes
At each visit, the following ten COVID-19 symptoms were collected (fatigue, dyspnoea at rest, joint pain, myalgia, headache, rhinorrhoea, cough, sore throat, ageusia and anosmia). In addition, a physical examination and a 6-minute walking test (6MWT) were performed.
At the M12-visit, a measure of the functional independence using the modified Rankin scale (mRS) (0, no symptoms; 5 severe disability) and an assessment of muscle strength of each limb using the modified Medical Muscle Research Council Scale (score: 0–60) were also performed [18]. Additionally, patients were interviewed on health-related quality of life (HRQL) with a 12-items Short Form Health Survey and on their psychological distress (Health Anxiety Depression Scale (HADS)). For 12-items Short Form Health Survey, an individual was defined as having an impaired physical (or mental) HRQL if his physical (or mental) component summary (was lower than the 25th percentile of the distribution in the general French population of the same age and gender. HADS is divided into anxiety (HADS-A) and depression subscale (HADS-D). Each HADS item was scored on a 4-point Likert scale with high scores indicating more severe anxiety/depression. Scores ≥11 indicated abnormal levels.
Statistical analysis
All analyses were stratified by gender. Associations between presence of PACS at M12 (defined by the presence of ≥3 of the ten COVID-19 symptoms) and baseline characteristics were assessed through bivariate logistic regressions. The final multivariate models were developed by starting with a model that included all covariates with <10% of missing values and p < 0.20 and then excluding variables that did not improve the overall fit as measured by the -2 log likelihood ratio test.
Prevalence of symptoms was evaluated as 95%CI (exact Clopper-Pearson method). For patients who underwent evaluation at both M6 and M12, we compared the proportion of each symptom through McNemar paired tests. We compared the baseline characteristics between alive patients who attended the M12-visit with those of the eligible patients who did not (excluding deceased patients), using a χ2 test. We computed the observed proportion of ≥3 PACSs and its 95% CI according to each combination of the risk factors found in the multivariate model to impute patients without M12 visit. Finally, separately in women and men, as a sensitivity analysis, we obtained three estimations of the proportion of patients with ≥3 persistent PACSs on the overall population of eligible patients for the M12 visit using three imputations: the mean proportion and proportions from the lower and the upper bound of the 95% CI. All tests were two sided, and the analyses were performed using the R software.
Results
We focused on participants enrolled between 24 January 2020 and 15 July 2020, to allow for a 12-month follow-up. Out of the 3426 participants enrolled during this period, 391 died (11%) during initial hospitalization, 67 died (2%) between hospital discharge and M12. By September 2021, M12 data were available for 737 patients. The baseline and M12 characteristics for the 737 patients (262 women and 475 men), are summarized in Table 1 .
Table 1.
Characteristics at hospital admission and clinical symptoms at 12 months follow-up of 737 patients enrolled in the French COVID cohort
| Characteristics |
Missing |
AllN = 737 |
WomenN = 262 |
MenN = 475 |
|---|---|---|---|---|
| At hospital admission | ||||
| Age, (y); Median (IQR) | 0 | 61 (52; 70) | 60 (51; 70) | 61 (52; 70) |
| Age <65 y - no/total no (%) | 0 | 437/737 (59) | 155/262 (59) | 282/475 (59) |
| Comorbidities - no/total no (%) | ||||
| Chronic cardiac disease (not hypertension) | 58 | 108/679 (16) | 31/248 (12) | 77/431 (18) |
| Hypertension | 72 | 258/665 (39) | 86/243 (35) | 172/422 (41) |
| Chronic kidney disease | 55 | 55/682 (8) | 11/248 (4) | 44/434 (10) |
| Malignant neoplasm | 57 | 46/680 (7) | 15/248 (6) | 31/432 (7) |
| Moderate or severe liver disease | 70 | 7/667 (1) | 1/244 (0) | 6/423 (1) |
| Obesity (clinician definition) | 71 | 139/666 (21) | 63/240 (26) | 76/426 (18) |
| Chronic pulmonary disease (not asthma) | 55 | 78/682 (11) | 22/248 (9) | 56/434 (13) |
| Diabetes (type 1 and 2) | 67 | 129/670 (19) | 43/245 (18) | 86/425 (20) |
| No of comorbidities - no/total no (%) a | 54 | |||
| 0 | 188/683 (28) | 72/249 (29) | 116/434 (27) | |
| 1 | 202/683 (30) | 78/249 (31) | 124/434 (29) | |
| ≥2 | 293/683 (43) | 99/249 (40) | 194/434 (45) | |
| Symptoms - no/total no (%) b | 82 | |||
| None | 39/655 (6) | 19/241 (8) | 20/414 (5) | |
| 1–2 | 250/655 (38) | 86/241 (36) | 164/414 (40) | |
| ≥3 | 366/655 (56) | 136/241 (56) | 230/414 (56) | |
| Management during hospitalization | ||||
| ICU during acute phase | 90 | 242/647 (37) | 63/234 (27) | 179/412 (43) |
| Oxygen therapy - no/total no (%) | 105 | 482/632 (76) | 165/234 (71) | 317/398 (80) |
| Non-invasive ventilation (e.g. BIPAP, CPAP) - no/total no (%) | 115 | 126/622 (20) | 43/233 (18) | 83/389 (21) |
| Pharmacological treatment during acute COVID-19 - no/total no (%) | ||||
| Antiviral agent | 104 | 178/633 (28) | 56/234 (24) | 122/399 (31) |
| Hydroxychloroquine | 129 | 106/608 (17) | 37/222 (17) | 69/386 (18) |
| Immunomodulator (e.g. anti-IL6) | 146 | 17/591 (3) | 2/219 (1) | 15/372 (4) |
| Corticosteroids | 98 | 142/639 (22) | 48/238 (20) | 94/401 (23) |
| Length of hospital stay, Median (IQR) (d) | 77 | 9 (5; 17) | 8 (5; 13) | 11 (6; 19) |
| M12 follow-up after discharge | ||||
| Days from symptom onset to M12 visit - Median (IQR) (d) | 55 | 391 (374; 419) | 391 (374; 415) | 392 (373; 420) |
| Days from discharge to M12 visit - Median (IQR) (d) | 56 | 370 (352; 398) | 371 (355; 395) | 368 (350; 400) |
| Six-minute walk test performed at M12 visit - no/total no (%) | 195 | 264/542 (49) | 75/189 (40) | 187/351 (53) |
| Distance walked in % - Median (IQR) | 570 | 88 (74; 100) | 85 (75; 100) | 94 (74; 100) |
| Medical Research Council Scale <48 at M12 visit - no/total no (%) | 253 | 8/484 (2) | 3/168 (2) | 5/316 (2) |
| Simplified Modified Rankin Scale at M12 visit - no/total no (%) | 257 | |||
| 0 - No symptoms | 242/480 (50) | 76/170 (45) | 166/310 (54) | |
| 1 - No significant disability | 134/480 (28) | 49/170 (29) | 85/310 (27) | |
| 2 - Slight disability | 79/480 (16) | 34/170 (20) | 45/310 (15) | |
| 3 - Moderate disability | 22/480 (5) | 10/170 (6) | 12/310 (4) | |
| 4 - Moderately severe disability | 2/480 (0) | 1/170 (1) | 1/310 (0) | |
| 5 - Severe disability | 1/480 (0) | 0/170 (0) | 1/310 (0) | |
| HADS (no/total no (%)) | 317 | |||
| Anxiety score ≥11 | 59/420 (14) | 33/156 (21) | 26/264 (10) | |
| Depression score ≥11 | 35/420 (8) | 18/152 (12) | 17/268 (6) | |
| SF-12 - no/total no (%) | 335 | |||
| Impaired physical HRQL | 196/402 (49) | 76/141 (54) | 120/261 (46) | |
| Impaired mental HRQL | 126/402 (31) | 45/141 (32) | 81/261 (31) | |
| If applicable, back to work at M12 (no/total no (%)) | 398 | 248/339 (73) | 77/116 (66) | 171/223 (77) |
| C-reactive protein level at M12 visit - Median (IQR) (mg/L) | 323 | 3 (1; 4) | 3 (2; 7) | 2 (1; 4) |
| Persistent PACS 12 mo after hospital admission – no/total no (%) b | 27 | |||
| None | 236/710 (33) | 62/253 (25) | 174/457 (38) | |
| 1–2 | 280/710 (39) | 93/253 (37) | 187/457 (41) | |
| ≥3 | 194/710 (27) | 98/253 (39) | 96/457 (21) |
BIPAP, Bi-level Positive Arway Pressure; COVID, coronavirus disease; CPAP, Continuous Positive Airway Pressure; HADS, Health Anxiety Depression Scale; HRQL, health-related quality of life; IQR, interquartile range; M12, 12 months; PACS, post-acute coronavirus disease 2019 symptom; SF-12, 12-items Short Form Health Survey.
Comorbidities were defined using the Charlson comorbidity index, with the addition of clinician-defined obesity.
Number of symptoms among: fatigue, dyspnoea, joint pain, myalgia, headache, rhinorrhoea, cough, sore throat, ageusia and anosmia.
Global population
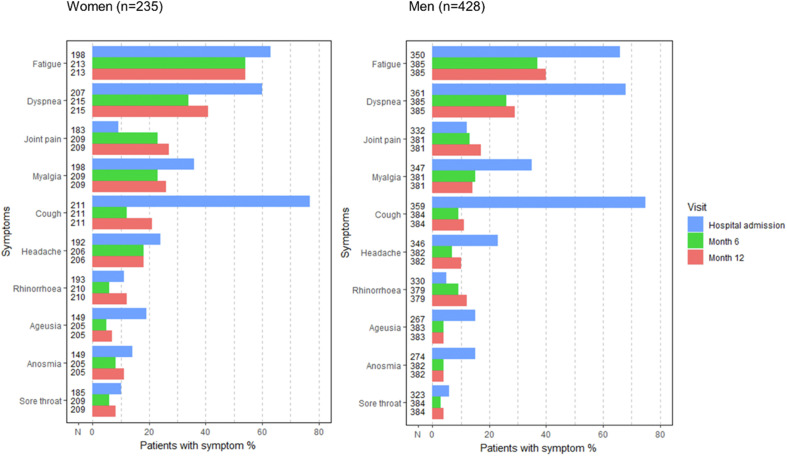
At M12 visit, 27% (194/710; 95% CI: 24–31%) participants had ≥3 persistent PACSs. Fatigue (327/705; 46%; 95% CI: 43–50%), dyspnoea (235/704; 33%; 95% CI: 30–37%) and joint pain (146/703; 21%; 95% CI: 17–24%) were the 3 most frequently reported symptoms individually or in combination. Women reported myalgia frequently in addition to the latter 3 symptoms (Fig. S1). Pulmonary auscultation was reported as ‘normal’ in 87% of the patients (507/634; 95% CI: 83–89%). In those with abnormal pulmonary auscultation, persistent crackles were reported in 26% (19/74) and wheezing in 14% (10/74) of the cases, respectively. The median percentage of predicted value of the 6MWT was 88% (interquartile range (IQR): 74;100) for 163 patients who underwent this test. Of note, this was lower in 61 patients who reported dyspnoea compared with those who did not (85%, IQR: 71;99 vs. 95%, IQR: 76,101; p 0.04). When focusing on dyspnoea at rest, persistent dyspnoea at M12 was reported in 32% (187/578) of the subset of individuals with no pulmonary chronic condition. Globally, the presence of ≥3 persistent PACSs was associated with female gender (data not shown because all analyses were presented by gender), both anxiety and depression, impaired HRQL (physical and mental), mRS ≥2 (Table S1). Anxiety at M12 was associated with female gender (odds ratio (OR) = 2.46; 95% CI: 1.41–4.32), not getting back to work (OR = 2.72; 95% CI: 1.17–6.27) and dyspnoea (OR = 3.49; 95% CI: 1.98–6.27) (Table S2). Six hundred and sixty-three patients attended both M6 and M12 visits. Between the two visits, there was no global evolution of the frequency of the ten PACS except for rhinorrhoea and cough that were more often reported at M12 in women only (Fig. 1 ). Some patients reported an onset of symptoms at M12 compared with M6: 95 out of 339 (28%, 95% CI: 33–46%) patients who did not have fatigue at M6 reported fatigue at M12, 101/425 (24%, 95% CI: 20–28%) for dyspnoea and 81/490 (17%, 95%CI: 13–20%) for joint pain.
Fig. 1.
Coronavirus disease 2019 (COVID-19) related symptoms during the acute phase and during follow-up visits of patients with M6 and M12 visits for women (n = 235) and for men (n = 428) enrolled in the French COVID cohort. Note: McNemar paired tests (M6 vs. M12) for each symptom among women and men: Women: fatigue (p 1, N = 213), dyspnoea (p 0.11, N = 215), joint pain (p 0.11, N = 215), myalgia (p 0.37, N = 209), cough (p 0.007, N = 211), headache (p 1, N = 206), rhinorrhoea (p 0.026, N = 210), ageusia (p 0.45, N = 205), anosmia (p 0.40, N = 205), sore throat (p 0.40, N = 209). Men: fatigue (p 0.31, N = 385), dyspnoea (p 0.29, N = 385), joint pain (p 0.22, N = 381), myalgia (p 1, N = 381), cough (p 0.55, N = 384), headache (p 0.090, N = 382), rhinorrhoea (p 0.093, N = 379), ageusia (p 0.82, N = 383), anosmia (p 0.65, N = 382), sore throat (p 0.45, N = 384).
Results according to gender
Compared with men, women more often reported the presence of ≥3 persistent PACSs (98/253; 39%; 95% CI: 33–45% vs. 96/455; 21%; 95% CI: 17–25%), depression and anxiety (18/152; 12%; 95% CI: 7–18% vs. 17/268; 6%; 95% CI: 4–10% and 33/156; 21%; 95% CI: 15–28% vs. 26/264; 10%; 95% CI: 7–14%, respectively), an altered physical HRQL (76/141; 54% vs. 120/261; 46%; 95% CI: 40–52%) and an mRS ≥2 (45/170; 26%; 95% CI: 20–34% vs. 59/310; 19%; 95% CI: 15–24%, respectively). For those who previously had an occupation, women were more often on sick leave than men (39/116; 34%; 95% CI: 25–43% vs. 52/223, 23%, 95% CI: 18–29%).
In women, factors associated with the presence of ≥3 persistent PACSs at M12 were age <65 years (adjustedOR (aOR) = 1.8; 95% CI: 1.0–3.2) and having ≥3 symptoms at admission during the acute phase (aOR = 2.2; 95% CI: 1.3–3.9). For men, only hospitalization in ICU and use of oxygen during the acute phase were significant factors (OR = 3.1; 95% CI: 1.4–7.9 and OR = 2.7; 95% CI: 1.2–7.0, respectively) (Table 2 ).
Table 2.
Univariate and multivariate association analyses with ≥3 symptoms at 12 month (M12) visit separately in women and in men
| <3 symptoms at M12 | ≥3 symptoms at M12 | Bivariate analysisa |
Multivariate analysisb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Missing | OR (95% CI) | p | Adjusted OR (95% CI) | p | |||||
| Women | Age <65 y, n (%) | 0 | 83 (54%) | 67 (68%) | 1.87 (1.11; 3.21) | 0.020 | 1.79 | (1.03; 3.15) | 0.042 |
| ≥3 symptoms at admission, n (%) | 19 | 69 (49%) | 64 (69%) | 2.30 (1.34; 4.02) | 0.003 | 2.21 | (1.28; 3.89) | 0.005 | |
| ≥2 comorbidities, n (%) | 11 | 54 (37%) | 41 (43%) | 1.31 (0.77; 2.22) | 0.32 | ||||
| Antiviral agent, n (%) | 26 | 37 (27%) | 17 (19%) | 0.63 (0.32; 1.19) | 0.16 | ||||
| Corticosteroids, n (%) | 22 | 28 (20%) | 18 (20%) | 0.99 (0.50; 1.90) | 0.97 | ||||
| ICU/non-invasive ventilation/oxygen | 31 | ||||||||
| No | 34 (25%) | 26 (30%) | 1 reference | ||||||
| Oxygen only (no ICU, no ventilation) | 58 (43%) | 33 (38%) | 0.74 (0.38; 1.45) | 0.38 | |||||
| ICU or non-invasive ventilation | 42 (31%) | 29 (33%) | 0.90 (0.45; 1.81) | 0.77 | |||||
| Men | Age <65 y, n (%) | 0 | 213 (59%) | 58 (60%) | 1.06 (0.67; 1.69) | 0.80 | |||
| ≥3 symptoms at admission, n (%) | 56 | 170 (54%) | 51 (60%) | 1.27 (0.78; 2.08) | 0.34 | ||||
| ≥2 comorbidities, n (%) | 37 | 144 (44%) | 40 (46%) | 1.11 (0.69; 1.78) | 0.68 | ||||
| Antiviral agent, n (%) | 73 | 84 (28%) | 31 (37%) | 1.46 (0.87; 2.41) | 0.15 | ||||
| Corticosteroids, n (%) | 71 | 64 (21%) | 23 (27%) | 1.39 (0.79; 2.40) | 0.24 | ||||
| ICU/non-invasive ventilation/oxygen | 70 | ||||||||
| No | 63 (21%) | 7 (9%) | 1 reference | 1 | reference | ||||
| Oxygen only (no ICU, no ventilation) | 98 (32%) | 30 (37%) | 2.77 (1.25; 7.03) | 0.019 | 2.70 | (1.17; 7.02) | 0.028 | ||
| ICU or non-invasive ventilation | 143 (47%) | 44 (54%) | 2.76 (1.20; 7.16) | 0.024 | 3.08 | (1.38; 7.85) | 0.010 | ||
CI, confidence interval; ICU, intensive care unit; M12, 12 month; OR, odds ratio.
Women: n = 253, 155 with <3 symptoms at M12 and 98 with ≥3 symptoms at M12. Men: n = 457, 361 with <3 symptoms at M12 and 96 with ≥3 symptoms at M12.
Women: n = 234, 141 with <3 symptoms at M12 and 93 with ≥3 symptoms at M12. Men: n = 385, 304 with <3 symptoms at M12 and 81 with ≥3 symptoms at M12.
The observed proportions of ≥3 persistent PACSs at M12 for each of the combinations of risk factors are reported in Fig S2. In women, these proportions ranged between 22% with no risk factor (age ≥65 years, <3 symptoms at admission) and 53% in those with both risk factors. In men, these proportions ranged between 10% with no risk factor (no oxygen, no invasive ventilation, no ICU stay) and 23% in those with both risk factors.
Comparison between eligible participants who attended M12 visit and those who did not, and sensitivity analysis on all eligible participants
Comparing the 737 patients who attended the M12 visit to the 2231 eligible patients who did not, significant differences were found for admitted/transferred to the ICU. Patients who attended the M12 visit had been more often admitted/transferred to ICU (242/654, 37% vs. 581/1937, 30%; p < 0.001) (Table 3 ).
Table 3.
Comparison between patients included in the analyses and patients not deceased who did not attend M12 visit
| Included in the analyses | Not included in the analyses | ||
|---|---|---|---|
| (N = 737) | (N = 2231) | pa | |
| Age ≥65 y | 300 (41%) | 973 (44%) | 0.12 |
| Female gender | 262 (36%) | 852 (39%) | 0.13 |
| ≥3 symptoms at admission | 366 (56%) | 1116 (57%) | 0.65 |
| Intensive care unit during acute phase | 242 (37%) | 581 (30%) | <0.001 |
| ≥2 comorbidities | 293 (43%) | 947 (45%) | 0.24 |
χ2 test.
In the sensitivity analysis, we obtained three estimations of the proportion of ≥3 persistent PACS among all eligible patients for the M12 visit. In women, the mean proportion was 39% (95% CI: 36–41), the imputed proportion from the lower bound of the 95% CI was 33%, and the imputed proportion from the upper bound of the 95% CI was 46%. In men, these proportions were 21% (95% CI: 19–23), 17% and 25%, respectively.
Discussion
Epidemiology and natural history of PACSs are poorly understood. PACS subtypes are widely distributed and cover exercise intolerance, pain syndromes, cognition, mood and sleep disorders and dysautonomia [19]. In this large national prospective cohort of patients hospitalized for confirmed diagnosis of COVID-19 during the acute phase, with 12-month follow-up after hospital discharge, one fourth of the participants reported the presence of ≥3 persistent PACSs. The prevalence of PACSs in our cohort is probably overestimated given the high proportion of participants not retained during the follow-up period, and given the fact that those still attending follow-up visits might be more prone to report concerns of PACS than those who did not attend. In addition, there was no change between M6 and M12 globally; however, in a same individual, some symptoms that were not reported at M6 could arise at M12. As these signs are unspecific, it is disputable whether they are linked with COVID-19. For example, the fatigue reported at M12 among patients who did not report fatigue at M6 may not be related to acute infection one year ago. Furthermore, 20% of participants stated that they had not regained full independence at M12. These symptoms had disabling consequences because one fourth of those who had a professional occupation before their hospitalisation for COVID-19 were still on sick leave at M12.
It has been previously shown that women reported symptoms more frequently than men, generally and in the COVID-19 setting [[12], [13], [14], [15]]; therefore, we chose to stratify our analyses according to gender. Indeed, factors associated with the presence of PACSs at M12 were different according to gender. In men, admission/transfer to ICU and oxygen therapy were associated with the presence of ≥3PACSs at M12, suggesting a potential role of initial severity of the disease in the persistence of symptoms. This could also suggest a role of the antiviral adaptive response, or of the innate immune response. However, in women, the persistence of ≥3 PACSs at M12 was associated with having ≥3 symptoms at admission and with younger age. Additionally, women reported more often anxiety and depression than men. Recently, it has been shown that cognitive complaints at one month after a hospitalization for COVID-19 were associated with psychological distress, independently of objective neuropsychological status [20]. Our results show that women are more likely to present to healthcare clinics with symptoms post discharge. Increase presentation is associated with severity of initial presentation and the presence of anxiety which may be associated with increased health seeking behaviour at M12 in this population. Our results at M6 were in keeping with those reported in a Chinese cohort of hospitalized patients with COVID-19; however, the proportion of individuals with ≥1 symptom and the proportion of those still on sick leave at M12 were lower in the Chinese cohort than in ours [7]. Of note, median age in the Chinese cohort was 59 years versus 61 in ours, and the proportion of women was higher in the Chinese cohort (47%) than in ours (34%). In addition, if 88% of participants were indeed back to work at M12 visit in the Chinese cohort, it is important to emphasize that 24% did not return to pre-COVID-19 level of work [7].
Interestingly, our results favourably compared with those reported in Dutch ICU patients at M12 post admission [8].
The proportion of patients still complaining from PACS at M6 post COVID-19 [6] was higher than that reported in matched patients who had influenza [21]. The pathophysiology underlying these persistent or fluctuant PACS long after the acute phase is still unknown. Chronic inflammation, initial cytokine storm, residual virus in lungs post recovery, activation of the complement system, microthrombi and macrothrombi formation have been suggested as potential causes for these persistent symptoms [22,23]. In our series, 21% of participants had an mRS ≥2, and the percentage of predicted value of the 6MWT was lower in the 61 patients who reported dyspnoea than those who did not. However, C-reactive protein level was low in all participants, but this marker might not be a good marker of prolonged/chronic inflammation. Additionally, no samples for identification of residual viral persistence were obtained. Indeed, a few studies reported detection of viral proteins and RNA in various tissues, by in situ methods, months after infection [24,25]. Chronic distress can also be associated with chronic inflammation [26].
Our study had some limitations. First, the severity of PACSs was not assessed. Indeed, in our cohort at M6, when focusing on self-reported symptoms (and not symptoms reported by the physician), the proportion of reported symptoms was roughly the same but most symptoms were grade 1 [27]. Second, is the potential bias in patients who attended M12 follow-up, such patients were more prone to exhibit symptoms and thus, continued to seek medical care, than those who had completely recovered. Indeed, patients who did not attend the M12 visit had been less frequently admitted/transferred to ICU than those who did attend; these characteristics being less frequently associated with persistent PACS far from the acute episode. This limitation might explain in part the differences between our results and those of the Chinese cohort in which the number of participants attending M6 and M12 visits was similar, whereas the number of those attending M12 visit in our cohort was not only lower than expected regarding the total number of eligible patients, but also lower than those who attended M6 visit. We performed a sensitivity analysis by computing the observed proportion of ≥3 PACSs at M12 according to each combination of the risk factors found in the multivariate model to impute patients without M12 visit. However, this approach, which takes into account the differences on the distribution of risk factors, assumes that there is no specific selection bias (i.e. it assumes that patients without visit behave as those with a visit according to the combination of risk factors). Of note, scheduling follow-up hospital visits in this time of saturation of the healthcare system was challenging. Third, we did not have the health status (HRQL, anxiety and depression) of patients before acute infection. Finally, the impact of vaccines, treatment and less virulent strains (such as Omicron variant) is unknown.
In conclusion, longitudinal follow-up of individuals with severe COVID-19 is warranted to precisely determine the nature and frequency of persistent PACSs, with self-reported online or telephone assessments to reduce the number of patients lost to follow-up, with additional questionnaires to address somatic symptom disorders and to better understand the pathophysiology underlying this long-term persistence.
Author contributions
Dr Laouenan had full access to all the data used in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Transparency declaration
Authors report no conflict of interest except J.G. who reports personal fees from Merck, grants and personal fees from ViiV healthcare, grants and personal fees from Gilead Sciences, personal fees from Roche, personal fees from AstraZeneca, personal fees from Janssen, outside the submitted work.
Funding
The French COVID cohort is funded by the REACTing (REsearch & ACtion emergING infectious diseases) consortium, by a grant of the French Ministry of Health (PHRC n°20-0424), and by the ORCHESTRA project which has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement N°101016167. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript as well as decision to submit the manuscript for publication.
Acknowledgement
Group Information: Please refer to the supplementary material for information on the members of the French COVID cohort study and investigators groups.
Additional Information: The study included scientific advisory board members Dominique Costagliola, Astrid Vabret, Hervé Raoul and Laurence Weiss.
Editor: M. Cevik
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.08.028.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V., WHO clinical case definition working group on post-COVID-19 condition A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petersen M.S., Kristiansen M.F., Hanusson K.D., Danielsen M.E., Á Steig B., Gaini S., et al. Long COVID in the Faroe Islands: a longitudinal study among nonhospitalized patients. Clin Infect Dis. 2021;73:e4058–e4063. doi: 10.1093/cid/ciaa1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehme M., Braillard O., Chappuis F., Courvoisier D.S., Guessous I., CoviCare Study Team Prevalence of symptoms more than seven months after diagnosis of symptomatic COVID-19 in an outpatient setting. Ann Intern Med. 2021;174:1252–1260. doi: 10.7326/M21-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosn J., Piroth L., Epaulard O., Le Turnier P., Mentré F., Bachelet D., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041. doi: 10.1016/j.cmi.2021.03.012. e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heesakkers H., van der Hoeven J.G., Corsten S., Janssen I., Ewalds E., Simons K.S., et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;32:559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim Y., Bitna-Ha, Kim S.W., Chang H.H., Kwon K.T., Bae S., et al. Post-acute COVID-19 syndrome in patients after 12 months from COVID-19 infection in Korea. BMC Infect Dis. 2022;22:93. doi: 10.1186/s12879-022-07062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera-Izquierdo M., Láinez-Ramos-Bossini A.J., de Alba I.G.F., Ortiz-González-Serna R., Serrano-Ortiz Á., Fernández-Martínez N.F., et al. Long COVID 12 months after discharge: persistent symptoms in patients hospitalised due to COVID-19 and patients hospitalised due to other causes-a multicentre cohort study. BMC Med. 2022;20:92. doi: 10.1186/s12916-022-02292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeßle J., Waterboer T., Hippchen T., Simon J., Kirchner M., Lim A., et al. Persistent symptoms in adult patients 1 year after coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin Infect Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otten D., Tibubos A.N., Schomerus G., Brähler E., Binder H., Kruse J., et al. Similarities and differences of mental health in women and men: a systematic review of findings in three large German cohorts. Front Public Health. 2021;9 doi: 10.3389/fpubh.2021.553071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tibubos A.N., Otten D., Zöller D., Binder H., Wild P.S., Fleischer T., et al. Bidimensional structure and measurement equivalence of the Patient Health Questionnaire-9: sex-sensitive assessment of depressive symptoms in three representative German cohort studies. BMC Psychiatry. 2021;21:238. doi: 10.1186/s12888-021-03234-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schou T.M., Joca S., Wegener G. Bay-Richter C. Psychiatric and neuropsychiatric sequelae of COVID-19 - a systematic review. Brain Behav Immun. 2021;97:328–348. doi: 10.1016/j.bbi.2021.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Kala M.P., Jafar T.H. Factors associated with psychological distress during the coronavirus disease 2019 (COVID-19) pandemic on the predominantly general population: a systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0244630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yazdanpanah Y., French COVID cohort investigators. study group Impact on disease mortality of clinical, biological, and virological characteristics at hospital admission and overtime in COVID-19 patients. J Med Virol. 2021;93:2149–2159. doi: 10.1002/jmv.26601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turan Z., Topaloglu M., Ozyemisci Taskiran O. Medical Research Council-sumscore: a tool for evaluating muscle weakness in patients with post-intensive care syndrome. Crit Care. 2020;24:562. doi: 10.1186/s13054-020-03282-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balcom E.F., Nath A., Power C. Acute and chronic neurological disorders in COVID-19: potential mechanisms of disease. Brain. 2021;144:3576–3588. doi: 10.1093/brain/awab302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gouraud C., Bottemanne H., Lahlou-Laforêt K., Blanchard A., Günther S., Batti S.E., et al. Association between psychological distress, cognitive complaints, and neuropsychological status after a severe COVID-19 episode: a cross-sectional study. Front Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.725861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taquet M., Dercon Q., Luciano S., Geddes J.R., Husain M., Harrison P.J. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirschenberger M., Hunszinger V., Sparrer K.M.J. Implications of innate immunity in post-acute sequelae of non-persistent viral infections. Cells. 2021;10:2134. doi: 10.3390/cells10082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakrishnan R.K., Kashour T., Hamid Q., Halwani R., Tleyjeh I.M. Unraveling the mystery surrounding post-acute sequelae of COVID-19. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung C.C.L., Goh D., Lim X., Tien T.Z., Lim J.C.T., Lee J.N., et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut. 2022;71:226–229. doi: 10.1136/gutjnl-2021-324280. [DOI] [PubMed] [Google Scholar]
- 26.Henningsen P., Zipfel S., Herzog W. Management of functional somatic syndromes. Lancet. 2007;369:946–955. doi: 10.1016/S0140-6736(07)60159-7. [DOI] [PubMed] [Google Scholar]
- 27.Eloy P., Tardivon C., Martin-Blondel G., Isnard M., Turnier P.L., Marechal M.L., et al. Severity of self-reported symptoms and psychological burden 6-months after hospital admission for COVID-19: a prospective cohort study. Int J Infect Dis. 2021;112:247–253. doi: 10.1016/j.ijid.2021.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.