Abstract
Event-related potentials (ERPs) are an ideal tool for measuring neural responses in a wide range of participants, including children diagnosed with neurodevelopmental disorders (NDDs). However, due to perceived barriers regarding participant compliance, much of this work has excluded children with low IQ and/or reduced adaptive functioning, significant anxiety symptoms, and/or sensory processing difficulties, including heterogeneous samples of children with autism spectrum disorder (ASD) and children with fragile X syndrome (FXS). We have developed a behavioral support protocol designed to obtain high-quality ERP data from children in a single session. Using this approach, ERP data were successfully collected from participants with ASD, FXS, and typical development (TD). Higher success rates were observed for children with ASD and TD than children with FXS. Unique clinical–behavioral characteristics were associated with successful data collection across these groups. Higher chronological age, nonverbal mental age, and receptive language skills were associated with a greater number of valid trials completed in children with ASD. In contrast, higher language ability, lower autism severity, increased anxiety, and increased sensory hyperresponsivity were associated with a greater number of valid trials completed in children with FXS. This work indicates that a “one-size-fits-all” approach cannot be taken to ERP research on children with NDDs, but that a single-session paradigm is feasible and is intended to promote increased representation of children with NDDs in neuroscience research through development of ERP methods that support inclusion of diverse and representative samples.
Keywords: autism spectrum disorder, child, event-related potentials, fragile X syndrome
1 ǀ. INTRODUCTION
Event-related potentials (ERPs) are an invaluable tool for measuring the timing and nature of neural responses in a wide range of participants, including children diagnosed with neurodevelopmental disorders (NDDs). Research utilizing ERPs to study pediatric participants with NDDs can increase understanding of connections between behavioral symptoms and neural deficits in general sensory systems or higher level processing (e.g., Jeste & Nelson, 2009). However, much of the current literature is limited to children with higher levels of cognitive functioning and behavioral compliance. This is due in part to (a) preemptive exclusion of specific participants due to perceived barriers (e.g., poor verbal comprehension), often based on an arbitrary threshold such as minimum IQ, and (b) post hoc exclusion due to behavioral noncompliance, inability to complete the experiment, or excessive data loss. To improve inclusion, representation, and generalizability of ERP studies in NDDs, we developed a behavioral support protocol designed to obtain high-quality ERP data from heterogeneous groups of participants with NDDs. Not only is the behavioral support protocol designed to support a more diverse group of participants, but it is carried out in a single session, minimizing the time that participants are required to spend in the laboratory.
Although difficult to conduct, past research utilizing ERPs has made important contributions to our understanding of the development of NDDs in childhood and has implications for treatment paradigms. The use of ERPs provides insight into aspects of neural function that are impacted by NDDs. For example, research measuring ERP responses to visual stimuli has shed light on social information processing (e.g., Apicella et al., 2013; Batty et al., 2011; Webb et al., 2006) and word–object matching (e.g., Cantiani et al., 2016; DiStefano, Senturk, et al., 2019) in children with autism spectrum disorder (ASD), orienting of attention (Mento et al., 2019) and face recognition (Pavlova et al., 2018) in children with Down syndrome, inhibition in attention-deficit/hyperactivity disorder (ADHD) (e.g., Kóbor et al., 2015; Liotti et al., 2010), social information processing in Williams syndrome (Mills et al., 2013), nonsocial information processing in Rett syndrome (Fabio et al., 2019), processing of printed text in dyslexia (Maurer et al., 2011), and implicit learning in children with specific language impairment (Zwart et al., 2018). Additionally, ERPs may prove to be an objective and valuable biomarker for assessing effectiveness of interventions, including pharmaceutical treatments, in NDDs (Berry-Kravis et al., 2018). For example, ERPs have been used to examine whether medication may be effective in producing more typical patterns of neural responses. Groen and colleagues (2008) studied ERP responses during a feedback-based learning task to show that medication contributed to successful monitoring of error responses in children with ADHD. ERPs have also been used to assess the effectiveness of early intensive behavioral interventions designed to address symptoms of ASD. Dawson and colleagues (2012) found more normalized ERP activity following an intensive, behavioral intervention compared with a less rigorous, community intervention.
To maximize the impact and generalizability of ERP research, it is important that study samples reflect the heterogeneity of NDDs and are not limited to homogenous subgroups that are not representative of the full spectrum of NDDs (e.g., children with average or above average cognitive functioning; children without comorbid anxiety symptoms). However, most prior ERP research has excluded substantial proportions of children with NDDs, including those with lower cognitive functioning, significant anxiety symptoms, and/or sensory processing difficulties. For example, most studies of ASD have included only children with higher cognitive functioning (e.g., IQ > 70). Therefore, much of what we know about neural responses in ASD comes from a subset of individuals representing only one end of the ASD spectrum. Furthermore, some NDDs, particularly those with high proportions of children with significant cognitive impairments and co-occurring symptoms such as anxiety, have been largely ignored in ERP research. One example is fragile X syndrome (FXS), a single-gene disorder that is characterized by moderate-to-severe intellectual disability and elevated rates of ASD, inattention, impulsivity, anxiety, and sensory processing difficulties (Bailey et al., 2008; Baranek et al., 2008; Ezell et al., 2019; Roberts et al., 2020; Sullivan et al., 2006). To our knowledge, no studies have measured ERPs to visual stimuli in young children with FXS, so a great deal remains undiscovered about neural responses in FXS. A thorough understanding of neural processes in diverse samples of children with FXS, ASD, and other NDDs is critical to the improvement of early identification and development of treatment targets.
In an effort to increase representation in ERP research with children with NDDs, we have developed a behavioral protocol intended to support successful data collection. This protocol has been informed by guidelines developed for ERP research with typically developing infants and children (e.g., Bell & Cuevas, 2012; Brooker et al., 2020) and children with ASD (e.g., Kylliäinen et al., 2014; Webb et al., 2015) and adapted for single-session data collection from a heterogeneous sample of participants with NDDs. Because FXS is a relatively rare disorder affecting approximately one in 7000 males and one in 11,000 females (Hunter et al., 2014), we recruit participants from across the United States. Thus, many of our participants travel significant distances to participate in research, so it is imperative that we maximize our success in collecting high-quality data within a single laboratory visit. In the current study, we detail our behavioral support protocol, describe rates of successful data collection in children with ASD and FXS, and utilize rich clinical–behavioral data collected from each participant to investigate characteristics associated with successful data collection.
Successful data collection is a challenge in pediatric EEG and ERP research, and high rates of attrition are common. Previous research has addressed ERP methods for typically developing children (e.g., Bell & Cuevas, 2012; Brooker et al., 2020; Debnath et al., 2020; Hoehl & Wahl, 2012; Kaatiala et al., 2014; Stets & Reid, 2011; Stets et al., 2012; Taylor & Baldeweg, 2002; van der Velde & Junge, 2020) and children with NDDs (e.g., DiStefano et al., 2019; Kylliäinen et al., 2014; Stahl et al., 2012) in an attempt to reduce attrition and promote greater methodological consistency. Guidelines have addressed methods for collection of ERP data, preparation for data collection, and processing and analyzing of data. For the current paper, the focus will be on preparation for and collection of ERP data. Several recommendations coming from this work have been widely adopted in the field, including enhancing the testing environment so that it is more child friendly (e.g., Bell & Cuevas, 2012; Brooker et al., 2020) and limiting testing demands through reduction of trials completed, passive experimental designs, and use of frequent breaks (e.g., Brooker et al., 2020; van der Velde & Junge, 2020).
Attrition is an especially significant concern for research on NDDs, as it may be systematic, which could limit the generalizability if the resulting sample no longer reflects the heterogeneity inherent in the diagnostic population. Brooker et al. (2020) predicted that 1%–10% of young children are likely to refuse EEG cap application, with elevated rates for children in special populations, such as those with high fearfulness and/or hyperactivity. Only a small handful of studies have explicitly examined attrition in ERP studies of NDDs, so child characteristics that are associated with successful ERP data collection remain poorly understood. For example, Dawson and colleagues (2002) collected ERP responses to visual stimuli in 3- to 4-year-old children with ASD, developmental delay, and typical development (TD), and utilized a desensitization procedure that included up to seven visits to the lab. Even with this procedure in place, useable data were collected from only 54% of their ASD sample and 59% of participants with developmental delay. Great variability in participant mental age and the inclusion of children with lower cognitive functioning likely contributed to attrition in this study. Additional work has attempted to investigate specific factors that may be associated with attrition in participants with ASD. For example, Dawson and colleagues (2012) found that successful data collection was associated with less severe social communication deficits and higher verbal abilities in an ERP study of 4- to 6-year-old children with ASD. Interestingly, repetitive and restricted behavior symptoms and nonverbal cognitive abilities were not associated with successful data collection. Another recent study examined the role of participant emotional state in successful ERP study completion in a sample of 5- to 11-year-old children with ASD and typically developing children (DiStefano, Dickinson, et al., 2019). They found that participants with ASD exhibited greater agitation during data collection than TD children. Additionally, greater agitation was associated with the power of the EEG data collected, suggesting that participant emotional state influences EEG data characteristics beyond just attrition and data quality. Although these studies have helped to shed light on participant characteristics associated with successful ERP data collection, they are restricted to participants with ASD and TD.
Past ERP research examining children with ASD has contributed to the development of standards for successful data collection in our work; however, expectations are much less clear for children with FXS. A 2015 review of EEG research in individuals with FXS reported that only 10 studies utilizing EEG had been published to date (McDevitt et al., 2015). Of the studies that have been published, nearly all utilized auditory stimuli and included only adult participants. Although EEG research with adult participants has since increased, there continues to be a dearth in the collection of ERP data from children with FXS (see Razak et al., 2020). Visual ERP responses have been examined in three groups of participants with FXS, including infants (Guy et al., 2018), adolescents (Knoth et al., 2014), and adults (Van der Molen et al., 2012). The studies of visual ERP responses in adolescents and adults with FXS report rates of successful data collection between 60% and 70%. In our own previous work on infants with FXS, we reported a success rate of over 90% (Guy et al., 2018); however, many of the more challenging behavioral characteristics associated with FXS that might impact EEG data collection (e.g., sensory sensitivities, aversion to novel experiences) are not present until the second year of life in FXS (Baranek et al., 2008). Therefore, it is very difficult to determine (a) what attrition rate to expect in an ERP study on children with FXS, (b) what child characteristics are associated with increased attrition, and (c) strategies for reducing attrition in this population.
1.1 ǀ. Behavioral protocol for single-session ERP recording
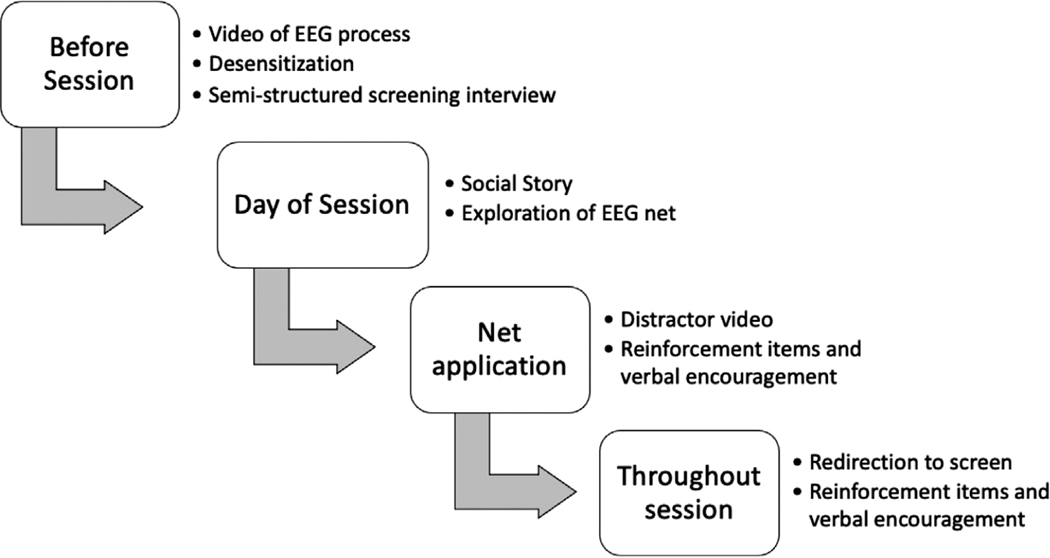
We developed a behavioral support protocol that is implemented in a single experimental session for collection of ERP data from children with NDDs. A flowchart presenting specific characteristics of our protocol is presented in Figure 1. Preparation for data collection begins prior to the participant’s lab visit. Upon scheduling an appointment, families are sent a video to familiarize them with the EEG setup and to help them to establish expectations for their visit. Parents are encouraged to share the video and discuss the visit with their child. Additionally, a lab member familiar with the experiment contacts the family to answer any questions and provide information about next steps, which include in-home desensitization. Desensitization protocols, which aim to reduce the intensity of emotional response to an aversive or fear-inducing stimulus through gradually increasing exposure, have been recommended for greater success in EEG/ERP data collection from children with ASD (DiStefano, Dickinson, et al., 2019; Kylliäinen et al., 2014; Webb et al., 2015). Webb and colleagues (2015) have suggested that sensory sensitivities and anxiety induced by novel experiences are the most common challenges to EEG data collection in children with ASD. Thus, desensitization is intended to increase familiarity and comfort with the EEG net and can be completed inside or outside of the lab setting. Desensitization that occurs in the EEG laboratory during a separate appointment prior to data collection is considered optimal; however, for studies like ours that recruit from large geographical areas, it is not feasible due to the distance that participants are required to travel for data collection. Thus, our desensitization procedure is completed by the parents in the participants’ homes. Families are sent desensitization materials, which include a child-sized silicone swim cap that mimics the sensation of wearing the EEG net and instructions encouraging the family to help their child practice wearing the cap on their head for increasing durations of time while talking to them about the upcoming EEG session. Desensitization instructions provided to families have been included in the Supporting Information.
FIGURE 1.
Behavioral support protocol
A week prior to the EEG data collection session, a lab member calls the family to conduct a semi-structured screening interview. Pre-appointment interviews have been recommended for EEG and ERP studies of children with ASD, during which researchers are encouraged to review the testing procedures in great detail, identify events that may be triggering, and consider accommodations that can be made (Kylliäinen et al., 2014; Webb et al., 2015). Additionally, our interview includes questions about preferred items to use for behavioral reinforcement (e.g., candy, toys), preferred videos to use as a visual distraction during the placement of the EEG net, and any other preferred behavioral support techniques the child may benefit from (e.g., visual schedules; for all questions, see the Supporting Information). The lab member also discusses the family’s progress with using the cap at home, offers suggestions for desensitization, and answers any remaining questions the parent has. Based on the information obtained in the screening interview, the EEG testing room is prepped with the preferred reinforcement items and any other necessary materials to best support the child. The notes from the interview are included in the participant’s materials to be reviewed by the experimental team on the day of the visit.
Prior to the experiment, the testing environment is prepared for the child. The lab space consists of multiple rooms, which allows for separation of the participant’s testing space from the experimental control space. This separation may reduce distraction and anxiety associated with a busier room and the presence of more staff. Preferred reinforcement items and behavioral support materials are placed in the testing room. Additional optional support tools, including a vest, weighted blanket, and visual schedule, are also placed into the testing for convenient access during testing.
The behavioral support protocol continues on the day of the visit. Participants’ families are greeted at the door and led to a waiting room adjacent to the EEG testing room. The participant is introduced to a clinically trained behavioral aid that assists them throughout the experiment and is given the opportunity to become familiar with the lab environment, which may reduce arousal and anxiety from being in the lab environment and interacting with experimenters (Kylliäinen et al., 2014). The behavioral aid reinforces positive behavior, promotes compliance, supports participant understanding and well-being, and serves as a liaison between the participant and the other experimenters (Webb et al., 2015). After welcoming the participant and their family to the waiting room, the behavioral aid reads the participant a social story, in the form of a picture book, which provides an overview of the EEG process. The participant is reminded that they will receive their preferred reinforcement items for listening to instructions and will get to choose a prize from a box of toys at the end of data collection. Frequent and regular reinforcement is recommended in EEG research conducted with children with ASD (Kylliäinen et al., 2014). Although the participant’s head measurements are taken, the lab members and family offer verbal encouragement and reinforcement. A behavioral aid checklist, detailing lab setup and behavioral support guidelines, and a copy of the social story are included in the Supporting Information.
Once the participant is ready and the EEG net has been prepped for application, they are led into the testing room. There they are seated either in a child’s highchair or their parent’s lap, dependent upon the family’s preference. Seats designed for children with physical disabilities are recommended for consideration in studies of children with ASD (Kylliäinen et al., 2014). We utilize a Keekaroo highchair that features a seat that can be adjusted for height and depth, an adjustable footrest, and a lap belt. This highchair provides physical support to the child and may reduce movement opportunities. The parent is seated in a chair behind the participant, if the highchair is selected. A child-friendly video plays during this time to keep the child’s attention and reinforce the behavior of sitting still in the chair. Before placement of the EEG net on their head, the child is shown the net and encouraged to touch it. Electrical Geodesics, Inc. (EGI) high-density Hydrocel geodesic sensor nets are used in this work due to their ease of application and technical benefits (Webb et al., 2015). If the child is hesitant to touch the net, the parent is encouraged to touch the net to reassure the child that the net is harmless. The child is then reminded that they will be given their preferred reinforcement item once the net is on. During net application, the behavioral aid offers the child verbal encouragement. After a brief break to receive the reinforcement item, the participant and their parent are reminded of expectations for the experiment. These include sitting quietly and watching the video, keeping hands off of the net, receiving breaks and reinforcement during the experiment, and receiving a prize at the end of the experiment. The behavioral aid and parent remain in the testing room for the duration of the experiment.
Behavioral support is continued throughout the experiment. A passive ERP paradigm is utilized to decrease demands on participants, which has been recommended for studies with young children (Brooker et al., 2020) and participants with intellectual disability (Kylliäinen et al., 2014). In the present study, experimental stimuli included brief presentations of upright and inverted faces and houses that were presented in eight blocks of 48 trials. The participant is rewarded with a short break to promote their engagement upon completion of each block of trials. Breaks include verbal encouragement, presentation of reinforcement items, and the opportunity for small movements. Breaks have been associated with decreased opposition to the experiment, decreased motor movements during data collection, decreased distress (Brooker et al., 2020), and decreased attrition more generally (van der Velde & Junge, 2020). Additionally, breaks may promote more consistent levels of arousal in participants (e.g., participants may be less likely to become drowsy during testing) (Noreika et al., 2020). The participant is reminded of the rules at the end of the break and then the next block begins. If the participant becomes inattentive during stimulus presentation, brief clips of Sesame Street are presented to attract attention to the monitor. The behavioral aid may also silently redirect the participant. Breaks may be initiated prior to completion of 48 trials, if necessary. The experiment is terminated upon completion of all blocks or when the participant becomes noncompliant (e.g., the experiment would be ended prematurely if the participant becomes agitated or is no longer responsive to redirection). A detailed log sheet is maintained during the experiment to better understand factors contributing to participant performance. This practice is recommended for research on children with ASD (Kylliäinen et al., 2014; Webb et al., 2015). Information on participant distress, attention, and compliance may promote insight into sources of attrition.
1.2 ǀ. Assessing successful data collection with the behavioral support protocol
In this paper, we systematically investigate factors associated with successful data collection in children with FXS and ASD. We first aim to assess whether our behavioral protocol is effective in achieving attrition rates similar to more intensive protocols involving repeated in-person desensitization. We then describe participants’ performance during the ERP experiment and examine relations between participant performance and clinical–behavioral characteristics including ASD symptom severity, nonverbal mental age (NVMA), attention problems, sensory hyperresponsivity, and anxiety symptoms. Due to a lack of previous systematic investigation, little is known about which clinical–behavioral features contribute to inclusion or attrition in psychophysiological studies of children with ASD or FXS. Thus, we selected clinical–behavioral features that represent common domains of impairment in ASD or FXS, based on the following sources of knowledge: (1) clinical expertise in early development in ASD and FXS; (2) research indicating that these clinical–behavioral features contribute to “challenging behaviors” in both ASD and FXS (Cervantes et al., 2013; Leader et al., 2021; Newman et al., 2015; O’Donnell et al., 2012); and (3) our anecdotal experience of collecting psychophysiological data (e.g., eye tracking, electrocardiogram) with children with ASD and FXS for more than a decade. We hypothesized that characteristics associated with greater impairments (e.g., lower NVMA, more inattention, greater sensory hyperresponsivity) would be associated with poorer compliance and fewer valid trials on the ERP task. Because children with FXS experience higher rates of many of these impairments, we expected that they would be more significantly impacted by low trials numbers than the ASD and TD groups.
2 ǀ. METHOD
2.1 ǀ. Participants
As part of a larger longitudinal study of development in young children, we enrolled and tested 111 children in this EEG experiment from several groups (TD, FXS, FMR1 premutation, Down syndrome, ASD, and non-ASD siblings of children with ASD). For the purpose of this paper, which focuses on the effectiveness of specific behavioral support procedures in children with NDDs, we present details on the children with FXS (n = 24) and children with ASD (n = 33), with typically developing children included for comparison (n = 25). We did not include the DS sample in analyses because of limited sample size (n = 6). Non-ASD siblings of children with ASD and children with the FMR1 pre-mutation were not included because they were not diagnosed with an NDD, which is intended to be the focus on the current study. Participants ranged in age from 35.84 to 114.36 months. Due to the nature of FXS and ASD, and the targeted recruitment of children with cognitive impairments as part of the larger longitudinal study, the FXS and ASD groups had high rates of intellectual disability (ID; 70.8% and 51.52%, respectively) as defined by IQ < 70. Additionally, 50% of participants in the FXS group received ASD diagnoses, which is consistent with prevalence rates of comorbid ASD in FXS (Roberts et al., 2020). Descriptive and demographic participant information is presented in Table 1. Recruitment strategies varied based on participant group. Children with FXS were recruited through local and national organizations that serve children with FXS. Diagnosis of FXS was confirmed through genetic report (i.e., ≥200 CGG repeats on the FMR1 gene). Children with ASD were recruited statewide through organizations that serve children with ASD. Diagnosis of ASD was confirmed through the study using gold-standard diagnostic measures, including the Autism Diagnostic Observation Schedule, Second Edition (Lord et al., 2012) and the Autism Diagnostic Interview-Revised (Lord et al., 1994), combined with clinical best estimate review by a licensed psychologist. Typically developing controls were recruited locally through advertisements placed in the community and were required to have no family history of ASD, FXS, or related disorders. TD was confirmed by clinical–behavioral testing and clinical best estimate review. Participants were excluded if they were born at <37 weeks gestation, had uncorrected vision or hearing impairments, or had parents who were not proficient in English.
TABLE 1.
Participant characteristics
| FXS (n = 24) | ASD (n = 33) | TD (n = 25) | F(df) = F, p = p | |
|---|---|---|---|---|
| Key variables | ||||
| Age (months), M (SD) | 66.39a (18.27) | 65.22a (18.14) | 61.41a (15.59) | F(2,79) = 0.559, p = .574 |
| Range | 36.93–105.18 | 42.38–114.36 | 35.84–85.07 | |
| Number of females (%) | 8 (33.3%) | 0 (0 %) | 4(16%) | - |
| IQ, M (SD) | 60.38 (17.44) | 62.91 (24.17) | 104.36 (14.36) | - |
| NVMA, M (SD) | 44.63a (13.87) | 45.58a (16.73) | 62.66b (18.54) | F(2,77) = 9.63, p < .001 |
| Range | 19–80.5 | 22–97 | 29.5–107 | |
| Proportion with intellectual disability (ID) (i.e., IQ < 70) |
70.83% | 51.52% | 0.00% | - |
| Expressive language | 26.21a (6.41) | 25.83a (6.62) | 32.54b (4.78) | F(2,69) = 9.56, p < .001 |
| Receptive language | 60.89a (23.45) | 58.48a (23.80) | 84.83b (16.12) | F(2,69) = 11.29, p < .001 |
| Valid trial count | 51.42a (69.85) | 116.24b (93.77) | 210.12c (79.63) | X2(2) = 51.46, p = < .001 |
| Clinical-behavioral variables | ||||
| ADOS-2 CSS | 5.25a (2.82) | 6.75b (1.98) | 1.88c (1.24) | F(2,78) = 38.84, p < .001 |
| ADOS-2 SA CSS | 5.17a (2.87) | 5.80a (1.73) | 2.52b (1.50) | F(2,76) = 18.29, p < .001 |
| ADOS-2 RB CSS | 6.58a (2.67) | 8.30b (1.66) | 2.04c (1.90) | F(2,76) = 63.77, p < .001 |
| SEQ hyperresponsivity total score | 1.94a,b (0.55) | 2.31a (0.50) | 1.80b (0.42) | F(2,41) = 4.10, p = .024 |
| SEQ hyporesponsivity total score | 1.78ab (0.57) | 2.13a (0.76) | 1.45b (0.33) | F(2,41) = 4.28, p = .021 |
| Spence total anxiety T score | 44.62a (6.27) | 44.30a (8.72) | 43.04a (5.74) | F(2,66) = 0.335, p = .716 |
| CBCL attention problems T score | 61.15a (9.12) | 60.28a (8.90) | 53.08b (4.97) | F(2,66) = 7.40, p = .001 |
| Descriptive variablesa | ||||
| Race, n (%) | ||||
| American Indian/Alaska Native | 2 (8.3%) | 0 (0.0%) | 0 (0.0%) | |
| Asian | 0 (0.0%) | 1 (3.0%) | ||
| Black or African American | 1 (4.1%) | 3 (9.1%) | 1 (4.0%) | |
| White | 18 (75.0%) | 28 (84.8%) | 24 (96.0%) | |
| More than one race | 2 (8.3%) | 1 (3.0%) | 0 (0.0%) | |
| Unknown/No response | 1 (4.1%) | 0 (0.0%) | 0 (0.0%) | |
| Ethnicity, n (%) | ||||
| Hispanic or Latino | 0 (0.0%) | 1 (3.0%) | 0 (0.0%) | |
| Not Hispanic or Latino | 24 (100.0%) | 31 (93.9%) | 25 (100.0%) | |
| No answer unknown/No response | 0 (0.0%) | 1 (3.0%) | 0 (0.0%) | |
| Household income, M (SD) | $109,010 ($87,627) | $78,349 ($35,197) | $95,524 ($48,841) | |
Descriptive variables were only used to characterize the sample. They were not used in any statistical analyses. Different subscripts represent significant differences on Tukey HSD corrected pairwise comparisons, p < .05.
2.2 ǀ. General procedure
Written informed consent was obtained from the parents prior to study enrollment. Consent was obtained as part of a large longitudinal study including behavioral and EEG tasks, and formal verbal assent was not collected from participants. Behavioral assessments occurred at the participants’ homes or in a research laboratory. Electroencephalogram data collection was conducted in a research laboratory on the same day or a different day as the behavioral assessments. For same-day sessions, EEG data were collected first, followed by behavioral measures. This was arranged to promote greater attention and decreased fatigue upon EEG data collection. This approach is consistent with recent research and recommendations by van der Velde and Junge (2020), who found that EEG and ERP data loss was decreased in infants and young children if they were tested in the morning. Parents received monetary compensation for their participation. All procedures were approved by the Institutional Review Boards at the University of South Carolina.
2.3 ǀ. Electroencephalogram procedures
2.3.1 ǀ. Apparatus
The testing room was equipped with a 29″ LCD monitor (NEC Multisync XM29) positioned approximately 55 cm away from the participant. Stimuli were presented on the monitor and participants’ looking behavior was recorded using a video camera that was positioned above the monitor. An experimenter in an adjacent experiment control room judged participant fixation online and controlled stimulus presentation using EGI Net Station software and an E-Prime experiment program.
2.3.2 ǀ. Stimuli
Stimuli included photographs of faces and houses that were presented in either upright or inverted orientations. Face stimuli came from the NimStim database (Tottenham et al., 2009) and included female and male faces of different races. House stimuli were selected for their symmetry and were gathered from web searches and pictures taken of houses in the local Columbia, SC area. Dynamic videos of 15 Sesame Street characters were used as attractor stimuli. All stimuli were presented on colorful, static variegated backgrounds containing simple patterns such as sand, water, and grass. The backgrounds stretched across the entire monitor and possessed more detail in the center of the screen. This part of the background was covered during stimulus presentation and was intended to attract attention toward the center of the screen during intertrial intervals.
2.3.3 ǀ. Procedure
Data were collected using the behavioral support protocol previously described. Three research staff members executed the protocol: a behavioral aid tasked with supporting the participant, a lab tech responsible for preparation of the EEG equipment and testing room, and an experimenter who controlled stimulus presentation and collection of EEG data. Additional staff were occasionally present for training and supervision purposes. The experiment began when the participant became fixated on the attractor stimulus at the center of the screen. An experimenter pressed a keyboard button to initiate brief stimulus presentations. Each stimulus presentation began with a blank screen for a period of 100 ms, followed by a 500-ms stimulus presentation, and a variable intertrial interval of 500–1500 ms. Forty-eight stimulus presentations were included in each block. Each block was followed by a short break, when the participant received reinforcement items. At the beginning of each block and during times of behavioral inattention (i.e., looking away from the monitor), a dynamic Sesame Street video was presented to attract visual fixation. The duration of each block varied based upon use of the attractor stimulus and occasionally blocks were terminated early to allow for a break. The blocks ranged from 51 to 223 s. The experiment was terminated after eight trial blocks or earlier if the participant became fatigued or noncompliant.
2.3.4 ǀ. EEG recording and segmentation
The EGI 128-channel EEG recording system was used to record EEG data (Tucker, 1993). Participants were fitted with an EGI Hydrocel geodesic sensor net (HGSN), selected based on participant head circumference. Net application took 5–10 min, during which participants viewed child-friendly attractor videos and were assisted by the behavioral aid. EEG was measured through 124 channels in the electrode net, two Ag–AgCl electrodes positioned at the outside of the eyes measured electrooculogram (EOG), and two Ag–AgCl electrodes positioned on the chest measured electrocardiogram (ECG). The EEG signal was referenced to the vertex electrode, recorded with 20 K amplification at a 250 Hz sampling rate, and with bandpass filters set from 0.1 to 100 Hz and 100 kΩ impedance.
Following EEG recording, the data were algebraically recomputed to an average reference. The EEG data were processed with the EEGLAB and ERPLAB toolboxes (Delorme & Makeig, 2004; Lopez-Calderon & Luck, 2014) within MATLAB. Video data were coded using Datavyu to confirm that the participant was looking at the monitor during stimulus presentation (Datavyu, 2014). Trials were discarded from further analysis if the participant looked away or blinked during stimulus presentation. Computational methods, including independent component analysis, were used on all data collected to detect and eliminate bad channels on individual trials. Manual visual review was also completed by at least one expert reviewer to confirm that all artifact was detected using the computational methods. Additional data were marked bad, if necessary. Participant diagnoses were not blinded from reviewers, as data were coded as they were collected, and many coders were members of the research team. Data in bad channels was substituted with average data from the five closest electrodes. The trial was rejected if it contained 12 or more bad channels. For the larger EEG study, participants are required to contribute at least 10 valid trials per condition to their individual average to be included in ERP analyses. For the purposes of the present study, we classify “successful” data collection using these standards (i.e., ≥10 valid trials per condition). Minimum trial numbers for stable ERP averages have not been established for ERP research with children; however, this minimum is in line with current estimates (for review, see Brooker et al., 2020). Additionally, reports indicate that there are no differences between observed artifact or number of rejected ERP trials between groups with ASD and TD (see Webb et al., 2015). We also computed the total number of valid trials contributed after processing and rejection, summed across the four conditions. Participants who were not able to be capped due to extreme fear and/or noncompliance were assigned a value of “0” for number of valid trials.
2.4 ǀ. Clinical–behavioral measures
2.4.1 ǀ. Nonverbal mental age
NVMA was computed via one of two measures. The Mullen Scales of Early Learning (MSEL; Mullen, 1995), a standardized assessment of cognitive development for children from birth to 68 months of age, was used in children ≤68 months. For the MSEL, NVMA was computed as the average of the visual reception and fine motor subscale age equivalents. In children older than 68 months, the Differential Ability Scales, Second Edition was used (DAS-II; Elliott, 2006). The DAS-II Early Years Battery (DAS-II-EY) was administered with participants between 69 and 83 months and the School-Age Battery (DAS-II-SA) was administered with participants >84 months old. Both DAS-II batteries consist of core subtests in the domains of verbal, nonverbal reasoning, and spatial abilities. NVMA was indexed by averaging the Matrix and Picture Similarities subscales age equivalents.
2.4.2 ǀ. ASD symptom severity
ASD symptom severity was measured using the Autism Diagnostic Observation Schedule, Second Edition (Lord et al., 2012), a semi-structured, play-based measure that is widely used as a gold-standard diagnostic measure of ASD in children and adults. The Calibrated Severity Score (CSS) was used as a continuous measure of overall ASD symptom severity. The Social Affect (SA) and Repetitive and Restricted Behavior (RRB) CSS scores were used as measures of domain-specific ASD symptom severity. CSS scores range from 1 to 10, with higher scores reflecting more severe ASD symptoms.
2.4.3 ǀ. Attention problems
The Child Behavior Checklist—1.5 to 5 (CBCL 1.5–5; Achenbach & Rescorla, 2001) Attention Problems subscale t-score was used as an indicator of attention difficulties. The CBCL 1.5–5 is a parent-report questionnaire used to screen for behavioral, emotional, and social problems in children between the ages of 1.5 and 5 years. Parents rate statements describing their children’s behavior on a Likert scale from 0 (Not true) to 2 (Very/Often true). The scores are totaled for each subscale and converted to t-scores, with higher scores indicating more severe symptoms.
2.4.4 ǀ. Sensory responsivity
Sensory hyper- and hyporesponsivity were measured using the Sensory Experiences Questionnaire (SEQ; Baranek et al., 2006), a parent-report questionnaire used to characterize sensory features in children with ASD and developmental disabilities aged 6 months to 6 years. Parents rate questions about their children’s responses to everyday stimuli on a Likert scale from 1 (Almost never) to 5 (Almost always). Items are grouped into categories corresponding to three separate sensory response pattern scales: Hyporesponsive, Hyperresponsive, and Sensory Seeking. Response pattern mean scores are computed, with higher scores indicating more intense sensory symptoms. The hyper- and hyporesponsive mean scores were used as measures of sensory responsivity in the current study.
2.4.5 ǀ. Anxiety symptoms
Anxiety symptoms were measured using the Preschool Anxiety Scale (PAS; Spence et al., 2001), a parent-report questionnaire designed to assess the presence of anxiety-related behaviors in preschool-aged children. Parents rate descriptions of their children’s behaviors on a Likert scale from 0 (Not at all true) to 4 (Very often true). The total t-score reflecting overall anxiety symptoms was used in the current study, with higher scores indicating more severe symptoms.
2.4.6 ǀ. Language ability
Expressive language (EL) and receptive language (RL) were measured utilizing the Vineland Adaptive Behavior Scales-II (VABS-II; Sparrow et al., 2005). The VABS-II is a semi-structured parent interview that measures adaptive functioning across four domains: Communication, Socialization, Daily Living Skills, and Motor Skills. Individual items are scored a 0 (never), 1 (sometimes), or 2 (usually). Raw scores for the subdomains of RL and EL were utilized in this study.
2.5 ǀ. Statistical analysis plan
The first aim of the study was to assess the effectiveness of our behavioral protocol in facilitating successful ERP data collection in children with FXS and ASD. For this aim, we describe the total number of valid trials collected (i.e., the total number of valid trials across all four conditions) and the rates of “successful” data collection (as defined by ≥10 valid trials per condition) across groups. Due to violation of the assumptions of ANOVA, nonparametric equivalents were utilized to compare the group differences on the number of valid trials. A Kruskal–Wallis test was utilized as the omnibus test of group differences. Pairwise Wilcoxon rank sum tests were utilized with Bonferroni correction to probe for specific group differences in the number of valid trials. Both of these tests were utilized as nonparametric equivalents due to a lack of normality within each group.
The second aim of the study was to determine which child characteristics were associated with successful EEG data collection. In preliminary analyses, group differences in chronological age and clinical–behavioral variables (i.e., NVMA, attention problems, anxiety symptoms, autism severity, sensory responsivity, and language ability) were tested using one-way ANOVAs and post hoc Tukey HSD pairwise comparisons between groups. Group means and results of these preliminary analyses are presented in Table 1. Spearman correlations were computed between the number of valid trials and both chronological age and NVMA. Because the groups showed different clinical–behavioral profiles (e.g., the ASD group exhibited higher ASD symptom severity than the FXS and TD groups; the ASD and FXS groups exhibited more attention problems than the TD group), and because NVMA is known to be associated with many other clinical–behavioral characteristics of interest, we chose to employ partial Spearman correlations, controlling for NVMA, separately within each group. We also examined relations between clinical–behavioral characteristics and the total number of valid trials collected in the full sample, the subsample of participants who successfully completed net application, and the subsample of participants with ID. Additionally, we controlled for participant sex in the FXS group correlations between the number of valid trials and clinical–behavioral variables due to the known sex differences associated with the FXS phenotype (Bartholomay et al., 2019).
3 ǀ. RESULTS
3.1 ǀ. Rates of successful ERP data collection
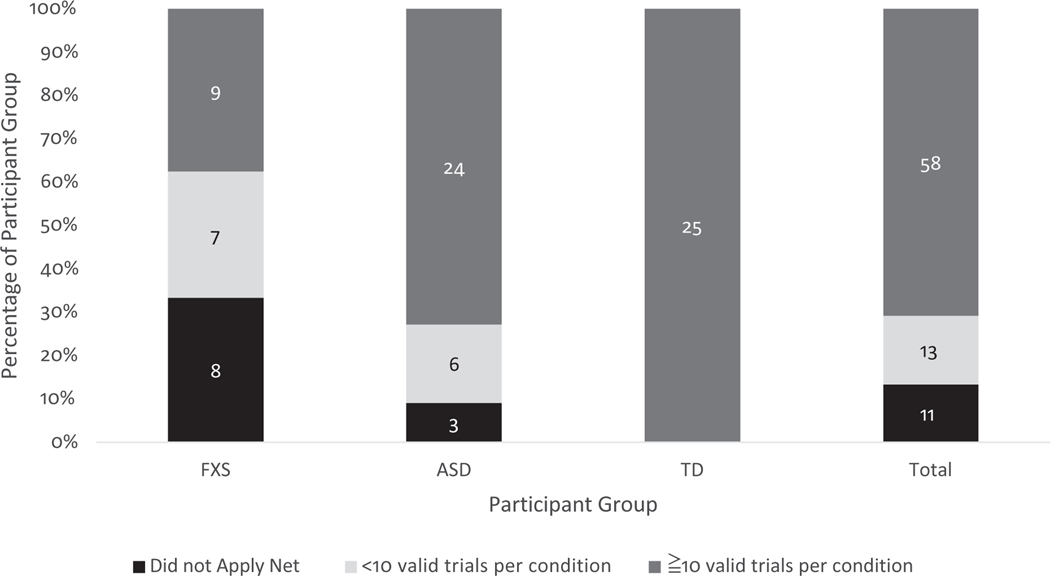
Implementation of the single-session behavioral support protocol resulted in the “successful” collection of ERP data (i.e., ≥10 good trials per condition) from 58 out of 82 participants (70.7%), including nine (four female) out of 24 participants with FXS (37.50%), 24 out of 33 (0 female) participants with ASD (72.72%), and 25 (four female) out of 25 TD children (100.00%). Figure 2 describes the proportion of participants who met standards for “successful” data collection, the proportion of participants who were able to tolerate net application but contributed <10 trials per condition, and the proportion of participants unable to complete net application. Of the three groups, children with FXS demonstrated the highest rates of unsuccessful net application (n = 8, 33.33%) and insufficient number of trials (i.e., <10 trials per condition; n = 7, 29.16%). In the ASD group, 9.09% of participants were unable to tolerate the net (n = 3), and an additional 18.18% of participants contributed an insufficient number of trials (n = 6).
FIGURE 2.
Proportion of participant data collection outcomes by group
3.2 ǀ. Number of valid trials by group
The number of valid trials was also compared across groups. Figure 3 illustrates the distribution of number of valid trials across groups. The TD group contributed the highest number of ERP trials (M = 210 trials, SD = 79.6), followed by children with ASD (M = 116 trials, SD = 93.8) and children with FXS (M = 51 trials, SD = 69.9). A Kruskal–Wallis rank sum test with post hoc Bonferroni pairwise comparisons indicated that number of trials was significantly different between groups (c2(2) = 51.46, p < .001) (see Table 1). Post hoc comparisons revealed that the TD group contributed significantly more trials than the ASD and FXS groups (ps < .001) and the ASD group contributed significantly more trials than the FXS group (p = .012).
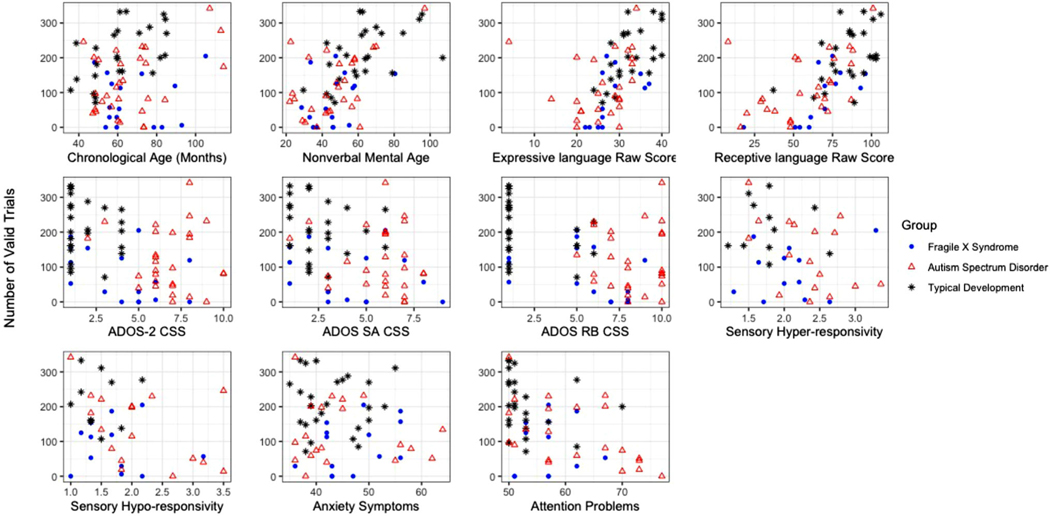
FIGURE 3.
Scatterplots of participant-level data by number of valid trials
3.3 ǀ. Relations between child characteristics and number of valid trials contributed
Figure 3 presents scatterplots of the number of valid trials completed in relation to chronological age and clinical–behavioral characteristics, including chronological age, NVMA, EL, RL, overall ASD symptom severity, ASD social affect symptom severity, ASD repetitive behavior symptom severity, sensory responsivity, anxiety symptoms, and attention problems. Partial correlations, controlling for NVMA, were computed between the number of valid trials and clinical–behavioral characteristics. Additionally, partial correlations for the FXS group controlled for sex, due to the known sex effects in FXS. The results of correlations are outlined by different participant samples and participant group in Table 2. In the following paragraphs, analyses are organized by variable and include the entire sample for each group (FXS n = 24, ASD n = 33, and TD n = 25), the subsample of participants who successfully completed net application (n = 71), and the subsample of participants with ID (n = 34).
TABLE 2.
Spearman correlations between child characteristics and number of valid trials
| FXS(n = 24) [95% CI] | ASD (n = 33) [95% CI] | TD (n = 25) [95% CI] | Full sample (n = 82) | Capped sample (n = 71) | Sample with ID (n = 34) | |
|---|---|---|---|---|---|---|
| Chronological age | .11 [−.30 .49] | .43** [.09 .67] | .55*** [.20 .77] | .23** [.01 .42] | .23* [.00 .44] | −.04 [−.37 .30] |
| NVMA | .33 [−.08 .65] | .52** [.20 .74] | .54*** [.18 .77] | .60*** [.44 .72] | .59*** [.41 .73] | – |
| Partial correlations | ||||||
| Expressive language | .67*** [.36 .84] | .18 [−.17 .50] | .43* [−.08 .76] | .38*** [.17 .55] | .31*** [.09 .51] | .28** [.05 .48] |
| Receptive language | .70*** [.42 .86] | .30* [−.05 .58] | .14 [−.38 .59] | .38*** [.18 .56] | .39*** [.17 .57] | .32*** [.10 .52] |
| ADOS-2 CSS | −.66*** [−.82 −.41] | −.06 [−.40 .29] | −.19 [−.50 .17] | −.39*** [−.56 −.19] | −.26** [−.47 −.03] | −.06 [−.29 .18] |
| ADOS-2 SA CSS | −.50** [−.75 −.12] | −.21 [−.52 .14] | −.31* [−.59 .043] | −.40*** [−.56 −.19] | −.26** [−.46 −.03] | −.08 [−.31 .16] |
| ADOS-2 RB CSS | −.71*** [−.865 −.427] | −.06 [−.39 .29] | −.21 [−.51 .15] | −.40*** [−.57 −.205] | −.30** [−.50 −.07] | −.08 [−.30 .16] |
| SEQ hyperresponsivity total score | .47* [−.03 .78] | −.11 [−.44 .25] | −.03 [−.52 .47] | .04 [−.18 .26] | −.08 [−.30 .16] | .58*** [.40 .76] |
| SEQ hyporesponsivity total score | .05 [−.36 .45] | −.25 [−.55 .10] | −.03 [−.52 .47] | −.08 [−.29 .14] | −.04 [−.27 .20] | .05 [−.19 .28] |
| Spence total anxiety T score | .55** [.19 .86] | .12 [−.24 .44] | −.25 [−.48 .52] | .01 [−.21 .23] | −.12 [−.34 .12] | .33*** [.10 .52] |
| CBCL attention problems T score | .01 [−.39 .41] | −.24 [−.54 .11] | −.43* [−.77 −.08] | −.34*** [−.52 −.14] | −.34*** [−.53 −.13] | −.06 [−.29 .18] |
Note: All correlations are between the behavioral variables listed and the number of valid trials. If not capped, the number of valid trials is 0. Partial correlations controlled for NVMA. In the FXS group, partial correlations controlled for NVMA and sex.
p < .10;
p < .05;
p < .01.
Abbreviations: ADOS-2 CSS, Autism Diagnostic Observation Schedule, Second Edition calibrated severity score; ADOS-2 RB CSS, Autism Diagnostic Observation Schedule, Second Edition Repetitive Behavior calibrated severity score; ADOS-2 SA CSS, Autism Diagnostic Observation Schedule, Second Edition Social Affect calibrated severity score; CBCL, Child Behavior Checklist; NVMA, nonverbal mental age; SEQ, Sensory Experiences Questionnaire.
3.3.1 ǀ. Chronological age
Chronological age was significantly correlated with the number of valid trials in the full sample (r = .23, p = .041). Chronological age and the number of valid trails for the subsample that completed net application was marginally significant (r = .23, p = .051). The subsample with ID demonstrated no relationship between chronological age and the number of valid trials (r = .04, p = .813). Within-group correlations between chronological age and the number of valid trials demonstrated a significant relationship for both the ASD (r = .43, p = .014) and TD group (r = .55, p = .004). In the FXS group, chronological age and the number of valid trials was not related (r = .11, p = .600).
3.3.2 ǀ. Nonverbal mental age
A significant correlation emerged between NVMA and the number of valid trials for the full sample (r = .60, p < .001) and the subset of the sample that successfully completed net application (r = .59, p < .001). Relations between NVMA and ID on successful data collection were not examined, as NVMA is used to define ID. Within-group correlations revealed a significant correlation for both the ASD (r = .52, p = .003) and TD (r = .54, p = .005) groups. In the FXS group, no significant relationship was observed (r = .33, p = .114).
3.3.3 ǀ. Language ability
EL and RL were significantly correlated with the number of valid trials within the full sample (EL: r = .38, p = .001; RL: r = .38, p < .001), subsample that successfully completed net application (EL: r = .31, p = .008; RL: r = .39, p = .001), and subsample with ID (EL: r = .28, p = .020; RL: r = .32, p = .006). Within-group correlations demonstrated a significant relationship between EL and the number of valid trials for the FXS group (r = .67, p < .001), a marginally significant relationship for the TD group (r = .43, p = .097), and a no relationship for the ASD group (r = .18, p = .307). In the RL domain, a significant relationship emerged for the FXS group (r = .70, p < .001). In the ASD group, a marginally significant relationship emerged (r = .30, p = .092). No relationship emerged for the TD group (r = .14, p = .606).
3.3.4 ǀ. ASD symptom severity
ADOS-2 overall CSS, ADOS-2 SA CSS, and ADOS-2 RRB CSS were significantly correlated with the number of valid trials for the full sample (r = −.39, p < .001; r = −.40, p < .001; r = −.40, p < .001; respectively) and the subsample that successfully completed net application (r = −.31, p = .028; r = −.26, p = .029; r = −.26, p = .011; respectively). In the subsample with ID, no significant relationship emerged between the measures of ASD symptom severity and the number of valid trials (rs <−.08, p > .509). Within the FXS group, correlations demonstrated significant relations between the ADOS-2 overall CSS (r = −.66, p < .001), the ADOS-2 SA CSS (r = −.50, p = .012), and the ADOS-2 RRB CSS (r = −.71, p <. 001) and the number of valid trials. In the TD group, a marginally significant relationship emerged between ADOS-2 SA CSS and the number of valid trials (r = −.31, p = .084); however, no significant relations emerged between ADOS-2 CSS or ADOS-2 RB CSS and the number of valid trials (rs < .21, ps > .250). The ASD group demonstrated no significant relations between any of the measures of ASD symptom severity and the number of valid trials (rs < .21, ps > .239).
3.3.5 ǀ. Sensory responsivity
In the full sample and the subsample that successfully completed net application, hyper- and hyporesponsivity were not significantly correlated with the number of valid trials (rs < −.08, ps > .475). For the sample of participants with ID, greater hyperresponsivity was associated with higher numbers of valid trials completed (r = .58, p < .001). Hyporesponsivity (r = .05, p = .696) was not significantly correlated with valid trials completed. Within-group correlations revealed that hyperresponsivity was only marginally significantly correlated with the number of valid trials completed for the FXS group (r = .47, p = .057). All other correlations between the sensory responsivity and the number of valid trials for the three groups were not significant (rs < −.25, p > .115).
3.3.6 ǀ. Anxiety symptoms
The full sample and the subsample that successfully completed net application did not display a significant relationship between anxiety symptoms and the number of valid trials (rs < −.12, p > .335). The subsample with ID demonstrated a significant relationship between anxiety symptoms and the number of valid trials (r = .33, p = .005). Within-group analyses demonstrated a significant relationship for the FXS group (r = .55, p = .006), but not for the ASD (r = .12, p = .524) or TD (r = −.25, p = .361) groups.
3.3.7 ǀ. Attention problems
The full sample and the subsample that successfully completed net application demonstrated a significant correlation between attention problems and the number of valid trials completed (rs = −.34, ps < .004). This relation was not significant for the sample with ID (r = −.06, p = .645). Within-group correlations for the TD (r = −.43, p = .093) group were marginally significant, but for the FXS (r = .01, p = .951) and ASD (r = −.24, p = .172) groups there was no relationship.
3.3.8 ǀ. The effect of controlling for sex in the FXS group
Additional partial correlations were completed without the additional control for sex to examine their effect within the FXS group partial correlations. The only observed difference between the two sets of partial correlations was that sensory hyperresponsivity went from marginally significant (r = .47, p = .057) to nonsignificant (r = .23, p = .277).
4 ǀ. DISCUSSION
We applied a single-session behavioral support protocol to the collection of ERP data in children with NDDs, specifically ASD and FXS, as well as TD children. Overall, our behavioral support protocol appears to have been highly effective for use with children with ASD and TD, as 73% of participants with ASD and 100% of TD participants successfully completed the study. It is less clear whether this protocol was effective for use with children with FXS, as only 33% successfully completed the study. Attrition rates vary greatly across ERP studies examining children with ASD; however, the vast majority of this research has been conducted with samples that exclude children with intellectual disability (i.e., IQ < 70). Approximately half of our participants with ASD also had intellectual disability, and we still successfully collected adequate data from 73% of the sample. This is considerably higher than other studies that have included children with ASD and intellectual disability. For example, Dawson and colleagues (2002, 2004, 2012) and Webb and colleagues (2006, 2011) have reported success rates of 46%–60% and 26%–43%, respectively, in studies of young children with ASD and high rates of intellectual disability. Dawson et al. (2002, 2004) and Webb et al. (2006) utilized data recruited from the same sample of sixty-three 30- to 60-month-old children with ASD. Participants in this sample had a mean mental age of approximately 28 months (specific data vary slightly by study based on inclusion criteria). Dawson and colleagues (2012) recruited children 49–77 months of age with mean IQs in the 45- to 48-point range. Finally, Webb and colleagues (2011) recruited children between 32 and 47 months of age with an average mental age of 28 months. In the development of our behavioral support protocol, we benefitted greatly from guidelines informed by these early studies (e.g., Webb et al., 2015). The results of the current study indicate that visual ERP studies with young children with ASD and comorbid intellectual disability are feasible. However, it is important to recognize that although our participants’ age range overlapped with ages studied by Dawson and colleagues (2002, 2004, 2012) and Webb and colleagues (2006, 2011), they were older on average, with mean ages of approximately 61–65 months across participant groups. Relatedly, the mental ages of our participants were higher than those from studies reviewed above. Still, 50%–70% of participants with NDDs in the current study were also impacted by intellectual impairment.
Although research has been published with infants, adolescents, and adults with FXS, this is the first study to our knowledge to examine ERP responses to visual stimuli in young children with FXS. Thus, it is difficult to assess the effectiveness of our protocol or evaluate our rate of attrition in young children with FXS, which likely represents one of the most challenging age ranges given the low communication and behavioral regulation skills at this age. In development of our behavioral protocol, we considered guidelines for ERP research with children with ASD and TD (e.g., Brooker et al., 2020; Kylliäinen et al., 2014; Webb et al., 2015) and worked with experts in both ASD and FXS to tailor our behavioral support protocol to a variety of NDDs. However, it is possible that despite these efforts, additional accommodations and supports may be needed in order to fully support children with FXS in ERP data collection. It is also possible that the demands of our ERP experiment were not ideal for the samples studied. For example, it is recommended that researchers use the minimum number of conditions necessary in studies of children with ASD (Kylliäinen et al., 2014; Webb et al., 2015), but our study required four stimulus conditions to answer our research questions. To promote future success in collection of valid data from children with FXS, in particular, we may limit our experiments to two stimulus conditions. For example, we may have reduced attrition rates in our current investigation by examining responses to only upright faces and upright houses or only upright and inverted faces. Indeed, three participants with FXS completed 10 or more valid trials in two or three conditions and may have been included in a final dataset if there were fewer stimulus conditions. Therefore, we could have potentially increased our success rate to 50% in the FXS group by decreasing the number of stimulus conditions included in the study. Alternatively, if a study paradigm requires a very large number of trials, modifications to the current protocol may be necessary.
Another recommendation calls for the elimination of desensitization procedures altogether, as some researchers have found that desensitization procedures are counterproductive to successful data collection in children with ASD (see Webb et al., 2015). For example, repeated exposure to the net (or swim cap, in our study) may serve to only heighten a child’s sensory aversion or anxiety, evoking a more intense negative reaction to the net at the time of data collection. If our desensitization was not effective for some of our participants, it may indicate that individuals’ sensitivities and level of comfort with desensitization materials should be considered in their implementation. If a child dislikes baths and swimming, a swim cap may increase their discomfort with the EEG procedure and another form of cap may be more effective. We may also consider shifting desensitization strategies to focus on the general experience instead, for example, using only the video, social story, and an introduction to the reinforcement schedule. It is suggested that these alternative desensitization strategies reduce participant fear and increase participant incentive, while avoiding sensory sensitivities (Webb et al., 2015) and anticipatory anxiety. It is important to acknowledge that desensitization compliance was not assessed in the current study, and it must be empirically considered before conclusions about its effectiveness can be reached.
In our examination of clinical–behavioral characteristics associated with successful data collection, correlations were significant for all characteristics. Children who were older and/or had greater nonverbal cognitive abilities successfully completed more trials among the full sample, participants who successfully completed net application, and participants with ASD and TD. Despite the ASD and FXS groups being similar in chronological age and NVMA, measures of chronological age and NVMA were not significantly correlated with trials completed in children with FXS. Intellectual disability is very common in FXS, affecting over 70% of our participants, and one possibility is that decreased variability in NVMA did not capture the variability in performance in this group. Indeed, chronological age was not related to the number of trials successfully completed for participants with ID. This may also explain why ASD severity was associated with the number of trials successfully completed in the full sample, the sample of participants who successfully completed net application, and children with FXS, but not ASD or TD. As shown in Figure 3, children with ASD were more likely to demonstrate high symptom severity, potentially decreasing variability in this measure for this group.
Results for the subsample of participants who successfully completed net application matched those of the full sample in the examinations of relations between clinical–behavioral characteristics and the number of trials successfully completed. Among these participants, higher numbers of trials successfully completed were associated with lower ASD symptom severity scores, fewer attention problems, and higher language ability. For the ID subsample, greater sensory responsivity, anxiety, and language ability were associated with higher numbers of successfully completed trials. Results were similar for participants with FXS, for whom higher anxiety scores, higher language ability, and lower ASD symptom severity scores were associated with greater numbers of successful trials. Correlations between clinical–behavioral measures and trials completed were not significant for the ASD and TD groups, except that greater language expression skill was associated with higher numbers of successful trials for those with ASD.
Language ability was associated with the number of trials successfully completed across more groups than any other clinical–behavioral characteristic examined. Greater RL and EL abilities were associated with higher numbers of successfully completed trials across the full sample, participants who successfully completed net application, participants with ID, and participants with FXS. For participants with ASD, greater EL scores were associated greater trials completed. Language ability was not significantly correlated with trial numbers for participants with TD, which may reflect their low variability in scores in this area. Participants with TD tended to score higher with lower standard deviations on the language ability subscales relative to the participants with NDDs.
Results of the clinical–behavioral characteristic comparisons were similar for participants with FXS and participants with ID. Surprisingly, greater anxiety symptoms and sensory sensitivity were associated with greater success in ERP data collection. Anxiety was positively correlated with trials completed for the group of participants with FXS and for participants with ID. Sensory hyperresponsivity was positively correlated with trials completed for participants with ID; this relation was marginally significant for participants with FXS. However, all participants with FXS or ID had scores in the normal or subclinical range (<60) on the Preschool Anxiety Scale, so this correlation represents variation in anxiety symptoms that is not clinically significant or impairing. It is possible that within the normal range of anxiety symptoms present in our sample of children with FXS and ID, having slightly higher anxiety symptoms contributed to better performance on the ERP task. Perhaps these children were more aware and sensitive to their surroundings in an adaptive manner. However, this positive correlation should be interpreted with caution.
We had expected that sensory hyperresponsivity would be associated with trials completed, as sensory sensitivities have been associated with participant performance in 18- to 30-month-old young children with ASD (Webb et al., 2011). Specifically, Webb and colleagues (2011) demonstrated that increased tactile sensitivity was associated with a decreased likelihood of successfully capping participants. In the current study, there was only a marginally significant correlation between sensory hyperresponsivity and number of valid trials completed for the FXS group. The pattern of results observed in the current study differed from the findings of Webb and colleagues (2011), in that greater hyperresponsivity was associated with an increased number of valid trials. One possible explanation for this discrepancy is that sensory sensitivity was measured using different assessment across these studies. Webb and colleagues utilized the Short Sensory Profile (SSP), whereas our study included the SEQ. Both measures rely on parent report, but the SSP allowed for specific examination of tactile sensitivity, whereas the SEQ is a general measure that assesses hyper and hyporesponsivity across multiple sensory systems. It is also possible that sensory hyperresponsivity affects children with FXS differently than those with ASD on ERP tasks, as we did not find that sensory hyperresponsivity was correlated with task performance in participants with ASD. Mean sensory hyperresponsivity scores were not at clinical levels for the participants with FXS in the current study, which may account for why sensory hyperresponsivity was not inhibiting to task performance.
Overall, our behavioral support protocol was less effective in facilitating successful data collection for participants with FXS than those with ASD, but the current study provides insight into why this discrepancy was observed, which is an important contribution. Across participants with ASD and FXS, different clinical behavioral characteristics were associated with successful data collection. Specifically, for participants with ASD, higher chronological age, NVMA, and RL skills were associated with increased successful trial completion, whereas greater language ability, decreased ASD symptoms severity scores, and increased anxiety were associated with greater numbers of trials successfully completed for participants with FXS. Although participants with FXS were very similar to participants with ASD in chronological age, NVMA, language ability, and clinical–behavioral characteristics, different factors were associated with study success for each group. Additional supports should be considered for future enhancements to the protocol, which may address factors associated with successful data collection in participants with FXS more directly. For example, parent modeling of the behavioral protocol may increase understanding and contribute to successful data collection despite decreased language ability among some participants with FXS. Currently, we request that participants’ parents touch the net prior to application, if the participant declines, but this type of behavior could be carried out prior to or simultaneously with requests for the participant and applied to extended range of steps in the protocol. It may also be more effective to provide participants with other forms of reinforcement during breaks, such as a clip of a video that is enjoyed by the participant. This may promote continued attention to the monitor, instead of reinforcement that is external to the experiment. Although our success rate was lower for children with FXS than ASD, this study represents an important starting point for visual ERP research with young children with FXS, and additional research in this area will enable us to better understand how best to support children with FXS in ERP study participation. Even with refinement of our behavioral protocol, it is unlikely that we will be able to fully eliminate attrition in these participant groups. Noreika and colleagues (2020) note that the development of new technology that is less sensitive to sources of noise, such as motion artifact, could really help to address some of the current limitations in the field.
There are additional limitations to the interpretation of results from the current study that could be addressed in future research. One limitation is the lack of a control group. The behavioral protocol was developed in an effort to promote successful data collection, and we did not attempt to collect additional data using a different protocol or without behavioral supports. Without these data, it is difficult to assess our success in collecting data from the children with FXS, in particular. We also did not correct for all comparisons as we believed that aspects of this study are exploratory and that the results can be useful for future studies. Another limitation is seen in the sample size. Greater confidence in the correlational results could be gained through replication with a larger sample, especially for the group of participants with FXS. Relatedly, low numbers of female participants were recruited across all participant groups, limiting our ability to understand whether the protocol was equally effective for male and female participants. Males are often more significantly affected by FXS and ASD than females (Bartholomay et al., 2019; Hagerman et al., 2017), so it is unclear whether we would also observe this pattern of results in our research. This should be examined in future research with greater representation of female participants. Additionally, participants in the current experiment were primarily recruited from a larger, longitudinal study and did not represent a high level of ethnic and racial diversity. We aspire to increasingly engage underrepresented families in our research and believe that an important step is to include greater diversity in our support documents (e.g., the social story and desensitization videos). These efforts are currently underway and are recommended for other researchers as well.
The results of the current study indicate that a “one-size-fits-all” approach cannot be taken to ERP research on children with NDDs, but that a single-session paradigm is feasible. Participants with ASD and ID showed a high level of success, even without in-person desensitization sessions. Our protocol was not equally successful across participants with ASD and FXS, although their clinical–behavioral profiles were strikingly similar. This work has important implications, as research on children with NDDs is valuable to promoting increased understanding of affected neural functions and informing effective treatments and interventions. It is our hope that this work will contribute to increased representation of all children with NDDs in neuroscience research, regardless of cognitive ability or challenging behaviors. We also believe that this work emphasizes the need for continued development of ERP methods and procedures that support inclusion of diverse and representative samples. To continue to promote greater understanding of factors associated with successful and failed data collection in children with NDDs, it is important for researchers to report detailed information about methods for data collection and attrition in their publications.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants R01HD018942 to J. E. Richards and NIMH-R01MH090194 and R01MH107573 to J. E. Roberts. The authors would like to express gratitude to James McPartland, Michael Murias, and Sara Jane Webb, who each provided consultation at the onset of this study in the development of our behavioral support protocol.
Funding information
National Institute of Mental Health, Grant/Award Numbers: R01MH090194, R01MH107573; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R01 HD018942
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
DATA AVAILABILITY STATEMENT
The data from this study will be available at the National Database for Autism Research following their publication.
REFERENCES
- Achenbach TM, & Rescorla LA (2001). Manual for the ASEBA school-age forms and profiles. University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Apicella F, Sicca F, Federico RR, Campatelli G, & Muratori F.(2013). Fusiform gyrus responses to neutral and emotional faces in children with autism spectrum disorders: A high density ERP study. Behavioural Brain Research, 251, 155–162. 10.1016/j.bbr.2012.10.040 [DOI] [PubMed] [Google Scholar]
- Bailey DBJ, Raspa M, Olmsted M, & Holiday DB (2008). Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American Journal of Medical Genetics Part A, 146A(16), 2060–2069. 10.1002/ajmg.a.32439 [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, 47(6), 591–601. 10.1111/j.1469-7610.2005.01546.x [DOI] [PubMed] [Google Scholar]
- Baranek GT, Roberts JE, David FJ, Sideris J, Mirrett PL, Hatton DD, & Bailey DB (2008). Developmental trajectories and correlates of sensory processing in young boys with fragile X syndrome. Physical and Occupational Therapy in Pediatrics, 28(1), 79–98. 10.1300/J006v28n01_06 [DOI] [PubMed] [Google Scholar]
- Bartholomay KL, Lee CH, Bruno JL, Lightbody AA, & Reiss AL (2019). Closing the gender gap in fragile X syndrome: Review on females with FXS and preliminary research findings. Brain Sciences, 9(1), 11. 10.3390/brainsci9010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty M, Meaux E, Wittemeyer K, Rogé B, & Taylor MJ (2011). Early processing of emotional faces in children with autism: An event-related potential study. Journal of Experimental Child Psychology, 109(4), 430–444. 10.1016/j.jecp.2011.02.001 [DOI] [PubMed] [Google Scholar]
- Bell MA, & Cuevas K.(2012). Using EEG to study cognitive development: Issues and practices. Journal of Cognition and Development, 13(3), 281–294. 10.1080/15248372.2012.691143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry-Kravis EM, Lindemann L, Jønch AE, Apostol G, Bear MF, Carpenter RL, Crawley JN, Curie A, Des Portes V, Hossain F, Gasparini F, Gomez-Mancilla B, Hessl D, Loth E, Scharf SH, Wang PP, Von Raison F, Hagerman R, Spooren W, & Jacquemont S.(2018). Drug development for neurodevelopmental disorders: Lessons learned from fragile X syndrome. Nature Reviews Drug Discovery, 17(4), 280–299. 10.1038/nrd.2017.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Bates JE, Buss KA, Canen MJ, Dennis-Tiwary TA, Gatzke-Kopp LM, Hoyniak C, Klein DN, Kujawa A, Lahat A, Lamm C, Moser JS, Petersen IT, Tang A, Woltering S, & Schmidt LA (2020). Conducting event-related potential (ERP) research with young children: A review of components, special considerations, and recommendations for research on cognition and emotion. Journal of Psychophysiology, 34(3), 137–158. 10.1027/0269-8803/a000243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantiani C, Choudhury NA, Yu YH, Shafer VL, Schwartz RG, & Benasich AA (2016). From sensory perception to lexical-semantic processing: An ERP study in non-verbal children with autism. PLoS ONE, 11(8), e0161637. 10.1371/journal.pone.0161637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes P, Matson JL, Tureck K, & Adams HL (2013). The relationship of comorbid anxiety symptom severity and challenging behaviors in infants and toddlers with autism spectrum disorder. Research in Autism Spectrum Disorders, 7(12), 1528–1534. 10.1016/j.rasd.2013.09.005 [DOI] [Google Scholar]
- Datavyu. (2014). Datavyu: A video coding tool. Databrary Project. http://datavyu.org [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, & Webb SJ (2002). Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development, 73(3), 700–717. 10.1111/1467-8624.00433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Kamara D, Murias M, Greenson J, Winter J, Smith M, Rogers SJ, & Webb SJ (2012). Early behavioral intervention is associated with normalized brain activity in young children with autism. Journal of the American Academy of Child and Adolescent Psychiatry, 51(11), 1150–1159. 10.1016/j.jaac.2012.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Carver L, Panagiotides H, & McPartland J.(2004). Young children with autism show atypical brain responses to fearful versus neutral facial expressions of emotion. Developmental Science, 7(3), 340–359. [DOI] [PubMed] [Google Scholar]
- Debnath R, Buzzell GA, Morales S, Bowers ME, Leach SC, & Fox NA (2020). The Maryland analysis of developmental EEG (MADE) pipeline. Psychophysiology, 57, e13580. 10.1111/psyp.13580 [DOI] [PubMed] [Google Scholar]
- Delorme A, & Makeig S.(2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- DiStefano C, Dickinson A, Baker E, & Spurling Jeste S.(2019). EEG data collection in children with ASD: The role of state in data quality and spectral power. Research in Autism Spectrum Disorders, 57, 132–144. 10.1016/j.rasd.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano C, Senturk D, & Jeste SS (2019). ERP evidence of semantic processing in children with ASD. Developmental Cognitive Neuroscience, 36, 100640. 10.1016/j.dcn.2019.100640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD (2006). Differential Ability Scales-II. Pearson Assessments. [Google Scholar]
- Ezell J, Hogan A, Fairchild A, Hills K, Klusek J, Abbeduto L, & Roberts JE (2019). Prevalence and predictors of anxiety disorders in adolescent and adult males with autism spectrum disorder and fragile X syndrome. Journal of Autism and Developmental Disorders, 49(3), 1131–1141. 10.1007/s10803-018-3804-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabio RA, Capri T, Buzzai C, Pittala V, & Gangemi A.(2019). Auditory and visual oddball paradigm evaluated through P300 in five girls with Rett syndrome. NeuroQuantology, 17(7), 40–49. 10.14704/nq.2019.17.7.2591 [DOI] [Google Scholar]
- Groen Y, Wijers AA, Mulder LJM, Waggeveld B, Minderaa RB, & Althaus M.(2008). Error and feedback processing in children with ADHD and children with Autistic Spectrum Disorder: An EEG event-related potential study. Clinical Neurophysiology, 119(11), 2476–2493. 10.1016/j.clinph.2008.08.004 [DOI] [PubMed] [Google Scholar]
- Guy MW, Richards JE, Tonnsen BL, & Roberts JE (2018). Neural correlates of face processing in etiologically-distinct 12-month-old infants at high-risk of autism spectrum disorder. Developmental Cognitive Neuroscience, 29, 61–71. 10.1016/j.dcn.2017.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Berry-Kravis E, Hazlett HC, Bailey DB, Moine H, Kooy RF, Tassone F, Gantois I, Sonenberg N, Mandel JL, & Hagerman PJ (2017). Fragile X syndrome. Nature Reviews Disease Primers, 3, 17065. 10.1038/nrdp.2017.65 [DOI] [PubMed] [Google Scholar]
- Hoehl S, & Wahl S.(2012). Recording infant ERP data for cognitive research. Developmental Neuropsychology, 37(3), 187–209. 10.1080/87565641 [DOI] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, & Leal J.(2014). Epidemiology of fragile X syndrome: A systematic review and meta-analysis. American Journal of Medical Genetics Part A, 164(7), 1648–1658. 10.1002/ajmg.a.36511 [DOI] [PubMed] [Google Scholar]
- Jeste SS, & Nelson CA (2009). Event related potentials in the understanding of autism spectrum disorders: An analytical review. Journal of Autism and Developmental Disorders, 39, 495–510. 10.1007/s10803-008-0652-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatiala J, Yrttiaho S, Forssman L, Perdue K, & Leppanen J.(2014). A graphical user interface for infant ERP analysis. Behavioral Research, 46, 745–757. 10.3758/s13428-013-0404-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth IS, Vannasing P, Major P, Michaud JL, & Lippé S.(2014). Alterations of visual and auditory evoked potentials in fragile X syndrome. International Journal of Developmental Neuroscience, 36(1), 90–97. 10.1016/j.ijdevneu.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Kóbor A, Takács Á, Bryce D, Szucs D, Honbolygó F, Nagy P, & Csépe V.(2015). Children with ADHD show impairments in multiple stages of information processing in a Stroop task: An ERP study. Developmental Neuropsychology, 40(6), 329–347. 10.1080/87565641.2015.1086770 [DOI] [PubMed] [Google Scholar]
- Kylliäinen A, Jones EJH, Gomot M, Warreyn P, & Falck-Ytter T.(2014). Practical guidelines for studying young children with autism spectrum disorder in psychophysiological experiments. Review Journal of Autism and Developmental Disorders, 1(4), 373–386. 10.1007/s40489-014-0034-5 [DOI] [Google Scholar]
- Leader G, Flynn C, O’Rourke N, Coyne R, Caher A, & Mannion A.(2021). Comorbid psychopathology, challenging behavior, sensory issues, adaptive behavior and quality of life in children and adolescents with autism spectrum disorder. Developmental Neurorehabilitation, 24, 397–407. 10.1080/17518423.2021.1898058 [DOI] [PubMed] [Google Scholar]
- Liotti M, Pliszka SR, Higgins K, Perez R, & Semrud-Clikeman M.(2010). Evidence for specificity of ERP abnormalities during response inhibition in ADHD children: A comparison with reading disorder children without ADHD. Brain and Cognition, 72(2), 228–237. 10.1016/j.bandc.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, & Luck SJ (2014). ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience, 8, 213. 10.3389/fnhum.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S, Gotham K, & Bishop S.(2012). Autism diagnostic observation schedule, second edition: ADOS-2. Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A.(1994). Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. [DOI] [PubMed] [Google Scholar]
- Maurer U, Schulz E, Brem S, van der Mark S, Bucher K, Martin E, & Brandeis D.(2011). The development of print tuning in children with dyslexia: Evidence from longitudinal ERP data supported by fMRI. NeuroImage, 57(3), 714–722. 10.1016/j.neuroimage.2010.10.055 [DOI] [PubMed] [Google Scholar]
- McDevitt N, Gallagher L, & Reilly R.(2015). Autism spectrum disorder (ASD) and fragile X syndrome (FXS): Two overlapping disorders reviewed through electroencephalography—What can be interpreted from the available information? Brain Sciences, 5(2), 92–117. 10.3390/brainsci5020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mento G, Scerif G, Granziol U, Franzoi M, & Lanfranchi S.(2019). Dissociating top-down and bottom-up temporal attention in Down syndrome: A neurocostructive perspective. Cognitive Development, 49, 81–93. 10.1016/j.cogdev.2018.12.004 [DOI] [Google Scholar]
- Mills DL, Dai L, Fishman I, Yam A, Appelbaum LG, St George M, Galaburda A, Bellugi U, & Korenberg JR (2013). Genetic mapping of brain plasticity across development in Williams syndrome: ERP markers of face and language processing. Developmental Neuropsychology, 38(8), 613–642. 10.1080/87565641.2013.825617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen E.(1995). Mullen scales of early learning. American Guidance Service. [Google Scholar]
- Newman I, Leader G, Chen JL, & Mannion A.(2015). An analysis of challenging behavior, comorbid psychopathology, and Attention-Deficit/Hyperactivity Disorder in Fragile X Syndrome. Research in Developmental Disabilities, 38, 7–17. 10.1016/j.ridd.2014.11.003 [DOI] [PubMed] [Google Scholar]
- Noreika V, Georgieva S, Wass S, & Leong V.(2020). 14 challenges and their solutions for conducting social neuroscience and longitudinal EEG research with infants. Infant Behavior and Development, 58, 101393. 10.1016/j.infbeh.2019.101393 [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Deitz J, Kartin D, Nalty T, & Dawson G.(2012). Sensory processing, problem behavior, adaptive behavior, and cognition in preschool children with autism spectrum disorders. American Journal of Occupational Therapy, 66(5), 586–594. 10.5014/ajot.2012.004168 [DOI] [PubMed] [Google Scholar]
- Pavlova MA, Galli J, Pagani F, Micheletti S, Guerreschi M, Sokolov AN, Fallgatter AJ, & Fazzi EM (2018). Social cognition in down syndrome: Face tuning in face-like non-face images. Frontiers in Psychology, 9, 2583. 10.3389/fpsyg.2018.02583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razak KA, Dominick KC, & Erickson CA (2020). Developmental studies in fragile X syndrome. Journal of Neurodevelopmental Disorders, 12(1), 1–15. 10.1186/s11689-020-09310-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Bradshaw J, Will E, Hogan AL, McQuillin S, & Hills K.(2020). Emergence and rate of autism in fragile X syndrome across the first years of life. Development and Psychopathology, 32(4), 1335–1352. 10.1017/S0954579420000942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Cicchetti DV, & Balla DA (2005). Vineland adaptive behavior scales (Vineland-II) (2nd ed.), San Antonio, TX: Pearson. [Google Scholar]
- Spence SH, Rapee R, McDonald C, & Ingram M.(2001). The structure of anxiety symptoms among preschoolers. Behaviour Research and Therapy, 39(11), 1293–1316. 10.1016/S0005-7967(00)00098-X [DOI] [PubMed] [Google Scholar]
- Stahl D, Pickles A, Elsabbagh M, Johnson MH, & the BASIS Team. (2012). Novel machine learning methods for ERP analysis: A validation from research on infants at risk for autism. Developmental Neuropsychology, 37(3), 274–298. 10.1080/87565641.2011.650808 [DOI] [PubMed] [Google Scholar]
- Stets M, & Reid VM (2011). Infant ERP amplitudes change over the course of an experimental session: Implications for cognitive processes and methodology. Brain & Development, 33, 558–568. 10.1016/j.braindev.2010.10.008 [DOI] [PubMed] [Google Scholar]
- Stets M, Stahl D, & Reid VM (2012). A meta-analysis investigating factors underlying attrition rates in infant ERP studies. Developmental Neuropsychology, 37(3), 226–252. 10.1080/87565641.2012.654867 [DOI] [PubMed] [Google Scholar]
- Sullivan K, Hatton D, Hammer J, Sideris J, Hooper S, Ornstein P, & Bailey D.(2006). ADHD symptoms in children with FXS. American Journal of Medical Genetics Part A, 140A(21), 2275–2288. 10.1002/ajmg.a.31388 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, & Baldeweg T.(2002). Application of EEG, ERP, and intracranial recordings to the investigation of cognitive functions in children. Developmental Science, 5(3), 318–334. [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, & Nelson C.(2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. 10.1016/j.psychres.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM (1993). Spatial sampling of head electrical fields - The Geodesic Sensor Net. Electroencephalography and Clinical Neurophysiology, 87, 154–163. 10.1016/0013-4694(93)90121-B [DOI] [PubMed] [Google Scholar]
- Van der Molen MJW, Van der Molen MW, Ridderinkhof KR, Hamel BCJ, Curfs LMG, & Ramakers GJA (2012). Auditory and visual cortical activity during selective attention in fragile X syndrome: A cascade of processing deficiencies. Clinical Neurophysiology, 123(4), 720–729. 10.1016/j.clinph.2011.08.023 [DOI] [PubMed] [Google Scholar]
- van der Velde B, & Junge C.(2020). Limiting data loss in infant EEG: Putting hunches to the test. Developmental Cognitive Neuroscience, 45, 100809. 10.1016/j.dcn.2020.100809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Bernier R, Henderson HA, Johnson MH, Jones EJH, Lerner MD, McPartland JC, Nelson CA, Rojas DC, Townsend J, & Westerfield M.(2015). Guidelines and best practices for electrophysiological data collection, analysis and reporting in autism. Journal of Autism and Developmental Disorders, 45(2), 425–443. 10.1007/s10803-013-1916-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Dawson G, Bernier R, & Panagiotides H.(2006). ERP evidence of atypical face processing in young children with autism. Journal of Autism and Developmental Disorders, 36(7), 881–890. 10.1007/s10803-006-0126-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Venema K, Greenson J, Murias M, & Dawson G.(2011). Developmental change in the ERP responses to familiar faces in toddlers with autism spectrum disorders versus typical development. Child Development, 82(6), 1868–1886. 10.1111/j.1467-8624.2011.01656.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart FS, Vissers CTWM, Kessels RPC, & Maes JHR (2018). Implicit learning seems to come naturally for children with autism, but not for children with specific language impairment: Evidence from behavioral and ERP data. Autism Research, 11(7), 1050–1061. 10.1002/aur.1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data from this study will be available at the National Database for Autism Research following their publication.