Abstract
Background
A biennial or perennial plant of the Apiaceae family, Eryngium caeruleum M. Bieb. is traditionally used in medicine as an antitoxic, diuretic, digestive, anti-inflammatory and analgesic drug. This plant is widely distributed in temperate regions around the world. Young leaves of the plant are used in cooking as aromatic cooked vegetables in various local products in Iran.
Purpose
The current review aimed to highlight complete and updated information about the Eryngium caeruleum species, regarding botanical, ethnopharmacological, phytochemical data, pharmacological mechanisms as well as some nutritional properties. All this scientific evidence supports the use of this species in complementary medicine, thus opening new therapeutic perspectives for the treatment of some diseases.
Methods
The information provided in this updated review is collected from several scientific databases such as PubMed/Medline, ScienceDirect, Mendeley, Scopus, Web of Science and Google Scholar. Ethnopharmacology books and various professional websites were also researched.
Results
The phytochemical composition of the aerial parts and roots of E. caeruleum is represented by the components of essential oil (EO), phenolic compounds, saponins, protein, amino acids, fiber, carbohydrates, and mineral elements. The antioxidant, antimicrobial, antidiabetic, antihypoxic, and anti-inflammatory properties of E. caeruleum have been confirmed by pharmacological experiments with extracts using in vitro and in vivo methods. The syrup E. caeruleum relieved dysmenorrhea as effectively as Ibuprofen in the blinded, randomized, placebo-controlled clinical study.
Conclusion
Current evidence from experimental pharmacological studies has shown that the different bioactive compounds present in the species E. caeruleum have multiple beneficial effects on human health, being potentially active in the treatment of many diseases. Thus, the traditional uses of this species are supported based on evidence. In future, translational and human clinical studies are necessary to establish effective therapeutic doses in humans.
Keywords: Eryngium caeruleum, Ethnobotany, Bioactive molecules, Pharmacological activities
Introduction
The genus Eryngium L. (Apiaceae) has approximately 250 species, being the most species-rich genus of the Apiaceae, and is distributed worldwide in temperate regions. The Eryngium spp. grow in North Africa, Australia, North and South America, and Eurasia [55]. Two centres of diversity are recognized for the genus. The first one is central-east South America and central-west Mexico whereas the second is the western Mediterranean and south-west Asia [113]. Approximately two-thirds of Eryngium spp. are located in Central, South and North America [13].
The species were grouped by Wolff [117] into 34 sections and numerous subsections. Eryngium caeruleum M.Bieb. (syn. Eryngium caucasicum Trautv. and Eryngium pskemense Pavlov) belongs to Plana section [117]. Subgeneric classification based on the morphology of the genus was introduced by Wörz [119], which recognizes five subgenera. The study of Calviño et al. [12, 13], based on phylogenetic analyses of DNA sequences corroborated some of the hypotheses of relationships and biogeography previously formulated [12, 13]. E. caeruleum is very close to the Western Mediterranean Eryngium dichotomum Desf. from South Italy, Southern Spain, and Northwestern Africa [15, 53, 54].
Eryngium caeruleum is an Oriental-Caucasian-Turcestanian floral component [58] that is widespread from North-Eastern Anatolia, Caucasia and Southern Russia across Central, Northern, Eastern Iran and Middle Asia (Southern Turkmenistan, Tyan Shan, Pamir-Alaj) to Afghanistan, Pakistan, and Western Himalaya [15, 54, 70, 71, 118]. The plant is indigenous to Kazakhstan, Afghanistan, Iran, North Caucasus, Kirgizstan, Tadzhikistan, Pakistan, Transcaucasus, Turkey, Uzbekistan, Turkmenistan, West Himalaya (Fig. 1) [74].
Fig. 1.
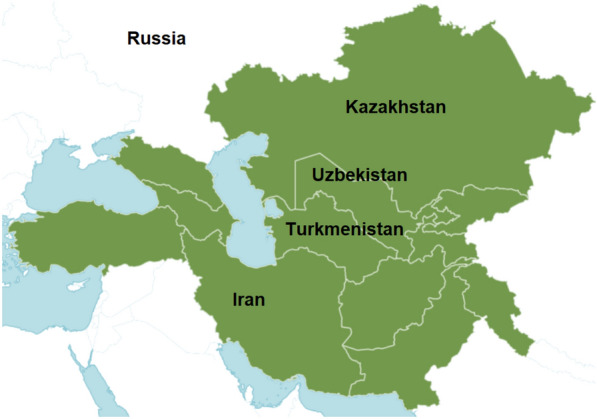
Distribution area of Eryngium caeruleum M. Bieb [74]
Several bioactive molecules, principally essential oils, terpenoids and phenolics, have been identified and purified from different parts of E. caeruleum, such as flavonoids and saponins [21, 41, 62]. Antioxidant, antidiabetic, antihypoxic, anticonvulsant, free radical scavenging, hepato-renoprotective, reproductive protection, and anti-inflammatory effects of E. caeruleum were reported [18, 23, 45, 46, 52, 59, 66, 66, 80]. For these reasons, it is important to note that understanding the bioactive constituents of plants that play an essential role in human healthcare, as well as scientific proof of their traditional use, should be made available to support traditional medicine and herbal treatments. The geographical pattern and botanical description of E. caeruleum, are discussed, as well as phytochemicals constituents, ethnopharmacology, biological activities and clinical studies are also more detailed.
In summary, this review summarizes the findings of previous E. caeruleum research and identifies information gaps that must be addressed to fully comprehend the mode of action of extracts used in traditional medical practice. It also suggests that a thorough investigation of the relationship between the pharmacological impact and the existence of the bioactive chemicals responsible for the action is required. The review synthesizes phytochemical analyses and biological studies that may be useful for researchers to conduct additional studies to supplement existing information and promote the discovery of new uses for these fascinating species.
Review methodology
Scientific databases and repositories, such as PubMed/Medline, ScienceDirect, Mendeley, Scopus, Web of Science and Google Scholar were used to search for information. The research was carried out using the next MeSH terms: Eryngium/classification, Eryngium /chemistry, Eryngium/Flavonoids, Plant Extracts/pharmacology, Plants, Medicinal, Anti-Infective Agents/pharmacology, Plant Oils/pharmacology. We obtained about 993 references according to these terms. References relating to E. caeruleum consist of 28 English and 15 Russian sources.
Inclusion criteria: publications which have focused on botanical description, geographical repartition, traditional uses, chemistry and pharmacological activities of E. caeruleum. These data have been arranged in tables and presented in this study according to each field.
Exclusion criteria: articles written in other languages than English, papers without pharmacological mechanisms included, meta-analyses, case studies or works that included pharmacological experiments associated with homoeopath products.
The most important data were summarized in tables and figures. The taxonomy of plants has been validated using the PlantList and chemical formulas according to PubChem [38, 72].
Botany
Eryngium caeruleum is a biennial or plurennial hapaxanthic plant turning glaucous-amethyst into a flower. Synflorescence (40–80 cm) has a dichotomous branch structure with spreading horizontal branches. The plant has basal leaves herbaceous, and long petiolate; the outer leaves decay early. The median cauline leaves are stalkless and profoundly palmate in spiny pinnatifid lobes [54]. In many tropical areas of the world, this genus has been extensively employed as an eatable plant and grown as an economic crop [27, 66, 69]. Cultivation was begun in some domestic gardens in Shirkala (Iran) and the undivided outer basal leaves are collected continuously in spring and summer to prevent flowering [54]. In the northern area of Iran, E. caeruleum is among the most prominent garden vegetables [27, 66] because it is easily grown in sandy, well-drained, dry soils, and under the full sun [25, 54, 64, 69]. Eliseeva et al. [24] studied the possibility of cultivating the E. caeruleum on the territory of the Caucasian Mineral Waters (North Caucasus, Russia). Seeds were sown at different times (in March and November), and the distance between the rows is 40–60 cm. Seeds germinated without a dormant period. Shoots appeared after 2–4 weeks, depending on climatic conditions. Seed germination ranged from 39 to 55% [24]. In the life cycle of E. caeruleum, the investigating authors identified several stages of development: germinant, juvenile, immature, adult vegetative and generative plants. The germinant had a height of up to 1 cm. The cotyledonous leaves are oval, up to 1.3 cm long, and up to 0.7 cm wide. The root has a length of up to 2 cm. At this stage, plants are 7–10 days. Juvenile plants have a height of 1.3–1.5 cm; the number of leaves is 2–3. Immature plants are characterized by the appearance of real leaves in the amount of 12–45. Leaves are parallel to the surface of the soil. Adult vegetative plants are fully formed on the 50–57 day of vegetation. They have a rosette of leaves with a diameter of 50–60 cm. The main root is 20–25 cm long, and 1.5–1.8 cm thick. The generative period of development is observed in the second year of vegetation. One to five peduncles are formed [24, 103]. The flowering period of plants was observed from May to July (40–50 days), plants reach up to 100–120 cm in height, and stems give an increase of 15–20 cm per week. E. caeruleum forms fruits in July–September. Fruits and seeds usually ripen in late August-early September [104]. Crop capacity (yield of raw materials from 1 m2) is on average: 0.72 kg for roots, overhead mass—1.2 kg, seeds—about 40 g [102]. The weight of the fresh aerial mass of one plant is on average 326.5 g. The roots for the second year are 38–45 cm long, 2.1–4.5 cm thick, an average weight of 117.6 g, seeds from one plant can produce up to 24.8 g [103].
Traditional and ethnomedicinal importance
The fresh leaves of E. caeruleum are used to flavor cooked vegetables in various local products of Iran and are also often used to flavor chicken and fish. Also, cooked leaves are mixed with yoghurt or used in soups [7, 54]. In Persian medicine, various therapeutic effects, and pharmacological actions of Eryngium had mentioned, including antitoxic, diuretic, emmenagogue, aphrodisiac, galactagogue, digestive, anti-flatulent, anti-inflammatory and analgesic properties. This plant was used for pulmonary diseases such as asthma and bronchitis, halitosis, snakebite and insect bites, cramps and influenza, early stages of lymphatic filariasis, hypochondriac, and epigastric pain [88]. The roots of the plant have a diuretic effect, a beneficial effect on vesical and kidney stones, as well as on peripheral oedemas. In traditional Iranian medicine, E. caeruleum increases lactation and sweating, as carminative, an aromatic agent and a fragrant. It decreases oedema and inflammation, promotes digestion, and removes phlegm from the digestive system [7, 8, 29]. The plant is currently of great scientific interest due to its historic use. E. caeruleum is also used to cure a variety of diseases in Islamic medicine; the roots, in particular, are used to treat inflammations, edema, urinary infections, and sinusitis [55]. In China, this plant is also used to treat cough, malaria, and animal poisoning [3]. The roots are used as an analgesic for rheumatoid arthritis patients [33].
Phytochemistry
Essential oils
Essential oils are concentrated hydrophobic liquids containing volatile chemical compounds from plants [93, 97, 99, 101]. Studies by various authors have established that the content of essential oils (Eos) in the aerial part of E. caeruleum is from 0.053 to 1.1% v/dry weight and increases during the growth and development of plants, reaching maximum values in the flowering phase. In the roots—up to 1.1 v/dry weight. The composition of EOs of dried samples of the aerial parts and roots of E. caeruleum growing in Iran according to Mohamadipour et al. [61] and Hamedi et al. [35] very close. As described, trans-pinocarvyl acetate and short-chain fatty acid esters are among the main components [35, 61]. It is noteworthy that the content of the polyacetylene compound Z-falcarinol in the composition of EOs derived from the roots and aerial parts of the plant is approximately the same (5.5% v/dry weight and 5.6% v/dry weight). Studies by Morteza-Semnani et al. [62], Assadian et al. [5] and Saeedi and Morteza-Semnani [81] found that limonene (52.1–60.5%), α-pinene (5.5–6.5%) and δ-2-carene (5.3–13.0%) accumulate as the main ingredients in the EO of the aerial parts of plants from Iran.
A study by Hashemabadi and Kaviani [36] showed that the composition of EOs from the leaves and stems of coastal and hill slope plants varies in different phases of their development. Unlike previous data, the main components of the EOs were sesquiterpenes (see Table 1). According to the authors, this study remarks that type and concentration of phytochemicals can be changed, based on the vegetation phase and location of E. caeruleum.
Table 1.
Essential oil analysis of Eryngium caeruleum M.Bieb
| Origin of materials (country, province, locality)/part used/phase of development | Method of extraction (extraction time)/yield (%) | Main components | References |
|---|---|---|---|
| Iran/aerial parts | Hydrodistillation | Limonene (52.1%), β-sesquiphellandrene (8.1%), α-pinene (5.5%) and δ-2-carene (5.3%) | [62] |
| Iran/aerial parts/flowering stage | Hydrodistillation (3 h)/0.3 air-dried weight | Limonene (60.5%), δ-3-carene (13.0%), α-pinene (5.6%) | [5] |
| Iran/dried aerial parts | Hydrodistillation (5 h)/0.65 w/w | Limonene (56.7%), β-sesquiphellandrene (8.9%), α-pinene (6.5%), δ-2-carene (5.9%) | [81] |
| Iran/dry leaves/vegetative phase | Hydrodistillation (4 h) | Coastal plants: 5-methyl-2-pyrimidone (61.69%), β-sesquiphellandrene (14.19%), 2,4-bis (1,1-dimethyl)-phenol (13.65%) | [36] |
| Iran/dry leaves/vegetative phase | Hill slope plants: 5-methyl-2-pyrimidone (41.74%), β-sesquiphellandrene (18.29%), β-bisabolene (12.30%) | ||
| Iran/dry stems/vegetative phase | Coastal plants: l-limonene (30.34%), β-sesquiphellandrene (28.53%), β-bisabolene (15.31%) | ||
| Iran/dry stems/vegetative phase | Hill slope plants: β-sesquiphellandrene (26.54%), 5-methyl-2-pyrimidone (23.89%), limonene (11.57%) | ||
| Iran/leaves/first vegetative stages | Hydrodistillation (4 h) | Coastal plants: β-sesquiphellandrene (44.21%), limonene (18.39%) and β-bisabolene (6.08%) | [36, 37] |
| Hill slope plants: 5-methyl-2-pyrimidone (53.83%), β-sesquiphellandrene (11.26%) and β-bisabolene (7.43%) | |||
| Iran/leaves/second vegetative phase | Coastal plants: β-sesquiphellandrene (27.32%), limonene (14.32%) and 5-methyl-2-pyrimidone (14.15%) | ||
| Hill slope plant: 4(5)-acetyl-1H-imidazole (50.14%), β-sesquiphellandrene (15.51%) and 4-(1,5-dimethylhex-4-enyl) cyclohex-2-enone (11.05%) | |||
| Iran/stems/generative phase | Coastal plants: hexadecahydrocyclobuta [1,2,3,4] dicyclooctene (45.46%), β-sesquiphellandrene (20.5%) and widdrene (19.06%) | ||
| Hill slope plant: piperiton (69.81%), 4-(1,5-dimethylhex-4-enyl) cyclohex-2-enone (18.38%) and β-sesquiphellandrene (4.54%) | |||
| Iran/dry leaves/pre-flowering stage | Hydrodistillation (3 h) | Cyclobuta, dicyclooctene, hexadecahydro (47.03%), n-hexadecanoic acid (11.16%), linoleic acid (5.41%), limonene (4.23%), cis-α-bisabolene (2.14%) | [18] |
| Iran/fresh flowers/early reproductive phase | Hydrodistillation/0.32 | Littoral location: allo-aromadendrene (66.3%), trans-calamenene (11%), dehydro abietal (6.7%), α-calacorene (6.1%) | [1] |
| Iran/fresh flowers/early reproductive phase | Hydrodistillation/0.38 | Unlittoral location: allo-aromadendrene (61.2%), trans-calamenene (13.4%), dehydro abietal (10.9%) | |
| Iran/fresh flowers/early reproductive phase | Hydro-steam distillation/0.176 | Littoral location: allo-aromadendrene (71.6%), trans-calamenene (12.8%), α-calacorene (4.5%) | |
| Iran/fresh flowers/early reproductive phase | Hydro-steam distillation/0.21 | Unlittoral location: allo-aromadendrene (69.1%), trans-calamenene (15.9%), dehydro abietal (4.5%) | |
| Iran/fresh flowers/early reproductive phase | Steam distillation/0.06 | Littoral location: allo-aromadendrene (48.7%), trans-calamenene (11.1%), α –eudesmol (4.4%), dehydro abietal (4.0%) | |
| Iran/fresh flowers/early reproductive phase | Steam distillation/0.09 | Unlittoral location: allo-aromadendrene (56.7%), trans-calamenene (18.2%), dehydro abietal (8.5%) | |
| Iran/fresh leaves/early reproductive phase | Hydrodistillation/0.13 | Littoral location: (E, E)-farnesol (24.3%), elemicin (12.2%), n-nonanyl acetate (9.1%), butyl acetate (6.4%), allo-aromadendrene (6.0%) | |
| Iran/fresh leaves/early reproductive phase | Hydrodistillation/0.19 | Unlittoral location: allo-aromadenderene (25.2%), α-calacorene (23.1%), (E,E)-farnesol (17.5%), dihydro tagetone (11.0%) | |
| Iran/dry leaves/early reproductive phase | Hydrodistillation/0.17 | Littoral location: alloaromadendrene (24%), dihydro tagetone (19.8%), (E,E)-farnesol (13.8%), elemicin (12.3%) | |
| Iran/dry leaves/early reproductive phase | Hydrodistillation/0.32 | Unlittoral location: allo-aromadenderene (33.2%), α-calacorene (14.4%), (E,E)-farnesol (16.7%), dihydro tagetone (8.3%) | |
| Iran/fresh leaves/early reproductive phase | Hydro-steam distillation/0.1 | Littoral location: allo-aromadenderene (30.6%), (E,E)- farnesol (28.3%), dihydro tagetone (12%) | |
| Iran/fresh leaves/early reproductive phase | Hydro-steam distillation/0.16 | Unlittoral location: allo-aromadenderene (30.3%), (E,E)-farnesol (29.1%), α-calacorene (11.5%) | |
| Iran/dry leaves/early reproductive phase | Hydro-steam distillation/0.1 | Littoral location: allo-aromadendrene (22.6%), dihydro tagetone (12.5%), (E,E)-farnesol (16.0%), elemicin (14.1%), methyl octadecanoate (10.9%) | |
| Iran/dry leaves/early reproductive phase | Hydro-steam distillation/0.16 | Unlittoral location: allo-aromadenderene (30.8%), dihydro tagetone (17.9%), (E,E)-farnesol (13.5%), α-calacorene (11.6%) | |
| Iran/fresh leaves/early reproductive phase | Steam distillation/0.1 | Littoral location: 1-phenyl pentan-3-one (23.8%), trans-chrysanthenyl acetate (11.3%), dihydro tagetone (8.7%), α-ylangene (8.5%) | |
| Iran/fresh leaves/early reproductive phase | Steam distillation/0.14 | Unlittoral location: allo-aromadenderene (13.0%), n-dodecanol (9.0%), (E,E)-farnesol (12.1%), α-calacorene (7.7%), methyl octadecanoate (7%) | |
| Iran/dry leaves/early reproductive phase | Steam distillation/0.053 | Littoral location: allo-aromadenderene (26.4%), (E,E)-farnesol (18%), elemicin (10.5%), methyl octadecanoate (8.3%) | |
| Iran/dry leaves/early reproductive phase | Steam distillation/0.087 | Unlittoral location: alloaromadenderene (32.3%), α-calacorene (15.5%), (E,E)-farnesol (11.2%) | |
| Iran/stems/early reproductive phase | Hydrodistillation/0.18 | Littoral location: allo-aromadenderene (56.4%), trans-calamenene (12%), dihydro tagetone (9.4%), dehydro abietal (9.6%) | |
| Iran/stems/early reproductive phase | Hydrodistillation/0.2 | Unlittoral location: allo-aromadenderene (36%), trans-calamenene (12.6%), dihydro tagetone (6.4%), dehydro abietal (31.5%) | |
| Iran/stems/early reproductive phase | Hydro-steam distillation/0.11 | Littoral location: allo-aromadenderene (55.5%), methyl octadecanoate (20.2%), trans-calamenene (8.3%), α- calacorene (5.8%) | |
| Iran/stems/early reproductive phase | Hydro-steam distillation/0.14 | Unlittoral location: alloaromadenderene (47.4%), dehydro abietal (19.5%), trans-calamenene (14.7%), dihydro tagetone (6.9%) | |
| Iran/stems/early reproductive phase | Steam distillation/0.09 | Littoral location: alloaromadenderene (67.4%), trans-calamenene (11%), dehydro abietal (6.3%), α- calacorene (6.0%) | |
| Iran/stems/early reproductive phase | Steam distillation/0.1 | Unlittoral location: allo-aromadenderene (54.9%), trans-calamenene (16.2%), dehydro abietal (11.7%), elemicin (3.9%) | |
| Iran/roots/early reproductive phase | Hydrodistillation/0.50 | Littoral location: n-octadecanol (91%), n-nonadecane (1.2%), 1-butyl acetate (1.2%) | |
| Iran/roots/early reproductive phase | Hydrodistillation/0.44 | Unlittoral location: n-octadecanol (95.6%), abietatriene (1.7%) | |
| Iran/roots/early reproductive phase | Hydro-steam distillation/0.33 | Littoral location: n-octadecanol (73.8%), 1-butyl acetate (4%) and trans-chrysanthenyl acetate (3.6%) | |
| Iran/roots/early reproductive phase | Hydro-steam distillation/0.28 | Unlittoral location: n-octadecanol (69.1%), α-terpinene (6.9%) and (E,E)-farnesol (5.2%) | |
| Iran/roots/early reproductive phase | Steam distillation/0.16 | Littoral location: n-octadecanol (74.6%), dihydro tagetone (4.9%) | |
| Iran/roots/early reproductive phase | Steam distillation/0.15 | Unlittoral location: n-octadecanol (43.5%), α-eudesmol (18.4%) and methyl octadecanoate (4.8%) | |
| Iran/fresh leaves/early reproductive phase | Hydrodistillation (3 h)/0.07 | Limonene (26.71%), cyclobuta[1,2:3,4]dicyclooctene, hexadecahydro (24.19%), β-sesquiphellandrene (15.25%), δ-3-carene (6.79%), trans-longipinocarveo (5.28%), n-hexadecanoic acid (2.69%), Z-α-bisabolene (2.57%), myrcene (1.95%), α-pinene (1,87%), β-bisabolene (1.84%), n-octanal (1.53%), E-β-ionone (1.23%), vervenene (0.84%), widdrol (0.83%), α-cis-bergamotene (0.72%), n-octanol (0.54%), trans-carveol (0.53%), α-acoradiene (0.46%), linalool (0.42%), n-heptanol (0.42%), citronellol (0.38%), p-cymene (0.37%), carvacrol (0.35%), n-octadecane (0.28%), p-mentha-2,4(8)-diene (0.27%), thymol (0.23%), β-elemene (0.21%), cis-p-mentha-2,8-dien-1-ol (0.18%), myristicin (0.17%), benzene acetaldehyde (0.16%), heptanal (0.15%), Z-4-decenal (0.13%) | [6] |
| Iran/air-dried aerial parts/flowering phase | Hydrodistillation (3 h)/1.1 v/dry weight | Trans-pinocarvyl acetate (15.6%), caryophyllene oxide (13.2%), n-hexyl isobutyrate (11.9%), hexyl isovalerate (9.1%), | [61] |
| Iran/dry roots | Hydrodistillation (3 h)/1.1 v/dry weight | Hexyl isovalerate (11.0%), hexyl valerate (10.1%), hexyl isobutyrate (7.3%), octyl octanoate (7.0%), octyl isovalerate (6.8%), trans-pinocarvyl acetate (6.6%) | [35] |
Dehghanzadeh et al. [18] isolated and identified EOs compositions from the aerial part of E. caeruleum at the pre-flowering stage and reported that among the fifty-six components cyclobuta[1,2,3,4]dicyclooctene, limonene (4.23%), n-hexadecanoic acid (11.16%), hexadecahydro (47.03%), linoleic (5.41%), and cis-α-bisabolene (2.14%) were the main components. Similarly, Mirahmadi et al. [59] also obtained EO from the aerial part of E. caeruleum and reported that twenty-three components contributed 96.7% of EO components and cyclobuta [1,2:3,4] dicyclooctene hexadecahydro (22.24%), limonene (25.42%), and δ-2-carene (16.19%) were the dominated components. Figure 2 shows the proven biological activities of the E. caeruleum extracts.
Fig. 2.
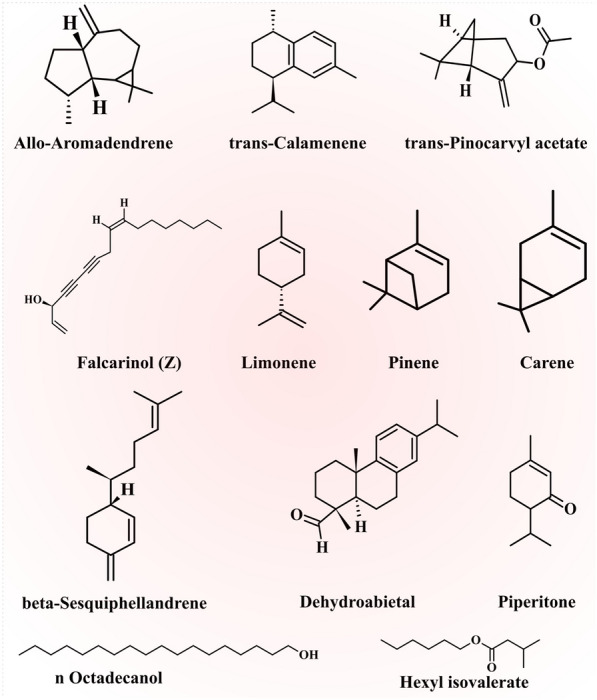
Chemical structure of essential oil constituents of Eryngium caeruleum M. Bieb
Phenolic compounds
Phenols are a family of organic compounds (alcohols) that are characterized by the fact that they have an aromatic ring in which one or more of the hydrogen atoms associated with the carbon atoms of the ring are replaced by one or more hydroxyl groups (–OH) [56, 73, 92]. Phenols and their derivatives are normally present in nature [10, 47, 98, 100]. The main phenol compound from E. caeruleum is represented by gallic acid. Nabavi et al. [65] determined total phenolic compound contents in a crude solid methanol extract from E. caeruleum leaves using the Folin–Ciocalteau reagent. The total phenolic contents of E. caeruleum leaves was 62.3 mg gallic acid equivalent (GAE)/g of extract powder. The total flavonoid contents of E. caeruleum leaves was 25.3 mg quercetin equivalent (QE)/g of extract powder.In another study, total phenolic contents in methanol extracts of E. caeruleum leaves and inflorescences at the flowering stage were 37.6 and 63.1 mg GAE/g of extract powder, respectively [21]. In leaves and inflorescences were 60.0 and 18.3 mg QE/g of extract powder, respectively. The aqueous extract of E. caeruleum leaves reported 80.2 mg GAE/g and the total flavonoid of this extract powder was34.92 mg/g [22].
Many researchers correlated the antioxidant activity of E. caeruleum extracts with the content of phenolic compounds. Therefore Nabavi et al. [66] using the previously described methods determined the phenolic composition of a flavonoid-rich fraction. They found that total phenolic contents of hexane, ethyl acetate and aqueous fractions of E. caeruleum were 29.06, 140.57, 214.18 mg GAE/g of extract, respectively. The total flavonoid contents were 97.37, 31.51, and 75.36 mg QE/g of extract powder, respectively. The aqueous fraction exhibited higher levels of total flavonoids and phenol compared to the other fractions. Ebrahimzadeh et al. [21] performed a comparative study of phenolic content in methanol extracts from E. caeruleum inflorescences obtained by percolation, Soxhlet extractor, and ultrasonic method. Total phenolic contents ranged from 58.8 to 105.5 mg GAE/g of extract. Total phenolic contents appeared in the order of Soxhlet extract > percolation method > ultrasonic extract, respectively. The total flavonoid content was between 11.9 and 18.7 mg QE/g of extract being Soxhlet method the extraction method with higher flavonoid contents [21].In the same year, Dehghan et al. [16] published an article in which the authors cite data from their research of E. caeruleum aerial parts. The authors sequentially extracted the dried aerial part with hexane, ethyl acetate and methanol. The content of the sum of phenolic substances as mg GAE/g dry weight of E. caeruleum aerial parts is presented in Table 2. The highest content of phenolic compounds was found in methanol extract. Phytochemical investigation of methanol extract from aerial parts of E. caeruleum revealed the identification of two novel flavone glycosides. [79].
Table 2.
The total phenolics and flavonoid contents of Eryngium caeruleum M.Bieb
| Country of origin/part used/phase of development | Method of extraction/extractant | Total phenolic contents (gallic acid mg/g of extract powder) | Total flavonoid contents (quercetin equivalent mg/g of extract powder) | Refs. |
|---|---|---|---|---|
| Iran/leaves | Percolation/methanol | 62.3 ± 0.2 | 25. 3 ± 0.2 | [65] |
| Iran/leaves | Percolation/methanol | 37.6 ± 1.5 | 63.1 ± 1.44 | [21] |
| Iran/inflorescences | Percolation/methanol | 60.0 ± 2.8 | 18.3 ± 0.9 | |
| Iran/leaves | Percolation/water | 80.2 ± 3.6 | 34.9 ± 1.1 | [22] |
| Iran/leaves | Percolation/acetone, n-hexane | 29.1 ± 1.8 | 97.4 ± 4.9 | [66] |
| Percolation/acetone, ethyl acetate | 140.6 ± 6.5 | 31.5 ± 1.2 | ||
| Percolation/acetone, water | 214.2 ± 11.5 | 75.4 ± 3.6 | ||
| Iran/inflorescences | Ultrasonic/methanol | 58.8 ± 1.5 | 18.2 ± 0.7 | [21] |
| Percolation/methanol | 60.1 ± 2.3 | 11.9 ± 0.5 | ||
| Soxhlet/methanol | 105.5 ± 2.8 | 18.7 ± 0.9 | ||
| Iran/aerial parts | Percolation/n-hexane | 7.1 ± 0.6 | [16] | |
| Percolation/ethyl acetate | 14.4 ± 3.3 | |||
| Percolation/methanol | 86.2 ± 5.0 |
Saponins
The main saponins of Eryngium species are represented by barrigenol, barringtogenol, cameliagenin, erynginol A, erynginol B, betulinic acid and steganogenin [26]. One of the goals of the study by Habibi et al. [34] was to determine the content of the total of saponins content in the aerial part of E. caeruleum. The raw material was collected mainly from the village of Kootena, on the outskirts of the city of Gaemshahr, Iran. According to the results of the research, the saponins content in the extract has been 5.4%.
Nutritional properties
In the world of medicinal plants there are many nutritious plants [91, 109, 110]. The main objective is to provide the body with the necessary nutrients and to diversify their range [50, 57, 73, 77]. Medicinal plants have a high content of bioactive compounds, phytochemical molecules, vitamins, minerals, polyphenols, carotenoids, and can be compared very easily with the nutritional elements of fruits and vegetables [83, 90, 111, 114]. Along with these, herbs and natural bioactive compounds can optimize nutrition and provide optimal health [85, 96, 106].
In the study by Ebrahimzadeh et al. [22] the elemental composition of the leaves of E. caeruleum was investigated by atomic absorption spectroscopy, and the levels of Fe, Zn, Ni, Cu, Mn, and Cr were established [22]. The concentration of elements analyzed in the ash of E. caeruleum leaves (μg/g) decreased in the order: Fe (17.18) > Zn (0.83) > Mn (0.50) > Cr (0.41) > Cu (0.08). Of the four elements studied by Sepanlou et al. [89], Fe prevailed in the mineral composition of the leaves and roots of E. caeruleum. Autumn leaves showed the greatest amounts of Mn, Cu and, Zn while the roots contained more Fe [89]. Ash, protein, moisture, carbohydrate, fiber, and fat contents of the samples were determined from autumn leaves, spring leaves, and roots of E. caeruleum gathered in Gilan province, Iran [89]. The content of protein, fiber, carbohydrates, ash and moisture in various parts of E. caeruleum varied significantly. Autumn leaves compared with spring leaves showed the highest amount (% dry weight) of fiber (22.6 against 16.7), protein (17.9 against 16.9) and moisture contents (8.6 against 7.5). Spring leaves contained more carbohydrates (49.8 against 39.6) and fats (1.6 against 1.5). While roots – the highest levels of ash (10.1) and carbohydrates (55.9) in % of dry weight. Nine non-essential and eight essential amino acids were found in spring and autumn leaves and dried roots of E. caeruleum. The most dominant essential amino acid in all parts of E. caeruleum appeared to be threonine [89].
Pharmacology: potential mechanisms of action
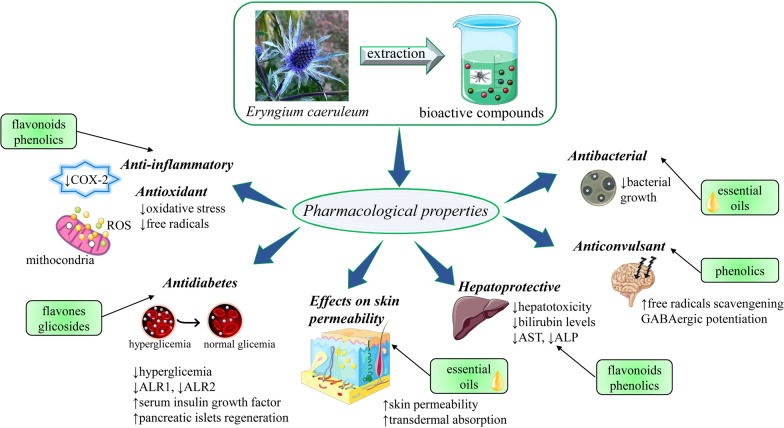
As evidenced in several preclinical studies in vitro, in vivo, E. caeruleum extracts or isolates attributed to different parts of this plant were revealed to exhibit a significant range of biological and pharmacological properties including antioxidant [22, 65, 66], antimicrobial [18, 46, 80], anti-diabetic anti-inflammatory, anticonvulsant [23], antihypoxic [52], and hepatic and renoprotective effects as well as other activities.
Figure 3 shows the proven biological activities of the E. caeruleum extracts.
Fig. 3.
Summarized scheme with the most important activities and biomedical applications of E. caeruleum extracts and correlation with its bioactive compounds. ↓, decrease, aldose reductase (ALR), ↑, increase, AST aspartate aminotransferase, ALP alkaline phosphatase, ROS reactive oxygen species, COX-2 cyclooxygenase-2
Antioxidant
Oxidative stress is one of the leading causes of cellular aging and the onset of chronic diseases [56, 67, 87]. Oxidative stress occurs as a result of an imbalance between free radicals and antioxidants [19, 45, 60, 121]. Natural antioxidants are substances that neutralize or eliminate free radicals by donating an electron [95, 112]. The neutralizing effect of antioxidants helps protect the body from oxidative stress [46, 60, 83, 99]. The antioxidant activity of E. caeruleum leaves (collected from Mazandaran forest) was investigated by Nabavi et al. [65] using a variety of in vitro test systems. For the methanol extract E. caeruleum leaves, the half-maximal inhibitory concentration (IC50) for DPPH (1,1-diphenyl-2-picryl hydrazyl radical) radical-scavenging activity was 0.27 mg/mL. The reducing power of methanol extract was equivalent to that of vitamin C (positive control, p > 0.05). The extract exhibited low Fe2+ chelation and nitric oxide-scavenging properties. The peroxidation inhibition by E. caeruleum extracts exhibited values from 93 (at 24 h) to 97% (at 72 h), comparable with vitamin C. The extract did not show good scavenging activity of hydrogen peroxide [65]. In another study, the antioxidant activities of inflorescence and leaf methanol extracts of E. caeruleum (collected from Khazar Abad area) were analyzed [21]. IC50 for DPPH assay was 0.39 mg/mL for inflorescences and 0.15 for leaves. The leaf extract demonstrated higher potency compared to vitamin C (p < 0.05). In nitric oxide-scavenging activity, methanol extracts showed weak activities. The leaf extract exhibited improved Fe2 + chelating capacity (IC50 = 0.25 mg/mL) that was similar to that of EDTA (ethylenediaminetetraacetic acid; IC50 = 18 μg/mL). Inflorescence extract was found to have very low potency. For hydrogen peroxide assay, IC50 was 177.2 and 25.5 mg/mL for inflorescence and leaves, respectively. Continuing their research Ebrahimzadeh et al. [22] examined the antioxidant activity of aqueous extract obtained from the leaves of E. caeruleum (collected from Panbeh Chooleh, Sari, Iran). IC50 for DPPH radical-scavenging activity of butylated hydroxyanisole (BHA) was 53.96, quercetin—5.28, and vitamin C—5.05 µg/mL. They found that IC50 for DPPH radical-scavenging activity of aqueous extract E. caeruleum was 7.99 mg/mL. The extract's reducing power also improved as its concentration increased but was found to be less than that of vitamin C. The activity of quercetin was more significant compared to the aqueous extract of E. caeruleum as evidenced by nitric oxide-scavenging activity analysis.
The leaf extract of E. caeruleum demonstrated an effective chelating ability, however, it could not be compared with that of EDTA (p < 0.01). For hydrogen peroxide scavenging activity, IC50 for quercetin and vitamin C were 52 and 21.4 mg/mL, respectively, which were higher than the extract of E. caeruleum (IC50 = 0.80 mg/mL). The investigated extract did not exhibit any activity in peroxidation inhibition in ferric thiocyanate assay in comparison with BHA and vitamin C used as controls [22]. The antioxidant effect of the flavonoid-rich part of this plant was subsequently examined [66]. Aqueous, ethyl acetate and n-hexane fractions showed an IC 50 values for DPPH scavenging activity in the range of 391.2, 706.6 and 779.7 μg/mL, respectively. IC50 for nitric oxide radical-scavenging activity was in the order: aqueous (133.5 μg/mL) > ethyl acetate (350.1 μg/mL) > and hexane (639.9 μg/mL) fractions, respectively. The extracts showed low hydrogen peroxide scavenging activity but excellent antioxidant effect against hemoglobin-induced peroxidation of linoleic acid. The fractions have delayed the initiation of hemolysis induced by cumene hydroperoxide. The study of ethanol and methanol extracts from the aerial parts, inflorescences and leaves of E. caeruleum, as well as their hexane, acetone, ethyl acetate and water fractions, showed their pronounced antioxidant properties (in vitro) [115]. The aim of a study conducted by Motallebi Riekandeh et al. [63] was an assessment of the efficiencies of three methods an extraction of antioxidants from E.caeruleum inflorescences: the ultrasonically assisted extraction, Soxhlet apparatus and percolation method. Four different in vitro tests were used to evaluate the antioxidant properties of the extracts. E.caeruleum inflorescences were gathered in Iran (Sari) in June 2012. Inflorescences were dried and extracted by the three above methods. The antioxidant capacities of extracts are presented in Table 3. Soxhlet extract exhibited a potent effect (IC50 = 83.1 μg/mL), then followed an extract obtained by percolation with IC50 = 177.3 μg/mL. Each extract demonstrated similar reducing potency (p > 0.05). The activities of these extracts were lower than the reducing power of vitamin C (p < 0.001).
Table 3.
The antioxidant activities of E. caeruleum inflorescence [63]
| Extraction method | Fe2+ chelating ability, IC50 (µg/mL) | NO scavenging activity, IC50 (µg/mL) | DPPH radical scavenging, IC50 (µg/mL) |
|---|---|---|---|
| Ultrasonic | 286 ± 9.2 | 2416 ± 69.6 | 188.7 ± 7.2 |
| Percolation | 421 ± 13.6 | 390 ± 11.4 | 177.3 ± 5.9 |
| Soxhlet | 272 ± 6.3 | 583 ± 9.8 | 83.1 ± 2.1 |
aIC50 of BHA (butylated hydroxyanisole) was 53.8 ± 3.7 μg/mL
bIC50 for quercetin was 155.0 ± 6.4 μg/mL
cEDTA used as control (IC50 = 17.4 ± 0.4 μg/mL)
The study of ethyl acetate, methanol, and n-hexane extracts of E.caeruleum aerial parts by another group of researchers from Iran showed that their 2,2-diphenyl-1-picrylhydrazyl scavenging activity (%) decreases in order: methanol extract (86.2) > ethyl acetate extract (14.4) > hexane extract (7.1) [16].
Antibacterial
Antibiotic-resistant bacteria is a global health threat and traditional antibiotics may lose their effectiveness over time due to antibacterial resistance [11, 30, 107, 120]. The development of new natural antimicrobial agents has become a topic of interest in recent years and medicinal plants are a source of antimicrobial compounds with vast therapeutic potential [4, 40, 96]. Secondary plant metabolites, terpenes, flavones, flavonols, some alkaloids and phenylpropanoids, isolated or as a constituent of extracts, have shown promising antimicrobial activity in several studies [4, 41]. The antibacterial activity from leaves and aerial parts of E. caeruleum was confirmed by several in vitro investigations. The EO obtained from the leaves of E. caeruleum showed high antibacterial activity against six bacterial strains (Staphylococcus aureus, Bacillus subtilis, Streptomyces scabies, Erwinia amylovora, Xanthomonas axonopodis pv. citri, and Klebsiella sp.) which are essential for plant and human pathogens [18]. In another investigation, Sadiq et al. [80] noted that methanolic extract and its different fractions of E. caeruleum aerial parts demonstrated remarkable antibacterial and antifungal activities against six bacterial strains (Proteus mirabilis, Enterococcus faecalis, Salmonella typhi, Klebsiella pneumonia, Pseudomonas aeruginosa, and Escherichia coli) and three fungal strains (Aspergillus flavus, Aspergillus fumigatus and Aspergillus niger). The standard drugs used in the antifungal and antibacterial assays were nystatin and ceftriaxone, respectively. All specimens showed pronounced antibacterial activity towards the strains tested. Among the other E. caeruleum fractions, the chloroform and ethyl acetate fractions showed superior antibacterial properties. It was observed by the authors that the chloroform fraction of this herb was the most potent one. It exhibited the minimum fungicidal concentration values of 250.64, 350.23, and 233.45 μg/mL against A. niger, A. fumigatus, and A. flavus respectively [80]. Dehghan et al. [17] used an extract of E. caucasicum to biosynthesize green nanoparticles of Ag/Fe3O4.which showed a high inhibition of Cryptococcus neoformans at 150 μg/mL. Antimicrobial activities of E. caeruleum EO were confirmed against S. aureus, E. coli, P. aeruginosa, and B. subtilis using a disc diffusion method by Mohamadipour et al. [61]. In the study of Hamedi et al. [35], the volatile oil of E. caeruleum roots showed the highest minimum bactericidal concentration and minimum inhibitory concentration against B. subtilis and S. aureus (500 μg/mL). According to research findings, EO of E. caeruleum contains high levels of trans-pinocarvyl acetate, short-chain fatty acid esters, and certain alcohols such as 1-hexanol and Z-falcarinol, which could play a role in the observed antibacterial activity of them [35].
Effect on skin permeability
The skin is the largest organ of the body and performs many functions [42, 48, 105]. The pathological conditions determine the change of the skin permeability, respectively it can increase or decrease it, and the effect is more obvious when the skin is damaged, lacking the stratum corneum, has altered keratinization, and the permeability is increased [43, 108]. If the skin is thickened, penetration is low [87]. In the case of dermatoses, characterized by the presence of a defective stratum corneum, there is an increase in absorption through the skin, influencing the local and systemic bioavailability of drug molecules [44, 94]. The investigation of Saeedi and Morteza-Semnani [81] was designed to investigate the ability of different levels of E. caeruleum EO to improve the permeation of piroxicam through rat skin and the transdermal absorption of piroxicam. The results showed that no linear correlation was found between the concentration of the EO of E. caeruleum and the penetration rate. The increased level of E. caeruleum EO (5% w/w) resulted in an increase in the permeability coefficient of piroxicam by nearly 8.56-fold.
Antihypoxic
Eryngium caeruleum inflorescences were collected in the 2012 year and isolated by percolation using methanol [52]. The extract obtained was treated with acetone/methanol/water (3.5/3.5/3) containing 1% formic acid. The aqueous phase was further extracted by ethyl acetate. The organic phase was used as a polyphenols fraction. The methanol extract and polyphenol fraction were used for the study antihypoxic activity (compared to phenytoin). Also, at 400 mg/kg, the polyphenolic fraction increased the death time of experimental animals. It had a comparative advantage over phenytoin (55 min, p > 0.05). The polyphenolic fraction was found to be more potent compared to the methanolic extract [52].
Anti-inflammatory
The study by Habibi et al. [34] showed that a dose of 200 and 400 mg/kg of ethyl acetate extract of E. caeruleum was effective in inducing an anti-inflammatory effect while reporting that a higher dose, causes a decrease in this effect. Furthermore, E. caeruleum exhibited a comparable anti-inflammatory effect to celecoxib as a cyclooxygenase (COX)-2 inhibitor (Fig. 4), however, its undesirable reaction is less than that of the chemical drugs, therefore, the Habibi et al. [34] believe that suggested some inflammatory diseases such as rheumatoid arthritis. It can be hypothesized that the anti-inflammatory properties of E. caeruleum might be linked to a high content of flavonoids and phenol like quercetin.
Fig. 4.
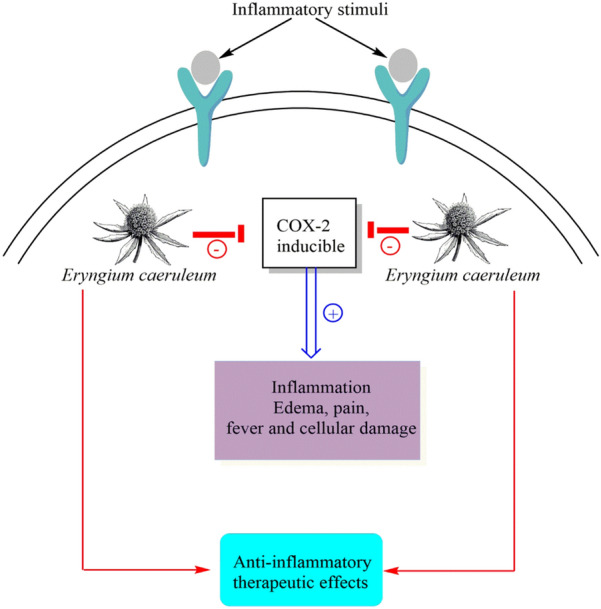
Anti-inflammatory effects of E. caeruleum
Anti-diabetes
Diabetes is a chronic non-communicable disease that occurs when the pancreas can no longer produce insulin, or when the body can no longer use insulin [76, 84]. The increasing frequency of hyperglycemia of various etiologies and the side effects of many synthetic hypoglycemic drugs have led to a shift toward phytotherapy [75, 91].
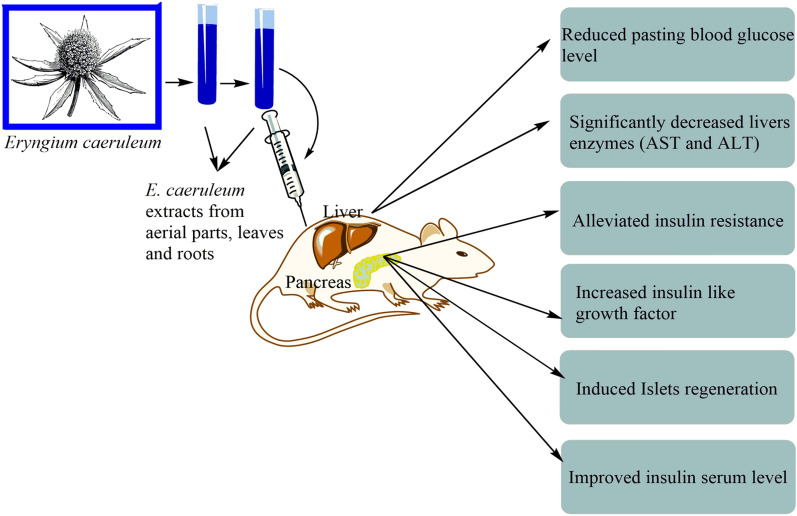
Dehghan et al. [16] have reported that ethyl acetate, methanolic, and n-hexane extracts of aerial parts of E. caeruleum possess an anti-diabetic effect. The methanolic extract showed a higher effect. Rehman et al. [79] reported two new flavone glycosides derived from E. caeruleum aerial parts. These compounds were analyzed in vitro against aldose reductase (ALR) of two types (ALR1 and ALR2). Also, flavonoid 2 showed a very significant inhibitory activity against both enzymes, while the effect on ALR1 was the highest [79]. Afshari et al. [2] studied the anti-diabetetes effect of E. caeruleum leaves hydro-alcoholic (70:30) extract in a model of type 2 diabetes mellitus induced by nicotinamide-streptozotocin in Wistar rats. Insulin-related indices, insulin secretion, and lipid profile were affected by the administration of E. caeruleum extract. It also revealed a significant decrease in liver enzymes. Additionally, E. caeruleum enhanced levels of serum insulin-like growth factor in a dose-dependent manner. Overall, it appears that E. caeruleum causes diabetes in rats in a dose-dependent manner, with increasing doses exhibiting greater benefits. In a study performed in a streptozotocin-induced diabetes rat model, the hydro-alcoholic (70:30) extract of aerial branches and roots of E. caeruleum improved glycemic and lipid profiles [86]. Pancreatic histology results revealed that extracts from the root and aerial branch (800 mg/kg) induced islets regeneration. In another investigation, the administration of metformin and E. caeruleum extract alone or in combination ameliorate types of morphologic injury to renal tubular cells in diabetic rats [31] (Fig. 5).
Fig. 5.
Antidiabetic effects of E. caeruleum
Hepatoprotective and renoprotective
The liver is an organ with a series of very important roles in the body: purification, detoxification, bile secretion necessary for intestinal digestion, having a decisive role in the synthesis processes, degradation and storage of organic and inorganic substances absorbed at the level of intestinal [20, 49, 116]. Some liver conditions can be treated with natural remedies and others with medication [14, 20, 51, 68]. Fattahi and Hemayatkhah Jahromi [28] investigated the protective role of E. caeruleum extract on hepatotoxicity in a mice model using tricyclazole (TCZ). The extract treatment reduced bilirubin levels and liver enzymes (aminotransferase and phosphatase alkaline) and increased albumin levels (p < 0.05) in TCZ-treated mice. Methanol/water (80/20 w/w) extract of E. caeruleum aerial parts (200 and 400 mg/kg/day). Intraperitoneally administered for ten consecutive days, provoked a renoprotective action [27].
Anti-convulsant
Epilepsy is a syndrome of cerebral distress, which is manifested by epileptic seizures [10, 98, 109]. There are two factors in its genesis: convulsive predisposition and the existence of an anatomical or biochemical brain injury [39, 47]. The medical term for seizures refers to abnormal, involuntary contractions of the muscles as a result of changes in the electrical activity of the brain and which generally occur in certain epileptic diseases [9, 98]. The objective of the study performed by Ebrahimzadeh et al. [23] was to assess the anticonvulsant effects of polyphenolic and methanolic extracts (250, 500 and 1000 mg/kg) of E. caeruleum inflorescences on pentylenetetrazole-induced seizures and maximal electroshock in mice. The effect of the polyphenolic extract was more potent. According to the authors, a mechanism of GABAergic potentiation or free radical scavenging might be possible for these pharmacological abilities [23].
Applications in the food industry
Raeisi et al. [78] demonstrated that E. caeruleum leaves extract (80% ethanol) had good potential for use as a natural preservative for shelf-life extension of fishery products. They examined the effects of E. caeruleum extract (after removing alcohol) on rancidity indices and lipid oxidation, sensory and microbiological qualities during refrigerated storage (4 °C) of silver carp (Hypophthalmichthys molitrix). The inclusion of plant extracts considerably retarded oxidative damage of silver carp fillets during the period of storage. Further, the findings indicated that the natural constituents from E. caeruleum extract were effective in decreasing bacterial growth within the fish fillets during the period of refrigerated storage. In addition, it was observed that 4% E. caeruleum had a beneficial effect on the sensory characteristics of the fillets [78]. Golmohammadi and Khademi shurmasti [32] investigated the effect of E. caeruleum extract on chicken fillets shelf life. The examination was carried out with uncoated chicken fillets (control), and fillets that were coated with xanthan and guar gums alone or in combination with E. caeruleum extract in during 12-day refrigerated storage. The findings revealed that E. caeruleum extract markedly (p < 0.05) enhanced the antibacterial function of guar coating. Also, the effect of E. caeruleum extract on improving the efficiency of edible guar coating resulted in a significant decrease in total volatile nitrogen compounds of the fillet (p < 0.05) [32].
Clinical studies
A blinded, randomized, placebo-controlled clinical trial involving 169 women between the ages of 15 and 30 years was aimed at studying the safety and effectiveness of syrup of E. caeruleum in the treatment of primary dysmenorrheal (Iranian Registry of Clinical Trials ID: IRCT2015082823789N1) [7]. The dosage was determined by the investigators to consist of 5 ml of syrup administered three times a day (15 ml/day), starting one day prior to the onset of bleeding and continuing for five days over two menstrual cycles. There have been no reported serious adverse reactions. E. caeruleum syrup provided relief from dysmenorrhea as efficiently as Ibuprofen.
Limitations
A limiting therapeutic aspect is the lack of studies on the E. caeruleum toxicity, the side effects that may occur during prolonged administration and the potential interactions with the medication administered concomitantly. In addition, the concentration of active ingredients varies depending on the cultivation area and soil characteristics. Also, the reduced bioavailability of the active ingredients is an important factor in the therapeutic efficacy. Therefore, the inclusion of phytocompounds in some pharmaceutical nanoformulations can increase their bioavailability. Saleh et al. [82] synthesized bio-compatible palladium nanoparticles via E. caeruleum leaves extract. These showed antibacterial activity against multidrug-resistant bacteria, high antioxidant activity and not showed hemolytic activity [82].
Conclusions and prospects
This article reports, explores and highlights published evidence on E. caeruleum regarding its botanical characteristics and traditional uses, its phytochemical composition, its cultivation, and its bioactivity and pharmacological properties as a promising medicinal plant. E. caeruleum leaves, traditionally are used to flavor cooked vegetables in various local products. In traditional medicine, the aerial parts of E. caeruleum are largely used for treating pulmonary disease, asthma, epigastric, and hypochondriac pain. The roots of the plant cause a diuretic effect, effective against kidney and bladder stones, as an analgesic for rheumatoid arthritis. Studies show that E. caeruleum is a potential preventative and medicinal plant. Extracts from aerial part, leaves and roots containing EO, phenolic compounds, saponins, protein, amino acids, fiber, carbohydrates, and mineral elements have antioxidant, antimicrobial, antidiabetic, antihypoxic, anti-inflammatory, and other properties. These effects show that E. caeruleum could be considere a novel herbal-derived medicine for the management of certain diseases. Nevertheless, stringent investigation studies are necessary to determine its effectiveness by examining its toxicological, pharmacological, chemical, and therapeutic effects. Future studies must evaluate new methods of obtaining and administering some drugs/nutritional supplements to increase the absorption and bioavailability of the bioactive compounds from the E. caeruleum species. Thus, new pharmaceutical forms such as nanoparticles should be evaluated in more detail in animals through preclinical in vivo and in vitro experimental studies. Translational medicine studies and clinical studies are also needed to accurately establish the effective dose and the method of administration in humans.
Acknowledgements
Authors acknowledge Pyatigorsk Medical-Pharmaceutical Institute (PMPI), Branch of Volgograd State Medical University, Ministry of Health of Russia, Pyatigorsk, Russian Federation. MM wants to thank ANID CENTROS BASALES ACE210012.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas. That is, revising or critically reviewing the article; giving final approval of the version to be published; agreeing on the journal to which the article has been submitted; and, confirming to be accountable for all aspects of the work. All authors read and approved the final manuscript.
Funding
No funding received.
Availability of data and materials
Yes.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dmitryi Alexeevich Konovalov, Email: d.a.konovalov@pmedpharm.ru.
Edgardo Avendaño Cáceres, Email: eavendanoc@unjbg.edu.pe.
Ekaterina Aleksandrovna Shcherbakova, Email: yeliseikina@mail.ru.
Deepak Chandran, Email: c_deepak@cb.amrita.edu.
Miquel Martorell, Email: mmartorell@udec.cl.
Muzaffar Hasan, Email: muzaffarhassan88@gmail.com.
Saad Bakrim, Email: s.bakrim@hotmail.com.
Abdelhakim Bouyahya, Email: boyahyaa-90@hotmail.fr.
William C. Cho, Email: chocs@ha.org.hk
Javad Sharifi-Rad, Email: javad.sharifirad@gmail.com.
Hafiz A. R. Suleria, Email: hafiz.suleria@unimelb.edu.au
Daniela Calina, Email: calinadaniela@gmail.com.
References
- 1.Abbaspour Z, Jaimand K, Mozaffari S. Comparison of essential oils compositions of eryngo (Eryngium caucasicum) in different parts of plant in two growth conditions. J Med Plants By-product. 2015;4:83–98. [Google Scholar]
- 2.Afshari M, Mohammadshahi M, Malayeri AR, Zaheri L. Antidiabetic, hepato-protective and hypolipidemic effects of Eryngium caucasicum extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iraq Med J. 2019;7(2):169–179. [Google Scholar]
- 3.Al-Azdadi AM. Ketab-al-Mae. Tehran: Iran University of Medical Science Publication, Research Institute for Islamic and Complementary Medicine; 1990. p. 412. [Google Scholar]
- 4.Alshehri MM, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Tutuncu S, Aydar EF, Topkaya C, Mertdinc Z, Ozcelik B, Aital M, Kumar NVA, Lapava N, Rajkovic J, Ertani A, Nicola S, Semwal P, Painuli S, González-Contreras C, Martorell M, Butnariu M, Bagiu IC, Bagiu RV, Barbhai MD, Kumar M, Daştan SD, Calina D, Cho WC. A review of recent studies on the antioxidant and anti-infectious properties of Senna plants. Oxid Med Cell Longev. 2022;2022:6025900. doi: 10.1155/2022/6025900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assadian F, Masoudi S, Nematollahi F, Rustaiyan A, Larijani K, Mazloomifar H. Volatile constituents of Xanthogalum purpurascens Ave-Lall., Eryngium caeruleum M. B. and Pimpinella aurea DC. Three umbelliferae herbs growing in Iran. J Essent Oil Res. 2005;17:243–245. doi: 10.1080/10412905.2005.9698889. [DOI] [Google Scholar]
- 6.Banikhademi S, Aminzare M, Hassanzad Azar H, Mehrasbi MR. Eryngium caeruleum essential oil as a promising natural additive: in vitro antioxidant properties and its effect on lipid oxidation of minced rainbow trout meat during storage at refrigeration temperature. SSRN J. 2021 doi: 10.2139/ssrn.3783481. [DOI] [Google Scholar]
- 7.Behmanesh E, Delavar MA, Kamalinejad M, Khafri S, Shirafkan H, Mozaffarpur SA. Effect of eryngo (Eryngium caucasicum Trautv) on primary dysmenorrhea: a randomized, double-blind, placebo-controlled study. Taiwan J Obstet Gynecol. 2019;58:227–233. doi: 10.1016/j.tjog.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi M, Lazzeri L, Perelli F, Reis FM, Petraglia F. Dysmenorrhea and related disorders. F1000Res. 2017;6:1645. doi: 10.12688/f1000research.11682.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buga AM, Docea AO, Albu C, Malin RD, Branisteanu DE, Ianosi G, Ianosi SL, Iordache A, Calina D. Molecular and cellular stratagem of brain metastases associated with melanoma. Oncol Lett. 2019;17:4170–4175. doi: 10.3892/ol.2019.9933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calina D, Buga AM, Mitroi M, Buha A, Caruntu C, Scheau C, Bouyahya A, El Omari N, El Menyiy N, Docea AO. The treatment of cognitive, behavioural and motor impairments from brain injury and neurodegenerative diseases through cannabinoid system modulation-evidence from in vivo studies. J Clin Med. 2020;9:28. doi: 10.3390/jcm9082395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calina D, Rosu L, Rosu AF, Ianosi G, Ianosi S, Zlatian O, Mitrut R, Docea AO, Rogoveanu O, Mitrut P, Nicolae AC, Dragoi CM, Gofita E. Etiological diagnosis and pharmacotherapeutic management of parapneumonic pleurisy. Farmacia. 2016;64:946–952. [Google Scholar]
- 12.Calviño C, Martínez S, Downie S. Unraveling the taxonomic complexity of Eryngium L. (Apiaceae, Saniculoideae): phylogenetic analysis of 11 non-coding cpDNA loci corroborates rapid radiations. Plant Divers Evol. 2010;128:137–149. doi: 10.1127/1869-6155/2010/0128-0006. [DOI] [Google Scholar]
- 13.Calvino CI, Martínez SG, Downie SR. The evolutionary history of Eryngium (Apiaceae, Saniculoideae): rapid radiations, long distance dispersals, and hybridizations. Mol Phylogenet Evol. 2008;46:1129–1150. doi: 10.1016/j.ympev.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Cioboată R, Găman A, Traşcă D, Ungureanu A, Docea AO, Tomescu P, Gherghina F, Arsene AL, Badiu C, Tsatsakis AM, Spandidos DA, Drakoulis N, Călina D. Pharmacological management of non-alcoholic fatty liver disease: atorvastatin versus pentoxifylline. Exp Ther Med. 2017;13:2375–2381. doi: 10.3892/etm.2017.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis P. Eryngium. Flora of Turkey. Edinburgh: Edinburgh University Press; 1972. pp. 292–304. [Google Scholar]
- 16.Dehghan H, Sarrafi Y, Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J Food Drug Anal. 2016;24:179–188. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dehghan Z, Ranjbar M, Govahi M. Green synthesis of Ag/Fe3O4 nanocomposite by aqueous extract of Eryngium caucasicum and evaluation of its’ antifungal activity. Qom Univ Med Sci J. 2021;15:28–37. doi: 10.52547/qums.15.1.28. [DOI] [Google Scholar]
- 18.Dehghanzadeh N, Ketabchi S, Alizadeh A. Essential oil composition and antibacterial activity of Eryngium caeruleum grown wild in Iran. J Essent Oil Bear Plants. 2014;17:486–492. doi: 10.1080/0972060X.2014.895186. [DOI] [Google Scholar]
- 19.Docea AO, Calina D, Buga AM, Zlatian O, Paoliello MMB, Mogosanu GD, Streba CT, Popescu EL, Stoica AE, Birca AC, Vasile BS, Grumezescu AM, Mogoanta L. The effect of silver nanoparticles on antioxidant/pro-oxidant balance in a murine model. Int J Mol Sci. 2020;21:17. doi: 10.3390/ijms21041233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Docea AO, Gofita E, Calina D, Ioan ZS, Valcea DI, Mitrut P. Autoimmune disorders due to double antiviral therapy with peginterferon and ribavirin in patients with hepatitis C virus infection. Farmacia. 2016;64:605–611. [Google Scholar]
- 21.Ebrahimzadeh M, Nabavi S, Nabavi S. Antioxidant activity of leaves and inflorescence of Eryngium Caucasicum Trautv at flowering stage. Pharmacogn Res. 2009;1:435–439. [Google Scholar]
- 22.Ebrahimzadeh M, Nabavi S, Nabavi S, Eslami S, Bekhradnia A. Mineral elements and antioxidant activity of three locally edible and medicinal plants in Iran. Asian J Chem. 2010;22:6257–6266. [Google Scholar]
- 23.Ebrahimzadeh MA, Chitsaz Z, Shokrzadeh M, Ataie A, Ataee R. Evaluation of anticonvulsant activities of Eryngium caucasicum with maximal electroshock and kindling model of seizure in Mice. Iran J Psychiatry Behav Sci. 2017;11:e3571. [Google Scholar]
- 24.Eliseeva LM, Scherbakova EA, Konovalov DA, Galkin MA. Active parts and seed efficiency of Eryngium planum from apiaceae family. Pharm Pharmacol. 2015 doi: 10.19163/2307-9266-2015-3-1(8)-14-16. [DOI] [Google Scholar]
- 25.Erdem SA, Nabavi SF, Orhan IE, Daglia M, Izadi M, Nabavi SM. Blessings in disguise: a review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. Daru. 2015;23:53. doi: 10.1186/s40199-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erdem SA, Nabavi SF, Orhan IE, Daglia M, Izadi M, Nabavi SM. Blessings in disguise: a review of phytochemical composition and antimicrobial activity of plants belonging to the genus Eryngium. DARU J Pharm Sci. 2015;23:53. doi: 10.1186/s40199-015-0136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eslami SH, Ebrahimzadeh M, Moghaddam A, Nabavi S, Jafari N, Nabavi S. Renoprotective effect of Eryngium caucasicum in gentamicin-induced nephrotoxic mice. Arch Biol Sci. 2011;63:157–160. doi: 10.2298/ABS1101157E. [DOI] [Google Scholar]
- 28.Fattahi E, Hemayatkhah Jahromi V. Protective effects of Eryngium caucasicum Trautv hydroalcholic extract on tricyclazole induced hepatotoxicity in mice. HBI J. 2016;19:67–78. [Google Scholar]
- 29.Ghanavati R, Namjoyan F, Zadeh HR. A review of possible herbal treatment in multiple sclerosis in traditional Persian medicine. Iran J Med Sci. 2016;41:S18. [PMC free article] [PubMed] [Google Scholar]
- 30.Ghenea AE, Cioboată R, Drocaş AI, Țieranu EN, Vasile CM, Moroşanu A, Țieranu CG, Salan A-I, Popescu M, Turculeanu A, Padureanu V, Udriștoiu A-L, Calina D, Cȃrţu D, Zlatian OM. Prevalence and antimicrobial resistance of Klebsiella strains isolated from a County Hospital in Romania. Antibiotics. 2021;10:868. doi: 10.3390/antibiotics10070868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golestaneh E, HasanpourDehkordi A, Yalameha B, Noorshargh P, Nasri P, Baradaran A. The nephroprotective effect of Eryngium caucasicum extract alone and in combination with metformin in adult male diabetic rats. J Nephropharmacol. 2022 doi: 10.34172/npj.2022.10379. [DOI] [Google Scholar]
- 32.Golmohammadi M, Khademi Shurmasti D. The effect of Eryngium caucasicum extract on chicken fillet shelf life coated with xanthan and guar gums during cold storage (4 ± 1 ºC) Iran J Food Sci Technol. 2019;16:253–261. [Google Scholar]
- 33.Goodier J. Encyclopedia of Islamic herbal medicine. Ref Rev. 2012;26:44–45. [Google Scholar]
- 34.Habibi E, Azami R, Fathi H, Yaghubi-Beklar S, Razzaghi F, Ataee R. Anti-inflammatory effects of Erynagium caucasicum Trautv extract in Carrageenan model of inflammation in rat. Glob J Intern Med. 2018;1:1–4. [Google Scholar]
- 35.Hamedi A, Pasdaran A, Pasdaran A. Antimicrobial activity and analysis of the essential oils of selected endemic edible Apiaceae plants root from Caspian Hyrcanian Region (North of Iran) Pharm Sci. 2019;25:138–144. doi: 10.15171/PS.2019.21. [DOI] [Google Scholar]
- 36.Hashemabadi D, Kaviani B. Seasonal and geographical variations in the essential oils of Eryngium caucasicum Trautv. growing in Iran. Am Eur J Agric Environ Sci. 2010;8:212–215. [Google Scholar]
- 37.Hashemabadi D, Kaviani Livani B, Erfatpour M, Larijani K. Comparison of essential oils compositions of eryngo (Eryngium caucasicum Trautv.) at different growth phases by hydrodistillation method. Plant Omics. 2010;3:135–139. [Google Scholar]
- 38.Heinrich M, Appendino G, Efferth T, Fürst R, Izzo AA, Kayser O, Pezzuto JM, Viljoen A. Best practice in research—overcoming common challenges in phytopharmacological research. J Ethnopharmacol. 2020;246:112230. doi: 10.1016/j.jep.2019.112230. [DOI] [PubMed] [Google Scholar]
- 39.Hossain R, Quispe C, Herrera-Bravo J, Beltrán JF, Islam MT, Shaheen S, Cruz-Martins N, Martorell M, Kumar M, Sharifi-Rad J, Ozdemir FA, Setzer WN, Alshehri MM, Calina D, Cho WC. Neurobiological promises of the bitter diterpene lactone andrographolide. Oxid Med Cell Longev. 2022;2022:3079577. doi: 10.1155/2022/3079577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hossain R, Quispe C, Herrera-Bravo J, Islam MS, Sarkar C, Islam MT, Martorell M, Cruz-Martins N, Al-Harrasi A, Al-Rawahi A, Sharifi-Rad J, Ibrayeva M, Daştan SD, Alshehri MM, Calina D, Cho WC. Lasia spinosa chemical composition and therapeutic potential: a literature-based review. Oxid Med Cell Longev. 2021;2021:1602437. doi: 10.1155/2021/1602437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hossain R, Quispe C, Saikat ASM, Jain D, Habib A, Janmeda P, Islam MT, Radha Daştan SD, Kumar M, Butnariu M, Cho WC, Sharifi-Rad J, Kipchakbayeva A, Calina D. Biosynthesis of secondary metabolites based on the regulation of microRNAs. Biomed Res Int. 2022;2022:9349897. doi: 10.1155/2022/9349897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ianoşi S, Ianoşi G, Neagoe D, Ionescu O, Zlatian O, Docea AO, Badiu C, Sifaki M, Tsoukalas D, Tsatsakis AM, Spandidos DA, Călina D. Age-dependent endocrine disorders involved in the pathogenesis of refractory acne in women. Mol Med Rep. 2016;14:5501–5506. doi: 10.3892/mmr.2016.5924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ianoși SL, Batani A, Ilie MA, Tampa M, Georgescu SR, Zurac S, Boda D, Ianosi NG, Neagoe D, Calina D, Tutunaru C, Constantin C. Non-invasive imaging techniques for the in vivo diagnosis of Bowen's disease: three case reports. Oncol Lett. 2019;17:4094–4101. doi: 10.3892/ol.2019.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iordache AM, Docea AO, Buga AM, Mitrut R, Albulescu D, Zlatian O, Ianosi S, Ianosi G, Neagoe D, Sifaki M, Rogoveanu OC, Branisteanu DE, Calina D. The incidence of skin lesions in contrast media-induced chemical hypersensitivity. Exp Ther Med. 2019;17:1113–1124. doi: 10.3892/etm.2018.7056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iordache AM, Docea AO, Buga AM, Zlatian O, Ciurea ME, Rogoveanu OC, Burada F, Sosoi S, Mitrut R, Mamoulakis C, Albulescu D, Vasile RC, Tsatsakis A, Calina D. Sildenafil and tadalafil reduce the risk of contrast-induced nephropathy by modulating the oxidant/antioxidant balance in a murine model. Food Chem Toxicol. 2020;135:9. doi: 10.1016/j.fct.2019.111038. [DOI] [PubMed] [Google Scholar]
- 46.Irfan M, Javed Z, Khan K, Khan N, Docea AO, Calina D, Sharifi-Rad J, Cho WC. Apoptosis evasion via long non-coding RNAs in colorectal cancer. Cancer Cell Int. 2022;22:280. doi: 10.1186/s12935-022-02695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Islam MS, Quispe C, Hossain R, Islam MT, Al-Harrasi A, Al-Rawahi A, Martorell M, Mamurova A, Seilkhan A, Altybaeva N, Abdullayeva B, Docea AO, Calina D, Sharifi-Rad J. Neuropharmacological effects of quercetin: a literature-based review. Front Pharmacol. 2021 doi: 10.3389/fphar.2021.665031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Islam MT, Salehi B, Karampelas O, Sharifi-Rad J, Docea AO, Martorell M, Calina D. High skin melanin content, vitamin D deficiency and immunity: potential interference for severity of COVID-19. Farmacia. 2020;68:970–983. doi: 10.31925/farmacia.2020.6.3. [DOI] [Google Scholar]
- 49.Jain D, Chaudhary P, Varshney N, Bin Razzak KS, Verma D, Zahra TRK, Janmeda P, Sharifi-Rad J, Dastan SD, Mahmud S, Docea AO, Calina D. Tobacco smoking and liver cancer risk: potential avenues for carcinogenesis. J Oncol. 2021 doi: 10.1155/2021/5905357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Javed Z, Khan K, Herrera-Bravo J, Naeem S, Iqbal MJ, Raza Q, Sadia H, Raza S, Bhinder M, Calina D, Sharifi-Rad J, Cho WC. Myricetin: targeting signaling networks in cancer and its implication in chemotherapy. Cancer Cell Int. 2022;22:239. doi: 10.1186/s12935-022-02663-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamal AM, Mitrut P, Docea AO, Sosoi SS, Kamal CK, Mitrut R, Margaritescu D, Calina D, Banciu C, Tica OS, Tica AA, Alexandru DO. Double therapy with pegylated interferon and ribavirin for chronic hepatitis C. A pharmacogenetic guide for predicting adverse events. Farmacia. 2017;65:877–884. [Google Scholar]
- 52.Khalili M, Dehdar T, Hamedi F, Ebrahimzadeh MA, Karami M. Antihypoxic activities of Eryngium caucasicum and Urtica dioica. Eur Rev Med Pharmacol Sci. 2015;19:3282–3285. [PubMed] [Google Scholar]
- 53.Khoshbakht K. Agrobiodiversity of plant genetic resources in Savadkouh, Iran, with emphasis on plant uses and socioeconomic aspects. Kassel: Kassel University Press; 2006. [Google Scholar]
- 54.Khoshbakht K, Hammer K, Pistrick K. Eryngium caucasicum Trautv. cultivated as a vegetable in the Elburz Mountains (Northern Iran) Genet Resour Crop Evol. 2007;54:445–448. doi: 10.1007/s10722-006-9121-5. [DOI] [Google Scholar]
- 55.Kikowska M, Dworacka M, Kędziora I, Thiem B. Eryngium creticum-ethnopharmacology, phytochemistry and pharmacological activity. A review. Rev Bras Farmacogn. 2016;26:392–399. doi: 10.1016/j.bjp.2016.01.008. [DOI] [Google Scholar]
- 56.Kitic D, Miladinovic B, Randjelovic M, Szopa A, Sharifi-Rad J, Calina D, Seidel V. Anticancer potential and other pharmacological properties of Prunus armeniaca L.: an updated overview. Plants. 2022;11:1885. doi: 10.3390/plants11141885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu Y, Li X, Chen C, Leng A, Qu J. Effect of mineral excipients on processing traditional Chinese medicines: an insight into the components, pharmacodynamics and mechanism. Chin Med. 2021;16:143. doi: 10.1186/s13020-021-00554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meusel H, Jäger E, Rauschert S, Weinert E. Vergleichende Chorologie der Zentraleuropäischen Flora. Bd. 2, G. Fischer, Jena. 1978.
- 59.Mirahmadi SS, Aminzare M, Azar HH, Kamali K. Effect of Eryngium caeruleum essential oil on microbial and sensory quality of minced fish and fate of Listeria monocytogenes during the storage at 4 °C. J Food Saf. 2020;40:e12745. doi: 10.1111/jfs.12745. [DOI] [Google Scholar]
- 60.Mititelu RR, Padureanu R, Bacanoiu M, Padureanu V, Docea AO, Calina D, Barbulescu AL, Buga AM. Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines. 2020;8:125. doi: 10.3390/biomedicines8050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohamadipour S, Hatamzadeh A, Bakhshi D, Pasdaran A. Antimicrobial activities of Caucalis platycarpos L. and Eryngium caucasicum Trautv. essential oils. Aust J Crop Sci. 2018;12:1357–1362. doi: 10.21475/ajcs.18.12.08.PNE1277. [DOI] [Google Scholar]
- 62.Morteza-Semnani KM, Azadbakht M, Houshmand A. Composition of the essential oils of aerial parts of Eryngium bungei Boiss. and Eryngium caeruleum MB. Pharm Sci. 2003;1:43–48. [Google Scholar]
- 63.Motallebi Riekandeh S, Mazandarani M, Ebrahimzadeh MA, Zargari M. Antioxidant activities of Eryngium caucasicum inflorescence. Eur Rev Med Pharmacol Sci. 2016;20:946–949. [PubMed] [Google Scholar]
- 64.Mozumder S, Hossain M. Effect of seed treatment and soaking duration on germination of Eryngium foetidum L. seeds. Int J Hortic. 2013;3:1046–1051. [Google Scholar]
- 65.Nabavi SM, Ebrahimzadeh M, Nabavi SF, Jafari M. Free radical scavenging activity and antioxidant capacity of Eryngium caucasicum Trautv and Froripia subpinata. Pharmacologyonline. 2008;3:19–25. [Google Scholar]
- 66.Nabavi SM, Nabavi SF, Alinezhad H, Zare M, Azimi R. Biological activities of flavonoid-rich fraction of Eryngium caucasicum Trautv. Eur Rev Med Pharmacol Sci. 2012;16(Suppl 3):81–87. [PubMed] [Google Scholar]
- 67.Padureanu R, Albu CV, Mititelu RR, Bacanoiu MV, Docea AO, Calina D, Padureanu V, Olaru G, Sandu RE, Malin RD, Buga A-M. Oxidative stress and inflammation interdependence in multiple sclerosis. J Clin Med. 2019;8:1815. doi: 10.3390/jcm8111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Painuli S, Quispe C, Herrera-Bravo J, Semwal P, Martorell M, Almarhoon ZM, Seilkhan A, Ydyrys A, Rad JS, Alshehri MM, Daştan SD, Taheri Y, Calina D, Cho WC. Nutraceutical profiling, bioactive composition, and biological applications of Lepidium sativum L. Oxid Med Cell Longev. 2022;2022:2910411. doi: 10.1155/2022/2910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul JH, Seaforth CE, Tikasingh T. Eryngium foetidum L.: a review. Fitoterapia. 2011;82:302–308. doi: 10.1016/j.fitote.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 70.Pimenov M. Umbelliferae. Vvedensky AI (ed), Opredelitel' rastenij Srednej Azii. T. VII, Taškent: Izd. Fan. 1983;167–322.
- 71.Pimenov M, Tamamschian S. 5. Eryngium, Akademische Druck- und Verlagsanstalt, Graz. 1987.
- 72.Plantlist T. http://www.theplantlist.org/. Accessed 2021.
- 73.Popović-Djordjević J, Quispe C, Giordo R, Kostić A, Katanić Stanković JS, Tsouh Fokou PV, Carbone K, Martorell M, Kumar M, Pintus G, Sharifi-Rad J, Docea AO, Calina D. Natural products and synthetic analogues against HIV: a perspective to develop new potential anti-HIV drugs. Eur J Med Chem. 2022;233:114217. doi: 10.1016/j.ejmech.2022.114217. [DOI] [PubMed] [Google Scholar]
- 74.Powo 2021. Eryngium caeruleum M.Bieb. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. http://powo.science.kew.org/taxon/841696-1. Accessed 06 June 2021.
- 75.Quetglas-Llabrés MM, Quispe C, Herrera-Bravo J, Catarino MD, Pereira OR, Cardoso SM, Dua K, Chellappan DK, Pabreja K, Satija S, Mehta M, Sureda A, Martorell M, Satmbekova D, Yeskaliyeva B, Sharifi-Rad J, Rasool N, Butnariu M, Bagiu IC, Bagiu RV, Calina D, Cho WC. Pharmacological properties of Bergapten: mechanistic and therapeutic aspects. Oxid Med Cell Longev. 2022;2022:8615242. doi: 10.1155/2022/8615242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Quispe C, Herrera-Bravo J, Javed Z, Khan K, Raza S, Gulsunoglu-Konuskan Z, Daştan SD, Sytar O, Martorell M, Sharifi-Rad J, Calina D. Therapeutic applications of curcumin in diabetes: a review and perspective. Biomed Res Int. 2022;2022:1375892. doi: 10.1155/2022/1375892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quispe C, Herrera-Bravo J, Khan K, Javed Z, Semwal P, Painuli S, Kamiloglu S, Martorell M, Calina D, Sharifi-Rad J. Therapeutic applications of curcumin nanomedicine formulations in cystic fibrosis. Prog Biomater. 2022 doi: 10.1007/s40204-022-00198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raeisi S, Ojagh SM, Sharifi-Rad M, Sharifi-Rad J, Quek SY. Evaluation of Allium paradoxum (M.B.) G. Don. and Eryngium caucasicum trauve. Extracts on the shelf-life and quality of silver carp (Hypophthalmichthys molitrix) fillets during refrigerated storage. J Food Safety. 2017;37:e12321. doi: 10.1111/jfs.12321. [DOI] [Google Scholar]
- 79.Rehman A, Hashmi M, Tehseen Y, Khan A, Khan S, Iqbal J, Perveen S, Khan S, Farooq U, Ahmad V. Antidiabetic flavonol glycosides from Eryngium caeruleum. Rec Nat Prod. 2017;11:229–334. [Google Scholar]
- 80.Sadiq A, Ahmad S, Ali R, Ahmad F, Ahmad S, Zeb A, Ayaz M, Ullah F, Siddique AN. Antibacterial and antifungal potentials of the solvents extracts from Eryngium caeruleum, Notholirion thomsonianum and Allium consanguineum. BMC Complement Altern Med. 2016;16:478. doi: 10.1186/s12906-016-1465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saeedi M, Morteza-Semnani K. Effect of the essential oil of Eryngium caeruleum on percutaneous absorption of piroxicam through rat skin. J Essent Oil Bear Plants. 2008;11:485–495. doi: 10.1080/0972060X.2008.10643657. [DOI] [Google Scholar]
- 82.Saleh EAM, Khan AU, Tahir K, Almehmadi SJ, Al-Abdulkarim HA, Alqarni S, Muhammad N, Dawsari AMA, Nazir S, Ullah A. Phytoassisted synthesis and characterization of palladium nanoparticles (PdNPs); with enhanced antibacterial, antioxidant and hemolytic activities. Photodiagn Photodyn Ther. 2021;36:102542. doi: 10.1016/j.pdpdt.2021.102542. [DOI] [PubMed] [Google Scholar]
- 83.Salehi B, Prakash Mishra A, Nigam M, Karazhan N, Shukla I, Kiełtyka-Dadasiewicz A, Sawicka B, Głowacka A, Abu-Darwish MS, Hussein Tarawneh A, Gadetskaya AV, Cabral C, Salgueiro L, Victoriano M, Martorell M, Docea AO, Abdolshahi A, Calina D, Sharifi-Rad J. Ficus plants: state of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35:1187–1217. doi: 10.1002/ptr.6884. [DOI] [PubMed] [Google Scholar]
- 84.Salehi B, Quispe C, Chamkhi I, El Omari N, Balahbib A, Sharifi-Rad J, Bouyahya A, Akram M, Iqbal M, Docea AO, Caruntu C, Leyva-Gómez G, Dey A, Martorell M, Calina D, López V, Les F. Pharmacological properties of chalcones: a review of preclinical including molecular mechanisms and clinical evidence. Front Pharmacol. 2021;11:592654–592654. doi: 10.3389/fphar.2020.592654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Salehi B, Sharifi-Rad J, Capanoglu E, Adrar N, Catalkaya G, Shaheen S, Jaffer M, Giri L, Suyal R, Jugran AK, Calina D, Docea AO, Kamiloglu S, Kregiel D, Antolak H, Pawlikowska E, Sen S, Acharya K, Bashiry M, Selamoglu Z, Martorell M, Sharopov F, Martins N, Namiesnik J, Cho WC. Cucurbita plants: from farm to industry. Appl Sci. 2019;9:21. doi: 10.3390/app9163387. [DOI] [Google Scholar]
- 86.Sardarbandeh A, Delnavazi MR, Sharifzadeh M, Sharifzadeh M, Ghajarieh Sepanlou M, Tamiji Z, Sadati Lamardi SN. Beneficial hypoglycemic, hypolipidemic effects of aerial branches and roots extract of Eryngium caeruleum M. Bieb on streptozotocin-induced diabetes model in rats. Tradit Integr Med. 2021 doi: 10.18502/tim.v6i2.6779. [DOI] [Google Scholar]
- 87.Scheau C, Caruntu C, Badarau IA, Scheau AE, Docea AO, Calina D, Caruntu A. Cannabinoids and inflammations of the gut-lung-skin barrier. J Pers Med. 2021 doi: 10.3390/jpm11060494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sepanlou M, Ardakani M, Hajimahmoodi M, Sadrai S, Amin G, Sadeghi N, Lamardi S. Ethnobotanical and traditional uses, phytochemical constituents and biological activities of Eryngium species growing in Iran. Tradit Med Res. 2019;4:148–159. [Google Scholar]
- 89.Sepanlou MG, Salami F, Mirabzadeh Ardakani M, Sadati Lamardi SN, Sadrai S, Amin G-R, Sadeghi N, Hajimahmoodi M. The proximate, mineral and amino acid composition of spring, autumn leaves and roots of Eryngium caeruleum M.Bieb. Res J Pharmacogn. 2019;6:1–7. [Google Scholar]
- 90.Sharifi-Rad J, Cruz-Martins N, López-Jornet P, Lopez EP-F, Harun N, Yeskaliyeva B, Beyatli A, Sytar O, Shaheen S, Sharopov F, Taheri Y, Docea AO, Calina D, Cho WC. Natural coumarins: exploring the pharmacological complexity and underlying molecular mechanisms. Oxid Med Cell Longev. 2021;2021:6492346. doi: 10.1155/2021/6492346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, Goloshvili T, Cirone Silva NC, Bouyahya A, Vitalini S, Varoni EM, Martorell M, Abdolshahi A, Docea AO, Iriti M, Calina D, Les F, López V, Caruntu C. Cinnamomum species: bridging phytochemistry knowledge, pharmacological properties and toxicological safety for health benefits. Front Pharmacol. 2021;12:600139. doi: 10.3389/fphar.2021.600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharifi-Rad J, Herrera-Bravo J, Kamiloglu S, Petroni K, Mishra AP, Monserrat-Mesquida M, Sureda A, Martorell M, Aidarbekovna DS, Yessimsiitova Z, Ydyrys A, Hano C, Calina D, Cho WC. Recent advances in the therapeutic potential of emodin for human health. Biomed Pharmacother. 2022;154:113555. doi: 10.1016/j.biopha.2022.113555. [DOI] [PubMed] [Google Scholar]
- 93.Sharifi-Rad J, Herrera-Bravo J, Semwal P, Painuli S, Badoni H, Ezzat SM, Farid MM, Merghany RM, Aborehab NM, Salem MA, Sen S, Acharya K, Lapava N, Martorell M, Tynybekov B, Calina D, Cho WC. Artemisia spp.: an update on its chemical composition, pharmacological and toxicological profiles. Oxid Med Cell Longev. 2022;2022:5628601. doi: 10.1155/2022/5628601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sharifi-Rad J, Kamiloglu S, Yeskaliyeva B, Beyatli A, Alfred MA, Salehi B, Calina D, Docea AO, Imran M, Kumar NVA, Romero-Roman ME, Maroyi A, Martorell M. Pharmacological activities of psoralidin: a comprehensive review of the molecular mechanisms of action. Front Pharmacol. 2020;11:11. doi: 10.3389/fphar.2020.571459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharifi-Rad J, Quispe C, Bouyahya A, El Menyiy N, El Omari N, Shahinozzaman M, Ara Haque Ovey M, Koirala N, Panthi M, Ertani A, Nicola S, Lapava N, Herrera-Bravo J, Salazar LA, Changan S, Kumar M, Calina D. Ethnobotany, phytochemistry, biological activities, and health-promoting effects of the Genus Bulbophyllum. Evid Based Complement Altern Med. 2022;2022:6727609. doi: 10.1155/2022/6727609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sharifi-Rad J, Quispe C, Durazzo A, Lucarini M, Souto EB, Santini A, Imran M, Moussa AY, Mostafa NM, El-Shazly M, Sener B, Schoebitz M, Martorell M, Dey A, Calina D, Cruz-Martins N. Resveratrol’ biotechnological applications: enlightening its antimicrobial and antioxidant properties. J Herb Med. 2022;32:100550. doi: 10.1016/j.hermed.2022.100550. [DOI] [Google Scholar]
- 97.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Akram M, Abbaass W, Semwal P, Painuli S, Konovalov DA, Alfred MA, Kumar NVA, Imran M, Nadeem M, Sawicka B, Pszczółkowski P, Bienia B, Barbaś P, Mahmud S, Durazzo A, Lucarini M, Santini A, Martorell M, Calina D. Phytochemical constituents, biological activities, and health-promoting effects of the Melissa officinalis. Oxid Med Cell Longev. 2021;2021:6584693. doi: 10.1155/2021/6584693. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 98.Sharifi-Rad J, Quispe C, Herrera-Bravo J, Martorell M, Sharopov F, Tumer TB, Kurt B, Lankatillake C, Docea AO, Moreira AC, Dias DA, Mahomoodally MF, Lobine D, Cruz-Martins N, Kumar M, Calina D. A pharmacological perspective on plant-derived bioactive molecules for epilepsy. Neurochem Res. 2021;46:2205–2225. doi: 10.1007/s11064-021-03376-0. [DOI] [PubMed] [Google Scholar]
- 99.Sharifi-Rad J, Quispe C, Kumar M, Akram M, Amin M, Iqbal M, Koirala N, Sytar O, Kregiel D, Nicola S, Ertani A, Victoriano M, Khosravi-Dehaghi N, Martorell M, Alshehri MM, Butnariu M, Pentea M, Rotariu LS, Calina D, Cruz-Martins N, Cho WC. Hyssopus essential oil: an update of its phytochemistry, biological activities, and safety profile. Oxid Med Cell Longev. 2022;2022:8442734. doi: 10.1155/2022/8442734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sharifi-Rad J, Quispe C, Rahavian A, Pereira Carneiro JN, Rocha JE, Alves Borges Leal AL, Bezerra Morais Braga MF, Melo Coutinho HD, Ansari Djafari A, Alarcón-Zapata P, Martorell M, Antika G, Tumer TB, Cruz-Martins N, Helon P, Paprocka P, Koch W, Docea AO, Calina D. Bioactive compounds as potential agents for sexually transmitted diseases management: a review to explore molecular mechanisms of action. Front Pharmacol. 2021 doi: 10.3389/fphar.2021.674682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharifi-Rad J, Quispe C, Turgumbayeva A, Mertdinç Z, Tütüncü S, Aydar EF, Özçelik B, Anna S-W, Mariola S, Koziróg A, Otlewska A, Antolak H, Sen S, Acharya K, Lapava N, Emamzadeh-Yazdi S, Martorell M, Kumar M, Varoni EM, Iriti M, Calina D. Santalum Genus: phytochemical constituents, biological activities and health promoting-effects. Zeitschrift für Naturforschung C. 2022 doi: 10.1515/znc-2022-0076. [DOI] [PubMed] [Google Scholar]
- 102.Shcherbakova A, Konovalov DA, Eliseyeva LM. Biologicheskiye osobennosti sinegolovnika kavkazskogo i sinegolovnika ploskolistnogo. Molodyye uchenyye i farmatsiya XXI veka. 216;113–7.
- 103.Shcherbakova EA, Konovalov DA, Eliseyeva LM. Rezultaty izucheniya vozmozhnosti introduktsii sinegolovnika kavkazskogo i sinegolovnika ploskolistnogo v usloviyakh Kavkazskikh Mineralnykh Vod. Aspirantskiy vestnik Povolzhia. 2017;1–2:221–227. [Google Scholar]
- 104.Shcherbakova EA, Konovalov DA, Eliseyeva LM, Galkin MA. Nekotoryye biometricheskiye dannyye sinegolovnika kavkazskogo semeystva seldereynyye (Apiaceae). Razrabotka. issledovaniye i marketing novoy farmatsevticheskoy produktsii. 2015;94–6.
- 105.Sifaki M, Calina D, Docea AO, Tsioumas S, Katsarou M-S, Papadogiorgaki S, Fragkiadaki P, Branisteanu DE, Kouskoukis K, Tsiaoussis J, Spandidos DA. A novel approach regarding the anti-aging of facial skin through collagen reorganization. Exp Ther Med. 2020;19:717–721. doi: 10.3892/etm.2019.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Su Y, Qin W, Wu L, Yang B, Wang Q, Kuang H, Cheng G. A review of Chinese medicine for the treatment of psoriasis: principles, methods and analysis. Chin Med. 2021;16:138. doi: 10.1186/s13020-021-00550-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taheri Y, Jokovic N, Vitorovic J, Grundmann O, Maroyi A, Calina D. The burden of the serious and difficult-to-treat infections and a new antibiotic available: cefiderocol. Front Pharmacol. 2021;11:18. doi: 10.3389/fphar.2020.578823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Taheri Y, Quispe C, Herrera-Bravo J, Sharifi-Rad J, Ezzat SM, Merghany RM, Shaheen S, Azmi L, Prakash Mishra A, Sener B, Kiliç M, Sen S, Acharya K, Nasiri A, Cruz-Martins N, Tsouh Fokou PV, Ydyrys A, Bassygarayev Z, Daştan SD, Alshehri MM, Calina D, Cho WC. Urtica dioica-derived phytochemicals for pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2022;2022:4024331. doi: 10.1155/2022/4024331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsoukalas D, Buga AM, Docea AO, Sarandi E, Mitrut R, Renieri E, Spandidos DA, Rogoveanu I, Cercelaru L, Niculescu M, Tsatsakis A, Calina D. Reversal of brain aging by targeting telomerase: a nutraceutical approach. Int J Mol Med. 2021;48:1. doi: 10.3892/ijmm.2021.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Thanasoula M, Spandidos DA, Tsatsakis A, Razgonova MP, Calina D. Discovery of potent telomerase activators: unfolding new therapeutic and anti-aging perspectives. Mol Med Rep. 2019;20:3701–3708. doi: 10.3892/mmr.2019.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsoukalas D, Fragkiadaki P, Docea AO, Alegakis AK, Sarandi E, Vakonaki E, Salataj E, Kouvidi E, Nikitovic D, Kovatsi L, Spandidos DA, Tsatsakis A, Calina D. Association of nutraceutical supplements with longer telomere length. Int J Mol Med. 2019;44:218–226. doi: 10.3892/ijmm.2019.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsoukalas D, Zlatian O, Mitroi M, Renieri E, Tsatsakis A, Izotov BN, Burada F, Sosoi S, Burada E, Buga AM, Rogoveanu I, Docea AO, Calina D. A novel nutraceutical formulation can improve motor activity and decrease the stress level in a murine model of middle-age animals. J Clin Med. 2021;10:624. doi: 10.3390/jcm10040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Turmel J. Répartition géographique des Eryngium. I. Ancien Monde Bull Mus Hist Nat. 1948;20:395–401. [Google Scholar]
- 114.Vasilopoulos E, Fragkiadaki P, Kalliora C, Fragou D, Docea AO, Vakonaki E, Tsoukalas D, Calina D, Buga AM, Georgiadis G, Mamoulakis C, Makrigiannakis A, Spandidos DA, Tsatsakis A. The association of female and male infertility with telomere length (review) Int J Mol Med. 2019;44:375–389. doi: 10.3892/ijmm.2019.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang P, Su Z, Yuan W, Deng G, Li S. Phytochemical constituents and pharmacological activities of Eryngium L. (Apiaceae) Pharm Crops. 2012;3:99–120. doi: 10.2174/2210290601203010099. [DOI] [Google Scholar]
- 116.Wojciechowski VV, Calina D, Tsarouhas K, Pivnik AV, Sergievich AA, Kodintsev VV, Filatova EA, Ozcagli E, Docea AO, Arsene AL, Gofita E, Tsitsimpikou C, Tsatsakis AM, Golokhvast KS. A guide to acquired vitamin K coagulophathy diagnosis and treatment: the Russian perspective. Daru. 2017;25:10. doi: 10.1186/s40199-017-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wolff H. Umbelliferae-Saniculoideae. Das Pflanzenreich. 1913; 4.
- 118.Wörz A. On the distribution and relationships of the South-West Asian species of Eryngium L. (Apiaceae-Saniculoideae) Turk J Bot. 2004;28:85–92. [Google Scholar]
- 119.Wörz A. A new subgeneric classification of the genus Eryngium L. (Apiaceae, Saniculoideae) Bot Jahrb Syst. 2005;126:253–259. doi: 10.1127/0006-8152/2005/0126-0253. [DOI] [Google Scholar]
- 120.Zlatian O, Balasoiu AT, Balasoiu M, Cristea O, Docea AO, Mitrut R, Spandidos DA, Tsatsakis AM, Bancescu G, Calina D. Antimicrobial resistance in bacterial pathogens among hospitalised patients with severe invasive infections. Exp Ther Med. 2018;16:4499–4510. doi: 10.3892/etm.2018.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zlatian OM, Comanescu MV, Rosu AF, Rosu L, Cruce M, Gaman AE, Calina CD, Sfredel V. Histochemical and immunohistochemical evidence of tumor heterogeneity in colorectal cancer. Rom J Morphol Embryol. 2015;56:175–181. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Yes.