Abstract
Background:
Acute myeloid leukemia (AML) is fatal in elderly patients who are unfit for standard induction chemotherapy. We aimed to evaluate the survival benefit of administering sapacitabine, an oral nucleoside analogue, in alternating cycles with decitabine, a low-intensity therapy, in elderly patients with newly diagnosed AML.
Methods
This randomized, open-label, phase 3 study (“SEAMLESS”) was conducted at 87 sites in 11 countries. Patients age 70 years or older who were not candidates for or chose not to receive standard induction chemotherapy were randomized 1:1 to receive decitabine 20 mg/m2 administered intravenously daily × 5 days of a 4-week cycle (control arm) or in alternating cycles (odd cycle of the study arm) with sapacitabine 300 mg p.o. b.i.d. × 3 days/week × 2 weeks of a 4-week cycle (even cycles of the study arm). Prior hypomethylating agent (HMA) therapy for pre-existing myelodysplastic syndromes (MDS) or myeloproliferative neoplasms (MPN) was an exclusion criterion. Randomization was stratified by antecedent MDS or MPN, white blood cell count (<10,000 vs. ≥10,000) and bone marrow blast percentage (≥50% vs. < 50%). The primary endpoint was overall survival (OS). Secondary endpoints were the rate of complete remission (CR), complete remission with incomplete platelet count recovery (CRp), partial remission (PR), hematological improvement (HI), stable disease (SD) and corresponding durations, transfusion requirements, number of hospitalized days, and one-year survival. The trial is registered at clinicaltrials.gov (NCT01303796).
Results
Between October 2011 and December 2014, 482 patients were enrolled and randomized to receive decitabine administered in alternating cycles with sapacitabine (study arm, n=241) or decitabine monotherapy (control arm, n=241). Median OS was 5.9 months on the study arm versus 5.7 months on control arm (p=0.8902). The CR rate was 16.6% on the study arm and 10.8% on the control arm (p=0.1468). In patients with WBC <10,000 (n=321), median OS was higher on the study arm vs. control arm (8.0 months vs. 5.8 months, p=0.145), as was CR rate (21.5% vs. 8.6%, p=0.0017).
Conclusion
The regimen of decitabine administered in alternating cycles with sapacitabine was active but did not significantly improve OS compared to decitabine monotherapy. Subgroup analyses suggested that patients with baseline WBC <10,000 might benefit from decitabine alternating with sapacitabine with improved CR rate and the convenience of an oral drug; these findings should be prospectively confirmed.
Keywords: AML, sapacitabine, decitabine, hypomethylation, therapy
Precis:
The regimen of decitabine administered in alternating cycles with sapacitabine was active but did not significantly improve OS compared to decitabine monotherapy. Subgroup analyses suggested that patients with baseline WBC <10,000 might benefit from decitabine alternating with sapacitabine with improved CR rate and the convenience of an oral drug; these findings should be prospectively confirmed.
INTRODUCTION
Acute myeloid leukemia (AML) is a life-threatening disease characterized by accumulation of clonal neoplastic hematopoietic precursor cells and impaired normal hematopoiesis. If untreated, patients usually die of infection or bleeding in a matter of weeks. 1
AML occurs more commonly in the older population. The median age at diagnosis is 64 years in Europe and 68 years in the US. 2, 3 Standard therapy is intensive induction chemotherapy consisting of an anthracycline and cytarabine (ara-C). Despite CR rate of 40–50%, intensive induction chemotherapy does not benefit most older, and in particular elderly patients.4 The 5-year survival for AML patients was 46.6% for those aged less than 65 years but only 7.9% for those aged 65 or older.5 The poor outcomes of older patients are caused by patient- and disease-related factors. Advanced age, poor performance status, comorbidities, and organ dysfunction significantly decrease the tolerance of cytotoxic therapy. Antecedent myelodysplastic syndromes (MDS) or myeloproliferative neoplasms (MPN), high peripheral white blood cell count (WBC), cytogenetic risk, and certain genetic mutations such as TP53, diminish the efficacy of cytotoxic therapy.6
To address poor tolerance of intensive induction chemotherapy, the European Medicines Agency (EMA) approved decitabine in 2012 as a low-intensity therapy for patients age 65 years or above who are not considered candidates for standard intensive induction chemotherapy by assessment of their treating physicians. The approval was based on a 2.7-month improvement in median overall survival (7.7 vs. 5.0 months) on the decitabine arm versus a control arm of low-dose cytarabine or best supportive care in a randomized phase III study. Secondary endpoints of response rate, progression-free survival, and tertiary endpoint of event-free survival were also in favor of decitabine.2
Sapacitabine, 1-(2-C-cyano-2-deoxy- ß-D-arabino-pentafuranosyl)-N4-palmitoylcytosine, (also known as CYC682, CS-682) is a rationally designed deoxycytidine analogue with a unique mechanism of action.7 Following oral administration, sapacitabine is converted to 2-C-cyano-2-deoxy- ß(-D-arabino-pentafuranosyl) cytosine (CNDAC). After phosphorylation to the triphosphate form and incorporation into DNA, replication is not inhibited at cytotoxic concentrations in contrast to cytarabine and clofarabine. Instead, after further polymerization, the strong electrophilic properties of the cyano group of CNDAC cause a rearrangement of the nucleotide to a form that lacks a 3’-hydroxyl moiety. This results in a single-strand DNA break that is repaired only to a small extent by the transcription-coupled nucleotide excision pathway. On a subsequent round of DNA replication unrepaired single-strand DNA breaks are converted to double strand breaks, causing cell death.8, 9 The palmitoyl side chain on CNDAC allows for improved oral absorption of sapacitabine and protects the N4 amino group from deamination, which is a major route of inactivation for other nucleoside analogues, such as cytarabine, azacitidine, decitabine and gemcitabine.10
Sapacitabine demonstrated single- agent activity in relapsed or refractory AML with a well-tolerated safety profile.11 Among 35 patients with relapsed or refractory AML enrolled on a phase 1 study of sapacitabine, 8 (23%) patients responded with 3 CR, 2 CRp and 3 CRi. All 8 patients had been previously treated with other nucleoside analogues such as cytarabine, decitabine, clofarabine or fludarabine. A follow-on, large randomized phase II study of single-agent sapacitabine evaluated 3 different dosing schedules in elderly patients age 70 years or over with newly diagnosed AML and established the schedule of sapacitabine administered orally twice daily for 3 days each week for 2 weeks, for a 28-day cycle as the schedule with the better efficacy profile.12 To minimize the overlapping toxicities of myelosuppression, a pilot study was designed to evaluate decitabine administered in alternating cycles with sapacitabine in the same elderly AML patient population. Among 23 patients treated with this regimen, 8 (35%) responded with 3 CRs, 3 PRs and 2 major hematological improvements in platelets.13
This phase 3 study was designed to evaluate the survival benefit of decitabine administered in alternating cycles with sapacitabine versus decitabine monotherapy in elderly patients with newly diagnosed AML.
METHODS
Study Design and Participants
This was a randomized, open-label, global phase 3 study conducted in 13 countries after approval by institutional review board (IRB) or ethics committee (EC). All patients provided written informed consent form in accordance with institutional guidelines at participating centers and the ethical principles of the Declaration of Helsinki.
Eligible patients were age 70 years or over with newly diagnosed AML and considered unsuitable candidates for intensive induction chemotherapy by assessment of their treating physician. Patients who were suitable candidates but unwilling to undergo induction chemotherapy could also participate in the study. Patients who had chemotherapy (except hydroxyurea) or hypomethylating agents for pre-existing MDS or MPN were excluded.
Other eligibility criteria included: Eastern Cooperative Oncology Group (ECOG) performance status 0–2; adequate hepatic function (bilirubin ≤ 1.5 × upper limit of normal (ULN) and alanine aminotransferase (ALT) ≤ 2 × ULN); adequate renal function (creatinine ≤ 1.5 × ULN). Exclusion criteria included acute promyelocytic leukemia (APL) or extramedullary myeloid tumor without bone marrow involvement, suspected or know central nervous system involvement by leukemia, uncontrolled illnesses including symptomatic congestive heart failure, unstable angina pectoris, cardiac arrhythmia, cancer requiring systemic therapy in the past 6 months, infection or HIV. Patients receiving intravenous antibiotics were allowed if infections were under adequate control.
This study initially included a sapacitabine monotherapy arm (Arm B) which was removed after a pilot study suggested that decitabine alternating with sapacitabine might be the best possible experimental arm because its sixty-day mortality rate of 12% was lower than that of single agent sapacitabine observed in the phase 2 study and those reported in the literature for intensive induction therapy, including clofarabine, decitabine, azacitidine, or low dose cytarabine. The protocol was amended after receiving agreement from the US Food and Drug Administration (FDA) according to the Special Protocol Assessment (SPA) procedure.
Randomization and Masking
There was a lead-in phase to confirm the safety and tolerability of the treatment regimen of decitabine alternating with sapacitabine prior to opening the randomization phase.14 Lead-in patients were not randomized and hence not counted in the intent-to-treat population.
Randomization was implemented at the International Drug Development Institute (IDDI, Louvain-la-Neuve, Belgium) using a fully validated Interactive Web-based Randomization Service (IWRS). Patients were randomized centrally to one of the treatment arms by the method of permuted blocks using the following stratification factors: presence of antecedent MDS or MPN (Yes vs. No), baseline peripheral white blood cells (<10 × 109/L vs. ≥ 10 × 109/L) and baseline bone marrow blast percentage (≥ 50% vs. < 50%). These stratification factors were chosen because they were reported to be prognostic factors for survival in AML patients.15, 16 As this was an open-label study, the investigators and patients were not masked.
Procedures
Treatments were administered in 28-day cycles. Patients assigned to the study arm of decitabine in alternating cycles with sapacitabine (Arm A) received 1-hour intravenous infusions of decitabine 20 mg/m2 once a day for five consecutive days every 8 weeks (first cycle and subsequent odd cycles); sapacitabine 300 mg b.i.d. × 3 consecutive days/week × 2 weeks every 8 weeks (second cycle and subsequent even cycles). Patients assigned to the control arm (Arm C) receive 1-hour infusions of decitabine 20 mg/m2 once a day for five consecutive days every 4 weeks.
Dosing on Day 1 of each treatment cycle and sapacitabine dosing on Day 8 of a sapacitabine treatment cycle did not start until clinically significant and drug-related non-hematologic toxicities had resolved to ≤ grade 1 or baseline. After recovery, a dose reduction of sapacitabine was required for grade 3–4 drug-related non-hematologic toxicities caused by sapacitabine. Dose reductions of sapacitabine for hematological toxicities were guided by findings from bone marrow and time to absolute neutrophil count (ANC) and platelet count recovery. A dose reduction of 50 mg twice daily was required for a delay in blood count recovery to best level on study beyond day 42 if bone marrow blasts decreased 25% or more from baseline but remained more than 10%. If blasts were less than 10%, dose reduction of 100 mg twice daily was required for persistent cytopenias. In addition, temporary dose reduction of sapacitabine was allowed for grade 2 toxicity in a frail patient.
Decitabine dose reduction was guided by commercial label or package insert approved by regulatory agencies.
Patients could continue treatment indefinitely as long as there was no evidence of clinically significant AML progression. After discontinuation from treatment, patients were contacted by the study staff for survival status approximately every 3 months.
Bone marrow biopsy and/or aspirate were performed at baseline, prior to starting cycle 2 and as clinically indicated thereafter.
Adverse events were graded according to National Cancer Institute Common Terminology for Adverse Events (CTCAE) version 4.0 and the relationship to decitabine or sapacitabine were determined by investigators. Safety was assessed by 30-day mortality rate, adverse events, serious adverse events (SAEs), and overall survival.
Outcomes
The primary endpoint was overall survival (OS), measured from the date of randomization to the date of death or censored at the last follow-up date when patients were known to be alive. Secondary endpoints were the rate of complete remission (CR), complete remission with incomplete platelet count recovery (CRp), partial remission (PR), hematological improvement (HI), stable disease (SD) and corresponding durations, transfusion requirements, number of hospitalized days, and one-year survival.
A CR was defined as normalization of the blood and marrow with 5% or fewer marrow blasts, independence of transfusions, a granulocyte count of 1 × 109/L or greater, and a platelet count of 100 × 109/L or greater.17, 18 A PR was defined by the same blood count as CR but with at least 50% decrease in marrow blasts to a level of 6% or more. A CRp was defined the same as CR but without platelet count recovery to 100 × 109/L or greater. Hematologic improvement (HI) was defined according to the International Working Group criteria.19 Stable disease was defined as no evidence of clinically significant progression for over 16 weeks without achieving at least HI. Transfusion requirement for each patient was defined as the number of units of packed red blood cells (PRBC) and/or platelet transfusions administered per 8-week period prior to the first dose of study drug and through the date of treatment discontinuation. Hospitalized days were days spent in the hospital for receiving decitabine or sapacitabine and/or the treatment of a medical condition regardless of its relationship to study drugs.
Survival analysis was performed in subgroups of patients with de novo AML vs those with antecedent MDS or MPN; baseline WBC ≥10 × 109/L vs. WBC <10 × 109/L; baseline bone marrow blast percentage ≥50% vs.<50%; unfavorable cytogenetics risk by SWOG 17 vs. those without unfavorable cytogenetic risk. These subgroups were selected because differences in treatment outcomes have been reported in the literature. 15,16
The following baseline patient and disease characteristics, which might be potentially related to survival, were selected as covariates for exploratory analysis of OS: age, ECOG performance status, treatment choice of low-intensity therapy as recommended by the investigator, significant concomitant medical illness measured by Hematopoietic Cell Transplantation-Comorbidity Index (HCTCI) 20 score, type of AML, time since AML diagnosis, peripheral WBC, absolute neutrophil count, platelet count, hemoglobin level, bone marrow blast percentage, bone marrow cytogenetic risk by SWOG, units of PRBC transfused, and units of platelets transfused.
Statistical Analysis
The planned sample size was 485 patients, about 243 per arm over an estimated accrual period of 24 months, requiring ≥424 events to detect a 27.5% reduction in the risk of death with ≥90% power and a significance level of 0.0249 (one-sided). The median survival was assumed to be 8 months on Arm C. An interim analysis was planned when approximately 212 deaths were observed. A Pampallona-Tsiatis boundary with power equal to 0.2 was used for the interim analysis. The boundary for futility would be reached if the P-value of the one-sided test comparing the overall survival of Arm A vs. Arm C was greater than 0.287, i.e., hazard ratios larger than 0.926 or a benefit of less than 0.6 month in median survival.21, 22
To prevent premature early termination, the data safety monitoring board (DSMB) was guided by a conservative criterion requiring a (one-sided) p-value < 0.0001 for extreme evidence of superiority of Arm A relative to Arm C on overall survival while monitoring the trial.
The intent-to-treat (ITT) population consisted of all randomized patients. The primary analysis compared overall survival between Arm A and Arm C in the ITT population. The safety population comprised all patients who had received at least one dose of sapacitabine or decitabine. Overall survival (OS) was measured from the date of randomization to the date of death. Patients alive at study closure were censored at the last follow-up date when they were known to be alive. The distribution of overall survival and one-year survival was estimated by the method of Kaplan and Meier. A log-rank analysis stratified by the presence of antecedent MDS or MPN (Yes vs. No), baseline peripheral white blood cells (<10 × 109/L vs. ≥ 10 × 109/L) and baseline bone marrow blast percentage (≥ 50% vs. < 50%) was used to compare OS between Arm A and Arm C.
The response rates of CR, CRp, PR, HI or SD were compared between the two arms using Fisher’s exact test. The mean number of transfusion-free weeks and mean number of units of PRBC and platelet transfusions were compared between the two arms using the two-sample Wilcoxon test. The mean number of hospitalized days was compared between the two treatment arms using the Wilcoxon test. Days alive and out of hospital over the first 90, 180, 240 and 360 days post randomization while on study for each patient were compared between arms using the Wilcoxon test. The percentage of days alive and out of hospital was defined as the number of days alive and out of hospital divided by the number of days alive on study for each patient was also compared among the two treatment arms at the above time points.
A multivariate Cox proportional hazard model was used for survival analysis in subgroups. The SIDES methodology was used in the exploratory analysis of predictive factors for survival. The optimal cutoffs for each covariate were based on the standard differential-effect slitting criterion which aimed at maximizing the difference between the test statistics in the subgroups associated with a particular split. 23 A two-sided p value of less than 0.1 was used to select the significant factors to be included in the multivariate analyses. A two-sided p-value of less than 0.05 was considered significant. Statistical computations were done with SAS 14.1.
Role of funding source
The sponsor of the study, HK, and MB designed the trial. Clinical data were collected by the investigators who had full access to raw data of their sites. Data were analyzed and interpreted by the sponsor and authors. The corresponding author (HK) had full access to the data and the final responsibility for the decision to submit for publication.
RESULTS
Between October 2011 and December 2014, 482 patients were randomized to receive decitabine administered in alternating cycles with sapacitabine (Arm A) or decitabine monotherapy (Arm C) at 87 sites in 11 countries.
At the planned interim analysis for futility in December 2014, the DSMB found that the planned futility boundary was crossed after 247 events had occurred and it would be unlikely for the study to reach statistically significant improvement in survival. The DSMB found no safety concerns in 470 randomized patients and recommended that all recruited patients stay on their assigned treatment to complete the study. Enrollment to study was stopped shortly after the DSMB meeting.
The primary analysis of OS was based on 424 projected deaths. There were 444 deaths at the time of clinical data cut-off in June 2017 which was approximately 2.5 years after the last patient was randomized in December 2014.
The efficacy analysis was based on the ITT population of 241 patients randomized to Arm A and 241 to Arm C. Thirteen patients did not receive treatment, 5 on Arm A and 8 on Arm C. Safety analysis was based on 469 patients, 236 on Arm A and 233 on Arm C.
Patient characteristics were similar between treatment arms in the ITT except that there were more patients aged 80 or older on Arm A than on Arm C (Table 1). Disease characteristics were similar between the treatment arms (Table 2).
Table 1.
Patient Characteristics
| Arm A - ITT (Arm A: Decitabine/sapacitabine, n=241) |
Arm C - ITT (Arm C: Decitabine, n=241) |
|
|---|---|---|
| Age (years), median | 78 (70, 92) | 77 (70, 92) |
| 70–74 | 77 (32.0%) | 70 (29.0%) |
| 75–79 | 69 (28.6%) | 99 (41.1%) |
| ≥80 | 95 (39.4%) | 72 (29.9%) |
| Gender | ||
| Male | 139 (57.7%) | 146 (60.6%) |
| Female | 102 (42.3%) | 95 (39.4%) |
| ECOG | ||
| 0–1 | 185 (76.8%) | 172 (71.4%) |
| 2 | 48 (19.9%) | 58 (24.1%) |
| HCTCI* | ||
| 0–2 | 124 (51.5%) | 129 (53.5%) |
| ≥ 3 | 117 (48.5%) | 112 (46.5%) |
| Low-intensity therapy recommended by investigator |
223 (92.5%) | 219 (90.9%) |
Hematopoietic Cell Transplantation-Comorbidity Index 20
Table 2.
Disease Characteristics
| Arm A – ITT (Arm A: Decitabine/sapacitabine, n=241) |
Arm C- ITT (Arm C: Decitabine, n=241) |
|
|---|---|---|
| Type of AML | ||
| De novo | 163 (67.6%) | 154 (63.9%) |
| Preceded by AHD | 66 (27.4%) | 70 (29%) |
| Treatment-related | 12 (5%) | 17 (7.1%) |
| WBC | ||
| <10 × 109/L | 157 (65.1%) | 162 (67.2%) |
| ≥ 10 × 109/L | 84 (34.9%) | 79 (32.8%) |
| Bone marrow blasts | ||
| < 50%). | 123 (51.0%) | 131 (54.4%) |
| ≥ 50% | 118 (49.0%) | 110 (45.6%) |
| Cytogenetic Risk (SWOG) | ||
| Favorable | 6 (2.5%) | 2 (0.9%) |
| Intermediate | 120 (49.8%) | 129 (53.5%) |
| Unfavorable | 100 (41.5%) | 94 (39%) |
| Unknown | 1 (.4%) | 0 |
| Fail to grow/Not done or Missing | 14 (5.8%) | 16 (6.6%) |
Survival
At the time of the final analysis, 444 patients had died: 226 on Arm A and 218 on Arm C. The median OS in the ITT population was 5.9 months in Arm A (95% CI: 4.7 to 8 months) versus 5.7 months in Arm C (95% CI: 4.9 to 8.2 months) which did not reach statistical significance (Figure 1). One-year survival was similar between the arms, 33.6% on Arm A (95% CI: 27.7% to 39.6%) and 34.7% on Arm C (95% CI: 28.8% to 40.8%).
Figure 1.
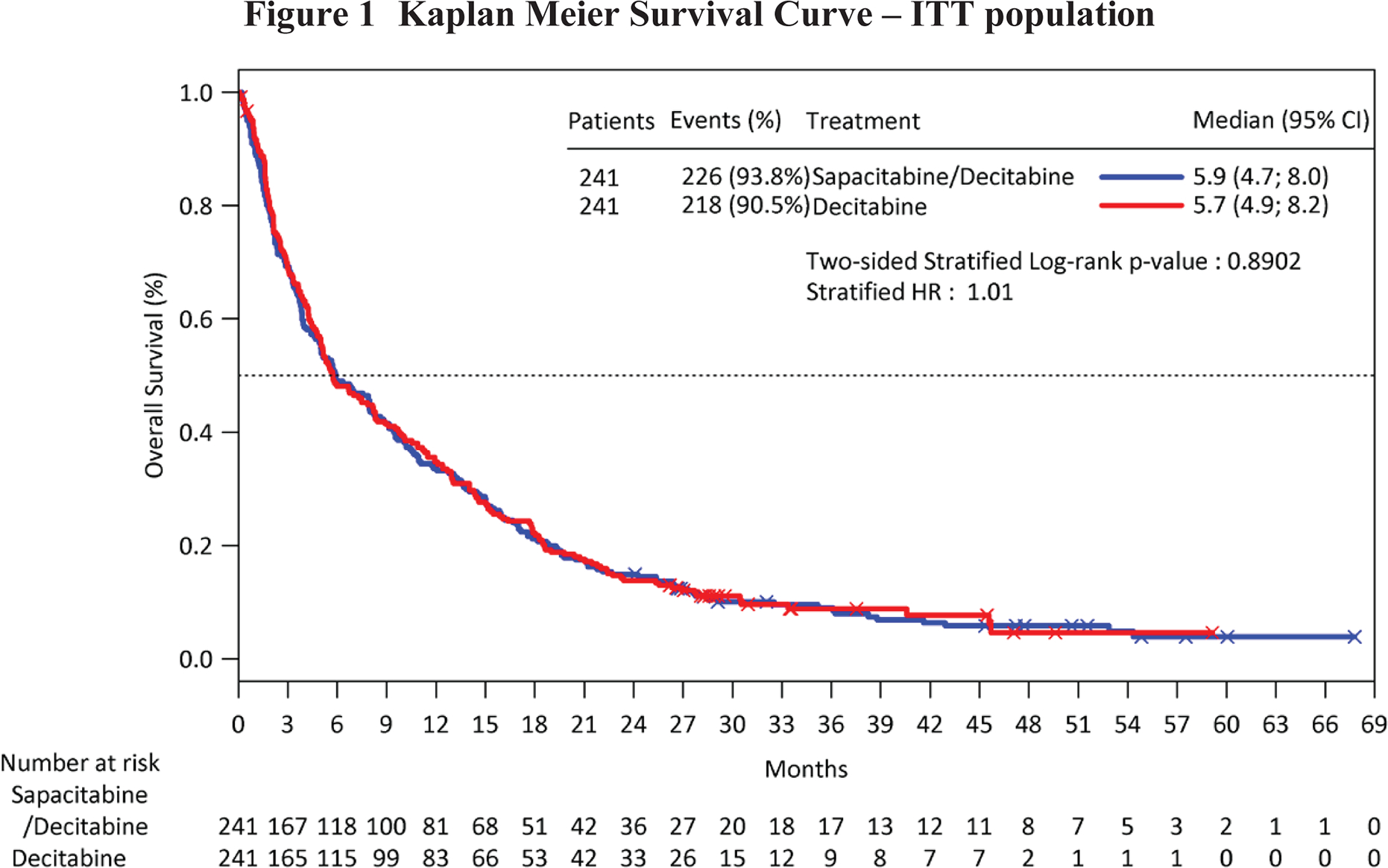
Kaplan Meier Survival Curve – ITT population
In an exploratory subgroup analysis using a multivariate Cox proportional hazard model, a trend of improved survival favoring Arm A was observed in patients with peripheral WBC <10,000. The opposite was observed in the subgroup of WBC ≥10,000, where longer survival was observed on Arm C (Figure 2)
Figure 2.
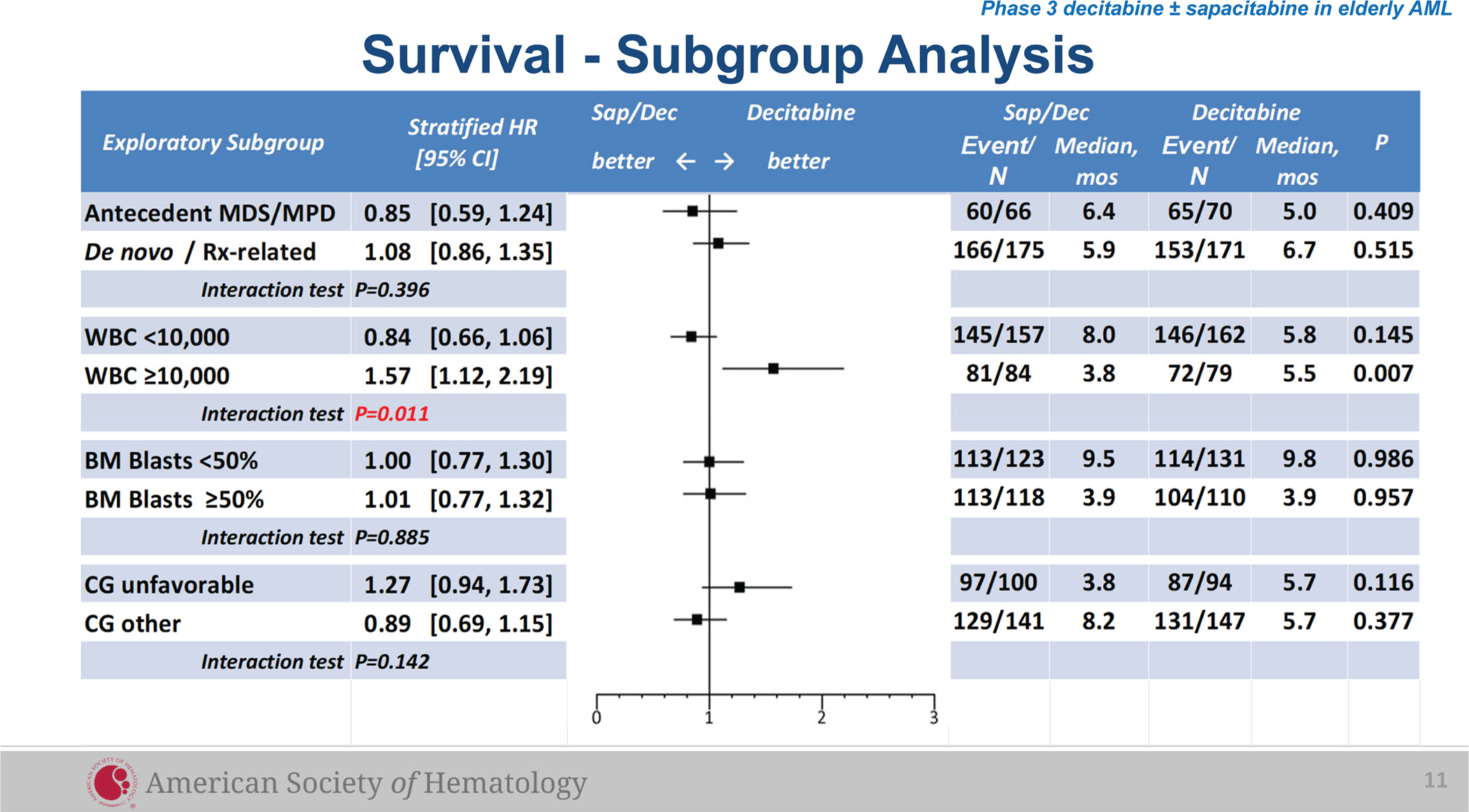
Survival Analysis in Subgroups
Responses
Forty patients achieved CR on Arm A (16.6%; 95% CI: 12.1% to 21.9%) and 26 achieved CR on Arm C (10.8%, 95% CI: 7.2% to 15.4%). The difference did not reach statistical significance. The median time to response was 2.6 months on Arm A and 3.4 months on Arm C. The 10.8% CR rate on Arm C was consistent with that reported in the DACO-016 study (15.7%) considering that this study enrolled more patients who were ≥80 years old and included patients with WBC >40,000 who were excluded from the DACO-016 study (Table 3).
Table 3.
Response Rate and Duration
|
Arm A – ITT (Arm A: Decitabine/sapacitabine, n=241) |
Arm C- ITT (Arm C: Decitabine, n=241) |
|||
| CR: [95% CI] Time to response, median Duration, median [95% CI] |
16.6% [12.1%, 21.9%]: p=0.1468 2.6 months 9.5 months [6.1, 13.6] |
10.8% [7.2%, 15.4%] 3.4 months 10.4 months [8.1, 14.0] |
||
| CRp: [95% CI] Time to response, median Duration, median [95% CI] |
2.1% [0.7%, 4.8%] 4.9 months 9.5 months [3.1, 20.7] |
2.1% [0.7%, 4.8%]: 4.5 months 5.7 months [3.0, 12.5] |
||
| PR: [95% CI] Time to response, median Duration, median [95% CI] |
5.0% [2.6%, 8.5%] 2.1 months 2.2 months [1.2, 9.9] |
3.3% [1.4%, 6.4%] 1.4 months 1.9 [0.5, 9.8] |
||
| HI: [95% CI] Time to response, median Duration, median [95% CI] |
17.0% [12.5%, 22.4%] 1.3 months 5.8 months [2.7, 17.0] |
15.8% [11.4%, 21.0%] 2.3 months 4.8 months [3.4, 7.2] |
||
| SD: [95% CI] Duration, median [95% CI] |
8.7% [5.5%, 13.0%] 23.3 months [9.1, 33.2] |
12.9% [8.9%, 17.8%] 14.8 months [10.6, absent] |
||
|
Arm A -WBC
<10,000 (n=157) |
Arm C - WBC
<10,000 (n=162) |
Arm A - WBC
≥10,000 (n=84) |
Arm C - WBC
≥10,000 (n=79) |
|
| CR: [95% CI] ; Time to response, median Duration, median [95% CI] |
21% [14.9%, 28.2%] P=0.0017 3.0 months 12.9 months [6.9, 16.4] |
8.6% [4.8%, 14.1%] 3.4 months 10.4 months [5.8, 22.8] |
8.3% [3.4%, 16.4%] 1.9 months 4.7 months [1.1, absent] |
15.2% [8.1%, 25%] P=0.1819 3.2 months 10.1 months [1.6, 13.1] |
| CRp Time to response, median Duration, median |
3.2% 4.9 months 9.5 months |
1.9% 1.8 months 7.7 months |
0% |
2.5% 4.5, 16 months 3.0, 5.7 months |
| PR: Time to response, median Duration, median |
3.8% 1.7 months 2.2 months |
2.5% 1.0 months 1.2 months |
7.1% 2.1 months 3.3 months |
5.1% 2.8 months 1.9 months |
| HI Time to response, median Duration, median |
18.4% 1.3 months 4.4 months |
15.4% 2.8 months 4.7 months |
14.3% 1.8 months 5.8 months |
16.5% 1.1 months 6.2 months |
| SD: [95% CI] Duration, median |
7.0% 33.2 months |
14.8% 14.8 months |
11.9% 11.0 months |
8.9% Non-estimable |
CR=complete remission. CRp=complete remission with incomplete platelet recovery. PR=partial remission. HI=hematological improvement. SD=stable disease. CI=confidence interval. ITT=intent to treat. WBC=white blood cell count.
In the WBC <10,000 subgroup, significantly more CRs occurred on Arm A compared to Arm C while the opposite was observed in the WBC ≥10,000 subgroup, consistent with the trends of OS in these subgroups (Table 3).
Transfusion and hospitalization
Transfusion and hospitalization requirements for patients who received at least one dose of study drug were similar between treatment arms (Table 4).
Table 4.
Transfusion and Hospitalization Requirements
| Arm A (Decitabine/sapacitabine, n=236) |
Arm C (Decitabine, n=233) |
|
|---|---|---|
| Average number of RBC units transfused per week while on treatment, median | 0.8 | 0.8 |
| Average number of platelets units transfused per week while on treatment, median | 0.3 | 0.2 |
| Transfusion-free weeks, median | 13 weeks | 12.3 weeks |
| Percentage Days Alive and out of Hospital While on Treatment, median | ||
| First 90 days | 83.3% | 81.1% |
| First 180 days | 86.4% | 83.3% |
| First 240 days | 86.9% | 83.3% |
| First 360 days | 87.8% | 84.0% |
Predictive Factors for Overall Survival and Response Rate
An exploratory analysis using the SIDES methodology23 found that patients with peripheral WBC< 4,100, SWOG risk of favorable, intermediate or unknown or HCTCI score ≤2 benefitted the most by being treated with decitabine administered in alternating cycles with sapacitabine as measured by OS, one-year survival and rate of CR/CRp.
Toxicity
Two hundred thirty-six patients on Arm A and 233 on Arm C received a median of 3 cycles. The median duration of treatment was 3.5 months for Arm A and 3.3 months for Arm C with 16.9% patients on Arm A and 15.5% on Arm C having received ≥12 cycles. Dose reduction for decitabine was similar on both arms. 18.2% of patients on Arm A had dose reduction for sapacitabine.
Four hundred sixty-eight patients (99.8%) reported at least one adverse event (AE). The most common grade 3 or 4 AE regardless of causalities were similar between the arms (Table 5).
Table 5.
Adverse Events of Grades 3 or 4 in ≥10% of Patients
| Arm A (Decitabine/sapacitabine, n=236) |
Arm C (Decitabine, n=233) |
|
|---|---|---|
| No of patients with at least one grade 3 or 4 TEAE | 205 (86.7%) | 213 (91.4%) |
| Hematological | ||
| Anemia | 114 (48.3%) | 103 (44.2%) |
| Neutropenia | 105 (44.5%) | 87 (37.3%) |
| Febrile neutropenia | 62 (26.3%) | 62 (26.6%) |
| Thrombocytopenia | 122 (51.7%) | 120 (51.5%) |
| Non-hematological | ||
| Pneumonia | 63 (26.7%) | 70 (30.0%) |
| Sepsis/Septic shock | 20 (8.5%) | 2 6 (11.2%) |
| Hyponatremia | 14 (5.9%) | 25 (10.7%) |
One patient could have multiple grade 3 or 4 TEAE; grade is of the worst severity regardless of cycles.
The most common serious adverse events were pneumonia (Arm A 26.7%, Arm C 27.9%), febrile neutropenia (Arm A 20.8%, Arm C 22.7%), sepsis or septic shock (Arm A 16.9%, Arm C 15.9%) and disease progression (Arm A 13.1%, Arm C 8.2%). Among 199 patients who had at least one SAE on Arm A, 44 only received a first cycle of decitabine and never received sapacitabine (Table 6).
Table 6.
Serious Adverse Events (SAE) in ≥5% Patients
| Arm A (Decitabine/sapacitabine, n=236) |
Arm C (Decitabine, n=233) |
|
|---|---|---|
| No of patients with at least one SAE | 199 (84.3%) | 188 (80.7%) |
| Anemia | 11 (4.7%) | 14 (6.0%) |
| Febrile neutropenia | 49 (20.8%) | 53 (22.7%) |
| Cellulitis | 10 (4.2%) | 11 (4.7%) |
| Pneumonia | 63 (26.7%) | 65 (27.9%) |
| Sepsis/Septic shock | 40 (16.9%) | 37 (15.9%) |
| Disease Progression | 31 (13.1%) | 19 (8.2%) |
The first cycle of treatment was decitabine on both arms. Twenty-one patients randomized to Arm A (8.9%) and 18 randomized to Arm C (7.7%) died within thirty days. Sixty-day mortality was 22.0% on Arm A and 20.6% on Arm C.
Eighty-five patients (36%) treated on Arm A and 57 (24.5%) treated on Arm C had adverse events with an outcome of death during treatment or within 28 days after last dose of study drug. Among 85 patients who died from adverse event on Arm A, 30 only received decitabine during the first cycle and did not receive sapacitabine (Table 7).
Table 7.
Adverse Events with Outcome of Death
| Arm A (Decitabine/sapacitabine, n=236) |
Arm C (Decitabine, n=233) |
|
|---|---|---|
| AE with outcome of death | 36% (12.7%*) | 24.5% |
| Disease progression | 12.3% (2.1%*) | 7.7% |
| Pneumonia | 2.5% (1.3%*) | 1.7% |
| Sepsis/septic shock | 9.7% (4.2%*) | 4.7% |
| Others | 11.4% (5.1%*) | 11.1% |
Died after receiving only decitabine in the first cycle.
DISCUSSION
This is the first large randomized, controlled, phase 3 trial designed to evaluate survival benefit of an oral drug, sapacitabine, given in alternating cycles with a best available standard of care therapy of an intravenous drug in elderly patients with newly diagnosed AML who were unfit for or refused intensive induction therapy. In the intent-to-treat population, the study arm of decitabine/sapacitabine with decitabine given in the first and subsequent odd cycles and oral sapacitabine in the second and subsequent even cycles did not reach statistically significant improvement in OS versus the control arm of decitabine monotherapy (median 5.9 months versus 5.7 months, p=0.8902). Complete remission rate was 16.6% on the study arm versus 10.8% on the control arm (p=0.15). Median durations of CR were similar between the two arms.
The study arm of decitabine/sapacitabine was well tolerated. Median number of treatment cycles was similar between the two arms; 16.9% of patients on decitabine/sapacitabine received at least 12 cycles versus 15.5% on the control arm. Grade 3 or 4 adverse events (regardless of causality) for the study arm were similar to those for the control arm and were consistent with the known safety profile of decitabine and sapacitabine. Eighty-five patients (36%) treated with decitabine/sapacitabine and 57 (24.5%) treated with decitabine monotherapy had adverse events with an outcome of death during treatment or within 28 days after the last dose. Among 85 patients randomized to receive decitabine/sapacitabine who died from treatment-emergent adverse event, 30 received only decitabine during the first cycle, and did not receive sapacitabine suggesting the presence of heterogeneity in patient and disease characteristics despite the use of stratification factors for randomization.
The strength of this study is the randomized assignment to 2 treatment arms with the control arm being the best available treatment in current clinical practice. The limitation of the study is the open-label design, which is necessary when the experimental treatment is an oral drug given in alternating cycles with an intravenously infused drug. The median OS of 5.7 months on the control arm of decitabine monotherapy was lower than the median OS of 7.7 months reported in the phase 3 decitabine study (DACO-016), possibly because of differences in patient populations shown in Table 8. This study had more patients age 75 years or older. Such patients had lower median OS (6.3 months) in the DACO-016 study. 24 In addition, this study included patients with WBC >40,000 who were excluded from DACO-016. Patients with proliferative AML (WBCs >10,000) are known to have worse outcomes. 15
Table 8.
Patients Randomized to Decitabine - DACO-016 and CYC682–12
| Decitabine Arm from DACO-016 n=242 |
Decitabine Monotherapy Arm from CYC682–12 n=241 |
|
|---|---|---|
| Age, median years (range) | 73 (64 – 89) | 77 (70 – 92) |
| 70–74 | 76 (31.4%) | 70 (29.0%) |
| 75–79 | 65 (26.9%) | 99 (41.1%) |
| ≥80 | 30 (12.4%) | 72 (29.9%) |
| WBC, median | 3.1 | 3.6 |
| 40,000 | Excluded from trial (eligibility criteria) | 29 (12.0%) |
It appears that the decitabine/sapacitabine arm performed better in patients with low peripheral WBC. In the less than 10,000 WBC subgroup (n=319) a trend towards improved overall survival (median 8.0 versus 5.8 months, HR=0.84 [0.66, 1.06], p=0.14) and a significantly higher CR rate (21.0% versus 8.6%, p=0.0017) was observed in patients who were randomized to the decitabine/sapacitabine arm. The opposite was observed in the subgroup of WBC ≥10,000 (n=163) where longer survival (median 5.8 versus 3.8 months, HR=1.57 [1.12, 2.19], p=0.007) and a trend toward higher CR rate (15.2% versus 8.3%, p=0.18) was observed on the decitabine monotherapy arm.
Decitabine dose density has been known to influence CR rate and median OS. In the phase 2 study of single-agent decitabine administered at 20 mg/m2/day for 5 days every 4 weeks, the CR rate was 24% and median OS 7.7 months.25 In the phase 2 study of decitabine administered at 20 mg/m2/day for 10 days every 4 weeks CR rate was 47% and median OS 13 months.26 It is possible that for highly proliferative disease i.e., high peripheral WBC, the dose density of decitabine must be at least 20 mg/m2/day × 5 days every 4 instead of every 8 weeks in order to control the disease. Treatment effect heterogeneity was further explored using the SIDES methodology. The optimal cut-off points for peripheral WBC, SWOG risk and HCTCI scores were identified which could be used to design future studies.
In conclusion, results of this large, multicenter, global study demonstrated that the regimen of decitabine administered in alternating cycles with sapacitabine was active and well tolerated but did not significantly improve overall survival as compared to decitabine monotherapy. Subgroup analyses suggested that patients with baseline WBC <10,000 might benefit from the regimen of decitabine alternating with sapacitabine which improved CR rate and had greater convenience of an oral drug. For patients with proliferative AML (WBCs ≥10,000), delivery of higher dose density of decitabine by concomitant administration of decitabine and sapacitabine should be considered.
On July 7, 2020, the Food and Drug Administration approved an oral combination of decitabine and cedazuridine (INQOVI, Astex Pharmaceuticals, Inc.) for adult patients with MDS based on decitabine exposure equivalence between oral combination and intravenous decitabine.27 The availability of oral decitabine administration may facilitate future development of an entirely oral treatment regimen for elderly patients with AML allowing them to enjoy good quality of life at home without being burdened with the inconveniences associated with intravenous infusions.
Acknowledgments
Study support: Cyclacel Limited, Dundee, Scotland, UK
Funding:
Cyclacel Limited is the sponsor of this study
Footnotes
Conflict of Interest Statement
Hagop M. Kantarjian reports research grants and honoraria from AbbVie, Amgen, Ascentage, BMS, Daiichi-Sankyo, Immunogen, Jazz, Novartis, Pfizer and Sanofi; honoraria from Actinium (Advisory Board), Adaptive Biotechnologies, Aptitude Health, BioAscend, Delta Fly, Janssen Global, Oxford Biomedical and Takeda. Stephen Strickland reports AbbVie, ArcherDx, Genentech, Incyte, Kura Oncology, Novartis, Pfizer, and Syros; Research Funding (paid t institution): Sunesis. Marc Buys reports being a shareholder of IDDI (International drug Development Institute, Belgium). Tapan M. Kadia reports consulting fees from AbbVie, Agios, Daiichi Sankyo, Genetech,Jazz, Liberum, Novartis, Phizer, Sanofi-Aventis; Grant Research Support from AbbVie, Amgen, BMS, Genetech, Jazz, Pfizer, Pulmotech, Cellenkos, Ascentage, Genfleet, Astellas, Astrazeneca; Speaker’s Bureau from Cure and honoraria from Genzyme. The other authors made no disclosures.
References
- 1.Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005; 106: 1154–1163. [DOI] [PubMed] [Google Scholar]
- 2.Nieto M, Demolis P, Behanzin E, et al. The European Medicines Agency Review of Decitabine (Dacogen) for the Treatment of Adult Patients with Acute Myeloid Leukemia: Summary of the Scientific Assessment of the Committee for Medicinal Products for Human Use. The Oncologist. 2016; 21: 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shallis RM, Wang R, Davidoff A, Ma X, Podoltsev NA, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019; 36: 70–87. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Ravandi F, O’Brien S, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010; 116: 4422–4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Cancer Institute. NCI Surveillance, Epidemiology, and End Results (SEER) Program Cancer Statistics Review (CSR) 1975–2014. https://seer.cancer.gov/csr/1975_2015/results_single/sect_13_table.14.pdf (accessed May 13, 2018)
- 6.Pettit K, Odenike O. Defining and treating older adults with acute myeloid leukemia who are ineligible for intensive therapies. Frontiers in Oncology. 2015; 5:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda J, Nakajima Y, Azuma A, Tanaka M, Sasaki T. Nucleosides and nucleotides: 100. 2’-C-cyano-2’-deoxy-1-β-arabinofuranosylcytosine (CNDAC): Design of a potential mechanism-based DNA-strand-breaking anti-neoplastic nucleoside. J Med Chem. 1991; 34: 2917–2919. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Liu X, Matsuda A, Plunkett W. Repair of 2’-C-cyano-2’-deoxy-1-β-D-arabino-pentofuranosyl-cytosine-induced DNA single-strand breaks by transcription-coupled nucleotide excision repair. Cancer Res. 2008; 68: 3881–3889. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Wang Y, Benaissa S, et al. Homologous recombination as a resistance mechanism to replication-induced double-strand breaks caused by the anti-leukemia agent, CNDAC. Blood. 2010; 116: 1737–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanaoka K, Suzuki M, Kobayashi T, et al. Antitumor activity and novel DNA self-strand-breaking mechanism of CNDAC (1-(2’-C-cyano-2-deoxy-β-D-arabino-pentofuranosyl) cytosine) and its N4-palmitoyl derivative (CS-682). Int J Cancer. 1999; 82: 226–236. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Garcia-Manero G, O’Brien S, et al. Phase I Clinical and pharmacokinetic study of oral sapacitabine in patients with acute leukemia and myelodysplastic syndrome. J Clin Oncol. 2010; 2: 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012; 30: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravandi F, Faderl S, Cortes JE, et al. Phase 1/ 2 study of sapacitabine and decitabine administered sequentially in elderly patients with newly diagnosed AML. J Clin Oncol. 2011; 29: 15_suppl; Abstract # 6587. [Google Scholar]
- 14.Ravandi F, Kadia TM, Borthakur G, et al. Pooled analysis of elderly patients with newly diagnosed AML treated with sapacitabine and decitabine administered in alternating cycles. Blood. 2012; 120: 2630. [Google Scholar]
- 15.Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009; 145: 598–605. [DOI] [PubMed] [Google Scholar]
- 16.Harousseau J, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009; 114:1166–1173. [DOI] [PubMed] [Google Scholar]
- 17.Lan KKG, Demets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983; 70: 659–663. [Google Scholar]
- 18.Jennison C, Turnbull BW. Group Sequential Methods with Applications to Clinical Trials. Boca Raton, FL: Chapman & Hall/CRC, 1999. [Google Scholar]
- 19.Slovak ML, Kopecky KJ, Cassileth PA, et al. Karyotypic analysis predicts outcome of preremission and postresmission therapy in adult myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 2000; 96: 4075–4083. [PubMed] [Google Scholar]
- 20.Cheson B, Bennett JM, Kopecky KJ, et al. Revised recommendation of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003; 21: 4642–4649. [DOI] [PubMed] [Google Scholar]
- 21.Cheson B, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000; 96: 3671–3674. [PubMed] [Google Scholar]
- 22.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106: 2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lipkovich I, Dmitrienko A. Strategies for identifying predictive biomarkers and subgroups with enhanced treatment effect in clinical trials using SIDES. J Biopharm Stat. 2014; 24: 130–153. [DOI] [PubMed] [Google Scholar]
- 24.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012; 30: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cashen A, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, Phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. J Clin Oncol. 2010; 28: 556–561. [DOI] [PubMed] [Google Scholar]
- 26.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A. 2010; 107: 7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astex Pharmaceuticals press release June 6, 2019.


