Abstract
Strains of Escherichia coli which lack the dam-encoded adenine methylase are mutators due to a reduction in the efficiency of postreplication mismatch repair. In this study, we show that Dam− strains are also defective in very-short-patch repair, the system which corrects T/G mismatches arising from the deamination of 5-methylcytosine. This defect is associated with decreased levels of Vsr, the endonuclease which initiates short-patch repair. We also show that production of the dcm-encoded cytosine methylase is unaffected in Dam− strains. Since the dcm and vsr genes are cotranscribed, the regulation of Vsr by Dam is probably posttranscriptional.
The methylation of GATC sites in the Escherichia coli K-12 genome by the dam-encoded adenine methylase is crucial for efficient methyl-directed mismatch repair (MMR) (reviewed in reference 19). MMR functions most effectively during the time period between the synthesis of a new strand of DNA and its methylation by Dam. The transient undermethylation of GATC's targets MMR to the new strand of the DNA, preserving the sequence of the template strand. The lesion itself, a mispaired or unpaired base, is recognized by MutS. In conjunction with MutL, this protein activates MutH, an endonuclease which cleaves the unmethylated strand of hemimethylated GATC sites. The repair process is completed by removal and resynthesis of the DNA between the nick and the errant base. dam strains, like mut strains, are mutators (16), characterized by an increased incidence of transition and frameshift mutations. Increased production of Dam is also mutagenic, presumably due to premature methylation of the newly synthesized DNA strand (9).
In contrast to MMR, the very-short-patch (VSP) repair system of E. coli is thought to be independent of adenine methylation. VSP repair corrects T/G mismatches caused by deamination of 5-methylcytosine to thymine (reviewed in reference 12). Repair is initiated by Vsr, an endonuclease which cleaves 5′ of the mismatched T (8). Strains lacking VSP repair have a high frequency of C-to-T mutations, primarily at CCWGG sites (W = A or T). The site specificity is due to the fact that Dcm, the sole cytosine methylase of E. coli K-12, methylates the second C of this sequence. VSP repair is reduced in mutS and mutL strains but is unaffected in mutH cells (10, 11, 24). The independence of MutH, combined with the fact that fully methylated and unmethylated C(T/G)AGG heteroduplexes are repaired as efficiently as hemimethylated DNA, suggested that Dam methylation is not important for VSP repair. However, the extent of VSP repair in a dam background has never been tested explicitly.
In this study, we used a Lac reversion assay to compare the frequency of cCagg-to-cTagg mutations in dam strains with that in mutS, mutL, and mutH strains. Mutation is increased far more in the dam strain than in any of the mut strains. Furthermore, the majority of mutations in the dam strain are dependent on the presence of the Dcm methylase and thus result from lack of VSP repair not from a defect in MMR. Western analysis suggests that the VSP repair defect is due to reduced production of Vsr.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains used in this study are described in Table 1. Note that all strains are lacI. Procedures for constructing strains containing mutS201::Tn5, mutH471::Tn5, mutL211::Tn5 or a dcm vsr deletion [Δ(supD-dcm-fla), zee3129::Tn10] were described previously (13). We used P1 transduction to introduce the dam-16::kan allele from GM3819 (21) into CSH142, CC110 and CC112; loss of adenine methylation was confirmed by the restriction of DNA with the methylation-sensitive enzyme MboI. Plasmids pDV101 (dcm+), pDV102 (dcm+ vsr+), pDV109 (trc-dcm+ vsr+), pDCM28 (vsr+), and pTP166 (dam+) have all been described previously (13, 17, 23).
TABLE 1.
Strains
| Strain | Genotype | Source or reference |
|---|---|---|
| CSH142 | ara(gpt-lac)5 thi | 18 |
| CC101–111 | CSH142 F′ lacZYA, proAB | 1, 2 |
| CC112 | CSH142 gyrA argE(Am) rpoB, F′ lacZYA, proAB, with amber suppressor plasmid | 22 |
| CC112V | CC112, vsr::kan | 5 |
| CC402 | CC112, dam-16::kan | This work |
| CC403 | CC110, dam-16::kan | This work |
| CC404 | CC112, mutS201::Tn5 | This work |
| CC405 | CC112, mutL211::Tn5 | This work |
| CC406 | CC112, mutH471::Tn5 | This work |
| CC110Δ | CC110, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC112Δ | CC112, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC402Δ | CC402, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC403Δ | CC403, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC404Δ | CC404, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC405Δ | CC405, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
| CC406Δ | CC406, Δ(supD-dcm-fla), zee3129::Tn10 | This work |
Assays.
The frequency of occurrence of specific base substitution and frameshift mutations was measured using Lac reversion assays (1, 2, 22). For quantitative assays, 100-μl aliquots of saturated overnight cultures were spread on minimal lactose plates, and the number of colonies was counted after 36 h of incubation. Viability was determined by spreading 100 μl of a 10−6 dilution of the culture on Luria-Bertani (LB) plates and incubating them overnight. For qualitative screening, 10-μl aliquots of the undiluted cultures were spotted onto papillation medium (20). All assays were done at least in triplicate. Cultures for Western analysis were grown in minimal glucose medium overnight. Equal amounts of total protein were run on a sodium dodecyl sulfate-polyacrylamide gel, and the blot was probed with antibodies to Dcm and Vsr as described previously (14).
RESULTS
Increased cCagg-to-cTagg mutations in dam strains of E. coli.
CC101 to CC112 revert from Lac− to Lac+ by unique base substitution or frameshift mutations in lacZ (1, 2, 22). Thus, the number of Lac+ revertants in cultures of each of these strains is an indicator of the frequency of occurence of a specific type of mutation in the cells. Table 2 shows the spectrum of mutations that occurs in a dam strain, CC402. As shown previously (17), cells with a Dam− phenotype are relatively weak mutators. Neither the frameshift (CC107 to CC111) nor the transition (CC102 and CC106) mutations are as high as those seen in a mutH strain (2). However, there is one anomaly: the relatively high numbers of Lac+ revertants that occur as a result of cCagg-to-cTagg mutations (CC112).
TABLE 2.
Mutational spectrum of dam strain
| lacZ allele | Mutation for Lac+ reversion | No. of Lac+ mutants/108 cells
|
||
|---|---|---|---|---|
| Wild type | dam | mutHa | ||
| CC101 | AT to CG | 0.56 | 1.52 | 0 |
| CC102 | GC to AT | 7.36 | 20 | 320 |
| CC103 | GC to CG | 0.06 | 0.1 | 0 |
| CC104 | GC to TA | 3.02 | 2.6 | 11 |
| CC105 | AT to TA | 1.08 | 0.27 | 3 |
| CC106 | AT to GC | 0.48 | 5.57 | 34 |
| CC107 | +G | 42.4 | 2874 | 12000 |
| CC108 | −G | 15.2 | 1308 | 5000 |
| CC109 | −CpG | 134 | 803 | ND |
| CC110 | +A | 2.59 | 36.7 | 300 |
| CC111 | −A | 16.7 | 56 | 500 |
| CC112 | CCAGG to CTAGG | 0.78 | 26.9 | ND |
Data from Cupples et al. (2). ND, not determined.
The frequency of cCagg-to-cTagg mutations is influenced by two factors in addition to MMR status: the rate of spontaneous deamination of the methylated cytosine and the efficiency of VSP repair. Neither of these factors should affect frameshift mutations or base substitution mutations in other sequence contexts. To determine the contribution that cytosine methylation and VSP repair make to mutations in CC402, the dam version of CC112, we deleted the dcm and vsr genes from the chromosome. The same Δ(dcm vsr) deletion was introduced into CC112, CC110, and CC403, the dam version of CC110. CC110 was chosen as the control strain because of the low frequency of mutation in the dam versions of CC102 and CC106 (Table 2).
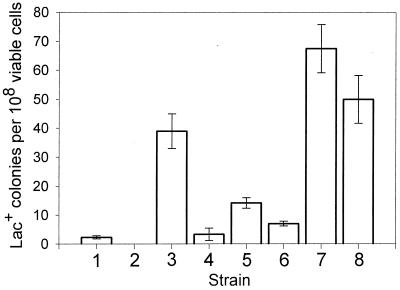
Figure 1 shows that the number of Lac+ revertants (due to cCagg-to-cTagg mutations) in cultures of both CC402Δ (bar 4) and CC112Δ (bar 2) are markedly reduced compared to CC402 and CC112 (bars 3 and 1, respectively). The number of mutants in CC402Δ cultures is reduced to the same level as that found in cultures of the MMR-proficient CC112 strain (bar 1), while the number of mutants in CC112Δ cultures is below detectable levels. Clearly, most of the cCagg-to-cTagg mutations in the dam strain are dependent on the presence of dcm and/or vsr. In contrast, comparison of the frequency of Lac+ revertants in CC403Δ (bar 8) and CC110Δ (bar 6) with that of the non-deleted strains, CC403 (bar 7) and CC110 (bar 5), shows that the Δ(dcm vsr) deletion has only a moderate effect on frameshift mutations.
FIG. 1.
Effect of dam and dcm inactivation on transition and frameshift mutations. The numbers of Lac+ mutants (± the standard error of the mean) per 108 viable cells due to cCagg-to-cTagg (strains 1 to 4) or (A)6-to-(A)7 (strains 5 to 8) mutations are shown. Strains: 1, CC112; 2, CC112Δ; 3, CC402; 4, CC402Δ; 5, CC110; 6, CC110Δ; 7, CC403; 8, CC403Δ.
dam strains have a higher frequency of cCagg-to-cTagg mutations than mutS, mutL, or mutH strains.
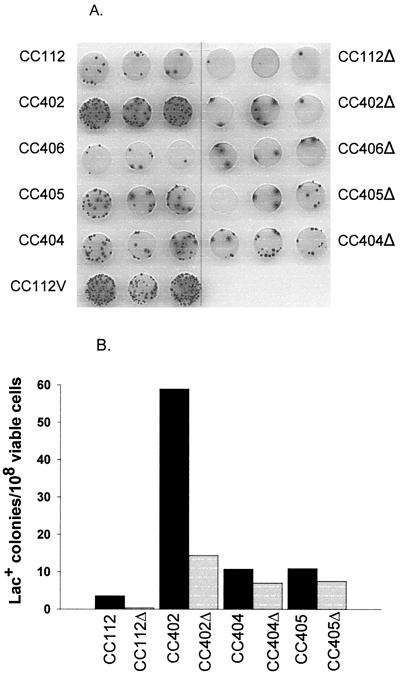
While the loss of Dam function reduces the efficiency of MMR by eliminating strand specificity, the loss of MutS, MutL, or MutH activity destroys MMR entirely. We therefore compared the frequency cCagg-to-cTagg mutations in mutS, mutL, and mutH versions of CC112 (CC404, CC405, and CC406 respectively) to that in CC402, the dam version of CC112. Mutation was measured by papillation (Fig. 2A) or as numbers of Lac+ colonies per 108 viable cells (Fig. 2B). Figure 2A (left) shows that mutation increases in CC404 (mutS) and CC405 (mutL) compared to CC112 but not in CC406 (mutH). However, the increase in mutations in the mutS and mutL strains is considerably less than it is in the dam strain. Lac reversion assays (black bars in Fig. 2B) confirm that the dam strain is a stronger mutator than the mutS and mutL strains.
FIG. 2.
Comparison of the effect of inactivation of dam, mutS, mutL, mutH, and vsr on cCagg-to-cTagg mutations. (A) Samples (5 μl) from saturated, overnight cultures spotted on papillation medium, with three separate transformants per row. (B) Number of Lac+ mutants per 108 viable cells for Dcm+ Vsr+ strains (black bars) or Δ(dcm vsr) strains (gray bars). All assays were done in triplicate.
To determine what proportion of the cCagg-to-cTagg mutations in CC404, CC405, and CC406 is due to Dcm and/or Vsr, we compared their mutation frequency (Fig. 2A, left) to that of isogenic strains with the Δ(dcm vsr) deletion (Fig. 2A, right). While CC406Δ is not noticeably different from CC406, both CC404Δ and CC406Δ mutate less than their parent strains, CC404 and CC405. However, the difference between the wild-type and deleted versions of CC404 and CC405 is not nearly as large as the difference between CC402 and CC402Δ. Lac reversion assays (Fig. 2B) confirm that cCagg-to-cTagg mutations decrease far more in dam strains than in mutS or mutL strains following the removal of dcm.
cCagg-to-cTagg mutations in a dam strain are not reduced by vsr.
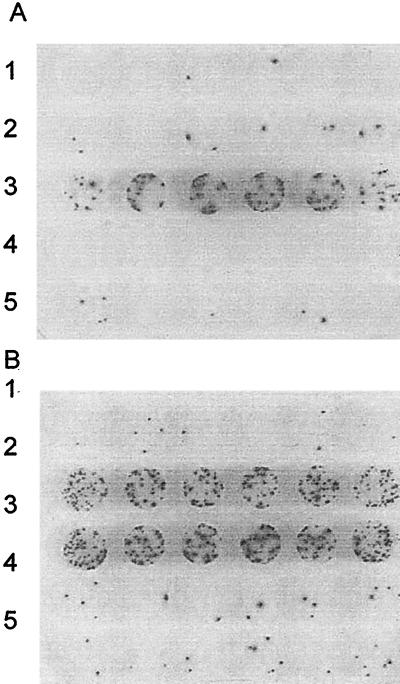
The mutation frequency in the dam strain is very similar to that of a vsr strain (Fig. 2A), suggesting that the dam strain has major defect in VSP repair. To test this possibility, we transformed CC112Δ (Fig. 3A) and CC402Δ (Fig. 3B) with plasmids containing dcm and/or vsr. As shown previously (13), transformation of CC112Δ with either a control plasmid (pACYC184) or a plasmid containing vsr alone (pDCM28) has no effect on mutation (rows 1 and 4 of Fig. 3A), while the addition of dcm alone (pDV101) sharply increases mutation (row 3). When the strain is transformed with pDV102, a plasmid which contains both genes (row 2), the mutagenic effect of dcm is nullified. As in CC112Δ, the mutation frequency in CC402Δ (Fig. 3B, row 5) is unaffected by pACYC184 (row 1) or pDCM28 (row 4) and is increased by pDV101 (row 3). However, transformation of CC402Δ with pDV102 results in a level of mutation comparable to that of the pDV101 transformants. Even pDV109, which produces considerably more Vsr than pDV102, had almost no effect on mutation (data not shown). Clearly, in the dam strain, the presence of the vsr gene on the plasmid does not counteract the mutagenic effect of Dcm.
FIG. 3.
Evidence for lack of Vsr activity in dam mutants. The Lac reversion in CC112Δ (A) and CC402Δ (B) due to cCagg-to-cTagg mutations is shown. Samples (5 μl) from saturated, overnight cultures were spotted on papillation medium with six separate transformants per row. Plasmids: row 1, pDCM28 (vsr+); row 2, pDV102 (dcm+ vsr+); row 3, pDV101 (dcm+); row 4, pACY184; row 5, no plasmid.
Vsr production is reduced in dam strains of E. coli.
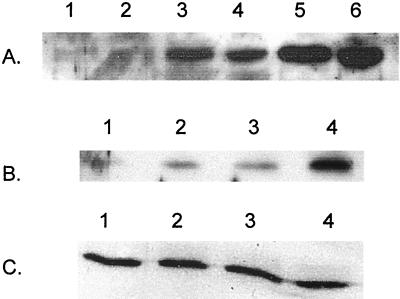
The apparent lack of VSP repair in pDV102-transformed CC402Δ suggested that dam cells are unable to make normal amounts of Vsr. We therefore used Western analysis to measure amounts of Vsr in cells producing no Dam (dam::kan) or producing excess Dam (transformed with the dam-containing plasmid, pTP166). For these experiments, we used a dam::kan version of CSH142 strain rather than CC112 since CC112 already contains a plasmid. Since we could not detect production of Vsr from pDV102 in dam cells (not shown), we used pDV109 for these experiments. In this plasmid, the dcm vsr operon is expressed from the trc promoter, raising the amounts of Vsr to easily detectable levels. Figure 4A shows that the Dam− mutants (lanes 3 and 4) make much less Vsr than the Dam overproducers (lanes 5 and 6).
FIG. 4.
Effect of Dam on Vsr and Dcm production during log-phase growth and stationary phase. Western analysis was performed on duplicate samples containing equal amounts of total protein and probed with an antibody to Vsr (A and B) or Dcm (C). (A) CC403 cotransformed with pACYC184 and pTP166 (lanes 1 and 2), pDV109 and pBR322 (lanes 3 and 4), or pDV109 and pTP166 (lanes 5 and 6). (B and C) CC110Δ (lanes 1 and 2) and CC110 (lanes 3 and 4) transformed with pDV109 and grown to mid-log (lanes 1 and 3) or stationary (lanes 2 and 4) phase. Plasmids: pDV109, wild-type dcm and vsr in pACYC184; pTP166, wild-type dam in pBR322.
We showed previously that the levels of Vsr from both the chromosomal gene and the pDV109-borne gene are growth phase dependent, while the levels of Dcm are constant (14). Therefore, we measured the amount of both proteins in dam and wild-type cells transformed with pDV109 in the log and stationary phases. Figure 4B shows that both cell types produce lower amounts of Vsr in the log phase (lanes 1 and 3) than in the stationary phase (lanes 2 and 4). (Note that the left side of the band in lane 1 is somewhat obscured by extraneous material.) However, the absolute amount of protein in both phases is lower in the Dam− cells than in the Dam+ cells. Meanwhile, the amount of protein produced by the dcm gene, cotranscribed with vsr, is independent of both Dam production and the growth phase (Fig. 4C). This steady production of Dcm and of the plasmid-encoded chloramphenicol acetyltransferase (data not shown) provides reassurance that the alterations in Vsr amounts seen in the dam cells are not due to changes in the plasmid copy number.
DISCUSSION
The T/G mismatches that cause cCagg-to-cTagg mutations arise primarily from two sources, errors in DNA replication and deamination of 5-methylcytosine to thymine. The mismatches are repaired, and the mutation is prevented, by two possible pathways: MMR and VSP repair. Thus, the mutator phenotype of the Dam− CC402 strain (Table 2, Fig. 1) could be due to any one of at least four causes: increased replication errors, increased deamination, decreased VSP repair, or untargeted MMR. Since the Dam methylase plays an important role in MMR, the last explanation was the most likely a priori. However, the data from this study indicates that the actual cause of the mutation is decreased VSP repair.
The first clue came from the finding that the numbers of Lac+ revertants in CC402 cultures (due to cCagg-to-cTagg mutations) are substantially reduced in cells deleted for dcm and vsr, while revertants in CC403 cultures (due to frameshift mutations) are not (Fig. 1). The decrease in mutation in CC402Δ could be due to the loss of any gene in the approximately 20-kb deleted region, but the fact that dcm and vsr alone modulate mutation in CC112Δ (Fig. 3) makes these two genes the most likely candidates. It is highly unlikely that the decrease in the numbers of Lac+ mutants in CC402Δ is due to the removal of vsr, since inactivation of VSP repair should lead to an increase in the number of cCagg-to-cTagg mutations (5). Thus, the probable cause of the reduced mutation is the removal of dcm, indicating that the vast majority of cCagg-to-cTagg mutations in dam strains are due to unrepaired deamination damage.
It is possible that CC402 lacks the ability to repair deamination damage due to its MMR defect. However, this hypothesis is counterintuitive given the decided preference of Vsr for T/G mismatches occurring at sites of Dcm methylation (6). It also contradicts previous evidence from our lab which shows that VSP repair is dominant over MMR at C(T/G)AGG sites (4). Nevertheless, we explored the role of MMR in the reversal of deamination damage further by measuring cCagg-to-cTagg mutations in other MMR− backgrounds. While MMR is effectively reduced in dam strains, it is eliminated entirely in mutS, mutL, and mutH strains. Thus, if MMR is an important player, the mut strains should all show a higher frequency of cCagg-to-cTagg mutations than the dam strain. In fact, the mutS and mutL strains (CC404 and CC405) are much weaker mutators than the dam strain (CC402), and mutation in the mutH strain (CC406) is hardly elevated at all (Fig. 2A). These results confirm that MMR is not a major factor in preventing mutations caused by deamination of 5-methylcytosine.
It is well established that MutS and MutL are accessory proteins in VSP repair, while MutH is not involved (10, 11, 24). Thus, CC404 and CC405 should be deficient in both VSP repair and MMR, while CC406 should be deficient only in MMR. The fact that cCagg-to-cTagg mutations are more frequent in CC404 and CC405 than in CC406 and that mutation decreases in CC404 and CC405 but not in CC406 upon deletion of dcm (Fig. 2B) is further evidence that VSP repair is the dominant pathway for preventing cCagg-to-cTagg mutations due to deamination damage. If VSP repair is the dominant pathway, then it follows that the Dam methylase, far from playing no role in VSP repair, must instead play an even larger role than MutS and MutL.
The low frequency of Lac reversion in CC112 and its derivatives (Fig. 2) is something that we have observed previously (13). We assume that T/G mismatches resulting from errors in DNA replication are not common at the particular CCAGG site in lacZ that we monitored. However, it is surprising that mutation in mutH strains is hardly elevated at all (Fig. 2A). It is possible that mutH strains are slightly weaker mutators than mutS and mutL strains.
The mutation frequency in the CC402 strain is very similar to that seen in CC112V, a vsr strain (Fig. 2A), suggesting that dam strains are completely lacking in VSP repair. Since Vsr interferes with MMR (4, 13), the modest but significant decrease in frameshift mutations in CC403Δ compared to CC403 (Fig. 1) is compatible with a substantial reduction in the amount of Vsr. The data in Fig. 3 support this hypothesis. The strain used in the experiments presented in panel A is Dam+, while that in panel B is Dam−. Both strains are Dcm− and Vsr− due to introduction of the Δ(dcm vsr) deletion. The dcm and vsr genes are added back individually or together on multicopy plasmids. While the addition of dcm alone increases mutation in both strains by comparable amounts, the concomitant addition of vsr lowers mutation only in the Dam+ strain. This suggests that the Dam− strain is unable to maintain the same concentrations of Vsr as the Dam+ one. The demonstration that Dam− cells transformed with pDV109 make reduced amounts of Vsr without a concomitant reduction in the amount of Dcm (Fig. 4) is consistent with this explanation.
We showed previously that production of Vsr is growth phase dependent, being present in very low amounts during log phase and increasing only as the cells enter stationary phase (14). The data presented in Fig. 4B show that Dam− strains follow the same pattern of expression as the wild-type cells but that the absolute amounts of Vsr are reduced in both phases in the mutant. Thus, it does not appear that Dam is controlling the growth-phase-dependent regulation of Vsr. Despite the artificial nature of the assay, the results are probably reliable given our previous demonstration that Vsr production from the trc promoter on a plasmid follows the same pattern of expression as that from the dcm promoter on the chromosome (14).
Dam has been shown to alter the transcription of a number of E. coli and Salmonella genes (7, 16). It is possible that the dcm vsr operon is one of them, although there are no GATC sites associated with either the putative dcm promoter (3) or the trc promoter of pDV109. The fact that Dcm levels are unaffected by deletion of dam (Fig. 4C) also makes this unlikely, although it is possible that maintenance of uniform Dcm levels is under separate, posttranscriptional control. Another possibility is that loss of Dam reduces the efficiency of vsr translation. However, a clear understanding of how production of Dcm and Vsr is affected in a dam strain will require more knowledge than currently exists about how production of the proteins is regulated in the wild-type strain.
In summary, the results of our experiments clearly show that VSP repair, like MMR, is dependent on the dam-encoded adenine methylase. Both forms of DNA repair are reduced in Dam− strains, although the effect on VSP repair is the more severe. The origin of the repair defect is fundamentally different in the two cases. In MMR, adenine methylation is used to distinguish the newly synthesized DNA strand (unmethylated) from the template strand (methylated), thereby targeting repair to the new strand and preserving the old one. In VSP repair, the methylation status of the substrate DNA is immaterial (10, 11, 24). Instead, Dam is probably required for maintenance of normal amounts of Vsr in the cell.
ACKNOWLEDGMENTS
We thank Gina Macintyre for technical advice and scientific input. Martin Marinus (University of Massachusetts) generously provided dam plasmids and strains.
This work was supported by grants to CGC from the Canadian Institutes of Health Research (CIHR) and from the Natural Sciences and Engineering Research Council of Canada (NSERC).
REFERENCES
- 1.Cupples C G, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc Natl Acad Sci USA. 1989;86:5345–5349. doi: 10.1073/pnas.86.14.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cupples C G, Cabrera C, Cruz C, Miller J H. A set of lacZ mutations in Escherichia coli that allow rapid detection of specific frameshift mutations. Genetics. 1990;125:275–280. doi: 10.1093/genetics/125.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dar M E, Bhagwat A S. Mechanism of expression of DNA repair gene vsr, an Escherichia coli gene that overlaps the DNA cytosine methylase gene, dcm. Mol Microbiol. 1993;9:823–833. doi: 10.1111/j.1365-2958.1993.tb01741.x. [DOI] [PubMed] [Google Scholar]
- 4.Doiron KMJ, Viau S, Koutroumanis M, Cupples CG. Overexpression of vsr in Escherichia coli is mutagenic. J Bacteriol. 1996;178:4294–4296. doi: 10.1128/jb.178.14.4294-4296.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doiron K M J, Lavigne-Nicolas J, Cupples C G. Effect of interaction between 5-azacytidine and DNA (cytosine-5) methyltransferase on C-to-G and C-to-T mutations in Escherichia coli. Mutat Res. 1999;429:37–44. doi: 10.1016/s0027-5107(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 6.Gläsner W, Merkl R, Schellenberger V, Fritz H-J. Substrate preferences of vsr DNA mismatch endonuclease and their consequences for the evolution of the Escherichia coli K-12 genome. J Mol Biol. 1995;245:1–7. doi: 10.1016/s0022-2836(95)80033-6. [DOI] [PubMed] [Google Scholar]
- 7.Heithoff D M, Sinsheimer R L, Low D A, Mahan M J. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 8.Hennecke F, Kolmar H, Bründl K, Fritz H-J. The vsr gene product of E. coli K-12 is a strand- and sequence-specific DNA mismatch endonuclease. Nature. 1991;353:776–778. doi: 10.1038/353776a0. [DOI] [PubMed] [Google Scholar]
- 9.Herman G E, Modrich P. Escherichia coli K-12 clones that overproduce dam methylase are hypermutable. J Bacteriol. 1981;145:644–646. doi: 10.1128/jb.145.1.644-646.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones M, Wagner R, Radman M. Mismatch repair of deaminated 5-methylcytosine. J Mol Biol. 1987;194:155–159. doi: 10.1016/0022-2836(87)90724-8. [DOI] [PubMed] [Google Scholar]
- 11.Lieb M. Bacterial genes mutL, mutS, and dcm participate in repair of mismatches at 5-methylcytosine sites. J Bacteriol. 1987;169:5241–5246. doi: 10.1128/jb.169.11.5241-5246.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieb M, Bhagwat A S. Very short patch repair: reducing the cost of cytosine methylation. Mol Microbiol. 1996;20:467–473. doi: 10.1046/j.1365-2958.1996.5291066.x. [DOI] [PubMed] [Google Scholar]
- 13.Macintyre G, Doiron K M J, Cupples C G. The Vsr endonuclease of Escherichia coli: an efficient DNA repair enzyme and a potent mutagen. J Bacteriol. 1997;179:6048–6052. doi: 10.1128/jb.179.19.6048-6052.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Macintyre G, Pitsikas P, Cupples C G. Growth phase-dependent regulation of Vsr endonuclease may contribute to 5-methylcytosine mutational hotspots in Escherichia coli. J Bacteriol. 1999;181:4435–4436. doi: 10.1128/jb.181.14.4435-4436.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinus M G. Methylation of DNA. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 782–791. [Google Scholar]
- 16.Marinus M G, Morris N R. Biological function for 6-methyladenine residues in the DNA of Echerichia coli K 12. J Mol Biol. 1974;15:309–322. doi: 10.1016/0022-2836(74)90366-0. [DOI] [PubMed] [Google Scholar]
- 17.Marinus M G, Poteete A, Arraj J A. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene. 1984;28:123–125. doi: 10.1016/0378-1119(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 18.Miller J H. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 19.Modrich P, Lahue R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 20.Nghiem Y, Cabrera M, Cupples C G, Miller J H. The mutY gene: A locus in Escherichia coli that generates G · C→T · A transversions. Proc Natl Acad Sci USA. 1988;85:2709–2713. doi: 10.1073/pnas.85.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker B O, Marinus G M. A simple and rapid method to obtain substitution mutations in Escherichia coli: isolation of a dam deletion/insertion mutation. Gene. 1988;73:531–535. doi: 10.1016/0378-1119(88)90517-3. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz S M, Létourneau S, Cupples C G. Isolation and characterization of an Escherichia coli strain with a high frequency of C-to-T mutations at 5-methylcytosines. J Bacteriol. 1993;175:4985–4989. doi: 10.1128/jb.175.16.4985-4989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohail A, Lieb M, Dar M, Bhagwat A S. A gene required for very short patch repair in Escherichia coli is adjacent to the DNA cytosine methylase gene. J Bacteriol. 1990;172:4214–4221. doi: 10.1128/jb.172.8.4214-4221.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zell R, Fritz H-J. DNA mismatch-repair in Escherichia coli counteracting the hydrolytic deamination of 5-methyl-cytosine residues. EMBO J. 1987;6:1809–1815. doi: 10.1002/j.1460-2075.1987.tb02435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]