Abstract
Background
Schistosomiasis is a global health problem affecting 250 million people, with 90% in Sub-Saharan Africa. In Botswana, the burden is high in the Okavango delta because of the water channels. WHO recommends integrated measures, including access to clean water, sanitation, health education, and drugs to control and eliminate schistosomiasis. Gauging knowledge and awareness of schistosomiasis for School-Aged Children (SAC) is crucial. Our study aimed at assessing knowledge and awareness of schistosomiasis among SAC in the Okavango Delta.
Methods
A cross-sectional survey assessing awareness and knowledge of schistosomiasis in schools was conducted. 480 questionnaires were administered to gather demographic profiles, awareness, and knowledge of risky behaviors. Chi-square and descriptive analysis determined the differences in SAC`s awareness and knowledge levels based on localities, gender, age, and health education.
Results
The results showed a low awareness level, with only (42%) of respondents having heard about the disease and (52%) knowing its local name. Younger children from Sekondomboro (83%) and Samochima lacked awareness, while children from Mohembo (77%) and those who had health education (70%) demonstrated significant awareness levels (P ≤ 0.001). Seventy-two percent (72%) lacked knowledge of the cause and (95%) did not know the disease life-cycle. Children from Xakao (91%), (85%) Sepopa, and (75%) of younger children did not know haematuria is a symptom of the disease. Older and SAC with health education were more likely to know that swimming is a risk factor (P ≤ 0.001) and (P ≤ 0.05) respectively.
Conclusions
Although respondents from four schools demonstrated some level of awareness of the disease, and knowledge of risky behaviors, the study showed a lack of in-depth knowledge on the life-cycle and cause of the diseases. We, therefore, recommend the implementation of an integrated approach to health education and improvement in access to clean water and sanitation in all study areas.
Keywords: Awareness, Knowledge, Schistosomiasis
Background
Schistosomiasis is a Neglected Tropical Disease (NTD) with negative effects on public health. Endemic in 78 tropical and sub-tropical countries, schistosomiasis afflicts approximately 250 million people worldwide, with more than 90% of cases occurring in Sub-Saharan Africa [1–3]. An estimated 20,000–200,000 people die of schistosomiasis each year [3]. The disease is ranked among the top ten causes of disability in six African countries [4] with an estimated 3, 7900 Years of Life with Disability (YLDs) in 2012 attributed to schistosomiasis. The highest prevalence of the disease in Africa is seen in Nigeria (29 million) followed by Tanzania (19 million) and Ghana and the Democratic Republic of Congo both at 15 million [5]. School-Aged Children (SAC) bear most of the burden of this disease in Africa [5, 6]. Botswana bears an overall prevalence of 38% for Schistosoma. Haematobium in the Okavango region carries the highest burden of the disease [7].
Schistosomiasis is a parasitic disease caused by blood flukes of the genius schistosome [8, 9]. Humans are usually infected by five species of Schistosoma mansoni, Schistosoma japonicum, Schistosoma mekongi, and Schistosoma intercalatum causing intestinal infection and haematobium responsible for urinary infection [10]. The sub-Saharan African infection is caused by Schistosoma mansoni and Schistosoma haematobium. The disease is often acquired during domestic, occupational, and livelihood activities which involve contact with water infested with cercariae from the intermediate host-the snail [5]. Climate change, ecological changes such as dam construction, and socio-economic factors have been crucial factors in the continuous transmission of schistosomiasis in endemic areas [5, 10]. In Botswana, the Okavango region presents a major threat of schistosomiasis because of its potential for a resurgence due to flood recession farming and the predicted increase in the seasonal flow of the delta [11, 12]. In their research, Appleton, and Madsen [13] indicated that hydrological factors seem to play an important role in disease transmission than livelihood activities in Botswana. The authors [13] noted that the population living near the river and children attending schools near the river in Botswana are at an increased risk of repeated epidemics. Recognizing the intense studies on the contribution of the aforementioned risk factors on the rate of infection with schistosomiasis, it is assumed that the complexity of the socio-economic, behavioral, and knowledge factors vary considerably at global, regional, and local levels, hence the need for in-depth risk factor analysis for their contextual understanding.
The World Health Assembly (WHA) has made several resolutions [14] and specified control and elimination measures of schistosomiasis. These include snail control, mass treatment, improved sanitation, provision of potable water, and health education. Disease control programs recommended for School-Aged Children (SAC) include Mass Drug Administration (MDA), sanitary methods, and health education. The WHO guidelines recommend every-other-year praziquantel treatment of all SAC in communities having moderate risk (prevalence between 10 and 49%), and annual SAC treatment for communities having high risk (prevalence ≥ 50%) [15]. Re-infection with Schistosoma haematobium among SAC is of global concern, especially with low (34.6%) MDA program coverage and low implementation of preventive therapy (40.8%) in the African region [16]. Several studies [17, 18] indicated that often after treatment new infection cases resurface to the point where baseline prevalence is reached within a short period. The 65.21 World Health Assembly resolutions [9] therefore called for the inclusion of all high-risk groups, including SAC, to eliminate schistosomiasis.
The schistosomiasis control program for Botswana was integrated into the Primary Health care services in 1983, and by 1985, the program existed in four districts of the country and was sustained until 1991 when Botswana reached elimination status. Despite, the huge success of the control program back then, Botswana still battles with transmission interruption and complete elimination of the disease, especially in the Okavango region. With a high-level prevalence of 38% [7] and impairing morbidity and the resultant complications, especially among the SAC, the disease has been undervalued in the past three decades in Botswana, thus qualifying it as a neglected disease. Moreover, the scarcity of data showing risk factors and their associated behaviours and rates of re-infections in Botswana poses scientific and ethical challenges in addressing the burden of this disease. Primary school-aged children in endemic foci are considered a high-risk group but there is a scarcity of data in Botswana showing the dynamics of re-infections among school children to inform current and future control strategies. Also, the Botswana schistosomiasis control program has heavily relied on the test and treats strategy, where cases detected through the Kato-Katz method are treated with 40 mg/kg of praziquantel [12].
Communities in the Okavango have low levels of knowledge and awareness of the disease [19]. Adequate knowledge and adoption of correct and consistent preventive behavior in endemic areas are necessary for an effective and sustainable control program owing to the elimination of the disease. Although health promotion through community education is an important aspect of the elimination of any disease, a review of literature in this area for Botswana SAC revealed a paucity of empirical data owing to decadal neglect of the disease.
There is insufficient knowledge in Botswana which mapped the knowledge and awareness of schistosomiasis and its risk factors. This calls for assessment knowledge of key risk factors for infections within endemic communities. This study aimed at assessing awareness and knowledge of risk factors for schistosomiasis among SAC in the Okavango Delta. The specific objectives are to determine the SAC`s i) awareness of the disease, ii) general knowledge, and iii) knowledge of risky behaviors of schistosomiasis among SAC in the Okavango Delta.
Methods
Study design This wasa cross-sectional survey that assessed students’ knowledge and awareness of schistosomiasis haematobium and its associated risky behaviours.
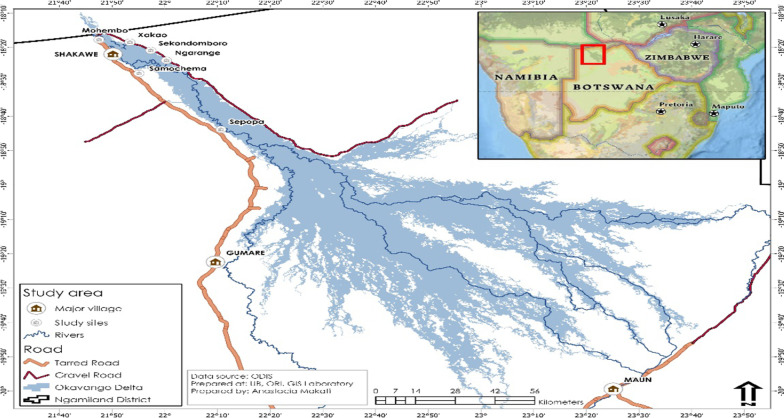
Study setting The study was conducted in the Ngamiland district in the Northwestern part of Botswana which has a population of 175,631 [20]. The site is part of the Okavango Delta which is one of the world`s largest wetlands. The delta comprises permanent, seasonal, and swampy areas which originate from upstream of Angola and Namibia. The Cubango River from Namibia enters Botswana and travels along with the stream passing into upper villages of the panhandle. As it travels along these villages it gives way to seasonal swamps. The flood is often at its peak at Mohembo between mid-March and mid-May immediately after the summer rains. The delta is the residence of a multi-ethnic group of people who include: Bayei, Hambukushu, San/Khoikhoi, and Batawana. More than 8000 people live in six villages along the Okavango River where the study was conducted (Fig. 1) [20, 21]. Six schools in villages along the Okavango watercourse were purposively sampled as sites for the study. Each village has one primary school. The proximity of the villages to the wetland and water contact livelihoods which often involve schoolchildren provided a good opportunity for studying schistosomiasis. The selected villages are proximal to the seasonal flood plains/permanent water channels status. The upstream schools of Samochima, Mohembo, Skondomboro, Ngarange, Xakao, and Sepopa were included in the study. Sepopa is one among the selected villages that are further at the mid-upper delta or where the Panhandle spread into the alluvial Delta.
Fig. 1.
Map of the Okavango delta and study sites. ODIS (2019); “okavango delta information system”, University of Botswana, Okavango Research Institute from https://www.odis.ub.bw/portal/home
Study population and sampling strategy
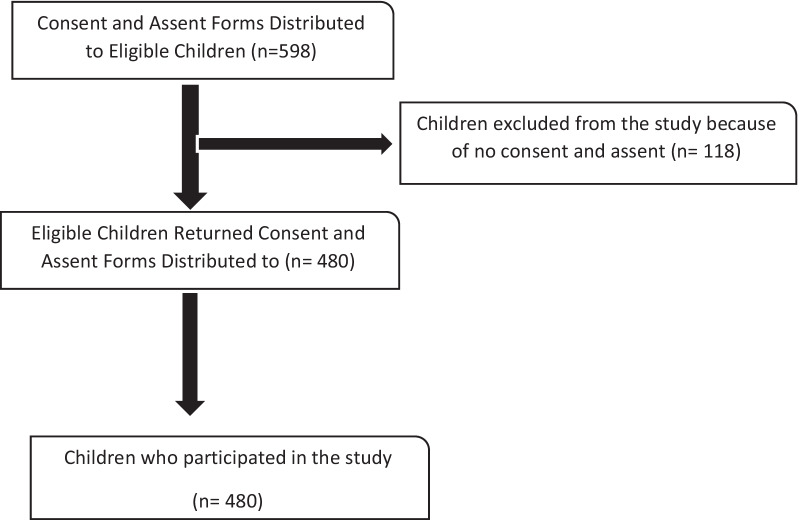
The sample for the study consisted of a population of 2417 children in standard 1 to 6, aged 6–13 years. Six Primary schools in the Okavango region were purposively selected to be included in the study due to their geographical location and being close to the river course. The sample size of 598 SAC was estimated using Yamane’s formula for the cross-sectional survey [22]. The eligible children were requested for assent and parental consent. One hundred and eighteen (118) of the 598 did not consent hence their exclusion from the study, (refer to Fig. 2). The response rate for the study was however notably high (80%).
Fig. 2.
Study participant flow diagram
A stratified random sample proportionate to size was drawn from each school as shown in Table 1. In each selected school, the total number of respondents per standard was obtained from the school head to determine the number required from each standard. The selection of children at each school was done through systematic random sampling using class lists obtained from class teachers and only 480 respondents participated in the study.
Table 1.
Proportions of required samples
| Villages | Total school enrolment | Required sample per school | Consented to the study |
|---|---|---|---|
| Sepopa | 250 | 62 | 55 |
| Samochima | 394 | 97 | 93 |
| Mohembo | 494 | 122 | 101 |
| Xakao | 659 | 163 | 100 |
| Skondomboro | 268 | 66 | 63 |
| Ngarange | 352 | 87 | 68 |
| Total | 2417 | 598 | 480 |
Survey questionnaire development and validation
A structured questionnaire was developed using the Theoretical Rational Approach (TRA), where targeted constructs of knowledge and awareness of schistosomiasis were adopted and adapted from literature and previous related research work [23]. The literature that guided the questionnaire construction included Xiao and colleagues` Protection Motivation Theory (PMT) [24] and the Knowledge Attitude and Practice (KAP) Theory [25–27].
Derived from previous similar studies [28–31], 13 subjective measures on knowledge of signs and symptoms, key risky behaviours, and the lifecycle of the disease were constructed. The items on knowledge did not comprise a score but assessed if the respondents possess the correct information and knowledge about the disease. The scales comprised “yes, no, or I don’t know” and probes with pre-coded responses. Awareness measures were also developed based on previous related studies [32–35] and comprised closed-end (yes and no) and open-ended items.
The questionnaire consisted of three main sections. The first section comprised 8 items on the respondents’ socio-demographic information. The second section comprised 10 questions, 8 of which were closed-ended (yes, no and I don’t know) and 2 open-ended measuring awareness. Questions measuring knowledge were also part of this section and comprised 2 open-ended and 6 close-ended items. The last section comprised 7 questions on the respondents’ knowledge of key risky behaviours. The Cronbach’s alpha coefficient for 8 close-ended questions on the domain of awareness was 0.81, indicating an adequate internal consistency. The Cronbach’s alpha score for the knowledge of risk factors items was almost 0.70 (0.69). While this is marginal, the mean inter-correlation for the items was 0.35, which according to Briggs and Cheeks is within the optimal ranges (0.2–0.4) that is recommended [36].
Pretesting in one non-selected school was done to assess content validity, appropriateness, and question comprehensiveness, and revisions were made based on the findings of the pre-test.
Data collection
Data was collected on January 18–28th, 2020 using the questionnaire directly administered by the researchers. Community research assistants received training to assist with the data collection. Mini-sized cellular tablets which had the Research Electronic Data Capture (REDCap) software installed in them were used for data collection. The researchers were onsite to ensure appropriate data capturing and to address any technical issues. Two data collectors and the principal investigator used 4 electronic tablets to collect data across the six study sites.
Data analysis
Data were exported from the REDCap and entered into Statistical Package for Social Scientist (SPSS) version 25.0 for management and analysis. Descriptive statistics (proportions and mean) were computed to summarise and describe the sample. Chi-squared tests of independence were used to determine the associations between demographic variables and variables of interest. Inferences using P ≤ 0.05 to test for statistical significance were made on the relationship between variables. All quantitative analyses were done at the 5% level of significance.
Ethical consideration
The study design and protocol were reviewed and approved by the Human Research Development Committee at the Botswana Ministry of Health and Wellness (letter referenced HPDME: 13/18/1) and the University of Botswana Institutional Review Board (referenced UBR/RES/IRB/BIO/154). Parental consent was sought and children were asked for assent at the beginning of the interview.
Results
Socio- demographics profile of respondents
A total of 480 respondents (80% response rate) 6–13 years were enrolled in the study, of which 51% were females and 41% were males. The mean age for the respondents was 9.13 years with a standard deviation of 1.66. Respondents were further divided into two age groups: 6–9 who formed the majority 59% and 10–13 years who were 41%. Most (21%) of respondents were from Mohembo and Xakao villages. Twenty percent (20%) of the respondents were in the fifth standard and the lowest number (10%) of respondents were from standard one (Table 2). Almost one-fifth of the respondents (38%) reported having received health education about the disease from different sources, while the majority (62%) had not.
Table 2.
Respondents` socio-demographic characteristics
| Variable | Category | Frequency (n) | Percentage (%) |
|---|---|---|---|
| Gender | Female | 245 | 51 |
| Male | 235 | 49 | |
| Age Group | 6–9 | 282 | 59 |
| 10–13 | 198 | 41 | |
| Standard | 1 | 48 | 10 |
| 2 | 83 | 17 | |
| 3 | 85 | 18 | |
| 4 | 92 | 19 | |
| 5 | 97 | 20 | |
| 6 | 75 | 16 | |
| School | Mohembo | 101 | 21 |
| Ngarange | 68 | 14 | |
| Samochima | 93 | 19 | |
| Sekondomboro | 63 | 13 | |
| Sepopa | 55 | 12 | |
| Xakao | 100 | 21 | |
| Health education about disease | No health education | 297 | 62 |
| Had health education | 183 | 38 |
Awareness of schistosomiasis among respondents
Majority of older respondents (10–13 years) (51%) were aware of Bilharzia compared to younger ones (6–9 years) (35%), and an almost equal proportion of females (42%) and males (41%) have heard about the disease. Over two-thirds of SAC in Sekondomboro (79%) and Samochima (69%) villages had not heard about the disease. The majority, (93%), of respondents who had no health education about the disease, indicated that they had not heard about the disease. A chi-square test conducted to determine if there were any significant differences among age groups and between those who received health education and those who did not, showed that older respondents and those with health education were more likely to have heard about the disease (P ≤ 0.001) and (P ≤ 0.001) respectively. A relatively higher proportion of respondents in the age group 10–13, (57%), and males (57%) knew the local name of the disease to be “bilharzia”. The majority of respondents who had health education (70%) knew the local name of the diseases than those with no health education (41%). Most respondents from Mohembo village (77%) demonstrated more awareness of the local name of the disease than those from other villages. A chi-square test indicated no significant differences in awareness of the local name of bilharzia between males and females in the four villages, (P = 0.09) and the age groups (P = 0.99). Respondents who had health education (P ≤ 0.001) were more likely to be aware of the local name of the disease. Similarly, respondents from Mohembo were more likely to be aware of the local name of the disease (P ≤ 0.001) than those from other villages.
The majority of younger respondents (75%) from, Sekondomboro (83%), and Samochima (80%) villages indicated that children from their schools lacked awareness of the disease. Similarly, respondents who had no health education about the disease (88%) were more likely to mention that other children from their schools lacked awareness of the disease than those who had health education. An almost equal proportion of respondents in the gender category indicate that other children from their school lacked awareness of the disease. Younger children (P ≤ 0.001), those who have no health education (P ≤ 0.001), and those from Sekondomboro and Samochima (P ≤ 0.001), were more likely to indicate that children from their schools lacked awareness of the disease. The results on the difference between males and females on this fact were not significant (P = 0.83), (refer to Table 3).
Table 3.
Awareness of Schistosomiasis by demographic characteristics
| Demographics | Frequency (n) | Heard about Bilharzia (%) | P-value | Know the local name of the disease (%) | P-Value | Think other children are aware of the disease (%) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | Don’t Know | Bilharzia | No | Yes | ||||||
| Age | 6–9 | 282 | 65 | 35 | 0.001 | 51 | 49 | 0.09 | 75 | 25 | ≤ 0.001 |
| 10–13 | 198 | 49 | 51 | 43 | 57 | 59 | 41 | ||||
| Total | 480 | 57 | 43 | 53 | 53 | 67 | 33 | ||||
| Gender | Females | 245 | 58 | 42 | 0.001 | 48 | 49 | 0.99 | 69 | 31 | 0.83 |
| Males | 235 | 59 | 41 | 48 | 52 | 68 | 32 | ||||
| Total | 480 | 59 | 42 | 48 | 51 | 69 | 32 | ||||
| Village | Mohembo | 101 | 49 | 51 | 0.001 | 23 | 77 | 0.001 | 66 | 34 | ≤ 0.001 |
| Ngarange | 68 | 47 | 53 | 47 | 53 | 52 | 48 | ||||
| Samochima | 93 | 69 | 31 | 50 | 50 | 80 | 20 | ||||
| Sekondomboro | 63 | 79 | 21 | 67 | 33 | 83 | 18 | ||||
| Sepopa | 55 | 47 | 53 | 64 | 36 | 47 | 53 | ||||
| Xakao | 99 | 59 | 41 | 53 | 47 | 73 | 27 | ||||
| Total | 480 | 58 | 42 | 51 | 49 | 67 | 33 | ||||
| Health education status | No health education | 297 | 93 | 19 | 0.001 | 59 | 41 | 0.001 | 88 | 12 | ≤ 0.001 |
| Had health education | 183 | 11 | 89 | 30 | 70 | 35 | 65 | ||||
| Total | 480 | 54 | 52 | 45 | 56 | 62 | 39 | ||||
General knowledge of schistosomiasis among respondents
The majority (72%) did not know that schistosomiasis was caused by parasites released during snail bites when one comes in contact with infested water. In terms of respondents’ knowledge of the effects of schistosomiasis on one’s health, a very small proportion (17%) and (23%) mentioned slow growth and poor concentration respectively. An overwhelming majority, (95%) of respondents did not know the life cycle of schistosomiasis in both humans and snails.
Concerning respondents’ knowledge of signs and symptoms of schistosomiasis, younger respondents (75%) did not know that blood in the urine (haematuria) is the main symptom of schistosomiasis compared with older children. The majority of respondents from Xakao (91%) and Sepopa (85%) and those with no health education (73%) did not know that haematuria is a symptom of schistosomiasis (see Table 4). The knowledge of fever as a symptom of schistosomiasis was very low (< 20%) across all the socio-demographics of the respondents. The results on fever as a sign were not significant between the age groups (refer to Table 4), of children who had and had no health education (P = 0.94). An overwhelming majority of older respondents (87%) than younger children (58%) did not know that lower abdominal pain is a symptom of schistosomiasis. Similarly, respondents from Xakao (99%), Sepopa (96%), and Mohembo (90%) didn’t know that abdominal pain was a symptom of the disease. A chi-square test for the difference in knowledge of abdominal pains showed that children from the three villages were more likely (P ≤ 0.001) to demonstrate a lack of knowledge of abdominal pain as a symptom than children from other villages. The results for the difference in knowledge of abdominal pain as a symptom between age groups (P = 0.29), gender (P = 0.75), and children with no or some health education (P = 0.97) were not significant.
Table 4.
Knowledge of signs and symptoms of schistosomiasis by demographic characteristics
| Socio-demographics | Frequency (n) | Fever (%) | P-value | Blood in Urine (%) | P-Value | Lower Abdominal Pain (%) | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | ||||||
| Age | 6–9 | 282 | 88 | 12 | 0.08 | 75 | 25 | 0.11 | 58 | 17 | 0.29 |
| 10–13 | 198 | 82 | 18 | 54 | 46 | 87 | 13 | ||||
| Total | 480 | 85 | 15 | 65 | 36 | 73 | 15 | ||||
| Gender | Female | 245 | 85 | 15 | 0.08 | 69 | 31 | 0.001 | 85 | 15 | 0.75 |
| Male | 235 | 86 | 14 | 63 | 37 | 84 | 15 | ||||
| Total | 480 | 86 | 15 | 66 | 34 | 85 | 15 | ||||
| Village | Mohembo | 101 | 81 | 13 | 0.02 | 75 | 25 | 0.001 | 90 | 10 | 0.001 |
| Ngarange | 68 | 82 | 18 | 47 | 53 | 87 | 13 | ||||
| Samochima | 93 | 75 | 25 | 35 | 65 | 61 | 39 | ||||
| Sekondomboro | 63 | 87 | 13 | 60 | 40 | 78 | 24 | ||||
| Sepopa | 55 | 93 | 13 | 85 | 15 | 96 | 4 | ||||
| Xakao | 99 | 91 | 9 | 91 | 9 | 99 | 1 | ||||
| Total | 480 | 85 | 15 | 60 | 35 | 85 | 15 | ||||
| Health education status | No health education | 297 | 86 | 14 | 0.94 | 73 | 27 | 0.00 | 85 | 15 | 0.97 |
| Had health education | 183 | 86 | 14 | 55 | 46 | 85 | 15 | ||||
| Total | 480 | 86 | 14 | 64 | 37 | 85 | 15 | ||||
Knowledge of risky behaviors for the disease among respondents
The majority (71%) of respondents aged 6–9 years knew that swimming was a risk factor for schistosomiasis, with (81%) of those who had health education possessing knowledge. An equal proportion of females (76%) and males (77%) knew that swimming poses a risk for the disease. One hundred and seventy of the respondents (85%) who had heard about schistosomiasis, knew that swimming was a risk for the disease. The majority (81%) of respondents from Samochima and Xakao (79%) villages knew swimming was a risk factor for Bilharzia. A chi-square test showed that older respondents and those with health education were more likely to know that swimming as a risk factor (P ≤ 0.001) and (P = 0.04) respectively. Similarly, children who have heard about schistosomiasis were more likely to know swimming was a risk factor for the disease (refer to Table 5). There were no significant differences within villages between those who know that swimming is a risk factor and those who did not know (P = 0.12).
Table 5.
Knowledge of swimming as a risk behaviour for Schistosomiasis by socio-demographics
| Factor | Variable | Frequency(n) | Swimming (%) | P-Value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Age | 6–9 | 282 | 29 | 71 | ≤ 0.001 |
| 10–13 | 198 | 16 | 84 | ||
| Total | 480 | 23 | 78 | ||
| Gender | Females | 245 | 24 | 76 | 0.86 |
| Males | 235 | 23 | 77 | ||
| Total | 480 | 24 | 77 | ||
| Village | Mohembo | 101 | 31 | 69 | 0.12 |
| Ngarange | 68 | 15 | 85 | ||
| Samochima | 93 | 19 | 81 | ||
| Sekondomboro | 63 | 29 | 71 | ||
| Sepopa | 55 | 29 | 71 | ||
| Xakao | 100 | 21) | 79 | ||
| Total | 480 | 24 | 76 | ||
| Health education | No education | 297 | 27 | 73 | 0.04 |
| Had education | 183 | 19 | 81 | ||
| Total | 480 | 23 | 77 | ||
| Heard about disease | Not heard | 280 | 30 | 70 | ≤ 0.001 |
| Heard | 200 | 15 | 85 | ||
| Total | 480 | 23 | 77 | ||
Children aged 10–13 years (71%) and those who received health education (74%) knew that walking barefooted in water poses a risk for schistosomiasis. Females (72%) were more knowledgeable about this fact than males (65%). The respondents who had received health education were more likely to know that walking in water barefooted was a risk factor for schistosomiasis (P = 0.03). There was no significant difference in age and gender (refer to Table 6) between those who did not know and those who know that walking barefooted in water was a risk factor for schistosomiasis Most (77%) of children who had heard about schistosomiasis knew that walking bearfooted in water was a risk for contarcting the disease compared to those who had not heard about it (63%). These results were significant (P = 0.001). Three quarters of children from Ngarange and Xakao (75% and 74% respectively) knew that walking barefooted in water was a risk for schistosomiasis. The comparison across villages were insignificant (P = 0.47) [see Table 6].
Table 6.
Knowledge Walking in Water Barefooted by Socio-Demographics
| Factor | Variable | Frequency (n) | Walking in water barefooted (%) | P-value | |
|---|---|---|---|---|---|
| No | Yes | ||||
| Age | 6–9 | 282 | 33 | 67 | 0.39 |
| 10–13 | 198 | 29 | 71 | ||
| Total | 480 | 31 | 69 | ||
| Gender | Females | 245 | 35 | 72 | 0.11 |
| Males | 235 | 35 | 65 | ||
| Total | 480 | 35 | 69 | ||
| Health education | No education | 297 | 35 | 65 | 0.03 |
| Had education | 183 | 26 | 74 | ||
| Total | 480 | 31 | 70 | ||
| Village | Mohembo | 101 | 35 | 65 | 0.47 |
| Ngarange | 68 | 25 | 75 | ||
| Samochima | 93 | 32 | 68 | ||
| Sekondomboro | 63 | 37 | 64 | ||
| Sepopa | 55 | 36 | 64 | ||
| Xakao | 100 | 26 | 74 | ||
| Total | 480 | 32 | 68 | ||
| Heard about disease | Not heard | 280 | 37 | 63 | 0.001 |
| Heard | 200 | 24 | 77 | ||
| Total | 480 | 31 | 70 | ||
The majority of female respondents (76%) considered drinking contaminated water as a risk for schistosomiasis compared to males (72%). Of the 198 respondents aged 10–13, (78%) mentioned drinking contaminated water was a risk for the disease compared to (71%) of those aged 6–9 years. Respondents from Samochima (59%) and Sekondomboro (60%) did not mention this factor as a risk for the disease. Respondents who had health education (83%) on the diseases mentioned drinking contaminated water as a risk factor to those who had no education (69%) of the disease. Health education (P = 0.01), and village (P = 0.001) were significantly associated with mentioning drinking contaminated water as a risk for the disease.
Discussion
Active participation of local populations in prevention and control programs of schistosomiasis in endemic areas, is highly dependent on adequate knowledge and awareness of the disease, especially among children who are often the target of control programs. Our study findings show low levels of awareness of the disease, where only a few of the respondents had heard about the disease and just above half knew the local name of schistosomiasis. These findings corroborate those of other studies [37]. Respondents who had health education at least knew that haematuria was a sign of the disease. However, having health education did not influence knowledge of the signs and symptoms of the disease because our study records low levels of knowledge of the signs and symptoms. It is also important to note that the few respondents who had heard about the disease got information from school, while the health sector's role in educating children was very insignificant. This may be attributed to the decadal neglect of the disease since 1993 when the national control program was terminated. A high proportion of respondents had no health education on the disease. This shows that health education as a control strategy has not been implemented for over two decades. Our results are similar to a Yemen study [38], which reported a lower knowledge of signs and symptoms but a higher level of awareness. A systematic review of the knowledge, attitude and practices on schistosomiasis found that knowledge of signs and symptoms is often low [39]. This poses challenges and threats to the uptake of most prevention and control strategies.
Our study found that males and those who had health education significantly knew haematuria as a symptom compared to females and those without health education. This could be explained by the fact that the universal local name for urinary schistosomiasis in Botswana is “thuti-sa-madi" which means blood in the urine. This finding is similar to that obtained in Swaziland [2] where 74% mentioned haematuria as a symptom. Again Folefac and collegues [40] in their study, recorded that among respondents who had prior knowledge of the disease, 80% mentioned haematuria as a symptom. With regards to males possessing more knowledge than females, a systematic review in sub-Saharan Africa [39], found eight out of twelve studies that reported high levels of knowledge among males compared to females. The authors of a review [39] attributed better knowledge among males to health education provided to fishermen and farmers because of their exposure to risky water practices. The difference between the review and the Cameroon study [40] and our study is that our respondents were SAC while the two studies focused on adults.
Our study also revealed that respondents do not know the cause and the life cycle of schistosomiasis. This finding is similar to what Swaziland's [2] study obtained. However, Kitalile [41] reported a higher proportion (77.6%) of respondents who knew the cause of schistosomiasis. The difference may be that we asked closed-ended questions which limited exploration of possible responses which could have unearthed the real causes of the disease. Part of these differences might have been related to the fact that these studies used a different study method (that is, non-probability purposive sampling technique) than ours [42].
Regarding the mode of transmission of the disease, which is indicated as a proxy from the question on drinking contaminated water, our study found some misconceptions from the respondents. A significant proportion of pupils across all villages mentioned that drinking contaminated water poses a risk of contracting schistosomiasis. This can be attributed to the fact that residents in the Okavango area often depend on contaminated river water for domestic purposes [43]. Consequently, outbreaks of water-borne disease (diarrhea) are common, especially during the dry season [44]. In addition to this, it is worth noting that the recurring unavailability and unreliability of water due to nonfunctioning taps and pumps and leaks experienced by villages in the Okavango delta, often prompt the utilization of river water for domestic use even in schools [45]. This may have prompted the respondents in our study to believe that schistosomiasis may be caused by drinking contaminated water. Moreover, this could suggest the lack of integrative approaches toward schistosomiasis and STH programming. Of greater concern is how these fears translate into preventive practices.
Regarding the knowledge of risk behaviours for schistosomiasis, our study found that younger pupils (aged 6–9 years) were more likely to know that swimming is a risk for the disease. This may be the influence of the teaching of content on schistosomiasis which is done at lower than upper school grades. Moreover, the health education in schools during MDA for STH in the past two years has contributed to this finding. Other studies [39] concurred that programs such as MDA improve awareness, knowledge, and uptake of preventive chemotherapy.
Notwithstanding the important findings from our study, we acknowledge the following limitations encountered during the implementation of the study; firstly, there was an issue with the subjectivity of responses. The self-reported data obtained from respondents lacked objectivity. Secondly, the study did not provide in-depth information about reasons for not having knowledge about schistosomiasis. It is important to note that being a cross-sectional survey, the study could not establish causal factors for the lack of knowledge and awareness which could be provided by analytic designs. There is a need, therefore, for a future qualitative and more analytic research design for this purpose.
The small sample size although adequate for statistical analysis used in the study, restricted the generalization of findings to the population, because the study was conducted in one sub-district, and hence may not be generalised to other sub-districts and districts elsewhere. Future studies must therefore target more districts using random sampling procedures for generalisation across the country. Not withstanding these limitations, the study results provided insights in knowledge and awareness of schistosomiasis among schools within the Okavango sub-district, which in turn informs and guided the following conclusions and recommendations.
Conclusions
This study found that school children in the sampled schools of the Okavango delta lack awareness and knowledge of schistosomiasis with the majority not knowing the life cycle and most of the symptoms of the disease. This provides a window of opportunity to re-instating prevention and control measures of the disease within the area. SAC demonstrated a fairly average knowledge of water contact and risky behaviors of swimming and walking barefooted in infested water. Health education has impacted positively on the children’s awareness and knowledge, but other socio-demographics of age and gender did not. This is a signal of the importance of health education as a key component of the control program for schistosomiasis. There is thus the need for intense integration of school-based health education to improve the level of knowledge and awareness to reduce children’s water contact behaviors. Further research focused on unearthing reasons for low levels of awareness and knowledge is required to inform policy on elimination. More consideration and appreciation of the socio-cultural and cognitive behaviors of children than a focus on their age, gender, and place of residence which are found to have very little influence on their knowledge of risk behaviours for schistosomiasis prevention in the present study.
Acknowledgements
The authors are grateful to the National Institute for Health Research (NIHR) Global Health Research Programme (16/136/33) for giving the first author scholarship using UK aid from UK Government. Our sincere thanks to the school children who volunteered to participate in the study.
Abbreviations
- MDA
Mass Drug Administration
- NTD
Neglected Tropical Disease
- SAC
School-Aged Children
- STH
Soil-Transmitted Helminths
- WHA
World Health Assembly
- WHO
World Health Organization
Author contributions
GPK conceptualized the study, obtained ethical clearance, did the formal analysis, and wrote the draft manuscript. PN acquired funding and together with DELP offered supervision and editing while TTO helped with the writing. All authors read and approve the manuscript.
Funding
This work was supported by National Institute for Health Research (NIHR) Global Health Research program (16/136/33). The corresponding author is ultimately responsible for the decision of submission and publication.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Ethical approval and consent for participation
The study was reviewed and approved by the Human Research Development Committee at the Botswana Ministry of Health and Wellness (letter referenced HPDME: 13/18/1) and the University of Botswana Institutional Review Board (referenced UBR/RES/IRB/BIO/154). Parental consent was sought and children were asked for assent to their participation in the study.
Consent for publication
The regional (Okavango) education office, parents, and children consented to participate in the study. Consent, assent forms, and letters are available from the corresponding author upon request.
Competing interests
DELP is the Deputy Editor-in-Chief for the Journal of Global Health Research and Policy. He is not invovled in the review of decision related to the manuscript.
References
- 1.Donohue RE, Mashoto KO, Mubyazi GM, Madon S, Malecela MN, Michael E. Biosocial determinants of persistent schistosomiasis among schoolchildren in Tanzania despite repeated treatment. Trop Med Infect Dis. 2017;2(4):61. doi: 10.3390/tropicalmed2040061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maseko TS, Mkhonta NR, Masuku SK, Dlamini SV, Fan C-K. Schistosomiasis knowledge, attitude, practices, and associated factors among primary school children in the Siphofaneni area in the Lowveld of Swaziland. J Microbiol Immunol Infect. 2018;51(1):103–109. doi: 10.1016/j.jmii.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Schistosomiasis and soil-transmitted helminthiases: number of people treated in 2015. Wkly Epidemiol Rec. 2016;91(49/50):585–595. [PubMed] [Google Scholar]
- 4.Chitsulo L, Engels D, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77(1):41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King CH, Galvani AP. Underestimation of the global burden of schistosomiasis. Lancet. 2018;391(10118):307–308. doi: 10.1016/S0140-6736(18)30098-9. [DOI] [PubMed] [Google Scholar]
- 6.Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis. 2015;19:196–205. doi: 10.1016/j.bjid.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colley D, Secor W. Immunology of human schistosomiasis. Parasite Immunol. 2014;36(8):347–357. doi: 10.1111/pim.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ministry of Health and Wellness. The mapping Exrecise for Schistosomiasis. 2015.
- 9.WHO Expert Committee on the Control of Schistosomiasis, World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. World Health Organization; 2002. [PubMed]
- 10.Kabuyaya M, Chimbari MJ, Manyangadze T, Mukaratirwa S. Schistosomiasis risk factors based on the infection status among school-going children in the Ndumo area, uMkhanyakude district, South Africa. South Afr J Infect Dis. 2017;32(2):67–72. [Google Scholar]
- 11.Appleton CC, Ellery W, Byskov J, Mogkweetsinyana S. Epidemic transmission of intestinal schistosomiasis in the seasonal part of the Okavango Delta, Botswana. Ann Trop Med Parasitol. 2008;102(7):611–623. doi: 10.1179/136485908X311867. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy TS, Bloem A, Larkin P. Observations on the hydrology and geohydrology of the Okavango delta. S Afr J Geol. 1998;101(2):101–117. [Google Scholar]
- 13.Appleton C, Madsen H. Human schistosomiasis in wetlands in southern Africa. Wetl Ecol Manag. 2012;20(3):253–269. doi: 10.1007/s11273-012-9266-2. [DOI] [Google Scholar]
- 14.World Health Organization Schistosomiasis: number of people receiving preventive chemotherapy in 2012. Wkl Epidemiol Rec. 2014;89(02):21–8. [PubMed] [Google Scholar]
- 15.World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. World Health Organization; 2006.
- 16.Tchuenté LAT, Rollinson D, Stothard JR, Molyneux D. Moving from control to elimination of schistosomiasis in sub-Saharan Africa: time to change and adapt strategies. Infect Dis Poverty. 2017;6(1):1–14. doi: 10.1186/s40249-017-0256-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Clercq D, Vercruysse J, Verle P, Kongs A, Diop M. What is the effect of combining artesunate and praziquantel in the treatment of Schistosoma mansoni infections? Trop Med Int Health. 2000;5(10):744–746. doi: 10.1046/j.1365-3156.2000.00628.x. [DOI] [PubMed] [Google Scholar]
- 18.Mutapi F, Ndhlovu P, Hagan P, Woolhouse M. A comparison of re-infection rates with Schistosoma haematobium following chemotherapy in areas with high and low levels of infection. Parasite Immunol. 1999;21(5):253–260. doi: 10.1046/j.1365-3024.1999.00227.x. [DOI] [PubMed] [Google Scholar]
- 19.Sokolow SH, Wood CL, Jones IJ, Swartz SJ, Lopez M, Hsieh MH, et al. Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl Trop Dis. 2016;10(7):e0004794. doi: 10.1371/journal.pntd.0004794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Motsholapheko M, Ngwenya B. Access to water resources and household vulnerability to malaria in the Okavango Delta, Botswana. In: African Handbook of Climate Change Adaptation. 2020:1–20.
- 21.Statistics Botswana Population and Housing Census 2011. Printed and obtainable from Department of Government Printing and Publishing Services, GABORONE Botswana; 2011.
- 22.Thakadu OT, Tau OS. Communicating environment in the Okavango Delta, Botswana: an exploratory assessment of the sources, channels, and approaches used among the delta communities. Sci Commun. 2012;34(6):776–802. doi: 10.1177/1075547012437277. [DOI] [Google Scholar]
- 23.Adam AM. A study on sample size determination in survey research. New Ideas Concern Sci Technol. 2021;4:125–134. doi: 10.9734/bpi/nicst/v4/2125E. [DOI] [Google Scholar]
- 24.Clark LA, Watson D. Constructing validity: new developments in creating objective measuring instruments. Psychol Assess. 2019;31(12):1412. [DOI] [PMC free article] [PubMed]
- 25.Xiao H, Peng M, Yan H, Gao M, Li J, Yu B, et al. An instrument based on protection motivation theory to predict Chinese adolescents’ intention to engage in protective behaviors against schistosomiasis. Glob Health Res Policy. 2016;1(1):1–9. doi: 10.1186/s41256-016-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Zhao A, Wang L-W, Zhang W-X, Huang C-Q, Tang Q-Q. Evaluation of health education in primary school students from schistosomiasis-endemic areas around Poyang Lake by KAP hierarchical evaluation method. Chin J Parasitol Parasit Dis. 2013;31(5):390–4. [PubMed] [Google Scholar]
- 27.Wang XY, He J, Juma S, Kabole F, Guo JG, Dai JR, Li W, Yang K. Efficacy of China-made praziquantel for treatment of Schistosomiasis haematobium in Africa: A randomized controlled trial. PLoS Neglect Tropic Disease. 2019;13(4):e0007238. [DOI] [PMC free article] [PubMed]
- 28.Vuoso K. Schistosomiasis: a review of other public health interventions. Undergrad J Public Health. 2022 doi: 10.3998/ujph.2309. [DOI] [Google Scholar]
- 29.Sacolo-Gwebu H, Kabuyaya M, Chimbari M. Knowledge, attitudes and practices on schistosomiasis and soil-transmitted helminths among caregivers in Ingwavuma area in uMkhanyakude district, South Africa. BMC Infect Dis. 2019;19(1):1–11. doi: 10.1186/s12879-019-4253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian R, Dan K, Sandrine M, Jorge A, Jamie T, Helen C, et al. Have you heard of schistosomiasis? Knowledge, attitudes and practices in Nampula province. Mozambique. PLoS Negl Trop Dis. 2016 doi: 10.1371/journal.pntd.0004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hambury SD, Grobler AD, Melariri PE. Knowledge, attitudes, and practices on urinary schistosomiasis among primary schoolchildren in Nelson Mandela Bay, South Africa. J Parasitol Res. 2021 doi: 10.1155/2021/6774434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mwanga JR, Lwambo NJ. Pre-and post-intervention perceptions and water contact behaviour related to schistosomiasis in north-western Tanzania. Acta Trop. 2013;128(2):391–398. doi: 10.1016/j.actatropica.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Yirenya-Tawiah DR, Ackumey MM, Bosompem KM. Knowledge and awareness of genital involvement and reproductive health consequences of urogenital schistosomiasis in endemic communities in Ghana: a cross-sectional study. Reprod Health. 2016;13(1):1–8. doi: 10.1186/s12978-016-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sow S, de Vlas SJ, Mbaye A, Polman K, Gryseels B. Low awareness of intestinal schistosomiasis in northern Senegal after 7 years of health education as part of intense control and research activities. Trop Med Int Health. 2003;8(8):744–749. doi: 10.1046/j.1365-3156.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- 35.Assefa A, Erko B, Gundersen SG, Medhin G, Berhe N. Low awareness and common misconceptions about schistosomiasis in endemic lowland areas in Western Ethiopia: a mixed-methods study. BMC Public Health. 2021;21(1):1–12. doi: 10.1186/s12889-021-11106-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odhiambo GO, Musuva RM, Atuncha VO, Mutete ET, Odiere MR, Onyango RO, et al. Low levels of awareness despite high prevalence of schistosomiasis among communities in Nyalenda informal settlement, Kisumu City, Western Kenya. PLoS Negl Trop Dis. 2014;8(4):e2784. doi: 10.1371/journal.pntd.0002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briggs SR, Cheek JM. The role of factor analysis in the development and evaluation of personality scales. J Pers. 1986;54(1):106–148. doi: 10.1111/j.1467-6494.1986.tb00391.x. [DOI] [Google Scholar]
- 38.Sady H, Al-Mekhlafi HM, Atroosh WM, Al-Delaimy AK, Nasr NA, Dawaki S, et al. Knowledge, attitude, and practices towards schistosomiasis among rural population in Yemen. Parasit Vectors. 2015;8(1):1–13. doi: 10.1186/s13071-015-1050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacolo H, Chimbari M, Kalinda C. Knowledge, attitudes and practices on schistosomiasis in sub-Saharan Africa: a systematic review. BMC Infect Dis. 2018;18(1):1–17. doi: 10.1186/s12879-017-2923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Folefac LN, Nde-Fon P, Verla VS, Tangye MN, Njunda AL, Luma HN. Knowledge, attitudes and practices regarding urinary schistosomiasis among adults in the Ekombe Bonji health area, Cameroon. Pan Afr Med J. 2018;29(1):1–9. doi: 10.11604/pamj.2018.29.161.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitalile J. Urinary schistosomiasis: knowledge. Attitude and practice among primary school children in Mtera dam area, Mpwapwa district. 2012.
- 42.Acharya S. Developing novel ways of studying motility in Schistosoma mansoni and its potential contribution towards inhibiting Schistosomiasis. 2019.
- 43.Tubatsi G, Bonyongo M, Gondwe M. Water use practices, water quality, and households’ diarrheal encounters in communities along the Boro-Thamalakane-Boteti river system, Northern Botswana. J Health Popul Nutr. 2015;33(1):1–12. doi: 10.1186/s41043-015-0031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexander KA, Heaney AK, Shaman J. Hydrometeorology and flood pulse dynamics drive diarrheal disease outbreaks and increase vulnerability to climate change in surface-water-dependent populations: a retrospective analysis. PLoS Med. 2018;15(11):e1002688. doi: 10.1371/journal.pmed.1002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngwenya B, Thakadu O, Phaladze N, Bolaane B. Access to water and sanitation facilities in primary schools: a neglected educational crisis in Ngamiland district in Botswana. Phys Chem Earth Parts A/B/C. 2018;105:231–238. doi: 10.1016/j.pce.2018.03.006. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.