Abstract
Scanning laser ophthalmoscopes (SLOs) are used widely for reflectance, fluorescence or autofluorescence photography and less commonly for retroillumination imaging. SLOs scan a visible light or near-infrared radiation laser beam across the retina, collecting light from each retinal spot as it’s illuminated. An SLO’s clinical applications, image contrast and axial resolution are largely determined by an aperture overlying its photodetector. High contrast, reflectance images are produced using small diameter, centered apertures (confocal apertures) that collect retroreflections and reject side-scattered veiling light returned from the fundus. Retroillumination images are acquired with annular on-axis or laterally-displaced off-axis apertures that capture scattered light and reject the retroreflected light used for reflectance imaging. SLO axial resolution is roughly 300 μm, comparable to macular thickness, so SLOs cannot provide the depth-resolved chorioretinal information obtainable with optical coherence tomography’s (OCT’s) 3 μm axial resolution. Retroillumination highlights and shades the boundaries of chorioretinal tissues and abnormalities, facilitating detection of small drusen, subretinal drusenoid deposits and subthreshold laser lesions. It also facilitates screening for large-area chorioretinal irregularities not readily identified with other en face retinal imaging modalities. Shaded boundaries create the perception of lesion elevation or depression, a characteristic of retroillumination but not reflectance SLO images. These illusions are not reliable representations of three-dimensional chorioretinal anatomy and they differ from objective OCT en face topography. SLO retroillumination has been a useful but not indispensable retinal imaging modality for over 30 years. Continuing investigation is needed to determine its most appropriate clinical roles in multimodal retinal imaging.
Keywords: Confocal, Light scattering, Optical coherence tomography, Pseudocolor, pseudo3D, Retinal imaging, Retroillumination, Scanning laser ophthalmoscope, Shape-from-shading, Transillumination, Visual illusion
Background
Confocal scanning laser ophthalmoscopes (SLOs) are widely used to produce conventional reflectance monochromatic or multiwavelength fundus images. Some confocal SLOs have an additional retroillumination mode that can produce “pseudo-three-dimensional” (pseudo3D) images [1–10]. In their widely-used reflectance-mode (direct-mode), SLOs record images from light reflected directly back (retroreflected) from chorioretinal structures. In their retroillumination-mode (indirect-mode), SLOs create images from light or infrared radiation that transilluminates chorioretinal structures as it returns indirectly from deeper choroidal and scleral layers.
Retroillumination highlights and shades the boundaries of chorioretinal tissues and abnormalities, facilitating detection of small drusen, subretinal drusenoid deposits and subthreshold laser lesions [1–10]. It also facilitates identification of large-area chorioretinal irregularities not readily displayed in other en face retinal imaging modalities [11–14]. Shaded boundaries create the perception of elevation or depression in imaged structures [15–22], a characteristic of retroillumination but not standard reflectance SLO imaging. This perception is an illusion rather than an objective three-dimensional representation of chorioretinal anatomy as provided by optical coherence tomography (OCT). We review and analyze the tissue-optics, clinical applications and limitations of SLO transillumination imaging.
Confocal SLOs
Clinical confocal SLOs sweep a 10–15 μm diameter laser spot across the fundus and collect light point-by-point from sequentially illuminated retinal sites [23–26]. The retina is exposed only to one small diameter, low irradiance laser spot at a time so SLO imaging is comfortable and safe for patients and provides high lateral image resolution [23–26].
An SLO’s laser beam enters the retina roughly perpendicular to the plane of the retina [23–26]. Light absorption and light scattering including reflection and refraction attenuate the laser beam progressively as it descends to greater chorioretinal depths [27–29]. Light scattering is most prominent at tissue interfaces and surfaces where there are changes in refractive index, including the sclera, retinal pigment epithelium (RPE) and internal limiting membrane (ILM) [30, 31]. Longer wavelengths (red and near-infrared) penetrate more deeply into the choroid than shorter wavelengths (blue) largely because optical radiation absorption by the primary chorioretinal absorbers (melanin, hemoglobin and macular pigment) is lower at longer wavelengths [27, 32, 33].
One or more lasers are used to produce monochromatic or multicolor SLO images, respectively. Monochromatic images are used in OCT systems to localize B-scans [34]. Multicolor images are created by combining monochromatic images [35, 36]. Multicolor confocal SLOs are useful alternatives for fundus cameras despite color imaging differences [37–40]. Some confocal SLOs can produce both (1) conventional reflectance images (reflectance-mode) as shown in Fig. 1A and (2) pseudo3D retroillumination-mode images as shown in Figs. 1B, 1C and 1D. We used a Mirante SLO (Nidek Co., Ltd., Gamagori, Japan) and a Cirrus 5000 HD-OCT (Carl Zeiss AG, Jena, Germany) to acquire the SLO and OCT images in this report, respectively.
Fig. 1.
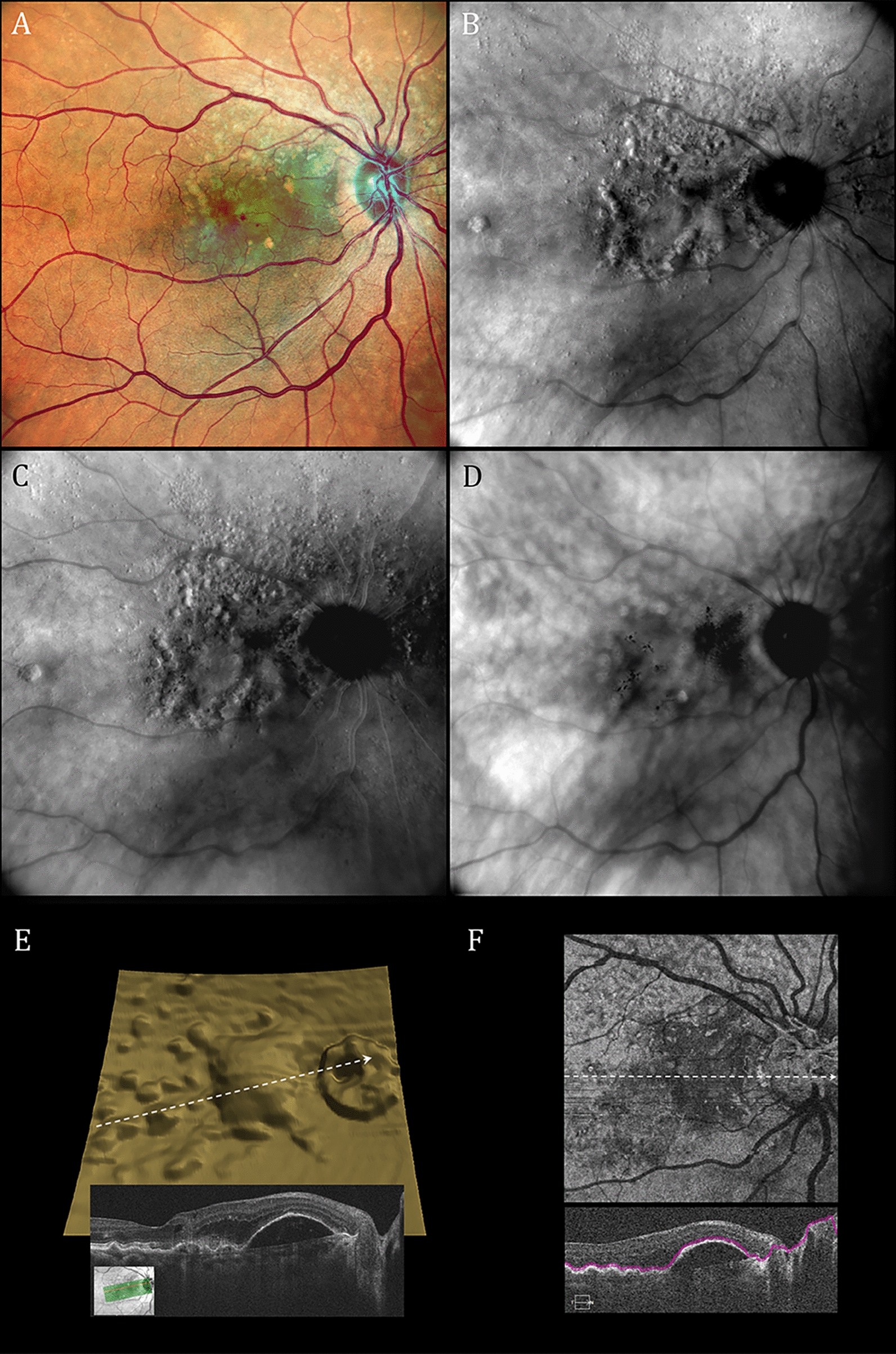
Reflectance and retroillumination scanning laser ophthalmoscope (SLO) as well as optical coherence tomography (OCT) images of the right retina of a 75-year-old female with neovascular age-related macular degeneration, small subretinal hemorrhages, a large vascularized retinal pigment epithelial detachment and numerous drusen. A Reflectance multiwavelength SLO image. B Retroillumination SLO image taken with a deviated-to-the-right (DR) confocal aperture. C Retroillumination SLO image taken with a deviated-to-the-left (DL) aperture. D Retroillumination SLO image taken with a ring aperture (RA; annular aperture). Prominent lesion highlighting and border shading in B and C are absent with the Mirante ring aperture but present in published images with annular apertures using other SLO retroillumination systems. [1–4, 49, 54] E Segmented retinal pigment epithelium three-dimensional OCT topography differing from retroillumination shading patterns in B and C (inset shows B-scan along white arrow). F En face structural OCT image corresponding roughly to tissue planes of RPE topography in E and drusen in B and C
Reflectance-mode imaging
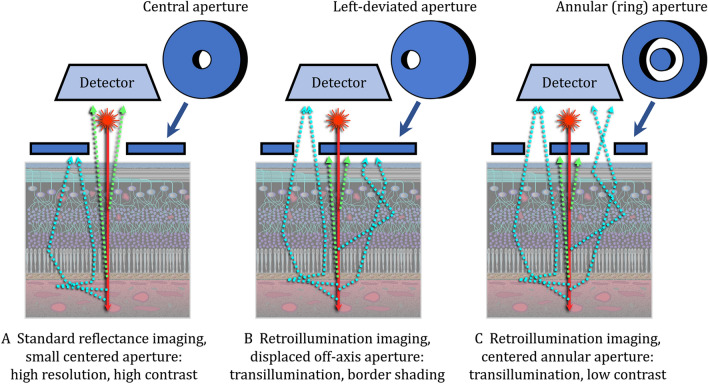
Confocal SLOs produce reflectance images (Figs. 1A and 2A) from laser light scattered directly back from the fundus (hence the term direct-mode for reflectance imaging) [4, 25, 41]. The system is called confocal because the scanning laser beam and a small aperture centered in front of the SLO’s photodetector are both focused on the same retinal location [25]. The small confocal aperture (1) restricts light collection to photons retroreflected from the illuminated retinal focal point, (2) blocks veiling glare that could be produced by photons scattered back to the photodetector from other retinal locations and (3) narrows depth of focus (increases axial resolution) [1, 25, 29, 42]. Reducing depth of focus (optical slab thickness) also eliminates eyelash artifacts encountered with larger aperture ultra-widefield SLO systems [43].
Fig. 2.
Scanning laser ophthalmoscopy (SLO) confocal apertures for reflectance (direct-mode) and retroillumination (indirect-mode) SLO imaging. A In standard reflectance imaging, a centered confocal aperture limits light collection only to photons reflected “directly” back to the photodetector from the illuminated retinal spot, [25] thereby increasing retinal image contrast by blocking photons from other fundus locations that could cause veiling glare at the photodetector. [25, 64] B and C. In retroillumination imaging, an aperture deviated laterally (B) or an annular aperture (C) blocks retroreflected light and collects only photons scattered “indirectly” back to the SLO’s photodetector. Laterally-deviated (B) or annular (C) apertures assure asymmetric or symmetric light collection in the retinal plane, respectively. In the Mirante system, asymmetric retroillumination imaging (B) transilluminates, highlights and shades borders of imaged chorioretinal structures whereas symmetric annular (ring) aperture light collection (C) provides low contrast transillumination images
Confocal apertures in SLO systems provide axial resolutions of only ~ 300 μm, [34, 44–47] equivalent to total macular thickness (285 μm) [48]. Thus, even tomographic SLO systems could not offer the intraretinal detail available with OCT’s 3 μm axial resolution [49, 50]. Larger area apertures used for retroillumination-mode or ultra-widefield SLO imaging have even lower axial resolutions (larger optical slab thickness) [1, 25, 42].
Retroillumination-mode imaging
Confocal SLOs produce pseudo3D images by replacing the small confocal aperture centered in front of the SLO’s photodetector (Fig. 2A, reflectance-mode) with a laterally-displaced (deviated) aperture (Fig. 2B) or a centered annular (ring-shaped) aperture (Fig. 2C) [1, 4, 5, 7, 25, 51]. Laterally-displaced and annular apertures both (1) collect initially scattered light that returns indirectly to the detector (hence the term indirect-mode for retroillumination imaging) and (2) reject light retroreflected directly back from the laser-illuminated retinal spot. In essence, retroillumination-mode imaging uses light that is rejected in reflectance-mode imaging and reflectance-mode imaging uses light rejected in retroillumination-mode imaging. Retroillumination images are created from light transilluminating chorioretinal structures (retroillumination) rather than reflected off their inner surfaces [29, 44]. The Mirante SLO is the only currently-available commercial SLO system that provides retromillumination-mode imaging. Past SLO transillumination studies have used earlier versions of this system, research SLOs and a commercial device that is no longer available [1–6, 25]. Retroillumination-mode Mirante imaging uses infrared laser radiation (790 nm) because it penetrates more deeply into the choroid than visible (400 – 700 nm) light [7, 27, 28, 32]. Chorioretinal structures are transilluminated by multiply-scattered photons returning from the sclera and deep choroidal layers. Structures are rendered visible by reflection and refraction, much in the same way that an empty, transparent wine glass is visualized [25, 29]. Tissue irregularities and abnormalities are visible as lesions or retinal areas shaded laterally from a light to a dark border. Tissue shading facilitates rapid detection of abnormalities that are more difficult to detect with other en face multimodal imaging methodologies. Figures 1B and 1C are typical Mirante pseudo3D images taken with deviated-to-the-right (DR) and a deviated-to-the-left (DL) apertures, respectively. Figure 1D was taken during the same imaging session using the Mirante annular ring aperture (RA) that produces low contrast images without the tissue shading needed for pseudo3D effects.
Pseudo3D imaging
Lesion shading and pseudo3D effects are often ascribed to the asymmetric off-axis (deviated right or left) positioning of the proprietary retro-mode apertures of the Mirante and its predecessor F-10 SLO system [5–7, 9, 52, 53]. Indeed, Mirante’s symmetric ring-aperture typically produces only low-contrast, non-pseudo3D images. Nonetheless, symmetric annular (ring) apertures in other commercial and experimental SLO systems create pseudo3D images with non-horizontal as well as horizontal shading gradients [1–4, 49, 54]. Contrast generated in retroillumination-mode imaging depends on aperture design, which the Mirante’s manufacturer does not disclose. In general, smaller area apertures produce higher image contrast and narrower depth of focus [25, 55, 56].
SLO retroillumination image shading has been compared to lunar mountain shadowing [7, 9]. For example, it was reported that “In retromode imaging, the lateral incident light generates a shadow on the side opposite to the incident light source, resulting in an appearance of the retina that shows some resemblance to the lunar landscape” [9]. That comparison is problematic because an SLO’s laser beam is incident perpendicular not oblique to the retinal surface so there is no “side opposite to the incident light source.” Moreover, the analogy is misleading clinically because accurate height information can be extracted from lunar shadows but not from SLO retroillumination image border shading. Specifically, photogrammetry can determine building, tree and impact crater-wall height from solar shadows but that is not possible with retromode boundary shading because it is caused by multiple intraretinal light scattering events.
Pseudo3D is shape-from-shading
The shape-from-shading percept causes the depth perceived in pseudo3D retroillumination-mode SLO images [57]. Shading and border structure help an observer identify a three-dimensional object’s shape but shape-from-shading percepts can produce false illusions of depth in two-dimensional objects [15, 16, 22]. Visual perception integrates sensory data with sensory biases acquired from prior experiences (“priors”), including the expectation that the shading of natural objects is caused by a single light source [15–22]. Shape-from-shading pseudo3D illusions have also been observed previously in scanning electron microscopy [58] and retinal differential interference contrast microscopy images. [59, 60]
Figure 3 demonstrates how the visual system uses shape-from-shading to perceive elevation and depth in intrinsically two-dimensional images. Perceived elevation or indentation depends on an observer’s belief that a light source is located superior, inferior or to the left or right of these hexagonal [18] or circular [15] patterns. There is no objective three-dimensional depth information encoded in Fig. 3’s patterns, as is present in a stereophotograph or hologram. Perceived depth in Fig. 3 is just a visual illusion. Apparent elevation or indentation can be reversed merely by rotating these objects 180 degrees in the horizonal plane or by an observer consciously changing the location of the light source believed to be causing the image shading.
Fig. 3.
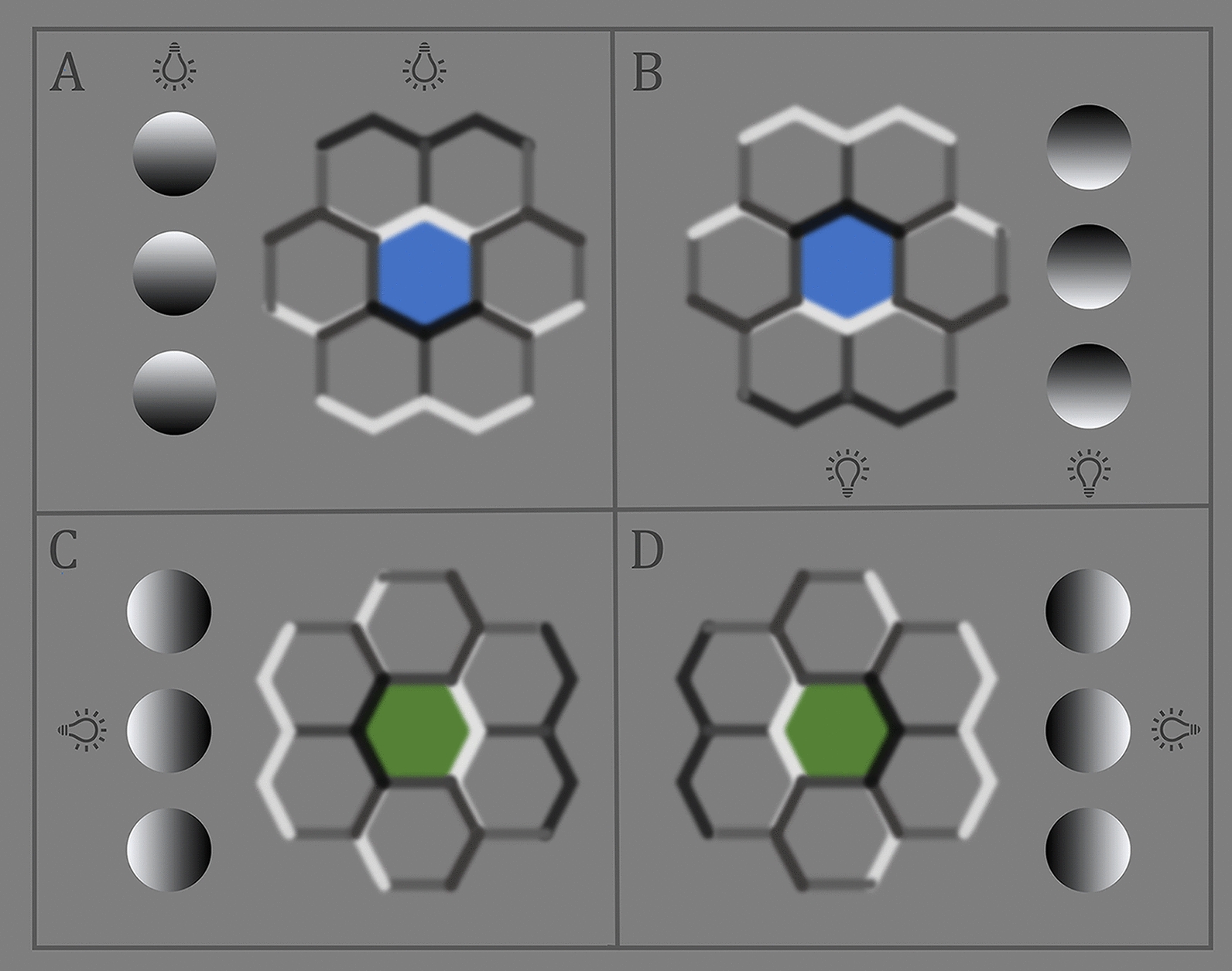
The visual system infers shape-from-shading of two-dimensional hexagonal [18] or circular [15] objects largely biased on the assumption that they are three dimensional objects illuminated by a single light source. There is no objective three-dimensional depth information in any of these two-dimensional patterns which are perceived to be elevated or depressed depending on whether the observer believes that the light source illuminating them is located superior or inferior (A and B), or to the left or right (C and D) of the image. The depth perceived in them is a visual illusion. Elevation and indentation can be reversed by rotating any of these objects 180 degrees in the horizontal plane or by an observer consciously changing the location of the light source believed to be responsible for the boundary shading
SLO pseudo3D and OCT
The exaggerated topographic irregularities perceived in Fig. 1B and C are illusory percepts that differ from OCT segmentation topography (Fig. 1E) and en face imaging (Fig. 1F) taken during the same clinical visit. En face OCT allows users to select the depth and thickness of an imaged retinal slab. Conversely, retroillumination-mode SLO’s full-thickness macula slab [44, 45, 47, 48] highlights only prominent chorioretinal disturbances, as shown in Figs. 1, 4 and 5. Border shading and accenting can identify abnormalities not readily visualized with other en face imaging modalities but they cannot provide OCT’s quantitative estimates of chorioretinal depth. Once identified, however, SLO retroillumination irregularities are readily evaluated with other forms of multimodal imaging.
Fig. 4.
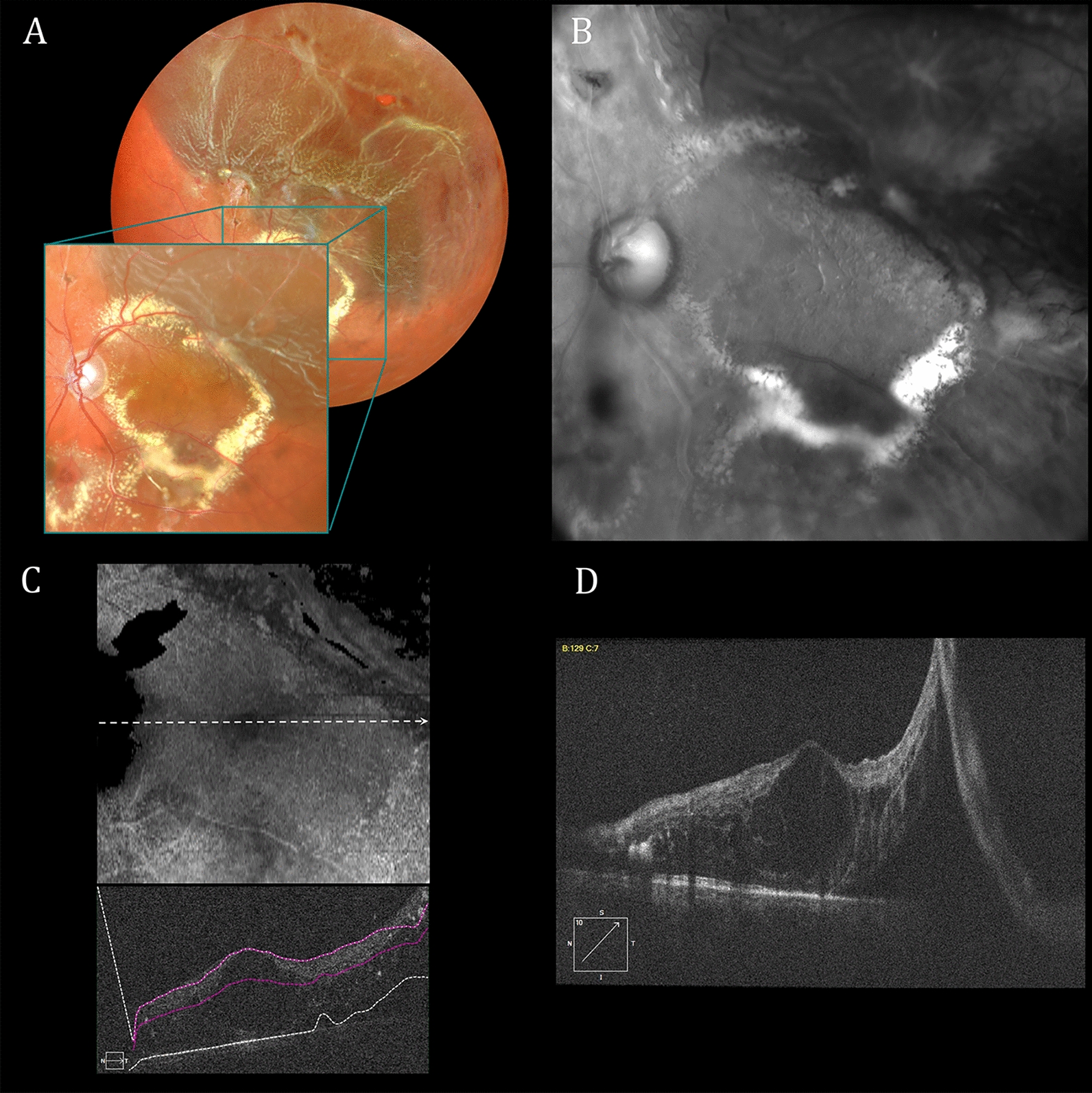
Reflectance and retroillumination scanning laser ophthalmoscope (SLO) as well as optical coherence tomography (OCT) images of the left retina of a 46-year-old man with Coats disease treated multiple times over a 15-year period with laser photocoagulation and intravitreal anti-VEGF therapy. A Reflectance multiwavelength SLO imaging shows a rhegmatogenous retinal detachment that extends centrally from a retinal hole in the superior-temporal mid-periphery. B Retroillumination SLO imaging with a deviated-to-the-left (DL) aperture shows widespread posterior pole retinal crinkling and cystoid changes associated with the macula-off detachment. C En face and associated B-scan OCT images display central wrinkling less prominently than in B. D OCT B-scan documenting a macula-off retinal detachment and prominent associated cystoid abnormalities
Fig. 5.
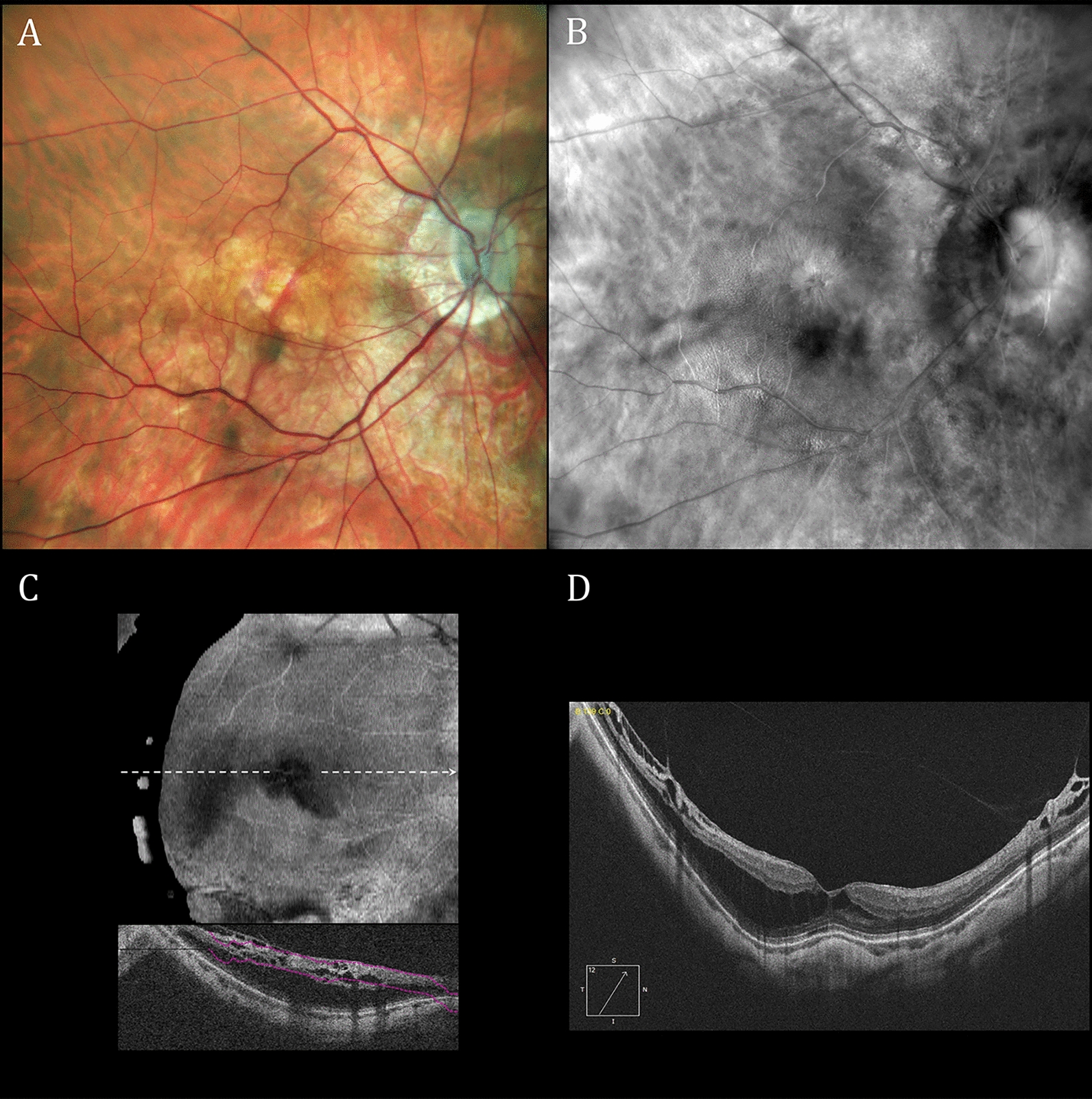
Reflectance and retroillumination scanning laser ophthalmoscope (SLO) as well as optical coherence tomography (OCT) images of the right retina of a 67-year-old male with myopic macular retinoschisis. A Reflectance multiwavelength SLO image shows a prominent myopic scleral crescent and reduced fundus pigmentation. B Retroillumination SLO image taken with a deviated-to-the-right (DR) aperture shows widespread posterior pole wrinkling due to macular retinoschisis. C En face and associated B-scan OCT images show less detailed and widespread cystic changes than in B. D OCT B-scan documents central and temporal macular retinoschisis
Tomography and stereoscopy have been used in the past to obtain chorioretinal depth information from confocal SLO technology. (1) Scanning laser tomography developed for optic disc topography analysis was used for volumetric macular edema [34, 61, 62]. (2) Paired reflectance SLO images taken at different angles were viewed stereoscopically to identify elevation in fluorescein angiographic lesion [63]. Neither method approaches OCT’s precision and clinical utility. Sequential confocal SLO and OCT imaging facilitates proper clinical interpretation of retroillumination SLO images.
Conclusions
Highlighting and shading of chorioretinal structure boundaries in SLO retroillumination images is useful for detecting small drusen, subretinal drusenoid deposits and subthreshold laser lesions. Additionally, it provides useful images of large-area chorioretinal irregularities not readily apparent with other en face retinal imaging modalities. Retroillumination SLO images are strikingly different from those produced by other en face imaging modalities. Their pseudo3D depth illusion caused by the shape-from-shading percept is perhaps their most distinguishing characteristic. It facilitates chorioretinal lesion screening and identification but does not provide objective depth information. Retroillumination-mode SLO imaging has been a useful but not indispensable retinal imaging modality for over 30 years. Continuing investigation is needed to determine its most appropriate clinical roles in multimodal retinal imaging.
Acknowledgements
The authors thank Christine A. Curcio, PhD, FARVO and Peter G. Harrington, BSc for helpful discussions.
Abbreviations
- ILM
Internal limiting membrane
- OCT
Optical coherence tomography
- RPE
Etinal pigment epithelium
- SLO
Scanning laser ophthalmoscope
- DR
SLO deviated-to-the-right aperture
- DL
SLO deviated-to-the-left aperture
- RA
SLO ring aperture
Declarations
Competing interests
The authors have no competing interests to declare.
Authors' contributions
Manuscript writing: M.A.M. Retinal photography: T.D. Image processing: G.L.-G. Artwork: M.A.M. Data, image and literature analysis: All authors. Review and approval of manuscript: All authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sharp PF, Manivannan A. The scanning laser ophthalmoscope. Phys Med Biol. 1997;42(5):951–966. doi: 10.1088/0031-9155/42/5/014. [DOI] [PubMed] [Google Scholar]
- 2.Ikeda T, Sato K, Katano T, Hayashi Y. Examination of patients with cystoid macular edema using a scanning laser ophthalmoscope with infrared light. Am J Ophthalmol. 1998;125(5):710–712. doi: 10.1016/S0002-9394(98)00008-7. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida A, Ishiko S, Akiba J, Kitaya N, Nagaoka T. Radiating retinal folds detected by scanning laser ophthalmoscopy using a diode laser in a dark-field mode in idiopathic macular holes. Graefes Arch Clin Exp Ophthalmol. 1998;236(6):445–450. doi: 10.1007/s004170050104. [DOI] [PubMed] [Google Scholar]
- 4.Remky A, Beausencourt E, Hartnett ME, Trempe CL, Arend O, Elsner AE. Infrared imaging of cystoid macular edema. Graefes Arch Clin Exp Ophthalmol. 1999;237(11):897–901. doi: 10.1007/s004170050383. [DOI] [PubMed] [Google Scholar]
- 5.Ohkoshi K, Tsuiki E, Kitaoka T, Yamaguchi T. Visualization of subthreshold micropulse diode laser photocoagulation by scanning laser ophthalmoscopy in the retro mode. Am J Ophthalmol. 2010;150(6):856–862. doi: 10.1016/j.ajo.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 6.Acton JH, Cubbidge RP, King H, Galsworthy P, Gibson JM. Drusen detection in retro-mode imaging by a scanning laser ophthalmoscope. Acta Ophthalmol. 2011;89(5):e404–411. doi: 10.1111/j.1755-3768.2011.02123.x. [DOI] [PubMed] [Google Scholar]
- 7.Diniz B, Ribeiro RM, Rodger DC, Maia M, Sadda S. Drusen detection by confocal aperture-modulated infrared scanning laser ophthalmoscopy. Br J Ophthalmol. 2013;97(3):285–290. doi: 10.1136/bjophthalmol-2012-302575. [DOI] [PubMed] [Google Scholar]
- 8.Parravano M, Querques L, Boninfante A, et al. Reticular pseudodrusen characterization by retromode imaging. Acta Ophthalmol. 2017;95(3):e246–e248. doi: 10.1111/aos.12948. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi M, Monteduro D, Parrulli S, et al. Sensitivity and specificity of multimodal imaging in characterizing drusen. Ophthalmol Retina. 2020;4(10):987–995. doi: 10.1016/j.oret.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Corradetti G, Byon I, Corvi F, Cozzi M, Staurenghi G, Sadda SR. Retro mode illumination for detecting and quantifying the area of geographic atrophy in non-neovascular age-related macular degeneration. Eye (Lond) 2021;36(8):1560–1566. doi: 10.1038/s41433-021-01670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka Y, Shimada N, Ohno-Matsui K, et al. Retromode retinal imaging of macular retinoschisis in highly myopic eyes. Am J Ophthalmol. 2010;149(4):635–640 e631. doi: 10.1016/j.ajo.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 12.Shin YU, Lee BR. Retro-mode Imaging for retinal pigment epithelium alterations in central serous chorioretinopathy. Am J Ophthalmol. 2012;154(1):155–163 e154. doi: 10.1016/j.ajo.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 13.Ito H, Takahashi A, Ishiko S, Nagaoka T, Yoshida A. Retinal cavernous hemangioma documented by spectral domain optical coherence tomography and confocal scanning laser ophthalmoscope retro-mode imaging. Retin Cases Brief Rep. 2016;10(4):373–376. doi: 10.1097/ICB.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 14.Corradetti G, Corvi F, Sadda SR. Bilateral retinal detachment imaged by Mirante color photography and retro mode illumination. Can J Ophthalmol. 2021;56(4):279. doi: 10.1016/j.jcjo.2020.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Ramachandran VS. Perception of shape from shading. Nature. 1988;331(6152):163–166. doi: 10.1038/331163a0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang R, Tsai P-S, Cryer JE, Shah M. Shape from shading: a survey. IEEE Trans Pattern Anal Mach Intell. 1999;21(8):690–706. doi: 10.1109/34.784284. [DOI] [Google Scholar]
- 17.Adams WJ, Graf EW, Ernst MO. Experience can change the 'light-from-above' prior. Nat Neurosci. 2004;7(10):1057–1058. doi: 10.1038/nn1312. [DOI] [PubMed] [Google Scholar]
- 18.Andrews B, Aisenberg D, d'Avossa G, Sapir A. Cross-cultural effects on the assumed light source direction: evidence from English and Hebrew readers. J Vis. 2013;13(13):2. doi: 10.1167/13.13.2. [DOI] [PubMed] [Google Scholar]
- 19.Chen CC, Chen CM, Tyler CW. Depth structure from asymmetric shading supports face discrimination. PLoS ONE. 2013;8(2):e55865. doi: 10.1371/journal.pone.0055865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pickard-Jones B, d'Avossa G, Sapir A. 3D shape-from-shading relies on a light source prior that does not change with age. Vis Res. 2020;177:88–96. doi: 10.1016/j.visres.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Fraser CL, Lueck CJ. Illusions, hallucinations, and visual snow. Handb Clin Neurol. 2021;178:311–335. doi: 10.1016/B978-0-12-821377-3.00014-3. [DOI] [PubMed] [Google Scholar]
- 22.Sapir A, Hershman R, Henik A. Top-down effect on pupillary response: evidence from shape from shading. Cognition. 2021;212:104664. doi: 10.1016/j.cognition.2021.104664. [DOI] [PubMed] [Google Scholar]
- 23.Webb RH, Hughes GW. Scanning laser ophthalmoscope. IEEE Trans Biomed Eng. 1981;28(7):488–492. doi: 10.1109/TBME.1981.324734. [DOI] [PubMed] [Google Scholar]
- 24.Mainster MA, Timberlake GT, Webb RH, Hughes GW. Scanning laser ophthalmoscopy. Clinical applications. Ophthalmology. 1982;89(7):852–857. doi: 10.1016/S0161-6420(82)34714-4. [DOI] [PubMed] [Google Scholar]
- 25.Webb RH, Hughes GW, Delori FC. Confocal scanning laser ophthalmoscope. Appl Opt. 1987;26(8):1492–1499. doi: 10.1364/AO.26.001492. [DOI] [PubMed] [Google Scholar]
- 26.Webb RH. Scanning laser ophthalmoscope. In: Masters BR, editor. Noninvasive diagnostic techniques in ophthalmology. Berlin: Springer; 1990. pp. 438–450. [Google Scholar]
- 27.Geeraets WJ, Williams RC, Chan G, Ham WT, Jr, Guerry D, 3rd, Schmidt FH. The loss of light energy in retina and choroid. Arch Ophthalmol. 1960;64:606–615. doi: 10.1001/archopht.1960.01840010608020. [DOI] [PubMed] [Google Scholar]
- 28.Mainster MA, White TJ, Allen RG. Spectral dependence of retinal damage produced by intense light sources. J Opt Soc Am. 1970;60(6):848–855. doi: 10.1364/JOSA.60.000848. [DOI] [PubMed] [Google Scholar]
- 29.Webb RH, Delori FC. How we see the retina. In: Marshall J, editor. Laser technology in ophthalmology. Amsterdam: Kugler & Ghedini; 1988. pp. 3–14. [Google Scholar]
- 30.Delori FC, Pflibsen KP. Spectral reflectance of the human ocular fundus. Appl Opt. 1989;28(6):1061–1077. doi: 10.1364/AO.28.001061. [DOI] [PubMed] [Google Scholar]
- 31.Lee BR, Bartsch DU, Kozak I, Cheng L, Freeman WR. Comparison of a novel confocal scanning laser ophthalmoscopy algorithm with optical coherence tomography in measurement of macular thickness and volume. Retina. 2009;29(9):1328–1334. doi: 10.1097/IAE.0b013e3181ac7d30. [DOI] [PubMed] [Google Scholar]
- 32.Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology. 1986;93(7):952–958. doi: 10.1016/S0161-6420(86)33637-6. [DOI] [PubMed] [Google Scholar]
- 33.Elsner AE, Burns SA, Weiter JJ, Delori FC. Infrared imaging of sub-retinal structures in the human ocular fundus. Vision Res. 1996;36(1):191–205. doi: 10.1016/0042-6989(95)00100-E. [DOI] [PubMed] [Google Scholar]
- 34.Fischer J, Otto T, Delori F, Pace L, Staurenghi G. Scanning laser ophthalmoscopy (SLO). In: Bille JF, ed. High resolution imaging in microscopy and ophthalmology: new frontiers in biomedical optics. Cham (CH); 2019. p. 35–57. [PubMed]
- 35.Manivannan A, Kirkpatrick JN, Sharp PF, Forrester JV. Novel approach towards colour imaging using a scanning laser ophthalmoscope. Br J Ophthalmol. 1998;82(4):342–345. doi: 10.1136/bjo.82.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manivannan A, Van der Hoek J, Vieira P, et al. Clinical investigation of a true color scanning laser ophthalmoscope. Arch Ophthalmol. 2001;119(6):819–824. doi: 10.1001/archopht.119.6.819. [DOI] [PubMed] [Google Scholar]
- 37.Bartsch DU, Freeman WR, Lopez AM. A false use of "true color". Arch Ophthalmol. 2002;120(5):675–676. [PubMed] [Google Scholar]
- 38.Meshi A, Lin T, Dans K, et al. Comparison of retinal pathology visualization in multispectral scanning laser imaging. Retina. 2019;39(7):1333–1342. doi: 10.1097/IAE.0000000000002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Rosa I, Ohayon A, Semoun O, et al. Real-color versus pseudo-color imaging of fibrotic scars in exudative age-related macular degeneration. Retina. 2020;40(12):2277–2284. doi: 10.1097/IAE.0000000000002771. [DOI] [PubMed] [Google Scholar]
- 40.Haridas S, Indurkhya S, Kumar S, Giridhar A, Sivaprasad S. Sensitivity and specificity of pseudocolor ultrawide field imaging in comparison to wide field fundus fluorescein angiography in detecting retinal neovascularization in diabetic retinopathy. Eye (Lond) 2021 doi: 10.1038/s41433-021-01772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Webb RH, Hughes GW, Pomerantzeff O. Flying spot TV ophthalmoscope. Appl Opt. 1980;19(17):2991–2997. doi: 10.1364/AO.19.002991. [DOI] [PubMed] [Google Scholar]
- 42.Manivannan A, Kirkpatrick JN, Sharp PF, Forrester JV. Clinical investigation of an infrared digital scanning laser ophthalmoscope. Br J Ophthalmol. 1994;78(2):84–90. doi: 10.1136/bjo.78.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ajlan RS, Barnard LR, Mainster MA. Nonconfocal ultra-widefield scanning laser ophthalmoscopy: polarization artifacts and diabetic macular edema. Retina. 2020;40(7):1374–1378. doi: 10.1097/IAE.0000000000002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woon WH, Fitzke FW, Bird AC, Marshall J. Confocal imaging of the fundus using a scanning laser ophthalmoscope. Br J Ophthalmol. 1992;76(8):470–474. doi: 10.1136/bjo.76.8.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartsch DU, Freeman WR. Axial intensity distribution analysis of the human retina with a confocal scanning laser tomograph. Exp Eye Res. 1994;58(2):161–173. doi: 10.1006/exer.1994.1004. [DOI] [PubMed] [Google Scholar]
- 46.Vieira P, Manivannan A, Sharp PF, Forrester JV. True colour imaging of the fundus using a scanning laser ophthalmoscope. Physiol Meas. 2002;23(1):1–10. doi: 10.1088/0967-3334/23/1/301. [DOI] [PubMed] [Google Scholar]
- 47.Podoleanu AG, Rosen RB. Combinations of techniques in imaging the retina with high resolution. Prog Retin Eye Res. 2008;27(4):464–499. doi: 10.1016/j.preteyeres.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Lee SS, Lingham G, Alonso-Caneiro D, et al. Macular thickness profile and its association with best-corrected visual acuity in healthy young adults. Transl Vis Sci Technol. 2021;10(3):8. doi: 10.1167/tvst.10.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharp PF, Manivannan A, Vieira P, Hipwell JH. Laser imaging of the retina. Br J Ophthalmol. 1999;83(11):1241–1245. doi: 10.1136/bjo.83.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spaide RF, Otto T, Caujolle S, et al. Lateral resolution of a commercial optical coherence tomography instrument. Transl Vis Sci Technol. 2022;11(1):28. doi: 10.1167/tvst.11.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamamoto M, Mizukami S, Tsujikawa A, Miyoshi N, Yoshimura N. Visualization of cystoid macular oedema using a scanning laser ophthalmoscope in the retro-mode. Clin Exp Ophthalmol. 2010;38(1):27–36. doi: 10.1111/j.1442-9071.2010.02193.x. [DOI] [PubMed] [Google Scholar]
- 52.Pilotto E, Sportiello P, Alemany-Rubio E, et al. Confocal scanning laser ophthalmoscope in the retromode imaging modality in exudative age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):27–34. doi: 10.1007/s00417-012-2031-7. [DOI] [PubMed] [Google Scholar]
- 53.Lee WJ, Lee BR, Shin YU. Retromode imaging: review and perspectives. Saudi J Ophthalmol. 2014;28(2):88–94. doi: 10.1016/j.sjopt.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishiko S, Akiba J, Horikawa Y, Yoshida A. Detection of drusen in the fellow eye of Japanese patients with age-related macular degeneration using scanning laser ophthalmoscopy. Ophthalmology. 2002;109(11):2165–2169. doi: 10.1016/S0161-6420(02)01227-7. [DOI] [PubMed] [Google Scholar]
- 55.Plesch A, Klingbeil U. Optical characteristics of a scanning laser ophthalmoscope. Proc SPIE. 1989;1161.
- 56.Manivannan A, Sharp PF, Forrester JV. Performance measurements of an infrared digital scanning laser ophthalmoscope. Physiol Meas. 1994;15(3):317–324. doi: 10.1088/0967-3334/15/3/010. [DOI] [PubMed] [Google Scholar]
- 57.Oshima S, Akita J, Mizuno K, Ishiko S, Yoshida A, van de Velde FJ. A novel imaging modality for the relaxed confocal SLO reveals pseudo-stereopsis in macular disease entities. Invest Ophthalmol Vis Sci. 2008;49(13):4277. [Google Scholar]
- 58.Wolf KW, Reid W, Schrauf M. Optical illusions in scanning electron micrographs: the case of the eggshell of Acrosternum (Chinavia) marginatum (Hemiptera: Pentatomidae) Micron. 2003;34(1):57–62. doi: 10.1016/S0968-4328(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 59.Hoang QV, Linsenmeier RA, Chung CK, Curcio CA. Photoreceptor inner segments in monkey and human retina: mitochondrial density, optics, and regional variation. Vis Neurosci. 2002;19(4):395–407. doi: 10.1017/S0952523802194028. [DOI] [PubMed] [Google Scholar]
- 60.Litts KM, Zhang Y, Freund KB, Curcio CA. Optical coherence tomography and histology of age-related macular degeneration support mitochondria as reflectivity sources. Retina. 2018;38(3):445–461. doi: 10.1097/IAE.0000000000001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fitzke FW, Masters BR. Three-dimensional visualization of confocal sections of in vivo human fundus and optic nerve. Curr Eye Res. 1993;12(11):1015–1018. doi: 10.3109/02713689309029228. [DOI] [PubMed] [Google Scholar]
- 62.Bartsch DU, Aurora A, Rodanant N, Cheng L, Freeman WR. Volumetric analysis of macular edema by scanning laser tomography in immune recovery uveitis. Arch Ophthalmol. 2003;121(9):1246–1251. doi: 10.1001/archopht.121.9.1246. [DOI] [PubMed] [Google Scholar]
- 63.Frambach DA, Dacey MP, Sadun A. Stereoscopic photography with a scanning laser ophthalmoscope. Am J Ophthalmol. 1993;116(4):484–488. doi: 10.1016/S0002-9394(14)71409-6. [DOI] [PubMed] [Google Scholar]
- 64.Mainster MA, Turner PL. Glare's causes, consequences, and clinical challenges after a century of ophthalmic study. Am J Ophthalmol. 2012;153(4):587–593. doi: 10.1016/j.ajo.2012.01.008. [DOI] [PubMed] [Google Scholar]