Abstract
The VirB8 protein of Agrobacterium tumefaciens is essential for DNA transfer to plants. VirB8, a 237-residue polypeptide, is an integral membrane protein with a short N-terminal cytoplasmic domain. It interacts with two transport pore proteins, VirB9 and VirB10, in addition to itself. To study the role of these interactions in DNA transfer and to identify essential amino acids of VirB8, we introduced random mutations in virB8 by the mutagenic PCR method. The putative mutants were tested for VirB8 function by the ability to complement a virB8 deletion mutant in tumor formation assays. After multiple rounds of screening 13 mutants that failed to complement the virB8 deletion mutation were identified. Analysis of the mutant strains by DNA sequence analysis, Western blot assays, and reconstruction of new point mutations led to the identification of five amino acid residues that are essential for VirB8 function. The substitution of glycine-78 to serine, serine-87 to leucine, alanine-100 to valine, arginine-107 to proline or alanine, and threonine-192 to methionine led to the loss of VirB8 activity. When introduced into the wild-type strain, virB8S87L partially suppressed the tumor forming ability of the wild-type protein. Analysis of protein-protein interaction by the yeast two-hybrid assay indicated that VirB8R107P is defective in interactions with both VirB9 and VirB10. A second mutant VirB8S87L is defective in interaction with VirB9.
DNA transfer from Agrobacterium tumefaciens to plants results in crown gall tumor disease. Tumor formation requires the presence of the tumor-inducing (Ti)-plasmid in the infecting bacterium. The transferred (T)-DNA is stably integrated into the plant nuclear genome and direct constitutive expression of the phytohormone biosynthetic genes in the transformed plant. The altered hormone level leads to the loss of cell division control, yielding a tumorous phenotype (8, 30). The virulence (vir) region of the Ti-plasmid is essential for DNA transfer. The vir region, a 35-kb DNA segment, is composed of five major loci, virA, virB, virD, virE, and virG (23). Proteins encoded in the vir region process the Ti-plasmid to produce a single-stranded T-strand DNA comprised of the bottom strand of the T-DNA (1, 24). The T-strand DNA is postulated to cross the bacterial membrane through a transport pore composed primarily of the proteins encoded in the virB operon (6, 15, 28). The virB operon encodes 11 proteins, VirB1 to VirB11 (15, 28). All except VirB1 are essential for DNA transfer (5). VirB1 is required for a high efficiency of DNA transfer.
Molecular characterization of the virB operon led to the hypothesis that the VirB proteins function in the biogenesis of a transport pore through which the T-strand DNA moves from the bacterium to the plant cell (15, 28). The subsequent discovery of the presence of homologs of the VirB proteins in other bacterial systems supports this hypothesis (7). Proteins essential for the conjugal transfer of Escherichia coli plasmids, the secretion of the Bordetella pertussis toxin protein, and the pathogenicity of Helicobacter pylori exhibit significant homology to the VirB proteins. Homologs of the VirB proteins in Brucella suis, Rickettsia prowazekii, Legionella pneumophila, and Bartonella henselae have also been identified. The conservation of these proteins and their role in various biological processes suggest that the VirB family of proteins function in the export of macromolecules to both prokaryotic and eukaryotic hosts.
The structure of the transport pore is not known. We proposed that VirB6, VirB7, VirB8, VirB9, and VirB10 are the primary constituents of the T-DNA transport pore (9). VirB7, a lipoprotein, is anchored to the outer membrane (13), while VirB8 and VirB10 are inner membrane proteins (9, 25, 29). VirB7 forms a disulfide-linked complex with VirB9 (2, 3, 22). We recently demonstrated that VirB8, VirB9, and VirB10 interact with one another (10). Chemical cross-linking and immunoprecipitation studies indicated that VirB7, VirB9, and VirB10 participate in the formation of oligomeric complexes (2–4, 22, 29). These studies support the proposed role of the VirB7 to VirB10 proteins in transporter assembly.
In a recent study we reported that VirB8, VirB9, and VirB10 are present in a protein complex (16). The subcellular location of two of the proteins, VirB9 and VirB10, changed dramatically in the presence of the other VirB proteins. Immunofluorescence and immunoelectron microscopy studies showed that the two proteins localized to a few sites on the membrane in the presence of the other VirB proteins. In immunoelectron microscopy, gold particles representing the two proteins were found in clusters in the presence of the VirB proteins. In contrast, gold particles were found mostly as a single particle all along the cell periphery in the absence of the other VirB proteins. The reorganization of cellular location of VirB9 and VirB10 was dependent on VirB8 since a deletion in virB8 abolished the reorganization. The important role of VirB8 in the assembly of the transporter complex led us to study this protein in detail. In the present study we report the identification of amino acids essential for VirB8 function and the role of interactions of VirB8 with the other VirB proteins in T-DNA transfer to plants.
MATERIALS AND METHODS
Strains and plasmids.
A. tumefaciens A348 contains the octopine Ti-plasmid pTiA6. PC1008 is a derivative of A. tumefaciens A348 with a nonpolar in-frame deletion in virB8 (5). A. tumefaciens A136 lacks a Ti-plasmid. The E. coli strains used in this study were DH5αF′ and CJ236 (relevant genotype: dut ung).
Plasmid pAD1420 contains a chimeric virDp-virB7-virB8 gene. It was constructed by cloning the AlwNI-SphI fragment (nucleotides 6131 to 7197) into plasmid pAD1416 digested with SalI and SphI. The AlwNI and SalI ends were blunt ended by treatment with T4 DNA polymerase prior to cloning. Plasmid pAD1416 is a pUC118 derivative containing the virD promoter (−384 to +7 [12]) in the polylinker region. Plasmid pAD1423 contains the virDp-virB7-virB8 gene in pUC119 (26) and was obtained by cloning the gene as a 1.5-kb SstI-HindIII fragment from pAD1420. Plasmid pAD1433 was constructed by cloning plasmid pAD1423 as a HindIII fragment into the HindIII site of the wide-host-range plasmid pTJS75 (21).
Mutagenesis of virB8.
Random mutations in virB8 were introduced by PCR mutagenesis (27). The virB8 coding region of plasmid pAD1423C15S (2) was amplified with primers B7C15S (dCGCTTTGAGCGGATCCCAGACAAATGAC) and the m13 reverse sequencing primer using Taq DNA polymerase. The C15S mutation in VirB7 is not present in the virB8 plasmids used in this study and was used here for convenience. The 0.94-kb amplified fragment was purified with QIAquick PCR purification kit (Qiagen, Inc.), digested with BglII and SphI, and cloned into similarly digested pAD1433. A mutant library was constructed by isolating plasmid DNA from a pool of E. coli transformants. The BglII-SphI fragment (nucleotides 6353 to 7197) encodes virB8 in its entirety and the C-terminal 14 residues of virB7.
Targeted mutagenesis.
Mutations at a specific site in virB8 were introduced by deoxyoligonucleotide-directed site-specific mutagenesis using uracil containing single-stranded pAD1420 DNA as a template for second-strand synthesis (17). The mutation and the mutagenic primers (complementary strand, with mutation underlined) were as follows: valine-52 to isoleucine, dCTTGAGCAATATTCCCCAAAA; serine-87 to leucine, dGGCAATCGCAAGATAGACAC; arginine-107 to alanine, dCTCTCACGCAGGGCTACGTACTCCCAC; valine-189 to methionine, dGTACTCACCATAGGCATTTTG; and threonine-192 to methionine, dGCGGTCCACATACTCACCAC. The mutations were identified by DNA sequence analysis (20). After linearization, the mutant plasmids pAD1420-V52I, -S87L, -R107A, -V189M, and -T192M were cloned into the HindIII site of the wide-host-range plasmid pTJS75 to construct plasmids pAD1630, -1631, -1688, -1632, and -1633, respectively.
Interactions of the VirB proteins.
Interactions of VirB8 and its mutants with the VirB proteins were monitored by the two-hybrid assay in yeast as described earlier (10, 11). Plasmids that express LexA-VirB and activator-VirB fusions were introduced into the yeast strain AD842 by transformation, and the transformants were tested for the expression of the reporter lacZ gene on solid medium containing the chromogenic substrate X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). A blue colony color indicates a lacZ+ phenotype and a positive interaction.
The LexA-VirB8 and the activator-VirB8, -VirB9, and -VirB10 fusion plasmids pAD1529, -1516, -1517, and -1493, respectively, have been described previously (10). For the construction of plasmids expressing the LexA-VirB8 mutant fusions, sequences encoding residues 60 to 237 (the periplasmic domain) were amplified by PCR and cloned as an EcoRI-XhoI fragment into plasmid pJK202. All fusions were confirmed by DNA sequence analysis (20).
Other methods.
Plasmid DNA was introduced into A. tumefaciens by electroporation (18). The virulence of A. tumefaciens was monitored by tumor formation assays on Kalanchöe daigremontiana leaves (11). The DNA sequence of the virB8 mutants and the gene fusions was determined by the dideoxy chain termination method using double-stranded DNA as a template and Sequenase (20; U.S. Biochemical Corp.). The level of VirB8 and its mutants was monitored by Western blot assays with purified anti-VirB8 antibodies (16).
RESULTS
Random mutagenesis of virB8.
Random mutations in virB8 were introduced by error-prone PCR amplification with Taq DNA polymerase (27). The mutant virB8 genes were cloned into the unmutagenized wide-host-range vector pAD1433 as a BglII-SphI fragment. Plasmid pAD1433 is a wide-host-range derivative of pAD1423 (2) that contains a chimeric virDp-virB7-virB8 gene. The virB7 sequences were included in the clone because the expression of virB8 requires upstream sequences (5). A virB8 mutant library was constructed by isolating plasmid DNA from approximately 2,600 independent E. coli transformants. The library DNA was introduced into the A. tumefaciens PC1008, a strain with a nonpolar deletion in virB8, by electroporation (18). Four hundred independent transformants were purified and tested for virB8 function by tumor formation assays on Kalanchöe leaves (11). A mutation that abolishes virB8 function will not form tumors at the site of infection. After several rounds of screening, we identified 13 mutations that failed to form tumors and were avirulent (Fig. 1 and Table 1).
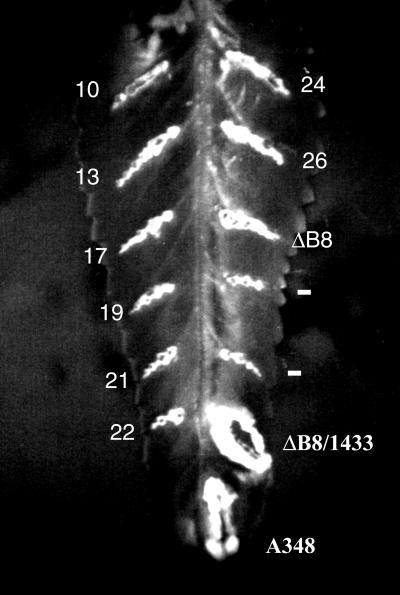
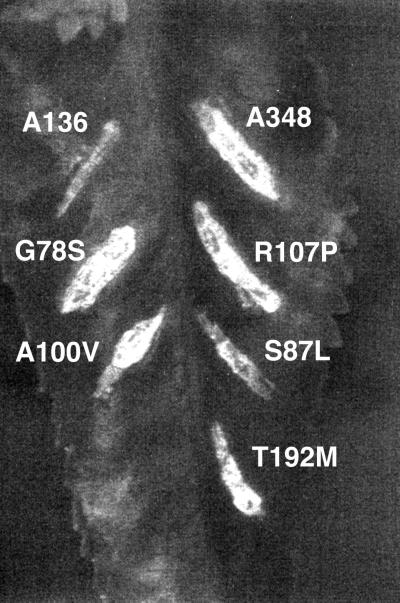
FIG. 1.
Phenotype of the virB8 mutants. The virB8 mutants were tested for the ability to complement a deletion in virB8. The mutants in A. tumefaciens PC1008 (ΔB8) were used to infect K. daigremontiana leaves and scored for tumor formation 3 weeks after infection. A subset of the mutants listed on Table 1 is shown. The numbers indicate the mutant number. Plasmid pAD1433 or its derivative harbors virB8 or the mutant. A348, wild-type strain; −, uninfected wound site.
TABLE 1.
Identification of the avirulent virB8 mutants
| Mutant(s) | Nucleotide change at positiona | Amino acid change at residue |
|---|---|---|
| 10, 11, 12, 23, and 25 | G 6616 → A | Glycine-78 → serine |
| 13 | G 6962 → A | Tryptophan-193 → stop |
| 17 and 18 | C 6683 → T | Alanine-100 → valine |
| 19 | C 6644 → T and G 6949 → A | Serine-87 → leucine and valine-189 → methionine |
| 21 | G 6613 → A and C 6703 → A | Aspartic acid-77 → asparagine and arginine-107 → serine |
| 22 | C 6484 → T | Arginine-34 → stop |
| 24 | G 6704 → C | Arginine-107 → proline |
| 26 | G 6538 → A and C 6959 → T | Valine-52 → isoleucine and threonine-192 → methionine |
Residue number is assigned according to Ward et al. (28).
Identification of the virB8 mutants.
DNA sequences of the entire mutagenized region of the 13 mutants were determined to identify the site(s) of mutation. Ten mutants had a single-base-pair change that led to a change in the amino acid sequence (Table 1). The remaining three mutants (mutants 19, 21, and 26) had changes in two positions that led to alterations in two amino acids. The collection of mutants contained five unique point mutations that led to the change of arginine-34 to a stop codon (R34X), glycine-78 to serine (G78S), alanine-100 to valine (A100V), arginine-107 to proline (R107P), and tryptophan-193 to a stop codon (W193X). Two mutants, G78S and A100V, were isolated more than once. One double mutant, mutant 21, contains a mutation in arginine-107, a residue identified as an essential one from the analysis of mutant 24. Consequently, this mutant was not characterized further. All virB8 genes were found to contain a C→G change in position 6425 of Ward et al. (28). The change alters amino acid 14 from threonine to serine.
To study whether a mutation destabilized the VirB8 protein, we performed Western blot assays (Fig. 2). All mutants, except mutants 13 and 24, accumulated VirB8 at a level comparable to that produced by the wild-type gene, indicating that in most cases a mutation did not affect protein stability (lanes 3 to 9 and 13). Mutant 24 accumulated a low level of the mutant protein; however, the level is similar to that in the wild-type strain A348 (compare lanes 2 and 8). This result suggests that the loss of function in mutant 24 is due to the effect of the change in amino acid sequence on protein function and not on protein stability. A double mutant, mutant 21, that contains a different substitution of arginine-107 expresses a stable protein, suggesting that all substitutions of residue 107 may not have a strong negative effect on protein stability. To confirm the requirement of arginine-107 in VirB8 function, we introduced an arginine-to-alanine change at position 107 by site-specific mutagenesis (17) and studied the effect of the mutation on VirB8 function and stability. The mutation abolished VirB8 function and was avirulent in a DNA transfer assay (Fig. 3). The mutation had no effect on protein stability (Fig. 2, lane 12).
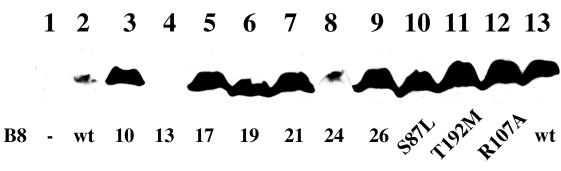
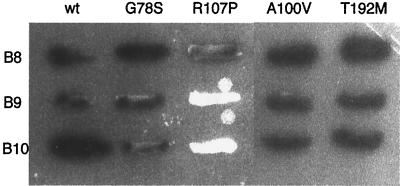
FIG. 2.
Effect of the virB8 mutations on protein stability. The level of VirB8 and its mutants were monitored by Western blot assays using purified VirB8 antibodies (16). Lanes 1, 2, and 13, uninduced A348, induced A348, and PC1008/pAD1433, respectively; lanes 3 to 12, virB8 mutants 10, 13, 17, 19, 21, 24, 26, virB8S87L, virB8T192M, and virB8R107A, respectively.
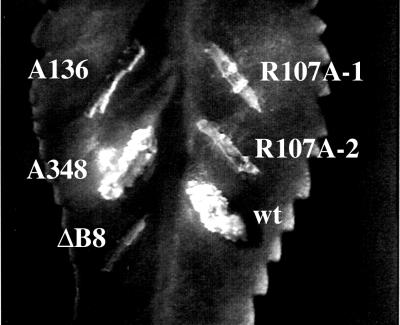
FIG. 3.
Role of VirB8 arginine-107 in DNA transfer to plants. An arginine at position 107 of VirB8 was changed to alanine by site-specific mutagenesis (17). The mutants were tested by complementation assays. wt, wild-type virB8; R107A, virB8R107A.
Identification of the mutation(s) conferring phenotype to the double mutants.
The avirulent phenotype of the other two double mutants, mutant 19 and mutant 26, can be due to a single-amino-acid change or both mutations. To identify the mutation(s) responsible for the phenotype, we introduced the individual mutations into virB8 by site-directed mutagenesis. Each virB8 mutant was introduced into the virB8 deletion mutant PC1008, and the effect of the mutation on virB8 function was tested by complementation assays (Fig. 4). Two mutations, the alteration of serine-87 to leucine (S87L) and threonine-192 to methionine (T192M), abolished virB8 function, indicating that a single-amino-acid change in both cases is sufficient to confer an avirulent phenotype. The other two mutations, valine-52 to isoleucine and valine-189 to methionine, had no effect on virB8 function. The loss of virB8 function in virB8S87L and virB8T192M is not due to protein stability because both mutant proteins were stable (Fig. 2, lanes 10 and 11). The phenotype of each of the double mutants is therefore due to a single amino acid change.
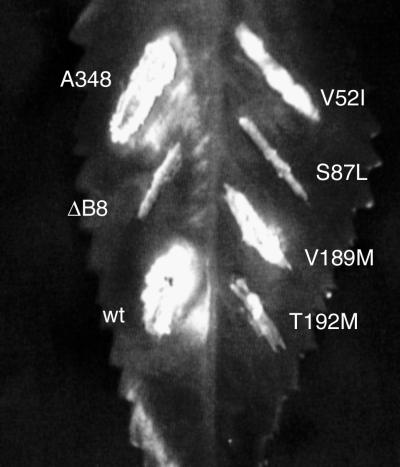
FIG. 4.
Identification of amino acids responsible for the avirulent phenotype of the double mutants. Mutations that led to single-amino-acid substitutions in virB8 were introduced by site-specific mutagenesis, and the mutants were introduced into A. tumefaciens PC1008. The resultant strains were used to infect K. daigremontiana leaves. The strains used for infection were A. tumefaciens A348 (A348), PC1008 (ΔB8), and PC1008 harboring a plasmid that expresses wild-type virB8 (wt), virB8V52I (V52I), virB8S87L (S87L), virB8V189M (V189M), or virBT192M (T192M).
virB8S87L is a semidominant mutant.
To study whether the presence of a mutant protein affects the function of the wild-type protein, we introduced the five mutants into A. tumefaciens A348 and studied the effect of the mutation on VirB8 function by virulence assays. One mutant, virB8S87L, had a semidominant phenotype. Three weeks after infection, A tumefaciens A348 virB8S87L consistently exhibited an avirulence phenotype (Fig. 5). After a prolonged infection (3 to 4 months), the mutant showed an attenuated phenotype, indicating that the mutant does not have a fully dominant phenotype. Three mutants, virB8G78S, virB8A100V, and virB8R107P, had no effect on the virulence of the wild-type strain. The phenotype of the virB8T192M mutant was variable from experiment to experiment. A tumefaciens A348 virB8T192M exhibited an attenuated phenotype, suggesting that this mutant may have a semidominant phenotype.
FIG. 5.
Dominant-recessive phenotype of the virB8 mutants. A plasmid expressing virB8 or its mutant was introduced into A. tumefaciens A348, and the resultant strains were used to infect K. daigremontiana leaves.
Mutations in virB8 affect its interaction with other VirB proteins.
VirB8 interacts with VirB9, VirB10, and itself (10). All or a subset of these interactions are likely to be essential for the assembly and function of the T-DNA transporter. A corollary to the hypothesis is that a mutant defective in an essential interaction will have an avirulent phenotype. We therefore tested whether an avirulent mutation identified in our study is defective in interactions of VirB8. The two-hybrid assay in yeast was used to study the interactions of the VirB8 mutants (10, 11). The activator-VirB8, -VirB9, or -VirB10 fusion was introduced into a yeast strain containing the LexA-VirB8 mutant fusion. Interaction between the two fusion proteins was determined by monitoring expression of the reporter lacZ gene. A positive interaction results in a blue colony color phenotype of the yeast strain when grown on solid medium containing the chromogenic substrate X-Gal. All mutants were proficient in interaction with VirB8 (Fig. 6, top row). These results indicate that the mutant fusion proteins are expressed in yeast, stable and properly targeted to the yeast cell nucleus. Two of the mutants were found to be defective in the VirB8-VirBx interactions. One, VirB8R107P, failed to interact with both VirB9 and VirB10, indicating that arginine-107 is essential for interactions of VirB8. A second mutant, VirB8S87L, is defective in interaction with VirB9 but not with VirB8 and VirB10 (10). Mutations at the other three sites, i.e., amino acids 78, 100, and 192, did not affect the ability of VirB8 to interact with VirB9 and VirB10.
FIG. 6.
Interaction of the VirB8 mutants with VirB8, VirB9, and VirB10. The interaction of VirB8 (wt) and its mutant with VirB8 (B8), VirB9 (B9), and VirB10 (B10) was monitored by the two-hybrid assay in yeast as described previously (10). A blue colony color indicates a positive interaction.
DISCUSSION
VirB8 is a bitopic membrane protein with a short cytoplasmic segment at its N terminus (9, 25). It is an essential virulence protein and is postulated to be a primary constituent of the T-DNA transporter (5, 16). The C-terminal periplasmic segment encodes domains essential for interaction with VirB9, VirB10, and itself (10). Random mutagenesis of virB8 led to the identification of five essential amino acids: glycine-78, serine-87, alanine-100, arginine-107, and threonine-192. All of the residues mapped to the large periplasmic domain. Two sets of mutations mapped within 10 or fewer residues of each other, and the four mutations lie within a 30-residue segment, i.e., amino acids 78 to 107. The mutations, however, probably affect different functions. Two mutations abolished interaction of VirB8 with another VirB protein in yeast two-hybrid assays. The substitution of arginine at position 107 with proline led to the loss of interactions with both VirB9 and VirB10. A mutation at residue 100, however, did not affect its interaction with VirB9, VirB10, or itself. Similarly, a serine-to-leucine change at residue 87 led to the loss of interaction with VirB9, but a mutation at residue 78 had no effect on the interaction. Therefore, the four mutations probably affect different functions of VirB8. Since two avirulent mutations are defective in the VirB8-VirB9 interaction, these results indicate that the VirB8-VirB9 interaction is essential for DNA transfer. The role of the VirB8-VirB10 interaction cannot be predicted from this study because of the pleiotropic nature of the virB8R107P mutation.
The loss of function of VirB8G78S, VirB8A100V, and VirB8T192M indicates that VirB8 has other functions in addition to its interaction with the three VirB proteins. These functions may include, among others, interaction with other transport pore protein(s) essential for the assembly of the transporter and/or interaction with a transported substrate. One virB8 mutant, virB8S87L, exhibited a semidominant phenotype. Semidominance is probably the result of partial activity of a VirB8-VirB8 mutant oligomer in DNA transfer. This property of the mutant protein suggests that VirB8 forms an oligomer, and oligomerization of VirB8 is required for its function. Studies using the two-hybrid assay and that on the subcellular localization of VirB8 presented here and previous studies (Fig. 6; see also references 10 and 16) support the hypothesis that VirB8 forms an oligomer.
VirB8 is found conserved in the family of type IV transport system proteins. Homologs of VirB8 are found in B. suis, B. henselae, B. pertussis, E. coli conjugal plasmids, H. pylori, L. pneumophila, Rhizobium etli, and R. prowazekii. One amino acid, the alteration of which led to an avirulent phenotype, glycine-78, is conserved in all of these proteins; however, the sequences around it show very little conservation (Fig. 7). This residue probably has an important role in the tertiary structure of the protein. Two mutations, R107P and T192M, mapped to areas that are found conserved in all homologs. One, arginine-107, falls within a nine-residue region that contains four invariant residues. This region (residues 105 to 113) has the consensus sequence YVnnREt/sYd/n (n, any residue; invariant residues are in large capitals, and highly conserved residues are in small capitals). The high degree of conservation of these sequences and the phenotype of the virB8R107P mutant suggest that this region in all homologs functions in interaction with other components of the transporter complex. A region with a significantly high homology among the VirB8 proteins is the C-terminal end. The C-terminal 18 residues of all but one homolog share a minimum of 50% identity.
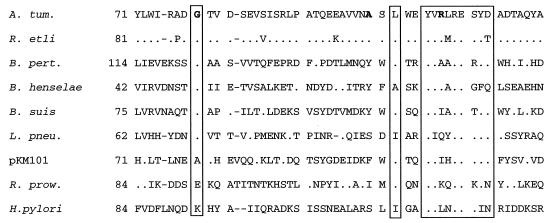
FIG. 7.
Conservation of glycine-78 and sequences around arginine-107 in the VirB8 homologs. The amino acid sequence of a segment of A. tumefaciens VirB8 and its homologs is shown. Residues identical to the A. tumefaciens VirB8 sequence are shown as dots. Sequences that exhibited a high degree of conservation are boxed. Glycine-78, alanine-100, and arginine-107 are shown in boldface. The numbers on the left indicate the position of the first amino acid residue shown in the figure. The gaps were introduced to achieve maximum homology.
We postulated that VirB8 identifies the site of transport pore assembly (16). This protein has the unique property of localizing to a few sites on the bacterial membrane. The interaction of VirB8 will target the other proteins to this site for the assembly of the transport pore. Two mutants, virB8S87L and virB8R107P, are expected to be defective in the assembly of the transporter because of their failure to interact with at least one pore constituent. The effect of the other mutations on transport pore cannot be predicted at this time. A mutant can form a nonfunctional transport pore, a transport pore that has no defect in assembly but cannot transport a substrate. Alternatively, a mutant can fail to assemble the transport pore. While a mutant is proficient in interaction with several constituents, it can fail to interact with another unidentified component of the pore or is defective in a higher-order interaction that is essential for pore assembly.
ACKNOWLEDGMENTS
We thank Peter Christie for the Agrobacterium sp. strain PC1008, Roger Brent for the plasmids and strains for the yeast two-hybrid assays, Paul Judd for discussions, and Alison Pirro and Xiao-Chao Ruan for excellent technical assistance.
This work was supported by grants from the University of Minnesota Agricultural Experiment Station and the University of Minnesota Graduate School.
REFERENCES
- 1.Albright L M, Yanofsky M F, Leroux B, Ma D Q, Nester E W. Processing of the T-DNA of Agrobacterium tumefaciens generates border nicks and linear, single-stranded T-DNA. J Bacteriol. 1987;169:1046–1055. doi: 10.1128/jb.169.3.1046-1055.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson L B, Hertzel A V, Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc Natl Acad Sci USA. 1996;93:8889–8894. doi: 10.1073/pnas.93.17.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron C, Thorstenson Y R, Zambryski P C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaupre C E, Bohne J, Dale E M, Binns A N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1997;179:78–89. doi: 10.1128/jb.179.1.78-89.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berger B, Christie P. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christie P. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 8.Das A. DNA transfer from Agrobacterium to plant cells in crown gall tumor disease. In: Biswas B B, Das H K, editors. Subcellular biochemistry: plant microbe interactions. London, England: Plenum Publishing Corp; 1998. pp. 343–363. [DOI] [PubMed] [Google Scholar]
- 9.Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. doi: 10.1046/j.1365-2958.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 10.Das A, Xie Y-H. Agrobacterium tumefaciens T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J Bacteriol. 2000;182:758–763. doi: 10.1128/jb.182.3.758-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das A, Anderson L, Xie Y-H. Delineation of the interaction domains of Agrobacterium tumefaciens VirB7 and VirB9 by use of the yeast two-hybrid assay. J Bacteriol. 1997;179:3404–3409. doi: 10.1128/jb.179.11.3404-3409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das A, Stachel S, Ebert P, Allenza P, Montoya A, Nester E. Promoters of Agrobacterium tumefaciens Ti-plasmid virulence genes. Nucleic Acids Res. 1986;14:1355–1364. doi: 10.1093/nar/14.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez D, Dang T A, Spudich G, Zhou X-R, Berger B, Christie P. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J Bacteriol. 1996;178:3156–3167. doi: 10.1128/jb.178.11.3156-3167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finley R, Brent R. Interaction trap cloning in yeast. In: Hames B, Glover D, editors. DNA cloning, expression systems: a practical approach. Oxford, England: Oxford University Press; 1995. pp. 169–203. [Google Scholar]
- 15.Kuldau G A, De Vos G, Owen J, McCaffrey G, Zambryski P. The virB operon of Agrobacterium tumefaciens pTiC58 encodes 11 open reading frames. Mol Gen Genet. 1990;221:256–266. doi: 10.1007/BF00261729. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Xie Y-H, Das A. Subcellular localization of the Agrobacterium tumefaciens T-DNA transport pore proteins: VirB8 is essential for the assembly of the transport pore. Mol Microbiol. 2000;36:608–617. doi: 10.1046/j.1365-2958.2000.01876.x. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel T. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA. 1985;82:488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mersereau M, Pazour G, Das A. Efficient transformation of Agrobacterium tumefaciens by electroporation. Gene. 1990;90:149–151. doi: 10.1016/0378-1119(90)90452-w. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 20.Sanger F, Nicklen S, Coulson A. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidhauser T, Helinski D. Regions of broad-host-range plasmid RK2 involved in replication and stable maintenance in nine species of gram-negative bacteria. J Bacteriol. 1985;164:446–455. doi: 10.1128/jb.164.1.446-455.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spudich G, Fernandez D, Zhou X-R, Christie P. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport pore. Proc Natl Acad Sci USA. 1996;93:7512–7517. doi: 10.1073/pnas.93.15.7512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stachel S, Nester E. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986;5:1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stachel S, Timmerman B, Zambryski P. Generation of single-stranded T-DNA molecules during the initial stages of T-DNA transfer from Agrobacterium tumefaciens to plant cells. Nature. 1986;322:706–712. [Google Scholar]
- 25.Thorstenson Y R, Zambryski P C. The essential virulence protein VirB8 localizes to the inner membrane of Agrobacterium tumefaciens. J Bacteriol. 1994;176:1711–1717. doi: 10.1128/jb.176.6.1711-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vieira J, Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- 27.Vogel A, Das A. Mutational analysis of Agrobacterium tumefaciens pTiA6 virD1: Identification of functionally important residues. Mol Microbiol. 1994;12:811–817. doi: 10.1111/j.1365-2958.1994.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 28.Ward J E, Akiyoshi D E, Regier D, Datta A, Gordon M P, Nester E. Characterization of the virB operon from Agrobacterium tumefaciens Ti plasmid. J Biol Chem. 1988;263:5804–5814. [PubMed] [Google Scholar]
- 29.Ward J E, Dale E M, Nester E W, Binns A N. Identification of a VirB10 protein aggregate in the inner membrane of Agrobacterium tumefaciens. J Bacteriol. 1990;172:5200–5210. doi: 10.1128/jb.172.9.5200-5210.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zupan J, Muth T, Draper O, Zambryski P. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 2000;23:11–28. doi: 10.1046/j.1365-313x.2000.00808.x. [DOI] [PubMed] [Google Scholar]