Abstract
Importunate high-risk HPV (HR-HPV) infection is the most common trigger for the cervical carcinogenesis process. In this respect, the presence of cancer can be imputed to telomere lengthening or shortening. This paper explores the possible correlation between relative telomere length and viral load in two groups of women, namely: those with high-risk HPV infection and those who do not have this infection. Thus, samples comprising of 50 women in each group were evaluated for this research. The Amplisens HPV HCR screen-titre-FRT PCR kite was employed for quantitative analysis. Relative telomere length was quantified by real-time PCR. In each of the two HPV load groups, there was no correlation between age and telomere length. Telomere shortening was found in the cervical cell samples of women with high HPV loads, compared with women in the control group. Telomere shortening is associated with elevated HPV loads.
Keywords: Telomere, Human papillomavirus, Viral load, Cervical cancer, Real-time PCR
INTRODUCTION
Telomeres are comprised of complexes of nucleoprotein that enclose chromosome ends and not only offer protection against fusion and other injuries but also contribute to the preservation of genomic stability [1]. Each subsequent division of the cell reduces the length of the 5′-TTAGGG-3′ sequence of DNA telomere repetitions, the outcome being either apoptosis or senescence [2]. When atypical conditions cause the telomeres to reduce in length, the possibility of telomere end fusions arises, which could result in an increased level of chromosome instability. This is a primary triggering event in many cancers, including colon, prostate, and cervical cancers [3,4,5]. Research in this field has postulated that a potential correlation exists between different cancer stages and telomere length. Specifically, telomeres that are shorter in length are linked to the onset of cancer. However, they gradually lengthen as cancer progresses as a consequence of telomerase activation or alternative telomere lengthening (ALT) [6,7]. Telomerase acts to safeguard the telomere against shortening. The telomere is comprised of two subunits, namely: TERC (telomerase RNA component) and hTERT (human telomerase reverse transcriptase). TERC functions as a blueprint for telomere sequence amplification, whereas hTERT serves as a reverse transcriptase subunit [8]. In 85–90% of human cancers, telomerase activation causes telomeres to expand in length [9]. Thus, it is reasonable to assume that the stabilization of telomere length occurs at a later point in the tumorigenesis process. Specifically, it takes place following a period of active proliferation cycles and reduced telomere length [10]. Sexual contact is the most common vehicle for the transmission of the human papillomavirus (HPV). HPV infection has affected a considerable number worldwide, although both men and women are infected with HPV, adolescent girls and women under the age of 25 are the most infected [11,12]. HPV is a diminutive virus. It penetrates cells with its capsid proteins, with the infection commencing at the basal cell layer, after which it disseminates to elevated tiers of the cervical epithelium [13]. Dysplasia resulting from the relentless HR-HPV infection can ultimately develop into cancer [14]. The principal causative agents for different cancer types are HR-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) [15]. HR-HPV has been implicated in cancers of the cervix, vulva, vaginal, penis, anus, and oropharyngeal cancers [16]. However, cervical cancer is the fourth most common malignant tumor in women globally, and it is associated with a significant mortality rate in women [17,18]. The cellular telomerase complex can be triggered by the hTERT transcription, which is stimulated by the HPV E6 protein [19]. Moreover, the cell immortalization that occurs during long-term high-risk human papillomavirus infection is dependent upon telomerase activation, which leads to cervical malignant neoplasm development [20]. The development of cancer passes through multiple stages, and one of the most common features is modifications to telomere length [21]. There is practically no data in the literature on the relationship between telomeric DNA length and the risk of the development of elevated viral load in cells infected with HPV. Therefore, the primary objective of the current study is to ascertain the existence of any disparities between comparative telomeric DNA lengths in the cervical epithelial cells of women with high HPV load and telomeric DNA lengths in the control group.
MATERIALS AND METHODS
Sample description
To conduct the molecular investigation, 100 samples were obtained from the epithelial cells scraped from the women's urogenital tracts of the participants in the two research groups. Material for the research was obtained from the clinical diagnostic laboratory Nauka (Rostov-on-Don, Russia). Participants who were HPV-positive were subdivided into two subgroups: participants with an HPV load of 4–5 lg HPV genomes per 100 thousand human cells (n = 21), and participants with an HPV load of over 5 lg HPV genomes per 100 thousand human cells (n = 29). HPV-negative women (n = 50) were in the control group. All participants in this research project were aged 30 or over. Three inclusion criteria were applied to participants in the control group, namely: uterus negative biopsy, normal colposcopy, and HPV negative PCR testing. The case study group included women with a range of symptoms, such as abnormal menstrual bleeding, abnormal vaginal discharge, a positive uterine biopsy, and an HPV-Positive PCR-test with an HPV viral load of over 104 DNA copies per 105 human cells. Informed written consent was required from all participants before their inclusion in this research. Ethical approval was sought and secured from the Bioethics Committee of the Academy of Biology and Biotechnology of the Southern Federal University (Protocol No. 2 of March 29, 2016). Any clinical testing included in this study was conducted according to the ethical protocols issued by the World Medical Association (Helsinki Declaration).
Quantitative analysis for HPV DNA
The total DNA was isolated from scrapings of epithelial cells from the cervical canal of women according to the protocols of the AmpliSens DNA-sorb-AM (InterLabService, Russia) reagent kit. The protocols specified for the AmpliSens-HPV HCR screen-titre-FRT and AmpliSens HPV HCR-genotype-FRT (Interlabservice, Russia) were employed for the genotyping and quantifying of the DNA for HR-HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59). The PCR mixture for the AmpliSens-HPV HCR screen-titre-FRT contains 7 μl of PCR mix-1-FRT HPV screen titer; 8 μl mix of PCR buffer Flu and Taq polymerase and 10 μl of a DNA solution. The amplification program is as follows: 1 cycle at 95 °C for 15 minutes, followed by 1 cycle at 65 °C for 2 minutes, 93 °C for 20 seconds, 64 °C for 25 seconds, 65 °C for 55 seconds, followed by 5 cycles at 95 °C for 15 seconds, 60 °C for 25 seconds, 65 °C for 25 seconds, and 25 cycles registration. Fluorescence is measured at 60 °C in 4 channels: Fam, Joe, Rox, and Cy5 [22]. The interpretation of the viral load was performed as follows: log ≤3 per 105 human cells indicated low clinical significance, 3–5 log per 105 human cells signified clinical significance and risk of dysplasia, whereas > 5 log per 105 human cells was clinically significant with a strong risk of dysplasia, as per the guidelines and clinical reports issued by the manufacturer [22]. The PCR mixture for the AmpliSens HPV HCR-genotype-FRT contains 3.5 μl of one of the four variants of PCR-mixture-1-FRT HPV (four variants of the mixture differ in primers for specific types of HPV), 4.5 μl of a mixture of PCR-buffer-FRT and polymerase (TaqF), and 5 μl of DNA. The amplification program is as follows: 95°C for 15 minutes; 45 cycles: 95°C for 15 seconds, then 60°C for 30 seconds. Fluorescence is measured at 60 ° C [23].
Telomere and real-time polymerase chain reaction (PCR)
With slight modifications, an approach was employed to ascertain telomere length according to the Cawthon method for telomere measurement by quantitative PCR [24]. The mix for the amplification reaction (25 ml) included 0.7 μl 10 mmol /L of either the telomere primers (teloF and teloR) or primers for the 36B4 gene (36B4F and 36B4R). The 36B4 gene, which functions as a reference gene that encodes acidic ribosomal phosphoprotein (PO). (Table 1) presents the primer sequence. The PCR mixture incorporated 2.5 μl 25 mM MgCl2, 2.5 μl 2, 5 мM of dNTP, 2.5 μl PCR buffer, 0. 5 μl Taq-polymerase (5 U/μl), universal SYBR-Green 0.3 μl, 14 μl ddH2O (Syntol, Russia) and 2 μl DNA. Test tubes for telomeres and reference gene (36B4) (single-copy gene) were tested individually, with the findings only being calculated once an equilibrium had been established for the reaction efficiency. A real-time system (Roter-Gene, QIAGEN) was adopted for the amplification. The (36B4) thermal cycling profile included one cycle (95°C for 15 seconds) followed by 40 cycles (95°C for15 seconds, 57°C for 1 minute), while the telomere cycling included one cycle (95°C for 10 minutes) followed by 50 cycles (95°C for 15 seconds, 58°C for 1 minute).
Table 1.
The sequence of primers used in real-time PCR.
| Primer/oligomer | Sequence |
|---|---|
| Primer Telo F | 5′- cgg-ttt-gtt-tgg-gtt-tgg-gtt-tgg-gtt-tgg-gtt-tgg-gtt-3′ |
| Primer Telo R | 5′- ggc-ttg-cct-tac-cct-tac-cct-tac-cct-tac-cct-tac-cct-3′ |
| Primer 36B4 F | 5′- cag-caa-gtg-gga-agg-tgt-aat-cc-3′ |
| Primer 36B4 R | 5′-ccc-att-cta-tca-tca-acg-ggt-aca-a-3′ |
Measurement of telomere length
In the case of each DNA sample, the following calculation was made: The T/S ratio (telomere (T) was calculated for a single-copy gene (S), Telomere length was expressed as the relative T/S ratio and the relative quantification (not the absolute length) of telomeric DNA was evaluated. In each cycle, the quantity of PCR product roughly doubles. The T/S ratio was computed via the Ct (cycle threshold), which was formulated as follows: [2Ct (telomeres)/2Ct (36B4)]−1 = 2−ΔCt. Furthermore, the fold-change was calculated following formula: 2−ΔΔCt = 2−(ΔCt(group 1) −ΔCt (group2)) from the average of group 1 (control group) subtracted from the average of group 2 (experimental group). The data from the 2−ΔΔCt method comprises the fold change, and the analysis of the 2−ΔΔCt for the case samples compares the changes in the telomere length fold with the control samples. 2−ΔΔCt > 1 indicates that the average telomere length for the case samples is longer than that of the control sample, whereas 2−ΔΔCt < 1 suggests that the DNA samples have an average telomere length that is shorter than the control DNA samples [25].
Statistical analysis
A Pearson rank correlation coefficient (rs) was employed to evaluate the correlation between the telomere length and different parameters, including age and viral load, with a p-value less than 0.05 being deemed significant. Thus, it was possible to identify statistical differences in the telomere length in the three groups (the control group, the case study group, and the viral load group) using Student's t-test. GraphPad InStat software (version 3.05) was utilized for all the statistical analyses.
RESULTS
The average age of the 50 participants in the HPV-positive group was 38.4 ±5.3 years, whereas the average age of the 50 HPV-negative participants was 42.8 ±6.6 years. The maximum HPV DNA loads among the 50 HPV-infected women were 8.5 log, the middle HPV DNA loads were 5.4 log, and the lowest loads were 4.1 log of HPV genomes per 105 human cells. The molecular analysis revealed that 8 participants in the case group (16% of the case group) had HPV co-infections. That is, these participants had two or more types of high-risk HPV. The most common type of mono-infection (44%) was HPV 16. HPV 18 accounted for 10% of the cases, as did HPV 33, whilst the HPV 31 type represented 8% of the cases. In addition, other HR-HPV genotypes, such as HPV 51, 56, 59, 45, and 39 were estimated to be present in 2–4% of cases (Figure 1). Comparatively longer telomeric DNA length (T/S ratios) was identified in participants in the cervical scrapings taken from the control group when a comparison was made with those from the HPV group (T/S ratios=1571.21 versus 1144.06 respectively). This is shown in (Table 2). Neither the risk HPV group (r=0.1976, p=0.1690) nor the control group (r= −0.1822, p=0.2052) revealed any significant correlation between age and telomere length (T/S ratio). However, a significant correlation was evident between HPV load and T/S ratio (r=0.4967, p=0.0002). The participants with HPV loads were subdivided into two groups to facilitate the evaluation of the extent to which the telomeric DNA length varied according to the viral load. The two groups were comprised of one group whose participants presented with an HPV load of 4-5lg and another group in which participants had HPV loads that exceeded 5lg. The analysis indicated that for participants with HPV loads of 4-5lg, the average telomere length in cervical epithelial cells (T/S ratios =1035.12) was less than that of the sample cells of participants in the HPV load (> 5lg) group (T/S=1222.93), as can be seen in (Table 2) (Figure 2). Despite this finding, it should be noted that the difference between the HPV load groups (4-5lg) and (> 5lg) was not statistically significant (p= 0.118).
Figure 1.
The prevalence of high-risk HPV types in HPV-positive women.
Table 2.
The relative telomere lengths among the studied groups.
| HPV group | Control group | 2−ΔΔCt | p-value | ||
|---|---|---|---|---|---|
| Number | Mean of T/S ± SDE | Number | Mean of T/S ± SDE | ||
| 50 | 1144.06 ± 64.085 | 50 | 1571.21 ± 111.55 | 2.5925 ×10−129 | 0.0014 |
| HPV load 4-5lg | |||||
| 21 | 1035.12 ± 65.926 | 4.1809 × 10−162 | 0.0001 | ||
| HPV load >5lg | |||||
| 29 | 1222.93 ± 98.052 | 1.4454 × 10−105 | 0.0217 | ||
SDE: Standard Error of mean; p-value <0.05 was regarded as statistically significant.
Figure 2.
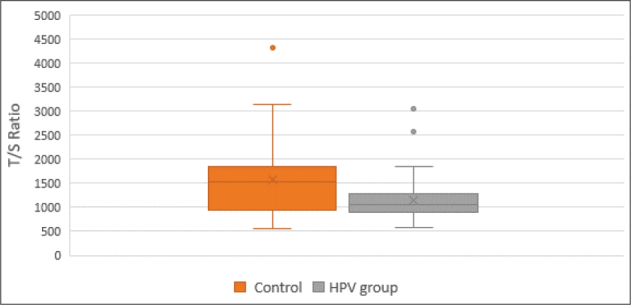
Relative telomere length in control and HPV-positive women. Data are shown as box plots which represent a five-number summary of the data (the minimum, lower quartile, median, upper quartile, and maximum). A small circle indicates an outlier sample.
DISCUSSION
Persistent high-risk human papillomavirus infection is the primary cause of cervical malignant neoplasm lesions [26]. As viral loads increase, in response to protracted exposure, the risk of epithelial cell dysplasia also increases. Thus, there is a discernible correlation between elevated viral loads and the incidence of abnormal lesions and cervical malignant neoplasms [27].
In the current study, cell samples taken from 50 participants with high HPV loads were investigated to ascertain whether telomere length could be employed as a potential diagnostic tool for women who are high-risk HPV positive. Thus, this study has enabled a comparative analysis of the relative telomere lengths in the DNA samples from cervical cell samples taken from participants with high HPV loads with those taken from participants in a control group. No significant differences were reported between participants with HPV loads of 4-5lg and those with HPV loads of >5lg. However, in both HPV load groups, the telomeres were shorter than what was observed in the control group. Thus, telomere shortening could be an initial molecular modification in advance of the development of high-risk HPV carcinogenesis [28]. The results emerging from the current study indicate that telemetric DNA undergoes shortening in cervical cell samples where the HPV load exceeds 4 lg. Early cervical tumorigenesis is characterized by either telomere dysfunction (the loss of telomere capping function) or excessive telomere erosion, both of which generate chromosomal aberrations that lead to oncogenesis [29].
Studies indicate that there is a link between substantially shortening telomeres and both the activation of the DNA damage response pathway (Chk2 phosphorylation) and an increase in cervical cell proliferation [30]. Shortened telomere length is associated with complex, nonreciprocal chromosomal translocations, genome amplification, deletion via breakage–fusion–bridge (BFB) cycles, and the development of epithelial carcinomas and other malignancies [31,32].
Marginal increases or predispositions to increase have been observed in association with viral loads that exceed 5 lg. The viral loads represent the number of infected cells and viral copies in individual cells. Thus, increased viral loads are linked to increased incidences of cervical cancer. Studies have shown that there is a rise in telomerase activity that corresponds with lesion development, ranging from low-grade squamous intraepithelial lesions (LSIL) to high-grade squamous intraepithelial lesions (HSIL), and onwards to invasive cervical cancer. This indicates that telomere length abnormalities constitute a common genetic change that transpires during the many stages in the development of malignant transformation. This correlates with dysplasia and telomerase activity. Furthermore, it increases with the progression of lesions [20,33,34,35,36,37].
There are several limitations to the current research project, the first of which is that the sample included no participants with either cervical cancer or histological lesions. This rendered it impossible to examine telomere alterations in lesions that were at a more advanced stage. The second major limitation pertains to the comparatively small size of the study sample, which might preclude generalization. It is necessary, therefore, to confirm the findings of this study in a larger population.
In conclusion, the current study has revealed that shortened telomeres are present in cervical samples taken from participants who are HR-HPV positive and have elevated viral loads. Thus, telomere length has a potential application in future triaging, monitoring, and screening of women with high-risk HPV infections. It must be reiterated, however, that additional research with a larger sample group is needed before this hypothesis can be confirmed.
ACKNOWLEDGMENTS
This study was performed with the equipment of the Center of Collective Use “High Technologies” (Southern Federal University).
Footnotes
Declaration of Interest.
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
REFERENCE
- 1.Herrmann M, Pusceddu I, März W, Herrmann W. Telomere biology and age-related diseases. Clin Chem Lab Med. 2018;56(8):1210–1222. doi: 10.1515/cclm-2017-0870. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong CA, Tomita K. Fundamental mechanisms of telomerase action in yeasts and mammals: understanding telomeres and telomerase in cancer cells. Open Biol. 2017;7(3):160338. doi: 10.1098/rsob.160338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernal A, Tusell L. Telomeres: Implications for Cancer Development. Int J Mol Sci. 2018;19(1):294. doi: 10.3390/ijms19010294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu X, Han W, Xue W, Zou Y, Xie C, Du J. et al. The association between telomere length and cancer risk in population studies. Sci Rep. 2016;6(1):22243. doi: 10.1038/srep22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caocci G, Greco M, Delogu G, Secchi C, Martino B, Labate C. et al. Telomere length shortening is associated with treatment-free remission in chronic myeloid leukemia patients. J Hematol Oncol. 2016;9(1):63. doi: 10.1186/s13045-016-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto K, Seimiya H. Revisiting Telomere Shortening in Cancer. Cells. 2019;8(2):107. doi: 10.3390/cells8020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivas N, Rachakonda S, Kumar R. Telomeres and Telomere Length: A General Overview. Cancers (Basel) 2020;12(3):558. doi: 10.3390/cancers12030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69. doi: 10.1186/s13073-016-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shay JW. Role of Telomeres and Telomerase in Aging and Cancer. Cancer Discov. 2016;6(6):584–593. doi: 10.1158/2159-8290.CD-16-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pańczyszyn A, Boniewska-Bernacka E, Głąb G. Telomeres and Telomerase During Human Papillomavirus-Induced Carcinogenesis. Mol Diagn Ther. 2018;22(4):421–430. doi: 10.1007/s40291-018-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vinodhini K, Shanmughapriya S, Das BC, Natarajaseenivasan K. Prevalence and risk factors of HPV infection among women from various provinces of the world. Arch Gynecol Obstet. 2012;285(3):771–777. doi: 10.1007/s00404-011-2155-8. [DOI] [PubMed] [Google Scholar]
- 12.Guan P, Howell-Jones R, Li N, Bruni L, de Sanjosé S, Franceschi S. et al. Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J cancer. 2012;131(10):2349–2359. doi: 10.1002/ijc.27485. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Huang X, Zhang Y. Involvement of Human Papillomaviruses in Cervical Cancer. Front Microbiol. 2018;9:2896. doi: 10.3389/fmicb.2018.02896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park Y, Kim T-J, Hwang C-S, Cho CH, Jeong DH, Seong SJ. et al. Risk of cervical dysplasia among human papillomavirus-infected women in Korea: a multicenter prospective study. J Gynecol Oncol. 2019;30(3) doi: 10.3802/jgo.2019.30.e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suligoi B, Vittori G, Salfa MC, Timelli L, Corsini D, Fattorini G. et al. Prevalence and incidence of external genital warts in a sample of Italian general female population. BMC Infect Dis. 2017;17(1):126. doi: 10.1186/s12879-017-2202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anorlu RI. What is the significance of the HPV epidemic? Can J Urol. 2008;15(1):3860–3865. [PubMed] [Google Scholar]
- 17.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 18.Kombe AJK, Li B, Zahid A, Mengist HM, Bounda G-A, Zhou Y. et al. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front Public Heal. 2020;8 doi: 10.3389/fpubh.2020.552028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Dakic A, Chen R, Dai Y, Schlegel R, Liu X. Direct HPV E6/Myc interactions induce histone modifications, Pol II phosphorylation, and hTERT promoter activation. Oncotarget. 2017;8(56):96323–96339. doi: 10.18632/oncotarget.22036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molano M, Martín D, Moreno-Acosta P, Hernández G, Cornall A, Buitrago O. et al. Telomerase activity in cervical scrapes of women with high-grade cervical disease: A nested case-control study. Oncol Lett. 2017;15(1):354–360. doi: 10.3892/ol.2017.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ustaoglu M, Bektas A, Bedir A, Bakir T, Duzgun A, Nar R. et al. The telomere length of gastric mucosal samples and peripheral blood lymphocytes in patients who have undergone Billroth II distal gastrectomy. Arch Med Sci. 2020;16(3):577–583. doi: 10.5114/aoms.2020.94656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Federal Budget Institute of Science Central Research Institute for Epidemiology. Guidelines to AmpliSens HPV HCR screen-titre-FRT PCR kit. Available at: https://interlabservice.ru/upload/iblock/a5b/Guidelines%20to%20AmpliSens%C2%AE%20HPV%20HCR%20screen-titre-FRT%20PCR%20kit%20(update%2028.07.2021).pdf.2018 .
- 23.Federal Budget Institute of Science Central Research Institute for Epidemiology. AmpliSens HPV HCR genotype-titre-FRT PCR kit Instruction Manual. available at: http://www.theranostica.co.il/wp-content/uploads/2015/12/HPV-HCR-genotype-FRT-211212.pdf . 2018.
- 24.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):47e–47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rao X, Huang X, Zhou Z, Lin X. An improvement of the 2^ (–delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat Bioinforma Biomath. 2013;3(3):71. [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Wang P, Ren Y, Du J, Jiang J, Jia X. et al. Prevalence of High-Risk Human Papillomavirus (HR-HPV) Genotypes and Multiple Infections in Cervical Abnormalities from Northern Xinjiang, China. PLoS One. 2016;11(8):e0160698. doi: 10.1371/journal.pone.0160698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo H, Belinson JL, Du H, Liu Z, Zhang L, Wang C. et al. Evaluation of Viral Load as a Triage Strategy With Primary High-Risk Human Papillomavirus Cervical Cancer Screening. J Low Genit Tract Dis. 2017;21(1):12–16. doi: 10.1097/LGT.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 28.Boscolo-Rizzo P, Rampazzo E, Polesel J, Giunco S, Menegaldo A, Mantovani M. et al. Predictive and prognostic significance of telomerase levels/telomere length in tissues and peripheral blood in head and neck squamous cell carcinoma. Sci Rep. 2019;9(1):17572. doi: 10.1038/s41598-019-54028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plug-DeMaggio AW, Sundsvold T, Wurscher MA, Koop JI, Klingelhutz AJ, McDougall JK. Telomere erosion and chromosomal instability in cells expressing the HPV oncogene 16E6. Oncogene. 2004;23(20):3561–3571. doi: 10.1038/sj.onc.1207388. [DOI] [PubMed] [Google Scholar]
- 30.Zhang A, Wang J, Zheng B, Fang X, Ångström T, Liu C. et al. Telomere attrition predominantly occurs in precursor lesions during in vivo carcinogenic process of the uterine cervix. Oncogene. 2004;23(44):7441–7447. doi: 10.1038/sj.onc.1207527. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph KL, Millard M, Bosenberg MW, DePinho RA. Telomere dysfunction and evolution of intestinal carcinoma in mice and humans. Nat Genet. 2001;28(2):155–159. doi: 10.1038/88871. [DOI] [PubMed] [Google Scholar]
- 32.O’Hagan RC, Chang S, Maser RS, Mohan R, Artandi SE, Chin L. et al. Telomere dysfunction provokes regional amplification and deletion in cancer genomes. Cancer Cell. 2002;2(2):149–155. doi: 10.1016/s1535-6108(02)00094-6. [DOI] [PubMed] [Google Scholar]
- 33.Dong L, Wang MZ, Zhao X, Feng R, Hu S, Zhang Q. et al. Human papillomavirus viral load as a useful triage tool for non-16/18 high-risk human papilloma-virus positive women: A prospective screening cohort study. Gynecol Oncol. 2018;148(1):103–110. doi: 10.1016/j.ygyno.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Schlecht NF, Trevisan A, Duarte-Franco E, Rohan TE, Ferenczy A, Villa LL. et al. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int J Cancer. 2003;103(4):519–524. doi: 10.1002/ijc.10846. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Chen J, Hong C, Yi D, Wang F, Cui W. High-risk human papillomavirus infection associated with telomere elongation in patients with esophageal squamous cell carcinoma with poor prognosis. Cancer. 2014;120(17):2673–2683. doi: 10.1002/cncr.28797. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Duque AF, Loango-Chamorro N, Ruiz-Hoyos BM, Landázuri P. Telomerase activity associated with progression of cervical lesions in a group of Colombian patients. Rev Bras Ginecol e Obs. 2015;37(12):559–564. doi: 10.1590/SO100-720320150005462. [DOI] [PubMed] [Google Scholar]
- 37.Pańczyszyn A, Boniewska-Bernacka E, Głąb G. Telomere length in leukocytes and cervical smears of women with high-risk human papillomavirus (HR HPV) infection. Taiwan J Obstet Gynecol. 2020;59(1):51–55. doi: 10.1016/j.tjog.2019.11.007. [DOI] [PubMed] [Google Scholar]



