Abstract
Background:
The role of eosinophils in thrombotic processes is well known, and the prognostic value of eosinophil to monocyte ratio had been determined in patients with ST elevated myocardial infarction and acute ischemic stroke in recent studies. We aimed to evaluate the impact of the eosinophil-to-monocyte ratio on short- and long-term all-cause mortality in patients with pulmonary embolism, which is another clinical condition closely related to the thrombotic pathway.
Methods:
In this study, a total of 212 retrospectively evaluated patients with intermediate-high risk and high-risk pulmonary embolism who underwent catheter-directed therapies with ultrasound-assisted thrombolysis or rheolytic thrombectomy (Angiojet©) and intravenous thrombolytic treatment were included.
Results:
The median Pulmonary Embolism Severity Index score was 105 (86-128; interquartile range: 25-75, min-max: 35-250). The intermediate-high status and high-risk status were noted in 83.5% and 16.5% of the patients, respectively. All of the reperfusion strategies resulted in significant improvements in the measures of pulmonary arterial pressure and right ventricular strain. Death was recorded in 42 (18.6%) patients during the follow-up period (median 1029 days, interquartile range: 651-1358). Multiple Cox regression analysis revealed that a higher pulmonary embolism severity index score (from 85 to 128; hazard ratio = 3.00; 95% CI: 2.11-4.29; P < .001) and a lower eosinophil-to-monocyte ratio (from 0.02 to 0.24; hazard ratio = 0.56; 95% CI: 0.34-0.98; P = .032) were 2 independent predictors for long-term all-cause mortality. The eosinophil-to-monocyte ratio at the admission of less than 0.03 was documented to be associated with higher mortality (P < .001).
Conclusion:
Our results revealed that a lower eosinophil-to-monocyte ratio and a higher pulmonary embolism severity index score independently predict the long-term mortality in patients with intermediate-high- and high-risk pulmonary embolism.
Keywords: Acute pulmonary embolism, ultrasound-assisted thrombolysis, eosinophil to monocyte ratio, rheolytic thrombectomy, prognostic biomarkers
Highlights
New biomarkers for risk stratification in patients with pulmonary embolism are important and will even affect the treatment choice.
Eosinophil-to-monocyte ratio is an easily calculated hematological biomarker without any additional cost and may help in early risk stratification for pulmonary embolism.
Our results revealed that a lower eosinophil-to-monocyte ratio independently predicts the long-term mortality in patients with intermediate-high- and high-risk pulmonary embolism.
Introduction
Acute pulmonary embolism (PE) is a potentially fatal cardiovascular emergency that can result in substantial morbidity and mortality, especially in the presence of acute pressure overload, right ventricular dysfunction (RVD), and hemodynamic instability.1 The RVD and its severity can be assessed by right ventricular to left ventricular diameter ratio (RV/LVr) on echocardiography and computed tomography (CT). Right ventricular dysfunction predicts clinical worsening and mortality risks within 30 days.2 The pulmonary embolism severity index (PESI), including 11 clinical and demographic variables and its simplified form (sPESI), has been validated for initial risk assessment in PE. However, the lack of echocardiographic and laboratory parameters might be considered a limitation of these risk models. Troponin I and RV/LVr > 1.0 by echocardiography and CT pulmonary angiography have also been included in the definition of high, intermediate-high, intermediate-low, and low-risk groups in the European Society of Cardiology/European Respiratory Society (ESC/ERS) 2019 PE Guidelines.3
A biomarker should predict either the progression or the risk and severity of the disease in any clinical condition. Therefore, many different biomarkers have been used in patients with cardiovascular diseases for diagnosis or risk stratification.4
Novel biomarkers of inflammation have also been proposed for risk assessment in PE. The neutrophil-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, and platelet-to-lymphocyte ratio were reported to predict mortality risk in PE and other cardiovascular diseases.5,6 Another blood count measure, namely eosinophil-to-monocyte ratio (EMR), has been shown to be associated with adverse clinical outcomes in acute ischemic stroke and acute myocardial infarction. The role of eosinophils in thrombus formation and thrombotic processes was evaluated, and high levels of eosinophils were detected in thrombus specimens.7,8
To the best of our knowledge, the prognostic impact of EMR has not been evaluated in PE so far. Hence, in this single-center study, we aimed to investigate whether EMR is a predictor of all-cause mortality in PE patients at high-risk (HR) or intermediate-high risk (IHR) status.
Methods
In this retrospective case record follow-up study, we evaluated 212 patients with a median age of 60 years [interquartile range (IQR): 47.8-72; females: 55.7%] who underwent reperfusion therapies including catheter-directed therapies with ultrasound-assisted thrombolysis (USAT) or rheolytic thrombectomy (Angiojet©) and intravenous (IV) thrombolytic treatments with the diagnosis of HR or IHR acute PE in our tertiary cardiovascular center between 2015 and 2021.
The diagnosis of acute PE, initial risk assessment, and management strategies was performed in accordance with the recommendations by the ESC/ERS 2019 acute PE Guidelines.3 Acute PE was confirmed by the presence of thrombus located in at least 1 main or proximal lower lobe pulmonary artery on CT imaging. Patients at intermediate-low risk and low-risk PE whose admission blood samples are unavailable were excluded. Patients with chronic thromboembolic pulmonary hypertension, atopic status, history of steroid use, and active infection (including parasitic infections) were also excluded from the study.
During the coronavirus disease 2019 (COVID-19) pandemic, nasal and oropharyngeal mucosal samples were taken from all patients on admission, and during the in-hospital stay in case of any suspicion. Patients with documented COVID-19 were excluded from the study (Figure 1).
Figure 1.
Flow diagram for inclusion and exclusion criteria.
The CONSERVE 2021 statement has been published to guide studies that started before the COVID-19 pandemic and were completed during the COVID-19 period. The difficulties in conducting a study during a pandemic were that only 18 patients were enrolled in our study after March 2020, and the patients were evaluated according to this statement.9
Chest CT pulmonary angiographic images were acquired before and after selected treatment regimens. The 64-slice helical CT angiography (Toshiba Aquilion 64™, Toshiba Medical Systems Corp., Tokyo, Japan) was used and the stored images were retrospectively evaluated. A validated CT score for pulmonary artery (PA) occlusion proposed by Qanadli score (QS), RV/LVr, main PA, and ascending aorta diameters were measured from CT images.
Echocardiographic evaluation, including tricuspid annular plane systolic excursion (TAPSE) along with tissue velocity and estimated PA systolic pressure from the tricuspid regurgitant jet, was also performed before and after each treatment. Lower extremity venous Doppler ultrasound reports were also available in all patients.
Venous blood samples were collected from all patients to perform laboratory analysis by an antecubital vein. Complete blood count, blood glucose, blood urea nitrogen, creatinine, and troponin-I levels were measured on an autoanalyzer. The patients in whom eosinophil and monocyte counts could be reached at admission were included in the study.
All-cause mortality was defined as the primary outcome. During the follow-up, patients were followed until death or up to January 01, 2021, as a result of the nature of retrospective cohort studies. All clinical data were obtained from the digital records system of hospital and clinic visit records. Survival status was obtained from the national health database records system, clinic visits, and telephone visits.
The Institutional Ethical Committee approved the study design (approval number: 2021.5/1-43), and signed informed consent was obtained from all patients.
Statistical Analysis
In the normality assessment of continuous variables made with Shapiro–Wilk’s test, continuous variables were presented as mean and standard deviation or median and IQR (25th-75th). Discrete data were depicted as absolute numbers and percentages. For comparison of variables between survivors and non-survivors, the t-test, Mann–Whitney U test, and chi-square test were used for continuous variables and categorical variables, respectively. To evaluate the correlation between EMR and QS, we used Spearman’s rank-order correlation and had given r-value and corresponding P-value. Before-after variables were assessed with paired t-test and Wilcoxon test, which were appropriate.
The candidate variables for mortality included focused variables and confounding variables which have been demonstrated in previous studies.3 The EMR was selected as a focused variable and was included in the model as a continuous variable. Three continuous adjustment variables were cardiac troponin-I measurements, PESI score, and TAPSE. To find the association between all-cause mortality and EMR, the Cox regression model was used. Because of overfitting risk, we only included 4 variables in the Cox proportional hazards regression model. In addition, to capture the nonlinearity of continuous variables, we used a restrictive cubic spline (3 knots). The hazard ratio represented an increase from the 25th percentile to the 75th percentile for continuous variables. The risk of all-cause mortality with follow-up was depicted by Kaplan–Meier curves. The survival data with maximally selected rank statistics reduce splitting variable selection bias and can capture non-linearity in the variables.10 The R 4.01 (R software, Vienna, Austria) was used with “rms,” “desctool,” “survival,” and “survminer” packages.
Results
In this study, 212 patients with PE met the inclusion criteria. Baseline characteristics and treatment strategies are summarized in Table 1. Initial risk assessment revealed that IHR and HR status were present in 83.5% and 16.5% of patients, respectively.
Table 1.
Baseline Clinical, Laboratory, and Treatment Characteristics of 212 Patients
| Variables | Overall Patients with PE (n = 212) |
|---|---|
| Age, years | 60 (47.8-72) |
| Male sex, n (%) | 94 (44.3%) |
| Diabetes mellitus, n (%) | 40 (18.9%) |
| Hypertension, n (%) | 84 (39.6%) |
| Atrial fibrillation, n (%) | 14 (6.6%) |
| Previous pulmonary embolism episode, n (%) | 18 (8.5%) |
| Concomitant deep vein thrombosis, n (%) | 123 (59.4%) |
| Precipitating factors | |
| Malignancy, n (%) | 22 (10.4%) |
| Orthopedic surgery/fractures, n (%) | 19 (9%) |
| Previous stroke history, n (%) | 11 (5.2%) |
| Long-haul traveling, n (%) | 17 (8%) |
| Early postoperative period of major surgery, n (%) | 66 (30.1%) |
| Baseline vital signs | |
| Heart rate, bpm | 112 (100-122) |
| Systolic arterial blood pressure, mm Hg | 118 (103-132) |
| Diastolic arterial blood pressure, mm Hg | 75 (64.8-86.3) |
| Systemic arterial oxygen saturation, % | 89 (85-92) |
| Baseline laboratory variables | |
| Hemoglobin, g/dL | 12.6 (11.2-13.7) |
| Troponin-I, ng/mL | 0.09 (0.04-0.22) |
| D-Dimer, U/mL | 9.99 (4.85-19.5) |
| Eosinophil, 103/µL | 0.03 (0.01-0.1) |
| Monocyte, 103/µL | 0.60 (0.50-0.80) |
| Eosinophil-to-monocyte ratio | 0.09 (0.02-0.24) |
| PESI score | 105 (86-128) |
| sPESI | 2 (1-2) |
| High-risk pulmonary embolism, n (%) | 35 (16.5%) |
| Intermediate-high risk pulmonary embolism, n (%) | 177 (83.5%) |
| Treatment regimens | |
| USAT | 132(62.2%) |
| Rheolytic thrombectomy | 25 (11.8%) |
| tPA-duration (hour) | 24 (6-24) |
| Systemic tPA infusion, n (%) Mean tPA dose (mg) |
58 (27.3%) 40 (25-50) |
n, number; bpm, beats per minute; mm Hg, millimeters of mercury; tPA, tissue plasminogen activator; USAT, ultrasound-assisted thrombolysis; PESI, pulmonary embolism severity index; sPESI, simplified pulmonary embolism severity index.
The CT measures of RV/LVr, QS, PA diameters, and echocardiographic measures of TAPSE and pulmonary artery systolic pressure (PAPs) estimates were significantly improved after the reperfusion treatments including USAT, rheolytic thrombectomy, and IV tPA infusions (Table 2).11-14
Table 2.
Change of the RV/LVr, Qanadli score, TAPSE, and Pulmonary Artery Systolic Pressure in 3 Treatment Groups (USAT, Rheolytic-Thrombectomy, and Intravenous t-PA)
| Before Therapy | After Therapy | Mean Change, 95% CI | P | |
|---|---|---|---|---|
| USAT group, n = 132 | ||||
| RV/LV ratio | 1.22 ± 0.19 | 0.91 ± 0.12 | 0.31 | <.001 |
| TAPSE (cm) | 1.78 ± 0.38 | 2.33 ± 0.39 | −0.55 | <.001 |
| Qanadli score | 23.4 ± 5.9 | 7.9 ± 5.2 | 15.6 | <.001 |
| PAPs | 55.2 ± 11.9 | 36.6 ± 9.6 | 18.9 | <.001 |
| Rheolytic thrombectomy group, n = 25 | ||||
| RV/LV ratio | 1.27 ± 0.14 | 0.92 ± 0.13 | 0.35 | <.001 |
| TAPSE (cm) | 1.79 ± 0.35 | 2.2 ± 0.49 | −0.41 | <.001 |
| Qanadli score | 25.4 ± 5.7 | 12.6 ± 7.2 | 12.8 | <.001 |
| PAPs | 56.6 ± 13.1 | 37 ± 11.3 | 19.5 | <.001 |
| tPA group, n = 58 | ||||
| RV/LVr | 1.23 ± 0.2 | 0.93 ± 0.14 | 0.30 | <.001 |
| TAPSE (cm) | 1.75 ± 0.44 | 2.27 ± 0.37 | −0.52 | <.001 |
| Qanadli score | 22.6 ± 7.2 | 8.2 ± 5.1 | 14.4 | <.001 |
| PAPs | 53.2 ± 12.9 | 33.6 ± 9.5 | 19.6 | <.001 |
USAT, ultrasound-assisted thrombolysis; TAPSE, tricuspid annular planary excursion; PAPs, pulmonary artery systolic pressure; RV, right ventricle; LV, left ventricle; RV/LVr, right ventricle-to-left ventricle ratio.
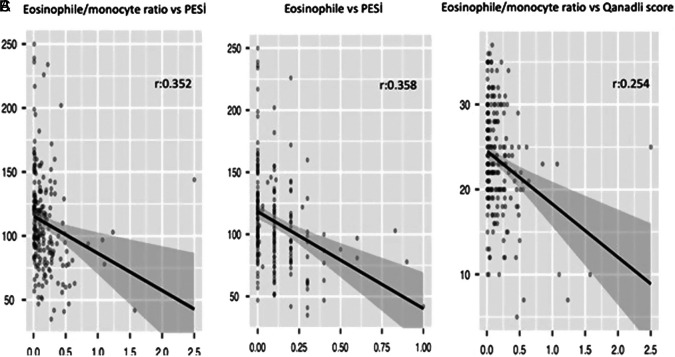
The EMR, but not eosinophil count, was found to be correlated with QS (r = 0.360, P = .014) (Figure 2A-C). However, neither EMR nor eosinophil count was related to the PESI score (Figures 3A and B).
Figure 2.
(A) Association between eosinophil-to-monocyte ratio and pulmonary embolism severity index (PESI) score. (B) Association between eosinophil amount and PESI score. (C) Association between eosinophil-to-monocyte ratio and Qanadli score.
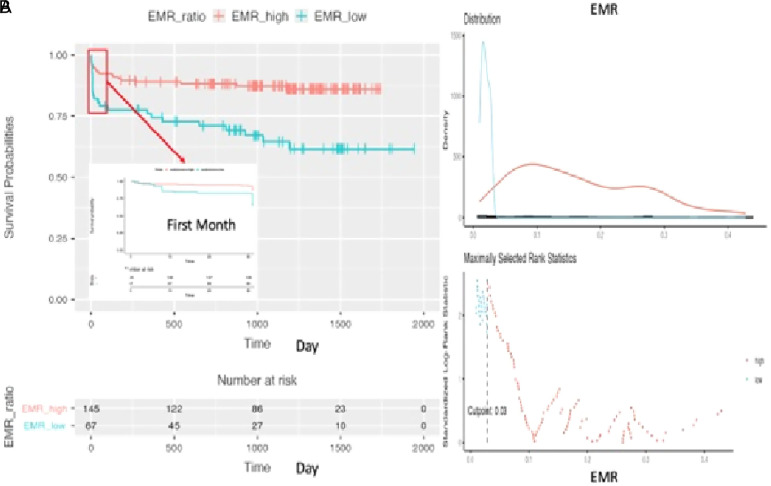
Figure 3.
(A and B) Difference between survival probabilities regarding high eosinophil-to-monocyte ratio (EMR) or low-EMR (cut-off: 0.03) during the follow-up.
Death was recorded in 42 (18.6 %) patients during the median of 1029 days (IQR: 651-1358) of the follow-up period.
According to the results of univariable Cox regression analysis (Table 3), age (from 47 to 72; hazard ratio = 2.19; 95% CI: 1.29-3.71; P = .003), malignancy (hazard ratio = 2.94; 95% CI: 1.44-5.99; P = .003), chronic obstructive pulmonary disease (hazard ratio = 3.61; 95% CI: 1.72-7.56; P < .001), heart rate (100-122 bpm; hazard ratio = 1.62; 95% CI: 1.10-2.36; P = .013), systolic BP (103-132 mm Hg; hazard ratio = 0.57; 95% CI: 0.40-0.83; P = .004), hemoglobin (11.1-13.7; hazard ratio = 0.60; 95% CI: 0.41-0.88; P = .009), oxygen saturation (85-92%; hazard ratio = 0.57; 95% CI: 0.47-0.73; P < .001), PESI score (85 to 128; hazard ratio = 2.27; 95% CI: 1.74-2.95; P < .001), risk status at admission (HR vs. IHR: hazard ratio = 2.31; 95% CI: 1.18-4.52; P = .012), and EMR (0.02-0.24; hazard ratio = 0.42; 95% CI: 0.20-0.89; P = .016) were found to be associated with all-cause mortality.
Table 3.
Univariate Cox Proportional Regression Analysis for Predicting Long-Term Mortality
| Variables | Crude Hazard Ratio | CI | P |
|---|---|---|---|
| Age (years; from 47 to 72) | 2.19 | 1.29-3.71 | .003 |
| Sex (male reference n %) | 0.61 | 0.33-1.13 | .112 |
| Diabetes mellitus | 0.72 | 0.30-1.72 | .474 |
| Atrial fibrillation | 1.48 | 0.53-4.14 | .455 |
| Malignancy | 2.94 | 1.44-5.99 | .003 |
| COPD | 3.61 | 1.72-7.56 | <.001 |
| Heart rate (beat per minute; from 100 to 122) | 1.62 | 1.10-2.36 | .013 |
| Systolic BP (mm Hg; from 103 to 132) | 0.57 | 0.40-0.83 | .004 |
| Oxygen saturation (%; from 85 to 92) | 0.59 | 0.47-0.73 | <.001 |
| PESI score (from 85 to 128) | 2.27 | 1.74-2.95 | <.001 |
| Creatinine (mg/dL; from 0.7 to 1.1) | 0.82 | 0.47-1.42 | .483 |
| Risk-status (intermediate-high reference) | 2.31 | 1.18-4.52 | .012 |
| Qanadli score (from 20 to 28) | 1.15 | 0.77-1.72 | .484 |
| RV/LVr | 0.96 | 0.92-1.02 | .297 |
| Main PA/aorta diameter ratio (from 0.80 to 0.99) | 1.13 | 0.68-1.89 | .631 |
| TAPSE (cm; from 1.5 to 2) | 0.80 | 0.54-1.18 | .266 |
| St (cm/s; from 9 to 12) | 1.12 | 0.62-2.02 | .701 |
| Troponin (ng/mL; from 0.04 to 0.22) | 0.94 | 0.86-1.03 | .174 |
| Hemoglobin (g/dL; from 11.1 to 13.7) | 0.60 | 0.41-0.88 | .009 |
| Eosinophil (from 0.01 to 0.102103/µL) | 0.77 | 0.43-1.35 | .425 |
| Monocyte (from 0.5 to 0.8 103/µL) | 1.02 | 0.51-2.07 | .172 |
| Eosinophil-to-monocyte ratio (from 0.02 to 0.24) | 0.42 | 0.20-0.89 | .016 |
n, number; mm Hg, millimeters of mercury; COPD, chronic obstructive pulmonary disease; BP, blood pressure; TAPSE, tricuspid annular planary systolic excursion; PA, pulmonary artery; RV, right ventricle; LV, left ventricle; RV/LVr, right ventricle-to-left ventricle ratio; St, systolic motion; PESI, pulmonary embolism severity index.
The results of multiple Cox regression analysis revealed that a higher PESI score (an increase from 85 to 128) (hazard ratio = 2.61; 95% CI: 1.67-4.10; P < .001) was an independent predictor for 30-day all-cause mortality, whereas a higher PESI (an increase from 85 to 128; hazard ratio = 3.00; 95% CI: 2.11-4.29; P < .001) and a lower EMR (from 0.02 to 0.24; hazard ratio = 0.56; 95% CI: 0.34-0.98; P = .032) were independent predictors for cumulative all-cause mortality (Table 4).
Table 4.
Multiple Cox Proportional Regression Analysis to Predict 30-Day and Long-Term All-Cause Mortality
| Variable | Adjusted Hazard Ratio | CI | P |
|---|---|---|---|
| 30-day all-cause mortality | |||
| PESI score (from 85 to 128) | 2.61 | 1.67-4.10 | <.001 |
| TAPSE (cm; from 1.5 to 2) | 1.42 | 0.81-2.50 | .213 |
| Troponin (ng/mL; from 0.04 to 0.22) | 0.86 | 0.68-1.08 | .172 |
| EMR (from 0.02 to 0.24) | 0.73 | 0.27-1.95 | .196 |
| Long-term all-cause mortality | |||
| PESI (from 85 to 128) | 3.00 | 2.11-4.29 | <.001 |
| TAPSE (cm; from 1.5 to 2) | 1.40 | 0.92-2.11 | .114 |
| Troponin (ng/mL; from 0.04 to 0.22) | 0.95 | 0.91-1.01 | .145 |
| EMR (from 0.02 to 0.24) | 0.56 | 0.34-0.98 | .032 |
TAPSE, tricuspid annular planary excursion; PESI, pulmonary embolism severity index; EMR, eosinophil-to-monocyte ratio.
The number of patients in our study before the COVID period was 194, and during the COVID-19 pandemic, only 18 patients were enrolled.
Additionally, it is not possible to evaluate if patients who had previous PE diagnoses died after a very long time due to COVID-19, because causality and clinical adjudication are not in the commit, and we also evaluate long-term mortality from the national registry system.
The EMR values for the pre-COVID period and COVID-19 pandemic were 0.17 ± 0.11 and 0.21 ± 0.27 (P = .497), respectively.
There was no difference in in-hospital mortality rates between pre-COVID 21 (5.6%) and COVID-19 periods 21 (10.8%) (P = .703).
The concomitant DVT was diagnosed in 118 patients, and the inclusion of acute deep vein thrombosis (DVT) into the model in Cox regression multiple analysis did not change the hazard ratio of EMR for 30-day and long-term mortality. The 30-day hazard ratios were 0.74 (CI: 0.26-1.97, P = .212) and 0.73 (CI: 0.27-1.95, P = .195) with and without the inclusion of the DVT into the model. The long-term hazard ratio for EMR was 0.55 (CI: 0.32-0.97, P = .032) and 0.56 (CI: 0.34-0.98, P = .031) with or without the inclusion of the DVT into the model.
The maximally selected rank statistics revealed the value of 0.03 at admission as an optimal cut-off for EMR in predicting the survival probability (Figure 2A). Kaplan–Meier survival estimates demonstrated that low versus high EMR at baseline assessment did not discriminate 30-day all-cause mortality, but it was associated with higher long-term all-cause mortality (log-rank test P < .001) (Figure 2B).
Discussion
The EMR, calculated as a simple ratio between eosinophil and monocyte, has been evaluated as an inflammatory response biomarker in various cardiovascular clinical scenarios except for PE. To the best of our knowledge, this study is the first to evaluate EMR for long-term outcomes in patients with IHR and HR PE, and our results revealed that an EMR less than 0.03 independently predicted a higher cumulative all-cause mortality during the median of 1029 days (IQR: 651-1358) of the follow-up period. Moreover, a higher PESI score independently predicted both 30-day and long-term all-cause mortality.
The role of inflammation in the pathogenesis of cardiovascular clinical conditions is well-known and studies have focused mainly on neutrophil-derived biomarkers for seeking additional clinical benefit and early treatment or intervention in patients with atherothrombotic cardiovascular diseases.15
White blood cells are the principal cells affected by inflammation and are considered to be responsible for undesirable situations in patients with cardiovascular diseases.16,17
Monocytes are pro-inflammatory cells and play an important role in inflammation, an immune-mediated process, by binding to adhesion molecules expressed on the damaged vascular endothelium and other different mechanisms in cardiovascular diseases.18-20 Recent studies have provided new evidence for the complexity of macrophage phenotypes found in the myocardium.21
In the past, eosinophils were thought to only be related to parasitic infections and allergies. But recent studies revealed that pro-inflammatory and cytotoxic granules could induce destructive properties of eosinophils.22 Therefore, the role of these cells in thrombosis and eosinophil-related vascular toxicity has attracted increasing attention in recent years.
Eosinophils migrate to the inflammatory areas and modulate immune responses through an array of mechanisms. Besides the inflammatory role, eosinophils are also considered to induce endothelial damage and exposure of endothelial cells to the tissue factor, which enhances thrombotic activity via platelet activation and aggregation. Eosinophils are activated by platelets and gathered in human thrombi and atherosclerotic plaques; they further promote thrombus formation by the release of eosinophil peroxidase, platelet activation factor, and some other proteins.23,24
The acute and marked decrease in eosinophil counts and percentages in peripheral blood in patients with acute coronary syndrome as compared to patients with stable angina and abundant eosinophil percentage in thrombus specimens extracted from occluded coronary arteries seem to be due to activation, accumulation, and capture of these cells into the fresh clot resulting in a possible “consumptive eosinopenia.” Hence, EMR could be a simple, useful, and inexpensive marker for risk stratification for PE. In a case report by Qiao et al25 it was reported that the migration of eosinophils to the extra-vascular area might explain the eosinopenia in peripheral blood.
The QS measured on CT pulmonary angiography has been used to assess clot burden, and some studies have indicated that QS correlates with risk stratification and prognosis in PE.26,27 In our study; the EMR, but not eosinophil count, was found to be correlated with QS and our results may reflect the role of eosinophil migration in the thrombus specimens.
Riegger et al28 demonstrated that neutrophils are present in thrombus samples, reflecting the important role of inflammation in-stent thrombosis, and eosinophils are also detected in all types of stent thrombus specimens. Notably, when compared to those thrombus cases detected in the first myocardial infarction, in patients with very late stent thrombosis, there was a significantly higher number of eosinophils.28
Although blood eosinophil counts decline, large numbers of eosinophils are detected in thrombus specimens and damaged tissue in the acute period of myocardial infarction, and low blood eosinophil counts have been documented to be associated with adverse outcomes.23
A lower EMR was associated with higher 30-day and long-term mortality in patients with acute ST-elevated myocardial infarction, and a low EMR was independently correlated with poor outcomes in patients with acute ischemic stroke.
Our results appear to be consistent with this hypothesis. The EMR was found to be correlated with pulmonary arterial obstruction as quantitated by QS, and more interestingly, in addition to the independent association between a higher PESI score, and 30-day and long-term all-cause mortality risk, a low EMR independently predicted long-term cumulative all-cause mortality. However, this association seemed to be independent of the concomitant DVT at the initial assessment.
Study Limitations
Our study has several limitations. First, it was a single-center study performed in a tertiary referral institution for PE. Second, due to the observational nature of the study, some treatment bias may exist. The treatment success for 3 reperfusion strategies was clinically relevant and statistically significant. The impact of EMR on reperfusion success might provide mechanistic insights into the importance of this measure in acute phase treatments of PE. Third, due to the nature of regression analysis, unmeasured confounders may still exist. Because of overfitting, we did not include malignancy as a separate variable in the multiple models. Although a higher PESI was associated with a higher mortality risk for 30-day and long-term periods, the impact of low EMR on 30-day mortality remained less clear. Further studies investigating the complex relationship between EMR and pro-inflammatory markers are needed to determine the value of EMR in this setting. The study covers both the pre-COVID period and the COVID-19 pandemic. During the COVID-19 pandemic, nasal and oropharyngeal mucosal samples were taken from all patients on admission, and during the in-hospital stay in case of any suspicion. Patients with documented COVID 19 were excluded from the study. The mechanisms and prognoses of PE during the COVID-19 period and before it might be different. Although patients with active infection (including parasitic infections) and a known history of atopic conditions or allergies were excluded, the possibility of any other clinical condition affecting eosinophil count may be a limitation of this study.
Conclusion
Our study revealed that a lower EMR independently predicted cumulative long-term all-cause mortality in patients with PE at IHR and HR. The cut-off value of 0.03 for EMR at admission was found to discriminate long-term survival whereas a higher PESI score predicted both 30-day and long-term mortality.
Footnotes
Availability of Data and Material: The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Ethics Committee Approval: This study was conducted in accordance with the Declaration of Helsinki. The study protocol was reviewed and approved by the Kartal Koşuyolu Heart Training and Research Hospital Institutional Ethics Committee (approval number: 2021.5/1-43).
Informed Consent: Written informed consent has been obtained from all patients prior to enrollment in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Ş.K.; Design - Ş.K. A.K.; Supervision - C.K., N.Ö.; Funding: None; Materials - D.Ç., K.B.; Data collection and/or processing - H.C.T., B.K.; Analysis and/or interpretation - İ.H.T., S.Ç.E.; Literature review - S.T., B.K.; Writing - Ş.K., A.K., C.K.; Critical review - Ö.Y.A., C.K.
Acknowledgments: None.
Declaration of Interests: No potential conflict of interest was reported by the author(s).
Funding: The authors did not receive any funding for this study.
References
- 1. Barco S, Mahmoudpour SH, Valerio L.et al. Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med. 2020;8(3):277 287. 10.1016/S2213-2600(19)30354-6) [DOI] [PubMed] [Google Scholar]
- 2. Sanders JL, Koestenberger M, Rosenkranz S, Maron BA. Right ventricular dysfunction and long-term risk of death. Cardiovasc Diagn Ther. 2020;10(5):1646 1658. 10.21037/cdt-20-450) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Konstantinides SV, Meyer G, Becattini C.et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): the Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54(3):1901647. 10.1183/13993003.01647-2019) [DOI] [PubMed] [Google Scholar]
- 4. MacNamara J, Eapen DJ, Quyyumi A, Sperling L. Novel biomarkers for cardiovascular risk assessment: current status and future directions. Future Cardiol. 2015;11(5):597 613. 10.2217/fca.15.39) [DOI] [PubMed] [Google Scholar]
- 5. Wang Q, Ma J, Jiang Z, Ming L. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in acute pulmonary embolism: a systematic review and meta-analysis. Int Angiol. 2018;37(1):4 11. 10.23736/S0392-9590.17.03848-2) [DOI] [PubMed] [Google Scholar]
- 6. Köse N, Yıldırım T, Akın F, Yıldırım SE, Altun İ. Prognostic role of NLR, PLR, and LMR in patients with pulmonary embolism. Bosn J Basic Med Sci. 2020;20(2):248 253. 10.17305/bjbms.2019.4445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu S, Luo Y, Zhang T.et al. Eosinophil-to-monocyte ratio is a potential biomarker in the prediction of functional outcome among patients with acute ischemic stroke. BMC Neurosci. 2021;22(1):8. 10.1186/s12868-021-00610-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deng X, Wang X, Shen L.et al. Association of eosinophil-to-monocyte ratio with 1-month and long-term all-cause mortality in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Thorac Dis. 2018;10(9):5449 5458. 10.21037/jtd.2018.09.27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Orkin AM, Gill PJ, Ghersi D.et al. Guidelines for reporting trial protocols and completed trials modified due to the COVID-19 pandemic and other extenuating circumstances: the CONSERVE 2021 statement. CONSERVE group. JAMA. 2021;326(3):257 265. 10.1001/jama.2021.9941) [DOI] [PubMed] [Google Scholar]
- 10. Wright MN, Dankowski T, Ziegler A. Unbiased split variable selection for random survival forests using maximally selected rank statistics. Stat Med. 2017;36(8):1272 1284. 10.1002/sim.7212) [DOI] [PubMed] [Google Scholar]
- 11. Kaymaz C, Akbal OY, Tanboga IH.et al. Ultrasound-assisted catheter-directed thrombolysis in high-risk and intermediate-high-risk pulmonary embolism: a meta-analysis. Curr Vasc Pharmacol. 2018;16(2):179 189. 10.2174/1570161115666170404122535) [DOI] [PubMed] [Google Scholar]
- 12. Kaymaz C, Akbal OY, Hakgor A.et al. A five-year, single-center experience on ultrasound-assisted, catheter-directed thrombolysis in patients with pulmonary embolism at high risk and intermediate to high risk. EuroIntervention. 2018;14(10):1136 1143. 10.4244/EIJ-D-18-00371) [DOI] [PubMed] [Google Scholar]
- 13. Kaymaz C, Akbal OY, Keskin B.et al. An eight-year, single-center experience on ultrasound-assisted thrombolysis with moderate-dose, slow-infusion regimen in pulmonary embolism. Curr Vasc Pharmacol. 2022;20. 10.2174/1570161120666220428095705) [DOI] [PubMed] [Google Scholar]
- 14. Akbal ÖY, Keskin B, Tokgöz HC.et al. A seven-year single-center experience on AngioJet rheolytic thrombectomy in patients with pulmonary embolism at high risk and intermediate-high risk. Anatol J Cardiol. 2021;25(12):902 911. 10.5152/AnatolJCardiol.2021.28303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Soehnlein O, Steffens S, Hidalgo A, Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. 2017;17(4):248 261. 10.1038/nri.2017.10) [DOI] [PubMed] [Google Scholar]
- 16. Malech HL, DeLeo FR, Quinn MT. The role of neutrophils in the immune system: an overview. Methods Mol Biol. 2020;2087:3 10. 10.1007/978-1-0716-0154-9_1) [DOI] [PubMed] [Google Scholar]
- 17. Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci. 2021;22(2):559. 10.3390/ijms22020559) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kang H, Li X, Xiong K.et al. The entry and egress of monocytes in atherosclerosis: a biochemical and biomechanical driven process. Cardiovasc Ther. 2021;2021:6642927. 10.1155/2021/6642927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shintani Y, Ito T, Fields L.et al. IL-4 as a repurposed biological drug for myocardial infarction through augmentation of reparative cardiac macrophages: proof-of-concept data in mice. Sci Rep. 2017;7(1):6877. 10.1038/s41598-017-07328-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Márquez AB, van der Vorst EPC, Maas SL. Key chemokine pathways in atherosclerosis and their therapeutic potential. J Clin Med. 2021;10(17):3825. 10.3390/jcm10173825) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bajpai G, Schneider C, Wong N.et al. The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med. 2018;24(8):1234 1245. 10.1038/s41591-018-0059-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fujita K, Ishimaru H, Hatta K, Kobashi Y. Hypereosinophilic syndrome as a cause of fatal thrombosis: two case reports with histological study. J Thromb Thrombolysis. 2015;40(2):255 259. 10.1007/s11239-014-1151-9) [DOI] [PubMed] [Google Scholar]
- 23. Shiyovich A, Gilutz H, Plakht Y. White blood cell subtypes are associated with a greater long-term risk of death after acute myocardial infarction. Tex Heart Inst J. 2017;44(3):176 188. 10.14503/THIJ-16-5768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol. 2014;10(8):474 483. 10.1038/nrrheum.2014.98) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Qiao L, Med BS, Gao D. China A case report and literature review of Churg–Strauss syndrome presenting with myocarditis. Medicine. 2016;95:e5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Qanadli SD, El Hajjam M, Vieillard-Baron A.et al. New CT index to quantify arterial obstruction in pulmonary embolism: comparison with angiographic index and echocardiography. AJR Am J Roentgenol. 2001;176(6):1415 1420. 10.2214/ajr.176.6.1761415) [DOI] [PubMed] [Google Scholar]
- 27. Shen C, Yu N, Wen L.et al. Risk stratification of acute pulmonary embolism based on the clot volume and right ventricular dysfunction on CT pulmonary angiography. Clin Respir J. 2019;13(11):674 682. 10.1111/crj.13064) [DOI] [PubMed] [Google Scholar]
- 28. Riegger J, Byrne RA, Joner M.et al. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J. 2016;37(19):1538 1549. 10.1093/eurheartj/ehv419) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a