Abstract
All primate lentiviruses (HIV-1, HIV-2, SIV) encode Nef proteins, which are important for viral replication and pathogenicity in vivo1-3. It is not known how Nef regulates these processes. It has been suggested that Nef protects infected cells from apoptosis and recognition by cytotoxic T lymphocytes4-6. Other studies suggest that Nef influences the activation state of the infected cell, thereby enhancing the ability of that cell to support viral replication7-10. Here we show that macrophages that express Nef or are stimulated through the CD40 receptor release a paracrine factor that renders T lymphocytes permissive to HIV-1 infection. This activity requires the upregulation of B-cell receptors involved in the alternative pathway of T-lymphocyte stimulation. T lymphocytes stimulated through this pathway become susceptible to viral infection without progressing through the cell cycle. We identify two proteins, soluble CD23 and soluble ICAM, that are induced from macrophages by Nef and CD40L, and which mediate their effects on lymphocyte permissivity. Our results reveal a mechanism by which Nef expands the cellular reservoir of HIV-1 by permitting the infection of resting T lymphocytes.
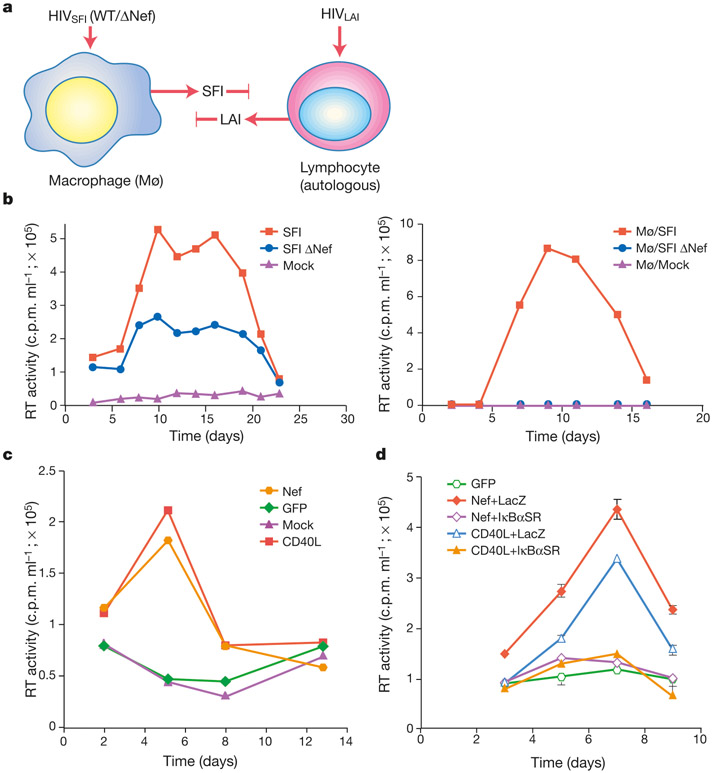
We have previously shown that human immunodeficiency virus (HIV)-1 Nef induces the release of CC- chemokines from infected macrophages, and we have proposed that Nef may promote the recruitment of substrate T lymphocytes to sites of infection11. We initiated the current study when we observed that lymphocyte/macrophage co-cultures supported the replication of wild-type HIV-1 more efficiently than did Nef-deleted virus, and that T lymphocytes within these co-cultures did not require exogenous stimulation to support viral replication. The ability of peripheral blood lymphocytes (PBLs) to support virus (HIV-1LAI) replication was examined after their incubation with macrophages harbouring awild-type (HIV-1SFIWT) or ΔNef virus (HIV-1SFIΔNef) (Fig. 1a). The level of virus production in macrophages infected with HIV-1SFI ΔNef approximated (twofold difference) that in wild-type HIV-1SFI-infected macrophages (Fig. 1b, left panel). Despite this, infectious HIV-1LAI (determined by virus titration on MT-4 cells) was detected only when autologous PBLs were co-cultured with wild-type HIV-1SFI-infected macrophages and not HIV-1SFI ΔNef-infected or uninfected macrophages (Fig. 1b, right panel). Similarly, lymphocytes cultured in the absence of infected macrophages remained refractory to HIV-1LAI replication (not shown). Thus, in infected macrophages, Nef induces a stimulus that renders lymphocytes permissive to infection. Nef activates the production of the CC- chemokines MIP-1α and MIP-1β11. These chemokines are also regulated, in a NF-κB-dependent manner, through the co-stimulatory receptor CD40 (ref. 12). We observed that Nef, in the context of viral replication and when expressed from an adenovirus vector, induced NF-κB activation (not shown), raising the possibility that signals generated by Nef and CD40 intersect.
Figure 1.
Macrophages expressing Nef render lymphocytes permissive to HIV-1 replication. a, Assay for permissivity. The tropism HIV-1SFI and HIV-1LAI is restricted to macrophages and T cells, respectively. HIV-1LAI production (detected by titration on MT4 cells) provides an indication that those lymphocytes were rendered permissive for viral replication by infected macrophages. b, Wild-type but not Nef-deleted viruses induce lymphocyte permissivity. Virus production from HIV-1 SFI WT- and ΔNef-infected macrophages in the absence of lymphocyte culture (left panel); MT4 titration of HIV-1LAI in supernatants of lymphocytes co-cultured with HIV-1SFI WT-infected, HIV-1SFI ΔNef-infected and mock-infected macrophages (right panel). c, CD40 stimulation induces lymphocyte permissivity. Viral replication was examined in resting lymphocytes exposed to supernatants from Nef- and GFP-expressing macrophages, or from CD40L-stimulated macrophages. d, Inhibition of Nef- and CD40-induced permissivity by an IκBα ‘super-repressor’. Macrophages were subject to CD40L stimulation/adenovirus infections as indicated, and supernatants were examined for induction of permissivity. RT, reverse transcriptase.
To examine the relationship between Nef and CD40 signalling, T lymphocytes were incubated with supernatants from macrophages activated by CD40L and examined for permissivity to infection. Whereas lymphocytes exposed to supernatants of untreated macrophages were completely refractory to HIV-1 replication, those exposed to culture supernatants from CD40L-stimulated macrophages were permissive to HIV-1 infection (Fig. 1c). This property of CD40L-stimulated macrophages was identical for Nef-expressing macrophages in that it required neither cell contact nor exogenous activation of substrate lymphocytes. We next examined whether the ability of CD40L and Nef to induce lymphocyte permissivity was dependent on NF-κB. We used an adenovirus expressing a ‘super-repressor’ variant of IκBα (IκBαSR)13 to inhibit NF-κB activation. In the presence of IκBαSR, the ability of Nef to induce lymphocyte permissivity was inhibited (Fig. 1d). Co-infection with an adenoviral vector expressing a control protein (LacZ) had no effect on Nef activity (Fig. 1d). The IκBαSR adenovirus, but not the control adenovirus, also inhibited the ability of CD40L-stimulated macrophages to induce lymphocyte permissivity (Fig. 1d). Macrophage function, as determined from the levels of macrophage-colony stimulating factor (MCSF) released into culture supernatants, was not impaired following transduction with the adenovirus vectors (not shown). Collectively, these results indicate that Nef induces lymphocyte permissivity to HIV-1 infection by intersecting a pathway that is regulated by the CD40 receptor, and that CD40 activation can functionally substitute for Nef.
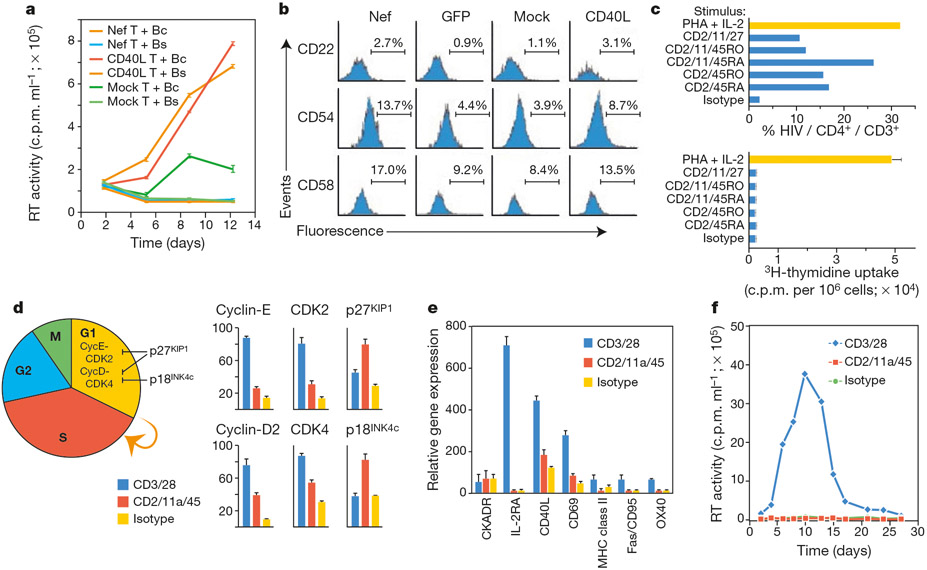
Because quiescent (G0) T lymphocytes are completely refractory to HIV-1 replication in vitro, we expected Nef and CD40L to induce permissivity by promoting lymphocyte activation. We reasoned that an analysis of specific CD4+ lymphocyte subsets stimulated by Nef or CD40L would assist in identifying the mechanism through which lymphocyte permissivity is effected. However, our experiments revealed that T lymphocytes, when separated from B lymphocytes, did not become permissive to HIV-1 infection on exposure to supernatants of Nef-expressing or CD40L-stimulated macrophages (Fig. 2). T-cell permissivity could be induced by B lymphocytes that were pretreated with supernatants from Nef-expressing or CD40L-stimulated macrophages (Fig. 2a). As supernatants from these pretreated B lymphocytes were inactive (Fig. 2a), this indicated a role for accessory molecules on B lymphocytes in the induction of T-lymphocyte permissivity by Nef and CD40L. Immunophenotyping revealed that B lymphocytes exposed to supernatants from Nef-expressing macrophages exhibited increased expression of the receptors CD22, CD54 and CD58—a pattern that was identical for B lymphocytes exposed to supernatants from CD40L-stimulated macrophages (Fig. 2b). Since antibody-blocking experiments indicated that these receptors were involved in the induction of permissivity by Nef and CD40L (not shown), we examined whether direct stimulation of the ligands for these receptors on T lymphocytes would render them permissive to HIV-1 infection.
Figure 2.
Nef and CD40 promote resting T-lymphocyte infection by regulating co-stimulatory receptors on B lymphocytes. a, Nef- and CD40-mediated permissivity requires the presence of B lymphocytes. B lymphocytes were incubated with supernatants from Nef-expressing or CD40L-stimulated macrophages. After 72 h, the B cells (Bc) or B-cell supernatants (Bs) were added to autologous lymphocytes, which were then examined for susceptibility to HIV-1 infection. b, Nef and CD40 upregulate similar receptors on B cells. B lymphocytes were incubated with supernatants from Nef- or GFP-expressing or CD40L-treated macrophages, and expression of CD22, CD54 and CD58 on CD19+ B cells was determined by FACS. c, B-lymphocyte signals induce cell-cycle-independent infection of T lymphocytes. Following ligation of the indicated receptors, resting CD3+ T lymphocytes were infected with HIV-1HSA and the level of infection and cell-cycle status determined by FACS (upper panel) and by thymidine incorporation (lower panel), respectively. Rabbit F(ab)2-cross-linked mouse IgG (isotype) is shown as a control. d, Expression profiles of cell-cycle regulatory genes in antibody-stimulated lymphocytes. Purified CD3+ lymphocytes were stimulated as in c and transcript levels for the indicated genes determined by RNase protection assay. e, Transcript profiles of cellular activation markers. f, Viral replication profiles in antibody-stimulated T lymphocytes. CycE, Cyclin-E; Cyc-D, Cyclin-D; MHC, major histocompatibility complex.
Purified T lymphocytes were stimulated with antibodies to CD45 RO/RA, CD11a and CD2 (the T-cell ligands for CD22, CD54 and CD58, respectively), then infected with a recombinant HIV-1 variant (HIV-1HSA)14. Expression of heat-stable antigen (HSA) indicates completion of steps in the viral life cycle up to, and including, de novo viral gene expression. CD2 and CD45 ligation on T lymphocytes was sufficient to render those cells highly susceptible to HIV-1 infection at rates approaching those obtained for T lymphocytes receiving a potent—PHA (phytohaemagglutinin)/interleukin-2 (IL-2)—activation stimulus (Fig. 2c). By contrast, other antibodies, such as CTLA-4, did not promote lymphocyte permissivity, nor did the cytokines IL-1 or IL-16 (not shown). The induction of lymphocyte permissivity minimally required a CD2 stimulus while the RO and RA isoforms of CD45 were interchangeable, and the CD11a stimulus augmented the CD2/CD45 stimulus (Fig. 2c). Contrary to our expectations, T lymphocytes rendered permissive to infection by the CD2/CD45 stimulus had not progressed through the cell cycle, on the basis of the level of thymidine uptake (Fig. 2c). Transition beyond G1 requires the cooperative action of Cyclin-D and Cyclin-E with their catalytic kinase partners CDK4 (cyclin-dependent kinase 4) and CDK2, which in turn are regulated by p18INK4c and p27KIP-1 (Fig. 2d). Although activated (CD3/CD28) lymphocytes exhibited higher levels of Cyclin/CDK transcripts relative to CDK-inhibitor transcripts, this relationship was inverted in CD2/CD11a/CD45-stimulated cells (Fig. 2d), indicating that this stimulus was insufficient to overcome blocks that prevent G1-to-S transition. CD2/CD11a/CD45-stimulated T lymphocytes also lacked transcripts of genes that are expressed in activated cells (Fig. 2e). Although CD2/11a/45 stimulation of CD4+ T lymphocytes allowed efficient viral infection and de novo expression of viral proteins, as evidenced by HSA expression (Fig. 2c), this stimulus did not allow the efficient release of virions (Fig. 2f). Thus, the CD2/CD11a/CD45 stimulus was sufficient to remove blocks leading up to the expression of viral proteins but not virus release.
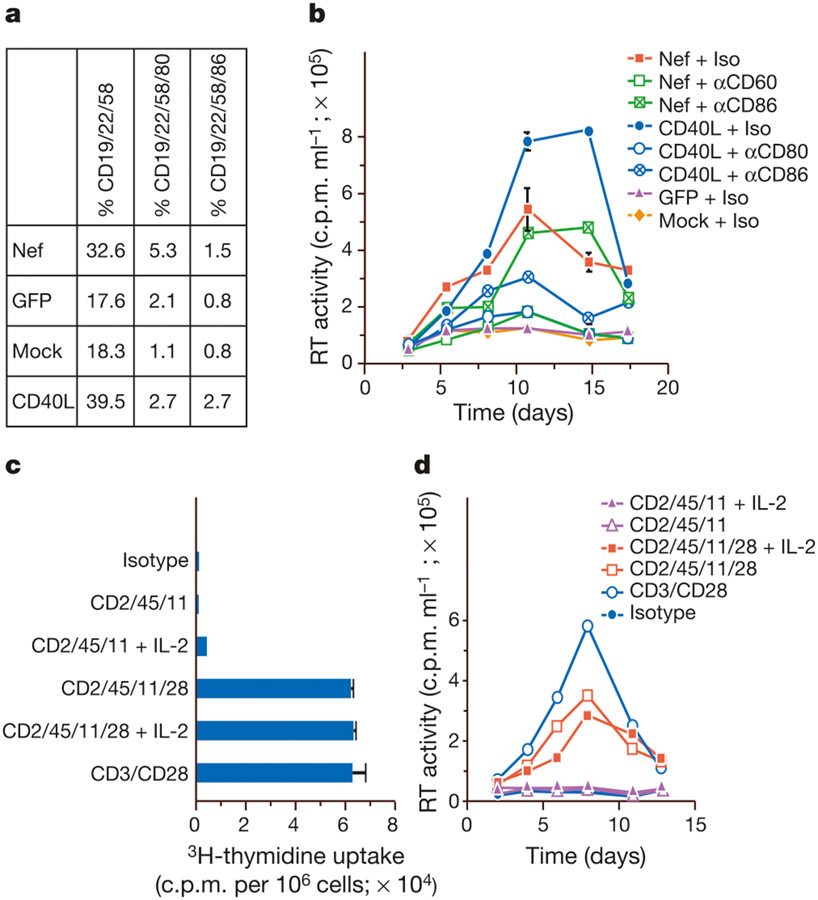
As ligation of CD2/CD45 on T lymphocytes only partially recapitulated the activities of Nef and CD40, we did further immunophenotyping of B cells to identify receptors that might promote a productive infection. FACS analysis further revealed the presence of distinct subpopulations of CD22/CD58-positive B cells that had differentially upregulated the CD80/CD86 receptors in response to Nef and CD40L (Fig. 3a). The induction of T-lymphocyte permissivity by Nef and CD40 was strongly inhibited when the B lymphocytes were pretreated with an anti-CD80 antibody (Fig. 3b). By contrast, antibody to CD86 partially impaired CD40L activity and had no effect on the activity of Nef (Fig. 3b). CD80/CD86 interaction with their T-cell ligand CD28 has a central role in T-lymphocyte co-stimulation15,16. Ligating CD28 on T lymphocytes augmented the CD2/CD11a/CD45 stimulus by inducing T lymphocytes to enter cell cycle (Fig. 3c), and recapitulated a productive viral infection (Fig. 3d).
Figure 3.
Identification of Nef and CD40-regulated B-lymphocyte receptors that overcome blocks to virus production. a, Nef and CD40 upregulate CD80/CD86 expression on B lymphocytes. B lymphocytes were immunophenotyped following incubation with supernatants from Nef- or GFP-expressing on CD40L-stimulated macrophages. b, Antibody to CD80 blocks induction of permissivity by Nef and CD40. Supernatants of Nef-expressing and CD40L-stimulated macrophages were incubated with autologous, resting lymphocytes in the presence of αCD80, αCD86 or isotype (Iso) antibodies, then examined for the ability to support HIV-1 replication. c, The additional CD80 stimulus induces cell-cycle progression. The isotype control comprises Rabbit F(ab)2-cross-linked murine IgG., The additional CD80 stimulus reconstitutes a productive infection.
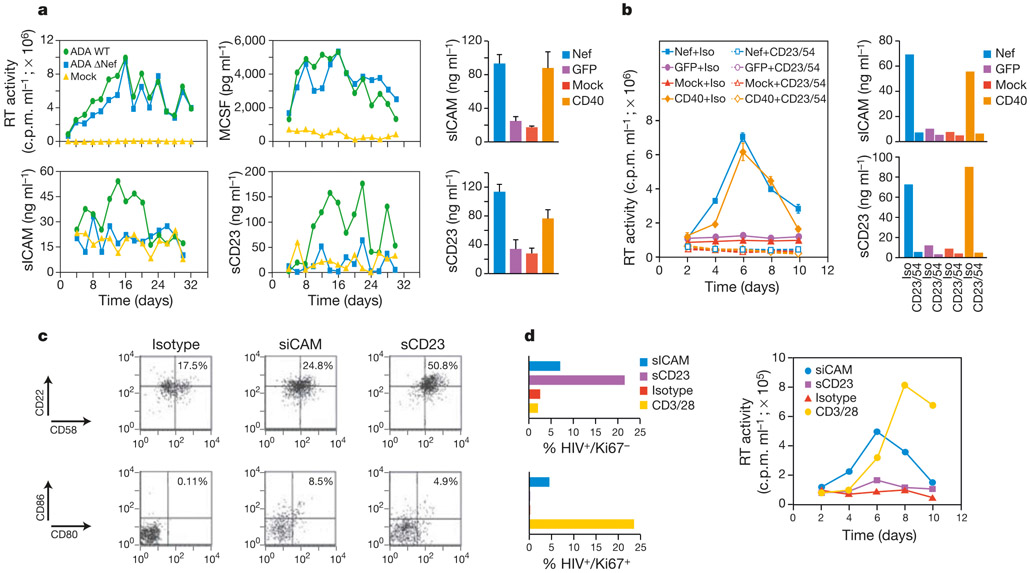
We next undertook a characterization of the factor(s) involved in the induction of permissivity by Nef and CD40L. We took advantage of our observation (Figs 1 and 2) that the induction of T-lymphocyte permissivity was recapitulated by CD40L stimulation and required the involvement of B-cell receptors. Therefore, we focused our analysis on soluble proteins implicated in CD40-mediated effector functions. We identified two proteins—the soluble form of the intercellular adhesion molecule ICAM-1 (sICAM) and the soluble form of the coactivation molecule CD23 (sCD23)—that were elevated in the supernatants of macrophages infected with wild-type HIV-1 (Fig. 4a). By contrast, sCD23 and sICAM levels in HIV-1 ΔNef-infected macrophage supernatants were similar to those in uninfected cultures (Fig. 4a). These differences in sCD23 and sICAM were apparent despite equivalent levels of wild-type and ΔNef HIV-1 replication. MCSF, which is induced by HIV-1 replication17 in a Nef-independent manner11, was present at comparable levels in wild-type and ΔNef HIV-1-infected cultures (Fig. 4a). sCD23 and sICAM were also induced from macrophages expressing Nef alone or those stimulated with CD40L (Fig. 4a, right panels). Immunodepletion of sCD23 and sICAM from supernatants of Nef-expressing or CD40L-stimulated macrophages abrogated the ability of the supernatants to induce T-lymphocyte permissivity (Fig. 4b). Purified B cells incubated with sCD23 at a concentration similar to that induced in wild-type infected macrophages and Nef-expressing macrophages exhibited increased expression of CD22 and CD58, and to a lesser extent CD80 and CD86 (Fig. 4c). By comparison, sICAM induced the expression of CD80/CD86, and to a lesser extent CD22/CD58.
Figure 4.
Induction of lymphocyte permissivity by Nef and CD40 is mediated by sCD23 and sICAM. a, Nef and CD40 induce sCD23 and sICAM release from macrophages. Replication profiles for wild-type and ΔNef viruses are indicated along with levels of sICAM, sCD23 and MCSF in wild-type-infected, ΔNef-infected and uninfected macrophage cultures. Results are representative of three independent donors. Right panels, sICAM and sCD23 levels in supernatants of macrophages 18 h after transduction with a Nef adenovirus vector or after CD40L stimulation. Levels in adeno-GFP-infected and mock-infected cultures are shown for comparison. b, Effects of sCD23 and sICAM immunodepletion on induction of permissivity by Nef or CD40L. Left panel, levels of viral replication. Right panels, levels of sICAM and sCD23 in immunodepleted macrophage supernatants. Supernatants from adeno-GFP-transduced and mock-infected cultures serve as controls. c, sICAM and sCD23 upregulate receptors on B lymphocytes. Purified B lymphocytes were immunophenotyped after incubation with recombinant sICAM (50 ng ml−1) or sCD23 (75 ng ml−1). Cells were gated on CD19+ and CD58+ B cells for CD22/CD58 and CD80/CD86 analysis, respectively. d, sCD23 and sICAM promote T-lymphocyte permissivity. T lymphocytes were incubated with sICAM, sCD23 or isotype-treated B cells then infected with HIV-1HSA. Infected resting () and cycling () T lymphocytes were identified by FACS (left panels). Levels of virus replication in T-lymphocyte cultures stimulated with sCD23, sICAM or isotype-treated B cells (right panel).
We next examined the impact of these proteins on the susceptibility of T lymphocytes to infection. When T lymphocytes were incubated with sCD23-treated B cells and subsequently challenged with HIV-1, infection (on the basis of HSA expression) was almost completely restricted to non-cycling (Ki67−) cells (Fig. 4d). By comparison, HIV-1 infection in T lymphocytes exposed to sICAM-treated B cells occurred in resting and cycling cells (Fig. 4d). The low level of resting-cell infection imparted by the isotype or CD3/CD28 stimulus (Fig. 4d, left panel) was probably due to the underlying presence of CD22/CD58-positive B cells in these cultures (Fig. 4c). Consistent with the requirement for B cells in Nef- and CD40-mediated permissivity, direct treatment of T lymphocytes with sCD23 or sICAM did not promote permissivity. Similarly, T cells exposed to sCD23 and sICAM and then incubated with untreated B cells did not support HIV-1 infection (not shown). Whereas the sICAM stimulus was sufficient to promote the entry of T lymphocytes into the cell cycle, as evidenced by an increase in the levels of Ki67+ cells, the sCD23 stimulus was not (not shown). This suggests a threshold in the B-cell stimulus that promotes the entry of T lymphocytes into the cell cycle. T lymphocytes incubated with sICAM-treated B cells also supported an efficient spreading infection (Fig. 4d, right panel). By contrast, low levels of viral replication were evident in T-lymphocyte cultures incubated with sCD23-treated B cells (Fig. 4d, right panel). This restricted pattern of infection in sCD23-stimulated cultures (Fig. 4d) parallels that invoked by the CD22/CD58 stimulus (Fig. 2). When sCD23 and sICAM were added in combination, their effect on lymphocyte permissivity was not additive (not shown). Collectively, these results suggest that the activities of sCD23 and sICAM are not completely redundant, and that although both proteins can, to differing degrees, promote resting-cell infection, the productive infection of cycling cells requires sICAM.
The results presented here support a model (Fig. 5) in which HIV-1 Nef intersects the CD40 signalling pathway in macrophages to promote the release of sCD23 and sICAM. These proteins, in turn, promote interactions between B cells and T cells that render the T cells permissive to HIV-1 infection. An unexpected finding was that this activity of Nef permitted the efficient infection of resting cells by HIV-1. Experimental systems using lymphoid-organ cultures have indicated that the infection of resting lymphocytes is independent of the infection frequency of macrophages18. However, the stimulation of B cells by adhesion molecules expressed on stromal cells within peripheral lymphoid tissues19 might contribute to an underlying level of resting-cell infection. Future studies should determine whether infection of resting cells in lymphoid-organ cultures requires the B cell–T cell interplay identified in this study.
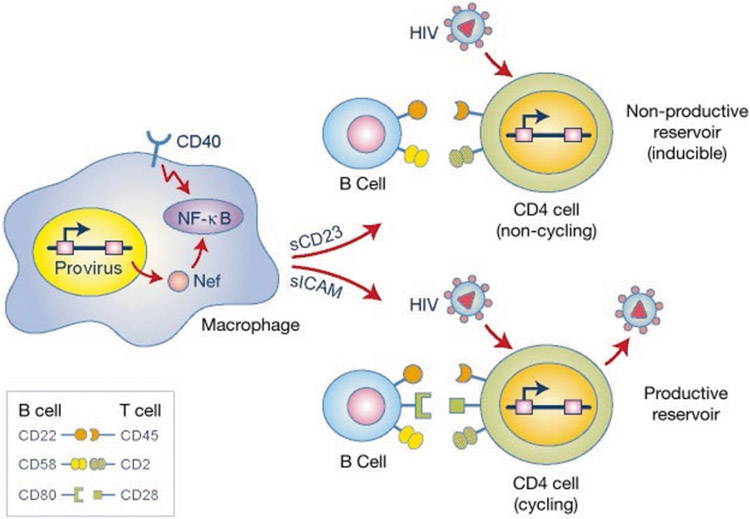
Figure 5.
Mechanistic model for induction of lymphocyte permissivity by Nef and CD40. Physiological stimulation of CD40 in macrophages leads to NF-κB activation. This signalling pathway is intersected by HIV-1 Nef. In macrophages, activation of CD40 or expression of Nef induces the release of sCD23 and sICAM, which upregulate the expression of co-stimulatory receptors on B lymphocytes. These, in turn, interact with their corresponding ligands on T lymphocytes, rendering them permissive to HIV-1 infection. The types of receptor that are upregulated on B cells dictate the outcome of the infection. The induction of CD22 and CD58 on B cells is mediated primarily by sCD23. The action of these receptors on T cells does not lead to T-cell proliferation, but is sufficient to permit virus entry and de novo expression of viral proteins, but not virion release. Induction of CD80 on B cells, as mediated primarily by sICAM, provides signals that promote entry of T cells into the cell cycle, thereby allowing the productive infection of these cells.
Our results could explain several enigmas regarding HIV-1 biology. As replication of HIV-1 in vitro is restricted to T lymphocytes that are in cycle20-23, many studies have focused on the possibility that Nef enhances viral replication by promoting T-lymphocyte activation24,25. However, this first requires the de novo expression of Nef in a cell that is refractory to infection. Although the expression of Nef from unintegrated DNA may occur in resting cells23, our results invoke an alternative mechanism in which Nef activates signals on antigen-presenting cells that, in turn, allow T lymphocytes to exit the G0 stage of the cell cycle, thereby rendering them susceptible to infection (Fig. 5). Our results further reveal that the central component of the Nef stimulus (CD58) was mediated not by classical (CD3) pathways of T-cell stimulation, but through the alternative pathway (CD2) of T-lymphocyte stimulation26. The CD2–CD58 interaction, which facilitates initial contact between the T lymphocyte and the antigen-presenting cell before specific antigen recognition, has not generally been viewed as one sufficient to drive T-lymphocyte proliferation26. Our studies show that this stimulus, although insufficient to promote cell-cycle progression, was sufficient to permit HIV-1 infection. These results may provide insight into the mechanism through which the reservoir of resting T lymphocytes is established in vivo. The infection of non-cycling CD4+ T lymphocytes, characterized by the expression of viral proteins, has been demonstrated in the setting of acute simian immunodeficiency virus (SIV) and HIV-1 infection in vivo27. We propose that Nef promotes conditions for resting-cell infection, thereby extending the cellular reservoir of HIV-1. Although the full significance of the resting-T-cell reservoir in HIV-1 pathogenesis is unclear, studies suggest that these cells are more stable than infected cycling cells27 and, as such, may serve as a more persistent viral reservoir.
Methods
Primary lymphocytes and macrophages
Lymphocytes and monocytes were obtained by leukapheresis from normal donors seronegative for HIV-1 and hepatitis B. Monocytes were further separated by counter-current centrifugal elutriation as detailed elsewhere28. T and B lymphocytes were further purified by negative selection with antibody-coupled para-magnetic beads (Dynal). Cell purity was determined by flow-cytometric staining with fluorochrome antibodies (Pharmingen) to CD3 (T cell), CD19 (B cell) and CD45 (leukocyte). Elutriated monocytes were cultured for 2 days in medium containing MCSF (R&D Systems) and for a further 5 days in medium lacking MCSF, and then used for virus infections.
Transcript profiles
For analysis of activation markers in lymphocytes stimulated with co-receptor antibodies, total RNA was isolated 72 h after antibody stimulation. RNA was reverse transcribed in the presence of 250 μCi α-32P-dATP and α-32P-dCTP using gene-specific primers according to the manufacturer’s protocol (Super Array). Filter arrays were hybridized and washed according to the manufacturer’s instructions. Filters were visualized on a Phosphor-Imager (Molecular Dynamics, ImageQuant) and hybridization intensity quantified by Image Master Software (Amersham). For analysis of Cyclin–CDK and CDK-inhibitor transcripts in antibody-stimulated lymphocytes, total RNA was isolated 72 h after antibody stimulation. RNase protection assays were performed using 32P-labelled riboprobes generated from assembled templates as detailed by the manufacturer (Pharmingen). Products were resolved on 5% acrylamide–urea gels, visualized on a Phosphor-Imager and quantified using ImageQuant.
Viruses
HIV-1 stocks were obtained after transfection of HeLa or 293T cells with 25 μg proviral DNA, normalized on the basis of reverse-transcription activity and used directly for cell infections. HIV-1HSA14 expresses mouse HSA in place of Vpr, allowing infected cells to be identified by flow cytometry. HSA expression indicates completion of steps in the viral life cycle up to, and including, de novo viral gene expression. Adenoviral vectors for Nef and GFP (green fluorescent protein) were prepared as described previously11. Adenoviral vectors for IκBαZ have previously been described13. These vectors were amplified and titred in 293T cells.
Lymphocyte assays
For the induction of T-lymphocyte permissivity by specific antibodies, T lymphocytes purified by negative selection were incubated with antigen-specific or isotype-control murine monoclonal antibodies (Pharmingen), (2 μg ml−1) and cross-linked with rabbit F(ab′)2 anti-mouse antibody (ICN Biomedicals) at 4 μg ml−1. DNA synthesis (3H-thymidine incorporation) and susceptibility to HIV-1 infection was determined 96 h after antibody stimulation. Recombinant soluble CD40L (10 μg ml−1) was mixed with an enhancing reagent (Alexis Corporation) at 1 μg ml−1 to promote trimer formation. For Ki67 and HSA analysis, cells were first stained for the surface antigens CD3–CyChrome and HSA–phycoerythrin, then permeabilized with 1% paraformaldehyde/1% Tween, incubated with Ki67–FITC (fluorescein isothiocyanate) fluorochrome and analysed by FACS. Isotype-matched non-specific immunoglobulin-γ (IgG) served as a staining control.
Permissivity assay
Monocyte-derived macrophages were infected with wild type (HIV-1SFIWT) or Nef-deleted (HIV-1SFI ΔNef) viruses. These viruses also lacked a functional envelope gene and were thus capable of a single round of infection when pseudo-typed with envelope glycoproteins of vesicular stomatitis virus (VSV). Fifteen days after HIV-1SFI infection, macrophages were co-cultured with autologous, resting PBLs that had been preinfected with the T-cell tropic virus HIV-1LAI. As resting PBLs are refractory to productive viral infection, replication of HIV-1LAI offers an indication that T lymphocytes are rendered susceptible to infection by HIV-1SFI-infected macrophages. One week after initiation of the co-cultures, culture supernatants were removed and titred on the T-cell line MT4. Although these supernatants contain a mixture of HIV-1SFI and HIV-1LAI, only HIV-1LAI virions will be scored in this assay.
sCD23/sICAM immunodepletion
Macrophage supernatants were incubated with 5 μg ml−1 anti-CD23 and 5 μg ml−1 anti-CD54 (Pharmingen), and 40 μl protein A/G beads (Santa Cruz Biotech) for 16 h at 4 °C. Residual antibody was removed by two successive treatments of culture supernatants with 40 μl protein A/G beads (2 h, 4 °C). This treatment was sufficient to remove the antibodies by approximately 99% as determined by IgG ELISA (enzyme-linked immunosorbent assay). As controls, macrophage supernatants were treated as above with isotype-matched IgG at the same concentrations. Immunodepleted supernatants were sterilized by filtration (0.22 μM) and analysed for sCD23, sICAM and MCSF by ELISA. Recombinant human sCD23 and the sCD23 ELISA were obtained from Pharmingen. Recombinant human sICAM-1 was obtained from BenderMed Systems; ELISAs for sICAM and MCSF were from R&D Systems.
Acknowledgements
We thank A. Dauphin, and K. Triques for research support, B. Blais for FACS analysis, B. Mellor for preparation of the figures, and T. Pinkos and N. Nelson for manuscript preparation. We also wish to acknowledge assay support provided by the University of Massachusetts Center for AIDS Research. The recombinant IκBα expression plasmid was kindly provided by R. Gaynor; the IκBαSR and LacZ adenovirus vectors were kindly provided by A. Baldwin. HIV-1SFI and HIV-1HSA were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. This study was supported by grants from the NIH and the Jenner Foundation to M.S.
Footnotes
Competing interests statement The authors declare that they have no competing financial interests.
References
- 1.Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL & Desrosiers RC Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med 332, 228–232 (1995). [DOI] [PubMed] [Google Scholar]
- 2.Deacon NJ et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270, 988–991 (1995). [DOI] [PubMed] [Google Scholar]
- 3.Kestler HW et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65, 651–662 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Johnson WE & Desrosiers RC Viral persistence: HIV’s strategies of immune system evasion. Annu. Rev. Med 53, 499–518 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Collins KL & Baltimore D HIV’s evasion of the cellular immune response. Immunol. Rev 168, 65–74 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Geleziunas R, Xu W, Takeda K, Ichijo H & Greene WC HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410, 834–838 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Saksela K, Cheng G & Baltimore D Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef + viruses but not for down-regulation of CD4. EMBO J. 14, 484–491 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fackler OT, Luo W, Geyer M, Alberts AS & Peterlin BM Activation of Vav by Nef induces cytoskeletal rearrangements and downstream effector functions. Mol. Cell 3, 729–739 (1999). [DOI] [PubMed] [Google Scholar]
- 9.Fackler OT & Baur AS Live and let die: Nef functions beyond HIV replication. Immunity 16, 493–497 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Simmons A, Aluvihare V & McMichael A Nef triggers a transcriptional program in T cells imitating single-signal T cell activation and inducing HIV virulence mediators. Immunity 14, 763–777 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Swingler S et al. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nature Med. 5, 997–1003 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Kooten C & Banchereau J CD40-CD40 ligand. J. Leukoc. Biol 67, 2–17 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Wang CY, Cusack JC Jr, Liu R & Baldwin AS Jr Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nature Med. 5, 412–417 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Jamieson BD & Zack JA In vivo pathogenesis of human immunodeficiency Virus Type 1 reporter virus. J. Virol 72, 6520–6526 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers CA The expanding world of co-stimulation: the two-signal model revisited. Trends Immunol. 22, 217–223 (2001). [DOI] [PubMed] [Google Scholar]
- 16.Frauwirth KA & Thompson CB Activation and inhibition of lymphocytes by costimulation. J. Clin. Invest 109, 295–299 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber MF, Weih KA, Boone EJ, Smith PD & Clouse KA Endogenous macrophage CSF production is associated with viral replication in HIV-1-infected human monocyte-derived macrophages. J. Immunol 154, 5528–5535 (1995). [PubMed] [Google Scholar]
- 18.Eckstein DA et al. HIV-1 Vpr enhances viral burden by facilitating infection of tissue macrophages but not nondividing CD4+ T cells. J. Exp. Med 194, 1407–1419 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisignoli G et al. In vitro cultured stromal cells from human tonsils display a distinct phenotype and induce B cell adhesion and proliferation. Eur. J. Immunol 26, 17–27 (1996). [DOI] [PubMed] [Google Scholar]
- 20.Stevenson M, Stanwick TL, Dempsey MP & Lamonica CA HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9, 1551–1560 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zack JA et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61, 213–222 (1990). [DOI] [PubMed] [Google Scholar]
- 22.Pierson TC et al. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol 76, 8518–8531 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y & Marsh JW Selective transcription and modulation of resting T cell activity by preintegrated HIV DNA. Science 293, 1503–1506 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Geyer M, Fackler OT & Peterlin BM Structure–function relationships in HIV-1 Nef. EMBO Rep. 2, 580–585 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greene WC & Peterlin BM Charting HIV’s remarkable voyage through the cell: Basic science as a passport to future therapy. Nature Med. 8, 673–680 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Davis SJ & van der Merwe PA CD2: an exception to the immunoglobulin superfamily concept? Science 273, 1241–1242 (1996). [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286, 1353–1357 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Kalter DC et al. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J. Immunol 146, 298–306 (1991). [PubMed] [Google Scholar]