Abstract
Angiogenesis, or the growth of new blood vessels from the preexisting vasculature, is a visible and important component of wound repair. When tissue damage occurs, disruption of the vasculature structure leads to hypoxia. The restoration of normoxia is essential for appropriate and durable tissue repair. Angiogenesis in wounds is regulated by endogenous proangiogenic mediators, which cause rapid growth of a new vascular bed that is much denser than that of normal tissue. Such rapid growth of the capillary bed results in capillaries that are abnormal, and the newly formed vessels are tortuous, dilated, and immature. During wound resolution, this substantial neocapillary bed is pruned back to normal density with attendant maturation. Many poorly healing wounds, including nonhealing ulcers and scars, exhibit an aberrant angiogenic response. The fine-tuning of capillary regrowth in wounds is an area of significant therapeutic potential.
Angiogenesis, defined as the growth of new capillaries and blood vessels from preexisting vasculature, occurs during development, as well as in pathologic situations (Eelen et al. 2020). The angiogenic response was one of the first processes to be identified as essential to wound repair. The concept that revascularization of wounds is an active process, and that the vasculature responds to the metabolic requirements of injured tissue, was originally suggested by John Hunter, a Scottish anatomist and surgeon. In 1794, Hunter suggested that when “considerable operations” occur in tissue, the vascular system becomes enlarged (Hunter 1794). Studies of wound angiogenesis began in earnest in the 1970s and 1980s, when Dr. Thomas K. Hunt began to examine the role of oxygen in wound healing. Dr. Hunt's studies demonstrated that wounds are hypoxic and that oxygen gradients regulate wound angiogenesis (Knighton et al. 1981). These studies set the stage for the observation that wound fluid contains soluble proangiogenic factors (Banda et al. 1982), and that hypoxia induces the expression of proangiogenic activity by macrophages (Knighton et al. 1983). This latter finding helped to explain the prior work of Leibovich and Ross (1975), who had shown that macrophages were essential for appropriate wound healing. Since these early studies, the field of wound angiogenesis has exploded with information about the pattern, regulation, and significance of the angiogenic process in healing outcomes. The composite research now shows that an adequate but not overabundant ingrowth of capillaries is required for tissue repair and that imperfect and/or inadequate angiogenesis is a common feature of poorly healing wounds (Galiano et al. 2004; DiPietro 2016; Okonkwo and DiPietro 2017).
THE PATTERN OF ANGIOGENESIS IN HEALING WOUNDS
Angiogenesis is commonly defined as the growth of blood vessels from the existing vasculature and can be distinguished from vasculogenesis, which is the growth of nascent vasculature from angioblasts during development (Iruela-Arispe and Dvorak 1997; Eming et al. 2007). Most of the new blood vessels that are formed during normal wound healing arise through the process of angiogenesis. Wound angiogenesis has been best studied in the context of skin wounds and follows a highly regular and reproducible pattern of growth and decline (Fig. 1; Nissen et al. 1998). The timing and pattern of wound angiogenesis has been best studied in mouse skin wounds, where it has been widely examined histologically (Nissen et al. 1998). More recently, angiogenesis has been examined in mouse wounds using microCT and in live animals using optoacoustic microscopy or two-photon imaging (Urao et al. 2016; Gurevich et al. 2018; Rebling et al. 2021). These newer techniques provide significant advantages in terms of quantification and the ability to image in live animals.
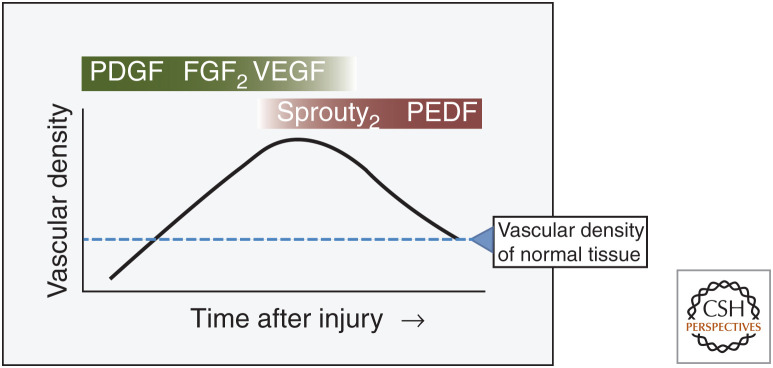
Figure 1.
Time course and pattern of wound angiogenesis. Over time, capillaries grow in the wound bed, reaching a density that is 3–10 times that of normal tissue. During the resolution phase, most of these vessels are removed. Studies in multiple systems, including mouse, human, and pig, have identified the major proangiogenic factors (green) and regression/maturation signals (red) in excisional wounds. Dashed line (blue) represents vascular density in normal unwounded tissue (Nissen et al. 1996, 1998; Artuc et al. 1999; Swift et al. 1999; Wietecha et al. 2011, 2015).
The precise timing of the angiogenic response depends upon the size of the wound; in general, capillary growth begins 3–4 d after injury (Hunt et al. 2000). Capillary density continues to markedly increase in wounds, reaching up to 10 times the level of normal tissue around days 7–10 postinjury. The process is driven by soluble proangiogenic factors, which are discussed further below. The time of maximum capillary density depends upon wound size and generally occurs within a few days after complete epithelial closure. Then, as wound healing resolves, capillary density decreases, eventually returning to normal levels.
When compared to normal capillary beds, the newly generated capillaries in wounds are poorly formed. As opposed to normal tissue, these new capillaries in wounds exhibit increased tortuosity, increased permeability, decreased pericyte coverage, and altered flow dynamics (Nagy et al. 2008; Erba et al. 2011; Urao et al. 2016; Chong et al. 2017). This abnormal architecture is similar to that found in solid tumors, supporting the idea that tumors are wounds that do not heal (Dvorak 1986, 2015). In wounds, an important study by Bluff et al. demonstrated that only a fraction of the newly formed capillaries are perfused (Bluff et al. 2006). The percent perfusion was found to vary by wound location and was as low as 30%–50% in much of the early wound healing response. Over time, perfusion rates returned to normal, nearly 90%, as healing resolved. Therefore, although the healing wound is filled with an enormous number of vessels, these new vessels provide relatively poor perfusion and oxygenation compared to the fewer but more physiologically normal vessels in healed or normal tissue.
THE REGULATION OF WOUND ANGIOGENESIS
The Proangiogenic Phase of Wound Healing
In adult wounds, the process of angiogenesis is initiated when cellular oxygen sensing mechanisms detect hypoxia, activating a pathway that results in the production of proangiogenic molecules. The most prominent proangiogenic mediator in wounds is vascular endothelial growth factor A (VEGF-A), which accounts for ∼50% of the proangiogenic stimulus in healing skin. The synthesis of VEGF-A lies downstream of the well described HIF-1α (hypoxia-inducible factor 1α) oxygen-sensing pathway (Hong et al. 2014). HIF-1 is a dimer that includes one α (HIF-1α) and one β ([HIF-1β] also known as the aryl hydrocarbon receptor nuclear translocator [ARNT]) subunit. With both subunits bound, HIF-1 can regulate many genes that control the response to hypoxia. In normoxic conditions, the HIF-1β subunit is constitutively expressed, while HIF-1α is rapidly degraded. Under hypoxic conditions, however, HIF-1α is stabilized, binds to HIF-1β, and the resulting HIF-1 molecule activates the hypoxic response (including the production of VEGF-A).
Many different cells are known to produce VEGF-A in wounds, with macrophages and epithelial cells described as prominent sources (Brown et al. 1992; Nissen et al. 1998; Spiller et al. 2014). As the gradient of VEGF-A is formed, VEGF-A acts on cells via two related receptor protein tyrosine kinases, VEGF receptor 1 (VEGFR1, encoded by the fms-related receptor tyrosine kinase 1, or FLT1 gene) and VEGF receptor 2 ([VEGFR2] encoded by the kinase insert domain receptor [KDR]), as well as via two coreceptor nontyrosine kinase transmembrane proteins, neuropilin-1 (NRP1) and neuropilin-2 (NRP2). This ligand-receptor binding activates downstream signaling and initiates the angiogenic process. Most of the proangiogenic activity in wounds is mediated via VEGFR2 as the primary receptor on endothelial cells (ECs). Interestingly, though, VEGF receptors are also present on wound epithelial cells and fibroblasts. Several studies suggest that VEGF-A, in addition to its proangiogenic effect in wounds, also modulates other cell types and functions in skin wounds, including epithelial cell repair, macrophage activity, and fibroblast function (Wilgus et al. 2005; Brem et al. 2009; Johnson and Wilgus 2014).
Whereas VEGF-A is clearly the dominant proangiogenic factor in wounds (Nissen et al. 1998), a large number of other proangiogenic factors have been shown to play a role in wound healing and contribute to the overall proangiogenic environment. Factors that have been shown to supplement the proangiogenic activity of VEGF-A in wounds include FGF-2 (fibroblast growth factor 2), TGF-β (transforming growth factor β), CARP (cardiac ankyrin repeat protein), IGF-1 (insulin-like growth factor 1), several CXC motif chemokines (such as CXCL8), and many others (Roberts et al. 1986; Brown et al. 1992; Franzen et al. 1995; Nissen et al. 1996, 1998; Devalaraja et al. 2000; Li et al. 2003; Shi et al. 2005; Balaji et al. 2014). In addition to soluble factors, nitric oxide, synthesized following the induction of inducible nitric oxide synthetase, also promotes wound healing and wound angiogenesis (Most et al. 2002; Chin et al. 2011). Nitric oxide stimulates angiogenesis but also modulates other aspects of repair, including collagen production and cellular proliferation (Witte et al. 2000). Therefore, as discussed above for VEGF, an important caveat to the cataloging of proangiogenic factors in wounds is that most factors have additional effects beyond the simple stimulation of capillary growth. This multiplicity of activity often makes it difficult to decipher the most significant functionality of any one component. As another example, nitric oxide has many effects beyond angiogenesis and can influence inflammation, cell proliferation, extracellular matrix (ECM) production, and ECM remodeling (Rizk et al. 2004). Whereas the primary functions of each mediator are not always clear, what is certain is that this very large number of proangiogenic factors, many of which exert additional effects on other cell types, supports complexity and redundancy in wound healing. In particular, the redundancy of proangiogenic factors ensures that the critical process of angiogenesis proceeds in wounds, even if production or activity of some factors is impaired.
Capillary Pruning and Maturation in Wounds
Following robust wound angiogenesis, the new capillary bed undergoes remodeling and maturation. In skin wounds, this process involves both the pruning of excess vessels and the maturation of the remaining capillaries (Fig. 2). Our previous studies have shown that pruning occurs following a switch to an antiangiogenic environment in wounds (Gosain et al. 2006). The mechanism of the switch is not known, but the environment generally changes once epithelial repair is complete (Wietecha et al. 2015). The factors that dictate vascular regression are complex, and several potently antiangiogenic factors are produced in resolving wounds. Pigment epithelium-derived factor (PEDF) and Sprouty2, both known antiangiogenic molecules, appear to be largely responsible for pruning of capillaries and contribute to capillary maturation in wounds (Wietecha et al. 2011, 2015; Michalczyk et al. 2018). PEDF is produced during vascular regression, induces endothelial cell apoptosis, and down-regulates proangiogenic factors, such as VEGF (Craword et al. 2013). Sprouty2, which is one of four members of the Sprouty family of homologous proteins, is an intracellular protein. When growth factors (GFs) bind to their cognate receptor tyrosine kinase (RTK) and activate the associated signaling pathway, Sprouty2 translocates to the inner plasma membrane, where it is activated (Impagnatiello et al. 2001; Cabrita and Christofori 2003; Edwin et al. 2009). Once activated, Sprouty2 interacts with various MAPK signaling pathway-associated proteins in a GF-specific manner. Functionally, then, Sprouty2 can inhibit endothelial cell proliferation and differentiation (Wietecha et al. 2011). Other modulating factors are also at play, including thrombospondins and the chemokine CXCL10 (DiPietro et al. 1996; Agah et al. 2002; Bodnar et al. 2009). CXCL10 and CXCL11, interacting with the chemokine receptor CXCR3, have been shown to contribute to the resolution of healing (Bodnar et al. 2006, 2009; Yates et al. 2009). CXCL10 and CXCL11 interact with CXCR3 on endothelial cells and keratinocytes, leading to a reduction in endothelial motility and increased keratinocyte movement (Satish et al. 2005; Bodnar et al. 2006). The inhibition of endothelial cell movement drives the wound toward resolution and remodeling. Recent studies suggest that macrophages may play a direct role in modulating both the pro- and antiangiogenic environment in wounds. Multiple studies demonstrate that the specific depletion of macrophages in wounds significantly perturbs the angiogenic process (Goren et al. 2009; Mirza et al. 2009; Lucas et al. 2010). Importantly, macrophages also influence blood vessel regression via intimate contact with the vasculature (Gurevich et al. 2018). Together, these studies of the resolution phase of repair have firmly established that the environment in the resolving wound is dominantly antiangiogenic and induces vascular regression. Moreover, vascular regression in wounds cannot be prevented by the administration of proangiogenic stimuli (Gosain et al. 2006).
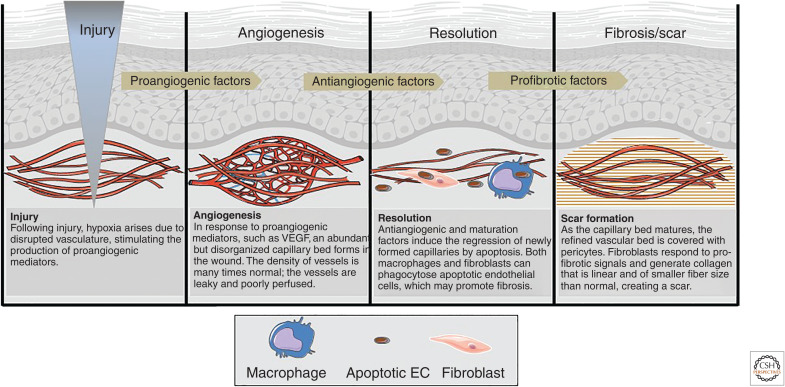
Figure 2.
The sequence of angiogenesis in healing wounds. Injury prompts the development of a robust but poorly perfused vascular bed in the wound bed. During resolution, most of the newly formed vessels regress. The apoptotic endothelial cells that are formed as the vascular bed is pruned can be engulfed by both macrophages and fibroblasts, orienting the wound toward a scar-forming outcome. (Figure adapted from Barakat et al. 2021 and graphics used in compliance with the terms of the Creative Commons Attribution 3.0 Unported License from Servier Medical Art images at smart.servier.com.)
As capillary pruning occurs, capillaries in wounds must undergo maturation to become fully functional (Ribatti et al. 2011; Dulmovits and Herman 2012). During maturation, vessel pericyte coverage increases, and endothelial cells produce a basement membrane. Studies in other systems have revealed that maturation is a complex process that relies not only on pericytes and endothelial cells but also on their interaction with the ECM. In vitro and in vivo studies suggest that the molecular mechanisms that guide pericyte recruitment and capillary maturation involve multiple pathways of endothelial cell–pericyte cross talk (Arboleda-Velasquez et al. 2015; Warmke et al. 2016). In addition to stabilizing capillaries, pericytes have been shown to reduce endothelial cell proliferation (Kutcher et al. 2007), suggesting that these cells may be important in the down-regulation of the angiogenic process. During pericyte recruitment and the development of EC–pericyte communication, PDGF/PDGFRβ (platelet-derived GF/platelet-derived GF receptor β) plays an important role in attracting pericytes. Numerous pathways are involved in the maturation of vessels, including TGF-β signaling pathways and Ang1/Tie2 (angiopoietin 1/tyrosine kinase with immunoglobulin-like and epidermal growth factor [EGF]-like domains-2) interactions (Gaengel et al. 2009; Kutcher and Herman 2009). Together, these signals and others support pericyte recruitment and proliferation, as well as the establishment of a stable basement membrane. Importantly, proangiogenic factors in wound healing, such as VEGF, cannot induce capillary maturation. Therefore, the final capillary functionality in wounds derives from the induction of capillary growth, the removal of most of the new capillaries, and capillary maturation. In short, adequate tissue perfusion, accomplished by the creation of a stable, well-perfused vascular bed, requires proangiogenic, antiangiogenic, and pericyte recruitment and maturation signals. Without satisfactory perfusion and oxygenation, wounds fail to develop a mature dermis, a feature that makes them susceptible to future breakdown and wound recurrence (Falanga 2005).
TYPES OF ANGIOGENESIS IN WOUNDS
Whereas the pattern and chronology of capillary growth and refinement in wounds is well appreciated, the vessel architecture during angiogenesis has been less well studied. Angiogenesis occurs in two basic forms—sprouting angiogenesis and intussusceptive angiogenesis (Fig. 3; Carmeliet and Collen 2000). Of the two, sprouting angiogenesis has been better studied and was the first form to be identified. Sprouting angiogenesis, as the name implies, involves the formation of new sprouts from existing vessels that grow toward an angiogenic stimulus. In contrast, intussusceptive angiogenesis involves the splitting of existing vessels to form new loops. Both types occur in healing wounds, although in most systems, including wounds, sprouting angiogenesis is better described. Interestingly, most wound healing studies do not distinguish between sprouting and intussusceptive forms of angiogenesis, and, frequently, the only measurement made in wound healing studies is that of vessel density. Indeed, in the study of wound repair, vessel architecture is often ignored. In addition to angiogenesis, a contribution from vasculogenesis has also been demonstrated in excisional wounds and ischemic skin flaps, but the importance of this vasculogenic component is also not yet well understood (Asahara et al. 1999; Tepper et al. 2005).
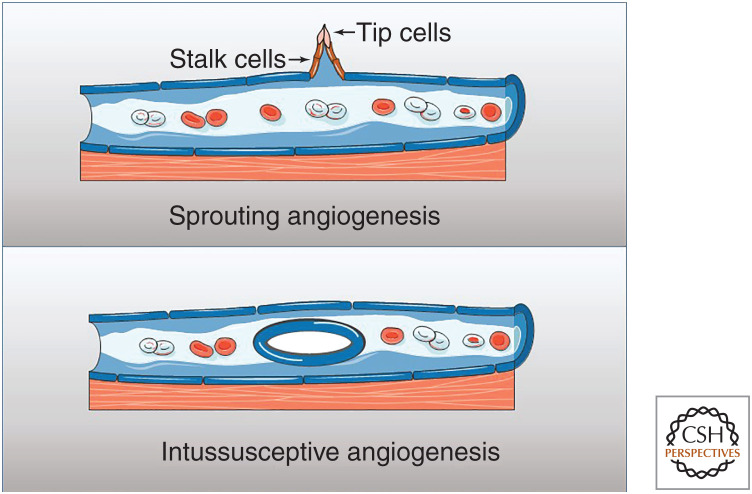
Figure 3.
The formation of new vasculature in wounds. Most of the new vasculature in wounds is created by sprouting angiogenesis, which involves budding from the existing vessel, with migration of the tip and stalk cells. A smaller component of wound angiogenesis is attributable to intussusceptive vessel formation, a situation in which the existing vessel splits to form additional tubular structure. Vasculogenesis (not shown) has been described to contribute to revascularization of skin tissue grafts. (Graphics created with Servier Medical Art images at smart.servier.com.)
Sprouting angiogenesis is a complex process, and many intricate details have now been described (for review, see Eelen et al. 2020). In brief, the process begins with degradation of the capillary basement membrane, followed by endothelial cell proliferation and migration of ECs toward the proangiogenic gradient. The migrating cells then form tubes, and the newly formed vessels fuse to provide a branching structure.
An endothelial tip cell leads the growth of the capillary sprout toward the proangiogenic stimulus. Endothelial tip cells have high levels of VEGF-A receptors and therefore align with the VEGF gradient (Apte et al. 2019). Behind this developing tip, stalk cells proliferate and push the sprout forward. Eventually, tip cells converge and fuse, creating a new loop through which blood can flow. Delta–Notch signaling is critical to sprout formation, as the Delta-like-4 ligand interacts with the Notch receptor on neighboring cells (Zarkada et al. 2015). The result is a dense network of new capillaries, which, as described above, are poorly formed in most wounds. Following this proliferative process, the wound capillary bed undergoes a period of pruning in which vessels are removed via apoptosis. Pruning occurs alongside the stabilization of the remaining new vessels, during which arriving pericytes wrap around the new tubes.
The mechanisms of sprouting angiogenesis have been described in detail, yet little is known about the regulation of in vivo intussusceptive angiogenesis in healing wounds. Intussusceptive angiogenesis has been shown to account for about 15%–25% of the newly formed vessels in a full-thickness excisional skin wound model in rats (Kiliçaslan et al. 2013). Treatment of these wounds with EGF increases the proportion of intussusceptive angiogenesis to more than 50% in this model. Studies that evaluate intussusceptive versus sprouting angiogenesis are not trivial undertakings, as they require detailed histologic and transmission electron microscopy (TEM) analysis. For this reason, nearly all analyses of wound angiogenesis exclude this parameter. As technology improves, the determination of the relative contributions of intussusceptive versus sprouting angiogenesis in both normal and impaired healing may be a promising area for further study. This type of information could significantly enhance our understanding of the angiogenic deficits in situations of impaired healing (see below).
ANGIOGENESIS AND HEALING FAILURES
Impaired Angiogenesis in Diabetic Wounds
Deficits in wound angiogenesis have been identified in many types of poorly healing wounds, including those due to aging, diabetes, and vascular disease (Cheng and Fu 2018). Among these wounds, the angiogenic deficit in diabetic ulcers is perhaps the best studied.
Described deficits in diabetic wounds include delayed wound closure, impaired inflammatory responses, modified ECM reconstitution, altered genomic responses/regulation, and compromised angiogenesis. More than 100 studies have examined angiogenesis in diabetic wound healing. These studies have shown that reduced angiogenesis is a critical element that underlies insufficient diabetic healing (Pham et al. 1998; Hadi and Suwaidi 2007; Avogaro et al. 2011). Deficits in angiogenic factors and receptors, and in the number and function of endothelial progenitors, have all been suggested to contribute to the impaired angiogenic response observed in diabetic wounds (Galiano et al. 2004; Hocking 2012; Warren et al. 2014). Prominent macrophage dysfunction in diabetes also contributes to the angiogenic deficit (Khanna et al. 2010; Barman and Koh 2020). Macrophages are an important source of proangiogenic factors in wounds, a function that is thwarted under the condition of diabetes. At a molecular level, a diabetes-associated dysregulation of specific miRNAs (microRNAs or miRs) that control wound angiogenesis has been demonstrated (Chan et al. 2012). This includes miRNAs that promote angiogenesis, such as miR-148b, and others that inhibit angiogenesis, such as miR-135a-3p (Miscianinov et al. 2018; Icli et al. 2019). Most interestingly, miR-200b, an miRNA that inhibits angiogenesis, has been shown to be down-regulated by wound hypoxia, thus providing a molecular switch that turns on wound angiogenesis (Chan et al. 2012). Moreover, the promoter of miR-200b is epigenetically modified in the condition of diabetes, resulting in the loss of this proangiogenic switch (Singh et al. 2017). Not surprisingly, research interest in the role of noncoding RNAs in the regulation of angiogenesis and in the modulation of noncoding RNAs to improve healing has grown tremendously in recent years (Luan et al. 2018; Ozdemir and Feinberg 2019).
As mentioned, both the density of capillaries and the level of angiogenic stimulus are decreased in diabetic wounds. In addition, the capillary architecture in diabetic wounds differs from normal wounds. MicroCT analysis shows that vessel surface area, branch junction number, total vessel length, and total branch number were significantly decreased in wounds of diabetic mice as compared to wild-type (WT) mice (Okonkwo et al. 2020). An examination of the capillary maturation phase demonstrated that just as capillary growth is delayed in diabetic wounds, capillary maturation is also impaired in diabetic wound healing (Okonkwo et al. 2020). Diabetic wounds show significant perturbations in factors known to be important to capillary maturation, including PEDF, LRP6 ([low-density lipoprotein (LDL) receptor-related protein 6] a PEDF receptor), Sprouty2, CXCL10, CXCR3, and TSP1 (thrombospondin 1) (Okonkwo et al. 2020). In addition, diabetic mouse wounds exhibit significantly increased capillary permeability and decreased pericyte coverage. Of note, capillary dysfunction, including limited pericyte coverage, has been described in uninjured tissues of diabetic subjects (Kutcher and Herman 2009). This suggests that both maturation deficits at baseline and during capillary refinement may contribute to impaired healing in diabetes.
The deficits in the proangiogenic phase of healing that occur in diabetes have been successfully reversed in multiple animal studies (for review, see Barakat et al. 2021). In such studies, improved angiogenesis generally occurs in tandem with improved wound closure in diabetic animals. For example, the addition of exogenous proangiogenic factors, such as VEGF, has been shown to restore angiogenesis and improve wound closure in diabetic mice in short-term experiments (Galiano et al. 2004). Beyond VEGF, a very large number of single therapeutics have been shown to be effective in improving diabetic wound healing in mouse models (Barakat et al. 2021). In human diabetic foot ulcers (DFUs), however, single-factor treatments have shown minimal efficacy. More specifically, there is no clear evidence that VEGF treatment alone improves healing of human DFUs (Marti-Carvajal et al. 2015). Studies in mice suggest that treatment with multiple factors, such as VEGF, PDGF, and HB-EGF (heparin-binding EGF-like GF), especially when engineered to bind to the ECM, may be much more effective than single factor approaches (Barakat et al. 2021; White et al. 2021), and this may well translate to human efficacy. Concerning proangiogenic therapeutics, one important yet often overlooked concept is that proangiogenic factors are generally not endothelial cell–specific. Instead, each these factors exhibit additional functionality and may influence inflammatory cells, fibroblasts, epithelial cells, and/or other stromal cells in the wound. For this reason, studies that show improvement of healing with single-factor application should consider the multifaceted effects of the GF. Whereas angiogenesis may be increased, the mechanism of enhanced healing is probably much more complex in nearly all scenarios.
Angiogenesis and Scar Formation
In contrast to the angiogenic deficit that underlies many poorly healing wounds, most normally healing wounds exhibit excessive capillary growth. Intuitively, robust angiogenesis would seem to be required to fulfill the cellular and metabolic needs of wounds. Yet, many studies suggest that in uncompromised skin wounds, wound closure proceeds normally when angiogenesis is reduced. In rodent studies, the reduction of wound angiogenesis by the neutralization of VEGF, the application of antiangiogenic agents, or the blockade of integrin signaling have each been shown to reduce wound angiogenesis without affecting wound closure rates (Jang et al. 1999; Klein et al. 1999; Berger et al. 2000; Bloch et al. 2000; Lange-Asschenfeldt et al. 2001; Roman et al. 2002; Wilgus et al. 2008; Wietecha et al. 2015).
The explanation for the finding that wounds can heal well with fewer capillaries probably relates to capillary architecture and performance (Dvorak 2015). As discussed above, many of the capillaries found in early healing skin wounds are not highly functional or well perfused (Bluff et al. 2006; Erba et al. 2011). The early wound capillary bed is highly permeable and tortuous, with many loops and blind-ended vessels (Nagy et al. 2008; Erba et al. 2011). An experimental reduction in wound angiogenesis seems likely to create a more effectively perfused and well-arborized capillary bed at an earlier time point, thus providing excellent oxygenation and nutrient support. Indeed, treatment of wounds with the endogenous antiangiogenic factor PEDF leads to reduced capillary content, decreased vascular leakage, decreased scar formation, and more rapid capillary maturation (Fig. 4; Wietecha et al. 2015).
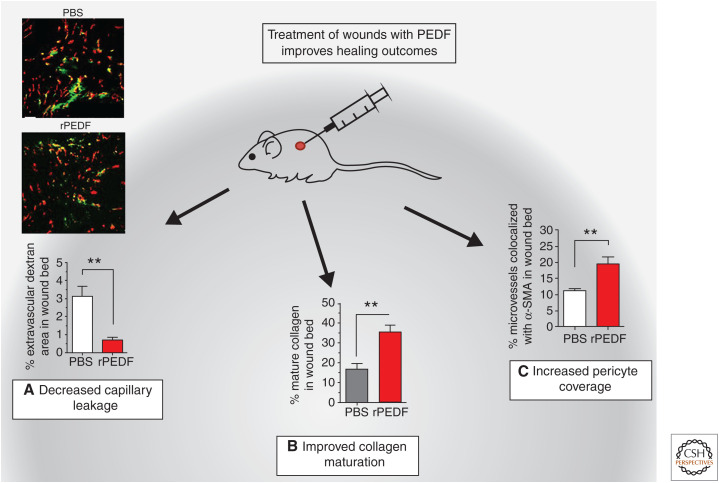
Figure 4.
Effect of treatment of normal wounds with the antiangiogenic pigment epithelium-derived factor (PEDF). Normally, healing wounds go through a phase of capillary dysfunction when the wound bed fills with poorly formed and leaky capillaries. The treatment of wounds with antiangiogenic factors, such as the endogenous antiangiogenic factor PEDF, improves healing and leads to (A) decreased blood vessel leakiness. Photomicrograph and quantification of FITC-dextran leakage from vessels in control versus recombinant PEDF (rPEDF)-treated wounds. Red = CD31, green = dextran. (B) Increased collagen maturity: collagen maturity determined using Picrosirius Red staining. (*) P < 0.05, (**) P < 0.01. (C) Increased pericyte coverage: capillary maturity assessed by % pericyte coverage of microvessels. (**) P < 0.01. (PBS) Phosphate buffered saline. (Figure adapted from Wietecha et al. 2015, courtesy of the authors and The American Physiological Society © 2015.)
The idea that wound healing can proceed without the typical excess capillary ingrowth is supported by studies of wounds that heal exceptionally well. Wounds placed on the fetus, if produced prior to the end of the second trimester, heal scarlessly (Longaker et al. 1990; Leung et al. 2012). The scar-free healing wound of the fetus also exhibits a refined and reduced angiogenic response (Wilgus et al. 2008). Similarly, wounds of the oral mucosa, a tissue that heals quickly and with little scar formation, also present with a diminished angiogenic response yet exhibit less hypoxia than skin wounds (Szpaderska et al. 2003, 2005; Chen et al. 2012). Overall, rapidly healing, non-scar-forming wounds do so with reduced capillary growth and a more rapidly maturing capillary network. In contrast, the pattern of skin wound angiogenesis in scar-forming wounds is crowded, poorly formed capillaries that do not effectively transport oxygen and nutrients.
The consequences of the overly exuberant angiogenic response in wounds are not yet completely understood. Some studies have linked increased vascular content to hypertrophic scar formation (Amadeu et al. 2003; van der Veer et al. 2011), and increased angiogenesis has been noted in keloids, a particularly vigorous type of skin scarring (Gira et al. 2004). Moreover, robust angiogenesis plays a role in fibrotic outcomes in tissues other than skin, including liver and lung (Farkas et al. 2011; Elpek 2015). Preclinical studies have shown that antiangiogenic approaches may be a possible therapy to reduce scar formation in skin wounds. For example, in a rabbit model of hypertrophic scar formation, administration of either endostatin or PEDF, both potent endogenous antiangiogenic agents, reduces scar formation (Ren et al. 2013; Ma et al. 2020). Thinking ahead to human therapeutics, a major obstacle to any antiangiogenic approach in wounds will be fine-tuning the response. An effective therapeutic would need to reduce capillary growth yet permit vessels to grow to normal density and with rapid maturation.
The linkage of robust angiogenesis to scar formation suggests that excess ECs may influence fibroblast phenotype in wounds and may somehow promote fibrosis. In particular, the pruning of the vasculature results in a large number of apoptotic endothelial cells (apoECs). In many systems, apoptotic cells seem to positively influence fibrosis (Laplante et al. 2010; Johnson and DiPietro 2013; Wang et al. 2016). In addition, recent studies suggest that the clearance of apoECs, by either immune cells or nonprofessional phagocytes, may promote a fibrotic response in wounds (Fig. 2).
As might be expected, macrophages can clear apoECs, and this capability can be demonstrated both in vitro and in vivo. Interestingly, the engulfment of apoECs causes macrophages to adopt a unique phenotype that has both M1 and M2 macrophage characteristics (Xu et al. 2021). More studies are needed to determine whether this type of macrophage phenotypic shift affects other aspects of wound resolution, such as collagen content and scar formation.
Macrophages are professional phagocytes, thus their ability to clear apoECs in wounds is easy to appreciate. Interestingly, recent studies have provided novel findings demonstrating that fibroblasts are also phagocytic in healing skin wounds, and that these nonprofessional phagocytes likely help to clear apoECs (Romana-Souza et al. 2021). Remarkably, after apoEC engulfment, fibroblasts develop a fibrotic phenotype with enhanced migration, α-smooth muscle actin (α-SMA) and TGF-β1 expression, and collagen deposition. This pathway—the phagocytosis of apoECs by fibroblasts—suggests a new mechanism by which the apoptotic load that develops in wounds during capillary pruning can influence fibrotic scar formation.
CONCLUDING REMARKS
The basic mechanisms that guide capillary growth and regression in wounds are now well understood. Following injury, the hypoxic environment stimulates the production of proangiogenic factors, and a dense capillary bed is created in the wound. During resolution, an actively antiangiogenic environment supports the refinement and maturation of the capillary bed. Many studies have demonstrated that the angiogenic process is impaired in poorly healing wounds, such as diabetic ulcers, and this is an area that is ripe for further investigation. Recently, communication between endothelial cells and fibroblasts in the wound bed has been clearly demonstrated. There is little doubt that future studies will provide new information about the importance of such cross talk in wounds. Most importantly, ongoing studies of wound angiogenesis will support the development of therapeutics to modulate the angiogenic process. These new therapeutics will guide optimal angiogenesis and, thus, optimal wound repair, fulfilling an important clinical need.
ACKNOWLEDGMENTS
This work was supported by the following National Institutes of Health grant awards: R01 GM50875 (L.A.D.), R35 GM139603 (L.A.D.), and F30 DK123989 (M.B.).
Footnotes
Editors: Xing Dai, Sabine Werner, Cheng-Ming Chuong, and Maksim Plikus
Additional Perspectives on Wound Healing: From Bench to Bedside available at www.cshperspectives.org
REFERENCES
- Agah A, Kyriakides TR, Lawler J, Bornstein P. 2002. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161: 831–839. 10.1016/S0002-9440(10)64243-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadeu T, Braune A, Mandarim-de-Lacerda C, Porto LC, Desmoulière A, Costa A. 2003. Vascularization pattern in hypertrophic scars and keloids: a stereological analysis. Pathol Res Pract 199: 469–473. 10.1078/0344-0338-00447 [DOI] [PubMed] [Google Scholar]
- Apte RS, Chen DS, Ferrara N. 2019. VEGF in signaling and disease: beyond discovery and development. Cell 176: 1248–1264. 10.1016/j.cell.2019.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Valdez CN, Marko CK, D'Amore PA. 2015. From pathobiology to the targeting of pericytes for the treatment of diabetic retinopathy. Curr Diabetes Rep 15: 573. 10.1007/s11892-014-0573-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artuc M, Hermes B, Steckelings UM, Grutzkau A, Henz BM. 1999. Mast cells and their mediators in cutaneous wound healing—active participants or innocent bystanders? Exp Dermatol 8: 1–16. [DOI] [PubMed] [Google Scholar]
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. 1999. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228. 10.1161/01.RES.85.3.221 [DOI] [PubMed] [Google Scholar]
- Avogaro A, Albiero M, Menegazzo L, de Kreutzenberg S, Fadini GP. 2011. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 34: S285–S290. 10.2337/dc11-s239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaji S, LeSaint M, Bhattacharya SS, Moles C, Dhamija Y, Kidd M, Le LD, King A, Shaaban A, Crombleholme TM, et al. 2014. Adenoviral-mediated gene transfer of insulin-like growth factor 1 enhances wound healing and induces angiogenesis. J Surg Res 190: 367–377. 10.1016/j.jss.2014.02.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda MJ, Knighton DR, Hunt TK, Werb Z. 1982. Isolation of a nonmitogenic angiogenesis factor from wound fluid. Proc Natl Acad Sci 79: 7773–7777. 10.1073/pnas.79.24.7773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat M, DiPietro LA, Chen L. 2021. Limited treatment options for diabetic wounds: barriers to clinical translation despite therapeutic success in murine models. Adv Wound Care (New Rochelle) 10: 436–460. 10.1089/wound.2020.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman PK, Koh TJ. 2020. Macrophage dysregulation and impaired skin wound healing in diabetes. Front Cell Dev Biol 8: 528. 10.3389/fcell.2020.00528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger AC, Feldman AL, Gnant MF, Kruger EA, Sim BK, Hewitt S, Figg WD, Alexander HR, Libutti SK. 2000. The angiogenesis inhibitor, endostatin, does not affect murine cutaneous wound healing. J Surg Res 91: 26–31. 10.1006/jsre.2000.5890 [DOI] [PubMed] [Google Scholar]
- Bloch W, Huggel K, Sasaki T, Grose R, Bugnon P, Addicks K, Timpl R, Werner S. 2000. The angiogenesis inhibitor endostatin impairs blood vessel maturation during wound healing. FASEB J 14: 2373–2376. 10.1096/fj.00-0490fje [DOI] [PubMed] [Google Scholar]
- Bluff JE, O'Ceallaigh S, O'Kane S, Ferguson MW, Ireland G. 2006. The microcirculation in acute murine cutaneous incisional wounds shows a spatial and temporal variation in the functionality of vessels. Wound Repair Regen 14: 434–442. 10.1111/j.1743-6109.2006.00142.x [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Yates CC, Wells A. 2006. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res 98: 617–625. 10.1161/01.RES.0000209968.66606.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar RJ, Yates CC, Rodgers ME, Du X, Wells A. 2009. IP-10 induces dissociation of newly formed blood vessels. J Cell Sci 122: 2064–2077. 10.1242/jcs.048793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H, Kodra A, Golinko MS, Entero H, Stojadinovic O, Wang VM, Sheahan CM, Weinberg AD, Woo SL, Ehrlich HP, et al. 2009. Mechanism of sustained release of vascular endothelial growth factor in accelerating experimental diabetic healing. J Invest Dermatol 129: 2275–2287. 10.1038/jid.2009.26 [DOI] [PubMed] [Google Scholar]
- Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de Water L. 1992. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 176: 1375–1379. 10.1084/jem.176.5.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrita MA, Christofori G. 2003. Sprouty proteins: antagonists of endothelial cell signaling and more. Thromb Haemost 90: 586–590. 10.1160/TH03-04-0217 [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Collen D. 2000. Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol 190: 387–405. [DOI] [PubMed] [Google Scholar]
- Chan YC, Roy S, Khanna S, Sen CK. 2012. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol 32: 1372–1382. 10.1161/ATVBAHA.112.248583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Gajendrareddy PK, DiPietro LA. 2012. Differential expression of HIF-1α in skin and mucosal wounds. J Dental Res 91: 871–876. 10.1177/0022034512454435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng B, Fu X. 2018. The focus and target: angiogenesis in refractory wound healing. Int J Low Extrem Wounds 17: 301–303. 10.1177/1534734618813229 [DOI] [PubMed] [Google Scholar]
- Chin LC, Kumar P, Palmer JA, Rophael JA, Dolderer JH, Thomas GP, Morrison WA, Penington AJ, Stewart AG, Mitchell GM. 2011. The influence of nitric oxide synthase 2 on cutaneous wound angiogenesis. Br J Dermatol 165: 1223–1235. 10.1111/j.1365-2133.2011.10599.x [DOI] [PubMed] [Google Scholar]
- Chong DC, Yu Z, Brighton HE, Bear JE, Bautch VL. 2017. Tortuous microvessels contribute to wound healing via sprouting angiogenesis. Arterioscler Thromb Vasc Biol 37: 1903–1912. 10.1161/ATVBAHA.117.309993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craword SE, Fitchev P, Veliceasa D, Volpert OV. 2013. The many facets of PEDF in drug discovery and disease: a diamond in the rough or split personality disorder? Exp Opin Drug Discov 8: 769–792. 10.1517/17460441.2013.794781 [DOI] [PubMed] [Google Scholar]
- Devalaraja RM, Nanney LB, Du J, Qian Q, Yu Y, Devalaraja MN, Richmond A. 2000. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol 115: 234–244. 10.1046/j.1523-1747.2000.00034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA. 2016. Angiogenesis and wound repair: when enough is enough. J Leukocyte Biol 100: 979–984. 10.1189/jlb.4MR0316-102R [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. 1996. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol 148: 1851–1860. [PMC free article] [PubMed] [Google Scholar]
- Dulmovits BM, Herman IM. 2012. Microvascular remodeling and wound healing: a role for pericytes. Int J Biochem Cell Biol 44: 1800–1812. 10.1016/j.biocel.2012.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. 1986. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 315: 1650–1659. 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- Dvorak HF. 2015. Tumors: wounds that do not heal—redux. Cancer Immunol Res 3: 1–11. 10.1158/2326-6066.CIR-14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwin F, Anderson K, Ying C, Patel TB. 2009. Intermolecular interactions of Sprouty proteins and their implications in development and disease. Mol Pharmacol 76: 679–691. 10.1124/mol.109.055848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G, Treps L, Li X, Carmeliet P. 2020. Basic and therapeutic aspects of angiogenesis updated. Circ Res 127: 310–329. 10.1161/CIRCRESAHA.120.316851 [DOI] [PubMed] [Google Scholar]
- Elpek GO. 2015. Angiogenesis and liver fibrosis. World J Hepatol 7: 377–391. 10.4254/wjh.v7.i3.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Brachvogel B, Odorisio T, Koch M. 2007. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem 42: 115–170. 10.1016/j.proghi.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Erba P, Ogawa R, Ackermann M, Adini A, Miele LF, Dastouri P, Helm D, Mentzer SJ, D'Amato RJ, Murphy GF, et al. 2011. Angiogenesis in wounds treated by microdeformational wound therapy. Ann Surg 253: 402–409. 10.1097/SLA.0b013e31820563a8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falanga V. 2005. Wound healing and its impairment in the diabetic foot. Lancet 366: 1736–1743. 10.1016/S0140-6736(05)67700-8 [DOI] [PubMed] [Google Scholar]
- Farkas L, Gauldie J, Voelkel NF, Kolb M. 2011. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 45: 1–15. 10.1165/rcmb.2010-0365TR [DOI] [PubMed] [Google Scholar]
- Franzen LE, Ghassemifar N, Nordman J, Schultz G, Skogman R. 1995. Mechanisms of TGF-β action in connective tissue repair of rat mesenteric wounds. Wound Repair Regen 3: 322–329. 10.1046/j.1524-475X.1995.30313.x [DOI] [PubMed] [Google Scholar]
- Gaengel K, Genové G, Armulik A, Betsholtz C. 2009. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol 29: 630–638. 10.1161/ATVBAHA.107.161521 [DOI] [PubMed] [Google Scholar]
- Galiano RD, Tepper OM, Pelo CR, Bhatt KA, Callaghan M, Bastidas N, Bunting S, Steinmetz HG, Gurtner GC. 2004. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am J Pathol 164: 1935–1947. 10.1016/S0002-9440(10)63754-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gira AK, Brown LF, Washington CV, Cohen C, Arbiser JL. 2004. Keloids demonstrate high-level epidermal expression of vascular endothelial growth factor. J Am Acad Dermatol 50: 850–853. 10.1016/j.jaad.2003.11.061 [DOI] [PubMed] [Google Scholar]
- Goren I, Allmann N, Yogev N, Schürmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. 2009. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 175: 132–147. 10.2353/ajpath.2009.081002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosain A, Matthies AM, Dovi JV, Barbul A, Gamelli RL, DiPietro LA. 2006. Exogenous pro-angiogenic stimuli cannot prevent physiologic vessel regression. J Surg Res 135: 218–225. 10.1016/j.jss.2006.04.006 [DOI] [PubMed] [Google Scholar]
- Gurevich DB, Severn CE, Twomey C, Greenhough A, Cash J, Toye AM, Mellor H, Martin P. 2018. Live imaging of wound angiogenesis reveals macrophage orchestrated vessel sprouting and regression. EMBO J 37: e97786. 10.15252/embj.201797786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi HAR, Suwaidi JA. 2007. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876. [PMC free article] [PubMed] [Google Scholar]
- Hocking AM. 2012. Mesenchymal stem cell therapy for cutaneous wounds. Adv Wound Care (New Rochelle) 1: 166–171. 10.1089/wound.2011.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong WX, Hu MS, Esquivel M, Liang GY, Rennert RC, McArdle A, Paik KJ, Duscher D, Gurtner GC, Lorenz HP, et al. 2014. The role of hypoxia-inducible factor in wound healing. Adv Wound Care (New Rochelle) 3: 390–399. 10.1089/wound.2013.0520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt TK, Hopf H, Hussain Z. 2000. Physiology of wound healing. Adv Skin Wound Care 13: 6–11. [PubMed] [Google Scholar]
- Hunter J. 1794. A treatise on the blood, inflammation and gunshot wounds (ed. Palmer JF). Raswell, Barrington, and Haswell, Philadelphia. [Google Scholar]
- Icli B, Wu W, Ozdemir D, Li H, Haemmig S, Liu X, Giatsidis G, Cheng HS, Avci SN, Kurt M, et al. 2019. MicroRNA-135a-3p regulates angiogenesis and tissue repair by targeting p38 signaling in endothelial cells. FASEB J 33: 5599–5614. 10.1096/fj.201802063RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello MA, Weitzer S, Gannon G, Compagni A, Cotten M, Christofori G. 2001. Mammalian sprouty-1 and -2 are membrane-anchored phosphoprotein inhibitors of growth factor signaling in endothelial cells. J Cell Biol 152: 1087–1098. 10.1083/jcb.152.5.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruela-Arispe ML, Dvorak HF. 1997. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Haemost 78: 672–677. 10.1055/s-0038-1657610 [DOI] [PubMed] [Google Scholar]
- Jang YC, Arumugam S, Gibran NS, Isik FF. 1999. Role of αv integrins and angiogenesis during wound repair. Wound Repair Regen 7: 375–380. 10.1046/j.1524-475X.1999.00375.x [DOI] [PubMed] [Google Scholar]
- Johnson A, DiPietro LA. 2013. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J 27: 3893–3901. 10.1096/fj.12-214189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Wilgus TA. 2014. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care (New Rochelle) 3: 647–661. 10.1089/wound.2013.0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. 2010. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE 5: e9539. 10.1371/journal.pone.0009539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliçaslan SM, Cevher SC, Peker EG. 2013. Ultrastructural changes in blood vessels in epidermal growth factor treated experimental cutaneous wound model. Pathol Res Pract 209: 710–715. 10.1016/j.prp.2013.08.005 [DOI] [PubMed] [Google Scholar]
- Klein SA, Bond SJ, Gupta SC, Yacoub OA, Anderson GL. 1999. Angiogenesis inhibitor TNP-470 inhibits murine cutaneous wound healing. J Surg Res 82: 268–274. 10.1006/jsre.1998.5551 [DOI] [PubMed] [Google Scholar]
- Knighton DR, Silver IA, Hunt TK. 1981. Regulation of wound-healing angiogenesis-effect of oxygen gradients and inspired oxygen concentration. Surgery 90: 262–270. [PubMed] [Google Scholar]
- Knighton DR, Hunt TK, Scheuenstuhl H, Halliday BJ, Werb Z, Banda MJ. 1983. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science 221: 1283–1285. 10.1126/science.6612342 [DOI] [PubMed] [Google Scholar]
- Kutcher ME, Herman IM. 2009. The pericyte: cellular regulator of microvascular blood flow. Microvasc Res 77: 235–246. 10.1016/j.mvr.2009.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher ME, Kolyada AY, Surks HK, Herman IM. 2007. Pericyte Rho GTPase mediates both pericyte contractile phenotype and capillary endothelial growth state. Am J Pathol 171: 693–701. 10.2353/ajpath.2007.070102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange-Asschenfeldt B, Velasco P, Streit M, Hawighorst T, Pike SE, Tosato G, Detmar M. 2001. The angiogenesis inhibitor vasostatin does not impair wound healing at tumor-inhibiting doses. J Invest Dermatol 117: 1036–1041. 10.1046/j.0022-202x.2001.01519.x [DOI] [PubMed] [Google Scholar]
- Laplante P, Sirois I, Raymond MA, Kokta V, Béliveau A, Prat A, Pshezhetsky AV, Hébert MJ. 2010. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ 17: 291–303. 10.1038/cdd.2009.124 [DOI] [PubMed] [Google Scholar]
- Leibovich SJ, Ross R. 1975. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 78: 71–100. [PMC free article] [PubMed] [Google Scholar]
- Leung A, Crombleholme TM, Keswani SG. 2012. Fetal wound healing: implications for minimal scar formation. Curr Opin Pediatr 24: 371–378. 10.1097/MOP.0b013e3283535790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhang YP, Kirsner RS. 2003. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 60: 107–114. 10.1002/jemt.10249 [DOI] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MW, Harrison MR. 1990. Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Pediatr Surg 25: 63–68; discussion 68–69. 10.1016/S0022-3468(05)80165-4 [DOI] [PubMed] [Google Scholar]
- Luan A, Hu MS, Leavitt T, Brett EA, Wang KC, Longaker MT, Wan DC. 2018. Noncoding RNAs in wound healing: a new and vast frontier. Adv Wound Care (New Rochelle) 7: 19–27. 10.1089/wound.2017.0765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, Roers A, Eming SA. 2010. Differential roles of macrophages in diverse phases of skin repair. J Immunol 184: 3964–3977. 10.4049/jimmunol.0903356 [DOI] [PubMed] [Google Scholar]
- Ma D, Chen L, Shi J, Zhao Y, Vasani S, Chen K, Romana-Souza B, Henkin J, DiPietro LA. 2020. Pigment epithelium-derived factor attenuates angiogenesis and collagen deposition in hypertrophic scars. Wound Repair Regen 28: 684–695. 10.1111/wrr.12828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti-Carvajal AJ, Gluud C, Nicola S, Simancas-Racines D, Reveiz L, Oliva P, Cedeno-Taborda J. 2015. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst Rev 2015: CD008548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalczyk ER, Chen L, Fine D, Zhao Y, Mascarinas E, Grippo PJ, DiPietro LA. 2018. Pigment epithelium-derived factor (PEDF) as a regulator of wound angiogenesis. Sci Rep 8: 11142. 10.1038/s41598-018-29465-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza R, DiPietro LA, Koh TJ. 2009. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 175: 2454–2462. 10.2353/ajpath.2009.090248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miscianinov V, Martello A, Rose L, Parish E, Cathcart B, Mitić T, Gray GA, Meloni M, Al Haj Zen A, Caporali A. 2018. MicroRNA-148b targets the TGF-β pathway to regulate angiogenesis and endothelial-to-mesenchymal transition during skin wound healing. Mol Ther 26: 1996–2007. 10.1016/j.ymthe.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Most D, Efron DT, Shi HP, Tantry US, Barbul A. 2002. Characterization of incisional wound healing in inducible nitric oxide synthase knockout mice. Surgery 132: 866–876. 10.1067/msy.2002.127422 [DOI] [PubMed] [Google Scholar]
- Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. 2008. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11: 109–119. 10.1007/s10456-008-9099-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Gamelli RL, DiPietro LA. 1996. Basic fibroblast growth factor mediates angiogenic activity in early surgical wounds. Surgery 119: 457–465. 10.1016/S0039-6060(96)80148-6 [DOI] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. 1998. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol 152: 1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Okonkwo UA, DiPietro LA. 2017. Diabetes and wound angiogenesis. Int J Mol Sci 18: 1419. 10.3390/ijms18071419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo UA, Chen L, Ma D, Haywood VA, Barakat M, Urao N, DiPietro LA. 2020. Compromised angiogenesis and vascular integrity in impaired diabetic wound healing. PLoS ONE 15: e0231962. 10.1371/journal.pone.0231962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir D, Feinberg MW. 2019. MicroRNAs in diabetic wound healing: pathophysiology and therapeutic opportunities. Trends Cardiovasc Med 29: 131–137. 10.1016/j.tcm.2018.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham HT, Economides PA, Veves A. 1998. The role of endothelial function on the foot. Microcirculation and wound healing in patients with diabetes. Clin Podiatr Med Surg 15: 85–93. [PubMed] [Google Scholar]
- Rebling J, Ben-Yehuda Greenwald M, Wietecha M, Werner S, Razansky D. 2021. Long-term imaging of wound angiogenesis with large scale optoacoustic microscopy. Adv Sci (Weinh) 8: 2004226. 10.1002/advs.202004226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren HT, Hu H, Li Y, Jiang HF, Hu XL, Han CM. 2013. Endostatin inhibits hypertrophic scarring in a rabbit ear model. J Zhejiang Univ Sci B 14: 224–230. 10.1631/jzus.B1200077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Crivellato E. 2011. The role of pericytes in angiogenesis. Int J Dev Biol 55: 261–268. 10.1387/ijdb.103167dr [DOI] [PubMed] [Google Scholar]
- Rizk M, Witte MB, Barbul A. 2004. Nitric oxide and wound healing. World J Surg 28: 301–306. 10.1007/s00268-003-7396-7 [DOI] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. 1986. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci 83: 4167–4171. 10.1073/pnas.83.12.4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman CD, Choy H, Nanney L, Riordan C, Parman K, Johnson D, Beauchamp RD. 2002. Vascular endothelial growth factor-mediated angiogenesis inhibition and postoperative wound healing in rats. J Surg Res 105: 43–47. 10.1006/jsre.2002.6444 [DOI] [PubMed] [Google Scholar]
- Romana-Souza B, Chen L, Leonardo TR, Chen Z, DiPietro LA. 2021. Dermal fibroblast phagocytosis of apoptotic cells: a novel pathway for wound resolution. FASEB J 35: e21443. 10.1096/fj.202002078R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish L, Blair HC, Glading A, Wells A. 2005. Interferon-inducible protein 9 (CXCL11)-induced cell motility in keratinocytes requires calcium flux-dependent activation of μ-calpain. Mol Cell Biol 25: 1922–1941. 10.1128/MCB.25.5.1922-1941.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Reitmaier B, Regenbogen J, Slowey RM, Opalenik SR, Wolf E, Goppelt A, Davidson JM. 2005. CARP, a cardiac ankyrin repeat protein, is up-regulated during wound healing and induces angiogenesis in experimental granulation tissue. Am J Pathol 166: 303–312. 10.1016/S0002-9440(10)62254-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh K, Pal D, Sinha M, Ghatak S, Gnyawali SC, Khanna S, Roy S, Sen CK. 2017. Epigenetic modification of microRNA-200b contributes to diabetic vasculopathy. Mol Ther 25: 2689–2704. 10.1016/j.ymthe.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. 2014. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35: 4477–4488. 10.1016/j.biomaterials.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift ME, Kleinman HK, DiPietro LA. 1999. Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 79: 1479–1487. [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. 2003. Differential injury responses in oral mucosal and cutaneous wounds. J Dental Res 82: 621–626. 10.1177/154405910308200810 [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Walsh CG, Steinberg MJ, DiPietro LA. 2005. Distinct patterns of angiogenesis in oral and skin wounds. J Dental Res 84: 309–314. 10.1177/154405910508400403 [DOI] [PubMed] [Google Scholar]
- Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. 2005. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood 105: 1068–1077. 10.1182/blood-2004-03-1051 [DOI] [PubMed] [Google Scholar]
- Urao N, Okonkwo UA, Fang MM, Zhuang ZW, Koh TJ, DiPietro LA. 2016. MicroCT angiography detects vascular formation and regression in skin wound healing. Microvasc Res 106: 57–66. 10.1016/j.mvr.2016.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer WM, Niessen FB, Ferreira JA, Zwiers PJ, de Jong EH, Middelkoop E, Molema G. 2011. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen 19: 292–301. 10.1111/j.1524-475X.2011.00692.x [DOI] [PubMed] [Google Scholar]
- Wang P, Koyama Y, Liu X, Xu J, Ma HY, Liang S, Kim IH, Brenner DA, Kisseleva T. 2016. Promising therapy candidates for liver fibrosis. Front Physiol 7: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke N, Griffin KJ, Cubbon RM. 2016. Pericytes in diabetes-associated vascular disease. J Diabetes Complications 30: 1643–1650. 10.1016/j.jdiacomp.2016.08.005 [DOI] [PubMed] [Google Scholar]
- Warren CM, Ziyad S, Briot A, Der A, Iruela-Arispe ML. 2014. A ligand-independent VEGFR2 signaling pathway limits angiogenic responses in diabetes. Sci Signal 7: ra1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MJV, Briquez PS, White DAV, Hubbell JA. 2021. VEGF-A, PDGF-BB and HB-EGF engineered for promiscuous super affinity to the extracellular matrix improve wound healing in a model of type 1 diabetes. NPJ Regen Med 6: 76. 10.1038/s41536-021-00189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietecha MS, Chen L, Ranzer MJ, Anderson K, Ying C, Patel TB, DiPietro LA. 2011. Sprouty2 downregulates angiogenesis during mouse skin wound healing. Am J Physiol Heart Circ Physiol 300: H459–H467. 10.1152/ajpheart.00244.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wietecha MS, Król MJ, Michalczyk ER, Chen L, Gettins PG, DiPietro LA. 2015. Pigment epithelium-derived factor as a multifunctional regulator of wound healing. Am J Physiol Heart Circ Physiol 309: H812–H826. 10.1152/ajpheart.00153.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Matthies AM, Radek KA, Dovi JV, Burns AL, Shankar R, DiPietro LA. 2005. Novel function for vascular endothelial growth factor receptor-1 on epidermal keratinocytes. Am J Pathol 167: 1257–1266. 10.1016/S0002-9440(10)61213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgus TA, Ferreira AM, Oberyszyn TM, Bergdall VK, Dipietro LA. 2008. Regulation of scar formation by vascular endothelial growth factor. Lab Invest 88: 579–590. 10.1038/labinvest.2008.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte MB, Thornton FJ, Efron DT, Barbul A. 2000. Enhancement of fibroblast collagen synthesis by nitric oxide. Nitric Oxide 4: 572–582. 10.1006/niox.2000.0307 [DOI] [PubMed] [Google Scholar]
- Xu M, Chen Z, Chen K, Ma D, Chen L, DiPietro LA. 2021. Phagocytosis of apoptotic endothelial cells reprograms macrophages in skin wounds. J Immunol Regen Med 12: 100038. 10.1016/j.regen.2021.100038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates CC, Whaley D, Hooda S, Hebda PA, Bodnar RJ, Wells A. 2009. Delayed reepithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair Regen 17: 34–41. 10.1111/j.1524-475X.2008.00439.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkada G, Heinolainen K, Makinen T, Kubota Y, Alitalo K. 2015. VEGFR3 does not sustain retinal angiogenesis without VEGFR2. Proc Natl Acad Sci 112: 761–766. 10.1073/pnas.1423278112 [DOI] [PMC free article] [PubMed] [Google Scholar]