DEATH MATTERS
Cell death has consequences beyond simply the loss of the cell itself. Dying cells trigger their removal by other cells, and this can be accompanied by an inflammatory response. The cells can also induce other cells to proliferate to compensate for the loss in cell number. And, in vertebrates, they can signal to the immune system to regulate adaptive immune responses to foreign or altered proteins associated with the dying cell. Dying cells are not simply debris to be discarded—they are resources to be recycled, and this process of waste management is tightly controlled.
In this review, we consider the ways in which dying cells are cleared from the body and the consequences for the organism after the cell is gone. As we will see, there are many possible outcomes, and these depend in part on the cell and how it dies.
DISPOSING OF THE CORPSE
In some cases, cells that die are simply sloughed off, and, in vertebrates, this happens to the many cells that die in the skin and the gut epithelium as a consequence of normal tissue homeostasis. But most cells that die in the body have no place to go; they must be cleared by other cells.
Regardless of the mode of cell death, the corpses are engulfed1 by phagocytic cells, by which they are digested. Presumably, some breakdown products of the dead cell are recycled, but others represent a problem for the cell doing the clearing and might require a form of waste management. The phagocytes (“eating cells”) are often “professionals,” such as macrophages (as well as dendritic cells and neutrophils in vertebrates). However, they can also be “amateurs,” such as epithelial cells. Some of the consequences of cell death depend on which cell does the eating.
Although any dead cell can be removed by phagocytosis, apoptosis provides a means to clear the dying cell before plasma membrane integrity is lost, thereby avoiding “secondary necrosis” (see Green 2022a). As we have seen, necrosis can promote inflammation through the release of intracellular contents. However, apoptosis involves effectively preparing the corpse for degradation by preprocessing some of its components while preserving the plasma membrane. The consequences of caspase activation—which leads to DNA fragmentation, compaction, and the “blebbing off” of pieces of the dying cell—can all be seen as preparation for clearance.
The signals produced by apoptotic cells echo Pete Townsend's “see me, feel me, touch me, heal me” from the classic rock opera Tommy.2 The steps here, however, are “find me, bind me, eat me, clear me.”
“FIND-ME” SIGNALS
Often, cells that die are simply engulfed and cleared by neighboring cells, and no recruitment of phagocytes is necessary. However, in the case of apoptosis, the facilitation of clearance can involve the production of find-me signals by the dying cell that recruit macrophages and other phagocytes.
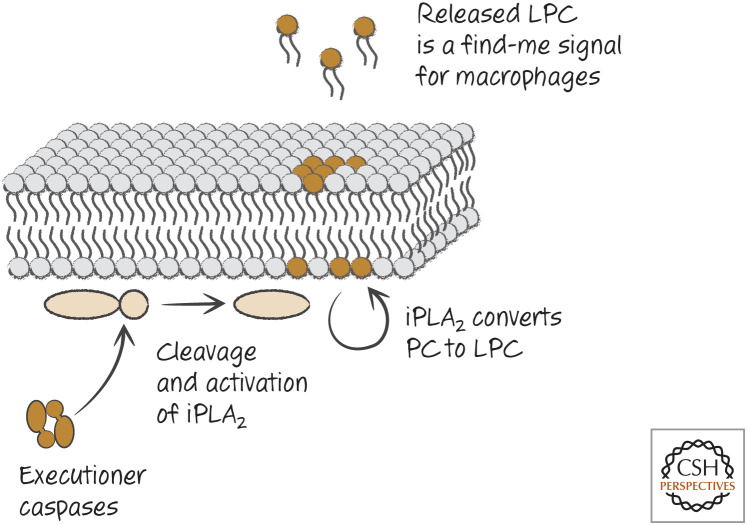
One such find-me signal is the phospholipid derivative lysophosphatidylcholine (LPC), which attracts macrophages, among its many other activities. Many cells that undergo apoptosis produce LPC in a caspase-dependent manner. Caspases cleave and activate calcium-independent phospholipase A2 by removing an inhibitory domain on the enzyme, and this catalyzes the production of LPC (Fig. 1). Although the generation of this find-me signal is by an elegant mechanism, high levels of LPC are required to attract macrophages, and there are already high levels of LPC in the circulation. Other find-me signals are likely to be important. Another lipid that attracts macrophages is sphingosine-1-phosphate, produced by apoptotic cells, although how apoptosis induces its production is not known.
Figure 1.
Executioner caspases induce production of the “find-me” signal LPC. LPC, lysophosphatidylcholine; iPLA2, calcium-independent phospholipase A2; PC, phosphatidylcholine.
Dying cells can also release damage-associated molecular patterns (DAMPs; see Green 2022b). Some examples that act (probably indirectly) as find-me signals are ATP and another nucleotide, UTP, that is converted to UDP in the extracellular environment. Both of these bind to receptors on phagocytic cells. Another DAMP, released from necrotic cells, is uric acid, and this can also generate find-me signals. It is likely that, in these cases, cells that are near the dying cell are activated by the DAMPs to produce molecules such as cytokines and chemokines, which are the actual signals that recruit more phagocytic cells. In general, the DAMPs that do this are released from necrotic cells, and whether they have roles in generating find-me signals for other forms of cell death has not been established.
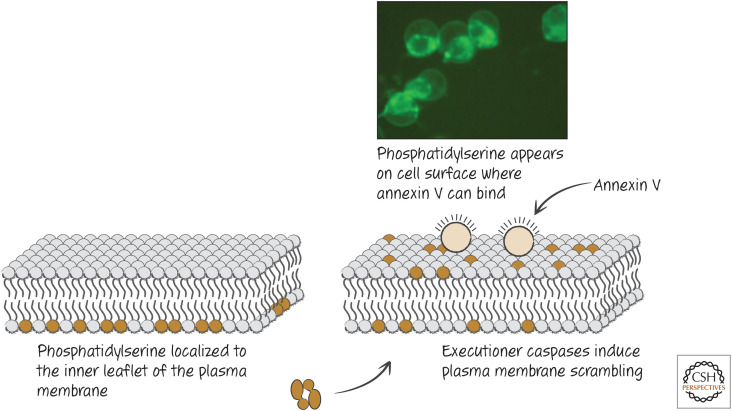
Figure 2.
Plasma membrane scrambling and its detection in dying cells. Phosphatidylserine “scrambled” from the inner to the outer membrane can be detected by probes, such as fluorescently labeled annexin V. (Reprinted from Brumatti et al. 2008, ©2008 with permission from Elsevier.)
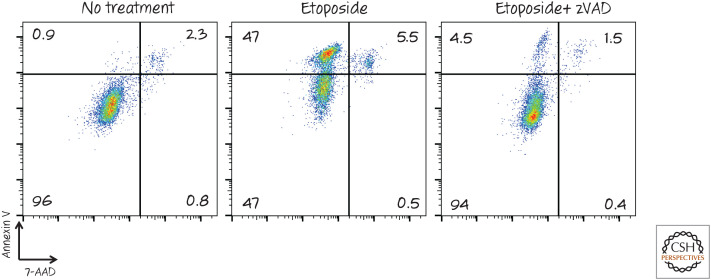
Figure 3.
Detection of phosphatidylserine exposure by fluorescence-activated cell sorting (FACS). Cells were treated with the chemotherapy agent etoposide to induce apoptosis (± the caspase inhibitor zVAD-fmk) and then stained with annexin V (coupled with a fluorescent dye) and the vital dye 7-AAD. The fluorescence intensity of each cell is represented by a dot. Notice that the cell population becomes positive for annexin V before membrane integrity (measured by 7-AAD) is lost. This annexin V staining, and by implication phosphatidylserine exposure, is dependent on caspases, as treatment with the inhibitor zVAD largely prevented it. 7-AAD, 7-amino actinomycin D; zVAD, zVAD-fmk.
“BIND-ME” SIGNALS ON DYING CELLS
As cells die, changes in the plasma membrane act as signals for other cells to engulf the corpse. The most important of these signals is a lipid—phosphatidylserine. In healthy cells, many plasma membrane lipids are arrayed asymmetrically, residing predominantly in either the inner or the outer leaflet of the membrane. Phosphatidylserine is kept to the inner leaflet by an ATP-dependent aminophospholipid translocase that flips phosphatidylserine and other aminophospholipids from the outer to the inner membrane. If energy in the form of ATP is unavailable, or if the plasma membrane is disrupted, phosphatidylserine accumulates by diffusion in the outer leaflet. In cells undergoing apoptosis, however, externalization of phosphatidylserine occurs in a rapid, caspase-dependent manner, before plasma membrane integrity is lost. The “scramblase” responsible for this phospholipid scrambling is the XK-related protein Xkr8 (Green 2022c). Interestingly, Xkr8 is homologous to a protein in the nematode Caenorhabditis elegans, CED-8, which was found to be important in dying cells for their removal. Caspases also destroy the phospholipid translocase (ATPC11) to prevent movement of phosphatidylserine from the outer to the inner leaflet of the plasma membrane. It turns out that the appearance of phosphatidylserine on the cell surface, before a loss in plasma membrane integrity, also occurs in cells undergoing necroptosis as a consequence of the active pseudokinase MLKL (see Green 2022a). In other forms of necrosis, phosphatidylserine is exposed passively, as the plasma membrane is disrupted. As a result, all dying cells display phosphatidylserine.
Although phosphatidylserine is an important bind-me signal (probably the most important one for engulfment), other bind-me signals also appear on dying cells. Oxidation of lipids and changes in carbohydrates also occur and can contribute to recognition. Calreticulin is a protein within the endoplasmic reticulum, and cells that die in different ways often expose this protein on the cell surface. This can also act as a bind-me signal for phagocytes. Calreticulin also appears to be important later on for subsequent responses to the corpse, as we will see.
DETECTING PHOSPHATIDYLSERINE BY USE OF ANNEXIN V.
The appearance of phosphatidylserine on apoptotic cells can be detected with a probe, annexin V, that binds to phosphatidylserine and can be coupled to fluorescent dyes for detection (Fig. 2). It is important to note, however, that annexin V is not how apoptotic cells are recognized by the body. Apoptotic cells can be stained with annexin V before they become permeable to other dyes, that is, before plasma membrane integrity is lost. This is also seen in necroptosis. In contrast, cells undergoing other forms of necrosis expose phosphatidylserine at the same time that they lose the plasma membrane barrier.
Annexin V is not the only way to detect phosphatidylserine on the surface of dying cells. The bridging molecule MGF-E8 (discussed below) can also be used as such a probe.
Because membrane disruption also causes the exposure of phosphatidylserine on the surface, a second dye that is membrane impermeable is generally used to monitor the integrity of the plasma membrane. Some examples are propridium iodide, 7-AAD, and Sytox Green (although several such dyes can be used) (Fig. 3).
During apoptosis, phosphatidylserine is exposed before any loss of plasma membrane integrity, and this condition is often used as a measure of apoptosis. As the dying cell continues to deteriorate, the plasma membrane loses its coherence, and the cell stains with both dyes.
However, in necroptosis (see Green 2022a), phosphatidylserine is also exposed on the cell surface before a loss of plasma membrane integrity. Therefore, to assess apoptosis in this way, the effects of caspase inhibitors on the staining should be evaluated to determine whether the effect is caspase dependent (Fig. 3)
“DON'T-EAT-ME” SIGNALS ON CELLS
If phosphatidylserine exposure were the only signal for removal of dying cells, then we would have a problem—plasma membrane scrambling and externalization of phosphatidylserine occurs in perfectly healthy cells as well and might have roles in cell signaling. One way this occurs is through the activation of a phospholipid scramblase—TMEM16F (also called anoctamin-6)—by calcium ions (as discussed in Green 2022c, this is not involved in phosphatidylserine exposure during apoptosis, and, as it turns out, it is also not involved in such exposure during necroptosis). Activation of lymphocytes, for example, induces a calcium flux that causes a transient exposure of phosphatidylserine.
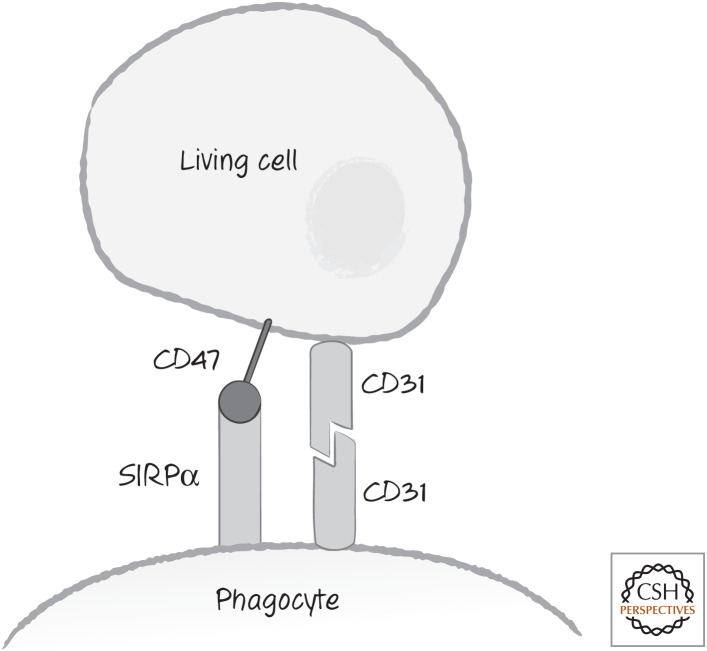
But healthy cells (at least in vertebrates) appear to produce “don't-eat-me” signals that are rapidly lost from dying cells (Fig. 4). One don't-eat-me signal is the leukocyte surface antigen CD47, a cell-surface molecule expressed on all healthy cells. CD47 is recognized by a receptor, SIRPα, on phagocytic cells, and this generates an inhibitory signal in the form of active tyrosine phosphatases. These signals appear to block phagocytosis as well as a range of other signaling pathways, and the CD47–SIRPα interaction is also involved in other processes. During cell death, CD47 is rapidly lost from the cell surface or partitioned away from externalized phosphatidylserine.
Figure 4.
“Don't-eat-me” signals. These include the leukocyte surface antigen CD47, recognized by phagocyte cell-surface receptor SIRPα, and the homotypic interaction between CD31 molecules. SIRPα, signal-regulatory protein alpha.
Mice lacking CD47 do not have a problem with spontaneous uptake of cells, other than a tendency of macrophages to remove living red cells from the circulation. However, neutralization of CD47 or SIRPα can cause living tumor cells to be engulfed by macrophages and destroyed, and this has been suggested as a novel route to cancer therapy. It seems that other signals (other than exposure of phosphatidylserine) cooperate with a loss of the don't-eat-me signal to cause this engulfment. One such signal on cancer cells is calreticulin (see above), perhaps exposed as a consequence of endoplasmic reticulum stress.
Another don't-eat-me signal is the cell-surface molecule CD31, which, when it interacts with CD31 on the phagocytic cell, generates an uncharacterized repulsion signal. The CD31 homotypic interaction is apparently disabled in dying cells.
There also appear to be “stay-away” signals that apoptotic cells produce. All of the find-me signals mentioned above attract neutrophils as well as macrophages, but neutrophils are not recruited to sites of apoptotic cell death. One stay-away signal is lactoferrin, which can selectively prevent neutrophil recruitment. How this is produced by apoptotic cells and how it acts on neutrophils are currently unknown.
BRIDGING MOLECULES RECOGNIZE “BIND-ME” SIGNALS
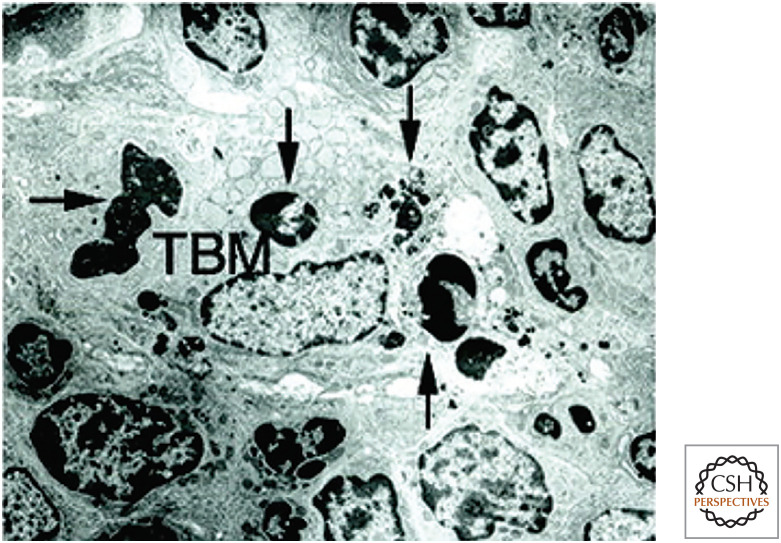
A number of soluble bridging molecules bind to dying cells because of the changes in the plasma membrane. Several of these bind to externalized phosphatidylserine on dying cells, and the most important of these is milk fat globulin-E8 (lactadherin/MFG-E8). Mice lacking MFGE8, or animals injected with a dominant-negative mutant protein, accumulate apoptotic corpses. This is especially evident in one region of the lymphoid tissues, the germinal centers, where B lymphocytes proliferate during immune responses. In normal animals, germinal centers contain what are known as “tingible body macrophages” that turn out to be macrophages with engulfed apoptotic bodies that they are digesting (Fig. 5). Mice lacking MFG-E8 do not have these cells.
Figure 5.
A single tingible body macrophage (TBM) in a germinal center engulfing four apoptotic cells (arrows). (Reprinted from Hanayama et al. 2004, ©2004 with permission from AAAS.)
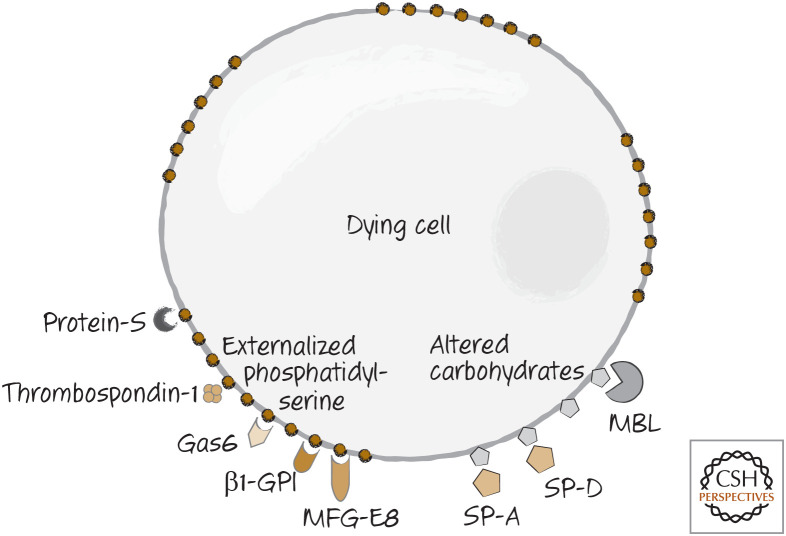
Several other bridging molecules bind to phosphatidylserine on dying cells. These include thrombospondin-1, Gas6, β1-GPI, and protein-S. Figure 6 illustrates the array of bridging molecules that can bind to phosphatidylserine on dying cells.3 Altered carbohydrates on the dying cells are bound by mannose-binding lectin (MBL) and the lung surfactant proteins A and D (SP-A and SP-D) (Fig. 6). It is likely that not all dying cells are bound by all of these bridging molecules, but clearly there is great redundancy in the system.
Figure 6.
Bridge molecules are soluble molecules that bind to dying cells because of the changes in the plasma membrane. MBL, mannose-binding lectin; MFG-E8, lactadherin.
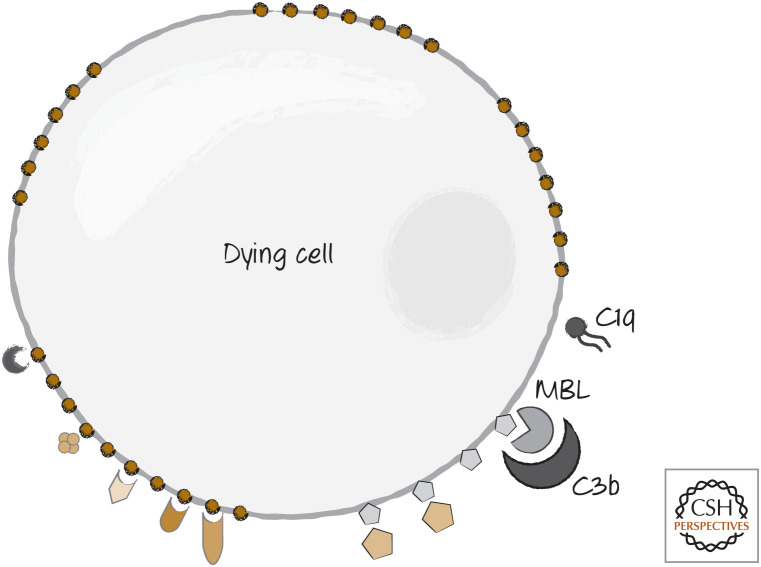
Two other bridging molecules might also be involved in the recognition of some dying cells. These are proteins in the complement pathway—a set of circulating proteins that function in immune responses to bacteria and other foreign invaders. C1q, which binds to antibody clusters, appears to be involved, as is C3b, which normally binds directly to bacteria to trigger their removal (Fig. 7). In the case of dying cells, C3b appears to be bound and activated by MBL.
Figure 7.
Complement components C1q and C3b can also bind to dying cells to act as bridge molecules.
Of course, bridging molecules that decorate dying cells must themselves be recognized by receptors on phagocytic cells if they are to aid engulfment. As we will see, there are also receptors that directly recognize the surface changes in the dying cells.
THE “TETHER AND TICKLE” MODEL
Why are there so many signals for uptake of dying cells? Relatively few of these actually trigger engulfment. A useful idea is that removal of cell corpses proceeds by a process of “tether and tickle.” In this model, many of the receptors on phagocytes that recognize bridging molecules and the dying cells themselves simply tether the corpse. Other receptors, then, actually generate the responses leading to engulfment. These “tickle”—that is, they produce intracellular signals that cause the phagocyte to eat the dying cell. This will prove a useful idea as we continue our survey to include the receptors on the engulfing cells.
PHAGOCYTES HAVE RECEPTORS FOR “BIND-ME” SIGNALS AND BRIDGING MOLECULES
Many different receptors on the phagocyte bind to bridging molecules; others appear to bind directly to phosphatidylserine and signals on the dying cells. No one phagocytic cell has all of the relevant receptors, and therefore the rather complex picture that emerges might be a bit misleading. It is worthwhile to keep this in mind in the discussion that follows.
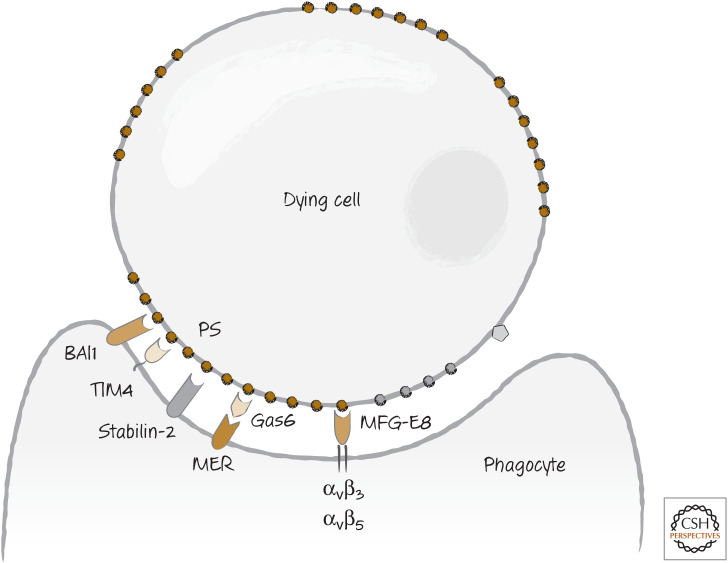
At least three receptors on phagocytes bind to phosphatidylserine and participate in the uptake of dying cells. These are TIM4 (T-cell immunoglobulin and mucin-domain-containing molecule 4—confusingly, residing on phagocytes), BAI1 (brain-specific angiogenesis inhibitor 1—confusingly, not brain specific), and stabilin-2 (Fig. 8). TIM4 appears to be particularly important; mice lacking TIM4 accumulate dying cells, and macrophages derived from bone-marrow precursors take up apoptotic cells less efficiently if they lack TIM4.
Figure 8.
Recognition of phosphatidylserine (PS), exposed on dying cells, by phagocytes occurs either directly (e.g., for BAI1, stabilin 2, TIM4) or indirectly through bridge molecules (e.g., for MER, integrins).
The phosphatidylserine-binding bridging molecule MFGE8 binds to two integrins on the phagocyte surface: αvβ3 and αvβ5. Gas6 binds to a different receptor on the phagocyte, a receptor tyrosine-protein kinase called Mer. Both the integrins and the Mer generate signals, and therefore these probably “tickle” the phagocytic cell to induce the subsequent engulfment. Macrophages from mice that lack these integrins or MER display at least partially defective binding to dying cells. TIM4 does not have a signaling domain in its intracellular region, and therefore if it “tickles” the phagocyte for uptake of corpses, how this happens is not clear.4
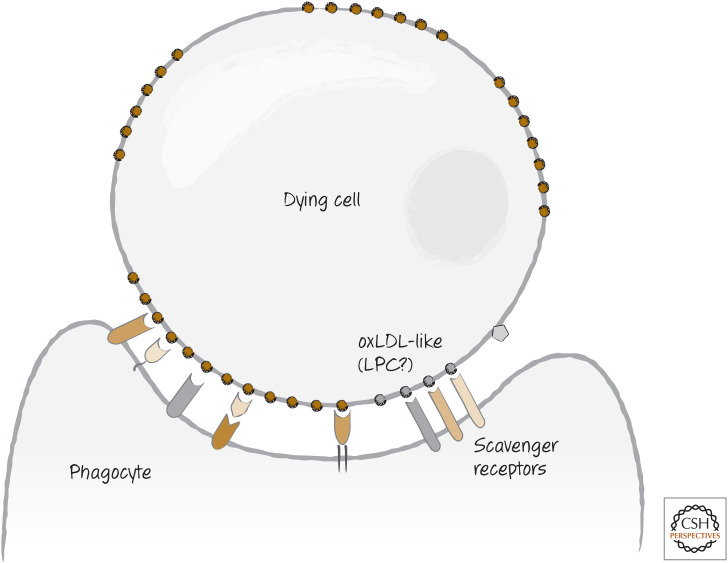
Some of the other phagocyte receptors that are probably involved in tethering (although they might also have “tickle” functions about which we do not know) are the scavenger receptors, which include scavenger receptor-A, LOX1, CD68, and CD36. These normally bind to oxidized low-density lipids (oxLDLs) and clear them from the circulation. OxLDL-like sites on dying cells are also bound by these receptors. It is possible that these sites are actually lysophosphatidylserine (a find-me signal) that was not released from the dying cell (Fig. 9).
Figure 9.
Scavenger receptors recognize dying cells expressing, for example, altered carbohydrates and exposed phosphatidylserine.
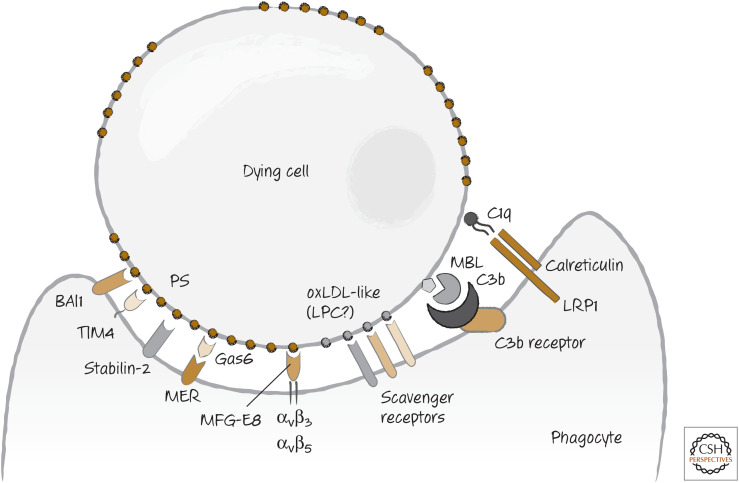
The complement molecule C3b is bound by complement receptors on the phagocyte. Because these clearly function in the engulfment of bacteria, it is likely that they similarly “tickle” phagocytes to engulf dying cells. In addition, C1q is also bound by a receptor comprising LRP1 (CD91) and the protein calreticulin (this time on the phagocyte). Calreticulin is also exposed on some dying cells, and it is likely that its interaction with LRP1 directly signals for engulfment. Figure 10 shows the array of receptors on phagocytes for dying cells and bridge molecules.
Figure 10.
The array of “bind-me” receptors that can directly, or indirectly through bridge molecules, bind to dying cells.
“BIND-ME” SIGNALS AND RECEPTORS IN OTHER ANIMALS
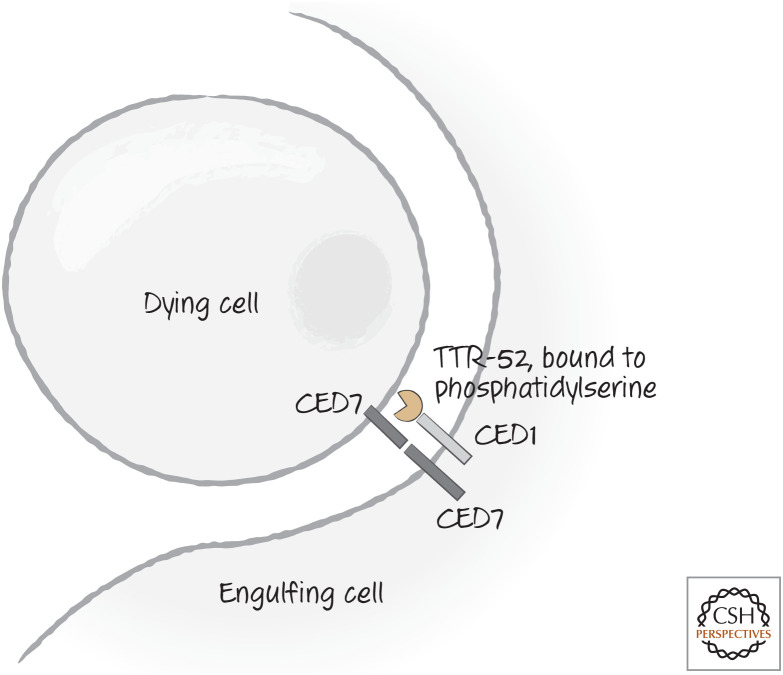
So far, we have focused entirely on the recognition of dying cells in vertebrate animals. However, much of what we know of the steps that follow binding has been gleaned from genetic studies of C. elegans. These have uncovered two complementary pathways for binding and engulfment of dying cells. Two of the genes identified (one for each pathway) encode the actual recognition receptors, CED1 and CED7. CED1 is the nematode homolog of LRP1, the potential receptor for cell-surface calreticulin, but we do not know whether calreticulin is the signal recognized by CED1. Alternatively, a bridging molecule that binds to phosphatidylserine, called transthyretin-like protein 52 (TTR-52), is bound by CED1 in nematodes. TTR-52 is a protein secreted by the endoderm; it forms clusters around dying cells and is required for their clearance.
CED7 is a cell-surface molecule in the ATP-binding cassette (ABC) family. It is important in both the dying and the engulfing cell and can engage in homotypic interactions (that is, CED7 binding to CED7; there are, of course, more-complex possibilities). Some studies suggest that, in mammals, two related proteins, ABC1 and ABC7, function similarly in engulfment of dying cells and contribute to externalization of phosphatidylserine.5 The recognition receptors of C. elegans are illustrated in Figure 11.
Figure 11.
Recognition receptors CED1 and CED7 in nematodes. CED7 is important in both the dying and the engulfing cells, whereas CED1 is important in the engulfing cell. CED1 can bind to a bridging molecule, TTR-52, which recognizes phosphatidylserine and is required for clearance of dying cells. There is at least one additional receptor (not illustrated) involved in recognition and engulfment of dying cells.
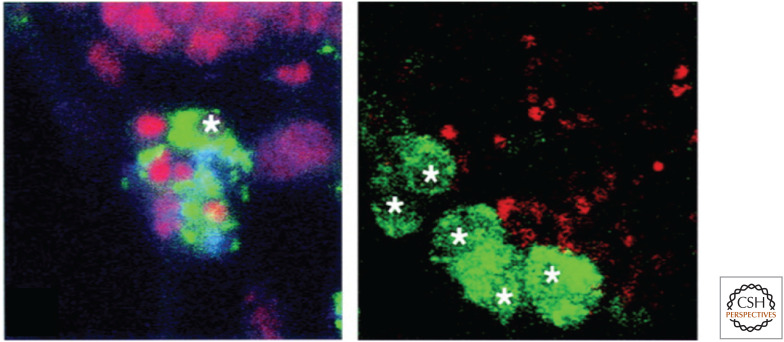
In Drosophila, an evocatively named protein, Croquemort (“undertaker”), can confer the ability to clear dying cells on a mammalian cell line on which it is otherwise lacking. Flies with mutations in the gene show defects in engulfment of apoptotic cells during development (Fig. 12). Croquemort is a homolog of the scavenger receptor CD36, which, as we have seen, is implicated in vertebrate recognition of dying cells.
Figure 12.
The Drosophila Croquemort mutant has defects in engulfment of dying cells. Here, phagocytic cells (green, with white stars) take up small dying cells (red) in wild-type flies (left) but not in mutants unable to express Croquemort (right). (Franc et al. 1999.)
“EAT-ME” SIGNALS PROMOTE ENGULFMENT
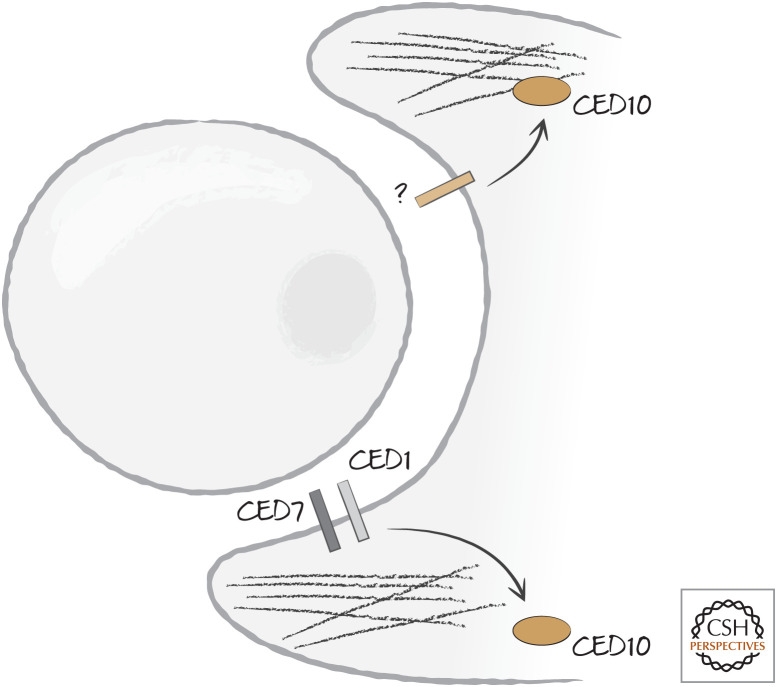
Engulfment requires extensive reorganization of the actin cytoskeleton of the engulfing cell to extend the phagocyte membrane around the corpse. The mechanisms that control this step are conserved in animals and involve small GTPases of the Rho family, especially RAC1 (CED10 in nematodes). This protein performs many functions in the cell, but we focus on its role in engulfment. In C. elegans, the two complementary pathways for engulfment converge on RAC1/CED10 (Fig. 13).
Figure 13.
Two pathways converge on the Ras-related protein CED10 (Rac1) to induce actin reorganization and phagocytosis. Although one pathway is linked to CED1 and CED7, the other pathway is initiated in the nematode Caenorhabditis elegans by an unknown receptor.
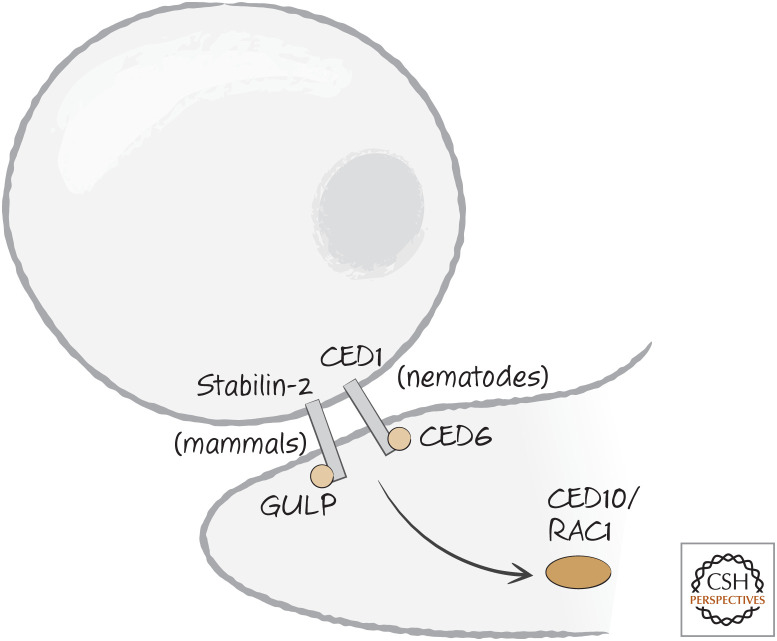
One of the pathways is linked to CED1 (LRP1 in vertebrates), which signals to RAC1/CED10 through the adapter molecule CED6, called GULP (get it?) in other organisms. Although we do not know whether LRP1 signals through GULP, another phosphatidylserine receptor, stabilin-2, appears to require GULP to induce engulfment (Fig. 14). How GULP–CED6 activates RAC1–CED10 is not known. In Drosophila, the CED1 homolog is called Draper, and this interacts with GULP–CED6 as well. But engulfment induced by Draper is also dependent on a tyrosine kinase,6 and this might in time provide a clue as to how this signaling complex functions.
Figure 14.
CED1 (nematodes) and stabilin-2 (mammals) engage an adapter molecule, CED6 or GULP, respectively, following recognition of dying cells. CED6/GULP activates CED10/RAC1 by an unknown mechanism.
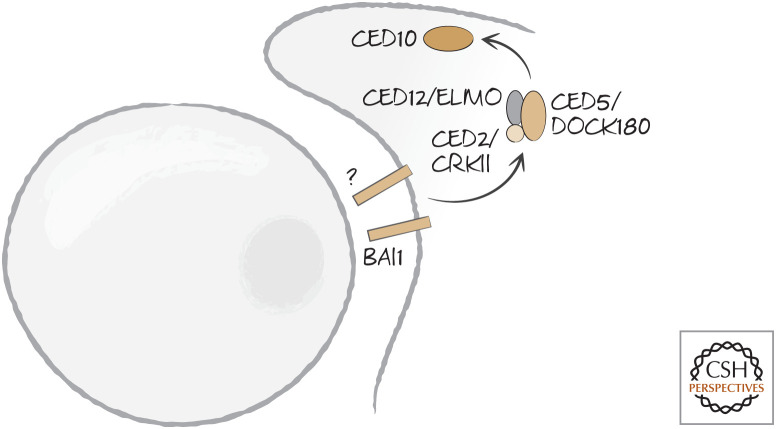
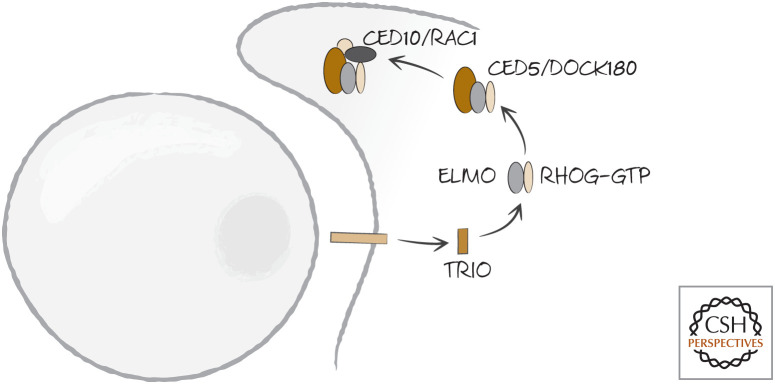
The other pathway that converges on RAC1–CED10 is linked to an unknown receptor in nematodes, but to BAI1 in mammals. This signals through a complex of three nematode proteins: CED2, CED5, and CED12. CED2 is CRKII in other organisms. It has two Src-homology 2 (SH2, phosphotyrosine binding) domains and a Src-homology 3 (SH3, proline-rich binding) domain and performs numerous functions in the regulation of actin. It interacts with CED5, called DOCK180 in other organisms, and CED12, called ELMO (for “engulfment and cell mobility”) (Fig. 15).
Figure 15.
BAI1 in mammals, and an unknown receptor in nematodes, engage a signaling complex (comprising CRKII–DOCK180–ELMO–Rac1 or CED2–CED5–CED12–CED10, respectively) following recognition of dying cells.
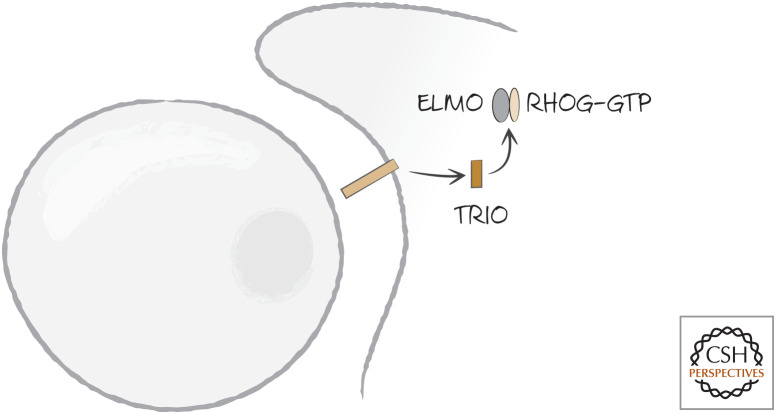
Here is how it all appears to work.7 DOCK180 is an unusual type of guanine nucleotide exchange factor (GEF) thought to load GTP onto RAC1 by forming a scaffold that binds several components, including RAC1. DOC180 is normally inhibited by the binding of its SH3 domain to its RAC1-binding region. When an appropriate receptor, such as the phosphatidylserine-binding receptor BAI1, is activated, a protein called TRIO (another GEF) loads GTP onto the small GTPase RHOG. RHOG–GTP then recruits ELMO (Fig. 16). ELMO then binds to the SH3 domain of DOCK180, allowing the latter to bind RAC1 as well as CRKII (Fig. 17). This complex, now localized to the contact site where the phagocyte binds to the dying cell, activates RAC1. This, in turn, reorganizes the actin cytoskeleton.
Figure 16.
The phosphatidylserine-binding receptor BAI1 (and other receptors) in mammals induce the GDP–GTP exchange factor TRIO, which activates the small GTPase RHOG, converting it to the GTP-bound active form.
Figure 17.
ELMO recruits DOCK180, which binds to RAC1, leading to the activation of this small GTPase. Engulfment by the phagocyte then proceeds.
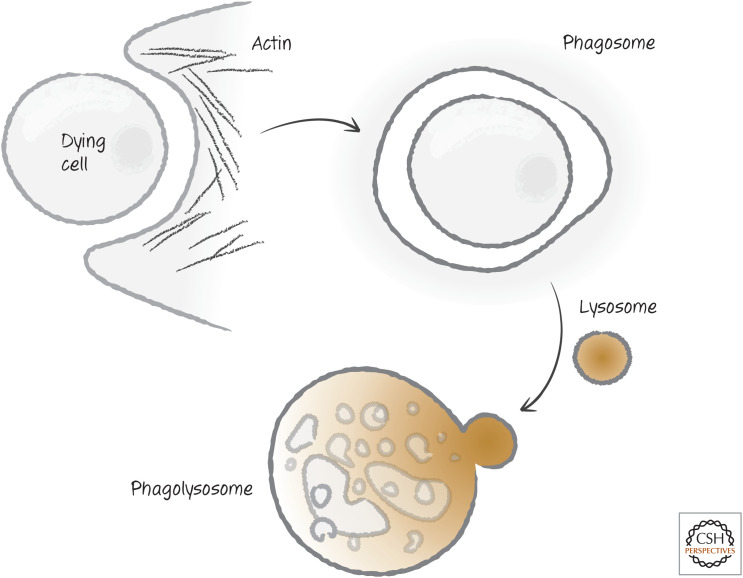
The phagocyte membrane envelops the corpse to form a vesicle called the phagosome. The actin is reorganized, and the phagosome now carries its cargo off to lysosomes for disposal (Fig. 18). We return to this part of the story later in this review.
Figure 18.
The phagosome and degradation. The phagocyte envelops the dying cell, forming an internal phagosome that, on rearrangement of the actin cytoskeleton and fusion with a lysosome, forms a phagolysosome, where disposal of the dying cell takes place.
ENGULFMENT CAN PROMOTE CELL DEATH
In some situations, cells that engage apoptotic (and perhaps other) pathways that promote their uptake might not be “dead” until the engulfment and degradation machinery acts on them. This has been most compellingly seen in the case of apoptotic cell death in nematodes, especially when the activity of the executioner caspase (CED3) is limited. In such cases, defects in engulfment can result in the appearance of living cells that would not otherwise be there. It appears that engulfment is necessary to enforce cell death in such cases.
In Drosophila, some cell death is mediated by autophagy (discussed in Green 2022a). One of the molecules involved in engulfment of dying cells, Draper, is somehow necessary for this phenomenon, but in this case it must be expressed in the cell that is destined to die. At this point, we do not understand how this molecule functions in the cell death.
As we discussed above, disruption of “don't-eat-me” signaling (e.g., CD47, SIRPα) can also promote the engulfment of stressed cells, such as tumor cells, and their death. In mammals, we do not know how much this process might function in physiological situations.
There is another phenomenon that promotes death of cells by engulfment, although, in this case, the process is not dependent on the mechanisms by which phagocytes clear dying cells. This process (entosis) is discussed in more detail below.
THE “CLEAR-ME” PROCESS AND WASTE MANAGEMENT
Once the dying cell is engulfed in a phagosome, the engulfing cell must deal with the debris. This begins in the phagosome with the action of enzymes that degrade proteins and lipids, but the real work is performed when the phagosome fuses with lysosomes to form a phagolysosome.
Lysosomes contain an array of degradative enzymes including a nuclease, DNAse II. Apoptotic cells chop up their DNA by the caspase-dependent activation of the nuclease CAD (see Green 2022c). Cells lacking CAD or its inhibitor-chaperone iCAD do not effectively fragment their DNA. However, when such cells are engulfed by phagocytes, the DNA is nevertheless degraded. In contrast, mice lacking both CAD function and DNAse II show no DNA fragmentation of dying cells.
The iCAD–CAD complex is highly conserved in animals but is not present in nematodes. In the worm, a DNase in the phagosome digests the DNA. Digestion of DNA is an important waste-management problem in the clearance of dying cells.
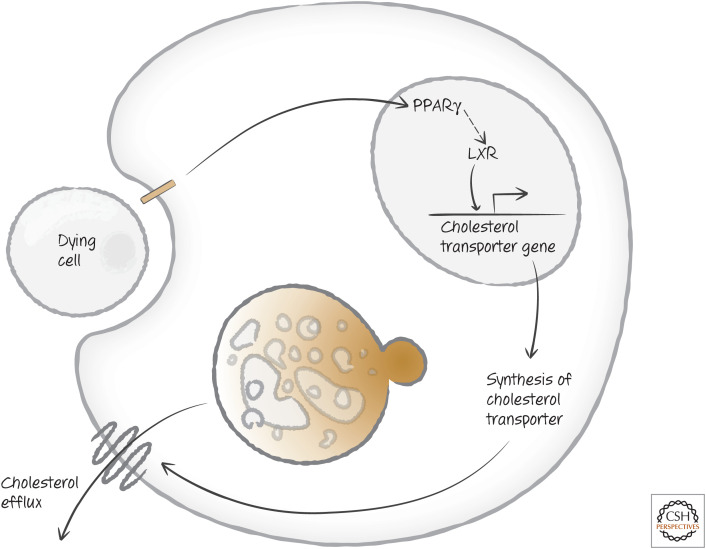
Another waste-management issue is cholesterol, the levels of which effectively double when a phagocyte takes up a dying cell. Recognition of phosphatidylserine on the corpse generates signals that activate PPARγ (peroxisome proliferator-activated receptor γ), and LXR (liver X receptor), which are important regulators of cholesterol and other lipids in the cell. PPARγ shuts down a number of processes, including generation of cholesterol; and LXR activates expression of a transporter to remove cholesterol from the cell (Fig. 19).
Figure 19.
Uptake of dying cells induces cholesterol management. Activation of the peroxisome proliferator-activated receptor γ (PPARγ) induces the expression of the nuclear oxysterols receptor LXR-alpha (NR1H3), which in turn induces the expression of a cholesterol transporter operating at the plasma membrane.
THE IMPACT OF DYING CELLS ON THE INNATE IMMUNE SYSTEM
Massive numbers of cells die in vertebrates all the time, and were we to generate immune responses to these, we would face the severe consequences of autoimmunity.8 But organisms must also be able to tell whether the cell death is a consequence of infection, so that an appropriate response can be rallied.
The innate immune response induced by dying cells involves Toll-like receptors (TLRs) and NOD-like receptors (NLRs), the pattern-recognition receptors (PRRs) discussed in Green (2022b). When these are engaged by pathogen-associated molecular patterns (PAMPs) or DAMPs, they generate signals that include inflammasome activation (see Green 2022b) as well as a variety of other effects. The latter include the production of cytokines and chemokines, molecules that control the differentiation and functions of immune and inflammatory cells.
Cells that die by necrosis release a number of DAMPs that trigger inflammatory responses. These DAMPs include uric acid, ATP, and another DAMP that induces the production of cytokines but not inflammasome activation, high-mobility group protein B1 (HMGB1). HMGB1 interacts with RAGE (“receptor for advanced glycation end products”) and, perhaps, one of the Toll-like receptors (TLRs), TLR4, on phagocytes to trigger such responses.9
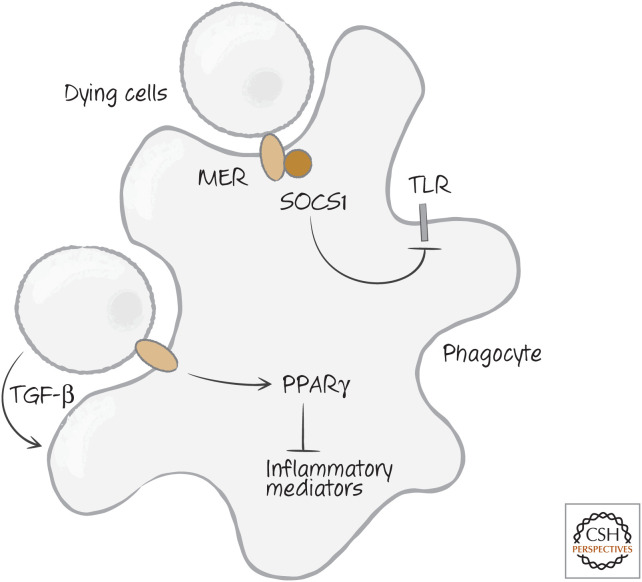
In contrast, apoptotic cells seem to have a calming effect on these responses. Not only do they not have a chance to release their DAMPs before engulfment, in some settings they also actively suppress inflammatory responses. There seem to be different ways in which they do this. Some apoptotic cells release the cytokine transforming growth factor β (TGF-β), which inhibits many immune responses. Alternatively, some phagocytic cells (macrophages and dendritic cells) produce TGF-β following interaction with apoptotic cells.
Another way apoptotic cells inhibit inflammatory responses may be through the activation of PPARγ (discussed above). PPARγ inhibits the production of several inflammatory mediators, including leukotrienes and prostaglandins. Yet a third mechanism involves Mer, the receptor for Gas6. Mer also interacts with the type I interferon receptor, and this activates transcription of an intracellular inhibitory protein, suppressor of cytokine signaling 1 (SOCS1), which can interfere with signaling by TLRs. Figure 20 shows the different ways in which apoptotic cells can inhibit inflammation.
Figure 20.
Apoptotic cells can inhibit inflammation. Mer, tyrosine-protein kinase Mer; PPAR γ, peroxisome proliferator-activated receptor γ; SOCS1, suppressor of cytokine signaling 1; TGF-β, transforming growth factor beta; TLR, Toll-like receptor.
By such means, the extensive apoptosis that occurs during tissue turnover does not evoke inflammatory responses. In contrast, what does do so is necrotic cell death, which occurs in response to damage or pathological conditions, and the inflammatory response is rallied to deal with any infectious agent that might as a consequence gain access to the body.
What happens with other forms of cell death? As we know, activation of the inflammasome and caspase-1 can lead to death in the cells that are stimulated, and this death is accompanied by the production of cytokines (interleukin-1 and interleukin-18) that can stimulate inflammatory responses. It is generally assumed that such cell death does not inhibit innate immune responses.
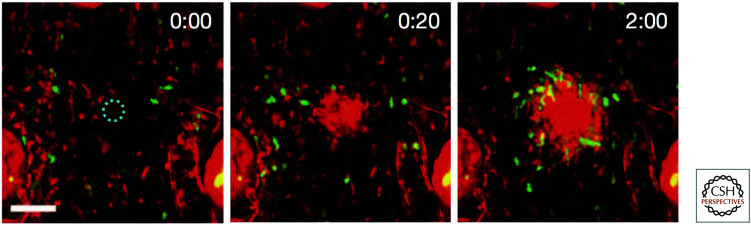
A dramatic demonstration of the effect of cell death on the innate immune system can be seen when a cell is damaged by infection or injury and dies. Very quickly, neutrophils swarm to the site of the damaged cell (Fig. 21), and this depends on the production of a lipid signaling molecule, leukotriene-B4, produced as the cell dies (how this occurs is not clear). These cells function to seal the wound, and in time macrophages enter the area to clear the debris.
Figure 21.
Neutrophils swarm to sites of cell damage. A single cell was damaged by laser light (dashed circle). Within seconds, the neutrophils (red) swarm to the site, followed by macrophages (green). The elapsed time is shown (minutes:seconds).
(Reprinted from Lämmermann et al. 2013, Fig. 1B.)
Cells undergoing necroptosis not only release DAMPs to induce inflammation, but also produce inflammatory cytokines (as a consequence of RIPK1 signaling). Finally, cells that undergo autophagic cell death are cleared from the body, but how these affect innate immune responses is not known.
CONSEQUENCES OF DYING CELLS FOR THE ADAPTIVE IMMUNE RESPONSE
When a phagocytic cell such as a macrophage or dendritic cell engulfs a particle, proteins associated with the particle are chopped into peptides and “presented” by the major histocompatibility complex (MHC) molecules on the cell surface. If a patrolling T lymphocyte recognizes the peptide, it can proliferate and generate an immune response, become refractory to stimulation, or actively inhibit immune responses (a state called immune tolerance). How it responds depends on other molecules on the presenting cell as well as the cytokines in the immediate environment.
PAMPs and DAMPs serve as maturation signals to dendritic cells, the “professionals” that present peptides to T cells and determine the T-cell response. Therefore, when cells that die by necrosis are eaten by dendritic cells, their proteins are processed to give peptides that are presented to T cells. Any peptides that are novel—for example, from an infectious organism that might have caused the necrosis—will be recognized, the T cells will proliferate, and an immune response will ensue.
Cells undergoing necroptosis are especially good at priming T-cell responses. This turns out to be dependent on the activation of NF-κB by RIPK1 during necroptosis. NF-κB induces a variety of cytokines in the dying cell that help to promote the T-cell response.
Apoptotic cells can similarly generate immune responses, but they can also actively inhibit them. The differences between immunogenic (the former) and tolerogenic (the latter) forms of apoptosis are not entirely clear. As with necrotic cells, the proteins associated with apoptotic cells are processed to give peptides that are presented on the surface of dendritic cells. Whether an immune response to any novel peptide occurs depends on several factors. One such factor is the presence of calreticulin on the dying cell. Some agents that induce apoptosis promote the exposure of calreticulin, whereas others do not. The reasons for this are not known, but the exposure of calreticulin appears to correspond with immunogenic cell death.
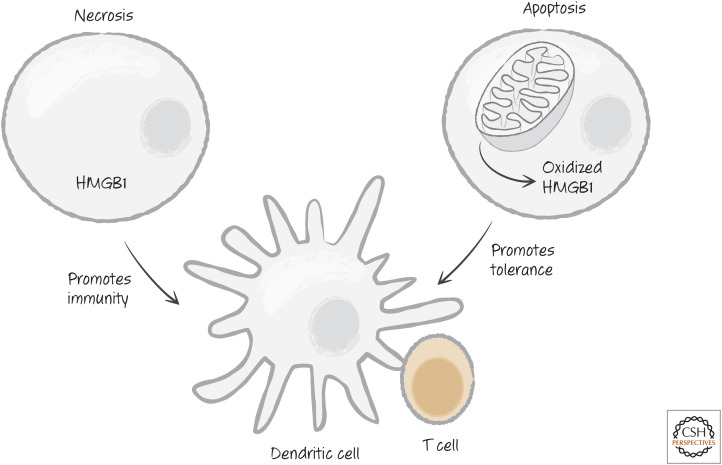
Another factor is the DAMP high-mobility group protein HMGB1. HMGB1 is expressed in all cells, and its release promotes immunity. However, this effect of HMGB1 can be blocked by oxidation (and perhaps other modifications) of the protein (Fig. 22). During apoptosis, a caspase-dependent burst of ROS can prevent the immunogenic effects, so that a tolerogenic response dominates. When they die, cells lacking HMGB1 tend to promote tolerance rather than an immune response, whether by apoptosis or necrosis (Fig. 22). In contrast, apoptotic cells in which the oxidation of HMGB1 is disrupted (e.g., by mutation of the caspase substrate NDUFS1 [see Green 2022c], which is responsible for the reactive oxygen burst) tend to promote immune responses rather than tolerance.
Figure 22.
The high-mobility group protein B1 (HMGB1) in immunity versus tolerance. Apoptotic cells, unlike necrotic cells, end up oxidizing HMGB1, preventing it from promoting an immune response.
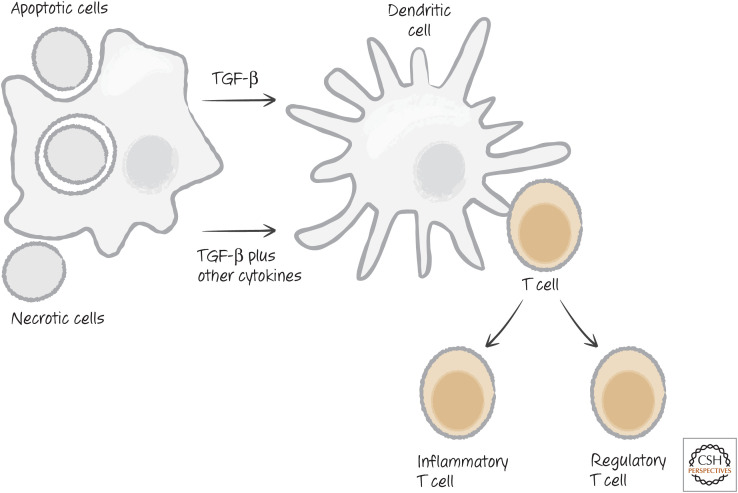
Cytokines also influence adaptive immune responses. As mentioned above, TGF-β is an inhibitory cytokine produced by some dying cells and by cells that engulf them. TGF-β induces T cells that encounter appropriate peptide antigens to differentiate into regulatory T cells (Treg), which actively inhibit immune responses. However, other cytokines that can be induced by PAMPs can skew T-cell differentiation toward a type of T cell that promotes, rather than inhibits, inflammatory responses (Fig. 23).
Figure 23.
Dying cells influence T-cell functional differentiation. TGF-β, transforming growth factor beta.
LC3-ASSOCIATED PHAGOCYTOSIS AND CLEARANCE
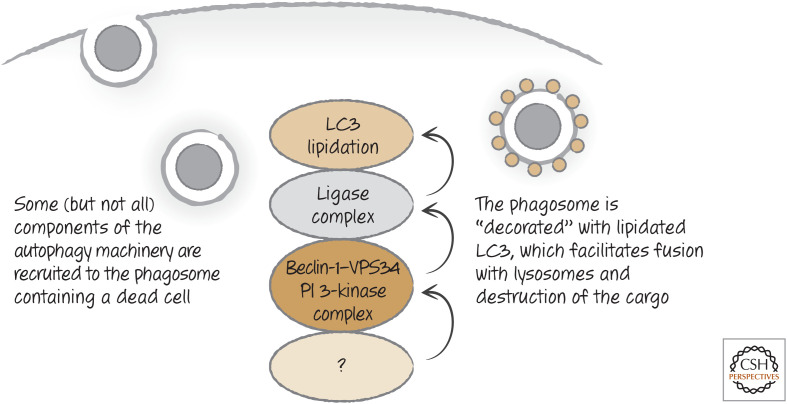
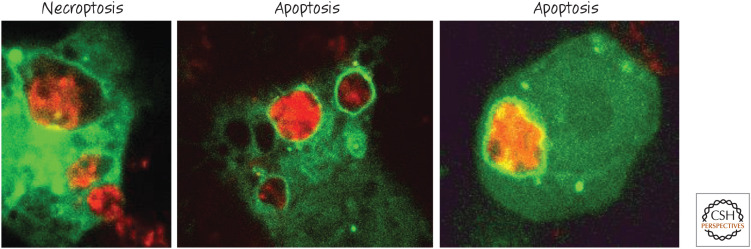
As we discussed, autophagy is a process that forms double-membrane structures (autophagosomes) that trap cytoplasm and deliver it to the lysosomes for digestion (see Green 2022a). When a dying cell is engulfed, some (but not all) components of the autophagy machinery are recruited to the membrane of the corpse-containing phagosome, where they decorate it with LC3 (on the single membrane of the phagosome). This process, called LC3-associated phagocytosis (LAP), is distinct from canonical autophagy,10 as some molecules required for autophagy are not required for LAP, and vice versa. For example, LAP proceeds without a requirement for the ULK1 serine kinase complex, and the elements of the VPS34 phosphoinositide 3-kinase complex are distinct from those of autophagy (Figs. 24 and 25). These differences are useful in discriminating roles for LAP versus canonical autophagy in the clearance of dying cells.
Figure 24.
LC3-associated phagocytosis occurs on engulfment of dying cells. As the phagosome forms, an unknown signal is generated that recruits a complex containing beclin-1 and the phosphoinositide 3-kinase VPS34, and other molecules distinct from those involved in autophagy. As a result, the ligase complex is recruited and LC3 is conjugated to lipids in the phagosome membrane. This facilitates fusion with lysosomes and the degradation of the corpse.
Figure 25.
LC3-associated phagocytosis occurs on engulfment of dying cells. Macrophages expressing an LC3 fused to green-fluorescent protein (GFP) were cocultured with cells (stained red) that were dying by means of different mechanisms. Note the ring of green LC3 around the corpse-containing phagosomes. (Provided by Jennifer Martinez and Clifford Guy, Department of Immunology, St. Jude Children's Research Hospital.)
Many of the events we have described so far in the clearance of apoptotic cells appear to depend on LAP. Apoptotic cells engulfed by macrophages that are defective for LAP are not rapidly degraded, do not induce the expression of the cholesterol efflux machinery, and do not induce the production of inhibitory cytokines. Instead, such engulfment induces the expression of a variety of inflammatory cytokines, such as those that are produced on engulfment of necrotic cells. How, exactly, the process of LAP promotes these responses to apoptotic cells is not known.
CLEARANCE AND DISEASE
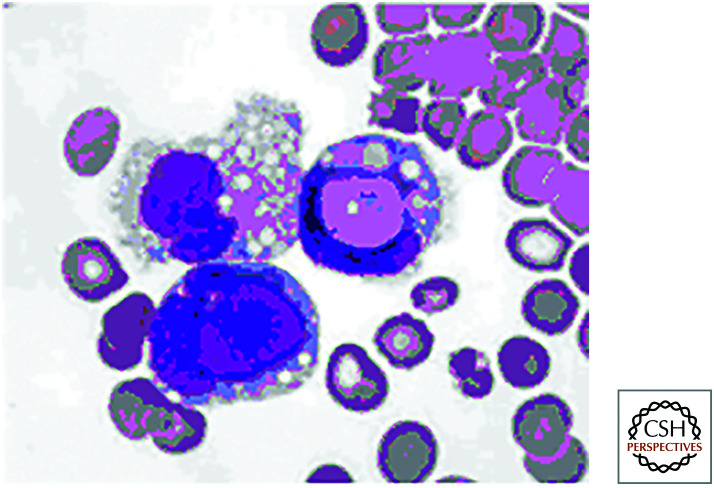
Do all these complex decisions really matter? The short answer is yes. Mice with defects in apoptotic cell clearance, such as those lacking MER, MFG-E8, or C1q, not only accumulate extra cell corpses but also show a form of autoimmunity resembling human systemic lupus erythematosus (SLE), a disease in which the immune system produces antibodies against DNA and other nuclear components. This effect is also seen in animals lacking LAP. In people with SLE, effective clearance of apoptotic cells frequently appears to be compromised. A diagnostic feature of SLE is the presence of so-called LE bodies in the circulation (see Fig. 26). These turn out to be free apoptotic corpses or corpses engulfed by macrophages (normally, clearance is sufficiently robust that such features are not seen in the circulation of healthy individuals). A feature of defective LAP is the persistence of the corpse in the phagosome. Intriguingly, polymorphisms in a gene involved in both canonical autophagy and LAP (ATG5) is associated with SLE in several human populations. This raises the interesting possibility that defects in LAP might be responsible for human SLE in some cases.
Figure 26.
LE bodies. These are apoptotic cells in the circulation and are diagnostic of systemic lupus erythematosus (SLE). (Reprinted from Holman 1951, with permission from BMJ Publishing Group Ltd.)
The inhibition of immune responses by apoptotic cells could have therapeutic value. A routine procedure in tissue transplantation is to infuse cells from the donor individual into the recipient. It is likely that these cells undergo apoptosis and they might dampen immune rejection of the graft.
Another therapeutic benefit involves the use of apoptotic cells to treat autoimmune diseases. In animal models, apoptotic cells coupled to proteins that are targets of the immune cells causing such diseases have proven effective in preventing or ameliorating autoimmunity in animals. Alternatively, it is possible to mimic the signals generated by apoptotic cells; drugs that activate a nuclear receptor downstream from PPARγ (see above) cure SLE in mice that have defective apoptosis. We will have to see whether these approaches work in humans.
Finally, when we treat cancers, we often use therapies designed to trigger apoptosis in the tumor cells. It is possible that, when such apoptosis is immunogenic, it contributes to eradication of the tumor.
ENTOSIS: EATEN TO DEATH11
Epithelial cells that lose contact with the basement membrane can undergo a process whereby they engulf each other, with the engulfed cell dying. In some developmental events and some cancers (discussed in Green 2022d,e, respectively), entosis is observed as the appearance of “cell-in-cell” structures, sometimes to such an extent that the engulfing cell is itself eaten, leading to a “Russian doll” situation—cell-in-cell-in-cell.
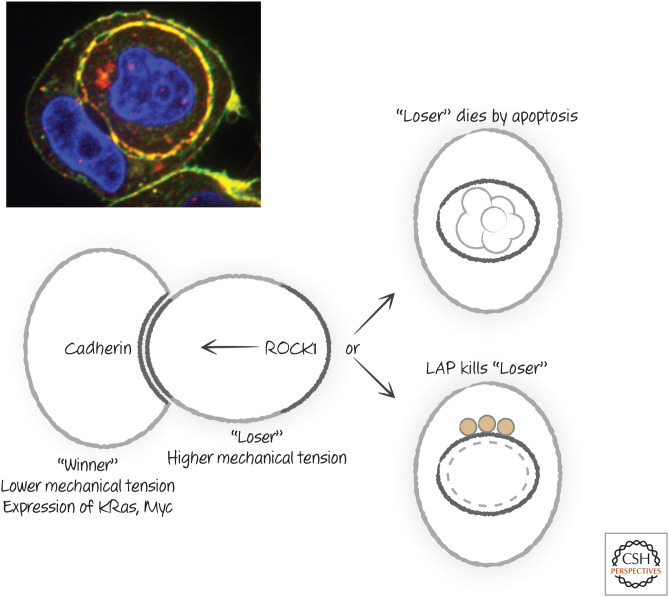
Unlike the engulfment of dying cells, entosis does not involve the usual machinery of eat-me signals and their receptors. Instead, the detached cells stick to each other by the interaction of adhesion molecules (cadherins), and the “losing” cell actively moves into the “winning” cell by actin rearrangement driven by ROCK1 (mentioned in Green 2022c in another context) (Fig. 27).
Figure 27.
Pathways and molecules of cell death by entosis. LAP, LC3-associated phagocytosis. (Michael Overholter, unpublished.)
Once eaten, the “loser” cell dies by a combination of processes. Because it is deprived of its normal growth factors, it can undergo apoptosis. Meanwhile, the “winning” cell engages LAP (see above) to promote the fusion of the cell-containing phagosome with lysosomes, ensuring the destruction of the “loser.” If cells are engineered to both prevent apoptosis (e.g., by expression of anti-apoptotic BCL-2 proteins) and to avoid LAP (e.g., by deletion of molecules required for LAP), the engulfed cell survives and can escape, to either be engulfed again or do the engulfing. Cells that are normally dependent on attachment can grow in an anchorage-independent manner if both apoptosis and LAP are prevented or if entosis is prevented (e.g., by a ROCK1 inhibitor).
Several factors determine which cell “wins” or “loses” during entosis. Cells that have a lower mechanical tension (i.e., they are more “deformable”) tend to engulf cells that have higher mechanical tension. Oncogenes, such as the GTPase K-Ras and Myc also promote a “winner” status, and indeed cells expressing these can engulf and kill normal cells that they recognize as “losers.” These observations have potentially important repercussions in situations of cellular competition, including cancer (Green 2022e).
COMPENSATORY PROLIFERATION
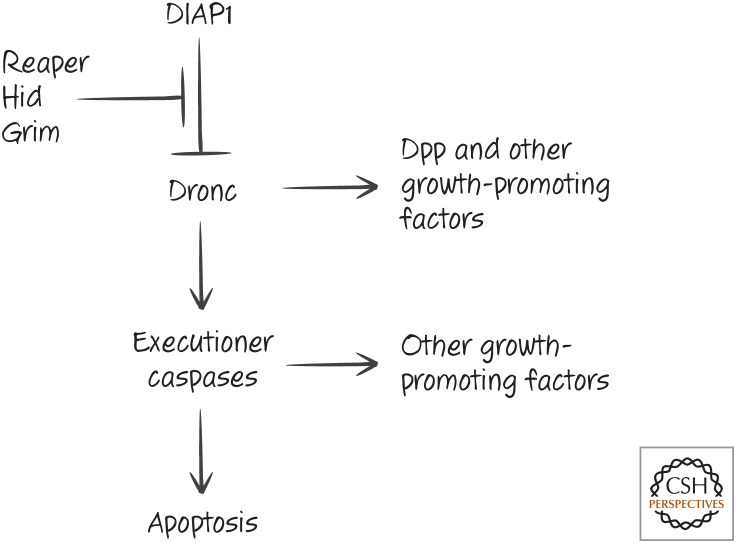
When cells die, we might expect this to generate a signal to make more of that cell type. Such compensatory proliferation has been verified in insects. Cells that undergo apoptosis in Drosophila produce factors that induce proliferation of surrounding cells. Interestingly, one of these factors, Decapentaplegic (Dpp), is a member of the TGF-β family (Fig. 28). This and the other factors12 responsible for compensatory proliferation are produced in a manner that depends on the activation of caspases, but the precise mechanisms of Dpp release are not known.
Figure 28.
Caspase activation promotes compensatory proliferation in the fruit fly Drosophila. DIAP1, death-associated inhibitor of apoptosis 1; Dpp, Decapentaplegic.
A different mechanism occurs during wound healing, but also involves cell death. When a wound occurs in Drosophila, macrophages are attracted to the wound by hydrogen peroxide, produced at the site, and these promote healing. It turns out that this process requires that the macrophage had at one time engulfed apoptotic cells, leaving a “memory” of the event that primes the macrophage for this subsequent function. Macrophages from animals that are defective for apoptosis do not migrate to wounds, and this failure can be “corrected” by exposing them to apoptotic cells.
In vertebrates, apoptosis induces compensatory proliferation of adult stem cells in several tissues. Although TGF-β is sometimes produced by dying mammalian cells, it does not appear to be important for this effect. Instead, caspase-mediated activation of phospholipase A2 (see Fig. 1) leads to the generation of prostaglandin E2 (PGE2), which is responsible for the effect. Although caspases are required for production of this mediator from dying cells, necrotic cells can induce PGE2 production by macrophages that engulf them,13 and therefore it is likely that necrotic cells, too, can induce compensatory proliferation of stem cells.
Compensatory proliferation in response to cell death can also occur less directly. Living cells rely on growth and survival factors, as shown in Green (2022f). It transpires that our cells do not only rely on these but, instead, compete for them; cells often take up and actively degrade such factors. When a cell dies, there are more of such factors available for the living cells, and these can then promote proliferation until the normal number of cells is achieved.
This process can be readily shown by removing cells from some tissues, such as surgical resection of part of the liver, or by introducing small numbers of normal lymphocytes into an animal in which they are lacking. In either case, rapid proliferation of the healthy cells occurs until normal tissue levels are attained.
Because dying cells are not directly responsible for the proliferation of healthy cells, this effect is not generally called “compensatory” but is, instead, often referred to as “homeostatic proliferation.” As in Green (2022e), even this indirect effect of cell death can have consequences for disease.
Footnotes
From the recent volume Cell Death: Apoptosis and Other Means to an End by Douglas R. Green
Additional Perspectives on Cell Death available at www.cshperspectives.org
A quick note on the use of our term “engulfment” is warranted. In general, when a cell eats a particle of any kind, this is referred to as phagocytosis (“cellular eating”). There are two main types of phagocytosis: opsonization and macropinocytosis. In opsonization, the phagocyte binds to the particle and effectively “zips” the phagocyte's membrane around it, stripping away anything loosely associated with it and excluding surrounding fluids. In contrast, macropinocytosis involves “gulping” the particle together with surrounding fluids and molecules. In the case of removal of cell corpses, both processes have been described (and have different consequences), leading to some confusion in the literature. For this reason, many who study the process prefer the term “engulfment” as a way to sidestep the issue. As we will see, there are several ways in which dying cells are removed by phagocytes. A general term for engulfment of dying cells is “efferocytosis.”
Readers unfamiliar with the reference are urged to listen to The Who's classic rock opera Tommy, while reading this review. Others who have not heard it for a long while might wish to do the same.
The bewildering variety of molecules and mechanisms for binding to dying cells can be taken as an indication of the importance of the process. It is also the consequence of an emerging field of research, in which the importance of particular interactions is not fully understood. If the reader prefers, it is enough for now to let the message be “there are lots of bridging molecules” that can decorate dying cells.
Another receptor for phosphatidylserine, PSR1, has also been described. However, subsequent studies have since shown that this is a nuclear factor and not directly involved in recognition of phosphatidylserine. It is mentioned here to help readers avoid unnecessary confusion when perusing the literature in this area.
How ABC1 and ABC7 might function in either phosphatidylserine externalization or engulfment is not known, and therefore these were not included in the mammalian bind-me scheme discussed above.
Intriguingly, this kinase is a homolog of the human kinase Syk, which is involved in the process of phagocytosis triggered by Fc receptors (the receptors on macrophages and other cells that bind antibodies). It is not known whether Syk participates in the uptake of dead cells in mammals.
This model is not quite complete. Our discussion assumes some understanding of GTPases and how they work; if this is obscure to the reader, it will not greatly matter for general understanding.
To fully appreciate the impact of dying cells on the immune response, the reader will require a much more complete understanding of the immune system than can be provided here. Therefore, our overview is necessarily cursory.
TLR4 is activated by bacterial lipopolysaccharides. Because these frequently contaminate proteins, there is always a concern that ligands that are identified for TLR4 are actually artifacts because of such contamination by the lipopolysaccharides. Therefore, the ability of HMGB1 to directly stimulate TLR4 is controversial.
LAP is induced not only by the uptake of dying cells, but also by engulfed particles engaging some TLRs or, if antibody is present, by the binding of receptors for the antibody (FcRs). LAP plays a variety of roles beyond those of clearance of dead cells, but it is only the latter that concerns us here.
As entosis is an alternative form of cell death in some cases, it might have been considered in Green (2022a). However, given the role of LAP in the process, as well as apoptosis, we have chosen to discuss it here. Nevertheless, it is certainly a pathway of cell death.
The other pathways signal proliferation through Wingless (Wg; mammalian WNT is the homolog) and Hedgehog (Hh). Curiously, Dpp and Wg are produced in a manner that depends on the initiator caspase Dronc, but apparently not executioner caspases, whereas Hh signaling is dependent on the latter.
However, as we noted, uptake of apoptotic cells seems to prevent production of prostaglandins. It is possible that, in addition to limiting inflammation, apoptosis in mammals tends to limit compensatory proliferation, whereas necrosis promotes it.
ADDITIONAL READING Engulfment of Dying Cells
Elliott MR, Ravichandran KS. 2016. The dynamics of apoptotic cell clearance. Dev Cell 38: 147–160.
Find-Me Signals
Medina CB, Ravichandran KS. 2016. Do not let death do us part: 'Find-me' signals in communication between dying cells and the phagocytes. Cell Death Differ 23: 979–989.
Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, et al. 2003. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113: 717–730.
Identification of lysophosphatidylcholine as a “find-me” signal and one way in which it is produced following caspase activation.
Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. 2009. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286.
Identification of ATP as a “find-me” signal.
Eat-Me Signals and Engulfment
Nagata S, Suzuki J, Segawa K, Fujii T. 2016. Exposure of phosphatidylserine on the cell surface. Cell Death Differ 23: 952–961.
Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, Green DR. 1995. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by overexpression of Bcl-2 and Abl. J Exp Med 182: 1545–1556.
An early paper describing the use of annexin V to detect apoptosis.
Wu YC, Horvitz HR. 1998. The C. elegans cell corpse engulfment gene ced-7 encodes a protein similar to ABC transporters. Cell 93: 951–960.
The original characterization of CED7.
Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, Earp HS, Matsushima GK. 2001. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411: 207–211.
Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. 2002. Identification of a factor that links apoptotic cells to phagocytes. Nature 417: 182–187.
The identification of MFG-E8 as a bridge molecule recognizing phosphatidylserine and its role in engulfment of dying cells.
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S. 2007. Identification of Tim4 as a phosphatidylserine receptor. Nature 450: 435–439.
Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, Klibanov AL, Mandell JW, Ravichandran KS. 2007. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/DOCK180/RAC module. Nature 450: 430–434.
Franc NC, Heitzler P, Ezekowitz RA, White K. 1999. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284: 1991–1994.
Reddien PW, Horvitz HR. 2004. The engulfment process of programmed cell death in Caenorhabditis elegans. Annu Rev Cell Dev Biol 20: 193–221.
A detailed review of the engulfment process in nematodes.
Park SY, Kang KB, Thapa N, Kim SY, Lee SJ, Kim IS. 2008. Requirement of adaptor protein GULP during stabilin-2-mediated cell corpse engulfment. J Biol Chem 283: 10593–10600.
Waste Management
Kiss RS, Elliott MR, Ma Z, Marcel YL, Ravichandran KS. 2006. Apoptotic cells induce a phosphatidylserine-dependent homeostatic response from phagocytes. Curr Biol 16: 2252–2258.
A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, et al. 2009. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31: 245–258.
In addition to illustrating the role of LXR in clearance of dying cells, this paper shows that activation of LXR may suppress disease consequences that arise due to defective apoptosis.
Dying Cells and Immunity
Elliott MR, Koster KM, Murphy PS. 2017. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J Immunol 198: 1387–1394.
Fond AM, Ravichandran KS. 2016. Clearance of dying cells by phagocytes: mechanisms and implications for disease pathogenesis. Adv Exp Med Biol 930: 25–49.
Rock KL, Latz E, Ontiveros F, Kono H. 2010. The sterile inflammatory response. Annu Rev Immunol 28: 321–342.
Green DR, Ferguson T, Zitvogel L, Kroemer G. 2009. Immunogenic and tolerogenic cell death. Nat Rev Immunol 9: 353–363.
A survey of the effects of dying cells on the adaptive immune system.
Gaipl US, Munoz LE, Grossmayer G, Lauber K, Franz S, Sarter K, Voll RE, Winkler T, Kuhn A, Kalden J, et al. 2007. Clearance deficiency and systemic lupus erythematosus (SLE). J Autoimmun 28: 114–121.
An overview of the possible role of engulfment defects in autoimmune disease.
Matzinger P. 1994. Tolerance, danger, and the extended family. Annu Rev Immunol 12: 991–1045.
The original review that triggered the idea that dying cells influence the adaptive immune response.
Martin SJ. 2016. Cell death and inflammation: the case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J 283: 2599–2615.
A perspective on how cell death engages the adaptive immune response.
Entosis
Krishna S, Overholtzer M. 2016. Mechanisms and consequences of entosis. Cell Mol Life Sci 73: 2379–2386.
Overholtzer M, Mailleux AA, Mouneimne G, Normand G, Schnitt SJ, King RW, Cibas ES, Brugge JS. 2007. A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell 131: 966–979.
The original paper describing entosis.
LC3-Associated Phagocytosis
Heckmann BL, Boada-Romero E, Cunha LD, Magne J, Green DR. 2017. LC3-associated phagocytosis and inflammation. J Mol Biol 429: 3561–3576.
Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. 2011. Microtubule-associated protein 1 light chain 3 α (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc Natl Acad Sci 108: 17396–17401.
The first paper showing LAP in the clearance of dying cells.
Compensatory Proliferation
Fan Y, Bergmann A. 2008. Apoptosis-induced compensatory proliferation. The cell is dead. Long live the cell! Trends Cell Biol 18: 467–473.
Ryoo HD, Gorenc T, Steller H. 2004. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7: 491–501.
Two pathways involved in compensatory proliferation in flies.
Weavers H, Evans IR, Martin P, Wood W. 2016. Corpse engulfment generates a molecular memory that primes the macrophage inflammatory response. Cell 165: 1658–1671.
A role for engulfment of apoptotic cells in wound healing in flies.
Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li CY. 2010. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Sci Signal 3: ra13.
A possible mechanism of compensatory proliferation in response to apoptosis in mammals.
Laplante P, Brillant-Marquis F, Brissette MJ, Joannette-Pilon B, Cayrol R, Kokta V, Cailhier JF. 2017. MFG-E8 reprogramming of macrophages promotes wound healing by increased bFGF production and fibroblast functions. J Invest Dermatol 137: 2005–2013.
Another mechanism linking clearance of dying cells to wound repair in mammals.
FIGURE CREDITS
Brumatti G, Sheridan C, Martin SJ. 2008. Expression and purification of recombinant annexin V for the detection of membrane alterations on apoptotic cells. Methods 44:235–240. doi:10.1016/j.ymeth.2007.11.010
Franc NC, Heitzler P, Ezekowitz RA, White K. 1999. Requirement for croquemort in phagocytosis of apoptotic cells in Drosophila. Science 284: 1991–1994. doi:10.1126/science.284.5422.1991
Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. 2004. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304: 1147–1150. doi:10.1126/science.1094359
Holman S. 1951. The lupus erythematosus cell inclusion phenomenon. J Clin Pathol 4: 290–295. doi:10.1136/jcp.4.3.290
Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 98: 371–375. doi:10.1038/nature12175
REFERENCES
*Reference is also in this collection.
- *.Green DR. 2022a. Nonapoptotic cell death pathways. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022b. Inflammasomes and other caspase-activation platforms. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022c. Caspases and their substrates. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022d. Cell death in development. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Green DR. 2022e. Cell death and cancer. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041103 [DOI] [Google Scholar]
- *.Green DR. 2022f. The mitochondrial pathway of apoptosis, Part II: the BCL-2 protein family. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a041046 [DOI] [PMC free article] [PubMed] [Google Scholar]