Abstract
Ketosynthases (KSs) catalyze carbon-carbon bond forming reactions in fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs utilize a two-step ping pong kinetic mechanism to carry out an overall decarboxylative thio-Claisen condensation that can be separated into the transacylation and condensation reactions. In both steps, an acyl carrier protein (ACP) delivers thioester tethered substrates to the active sites of KSs. Therefore, protein-protein interactions (PPIs) and KS-mediated substrate recognition events are required for catalysis. Recently, crystal structures of Escherichia coli elongating type II FAS KSs, FabF and FabB, in complex with E. coli ACP, AcpP, revealed distinct conformational states of two active site KS loops. These loops were proposed to operate via a gating mechanism to coordinate substrate recognition and delivery followed by catalysis. Here we interrogate this proposed gating mechanism by solving two additional high-resolution structures of substrate engaged AcpP-FabF complexes, one of which provides the missing AcpP-FabF gate-closed conformation. Clearly defined interactions of one of these active site loops with AcpP are present in both the open and closed conformations, suggesting AcpP binding triggers or stabilizes gating transitions, further implicating PPIs in carrier protein-dependent catalysis. We functionally demonstrate the importance of gating in the overall KS condensation reaction and provide experimental evidence for its role in the transacylation reaction. Furthermore, we evaluate the catalytic importance of these loops using alanine scanning mutagenesis and also investigate chimeric FabF constructs carrying elements found in type I PKS KS domains. These findings broaden our understanding of the KS mechanism which advances future engineering efforts in both FASs and evolutionarily related PKSs.
Keywords: Ketosynthase, Fatty Acid Synthase, Claisen Condensation Reaction, Fatty Acid Biosynthesis, Acyl Carrier Protein, Protein-Protein Interactions, Polyketide Biosynthesis
Graphical Abstract
Introduction
Fatty acid biosynthesis (FAB) is an essential primary metabolic pathway conducive to metabolic engineering.1–10 Fatty acid synthases (FAS) carry out biosynthesis by iteratively condensing and reducing malonyl-CoA derived 2-carbon units.1,2 FAS can be organized as either large, single gene, multidomain megasynthases, type I, or as discrete, multiple gene, monofunctional enzymes, type II.1,3,11–13 Despite differences in structural organization, the biochemistry of type I and II FAS is generally conserved (Figures S1–S2).2 Additionally, type II FASs are considered to be the evolutionary progenitors of polyketide synthases (PKSs), a class of enzymes known to produce a variety of structurally complex and biologically active compounds (Figure S1).14–18 Ketosynthases (KSs) initiate each round of FAS by condensing a growing acyl-chain with 2-carbon units via a decarboxylative Claisen condensation with the 3-carbon malonyl-CoA substrate to produce a β-keto intermediate. Subsequent reactions catalyzed by the ketoreductase (KR), dehydratase (DH), and enoylreductase (ER) fully reduce the β-carbon before another round of chain extension or offloading of the mature fatty acid (FA). Central to this process is the small 9 kDa acyl carrier protein (ACP). The ACP carries thioester-tethered pathway intermediates to each enzyme active site using a post-translationally installed 4’-phosphopantetheine arm (PPant).19,20 ACPs must form transient, productive protein-protein interactions (PPIs) with each respective partner enzyme (PE) in order to deliver their substrates in catalytic competent forms.21–30
KS-mediated carbon-carbon bond formation is the primary driving force of FAB. These enzymes use a two-step kinetic ping-pong bi-bi mechanism that can be viewed mechanistically as transacylation and condensation reactions (Figure 1A). 30–33 In the transacylation step, acyl-ACP binds to the KS and transfers its thioester-bound cargo to the KS active site cysteine residue, producing an acyl-KS thiointermediate.34–36 In the condensation step, malonyl-ACP associates with the KS and undergoes decarboxylation to produce a keto-enolate tautomerization, wherein the carbanion tautomer condenses with the thioester-bound FA to produce the β-ketoacyl-ACP product (Figure S2). Mechanistic elements of the transacylation and condensation steps are still contested, and a detailed molecular understanding for KS substrate specificity is therefore lacking.31–33 FA chain extension is energetically coupled to decarboxylation, making the forward reaction thermodynamically favored and essentially irreversible upon loss of CO2. Therefore, KSs play a central role in controlling metabolic flux and FA chain length.4,31 This point has been well demonstrated by recent successes in rationally retooling the product profile of the fungal FAS.6,37–39 Future engineering efforts, in both FAS and PKS platforms, will benefit from a more in depth understanding of the reaction mechanisms and substrate specificities of functionally diverse KSs.40,41
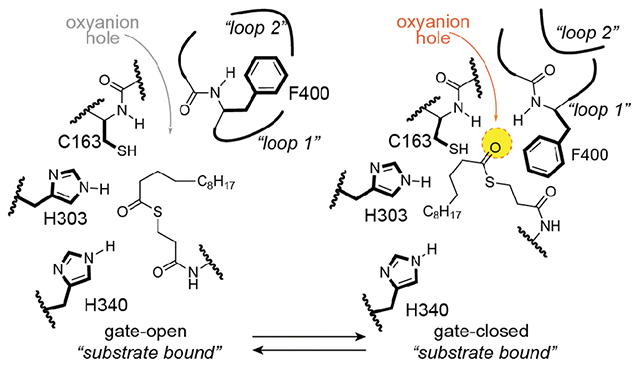
Figure 1: KS ping-pong reaction and gating mechanism overview.

A) Ketosynthase ping-pong mechanism with active site schematic showing oxyanion hole (yellow circle) stabilized tetrahedral intermediates that are formed during the transacylation and condensation half-reactions. B) Proposed ketosynthase gating mechanism coordinated by loops 1 and 2 and the Phe400 gating residue. Each active site represents a step forward during the transacylation half-reaction starting with the apo-KS active site and ending with the acyl-KS intermediate. (TWO COLUMN)
Escherichia coli type II FAS has long served as a model system for understanding the structure, function, and mechanism of FASs.1,42,43 E. coli possesses two related elongating KSs, FabB and FabF, that have broad overlapping substrate specificities and are representative of specific KS families, KASI and KASII, respectively.44,45 The active sites of elongating KSs are comprised of a Cys-His-His catalytic triad, where the cysteine, positioned at the N-terminus of a long α-helix, is the active site nucleophile and the two histidines participate in the decarboxylation step. The oxyanion hole formed by the backbone amides of the catalytic cysteine (Cys163) and a highly conserved phenylalanine gating residue (Phe400) stabilize oxyanion tetrahedral intermediates formed during both half-reactions (Figure 1A).34–36 Additionally, Phe400 is known to regulate access to the active site by sensing the acylation state of the catalytic cysteine.32,34–36
In our previous work, we determined crystal structures of FabB and FabF crosslinked to E. coli ACP (AcpP) loaded with either C12 or C16-α-bromo-pantetheinamide substrate mimetics.24 These structures, along with biochemical and computational studies, led to the proposal of a double drawbridge-like gating mechanism46 comprised of two active site loops, loop 1 and loop 2, that undergo conformational rearrangements to regulate KS substrate recognition and processing (Figure 1B). During substrate delivery, the gate opens to allow access to the KS active site, and in doing so, disrupts the oxyanion hole (Figure 1B). The active site machinery only reforms upon gate closure, thereby providing a means to regulate selectivity by organizing the oxyanion hole for acyl transfer when the correct substrate is bound. Loop 1 consists of a highly conserved GFGG motif, which includes the conserved phenylalanine gating residue, Phe400. Loop 2 is distal to the active site and abuts loop 1 in the gate-closed conformation. A conserved loop 2 aspartate residue, Asp265, stabilizes the gate-open state by coordinating a complex hydrogen-bonding interaction network with loop 1 (Figure 2A). Interestingly, much of the loop 2 motif appears to be only conserved within, but not between, different KS families (Figure 2B). In contrast, loop 1 shares similarities across other KS families, but the putative loop 1 gating residue notably diverges in cis-AT modular type I PKSs (TIPKS).47–49 Instead of the conserved GFGG motif, TIPKSs often possess GVSG or GISG motifs (Figure 2B). TIPKS KSs utilize the same Cys-His-His catalytic triad and likely the same, or similar, catalytic mechanism as type I and type II FAS (Figure S2B). However, they are capable of accepting a variety of α-branched and β-branched acyl-substrates and often use bulkier α-branched extender units than FAS KSs, such as methylmalonyl-CoA and ethylmalonyl-CoA.48,50 Therefore, the putative gating elements in PKSs may have diverged to facilitate catalysis with more structurally diverse substrates than FAS KSs.
Figure 2: Gating loop interaction network and conservation.

A) Schematic for loop 1–loop 2 interactions in the closed (apo-FabF) and open (C16-AcpP=FabF) states. Interactions between specific residues are shown by arrows. FabF loop 2 sequence is above the loop 2 peptide schematic and the FabF loop 1 sequence is below the schematic. B) Sequence alignment between FabF gating loops with the putative gating elements found in different classes of condensing enzymes. The loop 1 gating residue (Phe400) is outlined in a red box and the interaction between Asn404 and the conserved Asp265 is indicated by the connecting line segment. Loop 2 sequences from within 3 different classes of condensing enzymes (FabF, FabB, and modular type I PKS) are shown in separate alignments highlighting the conservation within, but not between, families of condensing enzymes. All residues above an identity threshold of 70% are highlighted. (TWO COLUMN)
Currently, the structure of the AcpP-bound gate-open conformation is available for FabF (PDB: 6OKG), while the structure of the AcpP-bound gate-closed conformation is available for FabB (PDB: 6OKC), making it difficult to directly compare the open and closed conformations in the same condensing enzyme family. Additionally, catalytic evidence that the proposed gating mechanism does indeed affect substrate turnover, and more specifically, the transacylation step, has not yet been demonstrated. In the work reported herein, we use mechanistic crosslinkers carrying different substrate mimetics to elucidate x-ray crystal structures of two additional acyl-AcpP=FabF (where the ‘=‘ denotes a covalent crosslink) complexes, one of which is in the missing gate-closed conformation. Next, we employ two HPLC-based kinetic assays to validate the importance of the KS gating machinery for the overall KS condensation reaction and transacylation half-reaction. We then explore the role of loop 2 using alanine scanning mutagenesis and demonstrate its conformational relevance to enzyme catalysis, providing additional evidence for an allosteric connection between AcpP binding and gating events. Finally, we characterize a panel of FabF-TIPKS KS loop 1 chimeras, where FabF’s GFGG motif is replaced by corresponding GVSG and GISG motifs found in the cis-AT modular TIPKS KS domains. Results from our study provide the missing AcpP-FabF gate-closed conformation structure as well as structural and biochemical support for the importance of gating in type II FAS KSs. These findings have broad implications for KS-directed metabolic engineering in FAS and provide additional insights into how these putative gating elements may operate in the evolutionarily related PKS condensing enzyme families.
Results and Discussion
Development and analysis of a C16:1-α-bromo-pantetheinamide crosslinking probe
In order to compare the open and closed states within a single family of condensing enzymes, we aimed to crystallize an acyl-AcpP=FabF complex in a catalytically relevant, gate-closed conformation. We first developed a pantetheinamide mechanistic crosslinker that we reasoned could trap the AcpP-FabF complex in a closed conformation. E. coli is the canonical paradigm for bacterial FAS and the two elongating KSs, FabF and FabB, perform different branchpoint reactions in FAS important to cell physiology. FabB, in concert with the dehydratase FabA, is responsible for elongation of cis-3-decenoyl-AcpP (cis-3-C10:1-AcpP) to cis-5-dodecenoyl-AcpP (cis-5-C12:1-AcpP) to establish the unsaturated fatty acid (UFA) branch of FAS.51–53 FabF is not able to perform this FabB specific extension in vivo, but previous studies regarding FabF’s substrate specificity show that, unlike FabB, FabF is responsible for elongation of cis-palmitoeloyl-ACP (C16:1) to cis-vaccenoyl-ACP (C18:1).44,54 While cis-vaccenate is not essential for E. coli survival, this FabF product is responsible for E. coli’s rapid homeoviscous adaptive response to regulate membrane fluidity in response to reductions in temperature.44,45,54–57 Therefore, we developed a synthetic route for a C16:1-α-bromo-pantetheinamide unsaturated crosslinking probe (Figure 3), designed to mimic FabF’s privileged substrate, palmitoleoyl-ACP (Figure S3). As with other crosslinkers developed to trap ACP-PE interactions21,23,24,25,57–64, we replaced the labile thioester in the native substrate with an amide linkage, which is necessary to trap crosslinked ACP-PE complexes. However, recent NMR data from our group suggests that the identity of the linkage affects the overall structure of AcpP in solution.66 These differences are mostly due to altered sequestration67–69 of non-natively tethered substrates and likely minimally affect conformation and structure in crosslinked complexes.
Figure 3: Crosslinking probes.

Comparison of acyl-AcpP with crosslinker-loaded crypto-AcpPs utilized in this study to trap and crystalize AcpP=FabF complexes. An acyl-ACP substrate is shown to demonstrate the chemical structure of the natural substrate. The two crosslinking pantetheinamide probes have an amide instead of a thioester linking their acyl substrates as well as either an α-bromo or chloroacrylamide warhead. The region that has been modified to facilitate stable crosslinkers is shown in red while the substrate mimetics are outlined in light green. (ONE COLUMN)
Using a one-pot chemoenzymatic method,59 we loaded the C16:1-α-bromopantetheinamide crosslinking probe onto apo-AcpP to produce C16:1αBr-crypto-AcpP (C16:1-AcpP) (Figure 3, Figure S4). We then tested the crosslinking efficiency and rate of C16:1-AcpP with wt FabF using a time course gel-based crosslinking assay. Similar to previously tested α-bromo crosslinkers, C16:1-AcpP crosslinks with FabF to completion in under ten minutes across a range of pHs (Figure S5). Synthesis of different chain length, unsaturated crosslinkers may aid in elucidating structures of ACP-partner enzyme complexes with additional metabolic enzymes, such as E. coli FabB51,53,70 or the FatA and FatB FAS thioestersases71–73 from plant plastids, to elucidate their respective substrate specificities and recognition mechanisms.
C16:1-AcpP=FabF captures distinct substrate conformation
Cross-seeding using C16-AcpP=FabF24 crystals nucleated diffraction-quality crystals of C16:1-AcpP=FabF for x-ray data collection, structure determination, and refinement to a nominal resolution of 2.0 Å (Table S1, Figure S6). The complex crystallized in the same space group and unit cell as that of C16-AcpP=FabF (PDB: 6OKG), with an asymmetric unit containing a FabF monomer crosslinked to a single AcpP. Structural alignment with C16-AcpP=FabF (PDB: 6OKG) shows that these two complexes are nearly identical and overlay with an RMSD of 0.124 Å. The gating loops in the C16:1AcpP=FabF complex are in the open conformation, indicating that the gate-open conformation is favored by FabF in the presence of a crosslinked AcpP bearing long-chain α-bromo crosslinkers. (Figure 4A–C, Figure S7) This is not the case for the previously published C12 and C16-AcpP-FabB structures, which instead mimic the AcpP-bound transacylation reaction intermediate.24 In these complexes, the carbonyl of the FA substrate analog is positioned in the oxyanion hole and the gating loops are found in the catalytically competent gate-closed conformation in a manner similar to the C12-FabF (PDB: 2GFY) and C12-FabB (PDB: 1EK4) acyl-enzyme intermediate complexes. Therefore, as in the C16-AcpP=FabF structure (PDB: 6OKG), the gating loops of C16:1-AcpP=FabF would need to return to the closed conformation to appropriately position the C16:1 FA substrate for transfer to the catalytic cysteine residue.
Figure 4: C16:1- and C8-AcpP=FabF active site organization.

A) 2D schematic of C16:1-AcpP=FabF active site. The oxyanion hole is not formed and Phe400 is rotated away from the active site. B) 3D rendering of C16:1-AcpP=FabF active site and crosslinker. C) Acyl-binding pocket of C16:1-AcpP=FabF compared to that of C12-FabF (PDB: 2GFY). D) 2D schematic of C8-AcpP=FabF active site. The oxyanion hole is formed and the gating loops are in the closed conformation. E) 3D rendering of C8-AcpP=FabF active site and crosslinker. F) Acyl-binding pocket of C8-AcpP=FabF compared to C12-FabF (PDB: 2GFY). In panels C and F structural alignments were performed over all atoms. (TWO COLUMN)_
Figure 5: C8-AcpP=FabF mimics a condensation reaction intermediate.

Superposition of C8Cl-AcpP=FabF and the C12-FabF acyl-enzyme intermediate (PDB: 2GFY) performed over all atoms, demonstrating that C8-AcpP=FabF mimics a condensation reaction intermediate between malonyl-AcpP and a hexanoate-bound FabF. The carbonyl of the C8Cl crosslinker is coordinated to the two catalytic histidines (dashed lines). The overlaid C12-FabF structure shows that the oxyanion hole (yellow circle) would be occupied by the carbonyl of the bound acyl substrate. Enolate attack on the bound FA would result in a tetrahedral intermediate stabilized by the oxyanion hole. A 2D schematic of the putative condensation half-reaction tetrahedral intermediate is provided for comparison. (ONE COLUMN)
Closer analysis of the crosslinker indicates that the stereocenter of the α-carbon at the thioether crosslink is in the R configuration, while that of the previously solved structures were assigned to the S configuration (Figure 4A, Figure S8). In the C16-AcpP=FabF complex, the C16 acyl chain extends away from the active site, whereas in C16:1-AcpP=FabF, the cis-double bond of the unsaturated probe is in a kinked conformation that redirects the polymethylene chain toward the acyl-binding pocket (Figure 4B–C, Figure S7). The terminal methyl moiety of the acyl chain is positioned in front of the back gate, comprised of residues Ile108 and Phe202, of the acyl-binding pocket (Figure 4C).35,74 When compared to the C12-bound acyl-enzyme intermediate structure of FabF (C12-FabF, PDB: 2GFY), the Ile108-Phe202 gate is in a closed conformation (Figure 4C). Despite the closed back gate, the tail of the kinked alkyl substrate is positioned at the entrance of the acyl binding pocket. This may indicate that the Ile108-Phe202 back gate of FabF, which is replaced by Gly107-Phe201 in FabB, plays a role in substrate-intermediate processing. Previous studies have shown that altering the identity of this gating residue results in antibiotic resistance75,76 and changes in KS chain length specificity in both bacterial and fungal FAS systems.37,76
The kinked C16:1 substrate favors a more compact conformation, which may be more readily accommodated and processed compared to the saturated C16 substrate. These observations, along with differences in the acyl-binding pocket and divergent loop 2 sequence found in the FabF condensing enzyme family (Figure 2B), may explain FabF’s substrate preference for C16:1.44,45 However, some FabF and FabB orthologs from related bacteria that do not fit within the canonical E. coli FAS framework. For example, Enterococcus faecalis, Lactococcus lactis, and Clostridium acetobutylicum each contain only a single elongating FAS KS, which is from the FabF family, that is able to recover unsaturated fatty acid auxotrophic growth defects in E. coli ΔfabB strains.77–79 Additionally, the FabB from Shewanella oneidensis is capable of elongating C16:1 to C18:1 and its two endogenous fabF genes are also capable of complementing ΔfabB growth defects.80,81 Currently, no structures are available for these FabF orthologs, making it difficult to infer conclusions based on sequence alone. However, the primary sequence of the two S. oneidensis FabFs (NCBI Accession: WP_011074037 and WP_011074034), which are capable of complementing E. coli ΔfabB strains, notably diverge from that of FabF for loop 1 (AFGG vs GFGG), loop 2, and the gating residues regulating entrance to the fatty acid binding pocket. Instead of the canonical Ile108-Phe202 back gate, S. oneidensis FabF possesses a Thr108-Phe202 back gate at the equivalent position based on primary sequence analysis. Future structural, computational, and mutagenic work probing the Ile108-Phe202 back gate and/or loops 1 and 2 may provide insights into FabF’s cryptic role in homeoviscous adaptation and its divergent function in other bacterial species.
C8-AcpP=FabF structure provides an ACP-bound gate-closed conformation
pP loaded with the trans-C8-chloroacrylate PPant probe, C8Cl-crypto-AcpP (C8-AcpP) (Figure 3), requires several hours to crosslink to FabF, but when loaded with a α-bromo crosslinkers or the smaller C3-chloroacrylate crosslinker that lacks an appended substrate analog, AcpP crosslinks in minutes.24 This significant difference in crosslinking rates, and the propensity for α-bromo crosslinkers to trap FabF in the gate-open conformation, led us to hypothesize that crosslinking to trans-C8-chloroacrylate may require a transition to the gate-closed conformation to appropriately position the warhead for attack by the catalytic cysteine residue. Therefore, we obtained and collected data on diffraction quality crystals of C8Cl-crypto-AcpP=FabF (C8-AcpP=FabF), which upon structure elucidation and refinement, resulted in a 2.65-Å-resolution structure. The asymmetric unit of C8-AcpP=FabF contains the FabF dimer crosslinked to two AcpPs. Analysis of the active sites shows reliable electron density for the PPant arm and the thiovinyl covalent crosslink between C3 and Cys163 (Figure S9). The gating loops are positioned in the catalytically competent gate-closed conformation and an organized, but unoccupied, oxyanion hole is formed by the backbone amides of Cys163 and Phe400 (Figure 4D,E). The PPant moiety of the probe is anchored by polar contacts with Thr270, Ser271, Thr305, and Thr307. The carbonyl oxygen of the substrate mimetic coordinates to His303 and His340, with the latter histidine serving an essential role for the condensation reaction.32,33 Similar probe-KS interactions were observed in the crystal structures of FabB-AcpP23,24 and IgaKS-IgaACP62, indicating an evolutionarily conserved PPant binding site.
The acylated C12-FabF structure (PDB: 2GFY) overlays with the KS domain from C8-AcpP=FabF with an RMSD of 0.314 Å (Figure 4F). The active sites of these complexes are in nearly identical conformations, including the gating loops, with the Phe400 gating residue rotated and translated away from Cys163 to form the presumptive malonyl-AcpP binding pocket.34,35,82 Interestingly, the chlorovinyl probe has the same carbon count as the putative condensation intermediate. The carbonyl group of the FA substrate mimetic interacts with His303 and His340, positioning the C2 carbon in close proximity to the Cys163-bound thioester carbonyl carbon (Figure 5). With the gating loops closed, the oxyanion hole is organized, which, as seen when overlaid with C12-FabF, would be occupied by the carbonyl oxygen of the bound FA. Even with the altered Cys163 position in C8-AcpP=FabF compared to C12-FabF, angle measurements in the overlay place the C2 carbon within 30 degrees of the Burgi-Dunitz angle (ca. 105°) (Figure 5). Given that the observed probe behavior matches the current knowledge of the condensation mechanism, we propose that the C8 chlorovinyl probe mimics a condensation reaction intermediate between malonyl-AcpP and a FabF-bound hexanoic acid. When taken in consideration with our recently published structures and the previously solved structures of KS acyl-enzyme intermediates, C8-AcpP=FabF provides an additional snapshot into KS-mediated catalysis (Figure S10). However, future structural studies using stable malonyl-ACP or malonyl-CoA83 mimetics would likely enhance our understanding of the decarboxylation reaction mechanism.
C8Cl-AcpP=FabF delineates a unique Loop 2 interaction network
The position of loop 2 in the gate-closed conformation suggests that movement of loop 2 is required to allow loop 1 to access the gate-open conformation. The interaction network that stabilizes the loop 2 closed conformation (apo-FabF PDB: 2GFW) is mediated by a Ser271(O)C-His268(H)N main-chain hydrogen bond, a Thr270-His268 side-chain interaction, and a Pro273-Tyr267 C–H π interaction (Figure 2A, Figure 6). Transition to the open conformation (C16-AcpP=FabF PDB: 6OKG) yields a distinct loop 2 interaction network, and interestingly, Thr270 swaps its interaction with His268 for Asp35 of the bound AcpP (Figure 2A, Figure 6).
Figure 6: Loop 2 interaction networks:

The apo-FabF, C16-AcpP-FabF, and C8-AcpP=FabF loop 2 interaction networks are distinct. Interactions between acyl-AcpP and loop 2 are present in the C8-AcpP=FabF (substrate bound, gate-closed) and C16-AcpP=FabF (substrate bound, gate-open)
Analysis of the C8Cl-AcpP=FabF crosslinked complex reveals a loop 2 that, while in a similar conformation as seen in apo-FabF and C12-FabF, nonetheless forms a distinct interaction network. The Pro273-Tyr267 C–H π interaction is maintained, but His268 instead interacts with Asp35 of AcpP and Thr270 and Ser271 form hydrogen-bonding interactions with the hydroxyl group of the PPant arm and the phosphoserine oxygen, respectively (Figure 6). Interestingly, similar loop 2 interaction networks are observed in the recently solved type II PKS ACP=KS-CLF complexes.62,84 Collectively, these findings suggest that unique AcpP-loop 2 interactions are present in both the closed and open conformations, providing additional evidence for an allosteric role in substrate binding and cargo delivery during KS gating events.
The gating mechanism is important for the FabF condensation reaction
To confirm that FabF gating events are important for catalysis, we subjected the panel of FabF gating mutants used in our previous study24 to an HPLC-based kinetic assay.28 These mutants were designed to test gate function by blocking access to the gate open conformation (pocket-block), destabilizing the gate open conformation (destabilization), or limiting the flexibility of loop 1 (flex-reduction) (Figures S11–S14). Briefly, the assay monitors the rate of holo-AcpP formation in a reaction utilizing acyl-AcpP substrates as acyl-donors in the transacylation half-reaction while taking advantage of FabF’s relaxed specificity for malonyl-CoA (extender unit) for the condensation half-reaction (Figures S15, S16). Given that proper gate function would be required for substrate turnover, especially during the transacylation step, these assay parameters should reliably test gate function. Additionally, previous studies have utilized similar assay formats that take advantage of the relaxed specificity of KSs towards CoA-based substrates28,36,85 or N-acetylcysteamine thioester substrates.86–89 Using this assay format, we monitored the overall FabF condensation reaction for all previously tested gating mutants as well as an additional destabilization mutant, N404A, using both C6-AcpP and C12-AcpP substrates.
Using C6 or C12-AcpP, the apparent turnover rates of wt FabF in the tested conditions are 1.57 min−1 and 1.41 min−1, respectively, which are comparable to previously reported values28 (Table 1). Similar to a previous study by Zhang et al., the F400A gate-removal mutant is 1-2% as active as wt FabF with both tested substrates.32 We then evaluated the activity for the two pocket-block mutants, G310M and G310F, where mutation of glycine to a bulky hydrophobic residue fills the pocket occupied by Phe400 when in the open conformation (Figure S12). These mutants demonstrated reduced condensation rates for both C6 and C12-AcpP substrates with an overall activity roughly 50-fold lower than that of wt FabF.
Table 1:
Quantification of condensation and transacylation rates of FabF mutants
| C6-AcpP | C12-AcpP | Transacylation | ||||
|---|---|---|---|---|---|---|
| FabF | Rate min−1 | % rel. to wt | Rate min−1 | % rel. to wt | Rate min−1 | % rel. to wt |
| wt | 1.569 ± 0.104 | 100.00 | 1.414 ± 0.083 | 100.00 | 3.686 ± 0.200 | 100.00 |
| F400A | 0.024 ± 0.006 | 1.56 | 0.026 ± 0.004 | 1.85 | 0.165 ± 0.010 | 4.48 |
| G310M | 0.027 ± 0.002 | 1.72 | 0.026 ± 0.005 | 1.83 | 0.014 ± 0.026 | 3.73 |
| G310F | 0.041 ± 0.003 | 2.59 | 0.029 ± 0.002 | 2.08 | - | - |
| D265A | 0.072 ± 0.009 | 4.61 | 0.059 ± 0.008 | 4.18 | 0.018 ± 0.005 | 0.49 |
| D265N | 0.216 ± 0.008 | 13.76 | 0.091 ± 0.003 | 6.41 | 0.009 ± 0.003 | 0.24 |
| N404A | 0.049 ± 0.025 | 3.14 | 0.025 ± 0.002 | 1.74 | 0.049 ± 0.011 | 1.32 |
| G399A | 1.284 ± 0.098 | 81.83 | 1.622 ± 0.046 | 114.75 | 1.643 ± 0.006 | 44.58 |
| G402A | 0.301 ± 0.046 | 19.18 | 0.201 ± 0.013 | 14.18 | 0.039 ± 0.033 | 10.65 |
| H268A | 0.566 ± 0.039 | 36.10 | 0.475 ± 0.013 | 33.62 | 0.379 ± 0.014 | 10.29 |
| M269A | 1.771 ± 0.028 | 112.90 | 1.897 ± 0.047 | 134.18 | - | - |
| T270A | 0.209 ± 0.012 | 13.30 | 0.150 ± 0.013 | 10.60 | 0.021 ± 0.005 | 0.58 |
| S271A | 1.085 ± 0.012 | 69.15 | 1.153 ± 0.059 | 81.57 | - | - |
| Y267A/P273A | 1.240 ± 0.048 | 79.01 | 1.139 ± 0.074 | 80.53 | - | - |
| Loop Swap | 0.010 ± 0.001 | 0.64 | 0.008 ± 0.001 | 0.59 | 0.003*± 0.001 | 0.08 |
Estimated rate from total product formation at 50 min.
- Indicates that the assay was not performed.
The destabilization class of mutants was designed to disrupt the hydrogen-bonding network coordinated by Asp265, a highly conserved loop 2 residue that interacts with the backbone amides of loop 1 (in the gate-open conformation) and the side chain of Asn404 (Figure 2A, Figure S13.). The D265A mutant is 4.6% (0.07 min−1) and 4.8% (0.06 min−1) as active as wild-type with C6-AcpP and C12-AcpP, respectively. Interestingly, the more conservative D265N mutation is 13.8% (0.22 min−1) as active as wild-type with C6-AcpP but only 6.4% (0.09 min−1) as active as wild-type with C12-AcpP. To further validate the importance of this interaction network, we mutated the conserved Asn404 residue (Figure 2) to alanine and evaluated its activity with both substrates. As seen with D265A and D265N, the N404A mutant is a poor catalyst with rates 3.1% (0.05 min−1) and 1.7% (0.03 min−1) that of wt FabF with C6-AcpP and C12-AcpP, respectively.
The two flex-reduction mutants, G399A and G402A, were designed to inhibit gate function by limiting the conformational space available to the loop 1 GFGG motif (Figure S14). The G399A mutant is as fast as wt FabF, while rates for the G402A mutant are 19.2% (0.301 min−1) and 11.0% (0.201 min−1) as active with C6 and C12-AcpP, respectively. These results are in line with our previous study as only G402A showed a reduction in crosslinking activity while G399A was as active as wt FabF. Taken together, our results indicate that all FabF gating mutants, with the exception of G399A, significantly decrease FabF catalyzed condensation rates.
Transacylation rates of FabF gating mutants
Results from our assays demonstrate that FabF gating mutants affect the overall condensation reaction. We next attempted to verify that the FabF gating mechanism is important for the transacylation half-reaction. To address this question, we modified our HPLC assay to monitor the transfer of the C12 FA from lauroyl-CoA to holo-ACP, effectively decoupling the transacylation and condensation reactions. Our proposed gating-mechanism posits that gating is required for acyl substrates to access the fatty acid binding pocket and catalytic cysteine. Therefore, transfer of lauric acid from lauroyl-CoA to holo-AcpP would also require similar active site transformations. Furthermore, acyl-CoA to holo-ACP transferase assays have been used previously to assess the role of FabF active site residues on the transacylation step32 and to evaluate substrate specificity and cooperativity in the murine FAS36. Using this assay design, we were able to reliably determine transacylation rates for wt FabF and FabF gating mutants by monitoring the formation of C12-AcpP (Table 1, Figure S15–S17).
Type I and II FAS KSs both possess the conserved Phe400 gating residue and only accept acyl-ACP substrates without β-carbon modifications (i.e. β-hydroxy-acyl-ACPs). The Phe400 gating residue was recently proposed to serve as a β-carbon sensor in the murine type I FAS KS domain.36 This model suggests that Phe400 is responsible for ensuring partially processed FAS intermediates are not accepted. However, previous work on type II FAS KS domains suggests that Phe400 minimally affects transacylation and primarily regulates the condensation step by rotating away from the catalytic cysteine to form the malonyl-ACP binding pocket after acylation of the catalytic cysteine.32,34,35,74,90 Despite these previous reports, we determined a transacylation rate 4.5% that of wt for the F400A gate-deletion mutant (Table 1). Therefore, our results demonstrate the importance Phe400 for the transacylation half-reaction in addition to its well-established function in the condensation step.
The transacylation rate of the pocket block mutant, G310M, shows a similar reduction in transacylation activity as F400A, demonstrating that either blocking gate function or deleting the central gating residue decreases acyl-transfer rates. Flex reduction mutants, G399A and G402A, show a 2 and 10-fold drop in transacylation activity, respectively, suggesting that G399A may have a minor role during the transacylation step despite not reducing the overall condensation reaction rate in the condensation assay. Interestingly, the destabilization mutants, D265A and D265N, which are part of loop 2 and distal to the KS active site, have the largest decrease in activity with rates 200 and 300-fold lower than wt FabF, respectively (Table 1). The additional destabilization mutant, N404A, also showed a strong reduction in transacylation rate, further indicating that disruption of this interaction network is detrimental to function. It is interesting that the rates of the destabilization class of mutants are more strongly reduced in the transacylation reaction than in the overall condensation reaction. However, differences in assay format and the use of non-native substrates affect absolute rates; therefore, direct comparisons between the condensation and transacylation rates should be avoided. Regardless, the proposed function of these residues is congruent with the reported results for both assays, indicating that the complex interaction network mediated by Asp265 is critical for gate function and the transacylation half-reaction.
Loops 1 and 2 were previously proposed to move in a coordinated manner to facilitate substrate processing during the transacylation half-reaction.24 Results from our condensation reaction assay demonstrate that blocking gate function significantly reduces overall KS-mediated substrate turnover. However, an argument could be made that these mutations alter malonyl-CoA binding or positioning of the catalytic histidines, thereby only disrupting the condensation half-reaction. Additionally, analysis is further complicated as Phe400 of loop 1 coordinates the oxyanion hole that stabilizes chemically distinct tetrahedral intermediates for both half-reactions, and as discussed above, previous work has shown the importance of Phe400 for the condensation half-reaction. By implementing an assay that eliminates the condensation half-reaction, we have demonstrated that the observed reductions in the overall KS condensation reaction for FabF gating mutants are correlated to reductions in transacylation rates, thus demonstrating the importance of gating events for the transacylation half-reaction.
Importance of loop 2 residues for gate function
To quantitatively measure the importance of loop 2 for gate function, we subjected it to alanine scanning mutagenesis and tested all resulting soluble protein constructs using our condensation and transacylation assays (Figure S11). In addition, we tested a FabF/FabB loop swap variant, where the loop 2 of FabB was swapped out for FabF’s loop 2. The Y267A, P272A, and P273A mutants were insoluble, but we determined rates for alanine mutants of all remaining loop 2 residues as well as a Y267A/P273A double mutant. The overall KS condensation assay results indicate that only the conserved His268 and Thr270 (Figure 2B) are important for FabF activity. The H268A variant has a 3-fold reduction in activity, while the T270A mutation results in a 10-fold reduction. All other constructs are as active as wt FabF with the exception of the FabF/FabB loop swap variant, which has greater than a 150-fold drop in activity for both substrates (Table 1).
To determine if these mutations have any effect on the transacylation half-reaction, we monitored transacylation rates for H268A, T270A, and the FabF/FabB loop swap variants. The H268A variant has a transacylation rate 10.3% that of wt FabF while the T270A variant has a rate 0.6 % that of wild-type. Interestingly, the transacylation rate for the FabF/FabB loop swap variant is too slow to be reliably measured (Table 1).
Results from the KS condensation and transacylation assays demonstrate that the conserved His268 and Thr270 residues, both of which interact with the bound acyl-AcpP in the closed and open states, respectively (Figure 6), are important for FabF activity. Mutation of these residues to alanine decreases KS transacylation rates, indicating that they likely play a role in gate function and that acyl-AcpP binding might participate in triggering or stabilizing gate transitions via loop 2 interactions, as suggested by previous molecular dynamics (MD) simulations.24 Interestingly, replacing FabF’s loop 2 with that of FabB results in an enzyme variant that is nearly inactive, providing additional evidence that loops 1 and 2 function in a coordinated manner, and may not be readily transferrable between different KS families. Therefore, additional elements, which may include ACP-KS PPIs, likely coevolve with loop 2 to fine-tune gate function. A dual loop gating mechanism suggests a dynamic and complex catalytic process, which is potentially coupled to ACP binding, chain-flipping of acyl cargo into the KS active site, and KS-substrate recognition. Therefore, future studies analyzing the putative gating loops in FabB, type I FAS, and PKS KS domains will be essential to conclusively address these latter hypotheses.
Evaluating Type II FAS-TIPKS loop 1 chimeras
Unlike FAS KSs, TIPKS KS domains readily accept α and β-modified acyl-ACP substrates as well as a variety of α-branched extender units (Figure S1–S2).17,91 Comparison between KS domains from TIPKS and type II FAS indicates that TIPKS KSs possess less bulky GISG and GVSG loop 1 motifs (Figure 2B) that could provide additional space to accommodate β-carbon modifications or α-branched extender units. To evaluate this hypothesis, we modelled PKS-like acyl substrates into C12-FabF (PDB: 2GFY) or PKS-like extender units (2S-methylmalonyl-AcpP) into C8-AcpP=FabF. In both instances, potential clashes are identified with the native Phe400 FAS gating residue. However, mutating this gating residue into a PKS gating residue, such as isoleucine, could provide additional space for these PKS-like substrates (Figure S18). Therefore, the identity of the loop 1 gating residue may be tuned to enforce substrate specificity during the transacylation step by acting as a β-carbon sensor,36 but it may also exert specificity during the condensation step by providing additional space for bulkier α-branched extender units.
To determine how TIPKS gating elements would alter FabF activity and substrate specificity, we generated chimeric FabFs containing loop 1 elements from TIPKS, where the Phe400 gating residue was replaced with either isoleucine or valine. Additionally, we prepared two complete FabF-TIPKS KS loop 1 chimeras by mutating FabF’s GFGG motif to either GVSG (FabF-GVSG) or GISG (FabF-GISG). We first evaluated the overall condensation rates of our panel of loop 1 chimeras in assays containing either 0.25 mΜ or 1.0 mΜ malonyl-CoA (Table 2). The condensation rates for all mutants are significantly reduced compared to wt FabF for both concentrations of malonyl-CoA. The F400V and F400I mutations turn over substrate with rates 4.4% and 5.5% that of wt FabF, respectively, at 0.25 mΜ malonyl-CoA and 4.6% and 6.9% that of wt FabF, respectively, at 1.0 mΜ malonyl-CoA. These mutants are roughly 3 and 10-fold more active than the gate deletion mutant, F400A, at 0.25 mΜ or 1.0 mΜ malonyl-CoA, respectively, indicating that reintroducing a gating residue recovers some activity. Condensation rates for the FabF-GVSG and FabF-GISG constructs are more significantly reduced with activities 1-2% that of wt FabF for both tested concentrations of malonyl-CoA.
Table 2:
Quantification of condensation and transacylation rates of FabF gating mutants with malonyl-CoA (mCoA) or methylmalonyl-CoA (mmCoA) substrates.
| 0.25 mM mCoA | 1 mM mCoA | 1 mM mmCoA | Transacylation | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| FabF | Rate min−1 | % rel. wt | Rate min−1 | % rel. wt | Rate min−1 | % rel. wt | Rate min−1 | % rel. wt |
| wt | 1.569 ± .104 | 100.00 | 5.011 ± 0.142 | 100.00 | 0.281 ± 0.056 | 100.00 | 3.686 ± 0.200 | 100.00 |
| F400A | 0.024 ± .006 | 1.56 | 0.027 ± 0.002 | 0.54 | 0.020 ± 0.009 | 7.14 | 0.165 ± 0.010 | 4.48 |
| F400I | 0.069 ± .003 | 4.44 | 0.232 ± 0.033 | 4.63 | 0.040 ± 0.002 | 14.33 | 0.371 ± 0.018 | 10.06 |
| F400V | 0.087 ± .013 | 5.53 | 0.346 ± 0.103 | 6.90 | 0.070 ± 0.034 | 25.03 | 0.400 ± 0.084 | 10.87 |
| GISG | 0.037 ± .009 | 2.34 | 0.084 ± 0.018 | 1.67 | 0.037 ± 0.007 | 13.09 | 0.016 ± 0.001 | 0.44 |
| GVSG | 0.023 ± .004 | 1.50 | 0.045 ± 0.005 | 0.90 | 0.012* ± 0.011 | 4.27 | 0.009 ± 0.002 | 0.25 |
Estimated rate from total product formation at 40 min
To ascertain if the measured reductions in activity were related to extender unity identity, we tested our FabF loop 1 chimeras with assays containing 1 mM methylmalonyl-CoA. We measured an apparent turnover number of 0.28 min−1 for wt FabF, an 18-fold reduction compared to assays with 1 mM malonyl-CoA (Table 2). Extending our analysis to the chimeric FabF constructs, we again found the condensation rates for all variants are decreased compared to wt FabF. Interestingly, the relative-to-wt reduction in activity for these mutants is less pronounced than in assays using malonyl-CoA; however, this may be due to the decreased activity of wt FabF with methylmalonyl-CoA rather than any gain of function by the FabF loop 1 chimeras. The F400V and F400I variants are 14% and 25% as active as wt FabF, respectively, and the GISG mutant is 13% as active. We were unable to reliably detect activity for the GVSG mutant in the tested conditions.
The overall reaction rates of FabF gating chimeras are slower than wt FabF with both malonyl-CoA and methylmalonyl-CoA, but we cannot identify which half-reaction is more impacted by these mutations. To determine if these mutations would have any effect on the transacylation step, we evaluated the transacylation rates of our panel of FabF loop 1 chimeras (Table 2). The F400V and F400I variants have transacylation rates 10% that of wt FabF, again demonstrating that Phe400 is important for the transacylation half-reaction. Although, it is worth noting that the F400V and F400I constructs have transacylation rates 2.5-fold higher than F400A. The transacylation rates of the FabF-GVSG and FabF-GISG variants are found to be more significantly reduced, with activities 0.3% and 0.4% that of wt FabF, respectively. The more than 200-fold drop in transacylation activity for FabF-GVSG and FabF-GISG chimeras is likely correlated with their low activities in condensation assays with either malonyl or methylmalonyl-CoA.
In summary, replacement of Phe400 with either valine or isoleucine results in an enzyme more active than the F400A gate-removal mutant, but still 20-fold less active than wild-type with malonyl-CoA substrates. Full replacement of FabF’s loop 1 with similar sequences to those found in TIPKS KS domains results in even larger reductions in activity. These results may indicate that loop 1 elements from other KS families are not readily compatible with the gating machinery of FabF. Indeed, the FabF loop 2 is divergent from that of TIPKS KSs and may not be tuned to coordinate gating transitions with GVSG and GISG loop 1 motifs (Figure 2B). This analysis is in agreement with our results from the FabF/FabB loop swap variant, suggesting any mutations to loop 1 may need to be evaluated in relationship to loop 2. Additionally, other residues in and near the acyl-binding pocket of the KS might provide favorable contacts to coordinate and position substrate for proper gate closure and transfer. These residues are hydrophobic in FabF but are often replaced by more polar residues in type I and type II PKS KS domains. Finally, due to the pleiotropic effects of modifying KS gating elements, it seems difficult to determine how swapping loop 1 residues between different KS families, or even just the Phe400 gating residue, alters extender unit substrate specificity in the condensation step. We have so far found no instances of a TIPKS KS domain utilizing a phenylanine gating residue, which instead is most often valine, isoleucine, leucine, or methionine. Additionally, there does not appear to be a direct relationship between the extender unit loaded by the TIPKS AT domain and loop 1 gating elements in the KS domain. The absolute conservation of Phe400 in FAS, and its general absence in TIPKS, may be linked to FASs role as a conserved, primary metabolic pathway that must efficiently and specifically synthesize FAs for cellular needs. PKSs, on the other hand, are part of specialized metabolism and have different evolutionary restrictions, suggesting that the gating elements of TIPKSs may have evolved to be more permissive to allow processing of diverse substrates in both the transacylation and condensation step. Duplications of these modules could then ensure at least some level of activity with the complex substrates processed in these assembly lines before evolutionary enhancement and fine tuning of turnover rates. Additional studies investigating the putative gating elements in related KS domains will be essential to addressing these unanswered questions.
Conclusions
To better understand the KS reaction mechanism, we sought to further characterize our recently proposed KS gating mechanism with additional structural and catalytic investigations. The data reported herein are congruent with a model wherein acyl-ACP binding and substrate transfer is mediated by the correlated movement of two KS active site loops, loops 1 and 2. We provide biochemical evidence that proper gate function is required for KS-mediated substrate turnover, and we further demonstrate its importance for the transacylation half-reaction. Importantly, our structural and biochemical data, along with previous MD simulation studies24, indicate a potential role for loop 2 in regulating loop 1 gating transitions. We observe distinct interaction networks for FabF’s loop 2 in both the open and closed state, which include contacts to the bound acyl-AcpP molecule, suggesting an allosteric role connecting acyl-AcpP binding and substrate delivery to gate function. Loop 2 is conserved within, but not between KS families, and our kinetic data indicates that neither loop 2 nor loop 1 is readily transferrable between evolutionarily divergent KS families. Therefore, loop 2 may coevolve with loop 1, or other KS elements such as ACP-mediated PPIs or KS-substrate interactions, to fine-tune KS gate function.
Despite the central role elongating KSs play in FAS and PKS dependent metabolic pathways, there are relatively few successful KS engineering attempts reported in the literature37,41,76,88,89. This challenge likely correlates with deficiencies in our fundamental understanding of the KS reaction mechanism. The manner in which KSs determine substrate preferences, for either the transacylation or condensation step, remains poorly understood. Using a combination of chemical biology, structural biology, and biochemistry, we provide compelling evidence in support of the KS gating mechanism and an additional structural framework governing its role in catalysis. Given the conserved nature of these gating elements in both FAS and PKS, the series of studies reported herein provide part of the necessary catalytic foundation to design and carry out future KS mechanistic studies. Moreover, this work also accelerates the development of strategies to engineer metabolic pathways in a predictable manner.
Supplementary Material
Acknowledgments.
This work was supported by NIH GM095970. J.T.M. was supported by T32 GM832626. R.N.R. was supported by NIH GM139327. Portions of the work were also funded by the Arthur and Julie Woodrow Chair at the Salk Institute (to J.P.N.) and the Howard Hughes Medical Institute (to J.P.N.). The authors thank A. Patel for suggestions regarding the preparation of Figure 2A and L. Misson for assistance editing the manuscript and advice on kinetic studies.
Footnotes
Supporting Information. Protein expression and purification; Site directed mutagenesis; Loading AcpP with probe molecules (one-pot); Preparation of AcpP=FabF crosslinked complexes; Crystallization, data collection, and refinement; Crosslinking assays with C16:1-crypto-ACP and FabF; Preparation of holo-AcpP and acyl-AcpP substrates; Ketosynthase condensation assay; Ketosynthase transacylation assay; Analysis of kinetic data; Synthesis of chlorovinylacrylamide-pantetheinamide crosslinker; Synthesis of C16:1-α-bromo-pantetheinamide crosslinker; General synthetic methods; Synthesis of C16:1 α-bromo-pantetheinamide; Reaction schemes of different modular synthases; Catalytic mechanism of type II FAS KS and type I PKS KS; Synthetic route for the synthesis of the C16:1-α-bromo-pantetheinamide probe; One-pot loading of C16:1αBromo pantetheineamide probe and crosslinking gels; Time course crosslinking gels of C16:1-crypto-AcpP with FabF; C16:1-AcpP-FabF substrate omit maps; Overlay of apo-FabF (PDB: 2GFW), C16-AcpP=FabF (PDB: 6OKG), and C16:1-AcpP=FabF; Stereochemistry at crosslinker α-carbon; C8-AcpP-FabF substrate omit maps; Trapped FAS KS catalytic intermediates throughout the KS catalytic cycle; SDS-PAGE gels of all FabF constructs used in kinetic assays; Rationale for FabF pocket block mutants; Rationale for FabF destabilization mutants; Rationale for FabF flex reduction mutants; Overview of the KS catalyzed condensation reaction assay; C12-AcpP calibration curve used for quantifying concentrations in kinetic assays; Overview of the KS catalyzed transacylation assay; FAS vs PKS loop 1 gating residues; Table 1 X-ray crystallography data collection and refinement statistics; Primers for site directed mutagenesis (SDM); This information is available free of charge on the ACS Publications website.
References
- (1).White SW, Zheng J, Zhang Y-M, and Rock CO (2005) The Structural Biology of Type II Fatty Acid Biosynthesis. Annu. Rev. Biochem 74, 791–831. [DOI] [PubMed] [Google Scholar]
- (2).Beld J, Lee DJ, and Burkart MD (2015) Fatty acid biosynthesis revisited: structure elucidation and metabolic engineering. Mol. Biosyst 11, 38–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Maier T, Leibundgut M, Boehringer D, and Ban N (2010) Structure and function of eukaryotic fatty acid synthases. Q. Rev. Biophys 43, 373–422. [DOI] [PubMed] [Google Scholar]
- (4).Torella JP, Ford TJ, Kim SN, Chen AM, Way JC, and Silver PA (2013) Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proc. Natl. Acad. Sci 110, 11290 LP – 11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Handke P, Lynch SA, and Gill RT (2011) Application and engineering of fatty acid biosynthesis in Escherichia coli for advanced fuels and chemicals. Metab. Eng 13, 28–37. [DOI] [PubMed] [Google Scholar]
- (6).Fischer M, and Grininger M (2017) Strategies in megasynthase engineering - fatty acid synthases (FAS) as model proteins. Beilstein J. Org. Chem 13, 1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Zhu Z, Zhou YJ, Krivoruchko A, Grininger M, Zhao ZK, and Nielsen J (2017) Expanding the product portfolio of fungal type I fatty acid synthases. Nat. Chem. Biol 13, 360. [DOI] [PubMed] [Google Scholar]
- (8).Lu X, Vora H, and Khosla C (2008) Overproduction of free fatty acids in E. coli: Implications for biodiesel production. Metab. Eng 10, 333–339. [DOI] [PubMed] [Google Scholar]
- (9).Wu H, Karanjikar M, and San K-Y (2014) Metabolic engineering of Escherichia coli for efficient free fatty acid production from glycerol. Metab. Eng 25, 82–91. [DOI] [PubMed] [Google Scholar]
- (10).Haushalter RW, Kim W, Chavkin TA, The L, Garber ME, Nhan M, Adams PD, Petzold CJ, Katz L, and Keasling JD (2014) Production of anteiso-branched fatty acids in Escherichia coli; next generation biofuels with improved cold-flow properties. Metab. Eng 26, 111–118. [DOI] [PubMed] [Google Scholar]
- (11).Maier T, Leibundgut M, and Ban N (2008) The Crystal Structure of a Mammalian Fatty Acid Synthase. Science (80-. ). 321, 1315 LP – 1322. [DOI] [PubMed] [Google Scholar]
- (12).Leibundgut M, Jenni S, Frick C, and Ban N (2007) Structural basis for substrate delivery by acyl carrier protein in the yeast fatty acid synthase. Science (80-. ). 316, 288. [DOI] [PubMed] [Google Scholar]
- (13).Johansson P, Wiltschi B, Kumari P, Kessler B, Vonrhein C, Vonck J, Oesterhelt D, and Grininger M (2008) Inhibition of the fungal fatty acid synthase type I multienzyme complex. Proc.Natl.Acad.Sci.USA 105, 12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Ridley CP, Lee HY, and Khosla C (2008) Evolution of polyketide synthases in bacteria. Proc. Natl. Acad. Sci. U. S. A 105, 4595–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Jenke-Kodama H, Sandmann A, Mü ller R, and Dittmann E (2005) Evolutionary Implications of Bacterial Polyketide Synthases. Mol. Biol. Evol 22, 2027–2039. [DOI] [PubMed] [Google Scholar]
- (16).Herbst DA, Townsend CA, and Maier T (2018) The architectures of iterative type I PKS and FAS. Nat. Prod. Rep 1046–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Hertweck C (2009) The Biosynthetic Logic of Polyketide Diversity. Angew. Chemie Int. Ed 48, 4688–4716. [DOI] [PubMed] [Google Scholar]
- (18).Chen A, Re RN, and Burkart MD (2018) Type II fatty acid and polyketide synthases: deciphering protein–protein and protein–substrate interactions. Nat. Prod. Rep 35, 1029–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Vagelos PR, Majerus PW, Alberts AW, Larrabee AR, and Ailhaud GP (1966) Structure and function of the acyl carrier protein. Fed. Proc 25, 1485–1494. [PubMed] [Google Scholar]
- (20).Larrabee AR, McDaniel EG, Bakerman HA, and Vagelos PR (1965) Acyl carrier protein. V. Identification of 4’-phosphopantetheine bound to a mammalian fatty acid synthetase preparation. Proc. Natl. Acad. Sci. U. S. A 54, 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Nguyen C, Haushalter RW, Lee DJ, Markwick PR, Bruegger J, Caldara-Festin G, Finzel K, Jackson DR, Ishikawa F, O’Dowd B, McCammon JA, Opella SJ, Tsai SC, and Burkart MD (2014) Trapping the dynamic acyl carrier protein in fatty acid biosynthesis. Nature 505, 427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Dodge GJ, Patel A, Jaremko KL, McCammon JA, Smith JL, and Burkart MD (2019) Structural and dynamical rationale for fatty acid unsaturation in Escherichia coli. Proc. Natl. Acad. Sci 116, 6775–6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Milligan JC, Lee DJ, Jackson DR, Schaub AJ, Beld J, Barajas JF, Hale JJ, Luo R, Burkart MD, and Tsai S-C (2019) Molecular basis for interactions between an acyl carrier protein and a ketosynthase. Nat. Chem. Biol 15, 669–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mindrebo JT, Patel A, Kim WE, Davis TD, Chen A, Bartholow TG, La Clair JJ, McCammon JA, Noel JP, and Burkart MD (2020) Gating mechanism of elongating β-ketoacyl-ACP synthases. Nat. Commun 11, 1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Misson LE, Mindrebo JT, Davis TD, Patel A, Andrew McCammon J, Noel JP, and Burkart MD (2020) Interfacial plasticity facilitates high reaction rate of E. coli FAS malonyl-CoA:ACP transacylase, FabD. Proc. Natl. Acad. Sci. U. S. A 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Agarwal V, Lin S, Lukk T, Nair SK, and Cronan JE (2012) Structure of the enzyme-acyl carrier protein (ACP) substrate gatekeeper complex required for biotin synthesis. Proc. Natl. Acad. Sci 109, 17406 LP – 17411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Masoudi A, Raetz CRH, Zhou P, and Pemble CW (2014) Chasing Acyl-Carrier-Protein Through a Catalytic Cycle of Lipid A Production. Nature 505, 422–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mindrebo JT, Misson Mindrebo LE, Johnson C, Noel JP, and Burkart M (2020) Activity Mapping the Acyl Carrier Protein - Elongating Ketosynthase Interaction in Fatty Acid Biosynthesis. Biochemistry 59, 3626–3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhang Y-M, Rao MS, Heath RJ, Price AC, Olson AJ, Rock CO, and White SW (2001) Identification and Analysis of the Acyl Carrier Protein (ACP) Docking Site on β-Ketoacyl-ACP Synthase III. J. Biol. Chem 276, 8231–8238. [DOI] [PubMed] [Google Scholar]
- (30).Mindrebo JT, Patel A, Misson LE, Kim WE, Davis TD, Ni QZ, La Clair JJ, and Burkart MD (2020) Structural Basis of Acyl-Carrier Protein Interactions in Fatty Acid and Polyketide Biosynthesis, in Comprehensive Natural Products III: Chemistry and Biology. Liu HW., and Begley TP. Eds.; Elsevier, Oxford; pp 61–122. [Google Scholar]
- (31).Heil CS, Wehrheim SS, Paithankar KS, and Grininger M (2019) Fatty Acid Biosynthesis: Chain-Length Regulation and Control. ChemBioChem 20, 2298–2321. [DOI] [PubMed] [Google Scholar]
- (32).Zhang YM, Hurlbert J, White SW, and Rock CO (2006) Roles of the Active Site Water, Histidine 303, and Phenylalanine 396 in the Catalytic Mechanism of the Elongation Condensing Enzyme of Streptococcus pneumoniae. J. Biol. Chem 281, 17390–17399. [DOI] [PubMed] [Google Scholar]
- (33).von Wettstein-Knowles P, Olsen JG, McGuire KA, and Henriksen A (2006) Fatty acid synthesis. Role of active site histidines and lysine in Cys-His-His-type beta-ketoacyl-acyl carrier protein synthases. FEBS J. 273, 695–710. [DOI] [PubMed] [Google Scholar]
- (34).Olsen JG, Kadziola A, von Wettstein-Knowles P, Siggaard-Andersen M, and Larsen S (2001) Structures of beta-Ketoacyl-Acyl Carrier Protein Synthase I Complexed with Fatty Acids Elucidate its Catalytic Machinery. Structure 9, 233–243. [DOI] [PubMed] [Google Scholar]
- (35).Wang J, Soisson SM, Young K, Shoop W, Kodali S, Galgoci A, Painter R, Parthasarathy G, Tang YS, Cummings R, Ha S, Dorso K, Motyl M, Jayasuriya H, Ondeyka J, Herath K, Zhang C, Hernandez L, Allocco J, Basilio Á, Tormo JR, Genilloud O, Vicente F, Pelaez F, Colwell L, Lee SH, Michael B, Felcetto T, Gill C, Silver LL, Hermes JD, Bartizal K, Barrett J, Schmatz D, Becker JW, Cully D, and Singh SB (2006) Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature 441, 358. [DOI] [PubMed] [Google Scholar]
- (36).Rittner A, Paithankar KS, Himmler A, and Grininger M (2020) Type I fatty acid synthase trapped in the octanoyl-bound state. Protein Sci. 29, 589–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Gajewski J, Pavlovic R, Fischer M, Boles E, and Grininger M (2017) Engineering fungal de novo fatty acid synthesis for short chain fatty acid production. Nat. Commun 8, 14650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gajewski J, Buelens F, Serdjukow S, Janßen M, Cortina N, Grubmüller H, and Grininger M (2017) Engineering fatty acid synthases for directed polyketide production. Nat. Chem. Biol 13, 363. [DOI] [PubMed] [Google Scholar]
- (39).Rossini E, Gajewski J, Klaus M, Hummer G, and Grininger M (2018) Analysis and engineering of substrate shuttling by the acyl carrier protein (ACP) in fatty acid synthases (FASs). Chem. Commun 54, 11606–11609. [DOI] [PubMed] [Google Scholar]
- (40).Klaus M, and Grininger M (2018) Engineering strategies for rational polyketide synthase design. Nat. Prod. Rep 35, 1070–1081. [DOI] [PubMed] [Google Scholar]
- (41).Klaus M, Buyachuihan L, and Grininger M (2020) Ketosynthase Domain Constrains the Design of Polyketide Synthases. ACS Chem. Biol 15, 2422–2432. [DOI] [PubMed] [Google Scholar]
- (42).Rock CO, and Cronan JE (1996) Escherichia coli as a model for the regulation of dissociable (type II) fatty acid biosynthesis. Biochim. Biophys. Acta - Lipids Lipid Metab. 1302, 1–16. [DOI] [PubMed] [Google Scholar]
- (43).Cronan JE, and Thomas J (2009)Chapter 17 Bacterial Fatty Acid Synthesis and its Relationships with Polyketide Synthetic Pathways, in Methods in Enzymology. Hopwood DA. ed.; Academic Press, Cambridge, MA; pp 395–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Edwards P, Sabo Nelsen J, Metz JG, and Dehesh K (1997) Cloning of the fabF gene in an expression vector and in vitro characterization of recombinant fabF and fabB encoded enzymes from Escherichia coli. FEBS Lett. 402, 62–66. [DOI] [PubMed] [Google Scholar]
- (45).Garwin JL, Klages AL, and Cronan JE (1980) Structural, enzymatic, and genetic studies of beta-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli. J. Biol. Chem 255, 11949–11956. [PubMed] [Google Scholar]
- (46).Gora A, Brezovsky J, and Damborsky J (2013) Gates of enzymes. Chem. Rev 113, 5871–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Khosla C (2009) Structures and Mechanisms of Polyketide Synthases. J. Org. Chem 74, 6416–6420. [DOI] [PubMed] [Google Scholar]
- (48).Robbins T, Liu Y-C, Cane DE, and Khosla C (2016) Structure and mechanism of assembly line polyketide synthases. Curr. Opin. Struct. Biol 41, 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Weissman KJ (2016) Genetic engineering of modular PKSs: From combinatorial biosynthesis to synthetic biology. Nat. Prod. Rep 33, 203–230. [DOI] [PubMed] [Google Scholar]
- (50).Dunn BJ, Cane DE, and Khosla C (2013) Mechanism and specificity of an acyltransferase domain from a modular polyketide synthase. Biochemistry 52, 1839—1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).D’Agnolo G, Rosenfeld IS, and Vagelos PR (1975) Multiple forms of beta-ketoacyl-acyl carrier protein synthetase in Escherichia coli. J. Biol. Chem 250, 5289–5294. [PubMed] [Google Scholar]
- (52).Cronan JEJ, Birge CH, and Vagelos PR (1969) Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J. Bacteriol 100, 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Heath RJ, and Rock CO (1996) Roles of the FabA and FabZ β-Hydroxyacyl-Acyl Carrier Protein Dehydratases in Escherichia coli Fatty Acid Biosynthesis. J. Biol. Chem 271, 27795–27801. [DOI] [PubMed] [Google Scholar]
- (54).de Mendoza D, Klages Ulrich A, and Cronan JEJ (1983) Thermal regulation of membrane fluidity in Escherichia coli. Effects of overproduction of beta-ketoacyl-acyl carrier protein synthase I. J. Biol. Chem 258, 2098–2101. [PubMed] [Google Scholar]
- (55).de Mendoza D, Garwin JL, and Cronan JEJ (1982) Overproduction of cis-vaccenic acid and altered temperature control of fatty acid synthesis in a mutant of Escherichia coli. J. Bacteriol 151, 1608–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Garwin JL, and Cronan JE (1980) Thermal modulation of fatty acid synthesis in Escherichia coli does not involve de novo enzyme synthesis. J. Bacteriol 141, 1457–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Dong H, and Cronan JE (2021) Temperature Regulation of Membrane Composition in the Firmicute, Enterococcus faecalis, Parallels That of Escherichia coli. Environ. Microbiol n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Ishikawa F, Haushalter RW, and Burkart MD (2012) Dehydratase-specific probes for fatty acid and polyketide synthases. J. Am. Chem. Soc 134, 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Worthington AS, and Burkart MD (2006) One-pot chemo-enzymatic synthesis of reporter-modified proteins. Org. Biomol. Chem 4, 44–46. [DOI] [PubMed] [Google Scholar]
- (60).Worthington AS, Rivera H, Torpey JW, Alexander MD, and Burkart MD (2006, December) Mechanism-based protein cross-linking probes to investigate carrier protein-mediated biosynthesis. ACS Chem. Biol United States. [DOI] [PubMed] [Google Scholar]
- (61).Worthington AS, Hur GH, Meier JL, Cheng Q, Moore BS, and Burkart MD (2008) Probing the compatibility of type II ketosynthase-carrier protein partners. Chembiochem 9, 2096–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Du D, Katsuyama Y, Horiuchi M, Fushinobu S, Chen A, Davis TD, Burkart MD, and Ohnishi Y (2020) Structural basis for selectivity in a highly reducing type II polyketide synthase. Nat. Chem. Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Gulick AM, and Aldrich CC (2018) Trapping interactions between catalytic domains and carrier proteins of modular biosynthetic enzymes with chemical probes. Nat. Prod. Rep 35, 1156—1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Davis TD, Michaud JM, and Burkart MD (2019) Active site labeling of fatty acid and polyketide acyl-carrier protein transacylases. Org. Biomol. Chem 17, 4720–4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Corpuz JC, Podust LM, Davis TD, Jaremko MJ, and Burkart MD (2020) Dynamic visualization of type II peptidyl carrier protein recognition in pyoluteorin biosynthesis. RSC Chem. Biol 1, 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Sztain T, Patel A, Lee DJ, Davis TD, McCammon JA, and Burkart MD (2019) Modifying the Thioester Linkage Affects the Structure of the Acyl Carrier Protein. Angew. Chem. Int. Ed. Engl 58, 10888–10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Epstein SC, Huff AR, Winesett ES, Londergan CH, and Charkoudian LK (2019) Tracking carrier protein motions with Raman spectroscopy. Nat. Commun 10, 2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Beld J, Cang H, and Burkart MD (2014) Visualizing the chain-flipping mechanism in fatty-acid biosynthesis. Angew. Chem. Int. Ed. Engl 53, 14456–14461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Crosby J, and Crump MP (2012) The structural role of the carrier protein – active controller or passive carrier. Nat. Prod. Rep 29, 1111–1137. [DOI] [PubMed] [Google Scholar]
- (70).Cronan JE, Birge CH, and Vagelos PR (1969) Evidence for Two Genes Specifically Involved in Unsaturated Fatty Acid Biosynthesis in <em>Escherichia coli</em> J. Bacteriol 100, 601 LP – 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Eccleston VS, Cranmer AM, Voelker TA, and Ohlrogge JB (1996) Medium-chain fatty acid biosynthesis and utilization in Brassica napus plants expressing lauroyl-acyl carrier protein thioesterase. Planta 198, 46–53. [Google Scholar]
- (72).Salas JJ, and Ohlrogge JB (2002) Characterization of substrate specificity of plant FatA and FatB acyl-ACP thioesterases. Arch. Biochem. Biophys 403, 25–34. [DOI] [PubMed] [Google Scholar]
- (73).Voelker T (1996) Plant Acyl-ACP Thioesterases: Chain-Length Determining Enzymes in Plant Fatty Acid Biosynthesis BT - Genetic Engineering: Principles and Methods (Setlow JK, Ed.), pp 111–133. Springer US, Boston, MA. [DOI] [PubMed] [Google Scholar]
- (74).Moche M, Schneider G, Edwards P, Dehesh K, and Lindqvist Y (1999) Structure of the Complex between the Antibiotic Cerulenin and Its Target, β-Ketoacyl-Acyl Carrier Protein Synthase. J. Biol. Chem 274, 6031–6034. [DOI] [PubMed] [Google Scholar]
- (75).Trajtenberg F, Altabe S, Larrieux N, Ficarra F, de Mendoza D, Buschiazzo A, and Schujman GE (2014) Structural insights into bacterial resistance to cerulenin. Febs J. 281, 2324–2338. [DOI] [PubMed] [Google Scholar]
- (76).Val D, Banu G, Seshadri K, Lindqvist Y, and Dehesh K (2000) Re-engineering ketoacyl synthase specificity. Structure 8, 565–566. [DOI] [PubMed] [Google Scholar]
- (77).Morgan-Kiss RM, and Cronan JE (2008) The Lactococcus lactis FabF fatty acid synthetic enzyme can functionally replace both the FabB and FabF proteins of Escherichia coli and the FabH protein of Lactococcus lactis. Arch. Microbiol 190, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Zhu L, Cheng J, Luo B, Feng S, Lin J, Wang S, Cronan JE, and Wang H (2009) Functions of the Clostridium acetobutylicium FabF and FabZ proteins in unsaturated fatty acid biosynthesis. BMC Microbiol. 9, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Wang H, and Cronan JE (2004) Functional replacement of the FabA and FabB proteins of Escherichia coli fatty acid synthesis by Enterococcus faecalis FabZ and FabF homologues. J. Biol. Chem 279, 34489–34495. [DOI] [PubMed] [Google Scholar]
- (80).Luo Q, Li M, Fu H, Meng Q, and Gao H (2016) Shewanella oneidensis FabB: A β-ketoacyl-ACP Synthase That Works with C16:1-ACP. Front. Microbiol 7, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Li M, Meng Q, Fu H, Luo Q, and Gao H (2016) Suppression of fabB Mutation by fabF1 Is Mediated by Transcription Read-through in Shewanella oneidensis. J. Bacteriol 198, 3060–3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Witkowski A, Joshi AK, Lindqvist Y, and Smith S (1999) Conversion of a beta-ketoacyl synthase to a malonyl decarboxylase by replacement of the active-site cysteine with glutamine. Biochemistry 38, 11643–11650. [DOI] [PubMed] [Google Scholar]
- (83).Stunkard LM, Dixon AD, Huth TJ, and Lohman JR (2019) Sulfonate/Nitro Bearing Methylmalonyl-Thioester Isosteres Applied to Methylmalonyl-CoA Decarboxylase Structure-Function Studies. J. Am. Chem. Soc 141, 5121–5124. [DOI] [PubMed] [Google Scholar]
- (84).Bräuer A, Zhou Q, Grammbitter GLC, Schmalhofer M, Rühl M, Kaila VRI, Bode HB, and Groll M (2020) Structural snapshots of the minimal PKS system responsible for octaketide biosynthesis. Nat. Chem 12, 755–763. [DOI] [PubMed] [Google Scholar]
- (85).Borgaro JG, Chang A, Machutta CA, Zhang X, and Tonge PJ (2011) Substrate recognition by β-ketoacyl-ACP synthases. Biochemistry 50, 10678–10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Kapur S, Lowry B, Yuzawa S, Kenthirapalan S, Chen AY, Cane DE, and Khosla C (2012) Reprogramming a module of the 6-deoxyerythronolide B synthase for iterative chain elongation. Proc. Natl. Acad. Sci 109, 4110–4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Kapur S, Chen AY, Cane DE, and Khosla C (2010) Molecular recognition between ketosynthase and acyl carrier protein domains of the 6-deoxyerythronolide B synthase. Proc. Natl. Acad. Sci. U. S. A 107, 22066–22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Jenner M, Frank S, Kampa A, Kohlhaas C, Pöplau P, Briggs GS, Piel J, and Oldham NJ (2013) Substrate Specificity in Ketosynthase Domains from trans-AT Polyketide Synthases. Angew. Chemie Int. Ed 52, 1143–1147. [DOI] [PubMed] [Google Scholar]
- (89).Murphy AC, Hong H, Vance S, Broadhurst RW, and Leadlay PF (2016) Broadening substrate specificity of a chain-extending ketosynthase through a single active-site mutation. Chem. Commun 52, 8373–8376. [DOI] [PubMed] [Google Scholar]
- (90).Price AC, Choi KH, Heath RJ, Li Z, White SW, and Rock CO (2001) Inhibition of beta-ketoacyl-acyl carrier protein synthases by thiolactomycin and cerulenin. Structure and mechanism. J. Biol. Chem 276, 6551–6559. [DOI] [PubMed] [Google Scholar]
- (91).Robbins T, Kapilivsky J, Cane DE, and Khosla C (2016) Roles of Conserved Active Site Residues in the Ketosynthase Domain of an Assembly Line Polyketide Synthase. Biochemistry 55, 4476–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


