Abstract
Purpose
Hyphema is a sequela of ocular trauma and can be associated with significant morbidity. Management of this condition is variable and can depend on individual institutional guidelines. We aimed to summarize current practices in hyphema management across ophthalmological institutions worldwide.
Methods
A cross-sectional online survey was conducted across North America, Asia, South America, Africa, Europe, and Australia from August 2020 to January 2021. The survey assessed the existing practices in the management of hyphema at each institution.
Results
For layered hyphema, topical steroids were routinely administered by 34 (of 36 respondents, 94.4%) institutions, of which prednisolone was the preferred choice (n = 32, 88.9%). Topical cycloplegics were used at 34 (94.4%) institutions. No institution reported routine use of antifibrinolytics. Head elevation was the most deployed procedure to promote hyphema reabsorption (n = 31, 86.3%), followed by partial bed rest (n = 21, 58.3%). The majority of institutions (n = 25, 69.4%) did not routinely pursue admission for hyphema patients, although 75.0% of institutions (n = 27) scheduled follow-up visits within 48 hours of presentation. Additionally, few institutions performed routine sickle cell trait testing for patients presenting with hyphema (n = 6, 16.7%). The decision to perform anterior chamber washout varied and was often based on intraocular pressure and the speed of hyphema resolution.
Conclusion
Unanimity of international institutions on hyphema management is lacking. As it stands, many current interventions have unconvincing evidence supporting their use. Evidence-based guidelines would be beneficial in guiding decision-making on hyphema management. Additionally, areas of consensus can be used as foundations for future standard of care investigations.
Keywords: current practices, hyphema, trauma, sickle cell, anterior segment
Introduction
Hyphema consists of blood build up in the anterior chamber (AC) of the eye resulting from injury to ciliary body and iris blood vessels.1 Hyphema most often occurs after trauma or surgery, but may also arise spontaneously due to coagulopathies, neovascularization, or sudden intraocular pressure changes.1,2
The level of hyphema is one of the critical factors for grading its severity, in addition to corneal blood staining and intraocular pressure.3 The mildest grade of hyphema is termed microhyphema, which consists of red blood cells in the AC that are only identifiable via microscopy and/or gonioscopy.4 When a hyphema progresses and erythrocytes precipitate, it is considered to be a gross or layered hyphema, which is graded based upon the extent to which erythrocytes fill the AC.4
In cases of traumatic ophthalmic injuries that induce hyphema, poor visual outcome (ie, <20/40) is often due to associated injuries (such as corneal laceration or retinal detachment), but can be due to the hyphema itself in 8–33% of cases.2 Current treatment regimens for hyphema include cycloplegics, steroids, antifibrinolytics, and non-steroidal anti-inflammatory medications, as well as activity restrictions, such as partial to complete bed rest, and protection with a rigid shield.2
While various studies have investigated the comparative efficacy of specific interventions for hyphema management, uncertainties persist regarding which medications and interventions are optimal.1 The American Academy of Ophthalmology (AAO) provides recommendations on management standards for many common conditions in ophthalmology called the Preferred Practice Patterns©.5 However, these guidelines do not cover most traumatic ocular injuries, including hyphema. Additionally, inconsistencies in traumatic hyphema treatment likely persist given the heterogeneity of institutional resources on an international level.
We surveyed ophthalmic trauma experts at international institutions to summarize generally accepted principles of hyphema management and to highlight areas of variation within clinical practice.
Methods
The development of this survey was described previously.6,7,31 Briefly, there were 123 questions in the survey, with 21 pertaining to the treatment of hyphema using medications, surgical interventions, and activity modifications. The hyphema-related survey questions are included in Supplemental Document 1. Respondents were advised to provide answers that reflect the management protocols at their respective institutions collectively rather than individual partialities. This study was deemed exempt by the Institutional Review Board at the Johns Hopkins School of Medicine due to the low risk posed to participants. Study activities observed the tenets of the Declaration of Helsinki and laws in the state of Maryland.
Email invitations for the survey were sent to experts in ophthalmic trauma who were members or affiliates of the American Society of Ophthalmic Trauma (ASOT) or the Asia Pacific Ocular Trauma Society (APOTS) and to the members of the International Globe and Trauma Epidemiology Study (IGATES). Invitees were reminded by email up to three times to complete the survey.
Data analysis was completed using Microsoft Excel (Microsoft Inc., Redmond, WA, USA), Stata (StataCorp, College Station, TX), and GraphPad Prism (GraphPad Software, San Diego, California, USA). Significant differences (p < 0.05) in proportions of responses were determined by Student’s t-test and chi-squared tests. Data were shown as percentages of all responses and exceptions were noted. For responses that included numerical ranges, the range mean was used.
Results
Characteristics of Responding Experts and Institutions
Responses from 37 institutions (response rate = 88.1%) were received; of which, one (2.4%) was excluded because the survey was not finished (Table 1). Locations and characteristics of respondents are outlined in Table 1.
Table 1.
Summary of Respondents and Institutional Characteristics
| Response Selection (N = 42) | |
| Responses Included in Analysis (N, %) | 36 (85.7%) |
| Non-Respondents (N, %) | 5 (11.9%) |
| Partial Responses (N, %) | 1 (2.4%) |
| Respondent Location and Experience (N = 36) | |
| Location of Institution | |
| Africa (N, %) | 3 (8.3%) |
| Asia (N, %) | 13 (36.1%) |
| Australia (N, %) | 1 (2.8%) |
| Europe (N, %) | 2 (5.6%) |
| North America (N, %) | 13 (36.1%) |
| South America (N, %) | 4 (11.1%) |
| Years practicing post-residency (avg. ± s.d.) | 14.81 ± 8.23 |
| Institutional Parameters (N = 36) | |
| Eye ED at Institution (N, %) | 20 (55.6%) |
| Center is Designated for Trauma (N, %) | 20 (55.6%) |
| In-House Ophthalmology Coverage Available at All Times (N, %) | 26 (72.2%) |
| Ophthalmologist Available on Hour Notice (N, %)a | 10 (100.0%) |
| No. of Patients Presenting with Hyphema at Responding Institutions per Year (N = 36) | |
| 0–50 | 12 (33.3%) |
| 51–100 | 7 (19.4%) |
| 101–150 | 7 (19.4%) |
| 151–200 | 4 (11.1%) |
| 201+ | 2 (5.6%) |
| Unknown | 4 (11.1%) |
Note: aProportion of respondents without in-house coverage (n = 10).
Abbreviations: ED, emergency department; avg, average; s.d, standard deviation.
The approximate volume of hyphema cases managed per year at each institution is shown in Table 1. Four responding experts (11.1%) were unable to estimate the caseload of patients with hyphema at their respective institutions. Sub-group analyses were completed to identify whether differences in practice paradigms exist between institutions where the caseloads of hyphema were known versus not known, and between experts at low-volume versus high-volume centers (<50 and 50+ cases per year, respectively, Supplemental Tables S1 and S2); these stratifications did not lead to any statistically significant differences in practices observed.
Medication Protocols for Hyphema Management
There was no significant difference in the frequency of administering topical steroids for gross versus microhyphema, with 94.4% of experts (n = 34) routinely administering steroids for gross hyphema and 80.6% (n = 29) for microhyphema (p = 0.079, Table 2). Prednisolone was the most used steroid for both hyphema types.
Table 2.
Preferences for Steroid Use in the Management of Different Types of Hyphema
| Gross Hyphema (N, %) | Microhyphema (N, %) | P-value | Total N | |
|---|---|---|---|---|
| Routinely Administer Topical Steroids | 34 (94.4%)a | 29 (80.6%)a | 0.079 | 36 |
| Prednisoloneb | 32 (88.9%) | 25 (69.4%) | N/A | 36 |
| Dexamethasoneb | 6 (16.7%) | 3 (8.3%) | ||
| Difluprednateb | 0 (0.0%) | 1 (2.8%) | ||
| Typical Steroid Duration (days ± s.d.)c | 17.8 ± 9.6 | 13.7 ± 8.3 | 0.093 | 36 |
Notes: aOne respondent selected “Do not know” for each gross and microhyphema, brespondents were able to select more than one response, for gross hyphema steroid preference, one respondent wrote in “prednefrin forte or maxidex”, and for microhyphema, one expert wrote in “lotepred”, cduration includes tapering of doses, two institutions responded that duration varies or was until resolved, one indicated duration depended on grade.
Most respondents (n = 34, 94.4%) administered topical cycloplegics for all patients presenting with hyphema. Of these, atropine 1% was the most preferred (n = 21, 58.3%), followed by cyclopentolate 1–2% (n = 13, 36.1% Table 3). Three of the experts who selected “other” wrote in tropicamide (8.3%), while two others wrote in homatropine (5.6%). Additionally, two experts (5.6% of all respondents) indicated cycloplegic selection was contingent on the age of the patient, their injury characteristics, and insurance coverage. No responding experts reported administering antifibrinolytics for hyphema (n = 0, 0.0%, Table 3). Fewer than half (n = 15, 41.7%) attested to discontinuing anticoagulation or anti-platelet medications if deemed appropriate by the patient’s primary care provider (PCP, Table 3).
Table 3.
Other Medication and Dispositional Preferences for the Management of Hyphema
| N=36 | All Hyphema (N, %) |
|---|---|
| Routinely administer topical cycloplegicsa | 34 (94.4%) |
| Atropine 1% | 21 (58.3%) |
| Cyclopentolate 1–2% | 13 (36.1%) |
| Otherb | 7 (19.4%) |
| Do not administer antifibrinolytics | 36 (100.0%) |
| Routinely discontinue anticoagulation or anti-platelet medicationsc | 15 (41.7%) |
| Activity modificationsd | |
| Elevate head and sleep upright | 31 (86.1%) |
| Partial bed rest | 21 (58.3%) |
| Shielding of eye | 19 (52.8%) |
| Complete bed rest | 8 (22.2%) |
| Minor activity limitations | 6 (16.7%) |
| Othere | 1 (2.8%) |
| No activity limitations | 0 (0.0%) |
| Routinely screen for sickle cell disease/trait | 6 (16.7%) |
| Routine disposition of hyphema patients | |
| Admitted to hospital | 8 (22.2%) |
| Discharged without admitting | 25 (69.4%) |
| Otherf | 3 (8.3%) |
Notes: aOne respondent selected “Do Not Know”, bother responses included homatropine, tropicamide (± phenylephrine), variable, cif deemed safe by primary care provider, other responses included depending on PCP advice, in patients with significant rebleeds, depending on the reason for anticoagulation, and that the decision is highly variable, drespondents could select more than one option, eother response stated that activity limitation depended on grade, fother responses included depending on IOP and blood amount.
Activity Limitation Protocols
While all responding experts routinely recommended limiting the activity of hyphema patients to at least some degree (n = 36, 100%), we observed variation in what limitations were routinely advised; 86.1% of experts routinely advised patients to elevate their head and sleep upright (n = 31), and 58.3% of experts restricted patients to partial bed rest (n = 21), while 22.2% required complete bed rest (n = 8). Around half (n = 19, 52.8%) recommended shielding of the eye, while 16.7% required minor activity limitations (n = 6).
Screening for Sickle Cell Disease or Trait in Hyphema Patients
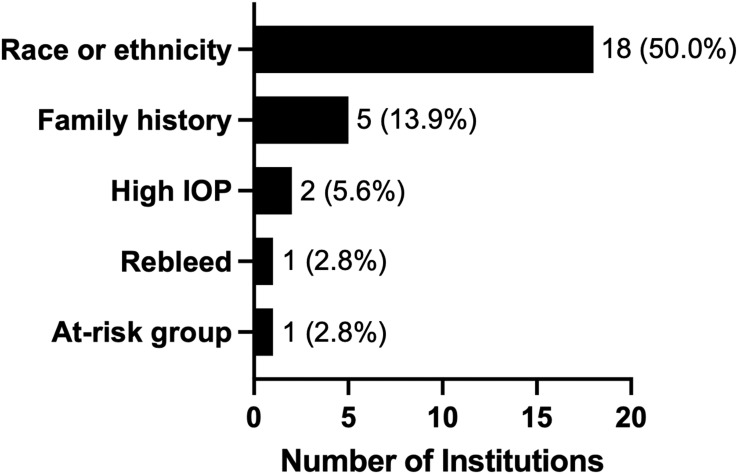
Approximately 16.7% of responding experts routinely screened all patients presenting with hyphema for sickle cell disease or trait (SCD/T, n = 6, Table 3), while 11.1% (n = 4) did not screen patients with only this presentation. Of those who did routinely screen for SCD/T, 2 centers were in Asia, 2 were in North America, 1 in Africa, and 1 in South America. The remaining survey respondents (n = 26, 72.2%) completed screening for SCD/T if certain indications were present (Figure 1), such as certain race or ethnicity (50.0%, n = 18), family history (13.9%, n = 5), and high IOP (n = 2, 5.6%). Information on indications for SCD/T screening was not provided by 3 respondents (8.3%).
Figure 1.
Indications for which screening for sickle cell disease/trait was performed in patients presenting with hyphema. Common races and ethnicities cited as potential indications for screening include African, Mediterranean, and Tharu descent. Percentages were shown as proportions of all respondents (n = 36). Three respondents who screened for certain patients did not provide indications. IOP = intraocular pressure.
Indications for Surgery in Patients with Hyphema
SCD appeared to affect the conditions under which the experts performed AC washouts in patients with hyphema. Many respondents had a lower threshold for surgical intervention when IOP was elevated in patients with SCD compared to patients without SCD. Specifically, 30.6% of experts (n = 11) reported performing AC washouts in patients with SCD when their IOP was greater than 24 mmHg for 1–2 days, while only 5.6% of experts (n = 2) performed the washout in patients without SCD with this indication. Rather, IOP >50 mmHg for two to five days was a commonly cited threshold to perform AC washouts in patients without SCD (16.7%, n = 6, Table 4). IOP criteria and thresholds for performing AC washouts in patients with and without SCD are summarized in Table 4.
Table 4.
Indications for Performing an Anterior Chamber Washout in Patients with Hyphema with and without Sickle Cell Disease
| Criteria, N (%)a | Patients with SCDb | Patients without SCD |
|---|---|---|
| IOP Indications | ||
| Uncontrolled for >1-2 days | 3 (8.3%) | 6 (16.7%) |
| >60 for 2 days | 0 (0.0%) | 3 (8.3%) |
| >50 for 2–5 days | 0 (0.0%) | 6 (16.7%) |
| >40 for 2 days | 1 (2.8%) | 1 (2.8%) |
| >35 for 5–7 days | 0 (0.0%) | 2 (5.6%) |
| >35-40 at any point | 0 (0.0%) | 2 (5.6%) |
| >30-35 for 2+ days | 4 (11.1%) | 4 (11.1%) |
| >30 at any point | 3 (8.3%) | 3 (8.3%) |
| >24 for 7+ days | 0 (0.0%) | 1 (2.8%) |
| >24 for 3–5 days | 2 (5.6%) | 4 (11.1%) |
| >24 for 1–2 days | 11 (30.6%) | 2 (5.6%) |
| >24 at any point | 2 (5.6%) | 1 (2.8%) |
| Other Indications | ||
| As soon as possible | 3 (8.3%) | 1 (2.8%) |
| Corneal blood staining | 2 (5.6%) | 8 (22.2%) |
| Not improving >1 week | 1 (2.8%) | 6 (16.7%) |
| Large or complete hyphema | 2 (5.6%) | 3 (8.3%) |
| Complete hyphema for 3–5 days | 1 (2.8%) | 1 (2.8%) |
| Physician or case-dependent | 1 (2.8%) | 4 (11.1%) |
| Do not see sickle cell cases | 6 (16.7%) | 0 (0.0%) |
Notes: aRespondents could provide more than one indication, btwo institutions did not provide indications for patients with SCD. SCD = sickle cell disease.
In terms of other indications for AC washout aside from elevated IOP, 8.3% of respondents (n = 3) performed AC washouts in all patients with hyphema and SCD as soon as possible, while only 2.8% of respondents did so in patients without SCD (n = 1, Table 4). A summary of indications aside from IOP thresholds for performing AC washouts is shown in Table 4.
Disposition for Patients with Hyphema
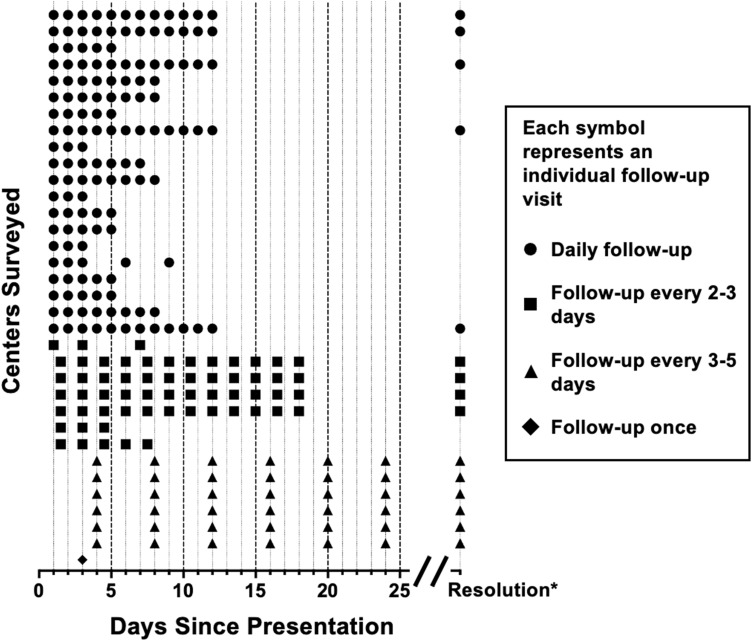
Patients with hyphema alone were not usually admitted to the hospital by 69.4% of experts (n = 25), while 22.2% routinely admitted them to the hospital for monitoring (n = 8). However, 8.3% of respondents (n = 3) indicated that admission practices were highly variable and dependent on the amount of blood present as well as intraocular pressure. The expected schedule of follow-up visits per center is shown in Figure 2.
Figure 2.
Follow-up visit schedules for patients with uncomplicated hyphema at each responding institution (for example, the patient could follow up daily for 5 days after presentation). Institutions are separated on the vertical axis. If no end date was given for follow-up, it was assumed that patients were followed until resolution of the hyphema. If a range of days were provided, the average was used for visualization. *Resolution may occur before 25 days.
Discussion
The data reported here highlight that the controversies previously described in hyphema management currently persist internationally.1 Although we witnessed several areas of general consensus, including the use of topical steroids and cycloplegics, and the recommendation for head elevation/sleeping upright, we observed areas of variation in preferences for the administration and types of medications used for hyphema cases, indications for surgical interventions, as well as activity limitations and typical disposition of patients. Previous investigations thus far have shown management techniques to affect secondary hemorrhage rates, but not necessarily improve visual outcomes.1,2,8,9,32 However, evidence-based management protocols are still needed to reduce clinical waste, limit unnecessary exposure to medication side effects or adverse surgical events, and ensure access to optimal care.
Steroids are widely considered a mainstay of hyphema treatment to prevent rebleeds and synechiae formation,2,10,11 but there is conflicting evidence for their effectiveness in this setting. Several controlled prospective studies found no association between systemic steroid use, particularly prednisone, and the incidence of secondary hemorrhage or final visual outcomes after traumatic hyphema.12,13 More recently, a systematic review was unable to determine efficacy due to a lack of statistical power in existing trials.11 In another meta-analysis, however, steroids (either topical or systemic) were found to effectively reduce secondary hemorrhage risk, but not to improve final visual acuity.2 Other trials have found that systemic and/or topical steroids may be beneficial for preventing rebleeds.8 Despite a lack of conclusive evidence, the vast majority of experts in our study routinely administered topical steroids to patients with gross hyphema or microhyphema (n = 34, 94.4%, and n = 29, 80.6%, respectively). Because topical steroids have limited side effects compared to systemic administration, they would be preferred if they are found to be similarly effective. Investigations are needed to compare the efficacy of topical steroids relative to control groups without steroid treatment and to systemic therapy to determine if topical steroids alone are sufficient, as well as determine an optimal dosing regimen.
Cycloplegics in the setting of hyphema have been shown to reduce pain resulting from iritis and ciliary spasm, prevent synechiae formation, and improve visualization of posterior eye structures.1 Several studies have shown cycloplegics to have no effect on preventing secondary hemorrhage or improving visual outcomes in patients with hyphema.1,14 A preventative effect of cycloplegics on rebleeding was found in only one study in patients who were also using aspirin.15 However, the use of topical cycloplegics was popular among survey respondents (n = 34, 94.4% routinely administered), with the most commonly used being atropine 1%. This may be due to the aforementioned benefits, including reduction of pain and improved visualization of the posterior segment. Clarification is needed on possible detrimental effects of cycloplegics on hyphema, as well as to ascertain benefit to their use.
A review of studies on hyphema management found antifibrinolytics tranexamic acid and ϵ-aminocaproic acid to be associated with moderately lower rates of rebleeding than control groups.2 This effect has potential benefits for patients, as rebleeds of hyphema are more likely to necessitate surgical intervention than primary hyphema.16 However, a more recent review has found no evidence that administering antifibrinolytics led to improved long-term outcomes including improved visual acuity after hyphema.11 Additionally, systemic antifibrinolytics incur the risk of clotting elsewhere in the body, and are contraindicated in patients with a history of thromboembolism.17 The topic of antifibrinolytic administration generated a consensus among the experts we surveyed, who agreed on a preference to not administer antifibrinolytics for patients presenting with hyphema.
We observed more controversy around discontinuing anticoagulation/anti-platelet medications in patients presenting with hyphema. Approximately 41.7% of experts (n = 15) in our study attested to routinely stopping these therapies if deemed safe by the patient’s PCP. Although there is a paucity of investigations exploring the role of discontinuing anticoagulation/anti-platelets in the management of traumatic hyphema, a study of these medications taken prior to and at the time of glaucoma surgery demonstrated a higher risk of post-operative hyphema.18 For this reason, around one-third of ophthalmologists surveyed stopped pro-bleeding therapies prior to glaucoma surgery.19 Further investigations into the risks and benefits of hemostasis-related medications in the setting of hyphema and their effects on long-term visual outcomes are needed to clarify optimal standards of care.
Another clinical consideration for patients with hyphema is the care of those with hemoglobinopathies. In particular, patients with SCD have a significantly increased risk of developing rebleeds, increased IOP, and non-resolving visual impairment because of hyphema.20 In these patients, red blood cells are prone to sickling in the AC and face greater resistance in passing through the trabecular meshwork, leading to increased intraocular pressures.21 It is therefore crucial that patients with hyphema and SCD are identified, monitored, and when appropriate, treated more aggressively. In fact, 16.7% of centers that responded to our survey screened all patients who presented with hyphema for this gene alteration. However, this may have been impacted by the relative prevalence of SCD/T in certain groups in the populations seen by the responding centers. For example, in the US, it has been estimated that 15.5 of 1000 newborns and 73.1 of 1000 Black newborns are identified to have SCT annually.22 Nine of 10 centers in the US screen for SCD/T if the patient presenting with hyphema identifies as Black or of African descent. One investigation recommended screening patients with microhyphema of African or Hispanic descent who had intraocular pressures greater than 21 mmHg because it was at this threshold that treatment would be more intensive.23 There are no prospective randomized studies regarding the indications for screening for SCD/T and further guidelines are needed to determine the scenarios in which screening for this allele is warranted.
SCD/T status not only impacts medication management decisions, but also thresholds for surgical interventions. Surgical interventions for hyphema are usually a last resort.2 One study that compared the medical and surgical treatment of hyphema found that the medically treated group had better final visual acuity while the surgically treated group had faster resolution.24 Surgical treatment has been associated with adverse effects such as bleeding and damage to the lens capsule or corneal endothelium.25 Hence, surgery is generally reserved for patients who are at meaningful risk for developing sight-threatening complications of hyphema. The criteria proposed by Read in 1975 are often used to determine when surgical management of hyphema is necessary. They are: i) presence of corneal blood staining, ii) total hyphema and IOP >50 mmHg for 5 days, ii) total hyphema that has not resolved by at least 50% after 5 days with IOP >25 mmHg, and iv) hyphema that has not cleared after 9 days.26,27 Deutsch et al proposed lower thresholds for surgical intervention in patients with SCD/T, including IOP >24 mmHg for 24 hours, or multiple transient pressures >30 mmHg.28 Respondents to our survey were generally more proactive than published recommendations in proceeding with surgery for patients without SCD. Twelve experts (33.3%) indicated that their institution would proceed with AC paracentesis if the patient’s IOP is >40 mmHg for approximately 2 days. Contrarily, respondents were more lenient with the IOP of patients with SCD; only 30.6% of responding experts (n = 11) adhered to the guideline of surgery when IOP was >24 mmHg for 1–2 days, while 27.8% of experts allowed SCD/T patients to reach higher IOPs or for longer periods of time (n = 10, Table 4).
In previous investigations, clots were shown by pathology to be well-formed and to retract from the iris at four days post-injury in hyphema patients.25,29 It was then hypothesized in these studies that disrupting the clots before this time point may disrupt healing of the vasculature.25 However, it was observed that delaying surgery past 8 days after presentation with hyphema was associated with the possible development of optic atrophy.30 It is notable, though, that most studies preceded the use of optical coherence tomography to sensitively detect retinal ganglion cell degeneration or modern automated perimetry to detect functional deficits.30 Due to the variation in indications we observed for performing AC taps in patients with hyphema, and the potential for time sensitivity of surgical interventions, further studies are needed to determine if and when surgery for hyphema should be performed.
Follow-up regimens were also highly variable, although three-quarters of experts conducted a first review within 48 hours of presentation. We did not investigate the rationale behind follow-up intervals, but a likely justification is the perceived likelihood and severity of IOP elevation at different time points and the rapidity with which this must be treated. Another consideration for timely follow-up is the rate of development of associated injuries such as retinal dialysis. The fact that delayed access to care is associated with poor outcomes highlights the importance of these decisions and the need for additional evidence.14
Several limitations exist when interpreting the results of this study. Firstly, in an effort to focus the survey, respondents were not asked for their preferences on the use of systemic steroids for hyphema, which may have impacted their preference for topical steroids or other management paradigms. Although there were no significant differences found between low- and high-volume centers, as well as centers with known and unknown volumes of patients, these estimated case volumes were based on physician reports rather than formal documentation. Additionally, while the study team made every effort to distribute the survey broadly, the survey sample size is small, and responses are not comprehensive geographically, limiting generalizability of these results. Similarly, survey invitees were members or affiliates of ASOT and APOTS, which may bias the results of the population surveyed. Because the survey was given exclusively in English, every attempt was made to write simple, plain-language questions; however, there remains a possibility that the wording of specific questions could have been incompletely understood by non-native English speakers. Lastly, despite explicit instruction for responding experts to provide responses that capture their institutional policies, individual preference and recency bias may have skewed responses.
Despite these limitations, this investigation has several pertinent strengths. We comprehensively outlined international practice patterns of hyphema management in ocular trauma centers. In doing so, we identified areas of variation that in future studies can be evaluated for their impact on visual outcomes. This work lays the foundation for more streamlined, evidence-based care for patients with hyphema compared to the current physician- and institution-level standards.
Acknowledgments
We would like to thank Nicholas C. Keith, BS, for his computational expertise and assistance. Contributing members of the International Globe and Adnexal Trauma Epidemiology Study (IGATES) include the writing authors, along with Dr. Sheri DeMartelaere, Dr. Thomas Hwang, Dr. Pradeep Prasad, Dr. Sulaiman Alsulaiman, Dr. Soon Ch’ng, Dr. Parisa Taravati, Dr. Sadik Taju Sherief, Dr. John Nkurikiye, Dr. Cecil McCollum, Dr. Anadi Khatri, Dr. Eli Pradhan, Dr. Meenu Chaudhary, Dr. Alok Sen, Dr. Sweta Singh, Dr. Purushottam Joshi, Dr. Chitaranjan Mishra, Dr. Aparna Rizyal, Dr. Rekha Khandelwal, Dr. Salma KC, Dr. Hugo Hernan Ocampo Dominguez, Dr. José Dalma-Weiszhausz, Dr. Carlos Wong, Dr. Felipe Morera, Dr. Cherie Fathy, Dr. Anne Murchinson, Dr. Seth Lartey, Dr. Rachel Patel, Dr. Stephanie Watson, Dr. Royce Chen, Dr. Shakeen Singh, Dr. Andres Rousselot, and Dr. Bartlett Hayes.
Funding Statement
This study was supported by grant P30EY001765 (Wilmer Biostatistics Core Grant) from the National Eye Institute, Bethesda, Maryland and Dean’s Funding for Summer Research, Johns Hopkins School of Medicine (SCM), Baltimore, Maryland. The sponsor or funding organization had no role in the design or conduct of this research.
Data Sharing Statement
Data are available upon request to the corresponding author.
Disclosure
AH was an employee of Essilor International and recipient of NHMRC Dora Lush PhD Scholarship (Sydney, Australia). YY was a consultant for Alcon (Philadelphia, USA). MG was an UpToDate contributor on Ocular Trauma (Boston, USA). AS holds equity in Pfizer Inc. and Medtronic, and reports potential royalties from Rebion, outside the submitted work. The authors report no other conflicts of interest in this work.
IRB/Ethics Committee ruled that this study was exempt due to the minimal risk incurred by subjects.
The abstract of this paper was presented at ARVO 2021 as a poster presentation talk with interim findings. The poster’s abstract was published in “Poster Abstracts” in the ARVO publication, Journal Investigative Ophthalmology and Visual Sciences: https://iovs.arvojournals.org/article.aspx?articleid=2775864.
References
- 1.Bansal S, Gunasekeran DV, Ang B, et al. Controversies in the pathophysiology and management of hyphema. Surv Ophthalmol. 2016;61(3):297–308. doi: 10.1016/j.survophthal.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Walton W, Von Hagen S, Grigorian R, Zarbin M. Management of traumatic hyphema. Surv Ophthalmol. 2002;47(4):297–334. doi: 10.1016/S0039-6257(02)00317-X [DOI] [PubMed] [Google Scholar]
- 3.Edwards WC, Layden WE. Traumatic hyphema: a report of 184 consecutive cases. Am J Ophthalmol. 1973;75(1):110–116. doi: 10.1016/0002-9394(73)90659-4 [DOI] [PubMed] [Google Scholar]
- 4.Anon. Hyphema - EyeWiki. Available from: https://eyewiki.aao.org/Hyphema. Accessed January 4, 2022.
- 5.Lum F, Feder RS, McLeod SD, Parke DW. The preferred practice pattern guidelines in ophthalmology. Ophthalmology. 2016;123(5):928–929. doi: 10.1016/j.ophtha.2016.01.024 [DOI] [PubMed] [Google Scholar]
- 6.Miller SC, Fliotsos MJ, Justin GA, et al. Global current practice patterns for the management of open globe injuries. Am J Ophthalmol. 2021;234:259–273. doi: 10.1016/j.ajo.2021.08.003 [DOI] [PubMed] [Google Scholar]
- 7.Wakabayashi T, Miller SC, Patel SN, et al. Global current practice patterns for the management of exogenous endophthalmitis: a survey by the American Society of Ophthalmic Trauma. Curr Eye Res;2022. 1–7. doi: 10.1080/02713683.2022.2103154 [DOI] [PubMed] [Google Scholar]
- 8.Yasuna E. Management of traumatic hyphema. Arch Ophthalmol. 1974;91(3):190–191. doi: 10.1001/archopht.1974.03900060198008 [DOI] [PubMed] [Google Scholar]
- 9.Shammas HF, Matta CS. Outcome of traumatic hyphema. Ann Ophthalmol. 1975;7(5):701–706. [PubMed] [Google Scholar]
- 10.Romano PE, Phillips PJ. Traumatic hyphema: a critical review of the scientifically catastrophic history of steroid treatment therefore; and A report of 24 additional cases with no rebleeding after treatment with the Yasuna systemic steroid, no touch PLUS protocol. Binocul Vis Strabismus Q. 2000;15(2):187–196. [PubMed] [Google Scholar]
- 11.Gharaibeh A, Savage HI, Scherer RW, Goldberg MF, Lindsley K. Medical interventions for traumatic hyphema. Cochrane Database Syst Rev. 2019;1(1):548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spoor TC. Traumatic hyphema: failure of steroids to alter its course: a double-blind prospective study. Arch Ophthalmol. 1980;98(1):116. doi: 10.1001/archopht.1980.01020030118011 [DOI] [PubMed] [Google Scholar]
- 13.Rahmani B, Jahadi HR. Comparison of tranexamic acid and prednisolone in the treatment of traumatic hyphema: a randomized clinical trial. Ophthalmology. 1999;106(2):375–379. doi: 10.1016/S0161-6420(99)90079-9 [DOI] [PubMed] [Google Scholar]
- 14.Fong LP. Secondary hemorrhage in traumatic hyphema: predictive factors for selective prophylaxis. Ophthalmology. 1994;101(9):1583–1588. doi: 10.1016/S0161-6420(94)31134-1 [DOI] [PubMed] [Google Scholar]
- 15.Gorn RA. The detrimental effect of aspirin on hyphema rebleed. Ann Ophthalmol. 1979;11(3):351–355. [PubMed] [Google Scholar]
- 16.Thomas MA, Parrish RK, Feuer WJ. Rebleeding after traumatic hyphema. Arch Ophthalmol. 1986;104(2):206–210. doi: 10.1001/archopht.1986.01050140060020 [DOI] [PubMed] [Google Scholar]
- 17.Fraser IS, Porte RJ, Kouides PA, Lukes AS, Benefit-Risk A. Review of systemic haemostatic agents. Drug Saf. 2008;31(3):217–230. doi: 10.2165/00002018-200831030-00003 [DOI] [PubMed] [Google Scholar]
- 18.Kojima S, Inatani M, Shobayashi K, Haga A, Inoue T, Tanihara H. Risk factors for hyphema after trabeculectomy with Mitomycin C. J Glaucoma. 2014;23(5):307–311. doi: 10.1097/IJG.0b013e3182741c85 [DOI] [PubMed] [Google Scholar]
- 19.Alwitry A, King AJ, Vernon SA. Anticoagulation therapy in glaucoma surgery. Graefes Arch Clin Exp Ophthalmol. 2008;246(6):891–896. doi: 10.1007/s00417-008-0792-9 [DOI] [PubMed] [Google Scholar]
- 20.Nasrullah A, Kerr NC. Sickle cell trait as a risk factor for secondary hemorrhage in children with traumatic hyphema. Am J Ophthalmol. 1997;123(6):783–790. doi: 10.1016/S0002-9394(14)71127-4 [DOI] [PubMed] [Google Scholar]
- 21.Shingleton BJ. Eye injuries. N Engl J Med. 1991;325(6):408–413. doi: 10.1056/NEJM199108083250607 [DOI] [PubMed] [Google Scholar]
- 22.Ojodu J, Hulihan MM, Pope SN, Grant AM. Incidence of sickle cell trait — United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63(49):1155–1158. [PMC free article] [PubMed] [Google Scholar]
- 23.Recchia FM, Saluja RK, Hammel K, Jeffers JB. Outpatient management of traumatic microhyphema. Ophthalmology. 2002;109(8):1465–1470. doi: 10.1016/S0161-6420(02)01091-6 [DOI] [PubMed] [Google Scholar]
- 24.Rakusin W. Traumatic hyphema. Am J Ophthalmol. 1972;74(2):284–292. doi: 10.1016/0002-9394(72)90546-6 [DOI] [PubMed] [Google Scholar]
- 25.Graul TA, Ruttum MS, Lloyd MA, Radius RL, Hyndiuk RA. Trabeculectomy for traumatic hyphema with increased intraocular pressure. Am J Ophthalmol. 1994;117(2):155–159. doi: 10.1016/S0002-9394(14)73070-3 [DOI] [PubMed] [Google Scholar]
- 26.Read J. Traumatic hyphema: surgical vs medical management. Ann Ophthalmol. 1975;7(5):659–62, 664–6, 668–70. [PubMed] [Google Scholar]
- 27.Read J, Goldberg MF. Comparison of medical treatment for traumatic hyphema. Trans Am Acad Ophthalmol Otolaryngol. 1974;78(5):OP799–OP815. [Google Scholar]
- 28.Deutsch TA, Weinreb RN, Goldberg MF. Indications for surgical management of hyphema in patients with sickle cell trait. Arch Ophthalmol. 1984;102(4):566–569. doi: 10.1001/archopht.1984.01040030444022 [DOI] [PubMed] [Google Scholar]
- 29.Caprioli J, Sears ML. The histopathology of black ball hyphema: a report of two cases. Ophthalmic Surg. 1984;15(6):491–495. [PubMed] [Google Scholar]
- 30.Weiss JS, Parrish RK, Anderson DR. Surgical therapy of traumatic hyphema. Ophthalmic Surg. 1983;14(4):343–345. [PubMed] [Google Scholar]
- 31.Thangamathesvaran L, Miller SC, Tsou B, et al. Global Current Practice Patterns for the Management of Central Retinal Artery Occlusion. Ophthalmology Retina. 2022;6(5):429–431. doi: 10.1016/j.oret.2022.01.014 [DOI] [PubMed] [Google Scholar]
- 32.Aylward GW, Dunlop IS, Little BC. Meta-analysis of systemic anti-fibrinolytics in traumatic hyphaema. Eye (Lond). 1994;8(4):440–2. doi: 10.1038/eye.1994.104 [DOI] [PubMed] [Google Scholar]