Abstract
Objective:
To compare outcomes associated with timing – early versus late – of any neurologic dysfunction during pediatric sepsis
Design:
Secondary analysis of a cross-sectional point prevalence study
Setting:
128 PICUs in 26 countries
Patients:
<18 years with severe sepsis on five separate days (2013-2014)
Interventions:
None
Measurements and Main Results:
Patients were categorized as having either no neurologic dysfunction or neurologic dysfunction (i.e., present at or after sepsis recognition), which was defined as Glasgow Coma Scale score <5 and/or fixed dilated pupils. Our primary outcome was death or new moderate disability (i.e., pediatric overall [or cerebral] performance category score ≥3 and change ≥1 from baseline) at hospital discharge and 87/567 (15%) severe sepsis patients had neurologic dysfunction within seven days of sepsis recognition (61 at sepsis recognition and 26 after sepsis recognition). Primary site of infection varied based on presence of neurologic dysfunction. Death or new moderate disability occurred in 161/480 (34%) without neurologic dysfunction, 45/61 (74%) with neurologic dysfunction at sepsis recognition, and 21/26 (81%) with neurologic dysfunction after sepsis recognition (p<0.001 across all groups). On multivariable analysis, in comparison with those without neurologic dysfunction, neurologic dysfunction whether at sepsis recognition or after were associated with increased odds of death or new moderate disability (adjusted odds ratio 4.9 [95%CI 2.3-10.1] and 10.7 [95%CI 3.8-30.5], respectively). We failed to identify a difference between these adjusted odds ratios of death or new moderate disability that would indicate a differential risk of outcome based on timing of neurologic dysfunction (p=0.20).
Conclusions:
In this severe sepsis international cohort, the presence of neurologic dysfunction during sepsis is associated with worse outcomes at hospital discharge. The impact of early versus late onset of neurologic dysfunction in sepsis on outcome remains unknown and further work is needed to better understand timing of neurologic dysfunction onset in pediatric sepsis.
Keywords: severe sepsis, children, neurologic dysfunction, outcomes, epidemiology, pediatric
Introduction
Sepsis remains an important cause of mortality and morbidity in critically ill children (1–5), with increasing number and severity of organ dysfunctions being a major risk factor for adverse outcomes (6,7). In particular, neurologic dysfunction at any time during sepsis occurs in up to 20% of children who die (8), with a five-fold increase in mortality among those who develop neurologic dysfunction at any point during sepsis (1,9). Survivors who have neurologic dysfunction during sepsis also have higher odds for experiencing a decline in health-related quality of life (HRQL) after discharge (2). Together, these data suggest that neurologic dysfunction has a significant impact on adverse outcomes in pediatric sepsis. However, there are limited data on whether the timing of neurologic dysfunction during pediatric sepsis is important.
We sought to determine the association between timing of neurologic dysfunction during pediatric sepsis with mortality and morbidity outcomes at hospital discharge. It has been previously shown that later onset of any organ dysfunction is associated with worse outcomes (1,8,9), which we hypothesize is due to differences in the pathophysiology of organ dysfunction before and after initiation of sepsis treatment. Thus, we hypothesized that neurologic dysfunction developing after sepsis recognition (i.e., on or after day 2 of sepsis), in comparison with development at the time of diagnosis, is associated with greater odds of death during hospitalization or, in survivors, new moderate disability at hospital discharge. An improved understanding of these relationships should inform future studies of explanatory risk factors and treatment of neurologic dysfunction during pediatric sepsis.
Methods
We performed a secondary analysis of patients with and without neurologic dysfunction during sepsis from the previously reported Sepsis Prevalence, Outcomes and Therapies (SPROUT) study data set (1). The SPROUT study was a prospective, cross-sectional point prevalence sepsis study performed in 128 pediatric intensive care units (PICUs) across 26 countries (1). Patients were enrolled on five separate days from June 2013 to June 2014. Ethics approval was obtained at all study sites (IRB #12-009784, Children’s Hospital of Philadelphia, main study site) (1). Waiver of consent was obtained at all sites for screening. Waiver of consent for data collection was approved at all but three sites where informed consent was required.
Study population
On each study day, all patients <18 years of age in a participating PICU were screened for eligibility at 9:00am local time. Severe sepsis was defined using the 2005 International Pediatric Sepsis Consensus criteria. Patients were classified as having severe sepsis if they had 1) two or more systemic inflammatory response syndrome criteria, 2) confirmed or suspected infection, and 3) cardiovascular dysfunction, acute respiratory distress syndrome, or at least two other organ dysfunctions (10). The cohort with septic shock, defined by the presence of cardiovascular dysfunction, is included within the term severe sepsis. Patients were only included if they met criteria for severe sepsis within the 24 hours prior to the study day. Exclusion criteria were gestational age <42 weeks corrected or surgery requiring cardiopulmonary bypass within 5 days leading up to study enrollment. Additional SPROUT methodology details have been previously published (1).
Data collection
Demographic, clinical, and infectious data were collected from the medical record. Day of sepsis recognition was determined as the first calendar day on which the patient met criteria for severe sepsis. Infectious data collected included primary site of infection and microbiological isolates (11). Illness severity was described using the Pediatric Index of Mortality-3 (PIM3) Risk of Mortality at PICU admission (12). For this analysis, we calculated the PIM3 Risk of Mortality without the neurologic component of the score for all analyses. Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores measured prior to septic illness were obtained as indicator of baseline functional status (13).
During the SPROUT study, organ dysfunctions were measured daily for 7 days from sepsis recognition based on criteria first published by Wilkinson et al and later modified by Proulx et al (1,14,15). Specifically, neurologic dysfunction was defined as Glasgow Coma Scale (GCS) <5 and/or fixed dilated pupils on the day of interest (1,15). Study investigators were instructed to assess the GCS in patients when not pharmacologically sedated and to not record abnormal pupillary responses secondary to drugs, toxins, or eye injury. Patients that met this definition on day of sepsis recognition (day 1) were classified as having neurologic dysfunction at sepsis recognition. If the neurologic dysfunction definition was not met on day 1 but was met on any of days 2-7 of sepsis, patients were classified as having neurologic dysfunction after sepsis recognition. Patients who did not meet criteria for neurologic dysfunction on days 1-7 were categorized as having no neurologic dysfunction.
The primary outcome was death or new moderate disability, defined as a composite of mortality or new moderate disability in survivors. Patients were followed until hospital discharge (or up to 90 days after the study day if still hospitalized) to determine all-cause mortality. For patients who survived, POPC and PCPC scores were calculated at the time of hospital discharge to characterize the development of a new disability in comparison to baseline POPC and PCPC scores. We defined new moderate disability as both a POPC or PCPC score ≥3 at hospital discharge and ≥ 1 increase from baseline in order to identify new functional decline even in those with moderate to severe baseline impairment (4). Although the initial SPROUT study analysis defined new disability only using the POPC score (1), we used a change in either POPC or PCPC to indicate new disability to more reliably include subtle cognitive deficits that might not be captured using the global POPC score alone (13). Secondary outcomes included hospital mortality, new moderate disability, new mild disability (defined as any increase in POPC or PCPC from baseline to discharge and did not meet definition for new moderate disability), PICU length of stay (LOS), and hospital LOS. Ventilator- and vasoactive-free days were determined from the day of sepsis recognition through day 28 or day of death (whichever came first) (1,16). Survivors who did not experience an increase in POPC and PCPC score were considered to have no new disability.
Statistical Analysis
Data were analyzed using STATA (Version 16.1, Statacorp, College Station, Texas). Categorical data, expressed as frequencies, were analyzed with Fisher’s exact test. Continuous data, expressed as median with interquartile range (IQR), were analyzed using the Mann-Whitney U or Kruskal-Wallis tests. We used logistic regression to create a bivariable model with neurologic dysfunction as the independent variable (modeled categorically as no neurologic dysfunction [reference], neurologic dysfunction at sepsis recognition, and neurologic dysfunction after sepsis recognition) and the primary outcome of death or new moderate disability as the dependent variable. Multivariable logistic regression was used to test for potential cofounding effects of covariates on the association of neurological dysfunction with death or a new moderate disability. To assess for confounding, covariates that were significantly different between patients with no neurologic dysfunction, neurologic dysfunction at sepsis recognition, and neurologic dysfunction after sepsis recognition were added to the bivariable model. Covariates that changed the base model odds ratio (OR) by 5% or greater were included in the final multivariable model as confounders (17). A Wald Test was used to test if there was a difference between the adjusted odds ratio based on timing of neurologic dysfunction. A base p-value <0.05 was used to indicate statistical significance with Bonferroni’s methods used to account for multiple comparisons.
Results
Comparison by Presence versus Absence of Neurologic Dysfunction
Of the 6,925 patients screened during the five SPROUT study days, 569 met consensus criteria for severe sepsis. Data for analysis was available for 567 patients (2 patients did not provide consent). From these 567 patients, 87 patients (15%) had neurologic dysfunction at any time during sepsis. Demographic and clinical characteristics for patients with and without neurological dysfunction during sepsis are shown in eTable 1. Patients with neurological dysfunction at any time during sepsis had higher PIM3 risk of mortality score compared to patients who never had neurologic dysfunction (p<0.001). Patients with neurologic dysfunction during sepsis had a higher number of non-neurologic organ dysfunctions on day 1 of sepsis but this was not statistically different from patients without neurologic dysfunction during sepsis after correction for multiple comparisons. Additionally, patients with neurologic dysfunction during sepsis had fewer ventilator- and vasoactive-free days compared to those who never had neurologic dysfunction (p<0.001, eTable 1). Seventy-six percent of patients experienced death or new moderate disability when neurologic dysfunction was present during sepsis compared to 34% in patients without neurologic dysfunction during sepsis (mean difference 42% [95%CI 31-51%], p<0.001; eTable 1).
Comparison by Presence and Timing of Neurologic Dysfunction
Among the 87 patients with neurologic dysfunction during sepsis, 61 patients (70%) had neurologic dysfunction at sepsis recognition (day 1) and 26 (30%) developed neurologic dysfunction after sepsis recognition (days 2-7; eFigure 1). Clinical and infectious characteristics for those with no neurologic dysfunction, neurologic dysfunction at sepsis recognition, and neurologic dysfunction after sepsis recognition are shown in Table 1. Across these three groups, there were no differences in age, sex, number of comorbidities, or type of isolates identified (all p>0.05). PIM3 Risk of Mortality was significantly different between the three groups, with higher scores in patients with neurologic dysfunction at or after sepsis recognition compared to patients without neurologic dysfunction (p<0.001). More non-neurologic organ dysfunctions were present in patients with neurologic dysfunction at or after sepsis recognition compared to patients without neurologic dysfunction (p=0.002). Primary site of infection was different between the three groups (p<0.001). In those without neurologic dysfunction during sepsis, respiratory and other site infections were most common, followed by bloodstream infections and then central nervous system (CNS) infections. However, for those with neurologic dysfunction at sepsis recognition, there were more CNS infections than bloodstream infections and for those with neurologic dysfunction after sepsis recognition bloodstream infections predominated above all sites of infections. Detailed microbiological isolate information is presented in eTable 2.
Table 1.
Clinical and Infectious Characteristics
| Neurologic Dysfunction Absent (n=480) | Neurologic Dysfunction Present | P value a,b (All groups) | P value b,c (Comparison between patients with Neurologic Dysfunction only) | ||
|---|---|---|---|---|---|
| At Sepsis Recognition (n=61) | After Sepsis Recognition (n=26) | ||||
| Age, years (median [IQR]) | 3 [0.7-10] | 7 [0.9-12] | 4 [0.5-14] | 0.18 | 0.47 |
| Male (n, %) | 257 (54) | 29 (48) | 16 (62) | 0.49 | 0.25 |
| Comorbid Conditions (n, %) | |||||
| Respiratory | 150 (31) | 12 (20) | 10 (36) | 0.11 | 0.10 |
| Gastrointestinal | 124 (26) | 10 (16) | 7 (27) | 0.27 | 0.38 |
| Cardiovascular | 121 (25) | 10 (16) | 5 (19) | 0.28 | 0.76 |
| Genetic | 103 (22) | 8 (13) | 4 (15) | 0.28 | 0.75 |
| Hematologic/Immunologic | 100 (21) | 9 (15) | 5 (19) | 0.56 | 0.75 |
| Neuromuscular | 80 (17) | 12 (20) | 5 (19) | 0.76 | 1.00 |
| Neoplastic | 70 (15) | 8 (13) | 2 (8) | 0.72 | 0.72 |
| Prematurity | 73 (15) | 2 (3) | 1 (4) | 0.008 | 1.00 |
| Metabolic | 52 (11) | 8 (13) | 2 (8) | 0.80 | 0.72 |
| Renal | 45 (9) | 7 (12) | 3 (12) | 0.72 | 1.00 |
| Solid organ/Stem cell transplant | 47 (10) | 4 (7) | 3 (12) | 0.67 | 0.42 |
| Number of comorbidities (n, %) | 0.09 | 0.61 | |||
| None | 100 (21) | 21 (34) | 7 (27) | ||
| 1 | 118 (25) | 17 (28) | 6 (23) | ||
| 2 or more | 262 (55) | 23 (38) | 13 (50) | ||
| Admission Type (n, %) | <0.001 | 0.09 | |||
| Medical | 389 (81) | 47 (77) | 24 (92) | ||
| Surgical | 80 (17) | 5 (8) | 2 (8) | ||
| Trauma | 11 (2) | 9 (15) | 0 (0) | ||
| Non-Neurologic PIM3 Risk of Mortality (%, median [IQR]) | 3.7 [1.6-7.2] | 8.7 [3.9-20.2] | 6.2 [4.3-11.1] | <0.001 | 0.09 |
| Non-Neurologic Organ Dysfunction, Day 1 (n, %) | 0.002 | 0.11 | |||
| None | 92 (19) | 3 (5) | 5 (19) | ||
| 1 | 151 (32) | 20 (33) | 10 (39) | ||
| 2 | 185 (39) | 23 (38) | 5 (19) | ||
| More than 3 | 52 (11) | 15 (25) | 6 (23) | ||
| Primary Site of Infection (n, %) | <0.001 | 0.11 | |||
| Respiratory | 199 (41) | 21 (34) | 8 (31) | ||
| Bloodstream | 89 (19) | 9 (15) | 10 (38) | ||
| Central Nervous System | 14 (3) | 10 (17) | 2 (8) | ||
| Other | 178 (37) | 21 (34) | 6 (23) | ||
| Bacterial Isolate (n, %) | 231 (48) | 35 (57) | 13 (50) | 0.38 | 0.64 |
| Viral Isolate (n, %) | 93 (19) | 16 (26) | 8 (31) | 0.17 | 0.79 |
| Fungal Isolate (n, %) | 60 (13) | 5 (8) | 6 (23) | 0.17 | 0.08 |
IQR = interquartile range, PIM = Pediatric Index of Mortality;
= Fisher’s exact test or Kruskal-Wallis test across groups;
= multiple comparisons p-value threshold for significance <0.0024;
= Fisher’s exact test or Mann-Whitney U test between groups
The distribution of baseline POPC scores was significantly different between the three groups (p=0.03), with a greater proportion of patients with neurologic dysfunction at and after sepsis recognition having POPC scores 3-5 compared to patients without neurologic dysfunction during sepsis (Figure 1). However, the distribution of baseline POPC scores did not differ between those with neurologic dysfunction at versus after sepsis recognition.
Figure 1. Baseline Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) Scores by presence and timing of neurologic dysfunction.
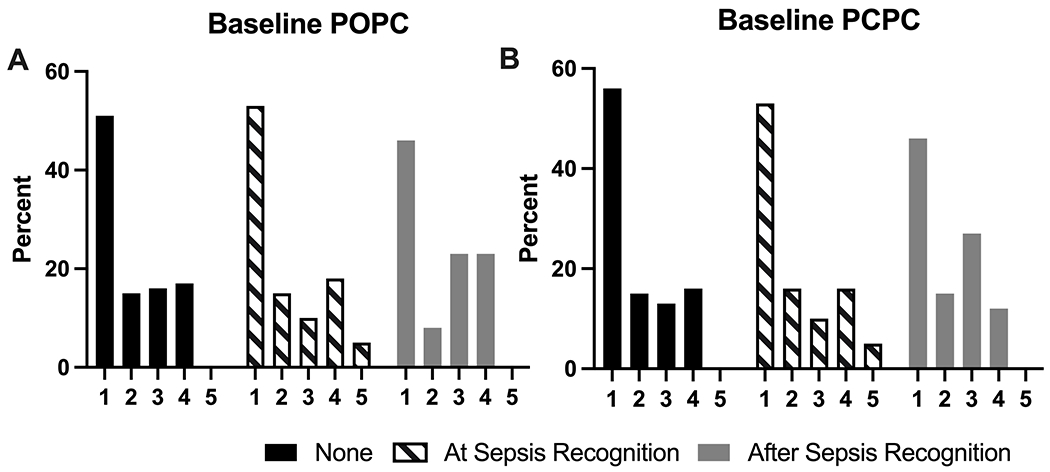
The distribution of POPC scores was different between the three groups (p=0.03, Fisher’s exact test), but there was no difference in the distribution when comparing neurologic dysfunction by timing of onset (p=0.38, Fisher’s exact test). The difference in PCPC score distribution was not different between the three groups (p=0.05, Fischer’s exact test).
Outcomes
Death or new moderate disability occurred in 161/480 (34%) patients without neurologic dysfunction during sepsis compared to 45/61 (74%) patients with neurologic dysfunction at sepsis recognition and 21/26 (81%) patients with neurologic dysfunction after sepsis recognition (p<0.001 across all three groups, Table 2). When further stratifying by day of neurologic dysfunction onset, we failed to identify a difference in the proportion of patients who experienced death or new moderate disability by timing of neurologic dysfunction (Figure 2).
Table 2.
Outcomes
| Neurologic Dysfunction Absent (n=480) | Neurologic Dysfunction Present | P value a, b (All groups) |
P value c (Comparison between patients with Neurologic Dysfunction only) | ||
|---|---|---|---|---|---|
| At Sepsis Recognition (n=61) | After Sepsis Recognition (n=26) | ||||
| Death or Moderate Disabilityd (n, %) | 161 (34) | 45 (74) | 21 (81) | <0.001 | 0.59 |
| Hospital Mortality (n, %) | 98 (20) | 32 (53) | 15 (58) | <0.001 | 0.82 |
| At Least Moderate Disabilityd (n, %) | 63 (16) | 13 (45) | 6 (55) | <0.001 | 0.73 |
| At Least Mild Disabilitye (n, %) | 107 (28) | 17 (59) | 7 (64) | 0.49 | 0.57 |
| PICU LOS, days (median [IQR]) | 15 [8-35] | 15 [5-32] | 18 [8-46] | 0.83 | 0.59 |
| Hospital LOS, days (median [IQR]) | 27 [14-57] | 18 [9-48] | 28 [10-54] | 0.11 | 0.59 |
| Ventilator-free days (median [IQR]) | 19 [1-26] | 0 [0-18] | 0 [0-7] | <0.001 | 0.32 |
| Vasoactive-free days (median [IQR]) | 24 [17-28] | 18 [0-25] | 13 [0-23] | <0.001 | 0.29 |
| Ventilator daysf (median [IQR]) | 7 [0-16] | 11 [3-28] | 24 [7-28] | 0.001 | 0.65 |
| Vasoactive daysf (median [IQR]) | 2 [0-7] | 4 [0-8] | 6 [1-13] | 0.09 | 0.51 |
PICU = pediatric intensive care unit; LOS = length of stay; IQR = interquartile range
= Fisher’s exact test or Kruskal-Wallis test across groups;
= multiple comparisons, p-value threshold for significance <0.005;
= Fisher’s exact test or Mann-Whitney U test between groups
= POPC/PCPC scores ≥ 3 and change ≥ 1 in survivors;
= POPC/PCPC change ≥ 1 in survivors;
= survivors only
Figure 2. Proportion of patients who experienced death or moderate disability based on day of neurologic dysfunction recognition.
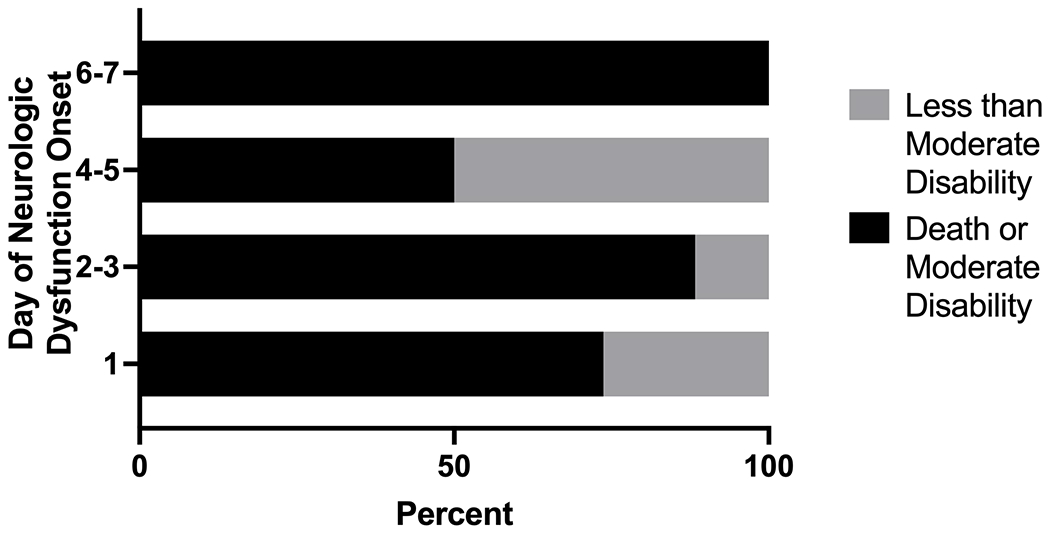
Graphical representation of outcomes based on timing of neurologic dysfunction onset in the first seven days of severe sepsis. Neurologic dysfunction at sepsis recognition is represented as day 1 of neurologic dysfunction onset (n=61). Neurologic dysfunction after sepsis recognition was stratified into three groups based on onset after sepsis recognition: days 2-3 (n=17), days 4-5 (n=6) and days 6-7 (n=3). The proportion of patients who died or had moderate disability was not different based on the specific day of sepsis recognition (p=0.22, Fisher’s exact test).
In assessing secondary outcomes, in comparison with patients without neurologic dysfunction, the presence of neurologic dysfunction whether at or after sepsis recognition were associated with death and new moderate disability (Table 2). We failed to find an association between presence of neurologic dysfunction and PICU and hospital LOS (all p>0.05). Neurologic dysfunction at and after sepsis recognition was associated with longer ventilator and vasoactive durations (lower ventilator- and vasoactive-free days) compared to those without neurologic dysfunction, although we failed to identify association in these outcomes and timing of neurologic dysfunction (Table 2). When mild dysfunction was also considered, the distribution of adverse outcomes remained different between those with neurologic dysfunction at or after sepsis recognition compared to patients without neurologic dysfunction (p<0.001, Figure 3). However, this difference was due to higher proportions of patients with either death or new moderate disability among those with neurologic dysfunction compared to patients without neurologic dysfunction, while mild disability was less common.
Figure 3. Proportion of outcomes following pediatric sepsis based on presence and timing of neurologic dysfunction.
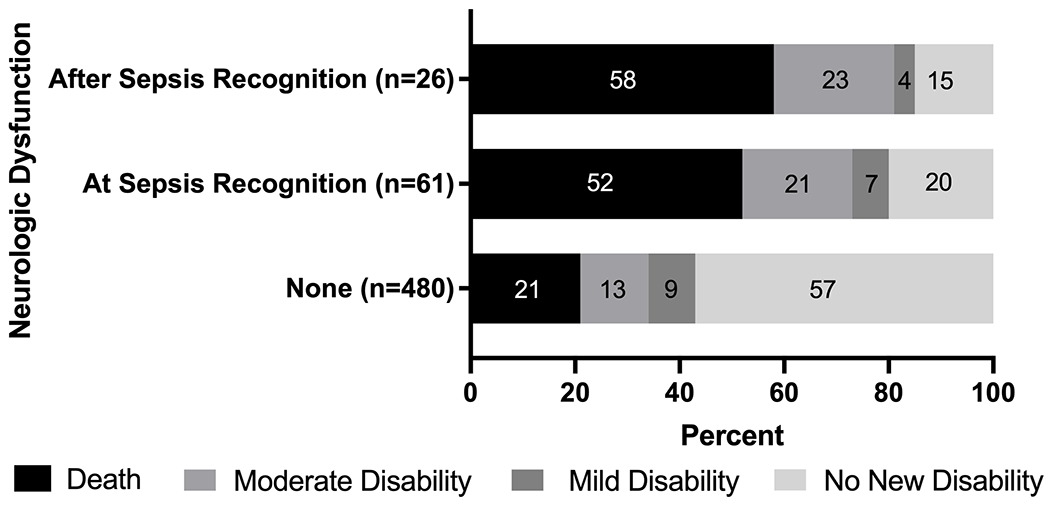
All outcomes were determined at hospital discharge. Moderate disability was defined as POPC or PCPC score ≥3 and ≥ 1 increase from baseline and mild disability was any increase in POPC or PCPC score from baseline that does not meet criteria for moderate disability. Survivors without an increase in POPC or PCPC score were labeled as no new disability. The bars are labeled with the percentage of patients within each group that experienced the outcome. The total size for each group is displayed. The proportion of patients with each of these outcomes was different between the three groups (p<0.001, Fisher’s exact test) but was no different based on the timing of neurologic dysfunction during sepsis (p=0.96, Fisher’s exact test).
After controlling for baseline POPC score, PIM3 Risk of Mortality, number of non-neurologic organ dysfunctions at sepsis recognition, number of co-morbidities, and primary site of infection, neurologic dysfunction at sepsis recognition (aOR 4.9, 95%CI 2.3-10.1) and neurologic dysfunction after sepsis recognition (aOR 10.7, 95%CI 3.8-30.5) were associated with increased odds of death or new moderate disability compared to patients without neurologic dysfunction (Table 3). We failed to identify a difference in odds of death or new moderate disability based on timing of neurologic dysfunction (p=0.20).
Table 3.
Multivariable Analysis Showing Association Between Neurologic Dysfunction and Confounders to Primary Outcome (Mortality or New Moderate Disability at Hospital Discharge)
| Variable | aOR | 95% CI | P value |
|---|---|---|---|
| Neurologic Dysfunction | |||
| None | Reference | ||
| At Sepsis Recognition | 4.9 | 2.3-10.1 | <0.001 |
| After Sepsis Recognition | 10.7 | 3.8-30.5 | <0.001 |
| POPC score, baseline | |||
| 1 | Reference | ||
| 2 | 1.3 | 0.8-2.4 | 0.32 |
| 3 | 0.8 | 0.4-1.4 | 0.39 |
| 4 | 0.2 | 0.1-0.4 | <0.001 |
| 5 | 0.3 | 0.0-4.5 | 0.42 |
| Non-Neurologic PIM3 Risk of Mortality | 1.1 | 1.0-1.1 | <0.001 |
| Number of non-neurologic organ dysfunctions, Day 1 | |||
| None | Reference | ||
| 1 | 2.5 | 1.3-4.8 | 0.004 |
| 2 | 2.8 | 1.5-5.2 | 0.001 |
| More than 3 | 3.7 | 1.6-8.2 | 0.002 |
| Number of co-morbidities | |||
| None | Reference | ||
| 1 | 1.2 | 0.7-2.2 | 0.53 |
| More than 2 | 2.7 | 1.5-5.1 | 0.001 |
| Primary Site of Infection | |||
| Respiratory | Reference | ||
| Bloodstream | 1.2 | 0.7-2.0 | 0.59 |
| Central Nervous System | 2.0 | 0.7-5.5 | 0.18 |
| Other | 1.1 | 0.7-1.7 | 0.67 |
POPC = Pediatric Overall Performance Category, PIM = Pediatric Index of Mortality
Discussion
In this secondary analysis of the SPROUT study, we found neurologic dysfunction at or after sepsis recognition – as compared with no neurologic dysfunction – was associated with greater odds of death or, in survivors, new moderate disability at hospital discharge. Our study expands on prior literature of neurologic dysfunction in sepsis to compare the differential impact of timing of neurologic dysfunction during sepsis on the risk of death or new moderate disability among survivors. Although we failed to identify a difference between the adjusted odds ratios for death or new moderate disability between patients with neurologic dysfunction at versus after sepsis recognition, the higher point estimate and upper limit of the 95% confidence interval for the group with neurologic dysfunction after sepsis recognition suggests that larger studies might identify a differential risk for poor outcomes based on the timing of neurologic dysfunction during pediatric sepsis.
The timing and evolution of organ dysfunction during sepsis has been shown to be related to outcomes in pediatric and adult sepsis cohorts (9,18). Both new and worsening organ dysfunction have been associated with higher mortality in sepsis (9,18–22). Given the significant impact neurologic dysfunction has on mortality and morbidity outcomes in septic patients and the potential to impact patient outcomes (1–5,9), differentiating between early and late onset of neurologic dysfunction is imperative. There are several proposed mechanisms for the development of neurologic dysfunction in sepsis including neuroinflammation, metabolic derangements, neurotransmitter dysregulation, mitochondrial and endothelial dysfunction, and cerebral blood flow alterations (23,24). Similarly, dysregulation of immunological, microvascular, mitochondrial, and metabolic pathways has been linked to ongoing organ dysfunction and mortality in sepsis (25–30). Further studies about the pathophysiological mechanisms underlying early versus late onset of neurological dysfunction in sepsis will help guide therapies to manage initial neurologic dysfunction and prevent the development of new neurologic dysfunction in sepsis.
It is notable that neurologic dysfunction was recognized in only 15% of patients in this study using the Proulx criteria (GCS <5 and/or fixed dilated pupils). Acknowledging that this definition of neurologic dysfunction is conservative, we suspect that the prevalence of true neurologic dysfunction during sepsis was underestimated in this study. Studies in critically ill adults suggest neurologic dysfunction in sepsis occurs in up to 70% of patients with more inclusive definitions that consider clinical findings, delirium, and/or neuroimaging or electroencephalogram findings. Yet, the lack of a standardized definition accounts for the varying prevalence of neurologic dysfunction seen in these adult studies (31–33). There are limited pediatric studies describing sepsis-associated neurologic dysfunction and thus the true prevalence of this complication is not well described. Several studies have shown that GCS is limited by poor interrater reliability and scoring inaccuracies in critically ill children and those with developmental disabilities (34,35). While the accuracy of GCS in classifying neurologic dysfunction specifically in pediatric sepsis has not been evaluated to date, GCS is universally included in the definition of neurologic dysfunction in these patients (10,36–38). An improved definition for neurologic dysfunction in pediatric sepsis is needed to improve epidemiological understanding of this disease entity and guide further prospective and observational studies. We anticipate that a neurologic dysfunction definition in sepsis for future prospective studies will involve input from multiple modalities to reflect clinician concerns for neurologic dysfunction. However, further studies are needed to validate any proposed definition for these future studies.
With reduction in mortality rates from pediatric sepsis, a shift to understanding risk factors for adverse outcomes is emerging as a significant public health concern. The Life After Pediatric Sepsis (LAPSE) study showed that one-third of pediatric sepsis survivors have a reduction in HRQL that persists for one year following hospital discharge (5). This study found that patients who experienced an acute, clinically diagnosed neurologic event or new pathology (e.g., seizure, new hypoxic ischemic injury) had a five-fold increased risk for death or persistent deficit in HRQL at 3 months following discharge (2). Although this study used a clinical diagnosis for neurologic events and not a specific neurologic dysfunction definition, the LAPSE results are consistent with our finding that neurologic dysfunction at or after sepsis recognition is a risk factor for both mortality and morbidity. In addition, a single-center study found that children with clinically diagnosed sepsis-associated encephalopathy had delayed development, lower intelligence, and decline in school function (39). Neither of these studies discussed the association of timing of the neurologic dysfunction onset with outcomes, further highlighting the gaps in our understanding of the difference between early and late onset of neurologic dysfunction as it pertains to long-term patient outcomes.
Our study has several strengths, including the multicenter and international study population, detailed data on timing of neurologic dysfunction onset despite the point prevalence study design, and data on new disability in survivors based on functional outcomes. This study also has several limitations. First, a small number of patients were categorized as having neurologic dysfunction during sepsis due to the restrictive yet accepted definition for neurologic dysfunction. The resulting small sample size limited statistical power and increased the risk of a type II error to fail to reject the null hypothesis of no difference in death or new moderate disability between patients with neurological dysfunction at versus after sepsis recognition. In addition, data were not available regarding neuroimaging or electroencephalogram results, neurological examinations, or the presence of delirium, thus we were unable to further characterize the full spectrum of neurologic dysfunction in this analysis. Such detailed neurological evaluation data and the relationship to the patients’ clinical status would be best obtained in a prospective study aimed directly at evaluating the timing of neurologic dysfunction during sepsis. Although sites were asked to categorize neurologic dysfunction when the patient was not sedated, we were not able to ascertain if sedation use contributed to misclassification bias for the timing of neurologic dysfunction. This study only evaluated outcomes at the time of hospital discharge, further studies are needed to evaluate long-term morbidity outcomes following discharge based on the presence and timing of neurologic dysfunction in pediatric sepsis (40–42). Finally, although we were able to adjust for several potential confounding variables, the secondary nature of the analysis and limited sample size mean that our findings are best construed as exploratory rather than an effort to measure causal inference.
Conclusion
Neurologic dysfunction during sepsis is a significant risk factor for death or new moderate disability irrespective of timing of neurologic dysfunction development relative to sepsis recognition. Although the odds of death or new moderate disability were not significantly different for children with neurologic dysfunction at versus after sepsis recognition, the higher point estimate and upper end of the 95% confidence interval for later onset of neurologic dysfunction support need for consideration of the association of timing of neurologic dysfunction with morbidity and mortality. Understanding risk factors for and the pathophysiology of neurologic dysfunction in pediatric sepsis could help to target mitigation efforts and identify treatment strategies for neurologic dysfunction to improve outcomes from pediatric sepsis.
Supplementary Material
Report in Context:
Neurologic dysfunction during sepsis is associated with higher mortality and worse morbidity in survivors.
The impact of early versus late onset of neurologic dysfunction in sepsis on outcomes is unknown.
Understanding the association of timing of neurologic dysfunction onset to outcomes is imperative for future studies evaluating risk factors for and treatment of neurologic dysfunction in sepsis.
At the Bedside:
Fifteen percent of children with sepsis develop neurologic dysfunction, with the majority of these children experiencing neurologic dysfunction at the time of sepsis recognition.
Adjusting for confounders, our analysis showed that neurologic dysfunction at and after sepsis recognition is associated with higher odds of death or, in those who survive, development of a new moderate disability.
We failed to identify an association between timing of neurologic dysfunction onset and outcome, which may be because of study design. Hence, additional work is needed to better understand timing of neurologic dysfunction onset in pediatric sepsis.
Acknowledgements
We would like to acknowledge all of the SPROUT investigators who contributed to the dataset used for this study. North America: Canada: P. Fontela (Montreal Children’s Hospital-McGill), M. Tucci, M. Dumistrascu (Sainte Justine Hospital), P. Skippen, G. Krahn (BC Children’s Hospital); Puerto Rico: E. Bezares (Hospital Cardiovascular de Puerto Rico y el Caribe), G. Puig, A. Puig-Ramos (San Jorge Children’s Hospital), R. Garcia, M. Villar (University Pediatric Hospital); United States: M. Bigham, T. Polanski, S. Latifi, D. Giebner, H. Anthony (Akron Children’s Hospital), J. Hume, A. Galster, L. Linnerud (Amplatz Children’s Hospital), R. Sanders, G. Hefley (Arkansas Children’s Hospital), K. Madden (Boston Children’s Hospital), A. Thompson, S. Shein (Children’s Hospital of Pittsburgh), S. Gertz (Children’s Hospital-Hackensack), Y. Han, T. Williams, A. Hughes-Schalk (Children’s Mercy Hospital), H. Chandler (Children’s Healthcare of Atlanta), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital in Minnesota), A. Orioles, E. Zielinski, A. Doucette (Children’s Hospital St. Paul), C. Zebuhr, T. Wilson (Children’s Hospital Colorado), C. Dimitriades, J. Ascani, S. Layburn, S. Valley (Children’s Hospital New Orleans), B. Markowitz, J. Terry, R. Morzov (Children’s Hospital of Los Angeles), A. Mcinnes (Children’s Hospital of Monmouth), J. McArthur, K. Woods, K. Murkowski (Children’s Hospital of Wisconsin), M. Spaeder, M. Sharron (Children’s National Medical Center), D. Wheeler, E. Beckman, E. Frank, K. Howard (Cincinnati Children’s Medical Center), C. Carroll (Connecticut Children’s), S. Nett, D. Jarvis (Dartmouth Hitchcock), V. Patel (Dayton Children’s Hospital), R. Higgerson, L. Christie (Dell Children’s Medical Center), K. Typpo, J. Deschenes (Diamond Children’s Hospital), A. Kirby (Doernbecher Children’s Hospital), T. Uhl, K. Rehder, I. Cheifetz, S. Wrenn (Duke Children’s Hospital), K. Kypuros (El Paso Children’s Hospital), K. Ackerman (Golisano Children’s Hospital), F. Maffei, G. Bloomquist (Janet Weis/Geisinger), N. Rizkalla (Johns Hopkins), D. Kimura, S. Shah, C. Tigges (Le Bonheur Children’s Hospital), F. Su, C. Barlow (Lucile Packard Children’s Hospital), K. Michelson, K. Wolfe, D. Goodman, L. Campbell, L. Sorce (Lurie Children’s Hospital of Chicago), K. Bysani, T. Monjure (Medical City Children’s-Dallas), M. Evans (Medical University of South Carolina), B. Totapally, M. Chegondi, C. Rodriguez (Miami Children’s Hospital), J. Frazier, L. Steele (Nationwide Children’s Hospital), S. Viteri, A. Costarino (Nemours/ Alfred I. duPont Children’s Hospital), N. Thomas, D. Spear (Penn State Hershey Medical Center), E. Hirshberg, J. Lilley (Primary Children’s Medical Center), C. Rowan, C. Rider (Riley Hospital for Children), J. Kane (Rush Children’s Hospital), J. Zimmerman, C. Greeley (Seattle Children’s Hospital), J. Lin, R. Jacobs (St. Louis Children’s Hospital), M. Parker, K. Culver (Stony Brook University), L. Loftis, N. Jaimon, M. Goldsworthy (Texas Children’s Hospital), J. Fitzgerald, S. Weiss, V. Nadkarni, J. Bush, M. Diliberto (The Children’s Hospital of Philadelphia), C. Allen, M. Gessouroun (Oklahoma University Medical Center), A. Sapru, T. Lang, M. Alkhouli (University of California San Francisco), S. Kamath, D. Friel, J. Daufeldt (University of Iowa), D. Hsing, C. Carlo, S. Pon (Weill Cornell Medical Center), J. Scimeme, A. Shaheen (Wolfson Children’s Hospital), A. Hassinger, H. Qiao (Women and Children’s Hospital of Buffalo), J. Giuliano, J. Tala (Yale Children’s Hospital). South America: Argentina: D. Vinciguerra, A. Fernandez (Hospital Durand); Colombia: R. Carrero (Clínica Infantil Colsubsidio), P. Hoyos (Hospital de San Jose), J. Jaramillo, A. Posada (Hospital General de Medellín), L. Izquiierdo (Hospital Military Central), B.E. Piñeres Olave, J. Donado (Pablo Tobón Uribe); Chile: R. Dalmazzo, S. Rendich (Clínica Las Condes), L. Palma, M. Lapadula (Clínica Santa María), C. Acuna (Hospital Luis Calvo Mackenna), P. Cruces (Hospital Padre Hurtado) Europe: Belgium: S. Clement De Clety, M. Dujardin, C. Berghe, S. Renard (St. Luc University Hospital); Czech Republic: J. Zurek (Masaryk University); Germany: H. Steinherr (Klinikum Augsburg); Greece: K. Mougkou (Aghia Sophia Children’s Hospital), E. Critselis, K. Mougkou (P. & A. Kyriakou Children’s Hospital); Italy: M. Di Nardo, S. Picardo, F. Tortora (Bambino Gesu Area Rossa); E. Rossetti (Bambino Gesu Children’s Hospital); T. Fragasso, P. Cogo, R. Netto (Bambino Gesu Pediatrico); Lithuania: A. Dagys, V. Gurskis, R. Kevalas (Lithuanian University of Health Sciences); Netherlands: C. Neeleman, J. Lemson, C. Luijten (Radboud University Medical Centre); Poland: K. Wojciech, I. Pagowska-Klimek (Polish Mother Memorial Hospital), M. Szczepanska, J. Karpe (Szyszko Śląskiego University); Portugal: P. Nunes, H. Almeida (Hospital Prof Dr. Fernando Fonseca), J. Rios, M. Vieira (Centrol Hospitalar Lisboa Norte); Spain: J. P. Garcia Iniguez, P. Revilla (Childreńs Hospital Miguel Servet), J. Urbano, J. Lopez-Herce, A. Bustinza (Hospital General Universitario Gregorio Marañón), A. Palacios, S. Hofheinz (Hospital 12 de Octubre), A. Rodriguez-Nunez (Hospital Clínico Universitario), S. Sanagustin, E. Gonzalez (Hospital de la Sant Creu Sant Pau), M. Riaza, R. Piaya (Hospital Universitario Madrid), P. Soler (Hospital Carlos Haya Materno Infantil), E. Esteban (Hospital Sant Joan de Déu), J. Laraudogoitia, C. Monge (Hospital Universitario Donostia), V. Herrera, J. Granados (Hospital Universitario Salamanca), C. Gonzalez (Hospital Virgen de la Arrixaca); Turkey: T. Koroglu, E. Ozcelik (Dokuz Eylul University); United Kingdom: P. Baines (Alder Hey Children’s Hospital), A. Plunkett (Birmingham Children’s Hospital), P. Davis, S. George (Bristol Royal Hospital for Children), S. Tibby, J. Harris (Evelina Children’s Hospital), R. Agbeko, R. Lampitt (Great North Children’s Hospital-Newcastle), J. Brierley, M. Peters, A. Jones, T. Dominguez, T. Thiruchelvam, (Great Ormond Street), A. Deep, L. Ridley, W. Bowen (King’s College Hospital), R. Levin, I. Macleod (Royal Hospital for Sick Children), M. Gray, N. Hemat (St George’s Hospital), J. Alexander, S. Ali (University Hospital of North Staffordshire NHS Trust), J. Pappachan, J. McCorkell (University Hospital Southampton NHS Foundation Trust), P. Fortune, M. MacDonald, P. Hudnott (Royal Manchester Children’s Hospital). Asia: China: Q. Suyun (Beijing Children’s Hospital); India: S. Singhi, K. Nallasamy (Advanced Pediatrics), R. Lodha (All India Institute); Japan: N. Shime, Y. Tabata (Kyoto Prefectural), O. Saito, T. Ikeyama (Tokyo Metropolitan), T. Kawasaki (Shizuoka Children’s Hospital); Malaysia: L. Lum, A. Abidin, S. Kee (University Malaya Medical Center), S. Tang, R. Jalil (Kebangsaan Malaysia Medical Center); Singapore: Y. Guan, L. Yao (KK Women’s and Children’s Hospital), K. Lin, J. Ong (National University Hospital). Africa: South Africa: A. Salloo, L. Doedens, L. Mathivha (Chris Hani Baragwanath), G. Reubenson, S. Moaisi (Rahima Moosa), A. Pentz, R. Green (Steve Biko Academic Hospital). Australia: A. Schibler, A. Fernandez (Mater Children’s Hospital), S. Erickson (Princess Margaret Hospital), J. McEneiry, D. Long, T. Dorofaeff, M. Coulthard (Royal Children’s Hospital Brisbane), J. Millar, C. Delzoppo (Royal Children’s Melbourne), G. Williams, M. Morritt (Sydney Children’s Hospital), N. Watts, M. Morritt (Children’s Hospital Westmead). New Zealand: J. Beca, C. Sherring, T. Bushell (Starship Children’s Hospital)
Funding Information:
Supported by the Endowed Chair, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, and the Center for Pediatric Clinical Effectiveness at The Children’s Hospital of Philadelphia. Financial support for data collection in all U.K. centers was provided by the National Institute for Health Research (NIHR) Clinical Research Network and in Southampton by the Southampton NIHR Wellcome Trust Clinical Research Facility. None of the funders participated in the design and conduct of study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Copyright Form Disclosure:
Drs. Weiss and Fitzgerald’s institutions received funding from Children’s Hospital of Philadelphia Center for Pediatric Clinical Effectiveness. Dr. Fitzgerald’s institution received funding from the National Institutes of Health (NIH). Dr. Loftis received support for article research from the NIH. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
Article Tweet: Neurologic dysfunction during pediatric sepsis in the #PedsICU is associated with worse outcomes. Later neurologic dysfunction may confer worse outcomes.
REFERENCES
- 1.Weiss SL, Fitzgerald JC, Pappachan J, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015; 191:1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmerman JJ, Banks R, Berg RA, et al. Critical Illness Factors Associated With Long-Term Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Killien EY, Farris RWD, Watson RS, et al. Health-Related Quality of Life Among Survivors of Pediatric Sepsis. Pediatr Crit Care Med 2019; 20:501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farris RW, Weiss NS, Zimmerman JJ. Functional outcomes in pediatric severe sepsis: further analysis of the researching severe sepsis and organ dysfunction in children: a global perspective trial. Pediatr Crit Care Med 2013; 14:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimmerman JJ, Banks R, Berg RA, et al. Trajectory of Mortality and Health-Related Quality of Life Morbidity Following Community-Acquired Pediatric Septic Shock. Crit Care Med 2020; 48:329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Typpo K, Watson RS, Bennett TD, et al. Outcomes of Day 1 Multiple Organ Dysfunction Syndrome in the PICU. Pediatr Crit Care Med 2019; 20:914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson RS, Crow SS, Hartman ME, et al. Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med 2017; 18:S4–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss SL, Balamuth F, Hensley J, et al. The Epidemiology of Hospital Death Following Pediatric Severe Sepsis: When, Why, and How Children With Sepsis Die. Pediatr Crit Care Med 2017; 18:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JC, Spinella PC, Fitzgerald JC, et al. New or Progressive Multiple Organ Dysfunction Syndrome in Pediatric Severe Sepsis: A Sepsis Phenotype With Higher Morbidity and Mortality. Pediatr Crit Care Med 2017; 18:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 11.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–332. [DOI] [PubMed] [Google Scholar]
- 12.Straney L, Clements A, Parslow RC, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med 2013; 14:673–681. [DOI] [PubMed] [Google Scholar]
- 13.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992; 121:68–74. [DOI] [PubMed] [Google Scholar]
- 14.Wilkinson JD, Pollack MM, Ruttimann UE, et al. Outcome of pediatric patients with multiple organ system failure. Crit Care Med 1986; 14:271–274. [DOI] [PubMed] [Google Scholar]
- 15.Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med 1994; 22:1025–1031. [DOI] [PubMed] [Google Scholar]
- 16.Curley MA, Zimmerman JJ. Alternative outcome measures for pediatric clinical sepsis trials. Pediatr Crit Care Med 2005; 6:S150–156. [DOI] [PubMed] [Google Scholar]
- 17.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138:923–936. [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Nelson DR, Williams MD. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit Care Med 2011; 39:1050–1055. [DOI] [PubMed] [Google Scholar]
- 19.Russell JA, Singer J, Bernard GR, et al. Changing pattern of organ dysfunction in early human sepsis is related to mortality. Crit Care Med 2000; 28:3405–3411. [DOI] [PubMed] [Google Scholar]
- 20.Dhainaut JF, Shorr AF, Macias WL, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med 2005; 33:341–348. [DOI] [PubMed] [Google Scholar]
- 21.Kinasewitz GT, Zein JG, Lee GL, et al. Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit Care Med 2005; 33:2214–2221. [DOI] [PubMed] [Google Scholar]
- 22.Sood MM, Shafer LA, Ho J, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care 2014; 29:711–717. [DOI] [PubMed] [Google Scholar]
- 23.Chaudhry N, Duggal AK. Sepsis Associated Encephalopathy. Adv Med 2014; 2014:762320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung HY, Wickel J, Brunkhorst FM, et al. Sepsis-Associated Encephalopathy: From Delirium to Dementia? J Clin Med 2020; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss SL, Zhang D, Bush J, et al. Persistent Mitochondrial Dysfunction Linked to Prolonged Organ Dysfunction in Pediatric Sepsis. Crit Care Med 2019; 47:1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Backer D, Orbegozo Cortes D, Donadello K, et al. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence 2014; 5:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 2016; 126:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutroulis I, Batabyal R, McNamara B, et al. Sepsis Immunometabolism: From Defining Sepsis to Understanding How Energy Production Affects Immune Response. Crit Care Explor 2019; 1:e0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reitsema VA, Star BS, de Jager VD, et al. Metabolic Resuscitation Strategies to Prevent Organ Dysfunction in Sepsis. Antioxid Redox Signal 2019; 31:134–152. [DOI] [PubMed] [Google Scholar]
- 30.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol 2018; 14:417–427. [DOI] [PubMed] [Google Scholar]
- 31.Schuler A, Wulf DA, Lu Y, et al. The Impact of Acute Organ Dysfunction on Long-Term Survival in Sepsis. Crit Care Med 2018; 46:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran DD, Groeneveld AB, van der Meulen J, et al. Age, chronic disease, sepsis, organ system failure, and mortality in a medical intensive care unit. Crit Care Med 1990; 18:474–479. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Shi X, Diao M, et al. A retrospective study of sepsis-associated encephalopathy: epidemiology, clinical features and adverse outcomes. BMC Emerg Med 2020; 20:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirschen MP, Snyder M, Smith K, et al. Inter-Rater Reliability Between Critical Care Nurses Performing a Pediatric Modification to the Glasgow Coma Scale. Pediatr Crit Care Med 2019; 20:660–666. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. Interrater reliability and predictive validity of the FOUR score coma scale in a pediatric population. J Neurosci Nurs 2009; 41:261–267; quiz 268-269. [DOI] [PubMed] [Google Scholar]
- 36.Proulx F, Fayon M, Farrell CA, et al. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest 1996; 109:1033–1037. [DOI] [PubMed] [Google Scholar]
- 37.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013; 41:1761–1773. [DOI] [PubMed] [Google Scholar]
- 38.Matics TJ, Sanchez-Pinto LN. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr 2017; 171:e172352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaur J, Singhi P, Singhi S, et al. Neurodevelopmental and Behavioral Outcomes in Children With Sepsis-Associated Encephalopathy Admitted to Pediatric Intensive Care Unit: A Prospective Case Control Study. J Child Neurol 2016; 31:683–690. [DOI] [PubMed] [Google Scholar]
- 40.Wong HR, Reeder RW, Banks R, et al. Biomarkers for Estimating Risk of Hospital Mortality and Long-Term Quality-of-Life Morbidity After Surviving Pediatric Septic Shock: A Secondary Analysis of the Life After Pediatric Sepsis Evaluation Investigation. Pediatr Crit Care Med 2021; 22:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pollack MM, Banks R, Holubkov R, et al. Long-Term Outcome of PICU Patients Discharged With New, Functional Status Morbidity. Pediatr Crit Care Med 2021; 22:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sankar J, Moodu S, Kumar K, et al. Functional Outcomes at 1 Year After PICU Discharge in Critically Ill Children With Severe Sepsis. Pediatr Crit Care Med 2021; 22:40–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


