Abstract
Objective:
Autonomic nervous system is involved in many disorders, and changes in its modulation are the known risk factors for cardiovascular diseases. Its role in metabolic disarrangements in children at high cardio-metabolic risk is not known. Aim of the study is to analyze the relation between clinical-metabolic parameters and autonomic nervous system in children.
Materials and Methods:
Children affected by type 1 diabetes (group 1), obesity (group 2), and control group (group 3) were enrolled. Autonomic nervous system functionality was assessed with dynamic tests (The Expiration to Inspiration indexes—E/I delta and ratio, 30:15 Ratio Test, Systolic blood pressure response to standing—deltaPA), and ultrasonography was performed to evaluate Intima Media Thickness (cIMT). Clinical parameters were recorded.
Results:
The study popultaion had a total of 75 children with mean age of 12.5 ± 2.8 years: 26 in group 1, 28 in group 2, 21 in group 3. Obese children had higher cIMT z-score (P < .001). Diabetic patients had lower EI delta values (P < .001) and 30:15 ratio test (P = .01). There was an inverse correlation between E/I delta and microalbuminuria levels (rho −0.955, P < .001) and 30:15 ratio test and microalbuminuria (rho −0.936, P < .001) in group 1, even after adjustment for age. DeltaPA was higher in obese (P = .032) and correlated with BMI z-score and homeostatic model assessment.
Conclusion:
Our results highlight imbalances in sutonomic nervous system function in children at high metabolic risk, in particular with involvement of parasympathetic function in type 1 Diabetes Mellitus and sympathetic one in obesity. An early screening could lead to a prompt identification of these alterations and could have a predictive role on cardio-metabolic risk.
Keywords: autonomic nervous system, diabetes, obesity, children, cardio-metabolic risk
What is already known on this topic?
Autonomic nervous system is recognized to be one of the main modulators of the stress response, and its balance may influence various metabolic functions but its role in causing metabolic alterations has not been sufficiently studied in children.
What this study adds on this topic?
Modification pattern in autonomic nervous system functionality seemed to be involved in children affected by type 1 diabetes and obesity; so, monitoring early signs of autonomic nervous system imbalance could have a predictive role on cardiometabolic risk in pediatric population.
Introduction
The autonomic nervous system (ANS), a functional part of the peripheral nervous system, regulates several physiologic processes, including cardiovascular, respiratory, digestive, urinary, and reproductive functions.1 ANS is characterized by 3 anatomically separated sections: sympathetic, parasympathetic, and enteric. The efferent and afferent fibers of sympathetic and parasympathetic nervous system provide sensory input and motor output to the central nervous system, while the enteric nervous system is mainly responsible for the regulation of digestive processes, through an extensive, netlike structure, functioning autonomously from the rest of the nervous system.2
ANS is recognized to be one of the main modulators of the stress response,3 which is associated to the level of activity of either the sympathetic or the parasympathetic system.4 The net balance of such activity may influence various metabolic functions.3 Recent reports have shown that a long-term increase in stress response, causing increased sympathetic and decreased parasympathetic activity due to ANS disbalance, contributes to the development of disorders associated to high cardio-metabolic risk, including obesity, insulin resistance, dyslipidemia, diabetes, and hypertension.5 These pathological conditions could be further complicated by a secondary impairment of ANS, causing an important worsening of their clinical features and further comorbidities.6 In adults, an increased sympathetic activity, alongside a decreased parasympathetic activity, was found to be frequently associated with a higher prevalence of metabolic syndrome and its individual elements, recognized risk factors for cardiovascular disease.7 Various reports showed that ANS dysregulation was predictive of metabolic abnormalities.8 Moreover, diabetic neuropathy is a complication of type 1 and type 2 diabetes, and various studies emphasized that first signs of autonomic dysfunction can occur very early and in some cases at the diagnosis of diabetes. A significant number of type 1 and type 2 diabetic patients, 6% and 8%, respectively, may show alterations in cardiovascular tests or other indices of autonomic function at the onset.9,10
It is known that early in childhood, high levels of chronic stress may harm the development of the brain and can affect metabolic and endocrine systems.3 However, the effects of ANS disbalance and its role in causing metabolic alterations has not been sufficiently studied in children. In particular, data on ANS function in children affected by pathologic conditions exposing patients to high risk of cardio-metabolic disorders are scarce. We studied the autonomic functionality in children affected by type 1 diabetes mellitus (1DM) and obesity, clinical conditions at high cardio-metabolic risk, with the aim to investigate the relation between some of the main clinical-metabolic parameters and their role in early identification of ANS imbalances.
Materials and Methods
Caucasian children and adolescents, aged 5–18 years, were subsequently enrolled between July and October 2019 at our Pediatric Department.
Subjects were divided into 3 study groups according to the following inclusion criteria:
Group 1: subjects with confirmed diagnosis of 1DM (duration > 2 years) according to ISPAD guidelines11;
Group 2: subjects with diagnosis of overweight/obesity according to the CDC growth charts12;
Group 3: control population, including subjects with normal weight, admitted for recurrent abdominal pain or re-evaluation after hospitalization due to an acute event. They showed conditions not related to diabetes, obesity, or any metabolic or other disorder involving cardiovascular risk.
Exclusion criteria were age < 5 years or >18 years, acute or chronic conditions (except for 1DM in group 1 and obesity in group 2), genetic disorders, arrhythmias, vagotonia with an history of vaso-vagal syncope, secondary obesity, underweight patients, and use of drugs (except for insulin in group 1). Familiarity for cardiovascular diseases in first-degree relatives was recorded. The duration of illness and the onset of ketacidosis were also recorded for diabetic subject. Complete auxological and clinical evaluation were performed in accordance with standardized procedures.13
Routine laboratory data were collected. Furthermore, microalbuminuria and creatininuria were recorded in group 1 and HbA1c in groups 1 and 2. Alumin Creatinine ratio (ACR, mg/g) was also calculated in group 1.14
Basal Insulin levels were measured, after fasting, and Homeostasis model assessment of insulin resistance (HOMA index) was calculated in group 2.15
The study followed the requirements of the International Conference on Harmonization Good Clinical Practice and respected the principles outlined by the Declaration of Helsinki on the Ethical Principles for Medical Research Involving Human Subjects. The study was approved by the Independent Ethic Committee for Developmental Medicine of the Medical School of the University of Foggia for studies performed in subjects < 18 years of age (Ethics Committee approval number: Prot. n. MSHSCRC_19/1) and by the Ethics Committee of the European Paediatric Association, Union of National Paediatric Societies and Associations (Ethics Committee approval number: Prot. n.1-Apr.04/2019-EPA). Written informed consent to the study was obtained by the parents or caregivers for the participation of children in the study.
Ultrasonography
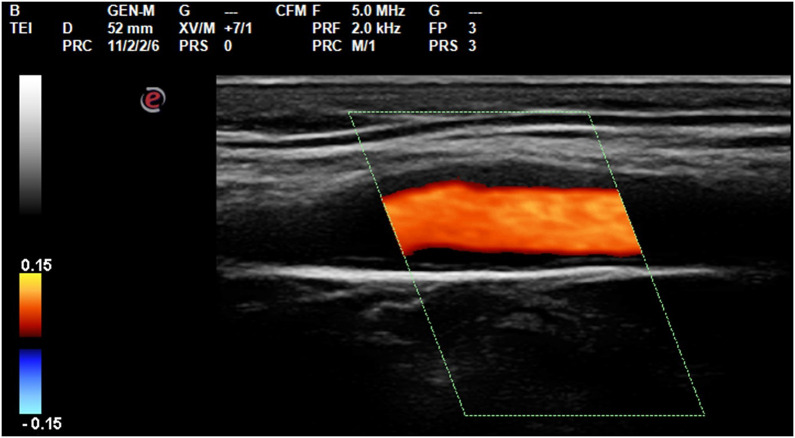
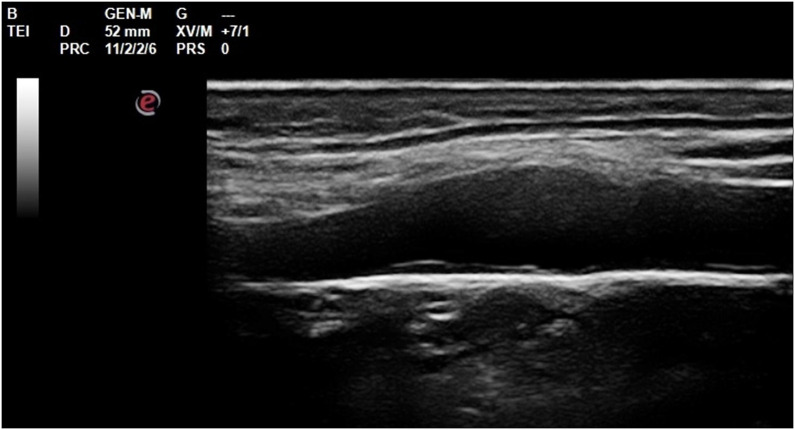
Carotid Intima-Media Thickness (cIMT) was assessed for each vascular sector according to Consensus Statement from the American Society of Echocardiography—Carotid Intima-Media Thickness Task Force Citeria, by a single operator in B-mode HD mode with a 7.5 Mhz Esaote My Lab 30 gold linear probe.16 Mean values were recorded and cIMT z-score was calculated17 (Figures 1 and 2).
Figure 1.
Longitudinal scan of the internal carotid at the bulb level for the measurement of cIMT.
Figure 2.
Distal wall of the vessel perfectly horizontal, cIMT evaluation at the end of diastolic phase at carotid bulb level.
The ankle-brachial-index measurements (ABI) were performed after a 20-minute rest in supine position using a standard sphygmomanometer with suitable bracelets size and a 7.5 MHz linear probe (Esaote My Lab 30 gold) by a single qualified operator. Each brachial artery, dorsal artery of the foot, posterior tibial artery at right and left sides were examined. ABI was calculated according to the American Heart Association Criteria.5,18
Autonomic Function
Autonomic function was evaluated by a single operator using the following tests:
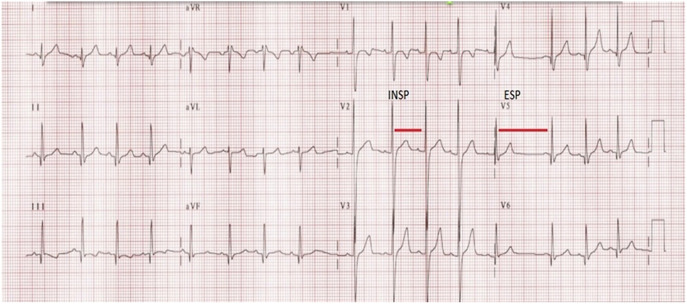
The Expiration to Inspiration Indexes (E/I): the heart rate response to respiration was evaluated according to the guidelines of the American Diabetes Association and the American Academy of Neurology of 2005.19 The beat-to-beat variation (RR variation) with deep breathing was obtained by calculating the ratio between the average of the 3 longest RR intervals in exhalation and the average of the 3 shortest RR intervals during inspiration (E/I ratio, Figure 3):
Figure 3.
Expiration to inspiration indexes: heart rate varies in relation to breathing, presenting an increase of heart beat during inspiration (Insp) and a decrease of heart beat during expiration (Exp). This phenomenon is controlled by parasympathetic nervous system. Beat-to-beat variation (R-R variation) with deep breathing is recorded by electrocardiography.
An E/ratio > 1.21 is considered to be normal in this group of age.20
The difference in heart rate was, however, calculated by measuring the delta between the average of the 3 highest frequencies and the average of the 3 lowest frequencies.
E/Delta: longest RR interval (expiration) − shortest RR interval (inspiration)
The 30:15 Ratio Test was performed according to the guidelines of the American Diabetes Association and the American Academy of Neurology of 2005.19 This is an alternative, less common, parasympathetic function test. During ECG monitoring, the RR interval is measured at beats 15 and 30 after active standing. The test results are expressed by the ratio between the value of the longest RR interval (nadir), measured between 25° and 35°, with the value of the shortest RR interval, measured between 10° and the 20° beat after standing. The ECG traces were assessed with ECG-1000 ECG analysis software for ECG Biocare. Normal value are considered > 1.1520:
- Systolic Blood Pressure Response to Standing (deltaPA): a first measurement of systolic pressure has been performed with the patient in a sitting position.21,22 Subsequently, the subject was placed in a supine position for at least 5 minutes, then 3 successive blood pressure measurements were performed at 1 minute intervals until stable values were found. Thereafter, the subject was asked to stand up as quickly as possible, if necessary with assistance, and 2 measurements were taken, respectively, after 60 and 120 seconds of orthostatism. The deltaPA was recorded considering the difference between the last of systolic pressure value measured in supine position and the lowest of orthostatic position’s one and values <20 mmHg are considered normal.21
Statistical Analysis
Results are expressed as mean ± standard deviation (95% confidence interval, CI) for continuous variables, and as relative frequencies for categorical and discrete variables. The Shapiro-Wilk test was used to evaluate data distribution. Data were analyzed by Student t-test, ANOVA, Mann-Whitney U-test, Kruskal Wallis for continuous variables, and chi-square test for categorical ones, according to their distribution. Bonferroni test and pairwise comparison according to Dunn-Bonferroni post hoc method were applied for multiple simultaneous comparisons, as appropriate. Spearman’s rank or Pearson’s correlation were performed to evaluate the association between variables: correlation coefficient was used to measure effect size.23 Partial correlations were also executed. A Simple linear regression analysis was designed to analyze the predictive capacity of E/I test (variable x) on microalbuminuria levels (variable y), variables used met the assumption. P value ≤ .05 were considered significant (2-sided level). The Statistical Package for Social Sciences version 22.0 software (IBM Corp.; Armonk, NY, USA).
Results
The study population consisted of 75 children, mean age of 12.5 ± 2.8 years (CI 95% 11.9–13.2), 44 females and 31 males. The description of the three groups is reported in Table 1.
Table 1.
Study Population: Type 1 Diabetes Mellitus (Group 1), Obese Children (Group 2), and Controls (Group 3)
| Subjects | Males/Females | Age (Mean ± SD) | BMI z-Score |
|---|---|---|---|
| Group 1 | 10/16 | 12.3 ± 3 | 0.68 ± 0.7*§ |
| Group 2 | 13/15 | 12.5 ± 2.7 | 2.1 ± 0.41*° |
| Group 3 | 8/13 | 12.8 ± 2.7 | 0.06 ± 0.7§° |
| Total | 31/44 | 12.5 ± 2.8 | 1.04 ± 1.06 |
*P < .001; § P = .004.
Cardio Metabolic Risk
Mean cIMT z-score was 0.7 ± 1.5: 0.5 ± 1.2 in group 1, 1.7 ± 1.3 in group 2, and - 0.33 ± 1.3 in group 3. Obese children had cIMT z-score statistically higher than group 1 (P = .002) and group 3 (P < .001). Median ABI value was 1.04 (IQR 1–1.06) in overall population: 1.02 (IQR 1–1.07) in diabetic children, 1.04 (IQR 1–1.06) in obese, 1 (IQR 1–1.105) in normal weight (P = .834). No pathological values were recorded.
Mean systolic z-score blood pressure was 0.28 ± 0.97 mmHg: obese patients had higher values than group 1 (P < .001) and group 3 (P < .001). Median diastolic z-score blood pressure values were 0.35 mmHg (IQR –0.15–0.82). No differences were recorded between the three groups (P = .325) (Table 2).
Table 2.
Z-Score Values of Systolic and Diastolic Blood Pressure
| Subjects | z-Score SBP (Mean ± SD) | z-Score DBP (Median and IQR) |
|---|---|---|
| Group 1 | 0.06 ± 0.8*§ | 0.3 (−0.19–0.85)#& |
| Group 2 | 0.8 ± 1.07*° | 0.4 (−0.02–0.97)#^ |
| Group 3 | −0.13 ± 0.6§° | 0.03 (−0.2–0.74)&^ |
| Total | 0.28 ± 0.97 | 0.35 (−0.15–0.82) |
SBP, systolic blood pressure; DBP, diastolic blood pressure.
z-Score SBP (Mean ± SD): * P = .09; § P = 1; °P = .001; z-Score DBP (Median and IQR): #,&,^ P > .05.
Cardio-vascular familiarity risk showed no statistical difference in the three groups.
E/I Delta Test: The mean E/I delta was 39.4 ± 8.99 bpm (range 22.34–58.08 bpm) in total population. No pathological values were recorded (i.e., <21 bpm). In group 1, mean E/I delta value was 34.22 ± 8.4 bpm: it was significantly lower than group 2 (P = .009) and group 3 (P = .001) (Table 3). No association was found between E/I delta values and BMI z-score, systolic and diastolic z-score, and cIMT z-score in overall population.
Table 3.
E/I Delta, EI Ratio, and 30:15 Ratio Test in Type 1 Diabetes Mellitus (Group 1) and Obese (Group 2) Children, and Controls (Group 3)
| Subjects | E/I Delta (Mean ± SD) | E/I Ratio (Mean ± SD) | E/I Ratio (Range) | 30:15 Ratio (Mean ± SD) |
|---|---|---|---|---|
| Group 1 | 34.22 ± 8.4*§ | 1.39±0.1#& | 1.22–1.59 | 1.3 ± 0.1∆ ø |
| Group 2 | 41.1 ± 8.1*° | 1.4 ± 0.1#^ | 1.27–1.62 | 1.4 ± 0.1∆α |
| Group 3 | 43.6 ± 7.9§° | 1.49 ± 0.1&^ | 1.35–1.72 | 1.5 ± 0.1øα |
| Total | 39.4 ± 8.99 | 1.44 ± 0.1 | 1.22–1.72 | 1.4 ± 0.12 |
E/I Delta (mean ± SD): *for comparison between group 1 and 2, P = .009; §for comparison between group 1 and group 2, P = .001; °for comparison between group 2 and 3, P = .870; E/I Ratio (mean ± SD): #P = .058; P = .002; ^P = .578; 30:15 Ratio (mean ± SD); ∆for comparison between group 1 and group 2, P = .071; øfor comparison between group 1 and 3, P = .003; ɑfor comparison between group 2 and 3, P = .548.
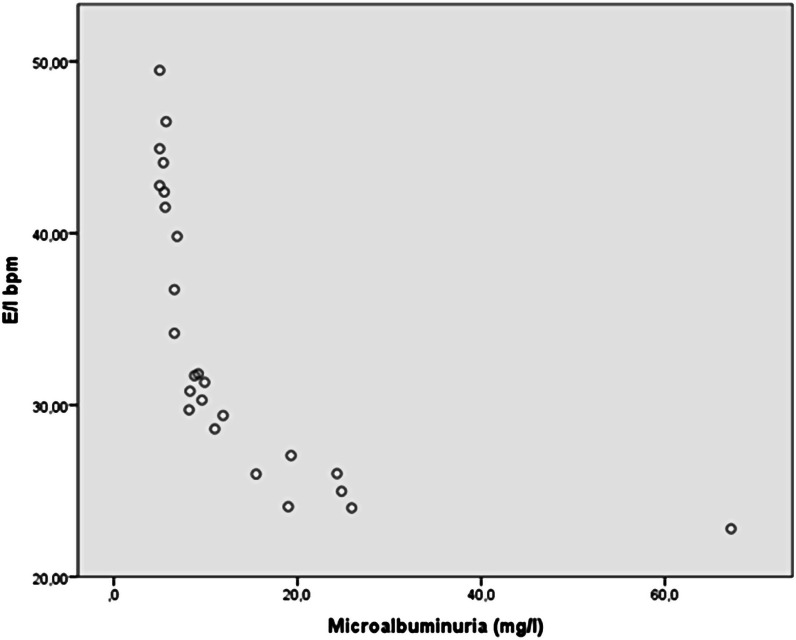
Spearman’s correlation between E/I delta and HbA1c (groups 1 and 2) was significant (rho: −0.405, P = .004). In group 1, a strong inverse association was found between E/I delta and microalbuminuria (rho: –0.955, P < .001), even after adjustment for age (rho: –0.643, P = .001) and for HbA1c (rho: –0.620, P = .001) (Figure 4). The same relation was observed between E/I delta and ACR (rho: –0.652, P = .01), but not between ACR and HbA1c (P = .432). No relation was observed between E/I delta and 1DM duration (P = .368) or onset with KAD (P = .68).
Figure 4.
Scatter plot: correlation of microalbuminuria and E/I delta test (bpm) in diabetic patients.
Simple linear regression analysis, in group 1, confirmed that E/I delta was predictive for microalbuminuria (F(1,23) = 15.132, P = .001; with R2: 0.397, standardized 0.371, beta –0.63).
E/I ratio: Mean E/I ratio was 1.44 ± 0.1 (range 1.22–1.72), although no subjects analyzed presented pathological values (≤1.21), a diabetic girl had E/I ratio of 1.22. The E/I ratio values are described in Table 3. Comparison between groups showed a statistical significance between mean E/I ratio in group 1 versus group 3 (P = .002). An inverse association was found between E /I ratio and HbA1c (rho: –0.374, P = .008) in diabetic and obese. In group 1, there was a strong association with microalbuminuria values (rho: –0.915, P < .001), even after adjustment for age (rho = –0.636, P = .001), HbA1c (rho: –0.617, P = .001) and ACR (rho: –0.594, P = .005).
The 30:15 ratio test: Mean 30:15 ratio test value was 1.41 ± 0.12 in overall population: group 1 had statistically lower values versus group 3 (Table 3). A pathological test (value 1.13) was observed in a diabetic girl. Correlation analysis highlighted an association between the 30 : 15 ratio test and HbA1c (groups 1 and 2) (rho: –0.371, P = .009) and 30:15 ratio test and microalbuminuria (rho: –0.936, P < .001) in group 1, even after adjustment for age (rho = –0.643, P = .001), HbA1c (rho: –0.647, P = .001), and ACR (rho = –0.650, P = .001).
Systolic Blood Pressure Response to Standing—DeltaPA: Median deltaPA value was 5 mmHg (IQR 3–9): 5.0 (IQR 3–7) in diabetic subjects, 7.5 (IQR 4–10.7) in obese and 3.0 (IQR 1.5–6.5) mmHg in normal weight patients (P = .032). None had pathological values, but the highest value (17 mmHg) was recorded in an obese patient. A significant correlation was observed between deltaPA and BMIz-score (rho: 0.314, P = .006), systolic blood pressure z-score (rho: 0.401, P < .001 ), cIMT z-score (rho: 0.315, P = .006) in overall population. In group 2, deltaPA showed a significant correlation with HOMA (rho: 0.667, P = .001), and fasting insulinemia (rho: 0.698, P < .001).
Discussion
Cardiovascular diseases are the main cause of death worldwide. The pathogenic mechanisms related to cardiovascular risk develop early in childhood, and diabetes and obesity are pathological conditions most frequently associated with cardiovascular risk.24 Obesity and diabetes are recognized as dysmetabolic processes involved in the pathogenesis of atherosclerosis in childhood.25 In recent years, increasing reports have emphasized the role played by imbalances of ANS as a cause of cardiometabolic risk.26 ANS is one of the main mediators of stress response and of the homeostasis of various systems. Sympathetic hypertonus contributes to hypertension, dyslipidemias, visceral obesity, and insulin resistance,27 while depressed sympathetic activity, shown by subjects with advanced dysautonomic diabetic neuropathy, is strongly associated with risk of sudden cardiac death,28 myocardial infarction due to early coronary damage,29 and ischemic stroke.30 Parasympathetic hypertonia is also associated with common syncopal events in childhood and adolescence.31 Furthermore, a vagal hypotone has been associated with serious arrhythmic events and myocardial infarction,32 and the dysregulation of sympathetic and parasympathetic systems has been described to cause hypoglycemia unawareness.33 Taken together, these observations emphasize the role of an imbalanced autonomic nervous system in the development of various disorders associated to cardiometabolic risk. Our data show that the average values of E/I indexes in diabetic children were significantly lower compared to controls, with no relation to the duration of the disease. Our results also indicate an inverse association of HbA1c levels in diabetic and obese patients, and a strong association with the levels of microalbuminuria in diabetic patients, which data confirm the importance to properly manage blood sugar levels in order to reduce the risk of evolution towards full-blown diabetic neuropathy.
The data shown by the 30:15 ratio test in diabetic and in obese subjects were comparable to the other test performed, as the values were significantly lower than in the control group and also showed an inverse correlation with HbA1c. Taken together, these results suggest that the afferent pathways are normal, while the initial imbalance involves the vagal efferent branch.
Most notably our results show an inverse correlation between microalbuminuria values and E/I tests, microalbuminuria, and 30:15 ratio test. The same relation was observed using ACR. To the best of our knowledge, these data has not been previously described. The role of ANS in the etiology and progression of renal damage has been described in patients with pure dysautonomia34 and in Shy Drager Syndrome.35 These conditions are characterized by an impairment of the ANS due to hyperglycemia and a recent study showed a faster progression toward the state of chronic renal failure in diabetic guinea pigs with renal denervation.36 In these conditions, the relative hypertonicity of the sympathetic system would determine an increased glomerular flow due to hyperfiltration resulting in microalbuminuria. In the long term, this process could result in proteinuria, leading to nephrotic syndrome and chronic renal failure. Although our data need to be confirmed by further studies including a larger number of subjects, they suggest that microalbuminuria could be considered a marker of autonomic dysfunction.
Systolic blood pressure response to standing test showed significantly higher values in obese subjects compared to controls. However, obese patients had also higher blood pressure values than control in supine position. This particular trend of systolic blood pressure, which tends to be higher in supine position and decrease in orthostatic position, mimics the peculiar characteristics of the Hyper-Hypo syndrome.22 In our group of obese patients, the greatest variations of pressure compared to control group indicate a defective pressure adaptation to postural changes, and it could be an early sign of baroreceptor dysfunction.26 To this regard, our results suggest that in obese subjects, there is a partial impairment of the reflex mediated by the carotid baroreceptors, causing a defective blood pressure response, leading to lower blood pressure compensation in supine and in orthostatic position.37 This possibility is further supported by the direct correlation between HOMA index and the deltaPA found in the patients studied, as a marked dysfunction of the carotid baroreceptors is typically described as insulin resistance.13,27 Obese children also showed increased cIMT, which alteration affect the arterial tract where the baroreceptors are located, causing a reduced receptor sensitivity. This mechanism is considered one of the main pathogenic factors in idiopathic and secondary hypertension in the adult,38 but it has never been described in children.
In diabetic patients, our results also show a greater imbalance of the parasympathetic function compared to the sympathetic, while obese show the opposite evidence. These data suggest that parasympathetic dysfunction may be considered an early complication of diabetes, which could accelerate renal injury and expose to cardiovascular accidents independently of other risk factors.
Finally, the reduced sensitivity of the carotid reflex, due to an increase in cIMT and HOMA index, could be considered responsible for the results documented by the autonomic function tests in the obese children studied, as described by previous reports in adult obese.39 Our results confirm that imbalances of this delicate mechanism begin already during the developmental years39 and suggest that early screening in children at high metabolic risk could lead to an early identification of these alterations.
In conclusion, although the results of this study need to be confirmed by further studies including a larger number of subjects, our data may contribute to clarify the importance of monitoring early signs of ANS imbalance and their predictive role in children with cardiometabolic risk.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee for Pediatric Studies of the Medical School of the University of Foggia (Approval No: MSHSCRC_19/1).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – I.R., S.C.; Design – I.R.; Supervision – M.S.; Materials – S.C., V.V.d.P., P.M.; Data Collection and/or Processing – S.C., V.V.d.P., P.M.; Analysis and/or Interpretation – I.R., M.S., M.P.M.; Literature Review – I.R., S.C., M.S.; Writing – I.R., M.P.M.; Critical Review – M.S., M.P.M.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1.Wehrwein EA, Orer HS, Barman SM. Overview of the anatomy, physiology and pharmacology of the autonomic nervous system. Compr Physiol. 2016;6(3):1239–1278.. 10.1002/cphy.c150037) [DOI] [PubMed] [Google Scholar]
- 2.Karemaker JM.An introduction into autonomic nervous function. Physiol Meas. 2017;38(5):R89–R118.. 10.1088/1361-6579/aa6782) [DOI] [PubMed] [Google Scholar]
- 3.Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism. 2012;61(5):611–619.. 10.1016/j.metabol.2011.10.005) [DOI] [PubMed] [Google Scholar]
- 4.Tentolouris N, Argyrakopoulou G, Katsilambros N. Perturbed autonomic nervous system function in metabolic syndrome. NeuroMolecular Med. 2008;10(3):169–178.. 10.1007/s12017-008-8022-5) [DOI] [PubMed] [Google Scholar]
- 5.Licht CM, Vreeburg SA, van Reedt Dortland AK.et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitaryadrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95(5):2458–2466.. 10.1210/jc.2009-2801) [DOI] [PubMed] [Google Scholar]
- 6.Joyner MJ, Charkoudian N, Wallin BG. Sympathetic nervous system and blood pressure in humans; individualized patterns of regulation and their implications. Hypertension. 2010;56(1):10–16.. 10.1161/HYPERTENSIONAHA.109.140186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jarczok MN, Koenig J, Schuster AK, Thayer JF, Fischer JE. Night time heart rate variability, overnight urinary norepinephrine, and glycemic status in apparently healthy human adults. Int J Cardiol. 2013;168(3):3025–3026.. 10.1016/j.ijcard.2013.04.147) [DOI] [PubMed] [Google Scholar]
- 8.Licht CM, de Geus EJ, Penninx BW. Dysregulation of the autonomic nervous system predicts the development of the metabolic syndrome. J Clin Endocrinol Metab. 2013;98(6):2484–2493.. 10.1210/jc.2012-3104) [DOI] [PubMed] [Google Scholar]
- 9.Tu E, Twigg SM, Semsarian C. Sudden death in type 1 diabetes: the mystery of the ‘dead in bed’ syndrome. Int J Cardiol. 2010;138(1):91–93.. 10.1016/j.ijcard.2008.06.021) [DOI] [PubMed] [Google Scholar]
- 10.Kahn R.Introduction. Proceedings of a Consensus Development Conference on Standardized Measures in Diabetic Neuropathy. Diabetes Care. 1992;15(8):1080–1103.. 10.2337/diacare.15.8.1080) [DOI] [Google Scholar]
- 11.Mayer-Davis EJ, Kahkoska AR, Jefferies C.et al. ISPAD Clinical Practice Consensus Guidelines 2018: definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr Diabetes. 2018;19(suppl 27):7–19.. 10.1111/pedi.12773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC Growth Charts: United States. Advance Data from Vital and Health Statistics; vol 314. Hyattsville, MD: National Centre for Health Statistics; 2000:2000–1250.. [Google Scholar]
- 13.Rutigliano I, Vinci R, De Filippo G.et al. Metabolic syndrome, hepatic steatosis, and cardiovascular risk in children. Nutrition. 2017;36:1–7.. 10.1016/j.nut.2016.10.017) [DOI] [PubMed] [Google Scholar]
- 14.Donaghue KC, Marcovecchio ML, Wadwa RP.et al. ISPAD Clinical Practice Consensus Guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19(suppl 27):262–274.. 10.1111/pedi.12742) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and b – cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419.. 10.1007/BF00280883) [DOI] [PubMed] [Google Scholar]
- 16.Stein JH, Korcarz CE, Hurst RT.et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111; quiz 189.. 10.1016/j.echo.2007.11.011) [DOI] [PubMed] [Google Scholar]
- 17.Jourdan C, Wühl E, Litwin M.et al. Normative values for intima-media thickness and distensibility of large arteries in healthy adolescents. J Hypertens. 2005;23(9):1707–1715.. 10.1097/01.hjh.0000178834.26353.d5) [DOI] [PubMed] [Google Scholar]
- 18.Aboyans V, Criqui MH, Abraham P.et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–2909.. 10.1161/CIR.0b013e318276fbcb) [DOI] [PubMed] [Google Scholar]
- 19.Boulton AJ, Vinik AI, Arezzo JC.et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–962.. 10.2337/diacare.28.4.956) [DOI] [PubMed] [Google Scholar]
- 20.Ziegler D, Laux G, Dannehl K.et al. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9(2):166–175.. 10.1111/j.1464-5491.1992.tb01754.x) [DOI] [PubMed] [Google Scholar]
- 21.Quadri R.Ipotensione ortostatica nel paziente diabetico. Il Diabete. 2005;17:47–54.. [Google Scholar]
- 22.Lagi A, Spini S. Clinostatic hypertension and orthostatic hypotension. Clin Cardiol. 2010;33(6):E10–E15.. 10.1002/clc.20722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–1768.. 10.1213/ANE.0000000000002864) [DOI] [PubMed] [Google Scholar]
- 24.Steinberger J, Daniels SRAmerican Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young), American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation. 2003;107(10):1448–1453.. 10.1161/01.cir.0000060923.07573.f2) [DOI] [PubMed] [Google Scholar]
- 25.Rose KM, Eigenbrodt ML, Biga RL.et al. Orthostatic hypotension predicts mortality in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2006;114(7):630–636.. 10.1161/CIRCULATIONAHA.105.598722) [DOI] [PubMed] [Google Scholar]
- 26.Quigley KS, Stifter CA. A comparative validation of sympathetic reactivity in children and adults. Psychophysiology. 2006;43(4):357–365.. 10.1111/j.1469-8986.2006.00405.x) [DOI] [PubMed] [Google Scholar]
- 27.Licht CM, Vreeburg SA, van Reedt Dortland AK.et al. Increased sympathetic and decreased parasympathetic activity rather than changes in hypothalamic-pituitary-adrenal axis activity is associated with metabolic abnormalities. J Clin Endocrinol Metab. 2010;95(5):2458–2466.. 10.1210/jc.2009-2801) [DOI] [PubMed] [Google Scholar]
- 28.Rose KM, Eigenbrodt ML, Biga RL.et al. Orthostatic hypotension predicts mortality in middle-aged adults. The Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2006;114(7):630–636.. 10.1161/CIRCULATIONAHA.105.598722) [DOI] [PubMed] [Google Scholar]
- 29.Rose KM, Tyroler HA, Nardo CJ.et al. Orthostatic hypotension and the incidence of coronary heart disease: the Atherosclerosis Risk in Communities Study. Am J Hypertens. 2000;13(6 Pt 1):571–578.. 10.1016/s0895-7061(99)00257-5) [DOI] [PubMed] [Google Scholar]
- 30.Eigenbrodt ML, Rose KM, Couper DJ, Arnett DK, Smith R, Jones D. Orthostatic hypotension as a risk factor for stroke. The Atherosclerosis Risk in Communities (ARIC) study, 1987-1996. Stroke. 2000;31(10):2307–2313.. 10.1161/01.str.31.10.2307) [DOI] [PubMed] [Google Scholar]
- 31.Moya A, Sutton R, Ammirati F, Blanc JJ, Brignole M, Dahm JB. Guidelines for the Diagnosis and Management of Syncope. 2009 version. The Task Force for the Diagnosis and Management of Syncope of the European Society of Cardiology (ESC). Developed in collaboration with European Heart Rhythm Association (EHRA), Heart Failure Association (HFA), and Heart Rhythm Society (HRS). Eur Heart J. 2009;30:2631–2371.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bauer A, Kantelhardt JW, Barthel P.et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: cohort study. Lancet. 2006;367(9523):1674–1681.. 10.1016/S0140-6736(06)68735-7) [DOI] [PubMed] [Google Scholar]
- 33.Goldstein DS, Robertson D, Esler M, Straus SE, Eisenhofer G. Dysautonomias: clinical disorders of the autonomic nervous system. Ann Intern Med. 2002;137(9):753–763.. 10.7326/0003-4819-137-9-200211050-00011) [DOI] [PubMed] [Google Scholar]
- 34.Elkayam L, Matalon A, Tseng CH, Axelrod F. Prevalence and severity of renal disease in familial dysautonomia. Am J Kidney Dis. 2006;48(5):780–786.. 10.1053/j.ajkd.2006.07.024) [DOI] [PubMed] [Google Scholar]
- 35.Huang HF, Guo YP, Feng YK. Shy-Drager syndrome. Chin Med J (Engl). 1982;95(9):679–686.. [PubMed] [Google Scholar]
- 36.Matsuoka H.Protective role of renal nerves in the development of diabetic nephropathy. Diabetes Res. 1993;23(1):19–29.. [PubMed] [Google Scholar]
- 37.Robertson D, Hollister AS, Biaggioni I, Netterville JL, Mosqueda-Garcia R, Robertson RM. The diagnosis and treatment of baroreflex failure. N Engl J Med. 1993;329(20):1449–1455.. 10.1056/NEJM199311113292003) [DOI] [PubMed] [Google Scholar]
- 38.Chi X, Li M, Zhan X.et al. Relationship between carotid artery sclerosis and blood pressure variability in essential hypertension patients. Comput Biol Med. 2018;92:73–77.. 10.1016/j.compbiomed.2017.03.012) [DOI] [PubMed] [Google Scholar]
- 39.Zeitler PS, Nadeau KJ. Insulin Resistance. Childhood Precursors of Adult Disease. 2020 ed. Cham, Switzerland: Springer Nature. [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a