Abstract
Objective:
The study aimed to show the clinical characteristics and prognosis of the L1 syndrome in patients with L1CAM mutations in the extracellular region.
Materials and Methods:
Three affected boys and their siblings and parents from a large family were included in this study. Genetic etiology was investigated by whole-exome sequencing in the index patient. The pathogenic variant was detected by whole-exome sequencing and was validated by Sanger sequencing in 3 patients and other family members.
Results:
We present 2 brothers and their cousin with prenatal onset hydrocephalus, severe developmental and speech delay, corpus callosum agenesis/hypogenesis, epilepsia, and adducted thumbs. A hemizygous missense mutation NM_024003 (c.A2351G:p.Y784C) on exon 18 of L1CAM gene was found in the 3 patients and their carrier mother. This missense mutation in the conserved region of the second fibronectin type III-like repeats located in the extracellular region of L1CAM resulted in the severe phenotype of X-linked inherited L1 syndrome in the patients.
Conclusion:
L1 syndrome should be considered in the differential diagnosis of male children with intellectual disability, hydrocephalus, and adducted thumbs. While truncating mutations of L1CAM may cause a more severe phenotype, missense mutations cause milder forms. However, pathogenic missense mutations affecting key amino acid residues lead to severe phenotype likely.
Keywords: L1CAM, hydrocephalus, adducted thumbs, corpus callosum agenesis, intellectual disability
What is already known on this topic?
L1CAM plays an important role in nervous system development including neuronal migration/differentiation, axonal growth/fasciculation, myelination, and synaptic plasticity. L1CAM gene mutation is the most common cause of congenital hydrocephalus.
What this study adds on this topic?
We have demonstrated the missense mutations that are located in the conserved region of the second fibronectin type III-like repeats in the extracellular region of L1CAM, affecting key amino acid residues, lead to the severe phenotype.
Introduction
L1 syndrome is an X-linked inherited group disorder caused by mutations in the L1CAM gene encoding the L1 cell adhesion molecule (L1CAM, MIM:308840). The spectrum of L1 syndrome includes HSAS (MIM:307000, X-linked hydrocephalus with stenosis of the aqueduct of Sylvius syndrome), MASA (MIM:303350, mental retardation, aphasia, spastic paraplegia, adducted thumbs) syndrome, X-linked complicated spastic paraplegia type 1 (MIM:303350), and X-linked corpus callosum agenesis (MIM:304100).1 Clinical features of L1 syndrome are characterized by varying degrees of congenital hydrocephalus with adducted thumb, corpus callosum agenesis, spasticity, and intellectual disability affecting 1/30 000 male births.1,2
L1 cell adhesion molecule protein is composed of a large extracellular domain, a single transmembrane, and a small cytoplasmic region. It plays an important role in nervous system development including neuronal migration and differentiation, axonal growth and fasciculation, myelination, and synaptic plasticity.3,4 L1CAM mutations is the most common cause of congenital hydrocephalus and accounts for 5%-10% of boys with the isolated congenital hydrocephalus.5 The phenotypic spectrum varies from severe to mild, depending on the affected domain of L1 protein. The truncated mutations in the extracellular part of L1CAM lead to a more severe phenotype while missense mutations of the extracellular part or cytoplasmic region can cause milder forms.6-8
We presented here 3 patients with severe form of L1 syndrome caused by missense mutation in L1CAM gene.
MATERIALS AND METHODS
Patients
We present 2 brothers (Figure 1A; IV-2 and 3) and their cousin (Figure 1A; IV-4) with X-linked hydrocephalus from a kindred family. The mother of proband also reported that both her brothers (Figure 1A) and maternal uncle (Figure 1A) were born with similar findings including severe macrocephaly with hydrocephalus and died at the first and third days of their life, respectively, before ventriculoperitoneal shunt operations.
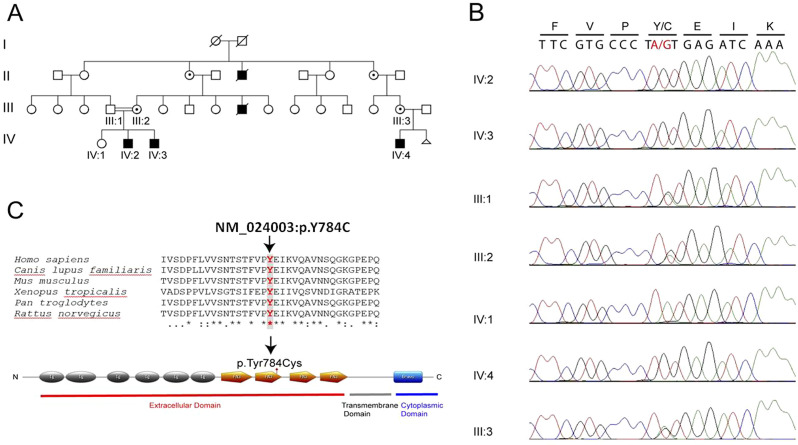
Figure 1.
Pedigree of the kindred family, affected members are shown with filled symbols (A). Sanger sequencing analysis confirmed missense mutation in our family (B). The mutation is indicated by a black arrow (c.A2351G:p.Y784C). Conservation of altered amino acid across species. Clustal Omega software was used to generate sequence alignment (C).
Methods
Written informed consent was obtained from the patient’s parents prior to sample collection. The study protocol was approved by the Yale Human Investigation Committee (protocol number 0908005592). Peripheral blood of all family members was collected and genomic DNA was isolated from peripheral blood leukocytes according to standard procedures. Genomic DNA of the affected case (Figure 1A; IV-1) was whole-exome sequenced at the Yale Center for Genome Analysis. Seq Cap EZ MedExome Target enrichment kit (Roche Sequencing, Pleasanton, Calif, USA) was used to produce an exome-captured sequencing library, and captured DNA was sequenced using Illumina Hiseq 2500 System (San Diego, Caif, USA). Raw reads from whole-exome sequencing (WES) were aligned to the human reference genome (GRCh38/hg38) using the BWA-MEM tool and Genome Analysis Tool Kit (GATK 1.1) was used to identify genetic variants, ANNOVAR and in-house programs were used for variant annotation. We filtered out variants with an allele frequency less than 1 in 1000 genomes, in dbSNP or in the Exome Aggregation Consortium’s browser.
Results
Clinical Report
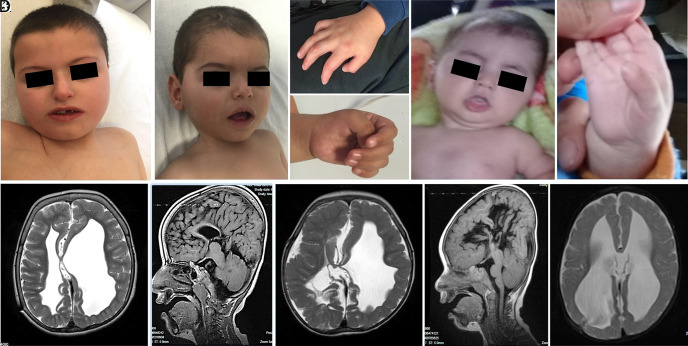
Patient 1 (Figure 1A; IV-2): The index case was diagnosed as intrauterine hydrocephalus at prenatal 6 months and was born at 40 weeks of gestation with a head circumference of 36 cm and a weight of 3000 g. He had a ventriculoperitoneal shunt operation at the age of 9 months. He gained head control at 3 years of age and achieved to sit without support at 4 years of age. He had his first epileptic seizure at 5 years of age. Since being on antiepileptic treatment, he had been completely free of seizures. In his first examination at 6 years of age, his head circumference was 52.5 cm (0.14 Standard deviation [SD]). The physical examination revealed frontal bossing, brachycephaly, hypertelorism, esotropia on the right eye, high palate, short neck, bilateral adducted thumbs, and axial hypotonia (Figures 2A and C). He was not making eye contact. Ophthalmologic evaluation was normal, and the audiometric evaluation was reported as moderate to severe hearing loss. Denver-II test result was compatible with severe developmental delay. Hemogram, routine serum biochemistry tests, and urinalysis were normal. In the evaluation of metabolic diseases, tandem mass, blood amino acid, and urine organic acid tests revealed normal results. Abdominal ultrasonography (USG) did not reveal any pathological features. The electroencephalography (EEG) recording showed a state of epileptic encephalopathy including periods of bioelectrical status and suppression activity. Cranial magnetic resonance imaging (MRI) showed corpus callosum hypogenesis, ventricular dilatation, cerebellar hypoplasia, and cerebellar folia organization dysfunction (Figures 2G and H). When re-evaluated at 8 years and 8 months of age; he could only walk 1-2 steps with support and speak only 1 word.
Figure 2.
Photography of patient 1 at 6 years (A), patient 2 at 20 months (B), and patient 3 at 8 months (E). Adducted thumbs were also noted in patient 1 (C), patient 2 (D), and patient 3 (F). Brain magnetic resonance imaging shows a corpus callosum hypogenesis, showing the left lateral ventricle larger than the right, cerebellar hypoplasia, cerebellar consentral folia organization dysfunction of patient 1 at 6 years of age (G and H), corpus callosum agenesis, dilatation in the left ventricle, cortical sulcation pattern disorder, organization dysfunction in cerebellum and thickening in dura mater at patient 2 of 20 months of age (I and J) and also prominent bilateral lateral ventriculomegaly with the third ventricle at 8 months of age in patient 3 (K). Informed consent to use these photographs was obtained from the patients’ fathers.
Patient 2 (Figure 1A; IV-3): This boy was diagnosed with intrauterine hydrocephalus in prenatal 5 months and was born at 38 weeks of gestation with a head circumference of 46 cm and a weight of 3700 g. He had a ventriculoperitoneal shunt operation on the first day of his life. The first seizure occurred on his third day of life. He smiled at 4.5 months. In his first examination at 20 months, his head circumference was 45 cm (−2SD). The physical examination revealed brachycephaly, hypertelorism, bilateral adducted thumbs, single palmar crease, axial hypotonia, and spasticity of the extremities (Figures 2B and D). The Denver-II test result was compatible with severe developmental delay. Ophthalmologic and audiometric evaluations were normal. Hemogram, routine serum biochemistry tests, and urinalysis were normal. In the evaluation of metabolic diseases, tandem mass, blood amino acid, and urine organic acid tests revealed normal results. Abdominal USG did not reveal any pathological features. Cranial MRI showed corpus callosum agenesis, dilatation in the left ventricle, cortical sulcation pattern disorder, organization dysfunction in the cerebellum, and thickening in the dura mater (Figures 2I and J).
He gained head control at 2 years and 6 months and was able to sit without support at the age of 4 years. In his last examination when he was 4 years and 3 months old, he could not walk or speak a word.
Patient 3 (Figure 1A; IV-4): The third patient was diagnosed with intrauterine hydrocephalus, born term, and had a ventriculoperitoneal shunt operation on the 45th day of his life. In his physical examination at the age of 8 months, head circumference was 48 cm (+2SD), oxycephaly, micrognathia, axial hypotonia, and bilateral adducted thumbs were observed (Figures 2E and F), but he had not gained head control yet. Ophthalmologic and audiometric evaluations were normal. Hemogram, routine serum biochemistry tests, and urinalysis were normal. In the evaluation of metabolic diseases, tandem mass, blood amino acid, and urine organic acid tests revealed normal results. Cranial MRI revealed prominent bilateral lateral ventriculomegaly with the third ventricle (Figure 2K). The EEG recording showed normal electrical activity.
The karyotype analysis performed on the proband was found to be normal (46XY). Since all patients had common phenotypic features, karyotype and/or chromosomal array analysis was not performed in the other 2 affected children, and WES was preferred as a second-line test.
The mothers of these patients had a lack of minor phenotypic features such as brachycephaly and adducted thumbs.
Molecular Studies
Whole-exome sequencing of patient 1 identified 1 shared hemizygous missense known variant NM_024003: c.2351A>G (p.Tyr784Cys) on exon 18 of L1CAM gene, located at chromosome Xq28. Sanger sequencing was performed on parents and children to confirm this variant identified by WES analysis. The pathogenic variant was detected in 3 affected boys (Figures 1B; IV-2, 3, and 4) and their carrier mothers (Figures 1B; III:2 and 3). The effect of this variant was shown using a meta-analytic support vector machine (https://omictools.co/metasvm- tool) and it was found to be pathogenic according to functional significance change in the amino acids. This missense mutation is located in a highly conserved region of the second fibronectin type III-like repeats in the extracellular region (Figure 1C).
Discussion
L1CAM mutations were divided into different phenotypic groups in studies investigating the phenotype–genotype relations. It has been reported that the children with a truncating mutation have a more severe phenotype than children with a missense mutation.7-11 The patients with prenatal onset hydrocephalus requiring a shunting and who died before the age of 3 years are classified as severe L1 phenotype, while the patients who live longer than 3 years of age without macrocephaly are classified as mild phenotype.1,2 Three patients presented here are classified as severe phenotype as they needed to have a ventriculoperitoneal shunt operation on the first day, 45 days, and 9 months of ages, respectively. Patients 1 and 2, who were followed up, gained head control at the age of 2.5 and 3 years, respectively, and could sit without support around the age of 4 years. Patient 1 could walk 1-2 steps with support and speak only 1 word at the age of 8 years and 8 months and lower extremity spasticity developed with aging.
L1 cell adhesion molecule is a member of the immunoglobulin adhesion protein superfamily and is composed of 6 immunoglobulin-like domains and 5 fibronectin type III-like repeats in the extracellular region and a short cytoplasmic tail.12 The majority of reported mutations are missense, nonsense, small insertions, or deletions and splice-site are distributed throughout the large extracellular domain of the L1 protein. The nonsense and frameshift variants lead to truncation of the L1 protein. Missense mutations account for over one-third of pathological L1 mutations described. The missense mutation (c.A2351G: p.Tyr784Cys) in the L1CAM we present here is pathogenic according to ClinVar. It is also in the moderate pathogenicity (PM1) group according to the ACMG Standards and Guidelines published by Richards et al.13 This mutation was reported by Mc Farlane14 in 5 children with prenatal hydrocephalus, all of whom had died before the age of 1. It is located in a highly conserved region of the second fibronectin type III-like repeats in the extracellular region (Figure 1C). L1 cell adhesion molecule may interact with itself (homophilic) and other molecules (heterophilic), such as axonin-1 and F11, which are known to be cell adhesion molecules of the immunoglobulin superfamily at the neuronal cell surface.3,12 Previous studies had shown the effect of 12 missense mutations on binding to L1, axonin-1, and F11 and many of those mutations affect all 3 interactions, while some only affects homophilic or heterophilic binding alone.15 It has been reported that missense mutations are subdivided as “key amino acid residues” and “surface residues.” The missense mutations which affect “key amino acid residues” are located in the extracellular part of L1CAM resulting in the diminution of both homophilic and 3 heterophilic bindings and this might produce more severe neurological problems.16,17 Since the variant (Tyr784Cys) presented here, located in the second Fn domain, is required for homophilic binding, it is defined as key residue mutation.
Adducted thumbs, which is a characteristic feature of this syndrome, were present bilaterally in 3 patients presented here.1,7-9 The pathological findings in the central nervous system are reported such as hydrocephalus with or without stenosis of the aqueduct of Sylvius (100%), corpus callosum agenesis/hypogenesis (68%), and/or cerebellar hypoplasia and small brain stem in the patients.10 Surgical interventions were required in infancy due to the ventriculomegaly and hydrocephaly accompanied by corpus callosum agenesis in our study group. Epilepsy, which was reported in a minority of patients with the L1 syndrome, was present in patient 1. Heterotopic epileptic foci formed by the disrupted and non-migrating neural stem cells are hypothesized to take part in the pathogenesis of epilepsy.10 In patient 1, epilepsy was also accompanied by hearing loss, which was not a part of the syndrome. The patients presented here had a more moderate course than the reported patients with the same mutation who died before the age of one.14 This may be due to early surgical interventions or interfamilial variability due to the effect of modifying genes.
In conclusion, L1 syndrome should be considered in both male fetuses with hydrocephalus and also in the differential diagnosis of male children with intellectual disability, hydrocephalus, and adducted thumbs. Besides the presence of severe clinical findings in this kindred family, our findings here support that pathogenic missense mutations which affect key amino acid residues are most likely to lead to a severe phenotype.
Footnotes
Ethics Committee Approval: Human subject study approval was obtained from the Yale School of Medicine Human Investigation Committee (HIC) (Approval No: 0908005592).
Informed Consent: Written informed consent was obtained from the patient’s parents prior to sample collection.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – B.T., A.G.E-S., K.B.; Design – B.T., A.G.E-S.; Supervision – B.T., A.G.E-S., K.B. Funding – K.B.; Materials – B.T.; Data Collection and/or Processing – B.T., A.G.E-S., N.G., C.Y.; Analysis and/or Interpretation B.T., A.G.E-S, N.G., C.Y.; Literature Review –B.T., A.G.E-S., E.Ö.; Writing – B.T., A.G.E-S, E.Ö.; Critical Review – B.T., A.G.E-S.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: This work is supported by the Yale-NIH Center for Mendelian Genomics (5U54HG006504).
References
- 1. Stumpel C, Vos YJ. L1 syndrome. In: Adam MP, Ardinger HH, Pagon RA.et al., eds. GeneReviews® [Internet]. Seattle: , WA: University of Washington; 2018. [Google Scholar]
- 2. Vos YJ, Hofstra RMW. An updated and upgraded L1CAM mutation database. Hum Mutat. 2010;31(1):1102 1109. 10.1002/humu.21172) [DOI] [PubMed] [Google Scholar]
- 3. De Angelis E, Watkins A, Schäfer M, Brümmendorf T, Kenwrick S. Disease-associated mutations in L1CAM interfere with ligand interactions and cell-surface expression. Hum Mol Genet. 2002;11(1):1 12. 10.1093/hmg/11.1.1) [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Bonilla M, McAllister JP, Limbrick DD. Genetics and molecular pathogenesis of human hydrocephalus. Neurol India. 2021;69(suppl):S268 S274. 10.4103/0028-3886.332249) [DOI] [PubMed] [Google Scholar]
- 5. Tully HM, Dobyns WB. Infantile hydrocephalus: a review of epidemiology, classification and causes. Eur J Med Genet. 2014;57(8):359 368. 10.1016/j.ejmg.2014.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otter M, Wevers M, Pisters M.et al. A Novel. mutation in L1CAM causes a mild form of L1 syndrome: a case report. Clinical Case Reports. 2017;5(8):1213 1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fransen E, Van Camp G, D'Hooge R, Vits L, Willems PJ. Genotype-phenotype correlation in L1 associated diseases. J Med Genet. 1998;35(5):399 404. 10.1136/jmg.35.5.399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weller S, Gärtner J. Genetic and clinical aspects of X-linked hydrocephalus (L1 disease): mutations in the L1CAM gene. Hum Mutat. 2001;18(1):1 12. 10.1002/humu.1144) [DOI] [PubMed] [Google Scholar]
- 9. Adle-Biassette H, Saugier-Veber P, Fallet-Bianco C.et al. Neuropathological review of 138 cases genetically tested for X-linked hydrocephalus: evidence for closely related clinical entities of unknown molecular bases. Acta Neuropathol. 2013;126(3):427 442. 10.1007/s00401-013-1146-1) [DOI] [PubMed] [Google Scholar]
- 10. Ortega E, Muñoz RI, Luza N.et al. The value of early and comprehensive diagnoses in a human fetus with hydrocephalus and progressive obliteration of the aqueduct of Sylvius: case Report. BMC Neurol. 2016;16(1):45. 10.1186/s12883-016-0566-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Michaelis RC, Du YZ, Schwartz CE. The site of a missense mutation in the extracellular Ig or FN domains of L1CAM influences infant mortality and the severity of X linked hydrocephalus. J Med Genet. 1998;35(11):901 904. 10.1136/jmg.35.11.901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Itoh K, Fushiki S. The role of L1cam in murine corticogenesis, and the pathogenesis of hydrocephalus. Pathol Int. 2015;65(2):58 66. 10.1111/pin.12245) [DOI] [PubMed] [Google Scholar]
- 13. Richards S, Az Richards S, Aziz N, Bale S, Bick D, Das S. ACMG Laboratory quality assurance committee, 2015. Standards and guidelines for the interpretation of se-quence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. 2015;17:405 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. MacFarlane JR, Du JS, Pepys ME.et al. Nine Novel L1CAM mutations in families with X-linked hydrocephalus. Hum Mutat. 1997;9(6):512 518. [DOI] [PubMed] [Google Scholar]
- 15. De Angelis E, MacFarlane J, Du JS.et al. Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J. 1999;18(17):4744 4753. 10.1093/emboj/18.17.4744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang R, Chen H, Wang X, Huang S, Xie A, Wu X. Prenatal diagnosis of a nonsense mutation in the L1CAM gene resulting in congenital hydrocephalus: A case report and literature review. Exp Ther Med. 2021;22(6):1416. 10.3892/etm.2021.10807) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang P, Liao H, Wang Q.et al. L1 syndrome prenatal diagnosis supplemented by functional analysis of one L1CAM gene missense variant. Reprod Sci. 2022;29(3):768 780. 10.1007/s43032-021-00828-4) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a