Abstract
Introduction
Opium dependence is a significant health concern in low and middle-income countries, leading to a considerable number of deaths annually. Opium has several detrimental effects on its consumers. Data regarding the impact of opium on stroke are controversial. The objective of this study is to evaluate the association between opium dependence and stroke.
Methods
I conducted a systematic search based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to evaluate the association between opium dependence and stroke. Following the extraction of qualitative findings from included studies, a meta-analysis was performed to assess the pooled estimate of odds ratios (ORs).
Results
Eight and four studies were included in qualitative and quantitative synthesis, respectively. Opium dependence increases the hazard of stroke mortality. Also, opium increases the odds of ischemic stroke by 127% (pooled OR = 2.27, 95% CI: 1.47–3.07).
Conclusion
Opium not only merely increases the odds of being diagnosed with ischemic stroke but also leads to a notable increase in the mortality rate following stroke.
Keywords: stroke, cerebrovascular, opium, Papaver somniferum, ischemic
Introduction
Opium (Lachryma papaveris) abuse has led to 1,000's of deaths annually. It is estimated that about 30 million people are addicted to opium or its derivatives (1). Not merely, these people are at increased risk of multiple diseases (2), but also they are at higher hazard of mortality as a result of malignancies, cardiovascular disease, respiratory disease, and infections. Golestan Cohort Study (GCS), a prospective study focusing on the effects of opium conducted in northern Iran, estimated that ever-use of opium results in an 86% higher hazard of all-cause mortality (3). They found that 16.95% of their participants are opium dependent. In a broader view, the World Health Organization estimates that about 2 million Iranians abuse opium, which is three times higher than the global average (4).
GCS also revealed that stroke accounts for 14.0% of premature death (5). The burden imposed by stroke increases as life expectancy rises, especially in low and middle-income countries where incidence or mortality rates have not been altered significantly (6–9).
Some studies justify these epidemiological findings by the surge in well-established risk factors such as hypertension, atrial fibrillation, diabetes, dyslipidemia, aging, and migraine (10–12). Moreover, several studies aimed to search for novel risk factors to explain the residual risk unaccounted by the set of these well-established risk factors (13, 14). With regards to opium dependence's medical and epidemiological features, I designed a systematic review focusing on the association between opium dependence and stroke.
Methods
This study is conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (15).
Eligibility criteria
I have used the following eligibility criteria: (I) studies should report the characteristics of patients diagnosed with opium dependence; (II) studies should report characteristics of patients diagnosed with any kind of stroke; (III) studies should report the association between opium with stroke incidence, prevalence, and outcome; and (IV) studies should be observational (namely, cohort, case-control, or cross-sectional studies).
Information sources
A systematic search was conducted through PubMed, Scopus, and Web of Science (WoS), from inception, until January 12, 2022. I also carried out a manual search using google scholar.
Search strategy
The search strategy for the systematic search was designed comprising two concepts; stroke and opium. I searched (“stroke” AND “opium”) OR (“cerebrovascular” AND “opium”) OR (“cva” AND “opium”) OR (“apoplexy” AND “opium”) OR (“stroke” AND “papaver”) OR (“cerebrovascular” AND “papaver”) OR (“cva” AND “papaver”) OR (“apoplexy” AND “papaver”). The search strategy algorithm is also shown in Supplementary Table 1.
Study selection
The study selection was carried out via the EndNote reference management software in three phases to manage identified records. Initially, duplicate articles were removed using the endnote software and manually, followed by the screening phase, in which the title and abstract of the studies were evaluated based on the inclusion criteria. Afterward, the screening of the full texts was conducted.
Data collection process and data items
I filled data extraction sheets containing variables such as author name, year of publication, age, gender, study type, sample size, the definition of exposure and outcome, the measure of association, and study findings.
Quality assessment
Quality assessment (QA) of included studies was conducted by STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guideline, comprising 22 sub-items. Each item's ratings are yes (1) or no (0), and the total QA score is the sum of these sub-items. This guideline restricts its recommendations to the three main analytical designs used in observational research (cohort, case-control, and cross-sectional studies) (16).
Data synthesis
The findings of this study are presented as odds ratios (ORs) and 95% confidence interval (95% CI). In order to perform the meta-analysis, STATA version 14.0 (StataCorp, College Station, TX) software was used. I restrict the meta-analysis when two or more records report the association of opium and stroke. The pooled estimate of ORs and their 95% CI was calculated based on the extracted datasheet. I2 statistic and chi-square-based Q-test were used for the assessment of heterogeneity. In the current study, the lack of heterogeneity was specified when the p-value was more than 0.10. Random models were used to pool the ORs. Publication bias was measured using Begg's test. Publication bias was considered substantial whenever the p-value was <0.1.
Results
Study selection
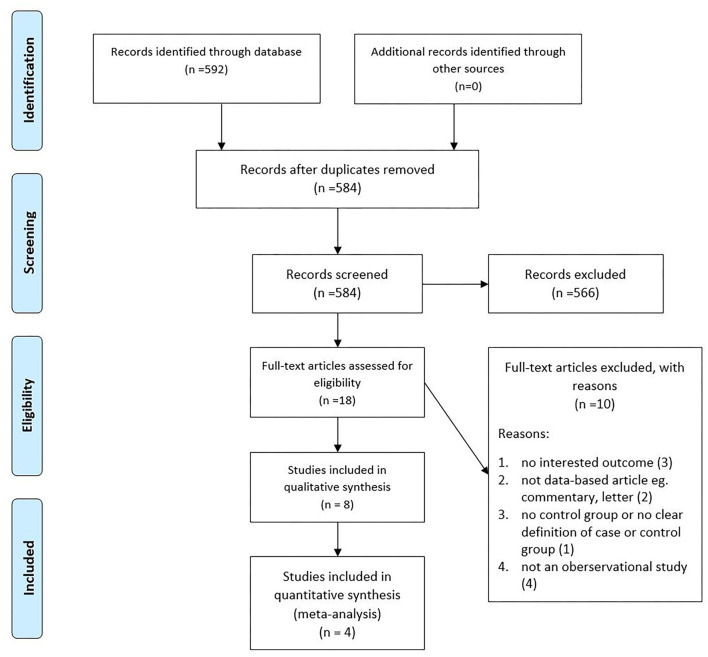
Our manual and systematic searches yielded 110 studies from PubMed, 88 studies from Scopus, 92 studies from Web of Science, and 302 from Google scholar. After duplicates removal, I evaluated 584 studies for eligibility, followed by the exclusion of 566 records based on their title and abstract. Finally, eight and four studies were included in the qualitative and quantitative syntheses. The detailed flow diagram is shown in Figure 1.
Figure 1.
PRISMA flow diagram of included studies.
Study characteristics
Table 1 demonstrates the characteristics of included studies (3, 17–23). Six (75%) studies included in the qualitative analysis were case-control, while the remaining two records were cohort studies. The sample size of included studies ranges from 133 in the Mousavi-Mirzaei et al. study (21) to 50,045 in the Khademi et al. study. The highest percentage of men in a paper was 57.8 in Saberi et al. study. QA of Studies based on STROBE showed that the QA score of included studies ranges from 15 to 20 out of 22.
Table 1.
Demographic characteristics of participants of included studies.
| References | Study type | Provenance | Sample size | Male (%) | Age | Demographic characteristics | Quality assessment score (out of 22) |
|---|---|---|---|---|---|---|---|
| Andalibi et al. (17) | Prospective cohort | Iran | 595 | 308 (51.76) | 64.6 ± 14.8 | • Study Name: Mashhad Stroke Incidence Study • Time period: 12-month period (2006–2007) • Study location: Mashad, an urban area of Iran • Other features of study population: 5 points follow-up, including 1 week, 1 month, 3 months, 1 year, and 5 years after their stroke |
20 |
| Ebrahimi et al. (18) | Case-control | Iran | 956 | 542 (56.69) | 67.19 | • Time period: 2014 • Study location: Alzahra Hospital in Isfahan, Iran • Other features of study population: case = patients with confirmed AIS, control = hospitalized patients with no history of AIS and cardiovascular disease, matched for age (±5 years) and sex. |
16 |
| Fallahzadeh et al. (19) | Case-control | Iran | 9,264 | 4,276 (46.15) | 52.6 ± 9.7 | • Study Name: The Pars Cohort Study • Time period: Fall 2012 to 2014 • Study location: Valashahr district, a rural area consisting of Valashahr city and 93 villages located in Fars province in southern Iran. • Other features of study population: study population was consist of a variety of ethnicities, mainly Fars and Turk. |
17 |
| Hamzehee Moghadam et al. (20) | Case-control | Iran | 210 | 100 (47.6) | 66.9 | • Study location: Shafa hospital, Kerman, Iran • Other features of study population: Patients diagnosed with Acute ischemic stroke. |
15 |
| Khademi et al. (3) | Prospective cohort | Iran | 50,045 | 21,019 (42.00) | 52.1 ± 8.9 | • Study Name: The Golestan Cohort Study • Time period: January 2004 to June 2008 • Study location: Gonbad City and 326 villages in Golestan province, north eastern Iran |
19 |
| Mousavi-Mirzaei et al. (21) | Case-control | Iran | 133 | 70 (52.63) | 71.52 | • Time period: June 2016 to April 2017 • Study location: Vali-Asr Hospital of Birjand, East of Iran. • Other features of study population: patients diagnosed with AIS. |
17 |
| Rezvani and Ghandehari (22) | Case-control | Iran | 558 | 251 (44.98) | 56.2 | • Time period: 2010 • Study location: Cardiology and Neurology clinics of Birjand, Province of Southern Khorasan, Iran. |
18 |
| Saberi et al. (23) | Case-control | Iran | 166 | 96 (57.8) | 68.2 | • Time period: April 2013 to March 2014 • Study location: two academic hospitals associated with Guilan University of Medical Science in north of Iran. |
15 |
AIS, Acute ischemic stroke was defined as a sudden focal neurologic deficit of presumed arterial origin lasting ≥24 h with or without corresponding ischemic lesion on brain imaging.
Age is described as mean or mean ± standard deviation.
Gender is described as number of males (percent).
Qualitative findings
Six included studies evaluated the relationship between opium consumption and stroke (18–23). The outcome of four papers was the diagnosis of ischemic stroke (18, 20, 22, 23), while in two other, indices regarding the formation of ischemic plaque (21) and diagnosis of any kind of stroke (19) were the outcome. Four (18, 19, 21, 22) out of these six studies revealed at least a significant measure concerning the association between stroke and opium use.
Moreover, a single paper was driven from GCS revealing that opium use increases the hazard of mortality due to stroke (3). Similarly, Andalibi et al.'s study showed that although opium use increases the hazard of death following stroke, it does not significantly alter the hazard of disability following stroke (17). The qualitative synthesis of included studies is shown in Table 2.
Table 2.
Qualitative findings of included studies.
| References | Study type | Exposure | Outcome | Measure of association | Findings | |
|---|---|---|---|---|---|---|
| Andalibi et al. (17) | Prospective cohort | Opium use (burned and | Mortality following | Adjusted hazard | 7 days | 1.36 (0.70–2.65) |
| the sap) | any type of stroke | ratio (95% CI) | 1 month | 1.51 (0.91–2.51) | ||
| Disability following | 3 months | 1.58 (1.01–2.49) | ||||
| stroke (mRS*) | 1 year | 1.78 (1.20–2.65) | ||||
| 5 years | 1.75 (1.28–2.40) | |||||
| 3 months | 0.63 (0.23–1.67) | |||||
| 1 year | 1.19 (0.42–3.37) | |||||
| 5 years | 0.54 (0.09–3.22) | |||||
| Ebrahimi et al. (18) | Case-control | Opium addiction (based on medical records and interview of the patient) | Ischemic stroke | Adjusted odds ratio (95% CI) | Not adjusted for tobacco use Adjusted for tobacco use |
3.95 (2.02–7.69) 2.36 (1.16–4.85) |
| Fallahzadeh et al. (19) | Case-control | Self-report opium use | Stroke (all types) | Unadjusted odds ratio (95% CI) | 1.60 (1.01–2.55) | |
| Hamzehee-Moghadam | Case-control | Constant use of opium for at least 1 year | Ischemic stroke | Logistic regression (95% CI) | β (SE) | 0.861(0.316) |
| et al. (20) | Adjusted odds ratio (95% CI) | Adjusted odds ratio | 2.36 (1.26–3.61) | |||
| Khademi et al. (3) | Cohort | Opium use (self-report) | Death as a result of | Adjusted hazard | Male | 1.49 (1.07–2.08) |
| any kind of stroke | ratio (95% CI) | Female | 1.97 (1.30–2.97) | |||
| Total | 1.68 (1.29–2.18) | |||||
| Mousavi-Mirzaei | Case-control | Opium dependence** | Atherosclerotic | Adjusted odds ratio | Number of plaques | 1.42 (1.11–1.81) |
| et al. (21) | plaques | (95% CI) | Vascular stenosis | 1.01 (0.98–1.03) | ||
| Calcified plaque | 1.37 (0.95–1.97) | |||||
| Internal media thickness | 2.48 (2.27–10.94) | |||||
| Rezvani et al. (22) | Case-control | Opium addiction** | Ischemic stroke | Adjusted odds ratio (95% CI) | Oral opium addiction | 0.21 (0.08–0.56) |
| Inhaled opium addiction | 1.76 (0.76–4.08) | |||||
| Saberi et al. (23) | Case-control | Opium addiction** | Ischemic stroke | Logistic regression | β (SE) | 0.67 (0.40) |
| Adjusted odds ratio (95% CI) | Adjusted odds ratio | 1.94 (0.89–4.36) | ||||
The Modified Rankin Scale (mRS) is used to measure the degree of disability in patients who have had a stroke.
Opium addiction is diagnosed when three of seven physiological (e.g., tolerance and withdrawal); behavioral (e.g., taking the drug in larger amounts than intended), and cognitive (e.g., persistent desire to cut down) symptoms are met within the previous 12 months.
CI, confidence interval.
Quantitative findings
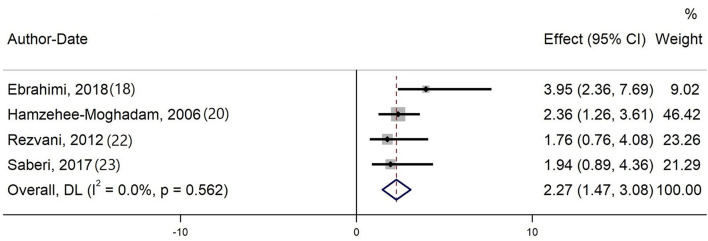
Four studies evaluating the effect of opium on ischemic stroke were included in the meta-analysis (18, 20, 22, 23). The quantitative synthesis proved that opium increases the odds of ischemic stroke by 127% (pooled OR = 2.27, 95% CI: 1.47–3.07). No heterogeneity was evident in current meta-analysis (Cochran's Q = 2.05, degree of freedom = 3, p-value = 0.562, I2 = 0.0%). Also, Begg's test did not show a significant publication bias (p-value = 0.192). Forest plot of the meta-analysis is shown in Figure 2.
Figure 2.
Forest plot of the meta-analysis.
Discussion
This study indicated that not only opium dependence almost doubles the odds of being diagnosed with a stroke but also it leads to a higher hazard of mortality due to stroke. This is the first systematic review and meta-analysis that proposes opium dependence as a notable risk factor for stroke to the best of my knowledge. It should be clarified that this study and included records are all focused on the cerebrovascular effects of opium abuse, rather than the effects of prescribed opium, as a component of a medical treatment plan.
Our meta-analysis on multi-variable adjusted case-control studies revealed that opium consumption independently increases the odds of ischemic stroke. Moadabi et al. proposed hypothetical pathophysiology underlying the impact of opium on cerebral hemodynamic parameters. They performed transcranial Doppler (TCD) ultrasonography, which revealed that patients with opium dependence are at higher odds of abnormal pulsatility index (OR = 9.615) and mean flow velocity (OR = 3.246). In other words, they indicated that opium affects both small and large vessels, which leads to a higher risk of ischemic stroke (24). Likewise, Mousavi-Mirzaei et al.'s study revealed that opium consumption leads to increased atherosclerotic plaques and internal media thickness, a well-established component of the pathogenesis of ischemic stroke (21). These findings are similar to studies showing an increased risk of myocardial infarction and coronary revascularization, among opium users (25). Opium consumption is a significant risk factor for coronary artery disease, as it increases the progression of atherosclerosis, and leads to an increase in low-density lipoprotein cholesterol (LDL-C) level and a decrease in high-density lipoprotein cholesterol through opium-induced activation of hepatic pathways (26–28).
Moreover, the Asiabanha et al. study demonstrated that opium imposes brain cell damage by inducing apoptosis, which may prone the brain to cerebrovascular incidents (29). Opium addiction also affects endothelial function, leading to a decline in nitrous oxide level (30), a molecule that improves brain blood flow and neuronal survival in stroke (31).
Apart from previously mentioned pathways, opium addiction is a well-established risk factor for diabetes, hypertension, and hyperlipidemia (32), which can potentially elevate stroke hazards (33, 34). Studies, included in the current meta-analysis, were adjusted for potential confounders such as dyslipidemia, diabetes, and hypertension. Nevertheless, it should be noted that previous studies revealed that opium consumption worsens glucose metabolism in patients both with diabetes and non-diabetes (35, 36), and it results in a significant disturbance in lipid profile, which in turn may provoke stroke (28). To sum up, opium not only directly affects brain cells but also leads to unfavorable changes in the incidence of metabolic risk factors.
Another modifiable risk factor for stroke is tobacco smoking. Comparing the effects of tobacco smoking and opium as habitual risk factors can help understand the harmful but modifiable effects of opium. OR calculated for the effect of opium on stroke in this study was higher or similar compared to ORs calculated for tobacco smoking (37–41). Pan et al.'s meta-analysis revealed that current smokers are at higher odds of stroke (1.92, 95% CI: 1.49–2.48), while the association of being a former smoker and stroke was not significant (1.30, 95% CI: 0.93–1.81) (38). Likewise, Wannamethee et al. study showed that quitting smoking results in notable and rapid benefits in reducing the risk of stroke (42). Interestingly, smoking cessation even after stroke enhances the outcome (43). Beneficial effects of opium cessation are also evident in previous studies (3, 44), although there is no scientific evidence regarding the impact of opium cessation and a decline in stroke risk.
Smoking is generally more prevalent in men (45). Similarly, the prevalence of opium addiction is notably higher in men than women (3). As the number of studies included in my meta-analysis was few and no heterogeneity was evident, I could not conduct a meta-regression analysis to evaluate the effect of gender. However, it is worth noting that experimental studies showed that addicted men are at higher risk of ischemic stroke as men brain cells are more susceptible to opium than women (29).
Our review shows that opium predisposes patients to stroke and increases the hazard of mortality following any type of stroke. In other words, Khademi et al.'s study demonstrated that opium consumption leads to strongly increased risks of death due to a stroke. That is to say, in GCS, opium was the cause of mortality in 0.50% of non-users compared to 1.15% of participants who consume opium. These findings remained significant after adjustment for potential confounders (3).
Comparably, Andalibi et al.'s study illustrated that using opium increases mortality risk following stroke. More specifically, multi-regression models proved that patients who consume opium are at higher hazard of 3-months (HR = 1.58, 95% CI: 1.01–2.49), 1-year (HR = 1.78, 95% CI: 1.20–2.65), and 5-years (HR = 1.75, 95% CI: 1.28–2.40) mortality. Conversely, patients' disability was not significantly different in user and non-user groups. Participants' disability was measured using an objective index (Modified Rankin Scale), which can be influenced by the analgesic and euphoric effects of opium (17).
Given the remarkable effects of opium on stroke, addiction prevention programs should be seriously pursued in countries where opium dependence is common, namely, central Asian countries and Iran (46). Although I did not add a filter for the provenance of the study, all included studies were from Iran in the current review. Even though the single geographical provenance of included studies makes the generalization of my findings challenging, it can assist other low and middle-income countries, similar to Iran, in adopting better policies.
Strengths and limitations
This study had several strengths. Most importantly, this is the first systematic review and meta-analysis that evaluates the effects of opium on stroke. This study evaluates the effect of opium on stroke incidence and mortality, and it also reviewed papers that evaluated the outcome of patients dependent on opium following stroke. This study also had several limitations; first, the number of studies included in the quantitative analysis was limited, resulting in a bias. Second, all included studies were from a single country (Iran). Third, studies included in the meta-analysis were all case-control studies. Conduction of larger cohorts is suggested to assess the effect of opium more precisely as a risk factor. Fourth, due to the absence of randomization, p-values (even underlying the confidence intervals) must be considered as a possible approximation to reality.
Conclusion
This study shows that opium consumption significantly increases the odds of being diagnosed with stroke in the general population. Moreover, this study's findings indicate that not only might there be a significant relationship between opium and mortality as a result of stroke but opium may also increase the odds of death after stroke.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
PM conceived the idea for the topic, performed the review, conducted quantitative synthesis, and drafted the manuscript.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.855578/full#supplementary-material
References
- 1.Drugs U, Crime M. World Drug Report 2010. New York, NY: United Nations Publications; (2010). [Google Scholar]
- 2.Sheikh M, Kamangar F, Malekzadeh R. Fifty years of research and one conclusion: opium causes cancer. Arch Iran Med. (2020) 23:757–60. 10.34172/aim.2020.95 [DOI] [PubMed] [Google Scholar]
- 3.Khademi H, Malekzadeh R, Pourshams A, Jafari E, Salahi R, Semnani S, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50,000 adults in Iran. Br Med J. (2012) 344:e2502. 10.1136/bmj.e2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eskandarieh S, Jafari F, Yazdani S, Hazrati N, Saberi-Zafarghandi MB. Compulsory maintenance treatment program amongst Iranian injection drug users and its side effects. Int J High Risk Behav Addict. (2014) 3:21765. 10.5812/ijhrba.21765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nalini M, Oranuba E, Poustchi H, Sepanlou SG, Pourshams A, Khoshnia M, et al. Causes of premature death and their associated risk factors in the Golestan Cohort Study, Iran. BMJ Open. (2018) 8:e021479. 10.1136/bmjopen-2018-021479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katan M, Luft A. Global burden of stroke. Semin Neurol. (2018) 38:208–11. 10.1055/s-0038-1649503 [DOI] [PubMed] [Google Scholar]
- 7.Mensah GA, Norrving B, Feigin VL. The global burden of stroke. Neuroepidemiology. (2015) 45:143–5. 10.1159/000441082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collaborators GLRoS. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. 10.1056/NEJMoa1804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feigin VL, Krishnamurthi RV, Parmar P, Norrving B, Mensah GA, Bennett DA, et al. Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology. (2015) 45:161–76. 10.1159/000441085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Alebeek ME, Arntz RM, Ekker MS, Synhaeve NE, Maaijwee NA, Schoonderwaldt H, et al. Risk factors and mechanisms of stroke in young adults: the FUTURE study. J Cereb Blood Flow Metab. (2018) 38:1631–41. 10.1177/0271678X17707138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitew ZW, Alemu A, Ayele EG, Tenaw Z, Alebel A, Worku T. Metabolic syndrome among children and adolescents in low and middle income countries: a systematic review and meta-analysis. Diabetol Metab Syndr. (2020) 12:1–23. 10.1186/s13098-020-00601-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borch-Johnsen K. The metabolic syndrome in a global perspective. The public health impact–secondary publication. Dan Med Bull. (2007) 54:157–9. [PubMed] [Google Scholar]
- 13.Freedman B, Martinez C, Katholing A, Rietbrock S. Residual risk of stroke and death in anticoagulant-treated patients with atrial fibrillation. J Am Med Assoc Cardiol. (2016) 1:366–8. 10.1001/jamacardio.2016.0393 [DOI] [PubMed] [Google Scholar]
- 14.Pan Y, Li Z, Li J, Jin A, Lin J, Jing J, et al. Residual risk and its risk factors for ischemic stroke with adherence to guideline-based secondary stroke prevention. J Stroke. (2021) 23:51. 10.5853/jos.2020.03391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. (2018) 169:467–73. 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 16.Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. (2007) 4:e297. 10.1371/journal.pmed.0040297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andalibi MSS, Rezaei Ardani A, Amiri A, Morovatdar N, Talebi A, Azarpazhooh MR, et al. The association between substance use disorders and long-term outcome of stroke: results from a population-based study of stroke among 450,229 urban citizens. Neuroepidemiology. (2021) 55:171–9. 10.1159/000514401 [DOI] [PubMed] [Google Scholar]
- 18.Ebrahimi H, Javanmard SH, Asgary S, Dehghani L, Amiri M, Saadatnia M. Opium addiction and Ischemic stroke in Isfahan, Iran: a case-control study. Eur Neurol. (2018) 79:82–5. 10.1159/000485098 [DOI] [PubMed] [Google Scholar]
- 19.Fallahzadeh MA, Salehi A, Naghshvarian M, Fallahzadeh MH, Poustchi H, Sepanlou SG, et al. Epidemiologic study of opium use in pars cohort study: a study of 9,000 adults in a rural Southern Area of Iran. Arch Iran Med. (2017) 20:205–10. [PubMed] [Google Scholar]
- 20.Hamzehee Moghadam A, Mousavi A. The relationship between opium dependency and stroke. J Kerman Univ Medical Sci. (2006) 13:203–8. [Google Scholar]
- 21.Mousavi-Mirzaei SM, Talebi A, Amirabadizadeh A, Nakhaee S, Azarkar G, Mehrpour O. Increasing the risk of stroke by opium addiction. J Stroke Cerebrovasc Dis. (2019) 28:1930–5. 10.1016/j.jstrokecerebrovasdis.2019.03.044 [DOI] [PubMed] [Google Scholar]
- 22.Rezvani MR, Ghandehari K. Is opium addiction a risk factor for ischemic heart disease and ischemic stroke? J Res Medical Sci. (2012) 17:958–61. [PMC free article] [PubMed] [Google Scholar]
- 23.Saberi A, Ghayeghran AR, Janeshin S, Biazar G, Kazemnezhad E. Opium consumption prevalence among patients with ischemic stroke compared with healthy individuals in Iran. Int J High Risk Behav Addict. (2017) 6:e27264. 10.5812/ijhrba.27264 [DOI] [Google Scholar]
- 24.Moadabi Y, Saberi A, Hoseini S, Karimi A, Yousefzadeh-Chabok S. Cerebral hemodynamic abnormalities of patients with ischemic stroke who are opium addicted: a study by transcranial doppler ultrasonography. Iran J Neurol. (2019) 18:76–81. [PMC free article] [PubMed] [Google Scholar]
- 25.Chow SL, Sasson C, Benjamin IJ, Califf RM, Compton WM, Oliva EM, et al. Opioid use and its relationship to cardiovascular disease and brain health: a presidential advisory from the American Heart Association. Circulation. (2021) 144:e218–32. 10.1161/CIR.0000000000001007 [DOI] [PubMed] [Google Scholar]
- 26.Bryant HU, Kuta CC, Story JA, Yim GK. Stress- and morphine-induced elevations of plasma and tissue cholesterol in mice: reversal by naltrexone. Biochem Pharmacol. (1988) 37:3777–80. 10.1016/0006-2952(88)90415-7 [DOI] [PubMed] [Google Scholar]
- 27.Maron DJ. The epidemiology of low levels of high-density lipoprotein cholesterol in patients with and without coronary artery disease. Am J Cardiol. (2000) 86:11l−4l. 10.1016/S0002-9149(00)01462-4 [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi A, Darabi M, Nasry M, Saabet-Jahromi MJ, Malek-Pour-Afshar R, Sheibani H. Effect of opium addiction on lipid profile and atherosclerosis formation in hypercholesterolemic rabbits. Exp Toxicol Pathol. (2009) 61:145–9. 10.1016/j.etp.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 29.Asiabanha M, Asadikaram G, Rahnema A, Mahmoodi M, Hasanshahi G, Hashemi M, et al. Chronic opium treatment can differentially induce brain and liver cells apoptosis in diabetic and non-diabetic male and female rats. Kjpp. (2011) 15:327–32. 10.4196/kjpp.2011.15.6.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghanei AM, Saadatnia M, Javanmard SH. Effects of opium addiction on vascular endothelium. J Isfahan Medical School. (2013) 2084–90. Available online at: http://jims.mui.ac.ir/index.php/jims/about/article_13954.html?lang=en
- 31.Terpolilli NA, Kim SW, Thal SC, Kataoka H, Zeisig V, Nitzsche B, et al. Inhalation of nitric oxide prevents ischemic brain damage in experimental stroke by selective dilatation of collateral arterioles. Circul Res. (2012) 110:727–38. 10.1161/CIRCRESAHA.111.253419 [DOI] [PubMed] [Google Scholar]
- 32.Najafipour H, Beik A. The impact of opium consumption on blood glucose, serum lipids and blood pressure, and related mechanisms. Front Physiol. (2016) 7:436. 10.3389/fphys.2016.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong K-S, Bang OY, Kang DW, Yu KH, Bae HJ, Lee JS, et al. Stroke statistics in Korea: part I. Epidemiology and risk factors: a report from the korean stroke society and clinical research center for stroke. J Stroke. (2013) 15:2. 10.5853/jos.2013.15.1.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Wen X, Li W, Li X, Wang Y, Lu W. Risk factors for stroke in the Chinese population: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2017) 26:509–17. 10.1016/j.jstrokecerebrovasdis.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 35.Asgary S, Sarrafzadegan N, Naderi GA, Rozbehani R. Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? Lipids Health Dis. (2008) 7:1–5. 10.1186/1476-511X-7-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karam GA, Reisi M, Kaseb AA, Khaksari M, Mohammadi A, Mahmoodi M. Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol. (2004) 9:53–8. 10.1080/13556210410001674095 [DOI] [PubMed] [Google Scholar]
- 37.Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk. (1999) 6:207–11. 10.1177/204748739900600403 [DOI] [PubMed] [Google Scholar]
- 38.Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke: a meta-analysis. Medicine. (2019) 98L14872. 10.1097/MD.0000000000014872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah RS, Cole JW. Smoking and stroke: the more you smoke the more you stroke. Expert Rev Cardiovasc Ther. (2010) 8:917–32. 10.1586/erc.10.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shinton R, Beevers G. Meta-analysis of relation between cigarette smoking and stroke. Br Medical J. (1989) 298:789–94. 10.1136/bmj.298.6676.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolf PA, D'Agostino RB, Kannel WB, Bonita R, Belanger AJ. Cigarette smoking as a risk factor for stroke: the Framingham Study. J Am Med Assoc. (1988) 259:1025–9. 10.1001/jama.259.7.1025 [DOI] [PubMed] [Google Scholar]
- 42.Wannamethee SG, Shaper AG, Whincup PH, Walker M. Smoking cessation and the risk of stroke in middle-aged men. J Am Med Assoc. (1995) 274:155–60. 10.1001/jama.274.2.155 [DOI] [PubMed] [Google Scholar]
- 43.Epstein KA, Viscoli CM, Spence JD, Young LH, Inzucchi SE, Gorman M, et al. Smoking cessation and outcome after ischemic stroke or TIA. Neurology. (2017) 89:1723–9. 10.1212/WNL.0000000000004524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golshiri A, Mokhtaree MR, Shabani Z, Tabatabaee ST, Rahnama A, Moradi M, et al. Effects of opium smoking cessation on the nasopharyngeal microbial flora. Addict Health. (2009) 1:1. [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer T, Göhlmann S, Sinning M. Gender differences in smoking behavior. Health Econ. (2007) 16:895–909. 10.1002/hec.1259 [DOI] [PubMed] [Google Scholar]
- 46.Ray R, Kattimani S, Sharma H. Opium Abuse and Its Management: Global Scenario. New Delhi: World Health Organization Department of Mental Health and Substance Abuse Management of Substance Abuse. National Drug Dependence Treatment Centre All India Institute of Medical Sciences; (2006). p. 1–13. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.