Abstract
OBJECTIVE:
This study investigated the efficacy of high-flow nasal oxygen therapy in patients with severe acute exacerbation of chronic obstructive pulmonary disease admitted to the intensive care unit.
MATERIAL AND METHODS:
Totally, 23 patients were enrolled in the study. High-flow nasal oxygen therapy was administered with a predefined protocol. Vital signs, Visual Analog Scale for dyspnea, and arterial blood gas parameters were recorded at the beginning under low-flow oxygen support therapy and the 1st, 6th, 12th, and 24th hours of high-flow nasal oxygen therapy. High-flow nasal oxygen therapy duration, intensive care unit length of stay, and intensive care unit, in-hospital, and 60-day mortality were recorded as outcomes and compared according to the presence of pneumonia upon admission.
Results:
In 12 patients (52.2%), pneumonia was present. High-flow nasal oxygen therapy was applied for a median of 57 hours [49.2-104.5]. Overall decreases were detected in heart rate (P = .001), respiratory rate (P < .001), and Visual Analog Scale for dyspnea (P = .001) during the first 24 hours of the therapy. Although there was an increase in PaCO2 (P = .001), pH increased (P < .001) over time too. No change in partial arterial oxygen pressure (P = .63) and partial arterial oxygen pressure/fraction of inspired oxygen ratio (P = .22) was noted. Nineteen patients (77%) were successfully weaned from high-flow nasal oxygen therapy. While the high-flow nasal oxygen therapy failure rate was 23%, the in-hospital and 60-day mortality rates were 8.6%. Outcomes were not different between patients with and without pneumonia.
Conclusion:
High-flow nasal oxygen therapy was efficient in relieving respiratory distress and well-tolerated with no adverse outcome in severe acute exacerbation of chronic obstructive pulmonary disease patients admitted to the intensive care unit.
Keywords: Oxygen inhalation therapy, chronic obstructive pulmonary disease, pneumonia, critical care, intensive care, critically ill
Main Points
High-flow nasal oxygen therapy relives respiratory distress without worsening arterial corbon dioxide pressure in patients with chronic obstructive pulmonary disease exacerbation and is believed to be safe in the co-existence of pneumonia.
Chronic obstructive pulmonary disease is a life-threatening progressive lung disease with airflow limitation that predisposes to exacerbations.
Noninvasive ventilation has been proven to reduce intubation rate and mortality. However, NIV failure may occur in COPD patients especially in the presence of pneumonia.
High Flow Nasal Oxygen Therapy has some physiological advantages for AE-COPD patients (PEEP effect, decrease in dead space-tidal volume ratio, stable fraction of inspired oxygen and facilitation of excessive secretions).
This study investigated the efficacy of high flow nasal oxygen therapy (HFNO) in patients with severe acute exacerbation of chronic obstructive pulmonary disease (AECOPD) admitted to the intensive care unit and patients were compared according to the presence of pneumonia.
High flow nasal oxygen therapy relives respiratory distress without worsening arterial carbon monoxide pressure in patients with COPD exacerbation and safe in co-existence of pneumonia.
Introduction
Chronic obstructive pulmonary disease (COPD) is a life-threatening progressive lung disease with airflow limitation that predisposes to exacerbations. According to the Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease (GOLD) 2017 Report, symptoms including sudden respiratory distress during rest, respiratory rate > 30/min, use of assisted respiratory muscles, paradoxical abdominal respiration, decrease in oxygen saturation, a tendency to sleep or confusion, and the absence of response to initial medical treatment are considered as severe acute exacerbation of COPD (AE-COPD).1 Non-invasive ventilation (NIV) has been proven to reduce intubation rate and mortality and is being widely used to support ventilation in these patients.2 Non-invasive ventilation increases tidal volume and minute ventilation by adding inspiratory pressure support so that dynamic hyperinflation resolves and respiratory workload and respiratory rate decrease.3 However, NIV failure may occur in COPD patients, with common causes being intolerance to mask, agitation, excessive secretions, and presence of pneumonia.4 Some studies reported an association between pneumonia and NIV failure and the need for intubation in approximately two-thirds of patients with pneumonia treated initially with NIV.5 The optimal non-invasive respiratory support for patients with COPD and pneumonia remains unclear.6
Although high-flow nasal oxygen (HFNO) has been used widely in hypoxemic respiratory failure,7 its role in hypercapnic respiratory failure has been investigated. Chronic obstructive pulmonary disease being the most common cause of hypercapnic respiratory failure,8 some studies have evaluated the effectiveness of HFNO among COPD patients in clinically different situations, such as acute exacerbation9-15 and weaning from mechanical ventilation.16 Although the system is an open circuit and does not provide inspiratory support as in NIV, it has some theoretical advantages for COPD patients providing positive end-expiratory pressure effect,17 more stable fraction of inspired oxygen (FiO2), and comfort18 by delivering heated and humidified air-oxygen mixture at flow rates up to 60 L/min.
We investigated the efficacy of HFNO therapy in addition to standard medical treatment in patients admitted to the intensive care unit (ICU) because of severe AE-COPD. Our primary endpoints were changes in respiratory rate, heart rate, dyspnea, arterial blood gas (ABG) results, and the ratio of partial arterial oxygen pressure (PaO2) to FiO2 (PaO2/FiO2) within 24 hours, whereas our secondary endpoints were HFNO failure rate; in ICU, in hospital, and 60-day mortality after ICU admission and the effect of coexisting pneumonia on outcomes.
Material and Methods
Consecutive patients admitted to the medical ICU for severe AE-COPD between October 2017 and January 2019 were included. The study was conducted as a prospective feasibility study which was approved by the Hacettepe University Clinical Research Ethics Committee (2018/14-18), and written informed consent was taken from patients or their relatives.
Patients who had been diagnosed with COPD and admitted to our emergency department with symptoms including sudden respiratory distress during rest, respiratory rate > 30/min, use of auxiliary respiratory muscles, paradoxical abdominal breathing, oxygen saturation <90%, and lack of response to initial medical treatment were transferred to the ICU and included in the study. Exclusion criteria were pH < 7.30, hemodynamic instability, age under 18 years old, presence of active upper gastrointestinal system bleeding, and a recent history of upper airway surgery.
Pneumonia was defined as a new radiographic pulmonary infiltrate upon admission with signs or symptoms of lower respiratory tract infection.
Patients’ demographic data, body mass index, smoking history, comorbidities, Charlson Comorbidity Index,19 Acute Physiology and Chronic Health Assessment score (APACHE II),20 Sequential Organ Failure Assessment score,21 pulmonary function test results within the last 6 months, modified Medical Research Council (mMRC) dyspnea scale,22 number of exacerbation and hospitalization in the past year, GOLD COPD stages1, and treatments including long-term oxygen therapy (LTOT) or home-NIV therapy, were recorded.
The visual analog scale (VAS) for dyspnea was used to assess temporal changes in patient’s respiratory distress.23 Subjects were asked to rate the severity of dyspnea ranging from 0 to 10 with 10 being the maximum.
High-flow nasal oxygen therapy was administered in the ICU with Optiflow™ (Fisher and Paykel Healthcare Limited, New Zeland), which is set to deliver flow rate of 40 L/min with FiO2 of 30%, at a temperature of 37°C as the initial set-up. Then, the flow rate was increased by 10 L/min with an interval of 20 minutes up to 60 L/min that the patient could tolerate, and FiO2 was set to keep the patient’s SpO2 above 90%.
Vital signs, VAS for dyspnea, signs of respiratory distress, and ABG parameters were recorded at the beginning under low-flow oxygen support therapy via nasal cannula or face mask and at the 1st, 6th, 12th, and 24th hours of HFNO therapy.
If deterioration in patients’ level of consciousness, worsening dyspnea (signs of respiratory muscle fatigue), pH < 7.30, malign arrhythmia, or hemodynamic instability without response to fluids were detected, it was recorded as treatment failure, commencing NIV or IMV as rescue therapy in accordance with indication, at the discretion of the primary treating physician. After 24 hours of follow-up, the flow was reduced by 50% if the patient’s respiratory rate decreased without paradoxical respiration or accessory muscle use and PaO2 > 60 mmHg with <35% FiO2 was noted. High-flow nasal oxygen therapy was discontinued if the patient was stable during the follow-up. Patients received proper medical treatments (nebulized short-acting beta-2 adrenergic agonist and muscarinic antagonists, systemic corticosteroids, empiric intravenous antibiotics) for COPD exacerbation and pneumonia.
Intensive care unit length of stay (LOS), hospital LOS, and ICU and in-hospital mortality were recorded. Patients were called to obtain 60-day mortality status. The Turkish Ministry of Health online death notification system was checked for patients who could not be reached by phone.
Statistical analyses were performed using the International Business Machines (IBM) Statistical Package for the Social Sciences software version 22. Descriptive analyses are presented using medians [25-75 percentiles] for ordinal variables and n (%) for categorical variables. Friedman tests were conducted to test whether a significant change in the vital signs, VAS, and ABG variables was noted. An overall 5% type 1 error level was used to infer statistical significance. The Wilcoxon test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons; P < .005 was considered statistically significant. The Mann–Whitney U test was used to compare the patient’s characteristics and outcomes according to the presence of pneumonia.
Results
Out of 43 screened patients, 23 patients who met the inclusion criteria had been enrolled in the study between October 2017 and January 2019. Twenty patients were excluded from the study because of pH < 7.30, hemodynamic instability, and refusal to written informed consent. Table 1 summarizes patients’ characteristics, COPD state, patient outcomes, and comparison according to the presence of pneumonia. In 12 patients (52.2%), pneumonia was present. Male gender (P = .046), smoking history (P = .03), and APACHE II score (P = .048) were higher in patients with pneumonia compared to those without pneumonia. Sixteen patients had a pulmonary function test in the last 6 months, 12 (75%) of whom had severe or very severe airflow limitation. The mMRC dyspnea index of 21 patients was 2 and above. All patients had 1 or more exacerbations and hospitalization history in the last year. Patients were mostly in stage D (n=17) according to the GOLD COPD staging. Eighteen patients had been using LTOT, and 6 of them had home therapy with NIV. At enrolment, the median pH was 7.37 [7.32-7.40], PaCO2 was 56.3 [48.3-63.3] mmHg, PaO2 was 65.1 [56.7-81.7] mmHg, and PaO2/FiO2 was 179.2 [150.8-222.0].
Table 1.
Patients’ Characteristics at Admission and Outcomes According to the Presence of Pneumonia at Admission to ICU
|
All,
n = 23 |
With Pneumonia, n = 12 | Without, Pneumonia n = 11 | P | |
|---|---|---|---|---|
| Age, year* | 68 [64-75] | 72 [62-75] | 65 [59-68] | .14 |
| Male sex, n (%) | 17 (74) | 11.0 (91.7) | 6.0 (54.5) | .046 |
| BMI, kg/m2* | 23.4 [21.5-28.0] | 23.1 [21.9-25.9] | 27.5 [20.7-32.4] | .23 |
| Smoking history, pack/year* | 40 [25-60] | 55 [30-100] | 30 [11-45] | .030 |
| Comorbidities, n (%)** | ||||
| Cardiovascular | 14 (60.9) | 9 (75) | 5(45.5) | .15 |
| Hypertension | 9 (40) | 5 (41.7) | 4 (36.4) | .56 |
| Diabetes mellitus | 6 (27) | 3 (25) | 3 (27.3) | .45 |
| Cancer | 4 (18) | 2 (16.7) | 2 (18.2) | .67 |
| Neurological disease | 1 (4.5) | 0 (0) | 1 (0.4) | .40 |
| Charlson Comorbidity Index | 5 [4-6] | 5 [4-7] | 5 [3-6] | .38 |
| APACHE II score* | 15 [12-17] | 17.0 [14.2-18.7] | 13.0 [10.0-15.0] | .048 |
| SOFA score* | 2 [2-4] | 3 [2-4] | 2 [2-4] | .32 |
| Visual Analog Scale for dyspnea | 6 [4-8] | 5 [4-9] | 7 [5-8] | .56 |
| Pulmonary function test | n = 16 | n = 8 | n = 8 | |
| FEV1 (L) | 0.99 [0.79-1.36] | 0.68 [0.53-1.45] | 1.05 [0.87-1.29] | .91 |
| FEV1, % | 37.5 [25.5-46.7] | 21.2 [18.0-43.0] | 34 [27.5-49.5] | .95 |
| FVC (L) | 2.0 [1.5-2.4] | 2.0 [1.2-2.5] | 1.9 [1.5-2.3] | .67 |
| FVC,% | 37.5 [36.7-69.2] | 50.5 [36.0-75.0] | 44.0 [37.0-56.7] | .75 |
| FEV1/FVC | 55.5 [48.5-64.7] | 57.0 [50.0-65.0] | 53.0 [44.2-63.8] | .37 |
| mMRC Dyspnea Scale, n (%) | .40 | |||
| 1 | 2 (8.7) | 0 (0) | 2 (18.2) | |
| 2 | 5 (21.7) | 2 (16.7) | 3 (27.3) | |
| 3 | 8 (34.8) | 6 (50.0) | 2 (18.2) | |
| 4 | 8 (34.8) | 4 (33.3) | 4 (36.4) | |
| AE-COPD* (per year) | 2 [1-3] | 2.5 [2-3] | 2 [1-3] | .52 |
| Hospitalization (per year)* | 2 [1-2] | 2 [1-2] | 2 [1-3] | .82 |
| GOLD COPD stage, n (%) | .90 | |||
| A | 0 (0) | 0 | 0 | |
| B | 2 (8.7) | 1 (8.3) | 1 (9.1) | |
| C | 4 (17.4) | 2 (16.7) | 2 (18.2) | |
| D | 17 (73.9) | 9 (75.0) | 8 (73.0) | |
| Baseline ABG parameters | ||||
| pH | 7.37 [7.32−7.40] | 7.37 [7.32−7.43] | 7.38 [7.32−7.40] | .97 |
| PaCO2 (mmHg) | 56.3 [48.3−63.3] | 52.8 [43.0−61.7] | 59.8 [54.3−64.5] | .17 |
| PaO2 (mmHg) | 65.1 [56.7−81.7] | 62.3 [52.9−66.2] | 71.0 [54.7−91.0] | .11 |
| PaO2/FiO2 (mmHg) | 179 [150−222] | 167 [142-208] | 193 [159-303] | .17 |
| HFNO failure rate, n (%) | 5 (21.7) | 3 (25) | 2 (18) | .67 |
| ICU length of stay, day* | 9 [6-9] | 9.0 [8.0-9.0] | 8.0 [5.0-10.2] | .24 |
| Hospital length of stay, day* | 10 [8-11] | 11.0 [9.0-29.0] | 10.0 [6.7-12.5] | .23 |
| ICU mortality, n (%) | 1 (4.3) | 1 (8.3) | 0 | .70 |
| Hospital mortality, n (%) | 2 (8.7) | 1 (8.3) | 1 (9.1) | .95 |
| 60-day mortality, n (%) | 2 (8.7) | 1 (8.3) | 1 (9.1) | .99 |
BMI, body mass index; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, sequential organ failure assessment; FEV1, forced expiratory volume; FVC, forced vital capacity; mMRC, Modified Medical Research Council; AE-COPD, acute exacerbation of chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ABG, arterial blood gas; PaCO2, partial arterial carbon dioxide pressure; PaO2, partial arterial oxygen pressure; FiO2, fractional oxygen; HFNO, high-flow nasal oxygen; ICU, intensive care unit.
*Median [25-75 percentile];**22 patients had one or more coexisting conditions. P < 0.05 values are bolded.
High-flow nasal oxygen was applied for a median of 57 hours [49.2-104.5]. Sixteen patients (70.0%) tolerated 60 L/min flow rate in the first hour of the therapy, and the median flow rate was 60 L/min [40-60] at the 24th hour. High-flow nasal oxygen duration was not different in patients with pneumonia (54.0 [43.5-84.0]) than those without pneumonia (75.0 [49.5-111.2]) (P = .55). While 19 patients were successfully weaned from HFNO, 5 (23.0 %) were not; 2 of them were intubated and the other 2 underwent NIV. A patient who was transferred to the ward was lost due to cardiac arrest within the first 72 hours, and it was recorded as a failure. The median ICU length of stay was 9 days [6-9], while the hospital length of stay was 10 days [8-11]. The ICU mortality rate was 4.3%, while the in-hospital and 60-day after ICU admission mortality rates were 8.6%. The HFNO failure rate, ICU LOS, and 60-day mortality were not different between the groups.
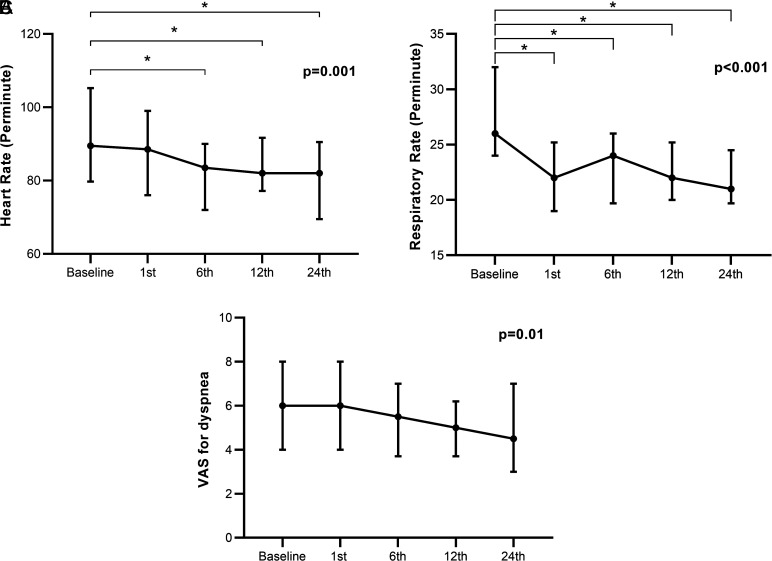
Figure 1 shows the temporal changes of heart rate, respiratory rate, and VAS for dyspnea. There was an overall decrease in heart rate (P = .001). Pairwise analyses revealed differences between baseline (89.5 [79.7-105.2]) and 6th hour (83.5 [72.0-90.0]) (P = .001), baseline and 12th hour (82.0 [77.2-91.7]) (P = .002), and baseline and 24th hour heart rate (82.0 [69.5-90.5]) (P = .001). Respiratory rate also decreased over time (P < .001) from baseline (26.0 [24.0-32.0]) to the 1st hour (22.0 [19.0-25.2]) and remained stable during the 6th (24.0 [19.7-26.0]), 12th (22.0 [20.0-25.2]), and 24th (21.0 [19.7-24.5]) hours. There was an overall decrease in VAS for dyspnea during follow-up (P = .001); however, no significant differences were found in pairwise comparisons.
Figure 1.
Temporal changes in heart rate (A), respiratory rate (B), and Visual Analogue Scale for dyspnea (C) at baseline, 1st, 6th, 12th, and 24th hours. Data points represent medians with 25-75 percentiles, and P values were assessed by Friedman test. *P < .005 in pairwise analyses with Wilcoxon test.
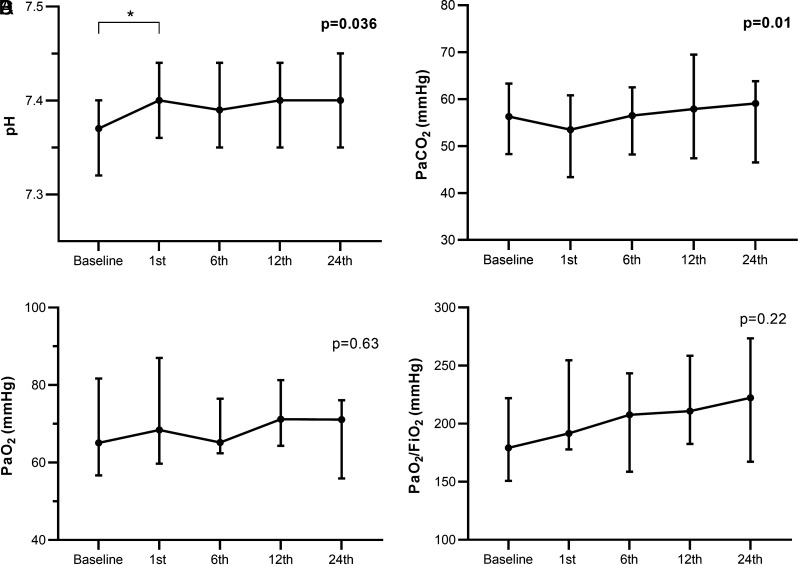
Figure 2 shows the ABG results. The difference in pH between baseline (7.37 [7.32-7.40]) and first hour (7.40 [7.36-7.44]) (P < .001) contributed to the overall significant variation (P = .036). Although there was an overall increase in PaCO2 (P = .001), there were not any differences in pairwise analyses. No change in PaO2 (P = .63) and PaO2/FiO2 ratio (P = .22) was noted.
Figure 2.
Temporal changes in pH (A), PaCO2 (B), PaO2 (C) and PaO2/FiO2 ratio (D) at baseline, 1st, 6th, 12th, and 24th hours. Data points represent medians with 25-75 percentiles, and P values were assessed by Friedman test. *P < .005 in pairwise analyses with Wilcoxon test.
Discussion
The results of this study revealed that HFNO therapy decreased respiratory rate and heart rate without deterioration of respiratory acidosis in patients with severe AE-COPD hospitalized in the ICU. Outcomes were not affected by the presence of pneumonia.
In AE-COPD, expiratory flow limitation and increased respiratory rate and respiratory muscle fatigue lead to dynamic hyperinflation and an increase in dead space–tidal volume ratio. By inspiratory pressure support, NIV diminishes respiratory workload and resolves dynamic hyperinflation.7 High-flow nasal oxygen system does not provide positive inspiratory pressure as in NIV; however, in stable COPD patients using LTOT, HFNO has been found to decrease respiratory rate and inspiration–expiration ratio which can reduce dynamic hyperinflation.24 Rezai et al25 compared the therapeutic effects of HFNO and NIV in severe AE-COPD patients. They found that NIV and HFNO with a flow rate of 20-30 L/min were similarly efficient in improving the respiratory rate and heart rate after 30 minutes. In a multicenter randomized trial comparing HFNO and NIV in mild to moderate hypercapnic respiratory failure in AE-COPD patients, respiratory rate decreased at the second and sixth hours (baseline mean, 27/min; at 2 hours, 22/min; and at 6 hours, 20/min) under HFNO support with a flow rate up to 60 L/min but not in a standardized therapy protocol. High-flow nasal oxygen was found to be non-inferior to NIV in improving patient’s condition regarding respiratory rate.15 Our study included patients with severe AE-COPD. Respiratory rate started to decrease in the first hour of the therapy and remained stable during the 24-hour duration. We also observed an apparent decrease in heart rate beginning at the sixth hour of the treatment, which might indicate a decreased respiratory workload and respiratory distress.
High-flow nasal oxygen was associated with improved VAS score for dyspnea and respiratory rate in patients with “do not intubate” order at 30 minutes outside the ICU setting.16 Lenglet et al26 investigated the efficacy of HFNO with VAS score in patients admitted to the emergency department for acute respiratory failure. They found that VAS score decreased within as early as 15 minutes. In this study, the main cause of respiratory failure was pneumonia, and none of the patients had COPD. In the study by Rezai et al.25 the level of dyspnea was evaluated using BORG scale and a decrease was found in 30 minutes of HFNO therapy. Similar to these studies, we found a temporal reduction in VAS for dyspnea during the first 24 hours.
Reduction of hypoxic vasoconstriction and increase in ventilation-perfusion mismatch by oxygen therapy in patients with acute exacerbation of COPD may aggravate hypercapnia. Therefore, both to prevent hypoxemia and to reduce the risk of hypercapnia, oxygen therapy should be titrated to achieve saturation of 88%-92%.27 Compared to low-flow oxygen delivery systems, high-flow systems can control the inhaled gas mixture without affecting the respiratory rate and therefore provide stable FiO2.28 Also, HFNO therapy has been considered to be safe in hypercapnic patients due to the reduction of carbon dioxide rebreathing by the wash-out effect of high flow through the dead space and enhance adequate alveolar ventilation with the decrease in dead space–tidal volüme ratio.18,29,30 Bräunlich et al31 investigated ABG results of stable hypercapnic COPD patients with HFNO therapy under 4 leakages and flow conditions up to 40 L/min. They found that by increasing the leakage and flow, hypercapnia decreased. In another study by the same group, in moderate and severe hypercapnic patients of AE-COPD who could not tolerate NIV, HFNO therapy was applied up to the flow that the patient can tolerate, and the most remarkable improvement in pH and PaCO2 was observed in patients with respiratory acidosis (baseline pH < 7.35).9 In a randomized–controlled multicenter trial, HFNO was found non-inferior to NIV in decreasing PaCO2 in life-threatening AE-COPD patients with moderate hypercapnia with a pH of 7.25-7.35.15 In this study, the device was set to a maximum flow of 60 L/min initially, and it was well tolerated by 15 (65%) patients during the first 24 hours. Since exclusion criterias of our study was based on ABG results; median pH and PaCO2 of the patients were lower than other studies. Although the PaCO2 improvement was expected to be higher with an increase in the gas flow, we observed a slight increase in PaCO2. Despite this, arterial pH was improved. It may be due to the initiation of renal compensation and the increase in PaCO2 was not excessive. Although the FiO2 was titrated to maintain a SaO2 > 90%, patients were also protected from hyperoxemia. Our patients were transferred from the emergency room to the ICU under low-flow oxygen support, and baseline blood gases were not in room air. Compared to the low flow systems, we did not observe any increase in PaO2 or PaO2/FiO2 ratio.
In our study, severe AE-COPD patients with HFNO therapy showed an acceptably lower rate of HFNO failure and mortality. Although more than half of our patients had pneumonia, HFNO failure rate and mortality were not different in subgroup analysis based on the presence of pneumonia.
Acute exacerbation of chronic obstructive pulmonary disease leads to substantial morbidity and mortality. In-hospital mortality due to respiratory causes was found as 25% in patients with severe AE-COPD32, and the presence of pneumonia was associated with increased mortality.33 How to manage respiratory failure in the presence of COPD and pneumonia together is a matter of debate. Non-invasive ventilation failure rate increases in patients with excessive secretions. Previously published studies have shown HFNO therapy failure rate of 17%-32%10,15 which was similar to NIV failure3 in AE-COPD patients and also similar to the HFNO failure observed in this study. Carillo et al.6 assessed the outcomes of patients with community-acquired pneumonia and acute respiratory failure treated with NIV. They found that NIV failure was more frequent (46% vs. 26%), and the mortality was higher (67% vs. 49%) in patients with previous respiratory disease compared to de novo Acute respiratory failure (ARF).6 Lee et al11 compared the outcome of HFNO and NIV in moderate hypercapnic respiratory failure with AE-COPD patients of whom 40% had concomitant pneumonia. Thirty-day mortality was not different between 2 groups (15.9% vs. 18.2 %, P = .85). They found pneumonia as the common cause of death (46%).11 In a similar study conducted in China, there was no difference between HFNO and NIV groups based on treatment failure and mortality, but the presence of pneumonia in patients with treatment failure was found to be 65% in the NIV group and 55% in the HFNO group (P = .268).10 Since that HFNO avoids mucosal dryness and facilitates the removal of secretions by delivering heated and humidified air, it might be better tolerated than NIV by these patients.
This study has some limitations. First, it is a single-center study with a small number of patients, with no comparative group. Respiratory workload was tested clinically. Lastly, clinical improvement of the patients could be due to adjunctive medical treatments (bronchodilators, corticosteroids, and antibiotics). Since the follow-up duration was 24 hours, it could be insufficient to detect medical treatment efficiency. However, it was shown that HFNO therapy could be beneficial even in severe AE-COPD, which needs to be tested in controlled studies with significant number of patients.
In conclusion, HNFO therapy was found to be useful to reduce tachypnea, dyspnea, and respiratory distress and was well tolerated with no adverse outcomes in severe AE-COPD patients admitted to ICU.
Footnotes
Ethics Committee Approval: This study was approved by Ethics committee of Hacettepe University, (Approval No: 2018/14-18).
Informed Consent: Written informed consent was obtained from the patients who agreed to take part in the study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – P.H., S.Ö.; Design – P.H., S.Ö., A.T.; SupervisiÖn – A.T.; Materials – P.H., E.K.K.; Data Collection and/or Processing – P.H., E.K.K.; analysis and/or Interpretation – P.H, S.Ö. A.T.; Literature Review – P.H., S.Ö.; Writing – P.H., S.Ö., A.T.; Critical Review – A.T.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Vogelmeier CF, Criner GJ, Martinez FJ.et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557 582. 10.1164/rccm.201701-0218PP) [DOI] [PubMed] [Google Scholar]
- 2. Crimi C, Noto A, Princi P, Esquinas A, Nava S. A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36(2):362 369. 10.1183/09031936.00123509) [DOI] [PubMed] [Google Scholar]
- 3. Murphy PB, Rehal S, Arbane G.et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death After an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177 2186. 10.1001/jama.2017.4451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moretti M, Cilione C, Tampieri A, Fracchia C, Marchioni A, Nava S. Incidence and causes of non-invasive mechanical ventilation failure after initial success. Thorax. 2000;55(10):819 825. 10.1136/thorax.55.10.819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jolliet P, Abajo B, Pasquina P, Chevrolet JC. Non-invasive pressure support ventilation in severe community-acquired pneumonia. Intensive Care Med. 2001;27(5):812 821. 10.1007/s001340100869) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carrillo A, Gonzalez-Diaz G, Ferrer M.et al. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38(3):458 466. 10.1007/s00134-012-2475-6) [DOI] [PubMed] [Google Scholar]
- 7. Oczkowski S, Ergan B, Bos L.et al. ERS clinical practice guidelines: high-flow nasal cannula in acute respiratory failure. Eur Respir J. 2022;59(4):2101574. 10.1183/13993003.01574-2021) [DOI] [PubMed] [Google Scholar]
- 8. Davidson AC, Banham S, Elliott M.et al. BTS Standards of Care Committee Member, British Thoracic Society/Intensive Care Society Acute Hypercapnic Respiratory Failure Guideline Development Group, On behalf of the British Thoracic Society Standards of Care Committee. BTS/ICS guideline for the ventilatory management of acute hypercapnic respiratory failure in adults. Thorax. 2016;71(suppl 2):ii1 i35. 10.1136/thoraxjnl-2015-208209) [DOI] [PubMed] [Google Scholar]
- 9. Bräunlich J, Wirtz H. Nasal high flow in acute hypercapnic exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:3895 3897. 10.2147/COPD.S185001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sun J, Li Y, Ling B.et al. High flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease with acute-moderate hypercapnic respiratory failure: an observational cohort study. Int J Chron Obstruct Pulm Dis. 2019;14:1229 1237. 10.2147/COPD.S206567). Erratum in: Int J Chron Obstruct Pulm Dis. 2019;14:1567-1568. (https://doi.org/10.2147/COPD.S222376)[CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee MK, Choi J, Park B.et al. High flow nasal cannulae oxygen therapy in acute-moderate hypercapnic respiratory failure. Clin Respir J. 2018;12(6):2046 2056. 10.1111/crj.12772) [DOI] [PubMed] [Google Scholar]
- 12. Plotnikow GA, Accoce M, Fredes S.et al. High-flow oxygen therapy application in chronic obstructive pulmonary disease patients With acute hypercapnic respiratory failure: A multicenter study. Crit Care Explor. 2021;3(2):e0337. 10.1097/CCE.0000000000000337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crimi C, Noto A, Cortegiani A.et al. High Flow Nasal Therapy Use in Patients with Acute Exacerbation of COPD and Bronchiectasis: A Feasibility Study, COPD. Int J Chronic Obstruct Pulm Dis. 2020;17(2):184 190. 10.1080/15412555.2020.1728736) [DOI] [PubMed] [Google Scholar]
- 14. Cong L, Zhou L, Liu H, Wang J. Outcomes of high-flow nasal cannula versus non-invasive positive pressure ventilation for patients with acute exacerbations of chronic obstructive pulmonary disease. Int J Clin Exp Med. 2019;12:10863 10867. 10.1080/15412555.2020.1728736) [DOI] [Google Scholar]
- 15. Cortegiani A, Longhini F, Madotto F.et al. High flow nasal therapy versus noninvasive ventilation as initial ventilatory strategy in COPD exacerbation: a multicenter non-inferiority randomized trial. Crit Care. 2020;24(1):692. 10.1186/s13054-020-03409-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zemach S, Helviz Y, Shitrit M, Friedman R, Levin PD. The use of high-flow nasal cannula oxygen Outside the ICU. Respir Care. 2019;64(11):1333 1342. 10.4187/respcare.06611) [DOI] [PubMed] [Google Scholar]
- 17. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care. 2011;39(6):1103 1110. 10.1177/0310057X1103900620) [DOI] [PubMed] [Google Scholar]
- 18. Itagaki T, Okuda N, Tsunano Y.et al. Effect of high-flow nasal cannula on thoraco-abdominal synchrony in adult critically ill patients. Respir Care. 2014;59(1):70 74. 10.4187/respcare.02480) [DOI] [PubMed] [Google Scholar]
- 19. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373 383. 10.1016/0021-9681(87)90171-8) [DOI] [PubMed] [Google Scholar]
- 20. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache II: a severity of disease classification system. Crit Care Med. 1985;13(10):818 829. 10.1097/00003246-198510000-00009) [DOI] [PubMed] [Google Scholar]
- 21. Vincent JL, Moreno R, Takala J.et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707 710. 10.1007/BF01709751) [DOI] [PubMed] [Google Scholar]
- 22. Hajiro T, Nishimura K, Tsukino M, Ikeda A, Koyama H, Izumi T. Analysis of clinical methods used to evaluate dyspnea in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(4):1185 1189. 10.1164/ajrccm.158.4.9802091) [DOI] [PubMed] [Google Scholar]
- 23. Noseda A, Carpiaux JP, Schmerber J, Yernault JC. Dyspnoea assessed by visual analogue scale in patients with chronic obstructive lung disease during progressive and high intensity exercise. Thorax. 1992;47(5):363 368. 10.1136/thx.47.5.363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomized crossover trial. Thorax. 2016;71(8):759 761. 10.1136/thoraxjnl-2015-207962) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. 26. Rezaei A, Fakharian A, Ghorbani F, Idani E, Abedini A, Jamaati H. Comparison of high-flow oxygenation with noninvasive ventilation in COPD exacerbation: A crossover clinical trial. Clin Respir J. 2021;15(4):420 429. 10.1111/crj.13315) Lenglet H, Sztrymf B, Leroy C, Brun P, Dreyfuss D, Ricard JD. Humidified high flow nasal oxygen during respiratory failure in the emergency department: feasibility and efficacy. Respir Care. 2012;57(11): 1873 1878. (https://doi.org/10.4187/respcare.01575) [DOI] [PubMed] [Google Scholar]
- 27. Abdo WF, Heunks LM. Oxygen-induced hypercapnia in COPD: myths and facts. Crit Care. 2012;16(5):323. 10.1186/cc11475) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care. 2005;50(5):604 609. [PubMed] [Google Scholar]
- 29. Sztrymf B, Messika J, Bertrand F.et al. Beneficial effects of humidified high flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med. 2011;37(11):1780 1786. 10.1007/s00134-011-2354-6) [DOI] [PubMed] [Google Scholar]
- 30. Pham TM, O’Malley L, Mayfield S, Martin S, Schibler A. The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis. Pediatr Pulmonol. 2015;50(7):713 720. 10.1002/ppul.23060) [DOI] [PubMed] [Google Scholar]
- 31. Bräunlich J, Mauersberger F, Wirtz H. Effectiveness of nasal highflow in hypercapnic COPD patients is flow and leakage dependent. BMC Pulm Med. 2018;18(1):14. 10.1186/s12890-018-0576-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925 931. 10.1136/thx.2005.040527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saleh A, López-Campos JL, Hartl S, Pozo-Rodríguez F, Roberts CM. European COPD Audit team. The Effect of incidental Consolidation on Management and outcomes in COPD Exacerbations: data from the European COPD Audit. PLOS ONE. 2015;10(7):e0134004. 10.1371/journal.pone.0134004) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a