Abstract
The coronavirus disease 2019 pandemic caused by severe acute respiratory syndrome-related coronavirus-2 continues its effects around the world with its new variants. Coronavirus disease 2019 infection may continue with a post-coronavirus disease period, which is characterized by high morbidity apart from the acute and subacute phases. Host immune response quality and inflammasome-induced uncontrollable inflammatory response take a role together in the pathogenesis of severe acute respiratory syndrome-related coronavirus-2 infection. Therefore, treatment of severe acute respiratory syndrome-related coronavirus-2 infection should basically include 3 measures: Viral replication, inflammation, and tissue damage control. Today, there is no effective therapy to control these points. At this point, preclinical studies have shown that mesenchymal stem cells can control inflammatory reactions and lung damage through both immune regulation and inflammasome control. Subsequently, controlled clinical studies on severe acute respiratory syndrome-related coronavirus-2 infection confirm their ability, indicating that mesenchymal stem cells may be a safe treatment option while reducing severe acute respiratory syndrome-related coronavirus-2-related morbidity and mortality. On the other hand, post-coronavirus syndrome is as important as acute coronavirus syndrome, it is a picture that can cause morbidity and mortality. Mesenchymal stem cell application can prevent the development of post-coronavirus syndrome through the mechanism of an inflammasome. However, there is no study that analyzes the effects of current treatments using mesenchymal stem cells in the post-coronavirus disease period, and that tests the use of mesenchymal stem cells when post-coronavirus syndrome develops. In this respect, studies that test the efficacy of mesenchymal stem cells in the post-coronavirus disease period are certainly needed.
Keywords: SARS-CoV-2, coronavirus disease 19, mesenchymal stem cells, immunity
Main Points
Post-COVID syndrome shows high morbidity rates. Therefore, it is as important as acute and subacute COVID syndromes.
Inflammation and inflammasome causing acute, subacute, and post-COVID syndromes play an active role in morbidity and mortality.
Mesenchymal stem cell therapy emerges as a safe treatment option with the ability to control the cytokine storm caused by the inflammasome.
Introduction
In the severe acute respiratory syndrome-related coronavirus-2 (SARS-CoV-2) pandemic, for which we are still far from being able to say that there is an effective treatment, another important problem is that even if patients recover after intensive care, they may face significant symptoms that can last for months.1,2 In particular, this picture, called post-coronavirus disease (post-COVID) syndrome, actually has consequences as important as the disease.2 After coronavirus disease 2019 (COVID-19), this syndrome is complex, the symptoms of which are muscle weakness (53%), respiratory distress (43%), anxiety, depression, cognitive disorders, confusion, neurological symptoms including sleep disorders (40%), joint pain (27%), hair loss (22%), and cardiovascular symptoms (12%), occurs at a rate of 55% and can continue to affect patients for more than 6 months.2 Infection-induced immune reactions and mitochondrial degeneration are thought to be the main mechanisms underlying these post-COVID 19 symptoms. Therefore, post-COVID 19 treatment should not only include controlling the virus, it should also be able to control the immune reactions induced by the virus. Previous studies have shown that mesenchymal stem cells (MSCs) are effective in the treatment of many diseases.3-6 Likewise, studies have reported that MSCs can suppress viral infection in the treatment of SARS-CoV-2 through the secretion of specific cytokines.3,7-11 In this review, we discussed molecular, mitochondrial, and immunological events involved in the pathogenesis of novel SARS-CoV-2 as well as the clinical perspective of MSC treatment from controlled studies to improve patients’ immunological response in the post-COVID period.
Coronavirus Disease 2019 Pathogenesis
Severe acute respiratory syndrome-related coronavirus-2 is a virus of the corona family and initiates the infection by its entry into the cell via the angiotensin-converting enzyme II (ACE-II) receptor. Infection begins with the synthesis of transmembrane protease serine 2 (TMPRSS2) enzyme in the host cell. The spike protein of the virus first decomposes the enzyme into 2 subunits and binds to the membrane at 2 points. Afterward, the virus is taken into the cell. Prognosis of the patient is determined by the tissue damage and the severity of the cytokine storm, which depends on the effectiveness and severity of the developing immune response.12
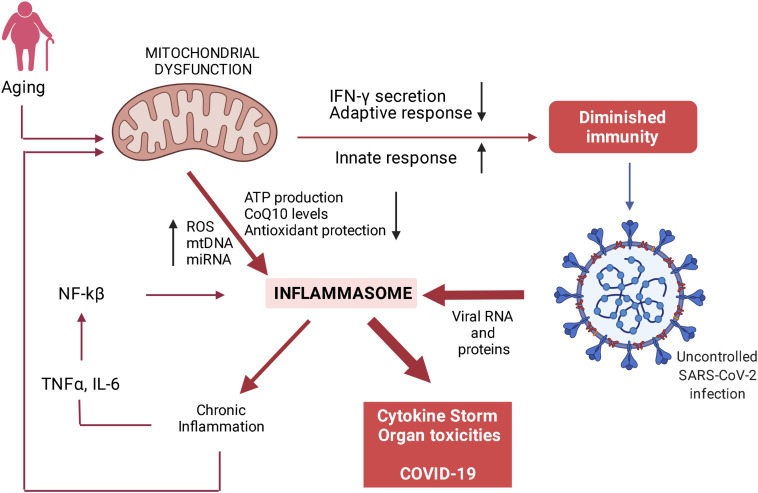
The most important factor that affects the level of success of the immune response is when the patients have a chronic active inflammation for another reason. Therefore, the disease progress more severely in older individuals. The underlying reason for this is the exaggerated immune response caused by chronic inflammation. Therefore, one of the agents widely used in the control of the disease is steroids.12 So why does aging go hand in hand with chronic inflammation? The most important mechanism here is thought to be mitochondrial aging or fatigue.13 Figure 1 summarizes this finding.14-19
Figure 1.
The mechanism of exaggerated but uncontrolled antiviral immunity observed in critically ill patients caused by mitochondrial aging/dysfunctioning (modified from Ayala DJMF, et al. 2020) ROS, reactive oxygen species; mtDNA, mitochondrial DNA; miRNA, microRNA; NF-kβ, nuclear factor kβ; TNFα, tumor ncrosis factor alpha; IL-6, interleukin 6; IFN-γ, interferon gamma.
The inability of aged mitochondria to prevent the formation of the inflammasome is due to the fact that cellular stress, which increases with age, activates the nuclear factor-κB pathway. The cascade formed by the direct stimulus of the virus causes an increased presence of inflammasome (Figure 1), which is the main cause of cytokine storm in the elderly or patients with chronic inflammation.13 For this reason, therapeutic agents that can be ideal elements of treatment in COVID-19 should not cause primary and opportunistic infections and tissue toxicities and should also prevent mitochondrial stress and post-COVID syndrome. Unfortunately, current steroid treatments do not have this feature and cease to be an ideal agent.
Functions and Pharmacokinetics of Mesenchymal Stem Cells
Mesenchymal stem cells are shown to have regenerative effects for as long as 25 years and have been used in the clinic for their different regenerative and immunomodulatory effects for 20 years. These cells are defined as cells that can be obtained from different tissue sources, adhere to plastic, can be shown to differentiate into at least 3 mesodermal cells, and express CD45, CD34, human leukocyte antigen DR negative, CD73, CD90, CD105, and CD106. In addition, since they do not carry ACE-II and TMPRSS2 receptors, which are necessary for SARS-CoV-2 infection,20 and the use of these cells seems to have an advantage in the treatment of COVID-19 infection. It is now almost accepted that umbilical cord-derived MSCs are more effective than adipose and bone marrow-derived stem cells when tested for their efficacy according to their source.21,22 However, another group of researchers published a report showing that especially menstrual mesenchymal stem cells can be more effective23 in the treatment of COVID-19.24 At this point, another important issue regarding the cell source and production method is that, MSCs tend to lead to thrombosis due to the tissue factor (TF-CD142) that they carry on their surface. The use of adipose tissue-derived MSCs is controversial, especially because of their high TF transport.25 Therefore, researchers recommend that MSCs shall be heparinized or intramuscularly (IM) administered in order to prevent post-infusional thrombosis and the formation of pulmonary aggregates.26 It has even been reported that the use of the IM route can increase the duration of MSC effectiveness by 4 times, and therefore IM application will be more effective and harmless.25-28 A group of researchers also reported that extra vesicles or exosomes of MSC can be preferred over MSCs because they are independent of the risk of pulmonary aggregates and are also effective.29
Mesenchymal Stem Cells can Exert Their Regenerative and Immunomodulatory Effects by the Following Mechanisms
Regenerative Mechanisms of Action
While MSCs stimulate healing in damaged tissue through mitochondria transfer, mRNA, mi-RNA transfers, and cytokines such as keratinocyte growth factor, hepatocyte growth factor, vascular endothelial growth factor, and insulin-like growth factor 1, can reduce apoptosis in tissue with the activation of antiapoptotic effect (B-cell lymphoma 2).30,31 In fact, the transformation of M1 macrophages into M2 macrophages, which starts with the phagocytosis of MSC or MSC extravesicles, is a part of the immune modulation process and is almost one of the main mechanisms of the regenerative process. Moreover, this reaction is especially responsible for the formation of the regenerative cytokine profile.30-32 It has been reported that it can prevent fibrosis in the post-COVID-19 period.9
Immunoregulatory Mechanisms of Action
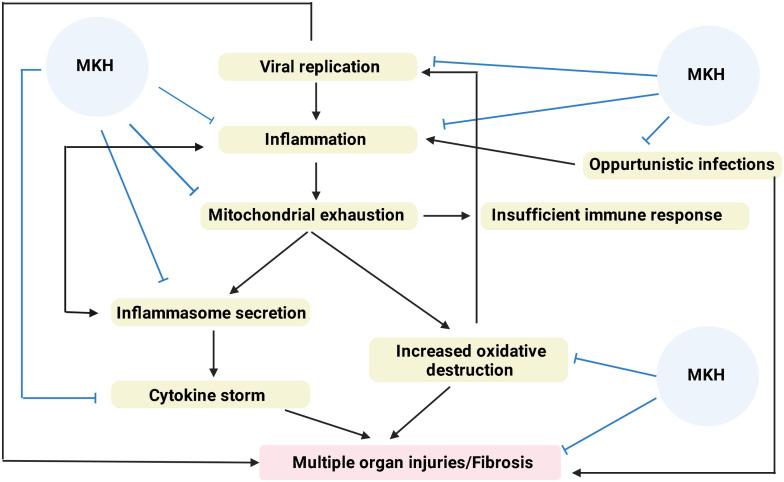
While MSCs decrease the functions of T and B lymphocytes, they can increase apoptosis in these cells.30,31 In addition, they suppress tissue-specific immune responses by increasing T-regulatory (Treg), B-regulatory, and DC-regulatory levels.30-32 While the resulting M1-M2 conversion causes Treg activation, it is also responsible for the secretion of suppressor cytokines. These cytokines interleukin-10, transforming growth factor-β, indoleamine 2, 3-dioxygenase, prostaglandin E2, and arginase secretion, and they suppress T lymphocyte functions in particular.31 Another immunomodulatory effect of MSCs is that they can also block the alternative complement pathway because they secrete substance H.32 In particular, the ability of MSCs to block the development of inflammasome plays an extremely important role in controlling chronic inflammatory diseases.33 Another feature that makes MSCs unique is that these cells, which can control the immune system at every step, do not cause infection at the same time. It is even possible to talk about antibacterial, antifungal, and antiviral effects through the transfer of Hepcidin, β-defensin 2, and Lipocallin 2 small interfering RNAs.34 Figure 2 summarizes the role MSCs can play in the pathogenesis of COVID.35,36
Figure 2.
Mesenchymal stem cells can control the reaction at every step in coronavirus disease 2019 infection progressing with cytokine storm.
Mesenchymal Stem Cells Pharmacokinetics
In a study conducted in the acute respiratory distress syndrome (ARDS) model, intravenous (IV) and endobronchial (EB) MSCs were administered to normal and ARDS-induced mice, and kinetic analyzes were evaluated.34 Kinetic analyzes for intravenous and endobronchial administration of MSCs show that, in case of lung damage, 80% of MSC remains in the lung tissue after intravenous administration. However, in this study, it is reported that in the normal mice group, a significant part of MSC administered intravenously is distributed throughout the body 5 hours after administration, and the majority of these dispersed cells are localized to the brain, liver, and kidney. Another finding in this study shows that MSCs given intravenously can be more localized in areas with aeration disorder. There was no difference between the effect of IV or EB administration on improving lung functions. In addition, studies comparing IV and IM applications reported that the effect of IM application may be longer and greater than IV application,26,27 it has been shown that IV administration in hypercoagulant conditions can cause microemboli in the lung.25 When all these are considered together it seems possible to say that EB and/or IM administration in COVID-19-related ARDS may be safer and even more effective.
Clinical Data
When the ClinicalTrials.gov site is examined, it is seen that more than 84 studies have been conducted on ARDS and COVID-19 infection, and 13 of them were performed with MSC exosomes (3 IV, 10 of them inhaled exosome application). As of today, it is seen that there are 9 studies whose results have been published in the control of COVID-19 ARDS. Of these, 5 were randomized placebo-controlled including 2 phase I/II and 1 phase II studies, 2 were non-randomized controlled, 1 was non-randomized without control and 4 were case studies. Two were uncontrolled studies including 1 phase I study and 1 non-randomized cohort study (Table 1). The common point of these studies is that the use of MSCs or exosomes is a safe practice.24,37-48
Table 1.
The Available Controlled Studies (https://pubmed.ncbi.nlm.nih.gov/: last accessed: March 11, 2022)
| Study | Center | Reference | Study design | Result |
|---|---|---|---|---|
| Lanzoni G, et al., 2021 | USA | 37 |
|
|
| Shu L, et al. 2020 | China | 38 |
|
|
| Dilogo IH, et al. 2021 | Indonesia | 39 |
|
2.5 times higher survival rate in the UC-MSCs group than that in the control group (P = .047; 10 patients vs 4 patients).
|
| Kouroupis D, et al. 2021 | USA | 40 |
|
In control group, levels of plasma sTNFR2, TNFα, and TNFβ were not significantly different between days 0 and 6. Significant decrease in TNFα and TNFβ levels (P = .005 and P = .002, respectively) in UC-MSC treatment group at day 6.Significantly higher levels of sTNFR2 (26.609 ± 846 pg/ml vs. 23.111 ± 760 pg/ml, P = .021) and significantly lower levels of TNFα (319 ± 40 vs. 950 ± 226 pg/mL, P = .048) and TNFβ (810 ± 126 vs. 2.944± 735 pg/mL, P = .032) in UC-MSC treatment group. |
| Shi L, et al. 2021 | China | 41 |
|
Improved whole-lung lesion volume with a difference of −10.8% (P = .030) after MSC administration on day 10.
|
| Saleh M, et al. 2021 | Iran | 42 |
|
Increased level of IL-10 and SDF-1 MSC therapy, but decreased level of VEGF, TGF-β, IFN-γ, IL-6, and TNFα.
|
| Xu X, et al. 2021 | China | 24 |
|
Significantly lower mortality rate in the MSC group (7.69% died in the experimental group vs 33.33% in the control group; P = .048).
|
| Sengupta V, et al. 2020 | USA | 43 |
|
|
| Meng F, et al. 2020 | China | 44 |
|
|
| Ercelen N, et al. 2021 | Turkey | 45 |
|
|
| Hashemian SMR, et al. 2021 | Iran | 46 |
|
|
| Zengin R, et al. 2020 | Turkey | 47 |
|
|
| Yalcin K, et al. 2020 | Turkey | 48 |
|
|
Summary Analysis of Published Randomized Controlled Studies in COVID-19
When only published controlled studies were examined, control (n = 12) and MSC (n = 12; 100 million/IV single dose) groups were compared in a phase I/II randomized controlled study conducted in the USA.37 In this study, investigators reported that cytokine storm could be easily controlled in the MSC group. The most important result of this study is that the survival rate in the MSC group was 91%, while it was reported as 42% in the control group.
Another double-blind, multicentered, randomized controlled trial (n = 40) evaluated the UC-MSCs (1 × 106/kg body weight) vs control group (0.9% saline solution). This study reported that the survival rate in the UC-MSCs group was 2.5 times higher than that in the control group (P = .047; 10 patients versus 4 patients. In addition, UC-MSC administration increased the survival rate by 4.5 times compared with controls in patients with comorbidities.39
In a publication from China,41 66 patients who received low-dose 40 million/IV single-dose MSCs were compared with 35 control patients. In this study, nonsignificant positive differences were observed in biochemical parameters, survival, and length of stay, but the reduction in lung lesions resulted in a statistically significant difference in the MSC group.
In the study by X et al24 with menstrual mesenchymal stem cells, 3 million/kg MSC was given to 26 patients every other day, while the placebo group was followed up with standard care. In this study, investigators reported that cytokine storm could be controlled with MSC, and this was reflected in the clinic, resulting in a significant decrease in mortality, which was 33% in the placebo arm, and 7.7% in the MSC group 10.
In another placebo-controlled study conducted by Shu L et al.38 12 patients who received 2 million cells/kg of single-dose MSCs originating from the human umbilical cord were compared with 29 patients who received a placebo. In this study, while C-reactive protein and interleukin-6 levels were significantly decreased in the MSC group, improvement in lymphocyte count and oxygenation levels were also detected in the MSC group. While the median recovery time was 7 days in the MSC group, it was significantly longer in the placebo group at 14 days. The 28-day mortality was 0% in the MSC group and 10.34% in the placebo group. However, these data are not statistically significant.
Contrary to these results, Meng F et al44 compared patients in whom they infused low-dose 30 million/IV dose of cord blood-derived MSCs 3 times sequentially with 9 control patients. However, although they found improvement in cytokine levels and respiratory functions in the MSC group, this was not statistically significant.
Apart from these controlled studies, MSCs have been reported to control cytokine storm in case–control series report (Table 1). In the study conducted by our group on this subject, 80 million MSCs originating from the umbilical cord were administered intravenously, 20 million MSCs were administered via endobronchial way, with an interval of 4 days, and it was reported that stem cells could significantly control the cytokine storm in patients and positively affect respiratory parameters.47,48
Considering all these existing studies, the most important shortcoming of these studies is that their analysis includes the acute and subacute periods. It seems that none of these studies analyzed the post-COVID period. Especially in these patients, oxidative stress, mitochondrial aging, and the presence of inflammasome continue after the disease and may cause post-COVID syndrome. In this respect, the use of MSCs during post-COVID treatment may have a positive effect. However, clinical evidence of theoretical knowledge has not yet emerged, as studies with MSC so far have not analyzed the effects of this treatment in the post-COVID period. Therefore, the effect of MSC use on post-COVID syndrome should be studied in particular. It should even be discussed in the treatment of post-COVID syndrome.49
Conclusion
In the treatment of COVID-19 infection, MSCs, which can interfere with the pathogenesis of the disease at many points, may be effective in accordance with pre-clinical data in clinical practice data. The most important point that can be said about this subject for now is that MSCs are a reliable treatment agent in the treatment of COVID-19. However, new, large and controlled studies to be planned in terms of the effectiveness of MSCs need to confirm the available data. In addition, the effects of MSCs used for treatment on COVID and post-COVID syndrome are promising and needs to be further studied. The following 2 questions must also be answered in these studies: First, what are the effects of MSCs used in the treatment of COVID on the post-COVID that will develop over a long period of time? Second, will there be efficacy of MSCs after post-COVID develops? Studies are needed to answer these questions.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept – E.O., D.D.K.; Design – E.O., D.D.K.; Supervision – E.O.; Data Collection and/or Processing – E.O., D.D.K.; Literature Review – E.O., D.D.K.; Writing – E.O., D.D.K.; Critical Review – E.O., D.D.K.
Declaration of Interests: The authors have no conflict of interest to declare.
Funding: The authors declared that this study has received no financial support.
References
- 1. Çelik D, Köse Ş. Erişkinlerde COVID-19: Klinik Bulgular. Tepecik Eğit Araşt Hast Derg. 2020;30(Ek sayı):43 8. [Google Scholar]
- 2. Nalbandian A, Sehgal K, Gupta A.et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601 615. 10.1038/s41591-021-01283-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao G, Fan C, Li W.et al. Mesenchymal stem cells: ideal seeds for treating diseases. Hum Cell. 2021;34(6):1585 1600. 10.1007/s13577-021-00578-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baykal B. Mesenchymal stem cells for the treatment of various diseases. J Stem Cell Res Med. 2016;1(2). 10.15761/JSCRM.1000110) [DOI] [Google Scholar]
- 5. Regmi S, Pathak S, Kim JO, Yong CS, Jeong JH. Mesenchymal stem cell therapy for the treatment of inflammatory diseases: challenges, opportunities, and future perspectives. Eur J Cell Biol. 2019;98(5-8):151041. 10.1016/j.ejcb.2019.04.002) [DOI] [PubMed] [Google Scholar]
- 6. Wang LT, Liu KJ, Sytwu HK, Yen ML, Yen BL. Advances in mesenchymal stem cell therapy for immune and inflammatory diseases: use of cell-free products and human pluripotent stem cell-derived mesenchymal stem cells. Stem Cells Transl Med. 2021;10(9):1288 1303. 10.1002/sctm.21-0021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dauletova M, Hafsan H, Mahhengam N, Zekiy AO, Ahmadi M, Siahmansouri H. Mesenchymal stem cell alongside exosomes as a novel cell-based therapy for COVID-19: a review study. Clin Immunol. 2021;226:108712. 10.1016/j.clim.2021.108712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monguió-Tortajada M, Bayes-Genis A, Rosell A, Roura S. Are mesenchymal stem cells and derived extracellular vesicles valuable to halt the COVID-19 inflammatory cascade? Current evidence and future perspectives. Thorax. 2021;76(2):196 200. 10.1136/thoraxjnl-2020-215717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vishnupriya M, Naveenkumar M, Manjima K.et al. Post-COVID pulmonary fibrosis: therapeutic efficacy using with mesenchymal stem cells–How the lung heals. Eur Rev Med Pharmacol Sci. 2021;25(6):2748 2751. 10.26355/eurrev_202103_25438) [DOI] [PubMed] [Google Scholar]
- 10. Häberle H, Magunia H, Lang P.et al. Mesenchymal stem cell therapy for severe COVID-19 ARDS. J Intensive Care Med. 2021;36(6):681 688. 10.1177/0885066621997365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bari E, Ferrarotti I, Saracino L.et al. Mesenchymal stromal cell secretome for post-COVID-19 pulmonary fibrosis: a new therapy to treat the long-term lung sequelae? Cells. 2021;10(5):1203. 10.3390/cells10051203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatterjee SK, Saha S, Munoz MNM. Molecular pathogenesis, immunopathogenesis and novel therapeutic strategy against COVID-19. Front Mol Biosci. 2020;7:196. 10.3389/fmolb.2020.00196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moreno Fernández-Ayala DJMF, Navas P, López-Lluch G. Age-related mitochondrial dysfunction as a key factor in COVID-19 disease. Exp Gerontol. 2020;142:111147. 10.1016/j.exger.2020.111147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019;20(13):3328. 10.3390/ijms20133328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGuire PJ. Mitochondrial dysfunction and the aging immune system. Biology. 2019;8(2):26. 10.3390/biology8020026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sandhir R, Halder A, Sunkaria A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim Biophys Acta Mol Basis Dis. 2017;1863(5):1090 1097. 10.1016/j.bbadis.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 17. Missiroli S, Genovese I, Perrone M, Vezzani B, Vitto VAM, Giorgi C. The role of mitochondria in inflammation: from cancer to neurodegenerative disorders. J Clin Med. 2020;9(3):740. 10.3390/jcm9030740) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pagliaro P. Is macrophages heterogeneity important in determining COVID-19 lethality? Med Hypotheses. 2020;143:110073. 10.1016/j.mehy.2020.110073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bektas A, Schurman SH, Franceschi C.et al. A public health perspective of aging: do hyper-inflammatory syndromes such as COVID-19, SARS, ARDS, cytokine storm syndrome, and post-ICU syndrome accelerate short-and long-term inflammaging? Immun Ageing. 2020;17(1):1 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jamalkhah M, Asaadi Y, Azangou-Khyavy M.et al. MSC-derived exosomes carrying a cocktail of exogenous interfering RNAs an unprecedented therapy in era of COVID-19 outbreak. J Transl Med. 2021;19(1):164. 10.1186/s12967-021-02840-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Bai J, Ji X, Li R, Xuan Y, Wang Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int J Mol Med. 2014;34(3):695 704. 10.3892/ijmm.2014.1821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bárcia RN, Santos JM, Filipe M.et al. What makes umbilical cord tissue-derived mesenchymal stromal cells superior immunomodulators when compared to bone marrow derived mesenchymal stromal cells? Stem Cells Int. 2015;2015:583984. 10.1155/2015/583984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6(1):199. 10.1186/s13287-015-0192-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xu X, Jiang W, Chen L.et al. Evaluation of the safety and efficacy of using human menstrual blood‐derived mesenchymal stromal cells in treating severe and critically ill COVID‐19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11(2):e297. 10.1002/ctm2.297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moll G, Drzeniek N, Kamhieh-Milz J, Geissler S, Volk HD, Reinke P. MSC therapies for COVID-19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11:1091. 10.3389/fimmu.2020.01091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Braid LR, Wood CA, Wiese DM, Ford BN. Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. 2018;20(2):232 244. 10.1016/j.jcyt.2017.09.013) [DOI] [PubMed] [Google Scholar]
- 27. Caplan H, Olson SD, Kumar A.et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645. 10.3389/fimmu.2019.01645) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qazi TH, Duda GN, Ort MJ, Perka C, Geissler S, Winkler T. Cell therapy to improve regeneration of skeletal muscle injuries. J Cachexia Sarcopenia Muscle. 2019;10(3):501 516. 10.1002/jcsm.12416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Askenase PW. COVID‐19 therapy with mesenchymal stromal cells (MSC) and convalescent plasma must consider exosome involvement: do the exosomes in convalescent plasma antagonize the weak immune antibodies? J Extracell Vesicles. 2020;10(1):e12004. 10.1002/jev2.12004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorman E, Millar J, McAuley D, O’Kane C. Mesenchymal stromal cells for acute respiratory distress syndrome (ARDS), sepsis, and COVID-19 infection: optimizing the therapeutic potential. Expert Rev Respir Med. 2021;15(3):301 324. 10.1080/17476348.2021.1848555) [DOI] [PubMed] [Google Scholar]
- 31. Sadeghi S, Soudi S, Shafiee A, Hashemi SM. Mesenchymal stem cell therapies for COVID-19: current status and mechanism of action. Life Sci. 2020;262:118493. 10.1016/j.lfs.2020.118493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dabrowska S, Andrzejewska A, Janowski M.et al. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. 2021;11:3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miteva K, Pappritz K, Sosnowski M.et al. Mesenchymal stromal cells inhibit NLRP3 inflammasome activation in a model of coxsackievirus B3-induced inflammatory cardiomyopathy. Sci Rep. 2018;8(1):2820. 10.1038/s41598-018-20686-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cardenes N, Aranda-Valderrama P, Carney JP.et al. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6(1):e000308. 10.1136/bmjresp-2018-000308) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kavianpour M, Saleh M, Verdi J. The role of mesenchymal stromal cells in immune modulation of COVID-19: focus on cytokine storm. Stem Cell Res Ther. 2020;11(1):404. 10.1186/s13287-020-01849-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh JY, Ko JH, Lee HJ.et al. Mesenchymal stem/stromal cells inhibit the NLRP3 inflammasome by decreasing mitochondrial reactive oxygen species. Stem Cells. 2014;32(6):1553 1563. 10.1002/stem.1608) [DOI] [PubMed] [Google Scholar]
- 37. Lanzoni G, Linetsky E, Correa D.et al. Umbilical cord mesenchymal stem cells for COVID‐19 acute respiratory distress syndrome: a double‐blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660 673. 10.1002/sctm.20-0472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shu L, Niu C, Li R.et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361. 10.1186/s13287-020-01875-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dilogo IH, Aditianingsih D, Sugiarto A.et al. Umbilical cord mesenchymal stromal cells as critical COVID‐19 adjuvant therapy: a randomized controlled trial. Stem Cells Transl Med. 2021;10(9):1279 1287. 10.1002/sctm.21-0046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kouroupis D, Lanzoni G, Linetsky E.et al. Umbilical cord-derived mesenchymal stem cells modulate TNF and soluble TNF receptor 2 (sTNFR2) in COVID-19 ARDS patients. Eur Rev Med Pharmacol Sci. 2021;25(12):4435 4438. 10.26355/eurrev_202106_26156) [DOI] [PubMed] [Google Scholar]
- 41. Shi L, Huang H, Lu X.et al. Effect of human umbilical cord-derived mesenchymal stem cells on lung damage in severe COVID-19 patients: a randomized, double-blind, placebo-controlled phase 2 trial. Signal Transduct Target Ther. 2021;6(1):58. 10.1038/s41392-021-00488-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Saleh M, Vaezi AA, Aliannejad R.et al. Cell therapy in patients with COVID-19 using Wharton’s jelly mesenchymal stem cells: a phase 1 clinical trial. Stem Cell Res Ther. 2021;12(1):410. 10.1186/s13287-021-02483-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29(12):747 754. 10.1089/scd.2020.0080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng F, Xu R, Wang S.et al. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020;5(1):172. 10.1038/s41392-020-00286-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ercelen NO, Pekkoc-Uyanik KC, Alpaydin N, Gulay GR, Simsek M. Clinical experience on umbilical cord mesenchymal stem cell treatment in 210 severe and critical COVID-19 cases in Turkey. Stem Cell Rev Rep. 2021;17(5):1917 1925. 10.1007/s12015-021-10214-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hashemian SR, Aliannejad R, Zarrabi M.et al. Mesenchymal stem cells derived from perinatal tissues for treatment of critically ill COVID-19-induced ARDS patients: a case series. Stem Cell Res Ther. 2021;12(1):91. 10.1186/s13287-021-02165-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zengin R, Beyaz O, Koc ES.et al. Mesenchymal stem cell treatment in a critically ill COVID-19 patient: a case report. Stem Cell Investig. 2020;7:17. 10.21037/sci-2020-024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yalcin K, Hemsinlioglu C, Zengin R.et al. Mesenchymal stromal cell therapy for critically ill patients With COVID-19. JMIR Prepr. 2020;20206. [Google Scholar]
- 49. Lage SL, Amaral EP, Hilligan KL.et al. Persistent oxidative stress and inflammasome activation in CD14highCD16− monocytes From COVID-19 patients. Front Immunol. 2021;12:799558. 10.3389/fimmu.2021.799558) [DOI] [PMC free article] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a