Abstract
Background
Canadian clinical pharmacy key performance indicators (cpKPIs) have been developed for inpatient hospital practice but are not established for ambulatory oncology. This study represents the first step in developing cpKPIs for ambulatory oncology.
Objectives
To describe the current landscape of pharmacy services in ambulatory oncology in Canada and to identify perspectives related to the development and implementation of cpKPIs in this practice setting.
Methods
In this national cross-sectional study, a web-based questionnaire was distributed to pharmacists working in ambulatory oncology settings. Potential participants who self-identified as pharmacists practising in an ambulatory oncology setting were eligible. Survey questions focused on participants’ demographic characteristics, oncology pharmacy services provided, metrics captured, and pharmacists’ perceptions of cpKPIs. All data were analyzed using descriptive statistics.
Results
A total of 44 responses were received, with most respondents practising in community hospitals in British Columbia, Ontario, and Atlantic Canada. The services most commonly provided were chemotherapy order verification, laboratory monitoring, identification and resolution of drug therapy problems, and counselling on anticancer medications. Twenty-six of the 44 respondents (59%) indicated that performance metrics or patient outcomes were tracked at their respective institutions, with none being universally captured. Overall, 43 (98%) of the respondents favoured the development of cpKPIs for ambulatory oncology practice.
Conclusions
Despite growing patient care needs in ambulatory oncology, there is significant heterogeneity in the scope of pharmacy services offered and the outcomes used to qualify their impact within this setting across Canada. This study demonstrates a clear need for national consensus cpKPIs to inform pharmacy resource utilization and patient-centred quality improvement initiatives.
Keywords: clinical pharmacy key performance indicator, key performance indicator, ambulatory oncology, oncology pharmacy, outpatient oncology, Canada
RÉSUMÉ
Contexte
Des indicateurs clés de performance de la pharmacie clinique canadienne (cpKPI) ont été élaborés pour la pratique hospitalière en milieu hospitalier, mais n’ont pas été définis pour l’oncologie ambulatoire. Cette étude constitue la première étape de l’élaboration de cpKPI pour l’oncologie ambulatoire.
Objectifs
Décrire le paysage actuel des services pharmaceutiques en oncologie ambulatoire au Canada et cerner les perspectives liées au développement et à la réalisation de cpKPI dans ce contexte de pratique.
Méthodes
Dans cette étude transversale nationale, un questionnaire en ligne a été distribué aux pharmaciens qui travaillent en oncologie ambulatoire. Les participants potentiels qui se sont identifiés comme des pharmaciens exerçant dans ce contexte étaient autorisés à participer. Les questions de l’étude portaient sur les caractéristiques démographiques des participants, les services de pharmacie offerts en oncologie, les paramètres saisis et les perceptions des pharmaciens à l’égard des cpKPI. Toutes les données ont été analysées à l’aide de statistiques descriptives.
Résultats
Au total, 44 réponses ont été reçues, la plupart des répondants exerçant dans des hôpitaux communautaires de la Colombie-Britannique, de l’Ontario et du Canada atlantique. Les services les plus couramment fournis étaient : la vérification des ordonnances de chimiothérapie, la surveillance en laboratoire, l’identification et la résolution des problèmes de pharmacothérapie et les conseils portant sur les médicaments anticancéreux. Vingt-six des 44 répondants (59 %) ont indiqué que les indicateurs de performance ou les résultats pour les patients faisaient l’objet d’un suivi dans leurs établissements respectifs, bien qu’aucun ne soit universellement saisi. Dans l’ensemble, 43 répondants (98 %) étaient favorables à l’élaboration de cpKPI pour la pratique de l’oncologie ambulatoire.
Conclusions
Malgré les besoins croissants des patients en oncologie ambulatoire, la portée des services pharmaceutiques offerts et les résultats utilisés pour qualifier leur effet dans ce contexte au Canada sont fortement hétérogènes. Cette étude démontre un besoin évident de consensus portant sur les cpKPI à l’échelle nationale pour éclairer l’utilisation des ressources pharmaceutiques et les initiatives d’amélioration de la qualité centrées sur le patient.
Mots-clés: indicateurs clés de performance de la pharmacie clinique, indicateur clé de performance, oncologie ambulatoire, pharmacie oncologique, oncologie ambulatoire, Canada
INTRODUCTION
Clinical pharmacy key performance indicators (cpKPIs) are quantitative measures of quality; they reflect pharmacy practice activities associated with evidence-based improvements in meaningful patient outcomes.1,2 Standardized metrics such as cpKPIs are valuable for several reasons, but ultimately they can measure progress toward minimum practice standards, demonstrate the value of pharmacy services, and justify resource allocation. They also allow for comparison within and between institutions and identification of opportunities for improvement and advancement, with the goal of ensuring that all patients are receiving the highest quality health care.
In 2015, the Canadian Society of Hospital Pharmacists (CSHP) published a Canadian consensus guideline, which detailed 8 cpKPIs relating to inpatient hospital pharmacy.1 However, these metrics are not generalizable to activities performed in an ambulatory pharmacy setting, which can differ significantly from inpatient care activities. In fact, very few international cpKPIs exist for ambulatory pharmacy, and, to our knowledge, there are none established for oncology pharmacy practice.3,4
In recent years, oncology pharmacy practice has evolved toward having a more specialized and patient-centred focus, to meet the increasing patient care needs that have resulted from the growth of complex anticancer therapies, multiple lines of therapy, and increased overall survival.5–7 Oncology pharmacists have become important members of multidisciplinary care teams, and their contributions to optimizing drug therapy have had meaningful impacts on patient outcomes.8–22 They are involved in routine direct patient care activities, such as medication reconciliation, but they also participate in services such as clinical trials, which indirectly affect patient care.5,8,14 Given the wide spectrum of adverse effects associated with anticancer therapies, oncology pharmacists also play a critical role in educating patients, preventing drug interactions, monitoring for toxicities, managing disease-related symptoms, and providing supportive care.8,12,23–26
In parallel with these advancements, there has been a notable shift toward providing cancer treatments in outpatient clinics and within the community.8 This has created opportunities for clinical pharmacy services within ambulatory oncology. For example, pharmacists may be involved in formal follow-up programs and adherence assessments.7,16,19,27,28 Nonetheless, the pharmacist’s role in ambulatory oncology remains largely undefined within and across organizations. Without benchmarks or metrics to capture the impact of pharmaceutical care activities, the evolution of this practice area has lacked a guiding direction. To ensure continued practice advancement that will translate into improved quality of care for oncology patients across Canada, it is imperative to define appropriate, meaningful, objective indicators.6 Thus there exists a need to reach consensus as to what constitutes a cpKPI for ambulatory oncology pharmacy.
Before cpKPIs can be established in this practice setting, it is crucial to first understand the current practice landscape in Canada. The primary objective of this study was to describe the ambulatory oncology pharmacy services provided across Canada and how their impact is currently being assessed. The secondary objective was to describe oncology pharmacists’ perceptions of the development, implementation, and evaluation of cpKPIs in this practice setting. It was anticipated that the results of this study would reveal gaps in the services provided by ambulatory oncology pharmacists, demonstrate a need for standardized metrics, and help inform future steps for developing candidate cpKPIs.
METHODS
An anonymous, online, cross-sectional survey was distributed to more than 650 oncology pharmacists in Canada from March 23 to September 14. 2020. The study protocol was reviewed and approved by the University of Waterloo Research Ethics Committee (ORE#41716).
Participants
The target survey population consisted of pharmacists in Canada providing care to patients with malignant disease treated in an outpatient setting. This definition encompassed pharmacists working in outpatient health care institutions and specialty community pharmacies. Participants self-identified as meeting the inclusion criteria and provided informed consent before beginning the survey. Pharmacy technicians, pharmacy students, and pharmacists working solely in an inpatient oncology practice were ineligible to participate, and survey responses that were incomplete were excluded from the analysis.
Survey Questionnaire
Data were collected through an online questionnaire, which was based on the study objectives and informed by relevant publications investigating pharmacist interventions in ambulatory oncology. The survey collected demographic information about the participants (e.g., years in oncology practice, practice site setting and province of work, oncology subspecialties, amount of direct oncology patient care), as well as pharmacy oncology services provided and details of any metrics captured by either individual pharmacists or their institution. Participants were asked to indicate how often they provided listed patient care activities according to a 4-point Likert scale (ranging from “never” to “often”). Lastly, participants were asked to provide feedback regarding the development and implementation of cpKPIs for ambulatory oncology.
Five oncology pharmacists in the study working group (L.H., J.W., M.L., S.E., T.M.) piloted the survey questionnaire for content validity, comprehensiveness, and clarity. These 5 pharmacists were excluded from participating in the survey.
Data Collection
The survey was conducted using Qualtrics Research Core software, version 05-09/2020 (© 2020, https://www.qualtrics.com). The survey was distributed to all members of the Canadian Association of Pharmacy in Oncology (CAPhO), a national voluntary organization of oncology pharmacy practitioners. An invitation detailing the purpose of the study and how to participate was featured in the news section of the CAPhO website and distributed through CAPhO’s social media page and e-newsletter, and was also distributed by personal communication from individual study team members to pharmacists in the field using a snowball technique. Responses to the survey were voluntary, and no compensation or other incentives were offered. Participants could withdraw from the survey at any time before their responses were submitted. Respondents were assured that all information was anonymous and that no individual could be identified from the results. Two email reminders were sent through the CAPhO distribution process described above, at 1 month into data collection and 1 month before the last day of survey availability.
Statistical Analysis
All data were synthesized and presented as descriptive statistics, including frequencies and means.
RESULTS
Of the 60 people who opened the survey, 4 did not meet the inclusion criteria and 12 submitted an incomplete response. Therefore, a total of 44 ambulatory oncology pharmacists self-identified as meeting the study inclusion criteria and submitted complete responses to the survey. The demographic and practice characteristics of these pharmacists are presented in Table 1. Survey responses were received from 9 provinces, and most respondents were practising in British Columbia, Ontario, or Atlantic Canada. On average, respondents had been practising in oncology care for 10 years (range 0.5–30 years). Almost half of respondents worked in community hospitals, with 12 (27%) working in university-affiliated teaching hospitals, 2 (5%) working in specialty oncology pharmacies, and 2 (5%) working in other settings such as a cancer centre or BC Cancer. The majority of respondents (n = 29, 66%) reported that they spent more than half of their day on direct oncology patient care services, and 26 (59%) reported that they saw 10 to 50 cancer patients per week in a direct patient care setting.
TABLE 1.
Characteristics of Respondents (n = 44)
| Characteristic | No. (%) of Respondentsa |
|---|---|
| Time in practice (years) (mean and range) | 9.7 (0.5–30) |
|
| |
| Province or territory | |
| British Columbia | 8 (18) |
| Alberta | 2 (5) |
| Saskatchewan | 1 (2) |
| Manitoba | 3 (7) |
| Ontario | 13 (30) |
| Quebec | 3 (7) |
| Newfoundland and Labrador | 6 (14) |
| Nova Scotia | 5 (11) |
| New Brunswick | 3 (7) |
| Prince Edward Island | 0 (0) |
| Northwest Territories, Yukon, Nunavut | 0 (0) |
|
| |
| Practice setting | |
| Community hospital, urban setting (population > 100 000) | 20 (45) |
| University-affiliated teaching hospital | 12 (27) |
| Rural hospital (population < 100 000) | 8 (18) |
| Specialty non–hospital oncology pharmacy | 2 (5) |
| Otherb | 2 (5) |
|
| |
| Type of patient | |
| Medical and/or hematologic oncology | 40 (91) |
| Radiation oncology | 26 (59) |
| Blood and bone marrow transplant | 23 (52) |
| Pediatric | 10 (23) |
|
| |
| Direct oncology patient care services per day (%) | |
| < 25 | 8 (18) |
| 25–50 | 7 (16) |
| 51–75 | 9 (20) |
| > 75 | 20 (45) |
|
| |
| No. of cancer patients seen per week | |
| < 10 | 8 (18) |
| 10–50 | 26 (59) |
| 51–100 | 6 (14) |
| > 100 | 3 (7) |
| Did not specify | 1 (2) |
Except where indicated otherwise.
Responses included BC Cancer, cancer centre.
Pharmacist Services
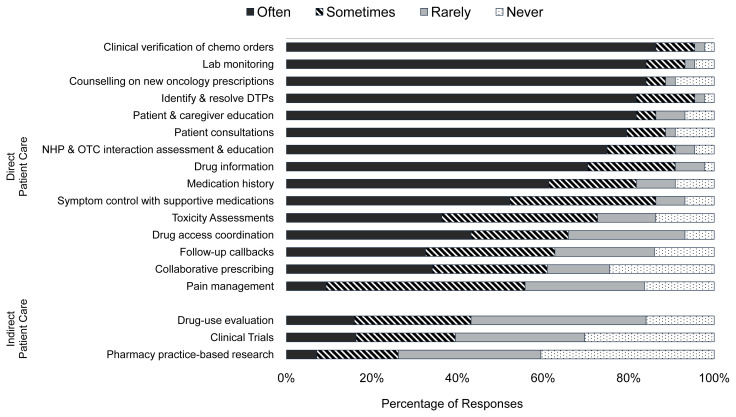
Twenty of the 44 respondents (45%) reported that their respective institutions currently had a formal pharmacist-led monitoring program for oncology patients, with the follow-up duration varying considerably, from 1 cycle to all treatment cycles. Figure 1 details the direct and indirect patient care activities reported by respondents. The direct patient care services most commonly reported as being provided by the oncology pharmacists were chemotherapy order verification, laboratory monitoring, counselling on new oncology prescriptions, and identification and resolution of drug therapy problems. In contrast, the direct patient care services provided least often included pain management, follow-up call-backs, and collaborative prescribing. Similar trends were observed when data were stratified according to the amount of time that respondents reported spending on direct patient care.
FIGURE 1.
Frequency of patient care activities performed by ambulatory oncology pharmacists. Other activities identified in the comments section of the survey: therapeutic drug monitoring, bedside rounds, education of learners and other health care providers, protocol development, hospital committee work such as formulary management, and software programming. DTP = drug therapy problem, NHP = natural health product, OTC = over the counter.
With respect to indirect patient care activities, 23% to 43% of pharmacists reported they were “sometimes” or “often” involved in activities such as drug-use evaluation, clinical trials, and practice-based research (Figure 1). However, a number of additional activities were recognized by survey respondents, such as education of pharmacy learners and other health care providers, protocol development, and participation in hospital committee work such as formulary management and software programming. When the data were stratified by the amount of time spent on direct patient care, oncology pharmacists who had less time for direct patient care were also less likely to be involved with the indirect patient care services specified in the survey.
Pharmacy Performance Metrics and Outcome Measures
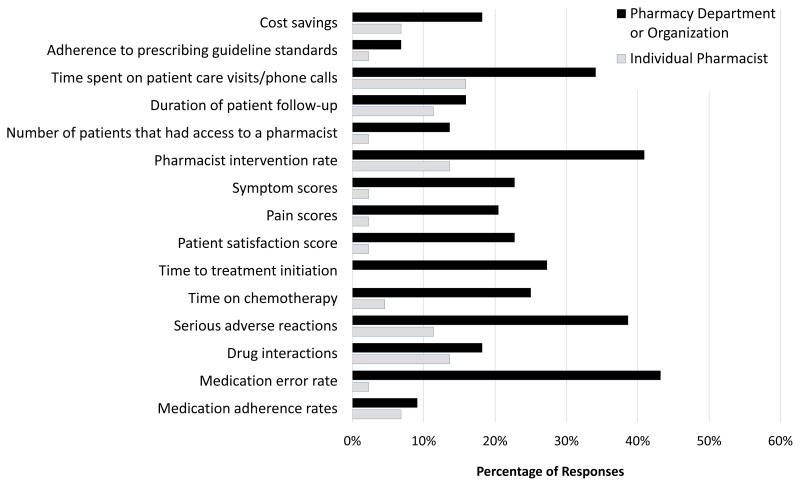
Twenty-six (59%) of the respondents stated that either they or their department currently tracked pharmacy performance metrics or statistics related to patient outcomes. Of the institutions that did such tracking, half collected data longitudinally across multiple clinic visits. Such metrics were usually captured by the pharmacy department; however, a handful of pharmacists reported that they personally tracked outcome measures (Figure 2). No metric was universally captured; the metrics most often collected included time spent on patient care visits and phone calls, pharmacist intervention rate, number of serious adverse events, and medication error rate. One respondent reported that both they and their institution tracked CSHP’s national consensus cpKPIs. Use of an electronic documentation system (n = 24, 55%) and self-reporting (n = 12, 27%) were the most common methods to capture this information; patient surveys (n = 7, 16%) and Microsoft Excel (n = 1, 2%) were less often used.
FIGURE 2.
Frequency of pharmacy performance metrics and patient outcomes captured by individual pharmacists or by their respective pharmacy departments or organizations. Other metrics identified in the comments section of the survey: Canadian Society of Hospital Pharmacists’ clinical pharmacy key performance indicators, intervention codes, number of new patients, total number of patients treated.
Pharmacists’ Perceptions of Key Performance Indicators for Outpatient Oncology
Overall, 43 (98%) of the respondents said they would favour the development of cpKPIs for ambulatory oncology. Respondents reported that cpKPIs were an opportunity to set practice standards across institutions, facilitate training of new staff, provide a tool to demonstrate the value of clinical pharmacy activities, and enable negotiations with management for increased staffing. Reported barriers were fairly consistent across responses; common themes included the lack of time and staffing to implement and document cpKPIs, difficulties with accurately capturing metrics across different electronic systems, lack of evidence in the literature to support clinical pharmacy activities in ambulatory oncology, and challenges in achieving consensus within and across provinces and institutions. Respondents also reported a number of enablers that could help to overcome these challenges, such as the use of technology and expansion of the role of registered pharmacy technicians.29 Furthermore, several respondents mentioned the strong network that exists within oncology pharmacy in Canada, which is supported by a national organization (i.e., the CAPhO) that could assist with value messaging and pharmacist buy-in.
DISCUSSION
To our knowledge, this is the first study attempting to describe pharmacist services provided in ambulatory oncology and to identify how these activities are being assessed. Our study captured pharmacists’ perspectives across a variety of ambulatory oncology practice settings in Canada—from hospitals to specialty community pharmacies.
Overall, the activities performed by pharmacists in this practice setting were heterogeneous, which was recognized by survey respondents as a potential barrier to the cpKPI development process. Nonetheless, pharmacists appeared to be involved in a core group of activities, namely, chemotherapy order verification, laboratory monitoring, identification and resolution of drug therapy problems, and counselling on new oncology prescriptions. A recent systematic review reported that the largest benefit of pharmacist activities in outpatient oncology was the improvement in medication safety.8 It is therefore reassuring that the majority of respondents were heavily involved in activities that contribute to this outcome, such as identification and resolution of drug therapy problems. The provision of patient education has also previously been reported as a key intervention by pharmacists in ambulatory oncology.9,30–32 This intervention significantly decreases symptoms related to cancer, reduces adverse events, and leads to improvement in patients’ quality of life.9 Pharmacist-led patient education is a valued service, as evidenced by its inclusion as a consensus cpKPI in other practice settings.3,4,33
Fewer than half of respondents (45%) reported that their institution had a formal pharmacist-led monitoring program, and even fewer reported that they are often involved in toxicity assessments. These results are comparable to findings in a previous study conducted in Atlantic Canada, which found that fewer than 60% of practice sites had a follow-up service facilitated by the oncology pharmacy team.7 This presents an opportunity for expanded pharmacy services, as such programs have been shown to reduce treatment-related adverse effects and improve patient adherence, and they are effective at identifying drug therapy problems.7,16,18,19,23,27,28,34
In our study, pharmacists who spent less time on direct patient activities were also less involved in indirect patient care activities. This finding seems counterintuitive; however, the list of services on the survey questionnaire was by no means exhaustive, so this result likely reinforces the extent of administrative responsibilities not captured by the survey in which ambulatory oncology pharmacists can be heavily involved. This result also highlights how involvement in direct patient care can lead to increased opportunities for pharmacists to contribute to system-level advancements in patient care. This aligns with the World Health Organization’s concept of a nine-star pharmacist, a concept detailing the goals for a robust and comprehensive role for pharmacists.35,36
In a recent US-based study, a Delphi expert panel was used to identify the clinical services that board-certified oncology pharmacists most frequently perform.31 Similar to our study, the panel found that pharmacists were highly involved in adjusting chemotherapy, providing patient education, and managing adverse events. Interestingly, the Delphi panel also identified frequent pharmacist involvement in pain management and toxicity assessments, which does not align with the results of our study. Unfortunately, the Delphi panel study did not appear to incorporate literature or patient outcomes to help guide the consensus activities and thus the panel’s conclusions may not represent evidence-informed practice.
Our results showed that pharmacy performance metrics were captured by only about half of the survey respondents. Clinical outcomes were most often evaluated indirectly through the use of pharmacist intervention rate, whereas direct clinical outcomes (e.g., symptom scores) were less commonly captured. Metrics pertaining to patient safety constituted a dominant theme, which is not surprising given that medication safety is a key and valuable role in which pharmacists are regularly involved.8 The time spent on patient care visits was also commonly collected, which likely pertains to pharmacy resource allocation. It is unclear exactly how these metrics are utilized in practice by pharmacy management or organizations, as that type of analysis was outside the scope of this study.
There was practically unanimous support from survey respondents for the development of cpKPIs for the ambulatory oncology setting. They recognized that to make a compelling case to management for increased pharmacy staffing, it is imperative to demonstrate that pharmacy services have significant value in terms of patient outcomes. Unfortunately, we found that high-quality evidence to support this case is limited, and future practice-based research is likely needed to bridge some of these evidence gaps.9 Relatively few published studies have focused on outpatient oncology pharmacy, and much of the literature consists of single-centre observational studies with small sample sizes. A commonly reported barrier to cpKPI implementation by survey respondents was staff shortages and lack of time to take on additional responsibilities. On the basis of these reported concerns, pharmacists will likely place value on cpKPIs that are practical to implement and efficient to measure. The increased use of electronic reporting platforms may also help facilitate the ease of use and feasibility of cpKPI tracking.
Limitations and Future Directions
This study had some limitations that should be highlighted. First, the number of survey responses was low, despite the survey being left open for an extended period. The study was conducted during the COVID-19 pandemic, and no incentives were offered, which may have negatively affected participation. We were also unable to calculate a true survey response rate for 2 reasons: CAPhO membership includes pharmacists working in areas outside the target population of this survey and CAPhO membership is voluntary, such that additional survey distribution relied on the snowball technique. For these reasons, we could not accurately determine the total number of eligible participants who received the survey. Moreover, because not all ambulatory oncology pharmacists are CAPhO members, there was likely an underrepresentation of pharmacists working in this specialty pharmacy setting.
Additionally, these data were primarily driven by participants in a few select provinces. It is therefore challenging to assess whether these results are generalizable to all Canadian pharmacists working in ambulatory oncology. We also recognize that the survey did not allow participants to delineate between rural and suburban practice areas, and the prespecified population threshold used to define these categories was somewhat arbitrary. More extensive subgroup analyses were limited by the relatively small sample size of this study and would be exploratory in nature. As such, we are unable to describe variation in workload or allocation of pharmacy resources across institutions and provinces. Similarly, we could not confidently determine workplace factors that may be affecting pharmacists’ activities or contributing to reported cpKPI barriers.
To address these limitations and move forward with the cpKPI development process, the next phases of this research will include structured interviews and focus group discussions with both pharmacy management and front-line pharmacists working in ambulatory oncology practice settings. The results of this survey will help inform the question development for these qualitative semistructured discussions, as well as future Delphi panel surveys.
CONCLUSION
These survey results suggest significant heterogeneity in the services that Canadian pharmacists provide for patients with malignant disease treated in an outpatient setting. Similarly, a wide range of metrics and patient outcomes are being captured by only a limited number of institutions. This study demonstrates a clear need for, and end user interest in, national consensus cpKPIs within this practice setting. However, further practice-based research is likely needed to fill evidence gaps and inform cpKPI development.
Acknowledgments
The authors would like to thank the survey participants, who have willingly shared their precious time.
Footnotes
Competing interests: For activities outside the study reported here, Lauren Hutton received an unrestricted research grant from Pfizer and Thomas McFarlane has received honoraria from AstraZeneca, ApoBiologix, Pfizer, GlaxoSmithKline, and Purdue. Jason Wentzell is the founder and co-owner of Extend Pharmacy, a privately owned oncology specialty pharmacy in Ottawa, Ontario. No other competing interests were declared.
Funding: None received.
References
- 1. Fernandes O, Gorman SK, Slavik RS, Semchuk WM, Shalansky S, Bussières JF, et al. Development of clinical pharmacy key performance indicators for hospital pharmacists using a modified Delphi approach. Ann Pharmacother. 2015;49(6):656–69. doi: 10.1177/1060028015577445. [DOI] [PubMed] [Google Scholar]
- 2. Lo E, Rainkie D, Semchuk WM, Gorman SK, Toombs K, Slavik RS, et al. Measurement of clinical pharmacy key performance indicators to focus and improve your hospital pharmacy practice. Can J Hosp Pharm. 2016;69(2):149–55. doi: 10.4212/cjhp.v69i2.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shawahna R. Development of key performance indicators to capture in measuring the impact of pharmacists in caring for patients with epilepsy in primary healthcare: a Delphi consensual study. Epilepsy Behav. 2019;98(Pt A):129–38. doi: 10.1016/j.yebeh.2019.07.034. [DOI] [PubMed] [Google Scholar]
- 4. Lima TM, Aguiar PM, Storpirtis S. Development and validation of key performance indicators for medication management services provided for outpatients. Res Soc Adm Pharm. 2019;15(9):1080–7. doi: 10.1016/j.sapharm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 5. Holle LM, Michaud LB. Oncology pharmacists in health care delivery: vital members of the cancer care team. J Oncol Pract. 2014;10(3):e142–5. doi: 10.1200/JOP.2013.001257. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt L, Klink C, Iglar A, Sharpe N. Implementation of performance metrics to assess pharmacists’ activities in ambulatory care clinics. Am J Health Syst Pharm. 2017;74(1):e76–82. doi: 10.2146/ajhp150698. [DOI] [PubMed] [Google Scholar]
- 7. Edwards S, Abbott R, Dranitsaris G. Patient monitoring programs in oncology pharmacy practice: a survey of oncology pharmacists in Atlantic Canada. J Oncol Pharm Pract. 2019;25(4):891–5. doi: 10.1177/1078155218790801. [DOI] [PubMed] [Google Scholar]
- 8. Maleki S, Alexander M, Fua T, Liu C, Rischin D, Lingaratnam S. A systematic review of the impact of outpatient clinical pharmacy services on medication-related outcomes in patients receiving anticancer therapies. J Oncol Pharm Pract. 2019;25(1):130–9. doi: 10.1177/1078155218783814. [DOI] [PubMed] [Google Scholar]
- 9. Colombo LRP, Aguiar PM, Lima TM, Storpirtis S. The effects of pharmacist interventions on adult outpatients with cancer: a systematic review. J Clin Pharm Ther. 2017;42(4):414–24. doi: 10.1111/jcpt.12562. [DOI] [PubMed] [Google Scholar]
- 10. Ruder AD, Smith DL, Madsen MT, Kass FH. Is there a benefit to having a clinical oncology pharmacist on staff at a community oncology clinic? J Oncol Pharm Pract. 2011;17(4):425–32. doi: 10.1177/1078155210389216. [DOI] [PubMed] [Google Scholar]
- 11. Shah S, Dowell J, Greene S. Evaluation of clinical pharmacy services in a hematology/oncology outpatient setting. Ann Pharmacother. 2006;40(9):1527–33. doi: 10.1345/aph.1H162. [DOI] [PubMed] [Google Scholar]
- 12. Valgus J, Jarr S, Schwartz R, Rice M, Bernard SA. Pharmacist-led, interdisciplinary model for delivery of supportive care in the ambulatory cancer clinic setting. J Oncol Pract. 2010;6(6):1–4. doi: 10.1200/JOP.2010.000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walter C, Mellor JD, Rice C, Kirsa S, Ball D, Duffy M, et al. Impact of a specialist clinical cancer pharmacist at a multidisciplinary lung cancer clinic. Asia Pac J Clin Oncol. 2016;12(3):e367–74. doi: 10.1111/ajco.12267. [DOI] [PubMed] [Google Scholar]
- 14. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549–57. doi: 10.2146/ajhp160475. [DOI] [PubMed] [Google Scholar]
- 15. Imamura M, Ogawa D, Takatori T, Yamaguchi M, Takata T, Hada T, et al. A retrospective study of the effects of oncology pharmacist participation in treatment on therapeutic outcomes and medical costs. Biol Pharm Bull. 2017;40(11):1956–62. doi: 10.1248/bpb.b17-00501. [DOI] [PubMed] [Google Scholar]
- 16. Lam MSH, Cheung N. Impact of oncology pharmacist-managed oral anticancer therapy in patients with chronic myelogenous leukemia. J Oncol Pharm Pract. 2016;22(6):741–8. doi: 10.1177/1078155215608523. [DOI] [PubMed] [Google Scholar]
- 17. Liekweg A, Westfeld M, Braun M, Zivanovic O, Schink T, Kuhn W, et al. Pharmaceutical care for patients with breast and ovarian cancer. Support Care Cancer. 2012;20(11):2669–77. doi: 10.1007/s00520-012-1385-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Parsons LB, Edwards K, Perez A, Letton C, Bondarenka C. Positive outcomes associated with a pharmacist-driven oral chemotherapy program. J Hematol Oncol Pharm. 2015;5(4):99–108. [Google Scholar]
- 19. Patel JM, Holle LM, Clement JM, Bunz T, Niemann C, Chamberlin KW. Impact of a pharmacist-led oral chemotherapy-monitoring program in patients with metastatic castrate-resistant prostate cancer. J Oncol Pharm Pract. 2016;22(6):777–83. doi: 10.1177/1078155215612541. [DOI] [PubMed] [Google Scholar]
- 20. Randolph LA, Walker CK, Nguyen AT, Zachariah SR. Impact of pharmacist interventions on cost avoidance in an ambulatory cancer center. J Oncol Pharm Pract. 2018;24(1):3–8. doi: 10.1177/1078155216671189. [DOI] [PubMed] [Google Scholar]
- 21. Ribed A, Romero-Jiménez RM, Escudero-Vilaplana V, Iglesias-Peinado I, Herranz-Alonso A, Codina C, et al. Pharmaceutical care program for onco-hematologic outpatients: safety, efficiency and patient satisfaction. Int J Clin Pharm. 2016;38(2):280–8. doi: 10.1007/s11096-015-0235-8. [DOI] [PubMed] [Google Scholar]
- 22. Wong SF, Bounthavong M, Nguyen CP, Chen T. Outcome assessments and cost avoidance of an oral chemotherapy management clinic. J Natl Compr Cancer Netw. 2016;14(3):279–85. doi: 10.6004/jnccn.2016.0033. [DOI] [PubMed] [Google Scholar]
- 23. Escudero-Vilaplana V, Ribed A, Romero-Jimenez RM, Herranz-Alonso A, Sanjurjo-Saez M. Pharmacotherapy follow-up of key points in the safety of oral antineoplastic agents. Eur J Cancer Care (Engl) 2017;26(3):e12463. doi: 10.1111/ecc.12463. [DOI] [PubMed] [Google Scholar]
- 24. Lopez-Martin C, Garrido Siles M, Alcaide-Garcia J, Faus Felipe V. Role of clinical pharmacists to prevent drug interactions in cancer outpatients: a single-centre experience. Int J Clin Pharm. 2014;36(6):1251–9. doi: 10.1007/s11096-014-0029-4. [DOI] [PubMed] [Google Scholar]
- 25. Caracuel F, Baños Ú, Herrera MD, Ramírez G, Muñoz N. Influence of pharmaceutical care on the delayed emesis associated with chemotherapy. Int J Clin Pharm. 2014;36(2):287–90. doi: 10.1007/s11096-014-9915-z. [DOI] [PubMed] [Google Scholar]
- 26. Gagnon L, Fairchild A, Pituskin E, Dutka J, Chambers C. Optimizing pain relief in a specialized outpatient palliative radiotherapy clinic: contributions of a clinical pharmacist. J Oncol Pharm Pract. 2012;18(1):76–83. doi: 10.1177/1078155211402104. [DOI] [PubMed] [Google Scholar]
- 27. Muluneh B, Schneider M, Faso A, Amerine L, Daniels R, Crisp B, et al. Improved adherence rates and clinical outcomes of an integrated, closed-loop, pharmacist-led oral chemotherapy management program. J Oncol Pract. 2018;14(6):e324–34. doi: 10.1200/JOP.17.00039. [DOI] [PubMed] [Google Scholar]
- 28. Simons S, Ringsdorf S, Braun M, Mey UJ, Schwindt PF, Ko YD, et al. Enhancing adherence to capecitabine chemotherapy by means of multidisciplinary pharmaceutical care. Support Care Cancer. 2011;19(7):1009–18. doi: 10.1007/s00520-010-0927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Read H, Ladds S, Rhodes B, Brown D, Portlock J. The impact of a supplementary medication review and counselling service within the oncology outpatient setting. Br J Cancer. 2007;96(5):744–51. doi: 10.1038/sj.bjc.6603634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krikorian S, Pories S, Tataronis G, Caughey T, Chervinsky K, Lotz M, et al. Adherence to oral chemotherapy: challenges and opportunities. J Oncol Pharm Pract. 2019;25(7):1590–8. doi: 10.1177/1078155218800384. [DOI] [PubMed] [Google Scholar]
- 31. Ignoffo R, Knapp K, Barnett M, Barbour SY, D’Amato S, Iacovelli L, et al. Board-certified oncology pharmacists: their potential contribution to reducing a shortfall in oncology patient visits. J Oncol Pract. 2016;12(4):e359–68. doi: 10.1200/JOP.2015.008490. [DOI] [PubMed] [Google Scholar]
- 32. Crespo A, Tyszka M. Evaluating the patient-perceived impact of clinical pharmacy services and proactive follow-up care in an ambulatory chemotherapy unit. J Oncol Pharm Pract. 2017;23(4):243–8. doi: 10.1177/1078155216634180. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes O, Toombs K, Pereira T, Lyder C, Bjelajac Mejia A, Shalansky S, et al. Canadian consensus on clinical pharmacy key performance indicators: quick reference guide. Canadian Society of Hospital Pharmacists; 2015. [cited 2021 Jul 25]. Available from: https://www.cshp.ca/docs/pdfs/CSPH-Can-Concensus-cpKPI-QuickReferenceGuide_June_2017.pdf. [Google Scholar]
- 34. Battis B, Clifford L, Huq M, Pejoro E, Mambourg S. The impacts of a pharmacist-managed outpatient clinic and chemotherapy-directed electronic order sets for monitoring oral chemotherapy. J Oncol Pharm Pract. 2017;23(8):582–90. doi: 10.1177/1078155216672314. [DOI] [PubMed] [Google Scholar]
- 35. Thamby SA, Subramani P. Seven-star pharmacist concept by World Health Organization. J Young Pharm. 2014;6(2):1–3. doi: 10.5530/jyp.2014.2.1. [DOI] [Google Scholar]
- 36. Sam AT, Parasuraman S. The nine-star pharmacist: an overview. J Young Pharm. 2015;7(4):281. doi: 10.5530/jyp.2015.4.1. [DOI] [Google Scholar]