Abstract
Homeobox genes are prominent regulators of neuronal identity, but the extent to which their function has been probed in animal nervous systems remains limited. In the nematode Caenorhabditis elegans, each individual neuron class is defined by the expression of unique combinations of homeobox genes, prompting the question of whether each neuron class indeed requires a homeobox gene for its proper identity specification. We present here progress in addressing this question by extending previous mutant analysis of homeobox gene family members and describing multiple examples of homeobox gene function in different parts of the C. elegans nervous system. To probe homeobox function, we make use of a number of reporter gene tools, including a novel multicolor reporter transgene, NeuroPAL, which permits simultaneous monitoring of the execution of multiple differentiation programs throughout the entire nervous system. Using these tools, we add to the previous characterization of homeobox gene function by identifying neuronal differentiation defects for 14 homeobox genes in 24 distinct neuron classes that are mostly unrelated by location, function and lineage history. 12 of these 24 neuron classes had no homeobox gene function ascribed to them before, while in the other 12 neuron classes, we extend the combinatorial code of transcription factors required for specifying terminal differentiation programs. Furthermore, we demonstrate that in a particular lineage, homeotic identity transformations occur upon loss of a homeobox gene and we show that these transformations are the result of changes in homeobox codes. Combining the present with past analyses, 113 of the 118 neuron classes of C. elegans are now known to require a homeobox gene for proper execution of terminal differentiation programs. Such broad deployment indicates that homeobox function in neuronal identity specification may be an ancestral feature of animal nervous systems.
Author summary
We address here how neuron types acquire their specific molecular identity features. We focus on a specific family of transcription factors, encoded by homeobox genes and analyze the function of more than a dozen homeobox gene family members throughout the entire nervous system of the nematode C. elegans, which is composed of 118 different neuron classes. We discover homeobox gene functions in 24 distinct neuron classes that are mostly unrelated by location, function and lineage history. Together with previous studies, this work demonstrates the wide-spread employment of this transcription factor family in neuronal identity specification, suggesting that these factors may have been recruited to neuronal cell differentiation early in evolution.
Introduction
Nervous systems are composed of diverse sets of neuron types, each characterized by the expression of specific gene batteries that define the structural and functional features of that mature neuron type. A fundamental question in developmental neurobiology is whether there are common organizational principles for how individual neuron types acquire their unique identities. One approach to uncover such common principles is to comprehensively analyze neuronal differentiation programs throughout a given nervous system and determine whether a specific set of rules or features recurs in the specification of different neuron types in different parts of the organism. We have engaged in such holistic analysis in the nematode C. elegans, which contains a nervous system with substantial cellular diversity but limited overall number: 302 neurons in the hermaphrodite which fall into 118 classes [1]. In our search for common principles in C. elegans neuron type specification, several themes have emerged: (1) the direct, coordinated control of neuron type specific gene batteries by so-called “terminal selectors” [2–4]; (2) the overrepresentation of homeodomain transcription factors as terminal selectors [5,6] and (3) the codification of individual neuron identities by distinct combinations of homeodomain proteins, unique for each individual neuron type [5–7].
Since the DNA binding site of a single transcription factor does not encode enough specificity to select downstream targets genes in vast genome sequence space, transcription factors usually operate in combination with other transcription factors [8–10]. In the context of homeodomain proteins in the C. elegans nervous system, combinatorial functions of co-expressed homeodomain proteins have usually been inferred genetically through removal of co-expressed homeobox genes, either in isolation or in combination, resulting in neuronal differentiation defects. In some cases, the biochemical basis for such combinatorial activity has been deduced: For example, in the case of the gentle touch receptor neurons, the POU and LIM homeodomain proteins UNC-86 and MEC-3 bind cooperatively to target gene promoters to determine the fully differentiated state of these neurons [11]; similarly, the Prd and LIM homeodomain proteins CEH-10 and TTX-3, whose expression uniquely overlaps in the cholinergic AIY interneurons, show cooperative binding to cis-regulatory elements of members of the gene battery that define the terminally differentiated state of the AIY neurons [12]. In other cases, homeodomain proteins appear to bind to target gene promoters independently of one another, but their joint presence is required for target gene expression [13,14].
In this paper, we set out to further probe the extent to which homeobox genes, and combinations thereof, are involved in neuronal identity specification. First, we further refine our atlas of homeodomain protein expression throughout the nervous system and, second, we define the impact of loss-of-function alleles of 12 homeobox genes on neuronal identity specification throughout the entire nervous system. One pillar of this mutant analysis is the recently described NeuroPAL transgene, which expresses more than 40 distinct neuronal identity markers throughout the entire C. elegans nervous system [15]. Together with other available molecular markers we identify 14 homeobox genes involved in the specification of 24 different neuron classes (of the total of 118 C. elegans hermaphrodite neuron classes), 12 of which had no previously known regulator assigned to them. This mutant analysis therefore expands our understanding of homeobox gene function in neuronal identity control, arguing that homeobox genes play a central and perhaps ancestral role in neuronal identity specification.
Results
Reporter alleles refine some homeodomain protein expression profiles
Precise knowledge of the expression pattern of a gene provides a useful guide for mutant analysis. In our previous, genome-wide analysis of homeodomain protein expression, we made use of both CRISPR/Cas9-engineered reporter alleles, as well as fosmid-based reporters to assess expression patterns [6]. Fosmids are generally 30–50 kb genomic fragments, usually containing several genes up/downstream of a gene of interest and can be expected to include all cis-regulatory information of a tagged locus. Indeed, the expression pattern of many fosmid reporters is successfully recapitulated by CRISPR/Cas9 genome-engineered reporter alleles [7,16–18]. A recently published nervous system wide scRNA transcriptome atlas, called CeNGEN [19] is also largely congruent with an atlas of homeodomain expression profiles that was based on either fosmid-based reporters or CRISPR/Cas9-engineered reporter alleles [6]. We nevertheless set out to compare the expression of 18 newly available CRISPR/Cas9-engineered reporter alleles, generated either by us or obtained from the Du lab [20], with previously described fosmid-based reporter patterns and the scRNA CeNGEN atlas (Table 1). Like the fosmid reporters, these reporter alleles fuse a reporter directly to the N- or C-terminus of the encoded homeodomain protein, thereby allowing the direct monitoring of protein expression.
Table 1. Comparing expression patterns of CRISPR/Cas9-engineered reporter alleles with fosmid-based reporter transgenes.
| Homeobox gene | Reporter allele 1 | CRISPR/Cas9-engineered reporter allele | Only observed with fosmid reporter 2, not with reporter allele | |
|---|---|---|---|---|
| also observed with fosmid reporter 2 | not observed with fosmid | |||
| vab-3 | devKi190 | ASK, BAG, OLQ, CEP, RIF, RIV, AS11, VA11, VD12 | ADA, OLL, URA, URB, URY | PVM*, SMB*, ALM, PLM, AVD, DBs, VBs, SAA |
| lim-7 | devKi125 | RIA, URY, OLL, PVN, PHC, AVB, AIB, AIM, BAG, M1, M5 | ALA, MI, AWC, I3, I4, BAG | AFD*, PVC*, RME |
| ceh-27 | syb2714 | I6, RIM, RIP, RME, RMF | AVL | none |
| ceh-43 | syb5073 | ADE, AIZ, ASJ, BDU, CAN, CEP, IL1, PDE, PVQ, SDQ | AIN | URB |
| ceh-45 | devKi191 | I1, MI | RIB | none |
| ceh-30 | syb4678 | SDQ | none | ubiquitous |
| ceh-31 | devKi250 | URA, URB, AVB, SDQ, PVR | none | none |
| ceh-16 | syb2880 | AIZ, RIF, RIG | none | none |
| ceh-36 | syb2934 | ASE, AWC, ASI, AWA | none | none |
| ceh-12 | devKi186 | VB | none | none |
| unc-30 | hzhCR1 | ASG, AVJ, DD, PVP, VD | none | none |
| ceh-17 | devKi180 | ALA, SIA | none | none |
| ceh-5 | devKi103 | embryo only | none | none |
| ceh-51 | devKi72 | embryo only | none | none |
| ceh-82 | devKi181 | none | none | ubiquitous |
| ceh-28 | devKi192 | M4 | none | none |
| ceh-2 | devKi17 | I3, M3 | none | NSM (dim) |
| ceh-10 | devKi101 | AIY, CAN, RID, DVC 3 | N/A 3 | N/A 3 |
Underlined neuron classed names indicate scRNA transcript found in this neuron in CeNGEN scRNA dataset [19]. When marked with *, transcript levels were very low, compared to other neurons that express the gene.
1 Generated by CRISPR/Cas9-based genome engineering. ot alleles were generated in our lab, syb alleles by Sunybiotech, dev and hzh alleles were kindly provided by Zhuo Du.
2 As described in [6]
3 the previously reported expression pattern [6] was not based on a fosmid but on a personal communication about another reporter allele.
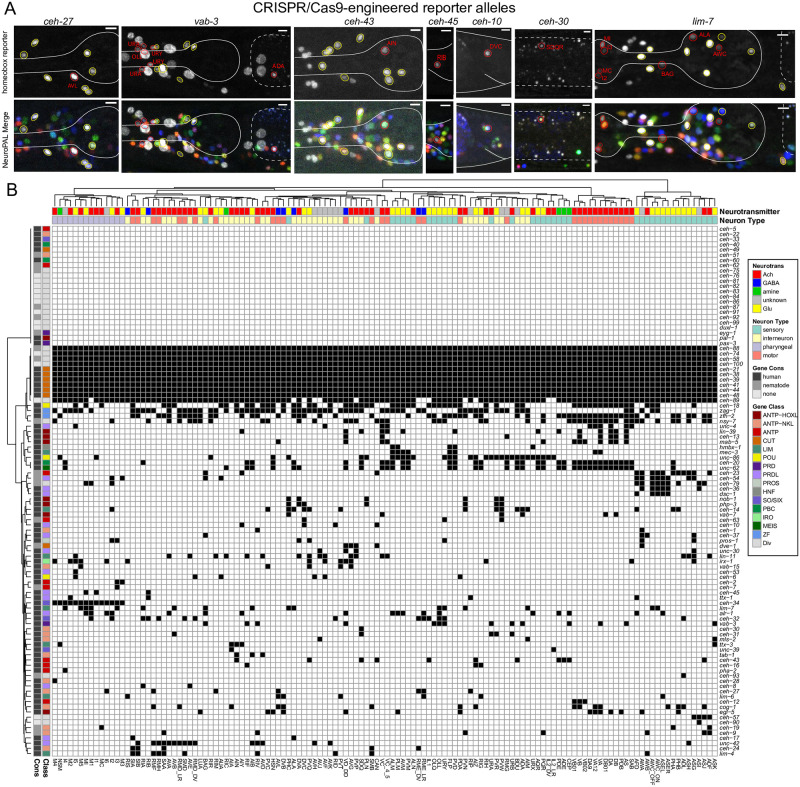
We observed expression patterns with the reporter alleles that are largely congruent between the fosmid-based expression profiles, but also observed some differences (Fig 1 and Table 1). Among the homeobox genes with the greatest difference in gene expression profiles are vab-3, the C. elegans Eyeless/PAX6 ortholog and ceh-30, one of the two C. elegans BarH1 homologs. In retrospect, the difference between the sites of expression of these fosmid reporters and the reporter alleles is not surprising. First, the previously used vab-3 fosmid reporter stood out for its weakness and variability in expression. Second, the fosmid-based reporter for ceh-30 covered all intergenic, non-coding regions, but it did not over all intergenic region of the neighboring paralogue ceh-31 with whom ceh-30 may share cis-regulatory control elements (Figs 1 and S1).
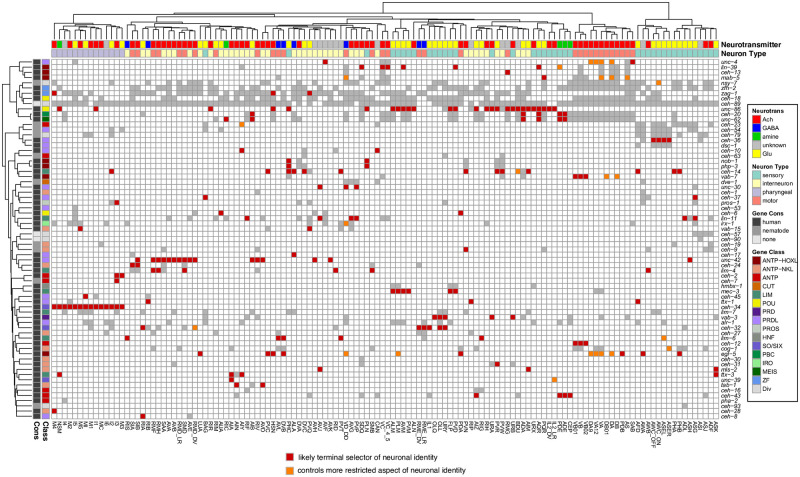
Fig 1. Updated expression of the homeobox gene family with reporter alleles.
Fig 1A: Representative images of homeobox reporter alleles, generated by CRISPR/Cas9 genome engineering (see strain list in S5 Table) with different expression than previously reported fosmid-based reporter transgenes. Neuron classes showing expression not previously noted were identified by overlap with the NeuroPAL landmark strain, and are outlined and labeled in red. Neuron types in agreement with previous reporter studies are outlined in yellow. Head structures including the pharynx were outlined in white for visualization. Autofluorescence common to gut tissue is outlined with a white dashed line. An n of 10 worms were analyzed for each reporter strain. Scale in bottom or top right of the figure represents 5 μm. See also S1 Fig for more information on ceh-30 and ceh-31. Fig 1B: Summary of expression of all homeobox genes across the C. elegans nervous system, taking into account new expression patterns from panel A and all previously published data [6]. Black boxes indicate that a homeodomain transcription factor is expressed in that given neuron type and white boxes indicate that a homeodomain transcription factor is not expressed in that given neuron type. Neuron types along the x axis are clustered by transcriptomic similarity using the Jaccard index (see methods) and homeobox genes along the y axis are clustered similarly by their similar expression profiles in shared neuron types. See S3 Fig for numerical representation of homeoboxes per neuron.
In two other cases, we observed that the reporter allele added expression in one additional neuron class (Fig 1 and Table 1). For example, a ceh-45 reporter allele is expressed in I1 and MI, as previously reported with a fosmid-reporter [6], but additional, albeit weaker expression is observed in the RIB interneurons with the reporter allele. In all these cases, the additional expression was supported by scRNA CeNGEN data [19], but the expression level was notably lower in the respective neuron class. In these cases, the fosmid reporter may have lacked cis-regulatory element(s) or may have been too weakly expressed.
In one case, the EMX homolog ceh-2, we recapitulated previously reported I2 and M3 expression, but failed to observe CEH-2 protein expression in the NSM neuron, which had shown expression with the fosmid reporter [6]. Since this neuron class also shows scRNA transcripts of the ceh-2 homeobox gene [19], it is conceivable that multicopy overexpression of the fosmid reporter may have titrated out a rate-limiting posttranscriptional regulatory event.
Altogether, the revised expression patterns of homeodomain proteins further refine the nature of combinatorial homeodomain expression codes that uniquely define each neuron class (Fig 1B and S1 and S2 Tables). In toto, 79 of the 102 C. elegans homeobox genes are expressed in the nervous system, 69 of which on a neuron type-specific manner, with neuron-type specific combinatorial codes [6,21] (this paper). We display this combinatorial coding data in a manner distinct from our previous study [6]. We first clustered neuronal cell types by similarity of scRNA-generated gene expression profiles (see Methods; S2 Fig) and then mapped homeodomain expression patterns onto this matrix. This representation provides a visually tractable way to discern whether transcriptionally similar neurons tend to express the same homeodomain protein(s) (Fig 1B and S1 and S2 Tables). There are indeed several instances of such co-clustering. For example, the set of neurons that co-express the homeodomain protein CEH-34 and the set of neurons that co-express the homeodomain protein UNC-42 share more molecular similarities among themselves than with other neuron classes (Fig 1B). This is particularly notable because both the UNC-42(+) and CEH-34(+) neurons are synaptically more interconnected than expected by chance [7, 17].
Mutant analysis of homeobox genes
Guided by homeodomain protein expression patterns, we sought to further implicate them in the process of terminal neuron differentiation using homeobox mutant strains. It is important to emphasize that our homeodomain protein expression profiles are entirely focused on those proteins that are continuously expressed throughout the life of a neuron and are therefore candidates to not only initiate but also maintain the differentiated state of a neuron, the original criterion for being a terminal selector of neuronal identity [2,4]. Our mutant analysis covered neurons that fall into three categories (Table 2): (1) neurons for which no transcriptional identity regulator (terminal selector) had been described before; (2) neurons for which only a non-homeobox identity regulator had been identified; (3) neurons for which no unique functional combination of homeobox genes has been identified. The neurons that we covered in this analysis are functionally diverse, many have different lineage histories and are located in distinct parts of the C. elegans nervous system (Table 2).
Table 2. Summary of newly identified homeobox regulators of neuronal differentiation.
| Neuron class | type | location | lineage history | Previously identified identity regulator for this neuron class | Newly described homeobox gene required for identity (this paper) |
|---|---|---|---|---|---|
| no identity regulator known before | |||||
| AVJ | peptidergic interneuron | lateral ganglion | ABalap(a/p)pppa | none | lin-11/LHX1 |
| unc-30/PITX | |||||
| mls-2/HMX | |||||
| ADA | glutamatergic interneuron | anterior deirid lineage | ABp(l/r)apaaaapp | none | ceh-14/LHX3 |
| unc-86/BRN3 | |||||
| unc-62/MEIS | |||||
| RMG | peptidergic interneuron | anterior deirid lineage | ABp(l/r)apaaapp | none | unc-86/BRN3 |
| ceh-13/HOX | |||||
| RIC | octopaminergic interneuron | lateral ganglion | ABp(l/r)ppaaaapp | none | unc-62/MEIS |
| RIR | cholinergic interneuron | ventral ganglion | ABprpapppaa | none | unc-86/POU |
| RIP | peptidergic interneuron | anterior ganglion | AB alpapaaaa, AB arappaaaa | none | ttx-1/OTX |
| RIB | GABAergic interneuron | lateral ganglion | AB p(l/r)paappap | none | ttx-1/OTX |
| AIN | cholinergic interneuron | lateral ganglion | ABalaaaalal ABalaapaaar |
none | tab-1/BSX |
| OLQ | glutamatergic sensory | anterior ganglion | ABalap(a/p)papaa ABp(l/r)paaappaa |
none | vab-3/PAX6 |
| IL1 | glutamatergic sensory | anterior ganglion | ABalapappaaa ABalappppaaa ABalapaappaa ABalaappppaa ABalppapppaa ABarapppppaa |
none 1 | ceh-32/SIX3/6 |
| PVW | peptidergic interneuron | lumbar ganglion | T(L/R).ppa |
none | ceh-14/LHX3 |
| LUA | glutamatergic interneuron | lumbar ganglion | ABp(l/r)pppaapap |
none | egl-5/HOX |
| only non-homeobox regulator known before | |||||
| AWA | peptidergic sensory | lateral ganglion | ABp(l/r)aapapaa |
odr-7 (nuclear receptor) |
egl-5/HOX |
| RME | GABAergic motor neuron | nerve ring | ABalapppaap ABalaaaar(l/r)p ABplpappaaa |
nhr-67/TLX (nuclear receptor) |
ceh-32/SIX3 |
| PDA | cholinergic motor neuron | ABprpppaaaa |
unc-3/COE (Zn finger) |
egl-5/HOX | |
| complements incomplete combination of previously described homeobox genes | |||||
| AIA | cholinergic interneuron | ventral ganglion | ABp(l/r)ppaappa |
ttx-3/LHX2 | unc-39/SIX4 |
| AIM | glutamatergic interneuron | ventral ganglion | ABp(l/r)paapppa |
unc-86/BRN3
|
mls-2/HMX
ceh-14/LHX3 |
| AIZ | glutamatergic interneuron | anterior deirid lineage | AB p(l/r)apaaapav | unc-86/BRN3 | ceh-43/DLX |
| AQR | glutamatergic sensory neuron | head | QR.ap |
unc-86/BRN3
lin-39/HOX egl-13/SoxD |
unc-62/MEIS
ceh-20/PBX |
| FLP | glutamatergic sensory neuron | anterior deirid lineage | ABp(l/r)apaaapad |
unc-86/BRN3
mec-3/LHX |
unc-62/MEIS
ceh-20/PBX |
| AVD | glutamatergic interneuron | lateral ganglion | ABalaaapalr ABalaaapprl |
unc-42/PROP1
unc-3/COE cfi-1/ARID |
tab-1/BSX |
| OLL | glutamatergic sensory | anterior ganglion | ABalppppapaa ABpraaapapaa |
vab-3/PAX6 | ceh-32/SIX3 |
| URY | glutamatergic sensory | anterior ganglion | ABalap(a/p)papp ABp(l/r)paaappp |
vab-3/PAX6 | ceh-32/SIX3 |
| URB | cholinergic interneuron | anterior ganglion | AB plaapaapa AB praapaapa |
unc-86/BRN3 | vab-3/PAX6 |
| URA | cholinergic interneuron | anterior ganglion | AB plaaaaaaa, AB arpapaaaa, AB plpaaapaa, AB prpaaapaa |
unc-86/BRN3 | vab-3/PAX6 |
1 we had previously assumed vab-3 to be a candidate terminal selector for the IL1 neurons [48]; however, more recent expression pattern analysis indicates that vab-3 is not continuously expressed in IL1 [6]. Hence, the reported IL1 differentiation defects of vab-3 mutants are likely a reflection of earlier function of vab-3 in the lineage.
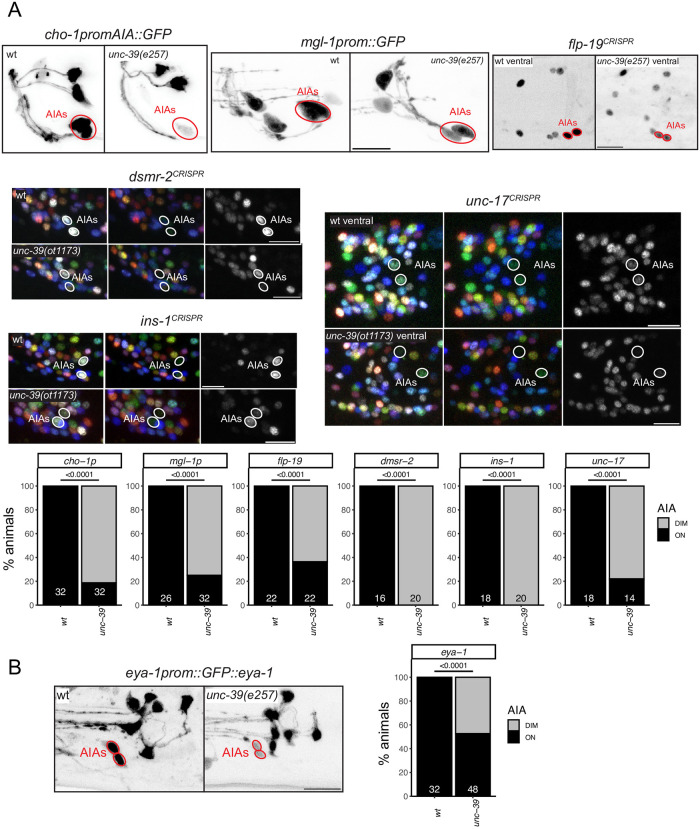
The SIX-type homeobox gene unc-39 affects differentiation of the AIA interneuron class
We have previously reported that the LIM homeobox gene ttx-3 is required for the proper differentiation of the cholinergic AIY and AIA interneurons [14]. However, unlike in the AIY interneuron class, where TTX-3 partners with the Chx10 homolog CEH-10 [12], no homeodomain protein was shown to be a functional partner for TTX-3 in the AIA neurons. The Six4/5 homeodomain protein UNC-39 is continuously expressed in the AIA interneurons from their birth, throughout the animal’s life [6], making it a candidate TTX-3 cofactor. The only other site of UNC-39 expression is the lateral IL2 neuron subtype [6]. We found that unc-39(e257) mutant animals display strong defects in the expression of six out of six tested molecular markers of AIA identity, two neuropeptides (ins-1 and flp-19), the ACh vesicular transporter, unc-17, the choline reuptake transporter cho-1, a neuropeptide receptor, dmsr-2 and a metabotropic glutamate receptor, mgl-1 (Fig 2A), indicating that UNC-39 may indeed cooperate with TTX-3 to specify AIA identity.
Fig 2. unc-39 controls differentiation of the AIA interneuron class.
unc-39R203Q mutant animals (either canonical e257 allele or CRISPR/Cas9 genome engineered ot1173 allele with identical nucleotide change) were analyzed. Fig 2A: unc-39 affects the cholinergic identity of the AIA interneuron class (unc-17 reporter allele syb4491 and a cho-1 promoter fragment which is part of the otIs653 array), and other AIA terminal identity markers: reporter alleles dmsr-2(syb4514), ins-1(syb5452) and flp-19(syb3278), and a mgl-1 promoter fragment otIs327. We did not quantify changes in AIA in the NeuroPAL color code, because it is variable in wild type. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of neurons examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 2B: unc-39 affects the expression of the tagged eya-1 locus (nIs352 transgene) in AIA.
The Eyes Absent protein is a transcriptional co-factor for many SIX domain-type homeodomain proteins across phylogeny [7,22–26]. Despite the expression of several SIX domain family members in many different parts of the C. elegans nervous system [6], a genomic fragment that contains the entire, gfp-tagged eya-1 locus [27] is only expressed in pharyngeal neurons [7] and one single extra-pharyngeal neuron class, the AIA neuron class (Fig 2B). This pattern is supported by scRNA analysis [19]. unc-39 is required for proper eya-1 expression in the AIA neurons (Fig 2B). However, using two different markers (unc-17, flp-19), we observe no AIA differentiation defects in eya-1 null mutants (S4 Fig).
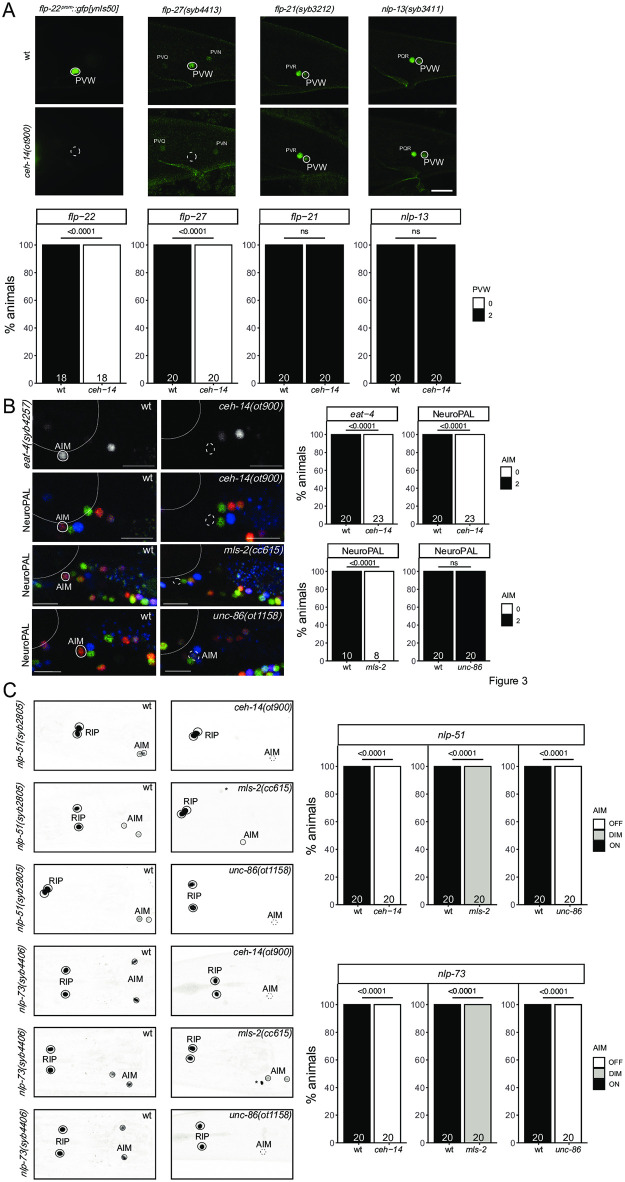
The LIM homeobox gene ceh-14 specifies distinct neuron classes
The PVW neuron pair is a peptidergic interneuron class located in the lumbar ganglion in the tail of the animal, with unknown function and no known identity regulator. The PVW neuron pair continuously expresses the C. elegans homolog of the vertebrate LHX3/4 LIM homeobox gene ceh-14 [6,28]. We assessed the peptidergic identity of PVW by analyzing the expression of four different neuropeptide-encoding genes that the CeNGEN scRNA atlas predicts to be expressed in PVW, flp-21, flp-22, flp-27 and nlp-13 [19]. To monitor flp-22 expression, we used a previously available promoter fusion transgene [29]. For the other three neuropeptides, an SL2::gfp::H2B or T2A::3xNLS::gfp cassette was inserted at the C-terminus of the respective loci. All four reporters show expected expression in PVW (Fig 3A). In ceh-14 null mutant animals, expression of two of the four neuropeptide-encoding genes (flp-22 and flp-27) is lost (Fig 3B).
Fig 3. ceh-14 affect differentiation of several neuron classes, in combination with different homeobox genes.
Fig 3A: ceh-14(ot900) mutant animals show a loss of neuropeptide-encoding gene expression in PVW, including a promoter fusion reporter transgene for flp-22 (ynIs50) and a flp-27 CRISPR reporter (syb4413), while expression of the neuropeptide CRISPR reporters for flp-21 (syb3212) and nlp-13 (syb3411) is unaffected. Neuron of interest is outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. Representative images of wild type and mutant worms are shown with 10 μm scale bar. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 3B: ceh-14(ot900) mutant animals show a loss of AIM marker expression, including an eat-4 CRISPR reporter (syb4257) and NeuroPAL (otIs669) in AIM. Additionally, unc-86(ot1158) as well as mls-2(cc615) mutant animals show expression defects of NeuroPAL (otIs669) in AIM. Neuron of interest is outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 3C: ceh-14(ot900) and unc-86(ot1158) mutant animals show a loss of neuropeptide-encoding gene expression in AIM using the CRISPR/Cas9-engineered reporter alleles nlp-51(syb2805) and nlp-73(syb4406). mls-2(cc615) mutant animals diminish, but do not extinguish expression in of nlp-51(syb2805) and nlp-73(syb4406) in AIM. Expression in RIP is unaffected by ceh-14(ot900), unc-86(ot1158), and mls-2(cc615). Neurons are outlined in solid black and a dashed black line represents loss of expression. Asterisks indicate ectopic expression of unidentified neurons. Graphs compare expression in wild type and mutant worms (on vs off or on vs dim) with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
We also analyzed ceh-14 function in a completely distinct set of interneurons in which CEH-14 protein is continuously expressed, the AIM neuron pair in the ventral ganglion of the head. We had previously shown that ceh-14 affects neurotransmitter identity of AIM [30]. We confirmed these defects using an eat-4/VGLUT reporter allele, generated by CRISPR/Cas9 genome engineering and a previously unavailable molecular null allele of ceh-14, ot900 (Fig 3B). We also analyzed expression of the NeuroPAL transgene, which expresses three markers for AIM identity, the ionotopic ACh receptor acr-5, the mbr-1 transcription factor and eat-4/VGLUT [15]. The NeuroPAL transgene also contains a synthetic panneuronal marker, which permits assessment of the generation of a neuron. We find that in ceh-14 null mutants, the neuron-type specific markers on the NeuroPAL transgene fail to be expressed in the AIM interneurons, while panneuronal markers are unaffected (Fig 3B). Lastly, using CRISPR/Cas9 genome engineering, we generated reporter alleles for two neuropeptide encoding genes, nlp-51 and nlp-73 for which the CeNGEN project detected expression in a small number of neurons, including AIM [19]. Both reporter alleles confirmed expression in AIM and showed loss of expression in AIM in ceh-14 mutants (Fig 3C). Together, this data strongly indicates that ceh-14 is a terminal selector of AIM identity.
Another homeobox gene continuously expressed in AIM is the HMX-type homeobox gene mls-2. mls-2 expression overlaps with ceh-14 exclusively in the AIM neurons. Previous work has shown that the neuropeptide-encoding gene, flp-10, fails to express in the AIM interneuron of mls-2 mutants [31]. To determine whether mls-2 has similar defects as ceh-14 mutants, we assessed expression of the NeuroPAL transgene in the AIM neurons of mls-2 mutants. We find that mls-2 mutants display the same color loss of the NeuroPAL transgene as ceh-14 mutants, suggesting that ceh-14 and mls-2 homeobox gene collaborate to instruct AIM identity (Fig 3B). Moreover, both AIM-expressed neuropeptide genes, nlp-51 and nlp-73 fail to be properly expressed in the AIM neuron of mls-2 mutants (Fig 3C).
Lastly, the BRN3 ortholog unc-86, a POU homeobox gene, was previously also shown to affect glutamatergic identity of the AIM neurons, as assessed by loss of eat-4/VGLUT fosmid reporter expression [32]. Consistent with a role of unc-86 in AIM differentiation, unc-86 null mutants also display NeuroPAL color code defects (Fig 3B). In this case, however, the wild type color composition of NeuroPAL in AIM (dim acr-5::mTagBFP, bright eat-4::mNeptune2.5, dim mbr-1::magenta) changes from bright red to a more blue color, which corroborates loss of eat-4/VGLUT expression, but indicates a potential increase of expression of acr-5 and mbr-1 expression. The two neuropeptides nlp-51 and nlp-73 lose expression in AIM in unc-86 mutants (Fig 3C). We conclude that unc-86 also affects AIM differentiation, albeit in a manner subtly distinct from the effect of ceh-14 and mls-2.
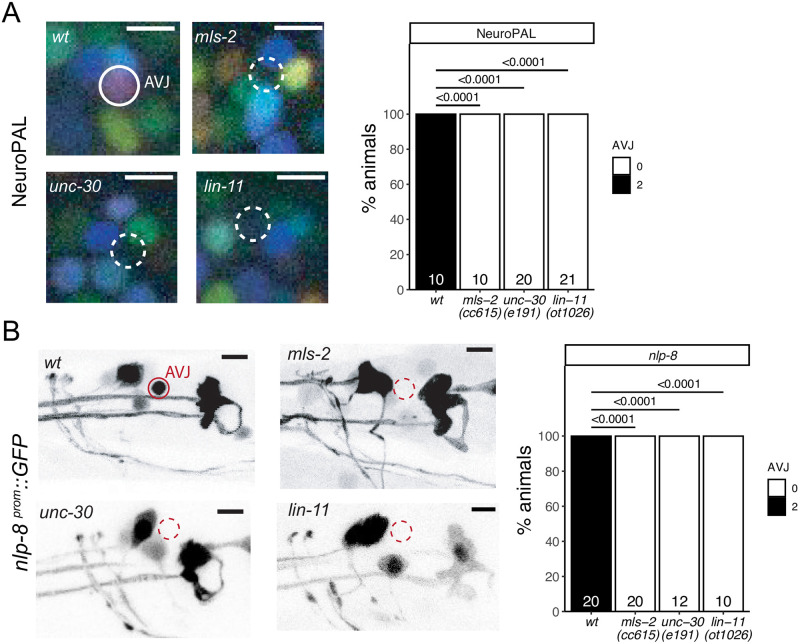
mls-2/HMX, unc-30/Pitx and lin-11/Lhx specify the previously uncharacterized AVJ neuron class
Apart from the AIM interneuron class, mls-2 is also continuously expressed in the AVJ neuron pair, an interneuron class in the lateral ganglion with no presently known function or identity regulator. The AVJ interneuron class appears morphologically very similar to its neighboring AVH neuron class, and AVJ has been commonly misidentified as AVH [33]. NeuroPAL provides a unique color code for AVJ, based on the expression of the neuropeptide-encoding flp-26 gene and the AMPA Glu receptor glr-1 [15,34]. mls-2 affects expression of these genes, but panneuronal marker expression remains unaffected, consistent with a role of mls-2 as a terminal selector of AVJ identity (Fig 4A). We further corroborated this notion by testing the expression of an additional neuropeptide gene, nlp-8, whose expression in AVJ was predicted by CeNGEN and confirmed with a reporter transgene (Fig 4B). We find proper nlp-8 expression in AVJ to be affected by loss of mls-2 as well (Fig 4B).
Fig 4. Three homeobox genes control the identity of the AVJ neuron class.
Fig 4A: mls-2 (cc615), unc-30 (e191) and lin-11(ot1026) mutant animals show defects in the expression of NeuroPAL (otIs669) in AVJ. Neuron of interest is outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. Representative images of wildtype and mutant worms are shown with 5 μm scale bars. Graphs compare expression in wildtype worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 4B: mls-2 (cc615), unc-30 (e191) and lin-11(ot1026) mutant animals show defects in the expression of an nlp-8 reporter transgene (otIs711) in AVJ. Neuron of interest is outlined in solid red when expressing wildtype reporter colors, and dashed red when one or all colors are lost. Representative images of wildtype and mutant worms are shown with 5 μm scale bars. Graphs compare expression in wildtype worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
In addition to mls-2, the AVJ neurons co-express the LIM homeobox gene lin-11 and the Pitx-type homeobox gene unc-30 [6]. This homeobox code is unique for the AVJ neurons [6]. While unc-30 and lin-11 were previously known to control the identity of other neuron classes [35–37], their function in AVJ has not been previously examined. Since the previously used lin-11 mutant alleles are not unambiguous molecular nulls [38], we generated such a null allele by deleting all coding sequences using the CRISPR/Cas9 genome engineering. Using NeuroPAL as a cell fate assessment tool, we found differentiation defects in unc-30 and lin-11 null mutants similar to those observed in mls-2 mutant animals (Fig 4A). Both unc-30 and lin-11 also affect expression of nlp-8 in AVJ (Fig 4B).
We also note that the AVJ gene battery shows an enrichment of phylogenetically conserved DNA binding sites of UNC-30 and MLS-2 [3], consistent with these factors acting as terminal selectors of neuron identity. LIN-11 binding sites are not specific enough to permit genome-wide analysis [3,39].
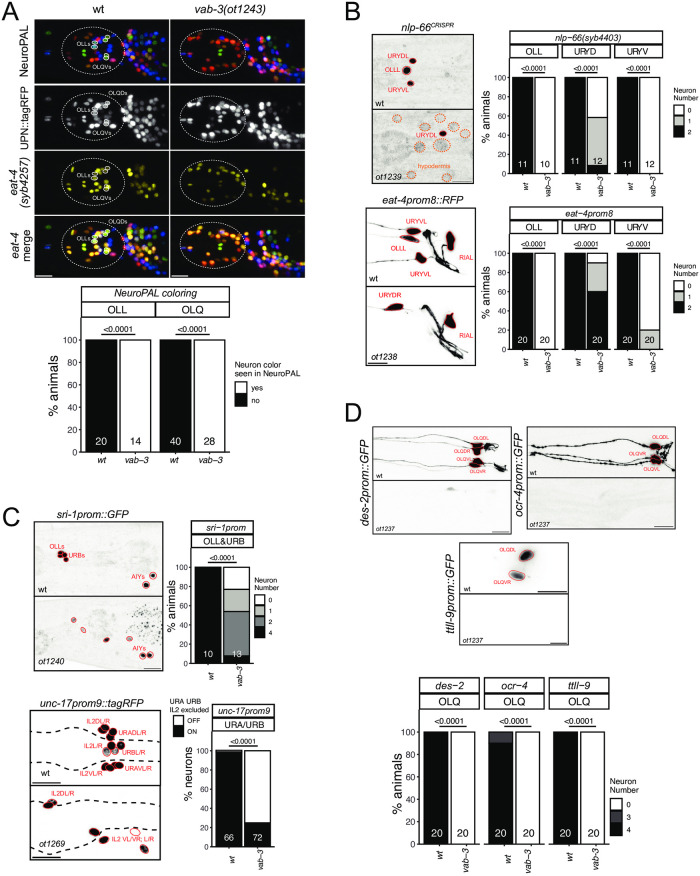
The Eyeless/Pax6 ortholog vab-3 is required for the differentiation of several anterior ganglion neurons
As described above (Fig 1), the Pax6/Eyeless ortholog vab-3 [40] is continuously expressed in a number of differentiating neurons, from their birth throughout their lifetime. vab-3 is most prominently expressed in many neuron classes of the most anteriorly located head ganglion, called the anterior ganglion (OLQ, URY, OLL, URA, URB, CEPV and, more strongly than in all other neurons, BAG). We have previously shown that vab-3 is required for the proper differentiation of the OLL neurons and the ventral URY neurons (URYV) [32], while others have shown a role in BAG neuron differentiation [41]. Seeking to extend this past analysis, we first used NeuroPAL to assess the differentiation program of anterior ganglion neurons in vab-3 mutant mutants. We note loss of neuron-type specific NeuroPAL color codes in many anterior ganglion neurons in vab-3 mutants, but still observe panneuronal marker expression Fig 5A), indicating that neurons are generated, but fail to properly differentiate into specific types. We could confidently identify the glutamatergic OLL and OLQ neurons to have lost their proper color code (Fig 5A). Consistent with this, an eat-4 reporter allele, expressed in all glutamatergic neurons in the anterior ganglion, shows loss of fluorescent signals in many neurons of this ganglion (Fig 5A).
Fig 5. The Eyeless/Pax6 ortholog vab-3 controls the identity of neurons in the anterior ganglion.
Fig 5A: In a vab-3(ot1243) mutant allele, many neurons in the anterior ganglion lose their NeuroPAL coloring (from otIs669) and expression of the eat-4 reporter allele (syb4257). Notably, there are much less blue neurons (URY/URA/URB but URX seem present), and the bright green OLQ and turquoise OLL are never seen. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of neurons (for n = 10 WT worms / 7 vab-3 mutant) examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Similar results were observed with a larger deletion allele, ot1269 (S7 Fig). Fig 5B: In vab-3 mutant worms (ot1239, ot1238, all carrying the same lesion, introduced into respective reporter background; S7 Fig), the OLL and URYmarkers nlp-66(syb4403), eat-4prom8 (otIs521) are affected. Markers are more frequently lost in the ventral URY than the dorsal URY. vab-3 mutants also ectopically express nlp-66 in hypodermal cells. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig. 5C: In vab-3 mutant worms (ot1240, ot1269; S7 Fig), URA and URB identities are affected as seen with the markers sri-1 (otIs879) (URB, OLL) and a promoter fragment of unc-17/VAchT (prom9; otEx7705) [105] expressed in IL2/URA/URB. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals (sri-1) or neurons (unc-17prom9) examined listed at the bottom of the bar. vab-3 does not affect expression of an unc-17 reporter allele in the IL2 neurons, and we can therefore infer that the remaining positive cells labeled with unc-17prom9, are the IL2 neurons and the lost expression is in URA/URB. P-values were calculated by Fisher’s exact test. Fig 5D: Markers of OLQ neuron identity (ocr-4 kyEx581, ttll-9 otIs850 and des-2 otEx7697) are fully lost in the vab-3(ot1237) mutant animals. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
Due to the overall disorganization of vab-3 mutant heads, a consequence of epidermal morphogenesis defects [42], we sought to generate and examine markers that are more specifically, if not exclusively expressed in vab-3(+) neurons, so that marker loss could be more unambiguously assigned to a specific neuron class. To identify such markers, we made use of the scRNA CenGEN atlas [19]. A neuropeptide encoding locus, nlp-66, shows highly enriched and restricted scRNA expression in URY and OLL. CRISPR/Cas9 genome engineering was used to insert an SL2::gfp::H2B reporter cassette at the 3’end of the nlp-66 locus. As predicted, this reporter allele showed strong expression in OLL and weaker expression in both dorsal and ventral URY neuron pairs (Fig 5B). Expression of this reporter is eliminated in vab-3 mutant animals (Fig 5B). Intriguingly, nlp-66::gfp reporter allele expression can be observed in what appear to be epidermal cells of vab-3 mutant animals (Fig 5B). Brandt et al. reported a similar ectopic expression of BAG markers ets-5 and flp-17 in epidermal cells of vab-3 mutants [41]. OLL and URY also show defects in expression of the glutamatergic marker eat-4 in vab-3 mutants (Fig 5B). This observation corrects previous analysis in which we had used a non-integrated version of the same eat-4 reporter construct, but had mistaken URY for OLQ [32].
According to the scRNA atlas [19], as well as previous reporter gene studies [43], the G-protein coupled receptor sri-1 is expressed in the OLL, URB and AIY neurons. We find that a chromosomally integrated sri-1 promoter fusion shows decreased expression in OLL and URB of vab-3 mutant animals (Fig 5C). Similarly, cholinergic identity of the URB neuron (measured with unc-17/VAchT expression) is affected, as is cholinergic identity of the URA neurons (Fig 5C).
The CeNGEN scRNA atlas also identified genes with very restricted expression in another vab-3(+) neuron class, the four radially symmetric OLQ sensory neurons. For example, transcripts for the ttll-9 gene, encoding a tubulin tyrosine ligase-like gene, appear to be exclusive to the OLQ neurons [19]. A reporter gene fusion using 500 bp of promoter sequences confirms OLQ-exclusive expression (Fig 5D). Expression of this reporter is completely lost in vab-3 mutant animals (Fig 5D). The ocr-4 gene, encoding a TRP channel, was previously reported to be exclusively expressed in the OLQ neurons [44] and we found this expression to be largely eliminated in vab-3 mutants (Fig 5D). Lastly, in the course of dissecting the cis-regulatory control regions of the des-2 locus, which encodes a degenerin-type ion channel [45], we isolated a 1.3 kb fragment that drives exclusive reporter expression in the OLQ neurons (Fig 5D). This transgene also fails to be expressed in vab-3 mutant animals. We conclude that vab-3 is required for the proper execution of most, if not all neuronal differentiation programs of the anterior ganglion in which vab-3 is normally continuously expressed.
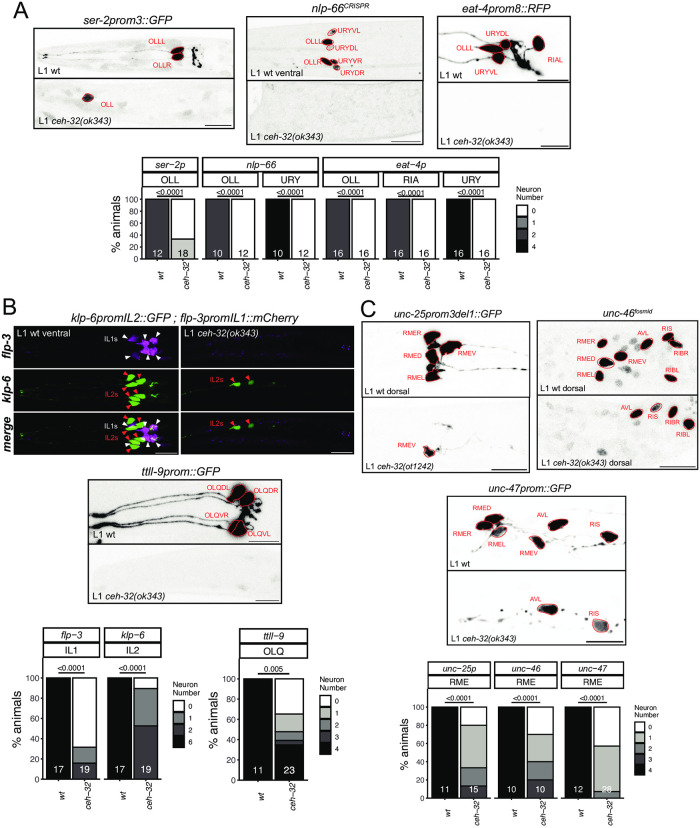
The SIX3/6 ortholog ceh-32 is also required for the differentiation of several anterior ganglion neurons
The function of PAX and SIX homeobox genes is tightly intertwined in the context of development of anterior sensory structures, e.g. in eye development [46]. In C. elegans, vab-3/PAX6 and ceh-32/SIX3/6 function have been linked in the context of head morphogenesis [47]. The function of ceh-32 in terminal neuron differentiation has only been reported in lateral ganglion neurons that express this gene (RIA, RMDD/V) [6, 18], but not in several anterior ganglion neuron classes in which ceh-32 and vab-3 are co-expressed throughout the neurons’ lifetime. We find that ceh-32 phenocopies the differentiation defects of the OLL and URY neurons observed in vab-3 mutants. Both nlp-66 expression, as well as expression of the glutamatergic marker eat-4 and the tyramine receptor ser-2 are lost in the OLL and URY neurons of ceh-32 mutants (Fig 6A).
Fig 6. The SIX3/6 ortholog ceh-32 controls the identity of neurons in the anterior ganglion.
Fig 6A: In ceh-32(ok343) null mutant animals, OLL, URY but also RIA markers are almost always lost (ser-2prom3 otIs138, nlp-66(syb4403), eat-4prom8 otIs521). Fig 6B: ceh-32 also controls IL1 identity (flp-3 / otIs703 marked with white triangles), and affects neurons where it is not expressed in adults (IL2: klp-6 myIs13 marked with red triangles; OLQ: ttll-9 otIs849). Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 6C: In ceh-32(ok343) and ceh-32(ot1242) null mutant animals, the GABAergic identity of all RME classes is affected (unc-25prom3del1 otIs837, unc-47 oxIs12, unc-46 fosmid otIs568). ceh-32 is expressed in adults in both RME and RIB, but ceh-32 loss did not affect RIB identity (unc-46 otIs568). Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
The glutamatergic IL1 sensory/motor neurons, another ceh-32(+) neuron class in the anterior ganglion, also display differentiation defects in ceh-32 mutants. Specifically, we find that expression of the neuropeptide gene flp-3 is lost in the IL1 neurons (Fig 6B). A similar defect has been observed in vab-3 mutants [48]; however, since vab-3 is not expressed throughout IL1 development [6], its function may be restricted to earlier progenitor stages, perhaps upstream of ceh-32. A potential progenitor role is also evident for ceh-32 in the OLQ and IL2 neuron classes. Both neuron classes fail to properly express differentiation markers in ceh-32 mutants (Fig 6B). While there is no robust expression of ceh-32 in postembryonic OLQ and IL2 neurons [6], ceh-32 expression is evident in the embryonic lineage that generates both OLQ and IL2 neurons [20].
Apart from the mature anterior ganglion neuron classes OLL, URY and IL1, ceh-32 is expressed in a number of additional postmitotic neurons, including the GABAergic RME motor neurons, which are also located in the anterior ganglion and which do not express vab-3. A nuclear hormone receptor, nhr-67, but no homeobox gene was previously shown to affect the identity of all RME neurons [49, 50]. Using three distinct markers of terminal GABAergic identity, unc-25/GAD, unc-46/LAMP and unc-47/VGAT, we find that ceh-32 affects the identity acquisition of the RME neuron class (Fig 6C). GABAergic neuron identity was not affected in the RIB neurons of ceh-32 mutant animals, which normally also express ceh-32 (Fig 6C). Similarly, expression of the RIB-specific sto-3 marker was also unaffected in the RIB neurons of ceh-32 mutants. We conclude that vab-3 and ceh-32 are required for the proper terminal differentiation of a partially overlapping set of neurons in the anterior ganglion.
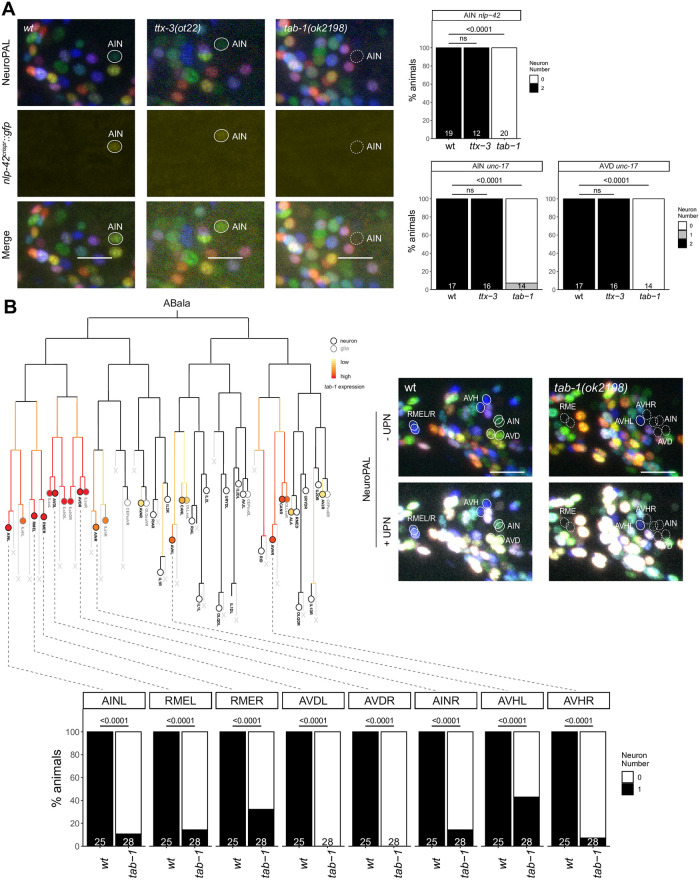
tab-1 functions in a lineage that produces the AIN and AVD neurons
The cholinergic AIN interneuron class is another neuron class for which no identity specifier has been previously identified. The AIN neuron pair is labeled with three markers by NeuroPAL (flp-19, cho-1 and mbr-1) and, being cholinergic, also expresses unc-17/VAChT [30]. In addition, the CeNGEN scRNA atlas reported very strong and selective expression of a neuropeptide, nlp-42, in AIN. We tagged the nlp-42 locus with a T2A-based reporter cassette and confirmed selective expression in AIN (Fig 7). Armed with these markers we first considered the LIM homeobox gene ttx-3, which is continuously expressed in AIN [6, 14] and which is known to control the cholinergic identity of two other amphid interneuron (“AI”) classes, the AIY and AIA interneurons [12,14]. We found no obvious AIN differentiation defects in ttx-3 mutants, as assessed with NeuroPAL, the nlp-42 reporter allele and unc-17 fosmid-based reporter (Fig 7A). Another homeobox gene expressed in the postmitotic AIN neurons is tab-1, a deeply conserved homeobox gene homologous to the Drosophila Brain-specific homeobox (Bsh) gene [51] and vertebrate Bsx genes [52]. We found that NeuroPAL as well as nlp-42 and unc-17/VAChT signals were absent in the AIN neurons of tab-1 mutants (Fig 7A). The only other cholinergic neuron that expresses tab-1 postmitotically throughout their life is the AVD command interneuron class. The NeuroPAL and unc-17/VAChT signals are absent in the AVD neurons of tab-1 mutants as well (Fig 7A).
Fig 7. tab-1 regulates the differentiation of various neurons in the ABala lineage.
Fig 7A: In tab-1(ok2198) mutants, expression of both nlp-42(syb3238) and NeuroPAL reporters in AIN is lost. tab-1(ok2198) mutants also showed defects in unc-17(otIs576) reporter expression in AIN and AVD. No loss of reporter expression was observed in ttx-3(ot22) mutants. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Fig 7B: tab-1 is expressed in various neurons derived from the ABala lineage (adapted from Ma et al., 2021). In tab-1(ok2198) mutants, defects in NeuroPAL reporter expression, including ultrapanneuronal (UPN) reporter expression, are seen in neurons which express tab-1 embryonically. Representative images of wild type and mutant worms are shown with 10 μm scale bars. In all panels, neurons of interest are outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. P-values were calculated by Fisher’s exact test.
Notably, the panneuronal signal from the NeuroPAL transgene is also not expressed in the AIN and AVD neurons of tab-1 mutants, suggesting a proneural role of tab-1. The AIN and AVD neurons are generated from the ABalaa progenitor lineage [53] (Fig 7B). Embryonic expression of tab-1 is observed very early throughout the entire ABalaa progenitor lineage [20] (Fig 7B), indicating that tab-1 may have an early role in controlling progenitor fate in this lineage. Consistent with this notion, another class of neurons generated from the ABalaa lineage, the lateral RME neuron pair also require tab-1 for their proper differentiation [50](Fig 7B). Outside the ABalaa lineage, tab-1 is also expressed in neuroblasts that generate the AVH neurons, a neuron class that employs unc-42 and hlh-34 as terminal selectors [17]. These neurons also do not acquire any NeuroPAL color code in tab-1 mutants (Fig 7B). Since tab-1 is not continuously expressed in AVH [6], we again surmise an early, rather than late terminal differentiation/maintenance function of tab-1.
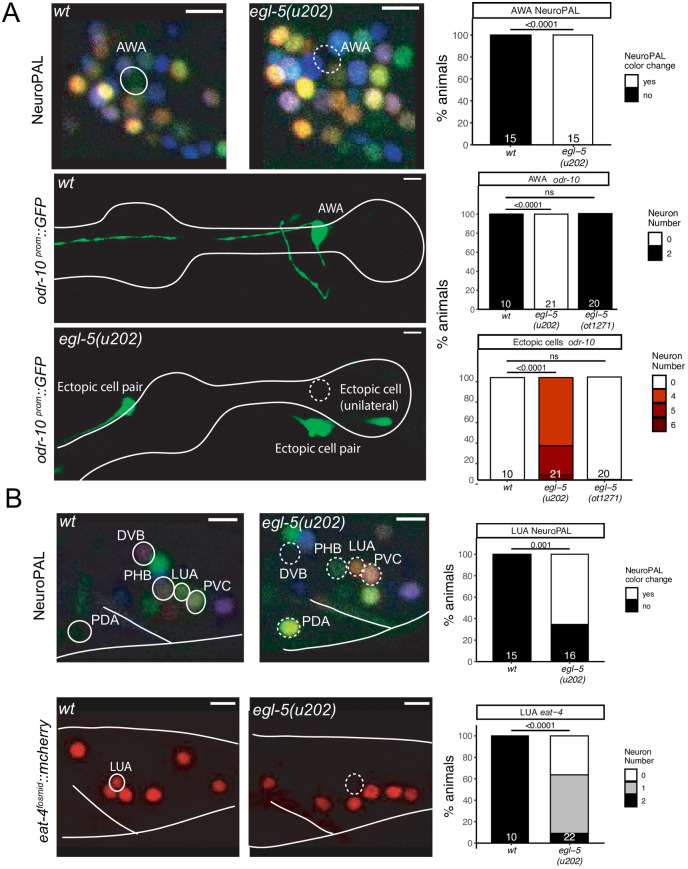
The egl-5 HOX gene is required for neuronal identity specification in head and tail neurons
The expression of HOX cluster genes is traditionally thought to be absent in anterior cephalic structures [54]. We were therefore intrigued to observe expression of the egl-5 HOX cluster gene, the C. elegans homolog of posterior Abdominal-B-type HOX genes that normally pattern posterior structures [55,56], in the AWA olfactory sensory neuron pair in the head of the animal [6]. The identity of this neuron was previously shown to be controlled by the odr-7 nuclear hormone receptor, which regulates the expression of the olfactory receptor ODR-10 [57], as well as other identity features of AWA [3]. We found that in animals carrying the canonical egl-5 allele u202 [55], odr-10 expression is also lost from AWA neurons and so is the characteristic NeuroPAL color code for AWA (Fig 8A). We instead observed ectopic expression of odr-10 in other, positionally very distinct neuron classes in the head of egl-5(u202) mutants, indicating neuronal identity transformations (Fig 8A). However, these defects may be due to a neo- or antimorphic activity of egl-5(u202) since we did not observe odr-10 expression defects in animals in which we deleted the entire egl-5 locus using CRISPR/Cas9 genome engineering (Fig 8A).
Fig 8. The HOX gene egl-5 affects the differentiation of head and tail neurons.
Fig 8A: egl-5(u202) mutant animals show a loss of AWA marker expression, including NeuroPAL (otIs669) and an odr-10 reporter transgene (kyIs37). Representative images of wildtype and mutant worms are shown with 5 μm scale bars. Graphs compare expression in wildtype and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 8B: egl-5(u202) mutant animals show changes in tail marker expression, including NeuroPAL (otIs669) in PDA, LUA, PVC and loss of eat-4 (otIs518) expression in LUA. Representative images of wildtype and mutant worms are shown with 5 μm scale bars. Graphs compare expression in wildtype and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. In all panels, neurons of interest are outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost.
Since our homeodomain protein expression atlas also detected the expression of two Otx-type homeodomain proteins in the AWA neurons, ceh-36 and ceh-37 [6], we tested whether they affect AWA differentiation, but found this not to be the case (S5 Fig). Also, ceh-36 and ceh-37 single mutants displayed no apparent defects in the differentiation of the ASI neurons, another neuron class in which our homeodomain protein expression atlas found these proteins to be expressed (S5 Fig).
As expected from its homology to AbdB, a posterior HOX gene, egl-5 is also expressed in a number of neurons in the tail of the worm [6, 56, 58] and we find that egl-5(u202) affects the proper specification of a number of them. Specifically, the LUA interneuron, for which no previous identity regulator was known, shows defects in NeuroPAL color coding, as well as a loss of expression of the eat-4 reporter allele (Fig 8B). NeuroPAL color code changes were also observed in the PDA and PHB neurons, suggesting that their identity is not properly executed either (Fig 8B). PDA and PHB differentiation defects of egl-5(u202) mutants were also recently reported [59], while this work was under preparation for publication. Other apparent NeuroPAL color changes in the tail of egl-5 mutant animals are likely reflections of neuronal identity changes in the most posterior class member of ventral nerve cord motor neurons (e.g., DA9, VA12), that we previously reported with more cell-type specific markers in egl-5 mutants [60] and that were corroborated by a more recent study [59].
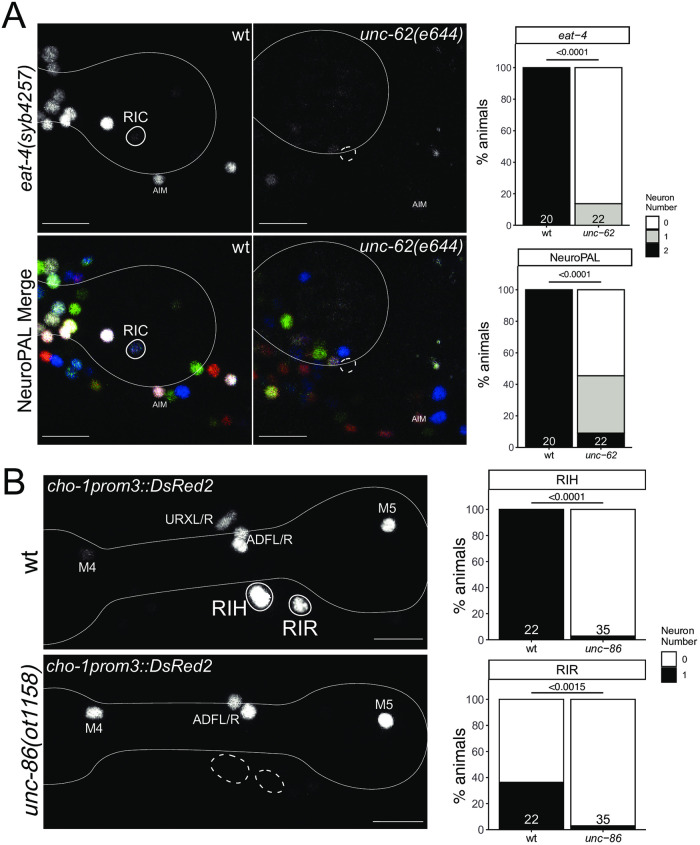
The RIC neuron class requires unc-62/MEIS for proper differentiation
The ring interneuron (“RI”) class RIC is the only octopaminergic neuron class in C. elegans [61]. In addition to synthesizing octopamine (using the biosynthetic enzymes TBH-1 and TDC-1), we also found that RIC uses glutamate as a neurotransmitter. Our original mapping of the sites of eat-4/VGLUT expression had not identified detectable expression in RIC [32]. However, prompted by the detection of eat-4/VGLUT transcripts in our scRNA brain atlas [19], we used CRISPR/Cas9 to insert an SL2::gfp:H2B reporter cassette into the eat-4/VGLUT locus. This reporter allele shows the same cellular sites of expression as the previously published fosmid-based reporter, but also revealed expression in RIC (Fig 9A).
Fig 9. Ring interneuron (RIC, RIH, RIR) differentiation defects in homeobox gene mutants.
Fig 9A: unc-62(e644) mutant animals show a loss of RIC marker expression, including an eat-4 CRISPR reporter (syb4257) and NeuroPAL (otIs669). Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 9B: unc-86(ot1158) mutant animals show loss of RIH and RIR marker expression of an extrachromosomal cho-1prom3 reporter (otEx4530) [105]. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. In all panels, neurons of interest are outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. P-values were calculated by Fisher’s exact test.
Neuronal differentiation defects of the RIC neurons were previously reported in animals lacking two non-homeobox genes, zip-5 and nhr-2 [62], but no regulator of the homeobox family was previously known. RIC expresses the MEIS homeobox gene unc-62 [6]. We found that in unc-62(e257) mutants, eat-4::gfp expression in RIC is lost (Fig 9A). Moreover, the fate signature of the RIC neurons in NeuroPAL (combination of ggr-3 and mbr-1) [15] is eliminated in unc-62 mutants. Panneuronal marker expression is unaffected, indicating that unc-62 does not control the generation, but rather the identity specification of the RIC neurons. We also note that expression of tbh-1, encoding a tyramine-beta hydroxylase involved in octopamine biosynthesis [61] is not affected in unc-62(e257) animals, but since this allele is a non-null allele (the null allele is early embryonic lethal; [63]), this lack of defect is difficult to interpret.
The cholinergic RIH and RIR interneurons require the unc-86 homeobox gene for proper differentiation
Unlike the bilaterally symmetric RIC neuron pair, two other ring interneuron classes, the RIH and RIR neurons, are unpaired single neurons. Both neurons have a branched main process that projects into both sides of the nerve ring, but are very distinct in synaptic connectivity [1] and molecular profile [19](S2 Fig). However, both neurons are cholinergic and express the POU homeobox gene unc-86/BRN3 [64]. Previous work has shown that the serotonergic co-transmitter identity of RIH is controlled by unc-86 [65]. Cholinergic neurotransmitter identity of RIH is also affected by loss of unc-86 (Fig 9B) [30]. Similarly, the RIR neuron, previously entirely unstudied, also fails to express a key cholinergic neurotransmitter gene (cho-1/ChT) in unc-86 mutants (Fig 9B). A neuropeptide that is expressed in RIR, nlp-52, is, however, not affected by unc-86 (n = 12).
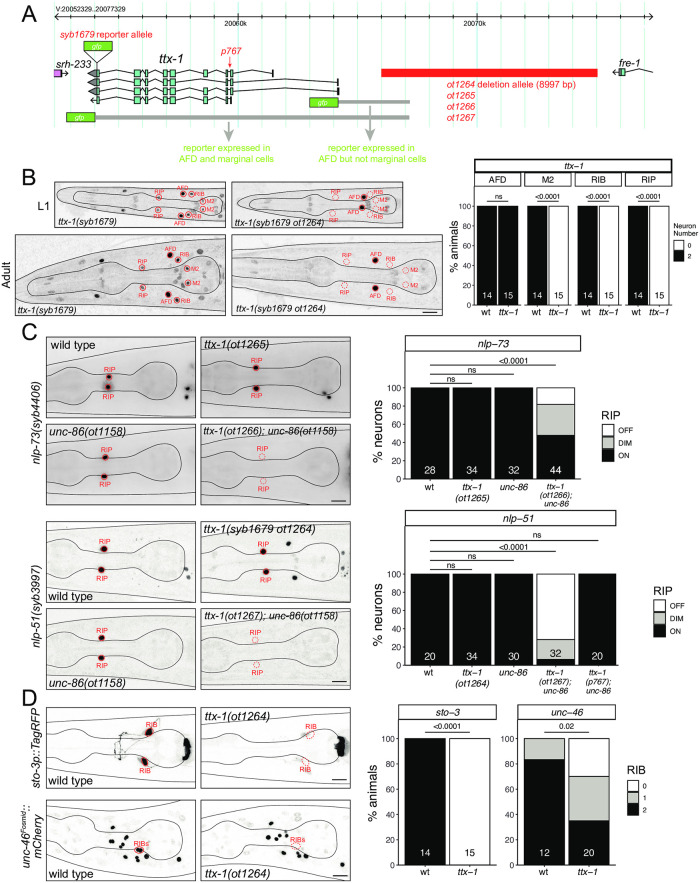
Differentiation of the ring interneurons RIP and RIB is affected by the OTX-type homeobox gene ttx-1
The RIP neurons are another ring interneuron pair that express unc-86. RIP is the only neuron that connects the main nervous system to the pharyngeal nervous system [1, 66], but both its function and its developmental specification have remained unexplored. Few molecular markers existed before the advent of the scRNA CeNGEN atlas. From the RIP-specific genetic signature that CeNGEN revealed [19], we selected two neuropeptide encoding genes that showed highly selective, strong transcript enrichment in RIP, the nlp-51 and nlp-73 genes, which aside from RIP, are also expressed in AIM (Fig 3C). CRISPR/Cas9 genome engineered nlp-51 and nlp-73 reporter alleles confirmed expression in RIP (Fig 10C). However, unc-86 null mutants displayed no defects in nlp-51 and nlp-73 expression in RIP (Fig 10C).
Fig 10. The OTX-type homeobox gene ttx-1 affects the differentiation of the ring interneurons RIP and RIB.
Fig 10A: ttx-1 locus showing different alleles used in this study. Fig 10B: The ttx-1(ot1264) cis-regulatory allele loses ttx-1(syb1679) expression in RIP, RIB and M2 neurons. Representative images of wild type and mutant worms at the L1 and adult stage are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 10C: ttx-1 and unc-86 show synergistic effects in RIP differentiation. Representative images of nlp-73(syb4406) and nlp-51(syb3997) reporter allele expression in wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of neurons examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. Fig 10D: The ttx-1(ot1264) cis-regulatory allele affects RIB differentiation. Representative images of sto-3 (otIs810) and unc-46 (otIs568) reporter transgene expression in wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
We found that the Otx-type homeobox gene ttx-1 is also expressed in RIP [6]. We first examined a previously described, viable splice site allele of ttx-1, p767, which displayed defects in AFD neuron differentiation [67]. In an unc-86(ot1158); ttx-1(p767) double mutant strain nlp-51 expression remains unaffected (Fig 10C). However, since we found that a complete deletion of the ttx-1 null locus results in embryonic lethality, the viable p767 allele is not a null allele. To circumvent embryonic lethality, we sought to eliminate ttx-1 function from the RIP neurons by generating cis-regulatory alleles of the ttx-1 locus. Previous reporter analysis had located cis-regulatory elements that drive ttx-1 expression in the AFD and the pharyngeal marginal cells to regions directly upstream of ttx-1 (AFD) and the introns of ttx-1 (marginal cells) [67](Fig 10A). Since the ttx-1 reporter allele that we generated revealed additional sites of ttx-1 expression in multiple neuron types in addition to AFD (RIP, RIB and M2) [6], we inferred that regulatory elements required for RIP, RIB and M2 expression are located more distally to the ttx-1 start site. Using CRISPR/Cas9 genome engineering, we deleted 9 kb of sequences 2 kb upstream of the ttx-1 start site, in a strain in which the ttx-1 locus is tagged with gfp (Fig 10A). We found that in the resulting animals, ttx-1(syb1679 ot1264), ttx-1::gfp expression in RIP, as well as the RIB and M2 neurons, is indeed eliminated (Fig 10B). While these animals still display no defects in nlp-51 and nlp-73 expression in RIP, expression of both neuropeptides is now indeed reduced upon simultaneous removal of unc-86 (Fig 10C). Similar redundant effects of unc-86 with another homeobox gene have been identified in other neuron classes as well, e.g. the NSM neurons [7, 14].
Since the cis-regulatory allele of ttx-1 also eliminates ttx-1 expression in RIB, a GABAergic interneuron class with no previously known identity regulator, we assessed the expression of two markers of RIB identity, unc-46 (involved in trafficking the vesicular GABA transporter) [68] and sto-3 (a stomatin-like gene) [69]. We found that expression of both reporters is severaly affected in animals carrying the ttx-1 cis-regulatory allele (Fig 10D).
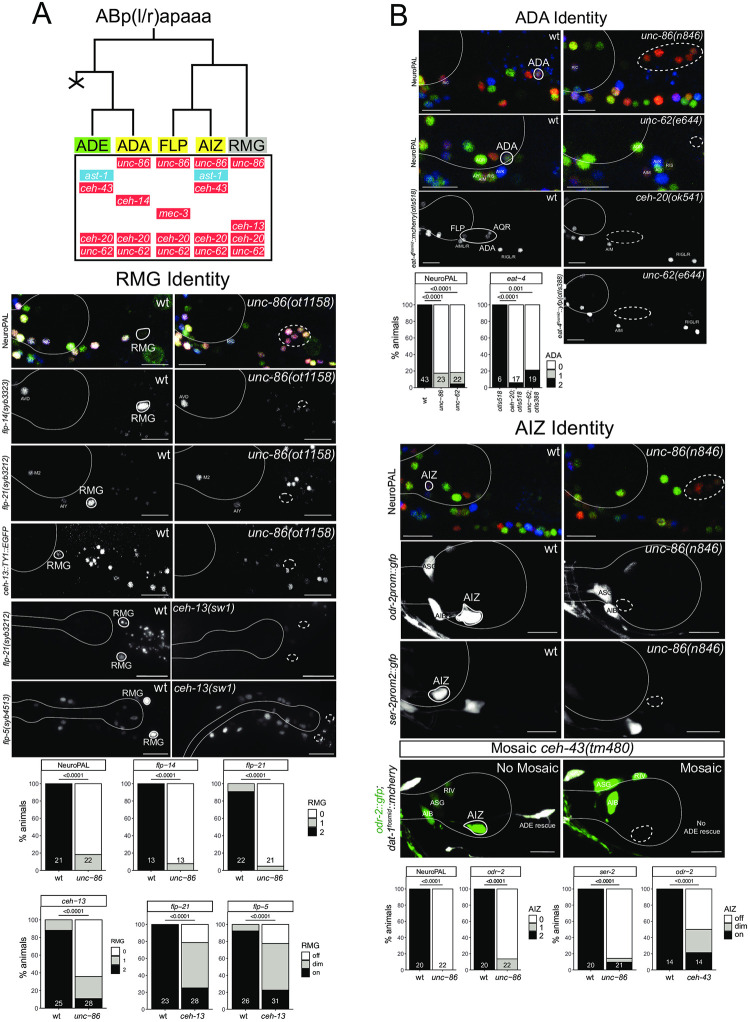
Homeobox genes affect the differentiation of neurons within the anterior deirid lineage
The glutamatergic, functionally and developmentally uncharacterized ADA neuron class is produced by the anterior deirid lineage (Fig 11A). The NeuroPAL transgene contains three molecular markers of ADA identity, eat-4/VGLUT, the acetylcholine receptor subunit acr-5 and the neuropeptide flp-26 [15]. The POU homeobox gene unc-86/BRN3 is expressed in ADA [64] and given the role of unc-86/BRN3 as terminal selector in several other neurons classes [70](this paper), we analyzed NeuroPAL expression in the ADA neurons of unc-86 null mutants. We found that the NeuroPAL fate signature for ADA is lost in unc-86 mutants (Fig 11B). Based on its continuous expression in all neurons of the anterior deirid, including ADA (Fig 11A), we considered the sole MEIS-type homeobox gene ortholog in C. elegans, unc-62, as a collaborator of UNC-86. Indeed, we observed the same NeuroPAL defects in the ADA neurons of unc-62 mutants as we observed in unc-86 mutants (Fig 11B). MEIS homeobox genes usually heterodimerize with PBX-type homeobox genes, of which there are three in C. elegans [63]. One of the three C. elegans PBX genes, ceh-20, is co-expressed with unc-62 in all neurons of the anterior deirid lineage, including the ADA neurons [6](Fig 11A). Using an eat-4/VGLUT fosmid reporter, we found that ADA displays differentiation defects in both unc-62 and ceh-20 mutant animals (Fig 11B). At least one other glutamatergic neuron class in the anterior lineage (FLP), as well as the adjacent AQR neuron also fail to acquire glutamatergic identity (i.e. eat-4/VGLUT expression) in unc-62/MEIS and ceh-20/PBX mutants (Fig 11B).
Fig 11. POU, MEIS and HOX genes control neuron identity in the anterior deirid lineage.
Fig 11A: Lineage diagram depicting the generation of the anterior deirid neurons. Shown below each neuron in the lineage are the transcription factors known to be expressed in each of the respective neurons. Expression patterns for homeodomain proteins (all except AST-1) in the lineage are from [6, 91] and AST-1 is from this paper (S6 Fig). Green shading: dopaminergic neuron; yellow shading: glutamatergic neuron, grey shading: peptidergic neuron, red shading: homeobox gene, blue shading: non-homeobox gene. Fig 11B: Marker analysis in the anterior deirid lineage of wild-type and mutant animals. ADA Identity: unc-62(e644) and unc-86(n846) mutant animals show a loss of NeuroPAL (otIs669) in ADA. Additionally, unc-86(n846) mutant animals show a loss of an eat-4 fosmid reporter (otIs518) in ADA. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. With two different eat-4 fosmid based reporters, unc-62 and ceh-20 mutants also show differentiation defects in other neurons in the location of the anterior deirid, besides ADA (namely, FLP and AQR neurons). RMG Identity: unc-86(ot1158) mutant animals show a loss of RMG marker expression, including NeuroPAL (otIs669), a flp-14 CRISPR reporter (syb3323), a flp-21 CRISPR reporter (syb3212), and a ceh-13 fosmid reporter (wgIs756). ceh-13(sw1) mutant animals show defects in the expression of two CRISPR reporters, flp-5(syb3212) and flp-5(4513), in RMG. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. AIZ Identity: unc-86(n846) mutant animals show a loss of expression of NeuroPAL (otIs669) and an odr-2 reporter transgene (kyIs51) and defects in the expression of a ser-2 reporter transgene (otIs358) in AIZ. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs for NeuroPAL and odr-2 compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. Graphs for ser-2 compare the brightness of expression (on, dim, off) in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. In all panels, neurons of interest are outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost. P-values were calculated by Fisher’s exact test.
Being part of the so-called anterior deirid lineage, the ADA neuron is lineally related to another interneuron whose differentiation program was previously uncharacterized, the RMG interneuron class (Fig 11A). RMG is a so-called “hub-and-spoke” neuron that integrates signals from various sensory neurons [71]. The RMG neuron pair expresses no classic, fast acting neurotransmitter pathway machinery, i.e. is neither cholinergic, glutamatergic, GABAergic or monoaminergic, but expresses a combination of neuropeptide-encoding genes [19, 29, 71]. Like ADA, the RMG neuron class also co-expresses unc-86 and the Meis and Pbx homologs unc-62 and ceh-20 [6] (Fig 11A). Like in ADA, we find that unc-86 is required for RMG differentiation, as assessed by its effect on neuropeptide gene expression (Fig 11B). Unlike ADA, the RMG neuron expresses the anterior most HOX cluster gene ceh-13, the C. elegans ortholog of Labial [6] (Fig 11A), which may act to distinguish ADA from RMG. We indeed found that ceh-13 null mutant animals fail to properly express the neuropeptides flp-5 and flp-21 (Fig 11B).
Lastly, the DLX ortholog ceh-43 is selectively expressed in two neuron classes of the anterior lineage, the dopaminergic ADE sensory neurons and the glutamatergic AIZ interneuron [6]. We had previously shown that ceh-43 is required to specify ADE differentiation [13] and we find that loss of ceh-43 also affects AIZ differentiation (Fig 11B).
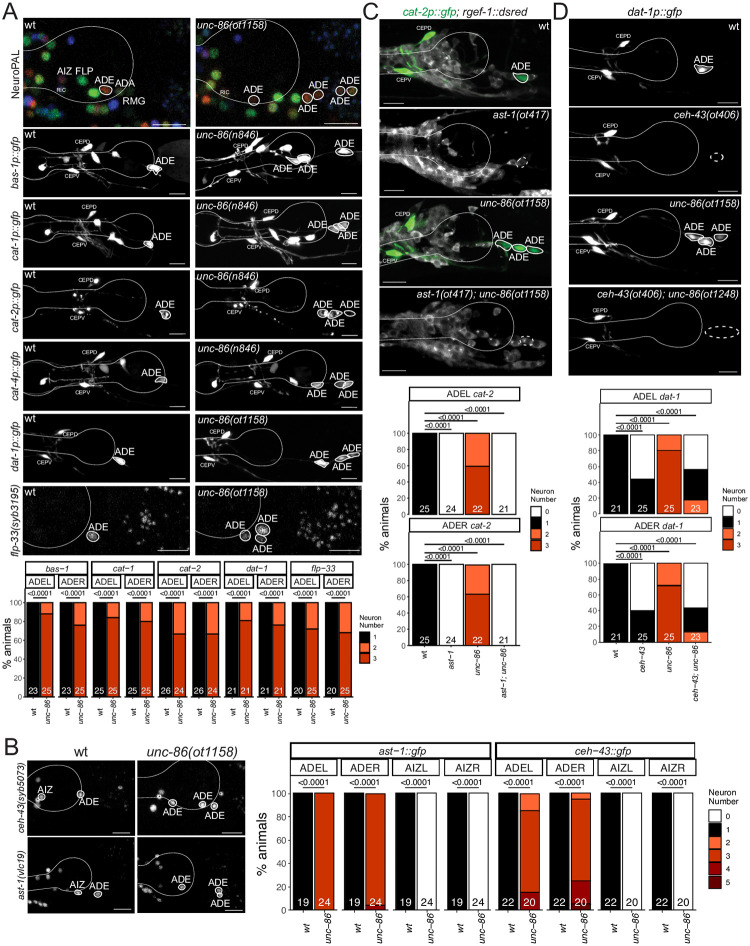
unc-86 mutants display homeotic identity transformations in the anterior deirid lineage
Apart from ADA and RMG, other neurons within the anterior deirid lineage, specifically the AIZ and FLP neurons, also express the unc-86/BRN3 homeobox gene (Fig 11A). Both neurons were previously shown to require unc-86 for their proper differentiation [32,72]. This notion is further corroborated with individual cell-type specific markers, as well as the NeuroPAL transgene; all normally UNC-86(+) neurons in this lineage fail to acquire the proper color code in unc-86 null mutants (Fig 11B). Notably, NeuroPAL reveals that these cells instead now adopt the color that is characteristic for the one non-UNC-86-expressing neuron in this lineage, the dopaminergic ADE neuron, marked with dat-1 in the NeuroPAL transgene (Fig 11B). Upon the initial isolation of unc-86 mutant alleles, additional cells with ectopic FIF staining, a chemical stain for dopamine, were noted in the anterior deirid lineage of unc-86 mutant animals [73].
We further probed the identity of the “ectopic” NeuroPAL/FIF-positive neurons by testing five markers of the dopamine biosynthesis and vesicular packaging pathway, bas-1/AAAD, cat-1/VMAT, cat-2/TH, cat-4/GTPCH, and dat-1/DAT. We found all genes to be ectopically expressed throughout the anterior deirid lineage of unc-86 mutants (Fig 12A). Another marker for ADE fate that is independent of the dopamine biosynthesis pathway is the flp-33 neuropeptide, which we also find to be ectopically expressed throughout the anterior deirid lineage of unc-86 mutants (Fig 12A). Similarly, the flp-5 gene, normally expressed in ADE, FLP and RMG also becomes expressed in more cells throughout the anterior deirid lineage.
Fig 12. Derepression of dopaminergic terminal feature and dopaminergic regulatory signature in unc-86 mutants.
Fig 12A: unc-86(ot1158) and unc-86(n846) mutant animals ectopically express markers of ADE identity, including NeuroPAL (otIs669), reporter transgenes for genes involved in dopamine synthesis, including bas-1 (otIs226), cat-1 (otIs224), cat-2 (nIs118), cat-4(otIs225) and dat-1(vtIs1), and a flp-33 CRISPR reporter (syb3195). Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression on the left side (ADEL) and the right side (ADER) in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. Fig 12B: unc-86(ot1158) mutant animals show a derepression of CRISPR/Cas9-engineered reporter alleles of ceh-43(syb5073) and ast-1(vlc19) in cells of the anterior deirid lineage. Representative images of wild type and mutant worms are shown with 10 μm scale bars (non-neuronal expression depicted with an asterisk). Graphs compare expression on the left side (ADEL and AIZL) and the right side (ADER and AIZR) in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. Fig 12C, FigD: ast-1 and ceh-43 are epistatic to unc-86. The derepression of expression of the cat-2 reporter transgene (otIs199) in unc-86(ot1158) mutant is suppressed in an ast-1(ot417) or an ast-1(ot406) mutant background. Note that both alleles are hypomorphic alleles (null alleles are lethal) [13,78]. Neurons of interest are outlined in solid white when expressing WT reporter colors, and dashed white when one or all colors are lost. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression on the left side (ADEL) and the right side (ADER) in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. In all panels, neurons of interest are outlined in solid white when expressing wildtype reporter colors, and dashed white when one or all colors are lost and P-values were calculated by Fisher’s exact test.
We examined whether ectopic dopaminergic fate execution in the anterior deirid linage are a reflection of aberrant cell divisions leading to lineage reiterations, akin to those observed in the postdeirid lineage of unc-86 mutants [73]. Using 4D embryonic lineage with the StarryNite package [74], we examined the cleavage patterns of six anterior deirid lineages (3 embryos) until the twofold stage in unc-86 mutants and found no differences in cellular cleavage patterns between these mutants and wildtype animals.
Taken together, UNC-86 not only induces specific differentiation programs in cells of the anterior deirid lineage, but also represses an alternative neuronal identity (dopaminergic neurons) in these cells, which is normally executed by the only cell in the anterior deirid lineage that does not express unc-86 (Fig 11A). Hence, unc-86 appears to act as a “homeotic gene” whose loss results in neuronal identity transformations, akin to those observed in other genetic and cellular contexts in C. elegans [75–77].
Derepression of a dopaminergic regulatory code in unc-86 mutants
What is the underlying molecular basis for homeotic identity transformation in unc-86 mutants? The terminal differentiation of all C. elegans dopaminergic neurons requires a combinatorial regulatory signature composed of at least four transcription factors, the ETS domain transcription factor ast-1, and the three homeobox genes ceh-43/Dlx, unc-62/Meis and ceh-20/Pbx [13,62,78](Fig 11A). unc-62 and ceh-20 are expressed in all neurons of the anterior deirid lineage (Fig 11A) and the data described above suggests that these factors are all required for each individual neuron fate in this lineage, apparently in combination with more restrictively expressed genes, namely unc-86 in ADA, FLP, AIZ and RMG, and ast-1 and ceh-43 in ADE (Fig 11A). Consequently, in unc-62 mutants, unlike in unc-86 mutants, no ectopic dopaminergic fate is observed in the anterior deirid lineage (Fig 11B).
With this information in mind, an ectopic execution of dopaminergic fate throughout the anterior deirid lineage in unc-86 mutants could mean that the complete regulatory signature for dopamine fate becomes de-repressed in unc-86 mutants. We therefore considered the expression of both ast-1 and ceh-43 in the anterior lineage in wild-type and unc-86 mutant animals. Our previous analysis of homeodomain protein expression showed that ceh-43 is expressed in one other neuron of the anterior deirid lineage, the AIZ neuron, but it is not expressed in ADA, FLP or RMG [6](Fig 11A). We examined the expression of a reporter allele of ast-1, in which gfp has been inserted at the C-terminus of ast-1 [79] and found that within the anterior deirid lineage ast-1 shows the same expression pattern as ceh-43. Like ceh-43, ast-1 is also expressed in AIZ, but not in ADA, FLP or RMG (Figs S6 and 11A). The dopamine regulatory signature therefore normally exists in AIZ, but unc-86 apparently antagonizes the ability of ast-1 and ceh-43 to promote dopaminergic fate in AIZ, as inferred from AIZ normally not being dopaminergic, but turning on dopaminergic markers in unc-86 mutants, as described above.
Furthermore, we observed that both ceh-43 and ast-1 expression becomes derepressed in additional neurons in the anterior deirid lineage in unc-86 mutants (Fig 12). To ask whether derepression of this regulatory signature can indeed be made responsible for the dopaminergic identity transformation in unc-86 mutants, we generated unc-86; ast-1, as well as unc-86; ceh-43 double mutant animals. We found that in these animals, the generation of ectopic dopaminergic neurons is suppressed (Fig 12). Hence, we conclude that in the absence of unc-86, a regulatory signature of dopamine fate becomes derepressed throughout the anterior deirid lineage to permit induction of dopaminergic identity. Therefore, unc-86 normally serves to antagonize dopaminergic fate specification, either by preventing the ability of ast-1 and ceh-43 to promote dopaminergic fate (in AIZ) or by preventing ast-1 and ceh-43 expression (in other cells in that lineage).
Several homeobox gene mutants display no apparent neuronal differentiation defects
While neuronal differentiation defects are easily apparent in many homeobox gene mutants, we also note that several neuron types display no obvious differentiation defects in single homeobox gene mutants (S3 Table). However, absences of defects are not straight-forward to interpret for multiple reasons: First, because our cell fate marker analysis only usually tested a small number of markers, one cannot exclude that other markers would show a defect in expression. Limited marker analysis may also not be able to capture a cell identity transformation phenotype, particularly if the alternative differentiation program involves the expression of similar molecular markers (e.g. expression of the eat-4/VGLUT marker is unaffected in cases in which one glutamatergic neurons switches its fate to another glutamatergic neuron type). Second, in several cases only hypomorphic alleles could be analyzed since null alleles displayed phenotypic pleiotropies that complicated the assessment of neuronal differentiation defects. Third, in several previously documented cases, even unrelated homeobox genes from different subfamilies (e.g., LIM, POU) can act redundantly in neuron identity specification [7,14]. For example, neither ttx-3 nor unc-86 single mutants affect expression of several different differentiation markers of the NSM neurons, but in a ttx-3; unc-86 double mutants these markers are completely lost [14]. A comprehensive analysis of neuronal cell fate in homeobox gene mutants may therefore require the generation of compound mutants.
Discussion
Homeobox genes have been implicated in nervous system differentiation throughout animal phylogeny [5, 80–90]. What sets apart the nematode C. elegans from other model systems is the extent to which homeobox genes have been implicated in neuron identity specification throughout the entire nervous system. First, expression pattern analysis of homeodomain proteins has shown that each of the 118 anatomically defined C. elegans neuron classes not only expresses at least one homeodomain protein, but expresses a unique combination of them [6]. Second, homeobox genes have been shown to act as terminal selectors of neuron identity in many neuron classes of the C. elegans nervous system (reviewed in [35]). The first described case was the heteromeric UNC-86/MEC-3 homeodomain complex that coordinates the expression of terminal identity features of touch receptor neurons [11,91,92]. Other examples, covering many parts of the C. elegans nervous system, followed (reviewed in [35]). However, many neuron classes, while expressing homeobox gene combinations, had not previously been shown to require a homeobox gene for proper identity specification. Moreover, while many neuron classes were known to be affected by a specific homeobox gene, neuron-type specific homeobox co-factors had not yet been identified. For example, unc-86 and ceh-14 were both known to specify distinct neuron classes [30,32], but it remained to be shown whether another homeobox gene dictates their ability to control distinct gene batteries in different neuron classes.
In this work, we expanded our view of homeobox gene function in the C. elegans nervous system. As summarized in Table 2, the present analysis has identified functions for 14 homeobox genes in 24 different neuron classes that are functionally and lineally diverse and located in different parts of the nervous system. In 12 of these neuron classes no identity regulator was previously known; in 3 neuron classes only a non-homeobox gene was previously implicated in controlling terminal differentiation; and for 9 of these neuron classes homeobox genes were previously known to be involved in their differentiation, but our analysis has now added additional homeobox gene function, thereby providing unique neuron type-specific functional combinations. For example, unc-86 and ceh-14 function in the PHC versus the AIM neuron can now be, at least in part, explained by unc-86 and ceh-14 apparently cooperating with mls-2 in the AIM neurons, but not the PHC neurons, where mls-2 is not expressed.
Do the homeobox genes that we describe here act as master-regulatory terminal selectors? Criteria for such a role are that the protein (a) is continuously expressed during differentiation and throughout the life of the neuron to maintain the differentiated state; (b) coordinately controls the expression of many, and not just a small subset of identity features; (c) exerts this effect by direct binding to terminal effector genes. For our cell fate analysis, we often used the NeuroPAL transgene, which, depending on neuron class, contains multiple differentiation markers and/or we used a core, hardwired feature of neuronal identity, the neurotransmitter/neuropeptidergic phenotype of a neuron. In several cases described here, every single one of multiple markers is affected and it seems reasonable to extrapolate these defects on other identity markers as well. Moreover, we note that several of the homeodomain proteins that we functionally characterized here have known and distinctive DNA binding sites (based on either ChIP and/or protein binding microarray) and those are overrepresented in the gene batteries of neurons whose identity they control [3], arguing for coordinated control of the gene battery and hence arguing for a terminal selector role of these homeodomain proteins. For example, the transcriptome of the neurons that we show in this paper to require UNC-62/MEIS for proper differentiation (ADA, FLP, RMG, AQR) show an enrichment for predicted, phylogenetically conserved binding sites of UNC-62, as determined by a phylogenetic footprinting pipeline, TargetOrtho [3]. A similar enrichment of UNC-86 binding sites is observed in the unc-86-dependent neurons that we describe here and the same holds for MLS-2/HMX binding sites and CEH-14 binding sites in mls-2- or ceh-14-dependent neurons. Hence, we assume that in many of the cases we describe here, the respective homeodomain protein indeed serve as terminal selectors. However, we also note that in some cases, the respective homeobox gene is unlikely to act as a terminal selector. For example, the LHX3/4 ortholog CEH-14 affects only two out of four tested neuropeptide markers in the PVW neurons. In other cases too few markers were tested to confidently predict a terminal selector function.
No matter whether each homeobox gene coordinates neuronal identity as a terminal selector or only affects specific aspects of a neuron identity, in combination with previous analysis of homeobox gene function in the C. elegans nervous system, we can draw a striking conclusion: most, and perhaps all, of the 118 neuron classes of the C. elegans hermaphrodite require at least one postmitotically expressed homeobox gene to properly execute at least some aspect of their terminal differentiation program. Specifically, according to the current tally of homeobox gene analysis (depicted in Fig 13; listed in S4 Table), 113 of the 118 neuron classes now have a homeobox identity regulator assigned to them. This tally takes into account not only homeobox genes acting as terminal selectors, but also homeobox genes that may “only” act as subtype selectors (e.g. the unc-4 gene, which diversifies VA from VB motor neuron fate, in combination with the non-homeobox gene unc-3) [93–96].
Fig 13. Regulation of neuron identity across the C. elegans nervous system by homeodomain transcription factors.
Functional analysis of homeobox gene family, overlayed onto the homeobox expression matrix from Fig 1B. Red boxes indicate that a homeodomain transcription factor is expressed in and likely acts as a terminal selector for a given neuron type (based on extent of functional marker analysis). Orange boxes indicate that a homeodomain transcription factor has a more restricted function in a given neuron class. Gray boxes indicate that a homeodomain transcription factor is expressed in that given neuron type, but not necessarily functionally analyzed and white boxes indicate that a homeodomain transcription factor is not expressed in that given neuron type. Panneuronal and non-neuronal homeoboxes were excluded from this representation because they do not contribute to unique neuron type codes. Neuron types along the x axis are clustered by transcriptomic similarity using the Jaccard index (see methods) and homeobox genes along the y axis are clustered similarly by their similar expression profiles in shared neuron types. See S4 Table for tabular list of genes and cells on which this matrix is based.
The 5 neuron classes that have not yet been found to require a homeobox gene for proper differentiation (ADF, ASI, ASJ, DVA, and RIM) do express combinations of homeobox genes, but their function still requires experimental validation. An assessment of the function of several of these homeobox genes is complicated by phenotypic pleiotropies (lethality) that several of these candidate genes display.
While in many cases, the loss of a homeobox gene results in the failure of a neuron to adopt an easily definable differentiated state, our analysis also provides a striking example of homeotic transformations in neuronal identity upon changes in homeobox codes. Specifically, we find that in the anterior deirid lineage, the POU homeobox gene unc-86 promotes, in conjunction with neuron-type specific cofactors, the execution of several neuronal differentiation programs (FLP, ADA, AIZ, RMG), but also represses an alternative differentiation program. This alternative program–a dopaminergic neuron differentiation program–is normally promoted by two other terminal selectors, the Distalless/Dlx ortholog ceh-43 and the ETS factor ast-1, but unc-86 either represses their expression in some neurons of the anterior deirid lineage or antagonizes their function in one neuron class, the AIZ neurons, which normally express ceh-43 and ast-1. One could imagine that a dopaminergic program may have been an “ancestral” state of several neurons in the anterior deirid, but the gain of unc-86 expression in a subset of these cells diverted the differentiation programs of these cells into a different direction.
The homeotic transformation that we observe in the anterior deirid lineage of unc-86 mutants is also observed in animals that lack the lin-32 Atonal-like transcription factor [97]. lin-32 has previously been shown to control unc-86 expression in the anterior deirid lineage [98] and, hence, the phenotype of both lin-32 and unc-86 appear indistinguishable. Yet another homeotic cell identity transformation observed in lin-32 mutants, an OLQ to URY identity transformation [97] can now also be explained with data presented here. Our updated expression, as well as functional data of vab-3/PAX6 reveals that the gene is required for OLQ and URY specification, and we have shown here that ceh-32/SIX is required for URY identity specification. Derepressed ceh-32 expression in OLQ observed in lin-32/ATO mutant [97], therefore generates a vab-3 + ceh-32 combination in OLQ that is normally instructive for URY fate and, hence, leads to a switch from OLQ to URY identity. Our data therefore indicates that precise allocation of homeobox genes to specific cell types, achieved by upstream regulators such as lin-32/ATO, are critical to proper neuronal identity specification.
In several cases, we also infer dual roles of a homeobox gene early at progenitor stages, but also later in terminal differentiation. Such a potential dual role may be played by the BSH/BSX homolog tab-1, a homeobox gene that is already expressed early during several progenitor phases in the ABala lineage [20]. Loss of tab-1 leads to an apparent failure to generate several neurons in this lineage. However, in a subset of these neurons (AIN and AVD), tab-1 is not only expressed during progenitor stages but is also continuously expressed during terminal differentiation throughout embryonic, larval and adult development and may be involved in initiating and maintaining the differentiated state as well. A good precedent for such a scenario is the function of the unc-86/BRN3 POU homeobox gene in the postdeirid lineage, where unc-86 acts at a progenitor state to prevent the progenitor from continuing to divide, but then also later in a descendant of the progenitor, the PVD harsh touch sensory neuron, to initiate and maintain its differentiated state [64,73,99]. We note that embryonic homeobox gene expression data indicates that several homeobox genes are more broadly expressed during embryogenesis than they are in terminally differentiating nervous system [20, 100], suggesting that earlier progenitor roles remain to be discovered for a number of these genes. In one case, it has been found that such a role may only become evident upon removal of two apparently redundantly acting homeobox genes [101].
In conclusion, we interpret the preponderance of homeobox genes in terminal neuronal identity control in C. elegans to be an indication of homeobox genes having become recruited as neuronal identity regulators early in nervous system evolution [5]. To further probe this issue, a systematic analysis of homeobox gene function in other organisms should be in order, but such analysis will critically depend on (a) the ability to properly assess mutant phenotypes (which, in vertebrates, may involve the experimental prevention of the execution of cell death programs often triggered after neuronal misspecification; [99]) and (b) the ability to conditionally target genes to avoid confounding issues such as early patterning versus late differentiation roles.
Clearly, other families of transcription factors have acquired important roles in neuronal identity specification as well, both in C. elegans and in vertebrates. In C. elegans, non-homeobox genes often act together with homeobox genes to affect neuronal identity specification [35]. For example, the ETS domain transcription factor AST-1 cooperates with the UNC-86/BRN3 POU homeodomain transcription factor to define the fate of multiple distinct monoaminergic neuron classes [78,79]. However, no individual transcription factor family seems to be as broadly involved in neuronal identity specification as homeobox genes, even though they make up less little more than 10% of transcription factors in animal genomes.
Material and methods
Mutant strains and transgenes
A list of all strains and transgenes can be found in S5 Table. vab-3, ceh-32, eya-1, unc-86, lin-11, egl-5 and ttx-1 mutant alleles were generated using CRISPR/Cas9 and homology-directed DNA repair with a precise repair template [102]. All these alleles are shown in Figs S7 or 10A (ttx-1). For several loci the same genomic deletion was independently generated in different reporter backgrounds, resulting in different allele names (Figs S7 and 10A).
CRISPR/Cas9 genome engineering
Reporter alleles were generated by CRISPR/Cas9 genome engineering inserting at the 3’end of the respective loci either a gfp tag (homeobox genes), an T2A::gfp::H2B cassette (eat-4), or, for neuropeptide-encoding genes, an SL2::gfp::H2B cassette or an T2A::3xNLS::gfp cassette. Reporter alleles were all generated by Sunybiotech. See S5 Table for each individual case. We generally found T2A::3xNLS::gfp to not give as bright a signal as other ensuing SL2::gfp::H2B cassette. We will report on systematic comparison between these cassettes elsewhere.
In addition to previously described reporter transgene (S5 Table), we generated a several additional transgene-based reporter construct. Promoter fusions for des-2, ttll-9 and sto-3 were Gibson-cloned into the pPD95.75 backbone, using either the SphI/XmaI multiple cloning sites. A PCR fusion reporter was generated for sri-1 following [103] by Manasa Basavaraju. Primer sequences are:
des-2: 1.3kb promoter: fwd gttggctgacagatcaggtg rev cctgtagtaaaagtaaatgtgtgttgtgtg
ttll-9: 500bp promoter: fwd ACCAAGTTCGCTTATCAGTTG rev cacaacaaaaaaaatccaaaaactagtcg
sto-3: 974bp promoter: fwd gaggaatcatagatgcccaatcag rev aagccaaaccaagtgagaagaag
sri-1: 800bp promoter: fwd Gaaaattgcattatattaatgttgttcaag rev gagtttagcatactaaaaaATG
Examination of expression reagents and neuron identification
For all homeobox genes where expression differences were noted relative to what was previously reported, we crossed the CRISPR/Cas9 generated reporter strain with the NeuroPAL landmark strain (otIs669 or otIs696) and determined neuronal expression by colocalization of landmark colors and position of the neurons.
Clustering of neuron types by transcriptomes
To cluster neurons by transcriptomic similarities (Figs S2, 1 and 12), we used the Jaccard index distance metric and clustered those distances using the Ward.D2 clustering method in R. The Jaccard distance metric is a binary determination of similarity between two groups. In this case, two neuron groups were assessed by the number of shared genes they express divided by the shared and unshared genes in those neuron groups. Thus, this analysis does not correlate levels of gene expression, but rather treats neuron types that express the same gene as similar. We then clustered those neurons based on their Jaccard distance using the Ward.D2 clustering method, which is agglomerative clustering mechanism where every neuron type starts in a separate cluster and is combined with similar neurons such that the internal cluster variance is minimized, until all neurons are part of a single cluster. We used the hclust() function in R to perform this analysis and the relationship of all neuron types is shown as a dendrogram.
Because each neuron type was sequenced at different depths there was an inconsistent number of transcripts picked up in each neuron type. Our initial clusterings using the complete transcriptome only showed relationships that reflected the depth of sequencing of each neuron type. To rectify this, we limited our transcriptomic analysis to only the top 500 expressed genes in each neuron type, assuming that those top genes were the important transcriptomic signature of that given neuron type. Therefore, our final clustering of the neuron types is based only on the 500 most highly expressed genes in each neuron type and whether or not those genes are similar to another neuron’s top 500 genes (S2 Fig).
Mutant analysis scoring and statistics
Reporter expression was scored as an all-or-nothing phenotype. In some cases, the number of neurons were counted per animal as 0, 1, or 2 neurons for bilateral pairs, 0 through 4 neurons for 4-member neuron classes, and 0 through 6 for 6-member neuron classes. Other times the level of reporter expression was counted for each individual neuron as ON, DIM or OFF. For both cases scoring data was processed in R and converted as number of expressing neurons by genotype contingency tables. Statistical analysis was then done using the Fisher.test function in R under the two-sided null hypothesis. For our data, the Fisher.test function used the Mehta and Patel FEXACT algorithm to treat our data as multiple groups in a row by column contingency matrix and calculated the corresponding p-values for each group. The resulting adjusted p-values are shown as less than 0.0001 when appropriate. No statistical methods were used to determine sample size prior to experiment. Based on the common standard in the field, we aimed for n greater than or equal to 10 per genotype.
Microscopy
Worms were anesthetized using 100mM of sodium azide (NaN3) and mounted on 5% agarose pads on glass slides. Images were acquired using confocal laser scanning microscopes (Zeiss LSM800 and LSM880) and processed using the ImageJ software [104]. For expression of reporters, representative maximum intensity projections are shown for GFP channel as gray scale and gamma and histogram were adjusted for visibility. For mutant functional analysis, representative maximum intensity projections are shown as inverted gray scale. NeuroPAL images provided in supplement are pseudocolored in accord with 28. All reporter reagents and mutants were imaged at 40x using fosmid or CRISPR reagents unless otherwise noted.
Supporting information
A: Comparison of expression of fosmid-based reporters (from [6]) to CRISPR/Cas9-engineered reporter alleles (see Fig 1 for expression pattern). The ubiquitous expression of the original ceh-30 fosmid reporter may have been an artefact. SDQ expression of the ceh-30 reporter allele syb4678 may be a result of cis-regulatory elements upstream of ceh-31, which were not included in the ceh-30 fosmid reporter, but were included in the ceh-31 fosmid reporter (which produces an expression pattern identical to that of the reporter allele). B: Transcript abundance of ceh-30 and ceh-31 from the CeNGEN project, at 4 different threshold values [19]. Matches between scRNA data and homeodomain expression data are indicated in red. ceh-31 transcripts completely match the sites of fosmid reporter (wg370) and reporter allele expression (devKi250). ceh-30 transcripts are indeed most strongly enriched in SDQ (the only neuron class where the reporter allele syb4678 shows expression), but is observed in other neurons as well. While those do not show reporter allele expression, it is notable that they partially overlap with the sites of ceh-31 expression. Perhaps these transcripts are produced from similar regulatory elements as the ceh-31 transcripts, but are somehow not translated into protein.
(EPS)
(EPS)
This data uses the expression data from S1 and S2 Tables.
(EPS)
We introduced the same CRISPR null deletion as in [7] in the unc-17 and flp-19 reporter alleles syb4491 and syb3278, yielding alleles ot1208 and ot1209. A small Z-stack (6–7 images at .8 micron) around the middle of the worm are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
(EPS)
A: ASI and AWA analysis in ceh-36(gj2127) and ceh-37(ok642) mutant animals. Markers used are CRISPR/Cas9-engineered reporter alleles for ins-3(syb5421), ins-6(syb5463), ins-24(syb5447), ins-30(syb5526), nlp-2(syb5697) and the odr-10(kyIs37) reporter transgene. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. B: ASE analysis in ceh-36(gj2127) mutant animals. Markers used are CRISPR/Cas9-engineered reporter alleles for ins-3(syb5421), ins-30(syb5526) and the flp-6(ynIs67) and flp-13(ynIs37) reporter transgenes. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
(EPS)
ast-1 CRISPR/Cas9-engineered reporter allele, vlc19, [79] is expressed in the following head neuron classes: ADE, AIN, AIZ, ASG, AVG, CEPD, CEPV, I4, I5, M3, M5, RIV, RMD, RMDD, RMDV, SMBD, SMBV, SMDD, and SMDV. Expression in the midbody, ventral nerve cord, and tail was not examined.
(EPS)
Deletions were generated by CRISPR/Cas9 genome engineering using an oligo-repair template. Identical deletions introduced into different reporter strain backgrounds get separate allele names. For lin-11, the deletions ot1025 and ot1026 are the same, but ot1241 is different by 4 nucleotides, even though the same oligo-mediated repair template was used.
(EPS)
This is an updated version of a table from [6].
(XLSX)
This is an updated version of a table from [6].
(XLSX)
(DOCX)
The basis for this data is taken mainly from [35] and supplemented with data from this paper, as well as others, including [6, 7, 17, 50, 59, 62, 79]. Criteria to be included in this list is that the transcription factor is expressed throughout embryonic and postembryonic development of the respective neuron type (i.e. is likely involved not only in initiation, but also maintenance of terminal differentiation programs) and the existence of mutant data that support a role in controlling marker genes. The homeobox part of this table is graphically presented in Fig 12.
(XLSX)
(DOCX)
Acknowledgments
We thank Chi Chen for generating transgenic animals, Zhuo Du for sending homeobox reporter alleles, Manasa Basavaraju from Marc Hammarlund’s lab for regenerating and providing an sri-1::gfp line, Seth Taylor in David Miller’s lab for sending and alerting us to a vab-3 reporter allele that showed expression in tail neurons, Maryam Majeed for a transgenic reporter line, Nuria Flames for comments on the manuscript and an ast-1 reporter allele, Ibnul Rafi for generating the nlp-52 reporter transgene and Neda Masoudi for its initial expression analysis. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs.
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This work was funded by the NIH (R01 NS039996 and R01NS110391 to O.H.; R01 NS116365 to P.K.; R24 OD016474 and R01 GM097576 to Z.B.) and the Howard Hughes Medical Institute (O.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B Biological Sciences. 1986;314:1–340. [DOI] [PubMed] [Google Scholar]
- 2.Hobert O. Terminal Selectors of Neuronal Identity. Curr Top Dev Biol. 2016;116:455–75. doi: 10.1016/bs.ctdb.2015.12.007 . [DOI] [PubMed] [Google Scholar]
- 3.Glenwinkel L, Taylor SR, Langebeck-Jensen K, Pereira L, Reilly MB, Basavaraju M, et al. In silico analysis of the transcriptional regulatory logic of neuronal identity specification throughout the C. elegans nervous system. eLife. 2021;10. Epub 2021/06/25. doi: 10.7554/eLife.64906 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobert O. Regulatory logic of neuronal diversity: terminal selector genes and selector motifs. Proc Natl Acad Sci U S A. 2008;105(51):20067–71. Epub 2008/12/24. doi: 10.1073/pnas.0806070105 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hobert O. Homeobox genes and the specification of neuronal identity. Nat Rev Neurosci. 2021;22(10):627–36. Epub 2021/08/28. doi: 10.1038/s41583-021-00497-x . [DOI] [PubMed] [Google Scholar]
- 6.Reilly MB, Cros C, Varol E, Yemini E, Hobert O. Unique homeobox codes delineate all the neuron classes of C. elegans. Nature. 2020;584(7822):595–601. Epub 2020/08/21. doi: 10.1038/s41586-020-2618-9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidal B, Gulez B, Cao WX, Leyva-Diaz E, Reilly MB, Tekieli T, et al. The enteric nervous system of the C. elegans pharynx is specified by the Sine oculis-like homeobox gene ceh-34. eLife. 2022;11. Epub 2022/03/25. doi: 10.7554/eLife.76003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63–101. doi: 10.1016/S0070-2153(09)88003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sousa E, Flames N. Transcriptional regulation of neuronal identity. Eur J Neurosci. 2022;55(3):645–60. Epub 2021/12/05. doi: 10.1111/ejn.15551 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiter F, Wienerroither S, Stark A. Combinatorial function of transcription factors and cofactors. Curr Opin Genet Dev. 2017;43:73–81. Epub 2017/01/23. doi: 10.1016/j.gde.2016.12.007 . [DOI] [PubMed] [Google Scholar]
- 11.Xue D, Tu Y, Chalfie M. Cooperative interactions between the Caenorhabditis elegans homeoproteins UNC-86 and MEC-3. Science. 1993;261(5126):1324–8. doi: 10.1126/science.8103239 [DOI] [PubMed] [Google Scholar]
- 12.Wenick AS, Hobert O. Genomic cis-Regulatory Architecture and trans-Acting Regulators of a Single Interneuron-Specific Gene Battery in C. elegans. Dev Cell. 2004;6(6):757–70. doi: 10.1016/j.devcel.2004.05.004 . [DOI] [PubMed] [Google Scholar]
- 13.Doitsidou M, Flames N, Topalidou I, Abe N, Felton T, Remesal L, et al. A combinatorial regulatory signature controls terminal differentiation of the dopaminergic nervous system in C. elegans. Genes Dev. 2013;27(12):1391–405. doi: 10.1101/gad.217224.113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang F, Bhattacharya A, Nelson JC, Abe N, Gordon P, Lloret-Fernandez C, et al. The LIM and POU homeobox genes ttx-3 and unc-86 act as terminal selectors in distinct cholinergic and serotonergic neuron types. Development. 2014;141(2):422–35. doi: 10.1242/dev.099721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yemini E, Lin A, Nejatbakhsh A, Varol E, Sun R, Mena GE, et al. NeuroPAL: A Multicolor Atlas for Whole-Brain Neuronal Identification in C. elegans. Cell. 2021;184(1):272–88 e11. Epub 2020/12/31. doi: 10.1016/j.cell.2020.12.012 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyva-Diaz E, Hobert O. Robust regulatory architecture of pan-neuronal gene expression. Curr Biol. 2022;32:1715–27. Epub 2022/03/09. doi: 10.1016/j.cub.2022.02.040 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berghoff EG, Glenwinkel L, Bhattacharya A, Sun H, Varol E, Mohammadi N, et al. The Prop1-like homeobox gene unc-42 specifies the identity of synaptically connected neurons. eLife. 2021;10. Epub 2021/06/25. doi: 10.7554/eLife.64903 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cros C, Hobert O. Caenorhabditis elegans Sine oculis/SIX-type homeobox genes act as homeotic switches to define neuronal subtype identities. Proc Natl Acad Sci U S A. 2022;119(37):e2206817119. Epub 2022/09/07. doi: 10.1073/pnas.2206817119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor SR, Santpere G, Weinreb A, Barrett A, Reilly MB, Xu C, et al. Molecular topography of an entire nervous system. Cell. 2021;184(16):4329–47 e23. Epub 2021/07/09. doi: 10.1016/j.cell.2021.06.023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Zhao Z, Xiao L, Xu W, Kou Y, Zhang Y, et al. A 4D single-cell protein atlas of transcription factors delineates spatiotemporal patterning during embryogenesis. Nat Methods. 2021;18(8):893–902. Epub 2021/07/28. doi: 10.1038/s41592-021-01216-1 . [DOI] [PubMed] [Google Scholar]
- 21.Reilly M, Hobert O. ceh-84, an unusual homeobox gene. MicroPubl Biol. 2020;2020. Epub 2020/12/10. doi: 10.17912/micropub.biology.000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, et al. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19(10):6815–24. Epub 1999/09/22. doi: 10.1128/MCB.19.10.6815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tadjuidje E, Hegde RS. The Eyes Absent proteins in development and disease. Cell Mol Life Sci. 2013;70(11):1897–913. Epub 2012/09/14. doi: 10.1007/s00018-012-1144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patrick AN, Cabrera JH, Smith AL, Chen XS, Ford HL, Zhao R. Structure-function analyses of the human SIX1-EYA2 complex reveal insights into metastasis and BOR syndrome. Nat Struct Mol Biol. 2013;20(4):447–53. Epub 2013/02/26. doi: 10.1038/nsmb.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin NM, Lim SE, Shi H, Chan TL, Liu J. A conserved Six-Eya cassette acts downstream of Wnt signaling to direct non-myogenic versus myogenic fates in the C. elegans postembryonic mesoderm. Dev Biol. 2009;331(2):350–60. Epub 2009/05/12. doi: 10.1016/j.ydbio.2009.05.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirose T, Galvin BD, Horvitz HR. Six and Eya promote apoptosis through direct transcriptional activation of the proapoptotic BH3-only gene egl-1 in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2010;107(35):15479–84. Epub 2010/08/18. doi: 10.1073/pnas.1010023107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuya M, Qadota H, Chisholm AD, Sugimoto A. The C. elegans eyes absent ortholog EYA-1 is required for tissue differentiation and plays partially redundant roles with PAX-6. Dev Biol. 2005;286(2):452–63. Epub 2005/09/13. doi: 10.1016/j.ydbio.2005.08.011 . [DOI] [PubMed] [Google Scholar]
- 28.Cassata G, Kagoshima H, Andachi Y, Kohara Y, Durrenberger MB, Hall DH, et al. The LIM homeobox gene ceh-14 confers thermosensory function to the AFD neurons in Caenorhabditis elegans. Neuron. 2000;25(3):587–97. doi: 10.1016/s0896-6273(00)81062-4 [DOI] [PubMed] [Google Scholar]
- 29.Kim K, Li C. Expression and regulation of an FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. The Journal of comparative neurology. 2004;475(4):540–50. doi: 10.1002/cne.20189 . [DOI] [PubMed] [Google Scholar]
- 30.Pereira L, Kratsios P, Serrano-Saiz E, Sheftel H, Mayo AE, Hall DH, et al. A cellular and regulatory map of the cholinergic nervous system of C. elegans. eLife. 2015;4. doi: 10.7554/eLife.12432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim K, Kim R, Sengupta P. The HMX/NKX homeodomain protein MLS-2 specifies the identity of the AWC sensory neuron type via regulation of the ceh-36 Otx gene in C. elegans. Development. 2010;137(6):963–74. Epub 2010/02/13. doi: 10.1242/dev.044719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serrano-Saiz E, Poole Richard J, Felton T, Zhang F, De La Cruz Estanisla D, Hobert O. Modular Control of Glutamatergic Neuronal Identity in C. elegans by Distinct Homeodomain Proteins. Cell. 2013;155(3):659–73. doi: 10.1016/j.cell.2013.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cook SJ, Vidal B, Hobert O. The bHLH-PAS gene hlh-34 is expressed in the AVH, not AVJ interneurons. MicroPubl Biol. 2021;2021. Epub 2021/10/05. doi: 10.17912/micropub.biology.000467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hart AC, Sims S, Kaplan JM. Synaptic code for sensory modalities revealed by C. elegans GLR-1 glutamate receptor. Nature. 1995;378(6552):82–5. doi: 10.1038/378082a0 . [DOI] [PubMed] [Google Scholar]
- 35.Hobert O. A map of terminal regulators of neuronal identity in Caenorhabditis elegans. Wiley interdisciplinary reviews Developmental biology. 2016;5(4):474–98. doi: 10.1002/wdev.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin Y, Hoskins R, Horvitz HR. Control of type-D GABAergic neuron differentiation by C. elegans UNC-30 homeodomain protein. Nature. 1994;372(6508):780–3. doi: 10.1038/372780a0 [DOI] [PubMed] [Google Scholar]
- 37.Hutter H. Extracellular cues and pioneers act together to guide axons in the ventral cord of C. elegans. Development. 2003;130(22):5307–18. doi: 10.1242/dev.00727 . [DOI] [PubMed] [Google Scholar]
- 38.Freyd G, Kim SK, Horvitz HR. Novel cysteine-rich motif and homeodomain in the product of the Caenorhabditis elegans cell lineage gene lin-11. Nature. 1990;344(6269):876–9. doi: 10.1038/344876a0 [DOI] [PubMed] [Google Scholar]
- 39.Narasimhan K, Lambert SA, Yang AW, Riddell J, Mnaimneh S, Zheng H, et al. Mapping and analysis of Caenorhabditis elegans transcription factor sequence specificities. eLife. 2015;4. doi: 10.7554/eLife.06967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chisholm AD, Horvitz HR. Patterning of the Caenorhabditis elegans head region by the Pax-6 family member vab-3. Nature. 1995;377(6544):52–5. doi: 10.1038/377052a0 [DOI] [PubMed] [Google Scholar]
- 41.Brandt JP, Rossillo M, Du Z, Ichikawa D, Barnes K, Chen A, et al. Lineage context switches the function of a C. elegans Pax6 homolog in determining a neuronal fate. Development. 2019;146(8). Epub 2019/03/21. doi: 10.1242/dev.168153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cinar HN, Chisholm AD. Genetic analysis of the Caenorhabditis elegans pax-6 locus: roles of paired domain-containing and nonpaired domain-containing isoforms. Genetics. 2004;168(3):1307–22. Epub 2004/12/08. doi: 10.1534/genetics.104.031724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal B, Aghayeva U, Sun H, Wang C, Glenwinkel L, Bayer EA, et al. An atlas of Caenorhabditis elegans chemoreceptor expression. PLoS Biol. 2018;16(1):e2004218. doi: 10.1371/journal.pbio.2004218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tobin D, Madsen D, Kahn-Kirby A, Peckol E, Moulder G, Barstead R, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35(2):307–18. Epub 2002/08/06. doi: 10.1016/s0896-6273(02)00757-2 . [DOI] [PubMed] [Google Scholar]
- 45.Yassin L, Gillo B, Kahan T, Halevi S, Eshel M, Treinin M. Characterization of the deg-3/des-2 receptor: a nicotinic acetylcholine receptor that mutates to cause neuronal degeneration. Mol Cell Neurosci. 2001;17(3):589–99. doi: 10.1006/mcne.2000.0944 . [DOI] [PubMed] [Google Scholar]
- 46.Wawersik S, Maas RL. Vertebrate eye development as modeled in Drosophila. Hum Mol Genet. 2000;9(6):917–25. Epub 2000/04/18. doi: 10.1093/hmg/9.6.917 . [DOI] [PubMed] [Google Scholar]
- 47.Dozier C, Kagoshima H, Niklaus G, Cassata G, Burglin TR. The Caenorhabditis elegans Six/sine oculis class homeobox gene ceh-32 is required for head morphogenesis. Dev Biol. 2001;236(2):289–303. doi: 10.1006/dbio.2001.0325 . [DOI] [PubMed] [Google Scholar]
- 48.Vidal B, Santella A, Serrano-Saiz E, Bao Z, Chuang CF, Hobert O. C. elegans SoxB genes are dispensable for embryonic neurogenesis but required for terminal differentiation of specific neuron types. Development. 2015;142(14):2464–77. doi: 10.1242/dev.125740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sarin S, Antonio C, Tursun B, Hobert O. The C. elegans Tailless/TLX transcription factor nhr-67 controls neuronal identity and left/right asymmetric fate diversification. Development. 2009;136(17):2933–44. Epub 2009/07/31. doi: 10.1242/dev.040204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gendrel M, Atlas EG, Hobert O. A cellular and regulatory map of the GABAergic nervous system of C. elegans. eLife. 2016;5. doi: 10.7554/eLife.17686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jones B, McGinnis W. A new Drosophila homeobox gene, bsh, is expressed in a subset of brain cells during embryogenesis. Development. 1993;117(2):793–806. Epub 1993/02/01. doi: 10.1242/dev.117.2.793 . [DOI] [PubMed] [Google Scholar]
- 52.Chu HY, Ohtoshi A. Cloning and functional analysis of hypothalamic homeobox gene Bsx1a and its isoform, Bsx1b. Mol Cell Biol. 2007;27(10):3743–9. Epub 2007/03/14. doi: 10.1128/MCB.01561-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- 54.Hombria JC, Garcia-Ferres M, Sanchez-Higueras C. Anterior Hox Genes and the Process of Cephalization. Front Cell Dev Biol. 2021;9:718175. Epub 2021/08/24. doi: 10.3389/fcell.2021.718175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chisholm A. Control of cell fate in the tail region of C. elegans by the gene egl-5. Development. 1991;111(4):921–32. doi: 10.1242/dev.111.4.921 . [DOI] [PubMed] [Google Scholar]
- 56.Wang BB, Muller-Immergluck MM, Austin J, Robinson NT, Chisholm A, Kenyon C. A homeotic gene cluster patterns the anteroposterior body axis of C. elegans. Cell. 1993;74(1):29–42. doi: 10.1016/0092-8674(93)90292-x [DOI] [PubMed] [Google Scholar]
- 57.Sengupta P, Bargmann CI. Cell fate specification and differentiation in the nervous system of Caenorhabditis elegans. Dev Genet. 1996;18(1):73–80. doi: [DOI] [PubMed] [Google Scholar]
- 58.Ferreira HB, Zhang Y, Zhao C, Emmons SW. Patterning of Caenorhabditis elegans posterior structures by the Abdominal-B homolog, egl-5. Dev Biol. 1999;207(1):215–28. Epub 1999/03/02. doi: 10.1006/dbio.1998.9124 . [DOI] [PubMed] [Google Scholar]
- 59.Zheng C, Lee HMT, Pham K. Nervous system-wide analysis of Hox regulation of terminal neuronal fate specification in Caenorhabditis elegans. PLoS Genet. 2022;18(2):e1010092. Epub 2022/03/01. doi: 10.1371/journal.pgen.1010092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kratsios P, Kerk SY, Catela C, Liang J, Vidal B, Bayer EA, et al. An intersectional gene regulatory strategy defines subclass diversity of C. elegans motor neurons. eLife. 2017;6. doi: 10.7554/eLife.25751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR. Tyramine Functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron. 2005;46(2):247–60. doi: 10.1016/j.neuron.2005.02.024 . [DOI] [PubMed] [Google Scholar]
- 62.Jimeno-Martin A, Sousa E, Brocal-Ruiz R, Daroqui N, Maicas M, Flames N. Joint actions of diverse transcription factor families establish neuron-type identities and promote enhancer selectivity. Genome Res. 2022;32(3):459–73. Epub 2022/01/26. doi: 10.1101/gr.275623.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Auken K, Weaver D, Robertson B, Sundaram M, Saldi T, Edgar L, et al. Roles of the Homothorax/Meis/Prep homolog UNC-62 and the Exd/Pbx homologs CEH-20 and CEH-40 in C. elegans embryogenesis. Development. 2002;129(22):5255–68. doi: 10.1242/dev.129.22.5255 . [DOI] [PubMed] [Google Scholar]
- 64.Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63(5):895–905. doi: 10.1016/0092-8674(90)90493-x [DOI] [PubMed] [Google Scholar]
- 65.Sze JY, Zhang S, Li J, Ruvkun G. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development. 2002;129(16):3901–11. doi: 10.1242/dev.129.16.3901 . [DOI] [PubMed] [Google Scholar]
- 66.Cook SJ, Crouse CM, Yemini E, Hall DH, Emmons SW, Hobert O. The connectome of the Caenorhabditis elegans pharynx. The Journal of comparative neurology. 2020. doi: 10.1002/cne.24932 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satterlee JS, Sasakura H, Kuhara A, Berkeley M, Mori I, Sengupta P. Specification of Thermosensory Neuron Fate in C. elegans Requires ttx-1, a Homolog of otd/Otx. Neuron. 2001;31(6):943–56. doi: 10.1016/s0896-6273(01)00431-7 . [DOI] [PubMed] [Google Scholar]
- 68.Schuske K, Palfreyman MT, Watanabe S, Jorgensen EM. UNC-46 is required for trafficking of the vesicular GABA transporter. Nat Neurosci. 2007;10(7):846–53. Epub 2007/06/15. doi: 10.1038/nn1920 . [DOI] [PubMed] [Google Scholar]
- 69.Turek M, Besseling J, Spies JP, Konig S, Bringmann H. Sleep-active neuron specification and sleep induction require FLP-11 neuropeptides to systemically induce sleep. eLife. 2016;5. doi: 10.7554/eLife.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leyva-Diaz E, Masoudi N, Serrano-Saiz E, Glenwinkel L, Hobert O. Brn3/POU-IV-type POU homeobox genes-Paradigmatic regulators of neuronal identity across phylogeny. Wiley interdisciplinary reviews Developmental biology. 2020:e374. doi: 10.1002/wdev.374 . [DOI] [PubMed] [Google Scholar]
- 71.Macosko EZ, Pokala N, Feinberg EH, Chalasani SH, Butcher RA, Clardy J, et al. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature. 2009;458(7242):1171–5. Epub 2009/04/08. doi: 10.1038/nature07886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Topalidou I, Chalfie M. Shared gene expression in distinct neurons expressing common selector genes. Proc Natl Acad Sci U S A. 2011;108(48):19258–63. doi: 10.1073/pnas.1111684108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chalfie M, Horvitz HR, Sulston JE. Mutations that lead to reiterations in the cell lineages of C. elegans. Cell. 1981;24(1):59–69. doi: 10.1016/0092-8674(81)90501-8 . [DOI] [PubMed] [Google Scholar]
- 74.Shah PK, Santella A, Jacobo A, Siletti K, Hudspeth AJ, Bao Z. An In Toto Approach to Dissecting Cellular Interactions in Complex Tissues. Dev Cell. 2017;43(4):530–40 e4. Epub 2017/11/22. doi: 10.1016/j.devcel.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arlotta P, Hobert O. Homeotic Transformations of Neuronal Cell Identities. Trends in neurosciences. 2015;38(12):751–62. doi: 10.1016/j.tins.2015.10.005 . [DOI] [PubMed] [Google Scholar]
- 76.Alqadah A, Hsieh YW, Vidal B, Chang C, Hobert O, Chuang CF. Postmitotic diversification of olfactory neuron types is mediated by differential activities of the HMG-box transcription factor SOX-2. EMBO J. 2015;34(20):2574–89. doi: 10.15252/embj.201592188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gordon PM, Hobert O. A Competition Mechanism for a Homeotic Neuron Identity Transformation in C. elegans. Dev Cell. 2015;34(2):206–19. doi: 10.1016/j.devcel.2015.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flames N, Hobert O. Gene regulatory logic of dopamine neuron differentiation. Nature. 2009;458(7240):885–9. Epub 2009/03/17. doi: 10.1038/nature07929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lloret-Fernandez C, Maicas M, Mora-Martinez C, Artacho A, Jimeno-Martin A, Chirivella L, et al. A transcription factor collective defines the HSN serotonergic neuron regulatory landscape. eLife. 2018;7. doi: 10.7554/eLife.32785 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubenstein JL, Puelles L. Homeobox gene expression during development of the vertebrate brain. Curr Top Dev Biol. 1994;29:1–63. Epub 1994/01/01. doi: 10.1016/s0070-2153(08)60546-3 . [DOI] [PubMed] [Google Scholar]
- 81.Krumlauf R, Marshall H, Studer M, Nonchev S, Sham MH, Lumsden A. Hox homeobox genes and regionalisation of the nervous system. J Neurobiol. 1993;24(10):1328–40. Epub 1993/10/01. doi: 10.1002/neu.480241006 . [DOI] [PubMed] [Google Scholar]
- 82.Holland PW, Takahashi T. The evolution of homeobox genes: Implications for the study of brain development. Brain Res Bull. 2005;66(4–6):484–90. Epub 2005/09/08. doi: 10.1016/j.brainresbull.2005.06.003 . [DOI] [PubMed] [Google Scholar]
- 83.Blochlinger K, Bodmer R, Jack J, Jan LY, Jan YN. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988;333(6174):629–35. doi: 10.1038/333629a0 . [DOI] [PubMed] [Google Scholar]
- 84.Doe CQ, Hiromi Y, Gehring WJ, Goodman CS. Expression and function of the segmentation gene fushi tarazu during Drosophila neurogenesis. Science. 1988;239(4836):170–5. Epub 1988/01/08. doi: 10.1126/science.2892267 . [DOI] [PubMed] [Google Scholar]
- 85.Doe CQ, Smouse D, Goodman CS. Control of neuronal fate by the Drosophila segmentation gene even-skipped. Nature. 1988;333(6171):376–8. Epub 1988/05/26. doi: 10.1038/333376a0 . [DOI] [PubMed] [Google Scholar]
- 86.Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–70. Epub 1994/12/16. doi: 10.1016/0092-8674(94)90027-2 . [DOI] [PubMed] [Google Scholar]
- 87.Joyner AL, Herrup K, Auerbach BA, Davis CA, Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991;251(4998):1239–43. Epub 1991/03/08. doi: 10.1126/science.1672471 . [DOI] [PubMed] [Google Scholar]
- 88.Qiu M, Bulfone A, Martinez S, Meneses JJ, Shimamura K, Pedersen RA, et al. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9(20):2523–38. Epub 1995/10/15. doi: 10.1101/gad.9.20.2523 . [DOI] [PubMed] [Google Scholar]
- 89.Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84(2):309–20. Epub 1996/01/26. doi: 10.1016/s0092-8674(00)80985-x . [DOI] [PubMed] [Google Scholar]
- 90.Hobert O, Westphal H. Function of LIM homeobox genes. Trends Genet. 2000;16:75–83. [DOI] [PubMed] [Google Scholar]
- 91.Finney M, Ruvkun G, Horvitz HR. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988;55(5):757–69. doi: 10.1016/0092-8674(88)90132-8 [DOI] [PubMed] [Google Scholar]
- 92.Duggan A, Ma C, Chalfie M. Regulation of touch receptor differentiation by the Caenorhabditis elegans mec-3 and unc-86 genes. Development. 1998;125(20):4107–19. doi: 10.1242/dev.125.20.4107 . [DOI] [PubMed] [Google Scholar]
- 93.Miller DM, Shen MM, Shamu CE, Burglin TR, Ruvkun G, Dubois ML, et al. C. elegans unc-4 gene encodes a homeodomain protein that determines the pattern of synaptic input to specific motor neurons. Nature. 1992;355(6363):841–5. doi: 10.1038/355841a0 [DOI] [PubMed] [Google Scholar]
- 94.Kerk SY, Kratsios P, Hart M, Mourao R, Hobert O. Diversification of C. elegans Motor Neuron Identity via Selective Effector Gene Repression. Neuron. 2017;93(1):80–98. doi: 10.1016/j.neuron.2016.11.036 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lickteig KM, Duerr JS, Frisby DL, Hall DH, Rand JB, Miller DM 3rd. Regulation of neurotransmitter vesicles by the homeodomain protein UNC- 4 and its transcriptional corepressor UNC-37/groucho in Caenorhabditis elegans cholinergic motor neurons. J Neurosci. 2001;21(6):2001–14. doi: 10.1523/JNEUROSCI.21-06-02001.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winnier AR, Meir JY, Ross JM, Tavernarakis N, Driscoll M, Ishihara T, et al. UNC-4/UNC-37-dependent repression of motor neuron-specific genes controls synaptic choice in Caenorhabditis elegans. Genes Dev. 1999;13(21):2774–86. doi: 10.1101/gad.13.21.2774 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Masoudi N, Yemini E, Schnabel R, Hobert O. Piecemeal regulation of convergent neuronal lineages by bHLH transcription factors in Caenorhabditis elegans. Development. 2021;148(11). Epub 2021/06/09. doi: 10.1242/dev.199224 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baumeister R, Liu Y, Ruvkun G. Lineage-specific regulators couple cell lineage asymmetry to the transcription of the Caenorhabditis elegans POU gene unc-86 during neurogenesis. Genes Dev. 1996;10(11):1395–410. Epub 1996/06/01. doi: 10.1101/gad.10.11.1395 [DOI] [PubMed] [Google Scholar]
- 99.Serrano-Saiz E, Leyva-Diaz E, De La Cruz E, Hobert O. BRN3-type POU Homeobox Genes Maintain the Identity of Mature Postmitotic Neurons in Nematodes and Mice. Curr Biol. 2018;28(17):2813–23 e2. doi: 10.1016/j.cub.2018.06.045 . [DOI] [PubMed] [Google Scholar]
- 100.Packer JS, Zhu Q, Huynh C, Sivaramakrishnan P, Preston E, Dueck H, et al. A lineage-resolved molecular atlas of C. elegans embryogenesis at single-cell resolution. Science. 2019;365(6459). Epub 2019/09/07. doi: 10.1126/science.aax1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Walton T, Preston E, Nair G, Zacharias AL, Raj A, Murray JI. The Bicoid class homeodomain factors ceh-36/OTX and unc-30/PITX cooperate in C. elegans embryonic progenitor cells to regulate robust development. PLoS Genet. 2015;11(3):e1005003. doi: 10.1371/journal.pgen.1005003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dokshin GA, Ghanta KS, Piscopo KM, Mello CC. Robust Genome Editing with Short Single-Stranded and Long, Partially Single-Stranded DNA Donors in Caenorhabditis elegans. Genetics. 2018;210(3):781–7. doi: 10.1534/genetics.118.301532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hobert O. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. BioTechniques. 2002;32(4):728–30. doi: 10.2144/02324bm01 . [DOI] [PubMed] [Google Scholar]
- 104.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Serrano-Saiz E, Gulez B, Pereira L, Gendrel M, Kerk SY, Vidal B, et al. Modular Organization of Cis-regulatory Control Information of Neurotransmitter Pathway Genes in Caenorhabditis elegans. Genetics. 2020;215(3):665–81. Epub 2020/05/24. doi: 10.1534/genetics.120.303206 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Comparison of expression of fosmid-based reporters (from [6]) to CRISPR/Cas9-engineered reporter alleles (see Fig 1 for expression pattern). The ubiquitous expression of the original ceh-30 fosmid reporter may have been an artefact. SDQ expression of the ceh-30 reporter allele syb4678 may be a result of cis-regulatory elements upstream of ceh-31, which were not included in the ceh-30 fosmid reporter, but were included in the ceh-31 fosmid reporter (which produces an expression pattern identical to that of the reporter allele). B: Transcript abundance of ceh-30 and ceh-31 from the CeNGEN project, at 4 different threshold values [19]. Matches between scRNA data and homeodomain expression data are indicated in red. ceh-31 transcripts completely match the sites of fosmid reporter (wg370) and reporter allele expression (devKi250). ceh-30 transcripts are indeed most strongly enriched in SDQ (the only neuron class where the reporter allele syb4678 shows expression), but is observed in other neurons as well. While those do not show reporter allele expression, it is notable that they partially overlap with the sites of ceh-31 expression. Perhaps these transcripts are produced from similar regulatory elements as the ceh-31 transcripts, but are somehow not translated into protein.
(EPS)
(EPS)
This data uses the expression data from S1 and S2 Tables.
(EPS)
We introduced the same CRISPR null deletion as in [7] in the unc-17 and flp-19 reporter alleles syb4491 and syb3278, yielding alleles ot1208 and ot1209. A small Z-stack (6–7 images at .8 micron) around the middle of the worm are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
(EPS)
A: ASI and AWA analysis in ceh-36(gj2127) and ceh-37(ok642) mutant animals. Markers used are CRISPR/Cas9-engineered reporter alleles for ins-3(syb5421), ins-6(syb5463), ins-24(syb5447), ins-30(syb5526), nlp-2(syb5697) and the odr-10(kyIs37) reporter transgene. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test. B: ASE analysis in ceh-36(gj2127) mutant animals. Markers used are CRISPR/Cas9-engineered reporter alleles for ins-3(syb5421), ins-30(syb5526) and the flp-6(ynIs67) and flp-13(ynIs37) reporter transgenes. Representative images of wild type and mutant worms are shown with 10 μm scale bars. Graphs compare expression in wild type and mutant worms with the number of animals examined listed at the bottom of the bar. P-values were calculated by Fisher’s exact test.
(EPS)
ast-1 CRISPR/Cas9-engineered reporter allele, vlc19, [79] is expressed in the following head neuron classes: ADE, AIN, AIZ, ASG, AVG, CEPD, CEPV, I4, I5, M3, M5, RIV, RMD, RMDD, RMDV, SMBD, SMBV, SMDD, and SMDV. Expression in the midbody, ventral nerve cord, and tail was not examined.
(EPS)
Deletions were generated by CRISPR/Cas9 genome engineering using an oligo-repair template. Identical deletions introduced into different reporter strain backgrounds get separate allele names. For lin-11, the deletions ot1025 and ot1026 are the same, but ot1241 is different by 4 nucleotides, even though the same oligo-mediated repair template was used.
(EPS)
This is an updated version of a table from [6].
(XLSX)
This is an updated version of a table from [6].
(XLSX)
(DOCX)
The basis for this data is taken mainly from [35] and supplemented with data from this paper, as well as others, including [6, 7, 17, 50, 59, 62, 79]. Criteria to be included in this list is that the transcription factor is expressed throughout embryonic and postembryonic development of the respective neuron type (i.e. is likely involved not only in initiation, but also maintenance of terminal differentiation programs) and the existence of mutant data that support a role in controlling marker genes. The homeobox part of this table is graphically presented in Fig 12.
(XLSX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.