Abstract
We investigated the impact of dietary patterns on the gut microbiota and concentration of short-chain fatty acids in the feces of Korean elementary school students. The dietary intake and ADHD assessment of 40 Korean elementary school students were analyzed using a dish-based semi-quantitative food frequency questionnaire. Analysis of gut microbiota and short-chain fatty acids composition were performed using the real-time polymerase chain reaction, metagenomics, and gas chromatography methods. The dietary patterns of participants were divided into four groups: healthy, processed food, fish and shellfish, and meat. The participants were also divided into two groups according to their ADHD scores: 0–30, control group; over 30, ADHD group. The ADHD score of the processed food group was significantly higher than that of the healthy group. The processed food and ADHD groups showed significantly higher abundance of harmful bacteria, such as the Enterobacter, Escherichia coli, and Clostridium strains, and markedly lower abundance of beneficial bacteria, such as the Bifidobacterium and Ruminococcus strains, than the control group. The heat maps of metagenomics indicated that each group was separated into distinct clusters, and the processed food and ADHD groups showed significantly lower α-diversity of gut microbiota than the control group. In these groups, the concentration of acetate or butyrate in the feces was significantly lower than that in the control group. These results may indicate that imbalanced diets can disturb the colonic microbial balance and are likely to become a potential risk factor for the prevalence of ADHD.
Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common mental disorders in childhood [1]. It is a neurodevelopmental disorder that leads to a failure in the control of one’s own behavior, causing various complications, such as depression, anxiety, and the bipolar disorder [2–4]. ADHD symptoms appear during childhood, being recognized as a medical condition, and tend to persist in adulthood [5]. ADHD is not only associated with neurotransmitters, like dopamine, but also with the immune system, which is greatly affected by the alteration of the gut microbiota composition [6, 7].
The genetic information of gut microbiota is known as the second genome of humans and several studies have reported that its abnormal alteration is associated with various diseases [8–10]. The interaction between the brain and gut is bidirectional communication because the gut is connected to the brain via 200–600 million neurons [11]. The gut microbiota can affect the enteric nervous system (ENS), which interacts with the central nervous system (CNS) of the brain [12]. The balanced gut microbiota composition contributes to health promotion, while its abnormal state can result in mental disorders by adversely affecting the ENS and CNS [13]. Therefore, the desirable modulation of gut microbiota may prevent and improve mental disorders [14]. Recently, studies have shown that the gut microbiota is associated with ADHD symptoms [15], which are related to the levels of neurotransmitters, such as dopamine and serotonin [16, 17]. Moreover, it was reported that beneficial bacteria, such as the Bifidobacterium strains, are associated with the increase of a dopamine precursor, indicating that the gut microbiota and their metabolites may modulate the release of neurotransmitters related to ADHD [18].
The composition of the gut microbiota is affected by various factors, such as region, race, and diet [19]. Dietary intake may have an especially important effect on the gut microbiota [20], the composition of which may be related to the short-chain fatty acids (SCFAs) produced by the metabolism of dietary ingredients [21]. SCFAs can modulate the intestinal environment to facilitate the growth of beneficial bacteria, such as the Lactobacillus and Bifidobacterium strains, resulting in the prevention and improvement of diseases through a balanced composition of the gut microbiota [22]. SCFAs can affect the brain function and the gut-brain interactions via direct or indirect pathways [17]. Alterations in the gut microbiota composition according to dietary intake are interrelated with health and disease [23, 24]. Millichap and Yee [25] revealed that the western dietary pattern is related to ADHD symptoms owing to the higher intake of saturated and refined sugars. Moreover, high intake of processed foods and snacks is associated with the aggravation of ADHD [26]. Therefore, this study aimed to investigate the correlation between the alteration of the gut microbiota composition and ADHD according to the dietary intake of Korean elementary school students.
Methods
Dietary intake investigation
This study was approved by the Sahmyook University Ethics Committee (2-7001793-AB-N-012018037HR). The dietary intake of 40 Korean elementary school students (11 years old) was analyzed using the Can Pro 4.0 (The Korean Nutrition Society, Seoul, Korea) database and a dish-based semi-quantitative food frequency questionnaire (FFQ), which was filled out by the parents. Based on the frequency of intake, the participants were divided into nine categories (more than three times a day, twice a day, once a day, five to six times a week, three to four times a week, one to two times a week, two to three times a month, once a month, and almost none), and based on the amount of intake, they were divided into three categories (usually less than normal, normal, and more than normal). The FFQ consists of 107 food items commonly consumed by Korean school-aged children. These foods are classified into 30 major groups by slight modifications to the method described by An et al. [27]: rice, 2 items; noodles, 2 items; instant noodles, 1 item; dumplings, 1 item; cereal, 1 item; rice cake, 1 item; bread, 2 items; fats, 2 items; fast foods, 3 items, Korean snacks, 3 items; potato, 1 item; sweet potato, 1 item; beef, 6 items; pork, 3 items; processed meat, 1 item; chicken, 3 items; egg, 1 item; fish, 8 items; shellfish, 4 items; fish cake, 1 item; milk/dairy foods, 6 items; beans, 5 items; vegetable, 18 items; mushroom, 1 item; seaweeds, 2 items; sweets, 7 items; nuts, 1 item; fruits, 9 items; beverage, 7 items; and kimchi, 4 items (Table 1). Dietary patterns were identified using principal component factor analysis, referring to the method of An et al [27] and Woo et al. [28]. The factors to be maintained for the division of dietary patterns were determined by the criteria of eigenvalues (above 1.0) and were rotated using a varimax procedure to achieve a structure with greater interpretability. Food groups with factor loading scores over 0.5, which represent the correlation coefficients between the individual food groups and dietary patterns, were considered to be the primary contributors.
Table 1. Lists of food items in a dish-based semi-quantitative food frequency questionnaire.
| Food groups | Number | Food items |
|---|---|---|
| Rice | 2 | White rice, multi-grain rice |
| Noodles | 2 | Noodle soup, noodles |
| Instant noodles | 1 | Instant noodles |
| Dumpling | 1 | Dumpling |
| Cereal | 1 | Cereal |
| Rice cake | 1 | Rice cake |
| Bread | 2 | Bread, red bean bread |
| Fats | 2 | Butter, mayonnaise |
| Fast foods | 3 | Pizza, hamburger, fried potatoes |
| Korean snacks | 3 | Gimbab, stir-fried rice cake, Korean sausage |
| Potato | 1 | Potato |
| Sweet potato | 1 | Sweet potato |
| Beef | 6 | Bulgogi, roast beef, roasted ribs, short rib soup, soy sauce braised beef, beef tripe |
| Pork | 3 | Grilled pork belly, pork cutlet, pork slices, |
| Processed meat | 1 | Ham |
| Chicken | 3 | Fried chicken, chicken stew, ginseng chicken soup |
| Egg | 1 | Egg |
| Fish | 8 | Fried white flesh fish, baked white flesh fish, fried external blue-coloured fish, etc. |
| Shellfish | 4 | Clam, squid, shrimp, crab |
| Fish cake | 1 | Fish cake |
| Milk/dairy foods | 6 | Milk, low fat milk, process milk, yogurt, fermented milk, cheese |
| Beans | 5 | Bean boiled in soy sauce, bean curd, soybean paste soup, soy milk, soybean paste |
| Vegetables | 18 | Lettuce, perilla leaf, cucumber, carrot, chili, garlic, onion, spinach, water parsley, a green pumpkin, etc. |
| Mushroom | 1 | Mushroom |
| Seaweeds | 2 | Laver, seaweed |
| Sweets | 7 | Ice cream, candy, chocolate, snack, soda, cocoa, coffee |
| Nuts | 1 | Nuts |
| Fruits | 9 | Strawberry, apple, orange, pear, banana, watermelon, melon, grape, peach |
| Beverages | 7 | Orange juice, tomato juice, fruit juice, tea, diet drinks, coffee, sports drink |
| Kimchi | 4 | Kimchi, kimchi stew, diced radish kimchi, water kimchi |
ADHD assessment
We estimated the ADHD scores of participants using Korean ADHD rating scale (K-ARS), which is commonly used in various institutions to assess the ADHD scores of children in Koreans. K-ARS, which was filled out by parents and class teachers, uses a four-point scale (0 = never, 1 = sometimes, 2 = often, 3 = very often) and consists of 18 questions, of which nine each are related to the attention deficit and hyperactivity disorders, respectively. The ADHD group was identified using the criteria of Lee [29] and the participants were divided into two groups according to their ADHD scores: 0–30, control group; above 30, ADHD group.
Anthropometric characteristics and sampling
We investigated the height, weight, body mass index (BMI), waist-to-hip ratio, and blood pressure of the participants. We also recorded the disease and medication history of the candidates and collected their feces to analyze the gut microbiota composition and fecal SCFA concentration.
Quantitative real-time polymerase chain reaction (qPCR)
qPCR was performed using DT-prime 5 (DNA-Technology, Moscow, Russia) with the power SYBR Green PCR master mix (Applied Biosystems, Foster City, CA, USA) and specific primers to measure the total number of bacteria (forward, 5′-GCAGGCCTAACACATGCAAGTC-3′; reverse, 3′-CTGCTGCCTCCCGTAGGAGT), Bifidobacterium (forward, 5′-CTCCTGGAAACGGGTGG-3′; reverse, 3′-GGTGTTCT-TCCCGATATCTACA-5′), Lactobacillus (forward, 5′-CGATGAGTGCTAGGTGTTG-GA-3′; reverse, 3′-CAAGATGTCAAGACCTGGTAAG-5′), Clostridium (forward, 5′-ATGCAAGTCGAGCGAKG-3′; reverse, 3′-TATGCGGTATTAATCTYCCTTT-5′), Enterobacter (forward, 5′-ATGGCTGTCGTCAGCTCGT-3′; reverse, 3′-CTACTTCT-TTTGCAACCCACTC-5′), and E. coli (forward, 5′-GTTAATACCTTTGCTCATTGA-3′; reverse, 3′-ACCAGGGTATCTAATCCTGTT-5′) strains based on the method described by Han et al [30]. The reaction mixture consisted of 10 μL SYBR green master mix, 1 μL each of specific primer (10 pM), and 2 μL DNA sample to make a final volume of 20 μL. An initial DNA denaturation step at 95°C for 10 min was followed by 40 cycles of amplification (95°C for 15 s, primer annealing at 55–61°C for 25 s, and extension at 72°C for 30–40 s) and cooling at 4°C. The calibration curve was constructed using Ct values depending on the serial dilutions of bacterial DNA isolated from each bacterial strain using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany).
Metagenomics analysis
The composition of the gut microbiota was determined at ChunLab Inc. (Seoul, Korea). PCR amplification was performed using fusion primers targeting the V3 to V4 regions of the 16S rRNA gene in the extracted DNA. For bacterial amplification, fusion primers 341F (5′-TGATACGGCGACCACCGAGATCTACACXXXXXXXXTCGTCGGCAG-CGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′; underlined sequence indicates the target region primer) and 805R (5′-CAAGCAGAAGACGGCA-TACGAGAT-X-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGACTACH-VGGGTATCTAATCC-3′). PCR was conducted using Master Mix and PTC-200 Peltier Thermal Cycler (Applied Biosystems, Foster City, CA, USA) using the following program: initial denaturation step at 94°C for 5 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 45 s, and extension at 72°C for 90 s. Each PCR product was purified using a QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and the refined PCR products were quantified using the Quant-iTTM PicoGreen dsDNA Assay Kit (Invitrogen, Carlsbad, CA, Austria). Each DNA sample was mixed in the same amount and electrophoresis was performed on the pooled DNA sample. After emulsion-based clonal amplification of the DNA library samples, sequencing was performed using the emPCR Amplification 7020 Thermal cycler (Applied Biosystems) using the following program: initial denaturation step at 94°C for 4 min; 50 cycles of denaturation at 94°C for 270 s, annealing at 58°C for 45 s, and extension at 68°C for 30 s. Data analysis was performed using the CLcommunityTM v.3.46 software (ChunLab Inc., Seoul, Korea) and the EZBioCloud database.
Measurement of fecal SCFAs
Fecal SCFAs were measured using gas chromatography. Dried feces (10 mg) were mixed with 1 mL of methanol (Sigma, Saint Louis, MO, USA) and then shaken at 200 rpm for 90 min at 25°C. After the mixture was centrifuged at 10,000 rpm for 10 min at 25°C, the supernatants were filtered through a 0.22 μm syringe filter (Advantec, Tokyo, Japan), and 2 μL of the filtrate was injected into YL 6100GC (Youngin Chromass, Gyeonggi-do, Korea) to analyze the SCFA content in the colonic digest. Calibration curves were obtained using standard reagents (Sigma-Aldrich, St. Louis, MO, USA).
Statistical analysis
The results are presented as the mean ± standard error of the mean (SEM). The significance of the differences among the groups was determined using the SAS/PROC GLM software (SAS v.9.1; SAS Institute Inc., Cary, NC, USA). The statistical significance according to dietary pattern was analyzed by one-way analysis of variance (ANOVA) with Duncan’s multiple range test. Statistical significance depending on the ADHD score was analyzed using Student’s t-test. Correlation analysis was used to analyze the relationship between the ADHD scores, intestinal microorganisms, and SCFAs according to the dietary patterns using the Pearson correlation coefficient.
Results
Dietary patterns
The dietary intake of the participants was investigated using FFQ. Factor analysis was performed to distinguish dietary patterns, and primary factors were separated through principal axis extraction and the varimax rotation method. As shown in Table 2, the four dietary patterns (healthy, n = 12; processed food, n = 8; fish and shellfish, n = 11; and meat, n = 9) were distinguished based on the foods or food groups with factor loading scores with absolute values over 0.5, according to the method of An et al. [27] and Woo et al. [28] and which reported the association between ADHD and dietary patterns. The "healthy" dietary pattern included high intakes of kimchi, fruits, vegetable, sweet potato, milk and dairy foods, and mushroom. The "processed food" dietary pattern was characterized by high intakes of fats, sweets, and fast foods. The "fish and shellfish" dietary pattern was identified by high intakes of shellfish, fish, and fishcake. The "meat" dietary pattern included high intakes of beef and pork. The four dietary patterns accounted for 44.65% of the variance in food intake, and each dietary pattern explained 14.39%, 10.09%, 12.09%, and 8.08% of the variation in food intake, respectively.
Table 2. Factor loadings for the major dietary patterns derived from the principal components analysis with orthogonal rotation.
| Foods/Food groups | Dietary patterns | |||
|---|---|---|---|---|
| Healthy (n = 12) | Processed food (n = 8) | Fish and shellfish (n = 11) | Meat (n = 9) | |
| Rice | .250 | -.214 | .099 | -.066 |
| Noodles | .221 | .406 | .215 | .044 |
| Instant noodles | .406 | .338 | .135 | -.309 |
| Dumpling | .338 | .422 | .436 | .263 |
| Cereal | -.102 | -.095 | .337 | .493 |
| Rice cake | .319 | .051 | .078 | .465 |
| Bread | .305 | -.016 | -.032 | .026 |
| Fats | -.104 | .680 | .076 | .049 |
| Fast foods | .039 | .638 | -.083 | .149 |
| Korean snacks | .400 | -.003 | .314 | .053 |
| Potato | .019 | .320 | .487 | -.051 |
| Sweet potato | .583 | .294 | .161 | .165 |
| Beef | .027 | -.084 | .010 | .677 |
| Pork | .095 | .369 | .230 | .596 |
| Processed meat | .397 | .385 | .479 | .094 |
| Chicken | .409 | .102 | .452 | .134 |
| Egg | .413 | -.069 | .139 | .160 |
| Fish | .377 | .004 | .794 | .208 |
| Shellfish | .142 | -.078 | .923 | -.069 |
| Fishcake | -.080 | .444 | .590 | .165 |
| Milk/dairy foods | .544 | .044 | .098 | .325 |
| Beans | .324 | .293 | -.079 | .437 |
| Vegetables | .622 | .441 | .190 | .172 |
| Mushroom | .512 | .235 | -.243 | -.004 |
| Seaweed | .481 | .192 | -.222 | .489 |
| Sweets | .353 | .679 | .192 | -.080 |
| Nuts | .438 | .039 | .203 | .004 |
| Fruits | .647 | .180 | .165 | -.015 |
| Beverages | .068 | .274 | .027 | .392 |
| Kimchi | .717 | -.036 | .514 | .266 |
| Variance explained (%) | 14.39 | 10.09 | 12.09 | 8.08 |
Dietary patterns were determined using a factor analysis. The values in bold refer to the factor loadings of over 0.5
ADHD Score and characteristics of participants
The ADHD score of participants was investigated using the K-ARS, which was examined by the parents and class teachers, using a four-point scale and consisted of 18 questions, containing nine questions each related to the attention deficit and hyperactivity disorders, respectively. ADHD scores according to the four dietary patterns are as follows: healthy group, 10.04 ± 2.83; processed food group, 21.44 ± 4.74; fish and shellfish group, 13.73 ± 3.82; meat group, 15.56 ± 4.23. No significant difference in ADHD scores was noted among the healthy dietary pattern, meat dietary pattern, and fish dietary pattern, whereas those of processed food dietary pattern were remarkably (p < 0.05) higher than those of the healthy dietary pattern. The ADHD group was identified using the criteria of Lee [29] and the participants were divided into two groups depending on their ADHD scores: 0–30, control group (n = 33); above 30, ADHD group (n = 7). In this study, we investigated the characteristics of participants, such as sex, age, BMI, parental smoking, parental income (Table 3). However, the basic characteristics did not significantly relate to the dietary patterns and ADHD scores.
Table 3. The characteristics of participants.
| Number | Percentage (%) | |
|---|---|---|
| Sex (n = 40) | ||
| Male | 26 | 65 |
| Female | 14 | 35 |
| Age (n = 40) | ||
| 11 years | 7 | 17.5 |
| 12 years | 33 | 82.5 |
| BMI (n = 40) | ||
| < 18.5 | 10 | 25.0 |
| 18.5–22.9 | 21 | 52.5 |
| 23.0–24.9 | 7 | 17.5 |
| > 25 | 2 | 5.0 |
| Parental smoking (n = 40) | ||
| Smoking | 18 | 45.0 |
| Non-smoking | 22 | 55.0 |
| Parental income (n = 40) | ||
| High | 12 | 30.0 |
| Middle | 21 | 52.5 |
| Low | 9 | 22.5 |
qPCR
The results of qPCR are shown in Table 4. No significant difference was noted among the four dietary pattern groups with regard to the number of total bacteria, Bifidobacterium, Lactobacillus, Clostridium, and Escherichia coli strains. However, the number of Enterobacter strains was significantly (p < 0.05) higher in the processed food group, and the ratio of Lactobacillus to Enterobacter strains was significantly (p < 0.05) lower in the processed food group than in the healthy group. The number of pathogenic bacteria, such as the Clostridium and Enterobacter strains, was significantly (p < 0.05) higher, whereas the ratio of Lactobacillus to Enterobacter strains was significantly (p < 0.05) lower in the ADHD group than in the control group.
Table 4. The counts (log10 16S rDNA gene copies g-1 of colonic digest) of different bacterial groups measured by quantitative real-time polymerase chain reaction (qPCR).
| Bacteria | Dietary patterns | ADHD scores | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | Processed food | Fish and shellfish | Meat | p-value | Control | ADHD | p-value | |
| Total bacteria | 11.40±0.311) | 11.41±0.21 | 11.24±0.23 | 11.17±0.21 | NS2) | 11.37±0.14 | 10.99±0.24 | NS4) |
| Bifidobacterium | 10.14±0.13 | 10.35±0.21 | 10.11±0.14 | 10.25±0.13 | NS | 10.16±0.08 | 10.41±0.15 | NS |
| Lactobacillus | 8.87±0.17 | 8.59±0.28 | 8.67±0.19 | 8.49±0.26 | NS | 8.67±0.12 | 8.70±0.29 | NS |
| Clostridium | 7.73±0.20 | 7.57±0.33 | 7.38±0.31 | 7.64±0.26 | NS | 7.39±0.13 | 8.51±0.16* | <0.055) |
| Enterobacter | 6.57±0.15b | 7.53±0.19a | 7.18±0.37ab | 7.20±0.26ab | <0.053) | 6.91±0.13 | 7.84±0.28* | <0.05 |
| Escherichia coli | 6.21±0.16 | 6.95±0.36 | 6.75±0.33 | 6.84±0.23 | NS | 6.53±0.14 | 7.22±0.41 | NS |
| Lactobacillus: Enterobacter | 2.30±0.17a | 1.06±0.19b | 1.49±0.41ab | 1.29±0.38ab | <0.05 | 2.09±0.14 | 0.86±0.30* | <0.05 |
1) Values represent the mean ± standard error of the mean (SEM)
2) NS, there were no significant differences by one-way analysis of variance (ANOVA) test
3) abc Groups with different letters in the same row are significantly different by one-way ANOVA with Duncan’s multiple range test
4) NS, there were no significant differences by Student’s t-test
5) The statistical significance between the control and ADHD groups was analyzed by the Student’s t-test (*p < 0.05)
Metagenomics analysis
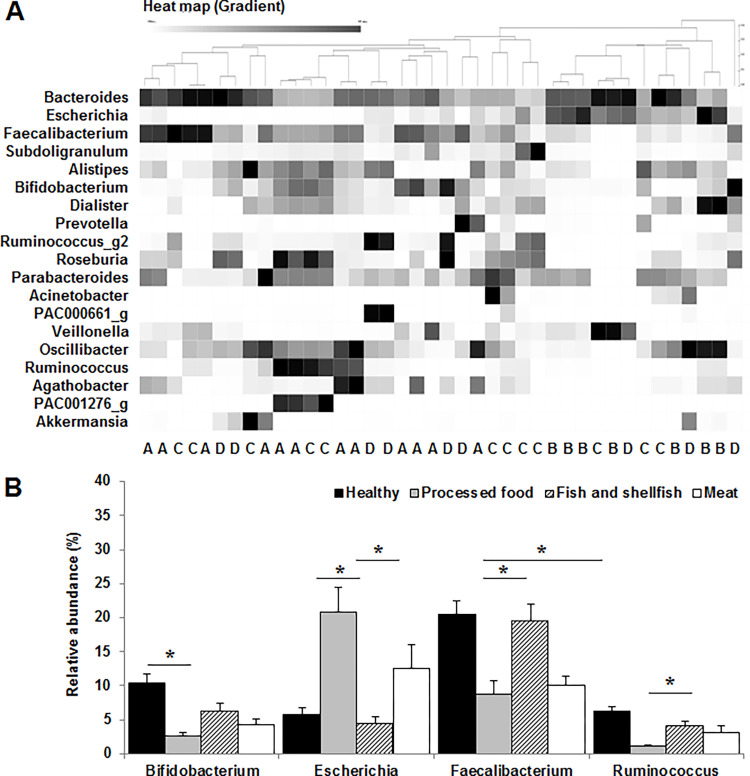
Metagenomic analysis was conducted to investigate the gut microbiota composition and diversity in the feces of the participants. Heat map analysis of different genera of the gut microbiota revealed that the microbiota profile of fish and shellfish dietary pattern and meat dietary pattern did not have distinct clusters, whereas that of the processed food dietary pattern mostly belonged to a separate cluster from that of the healthy group (Fig 1A). Moreover, the composition of the gut microbiota at the genus level according to the dietary pattern showed that the relative abundance of beneficial bacteria, such as the Bifidobacterium, Faecalibacterium, and Ruminococcus strains, was significantly (p < 0.05) lower in the processed food group or meat group, whereas that of harmful bacteria, such as E. coli, was markedly (p < 0.05) higher in the processed food group than in the healthy group (Fig 1B).
Fig 1. Comparison of the gut microbiota at the genus level according to the dietary patterns.
(A) Heat map of different genera in the gut microbiota. A, healthy; B, processed food; C, fish and shellfish; D, meat. (B) Relative abundance of the gut microbiota. Values are expressed as the mean ± standard error of the mean (SEM). Statistical significance was determined by one-way analysis of variance (ANOVA) with Duncan’s multiple range test (*p < 0.05).
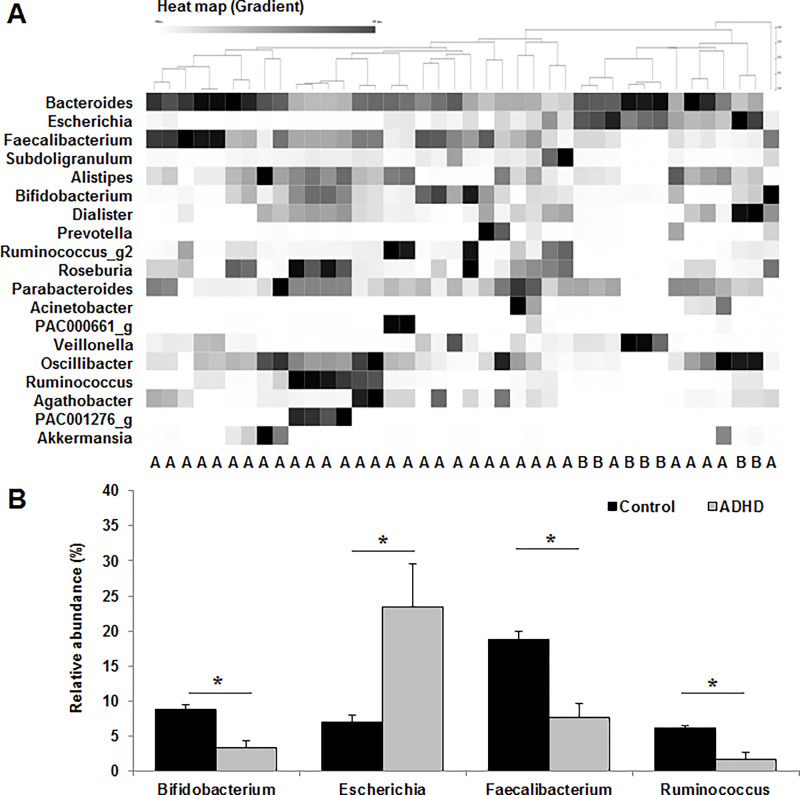
Heat map analysis of the different genera of the gut microbiota according to ADHD score showed that the microbiota profiles of the control and ADHD groups mostly belonged to distinct clusters (Fig 2A). The relative abundance of harmful bacteria, such as the Escherichia strains, was significantly (p < 0.05) higher in the ADHD group, whereas that of beneficial bacteria, such as the Bifidobacterium, Faecalibacterium, and Ruminococcus strains, was markedly (p < 0.05) lower in the ADHD group than in the control group (Fig 2B).
Fig 2. Comparison of the gut microbiota at the genus level between the control and attention deficit hyperactivity disorder (ADHD) groups.
(A) Heat map of different genera in the gut microbiota. A, control group; B, ADHD group. (B) Relative abundance of the gut microbiota. Values are expressed as the mean ± SEM. The statistical significance between the control and ADHD groups was analyzed by the Student’s t-test (*p < 0.05).
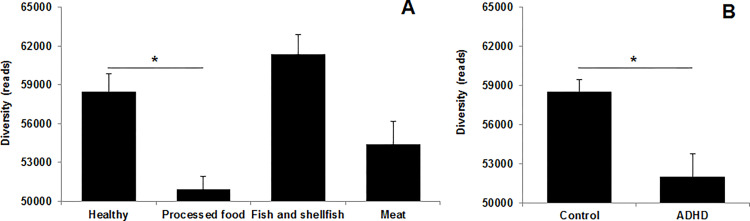
The α-diversity of the processed food group was significantly lower (p < 0.05) than that of the healthy group (Fig 3A, and that of the ADHD high-risk group was markedly (p < 0.05) lower than that of the control group (Fig 3B).
Fig 3. The α-diversity of the gut microbiota.
(A) Dietary patterns; (B) ADHD scores. Values are expressed as the mean ± SEM. Statistical significance was analyzed by (A) one-way ANOVA with Duncan’s multiple range test (*p < 0.05) and (B) Student’s t-test (*p < 0.05).
The results of the correlation analysis for ADHD scores, dietary patterns, and gut microbiota composition are shown in Table 5. The processed dietary pattern was positively (p < 0.05) correlated with the relative abundance of Escherichia strains, whereas it was negatively (p < 0.05) associated with the α-diversity and composition of Bifidobacterium, Faecalibacterium, and Ruminococcus strains. Furthermore, the ADHD score was significantly (p < 0.05) associated with increased composition of Escherichia strains, whereas markedly (p < 0.05) was associated with decreased α-diversity and the relative abundance of Bifidobacterium, Faecalibacterium, and Ruminococcus strains. These results showed that processed food dietary patterns may increase ADHD scores, disrupting the balance and diversity of the gut microbiota.
Table 5. Correlation analysis among the dietary pattern, attention deficit hyperactivity disorder (ADHD) score, and gut microbiota composition.
| AD score | HD score | Healthy | PF | FS | Meat | Diversity | BIF | ESCH | FAE | RUM | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD score | 1 | ||||||||||
| HD score | .975** | 1 | |||||||||
| Healthy | -.309 | -.314 | 1 | ||||||||
| PF | .387* | .372* | -.046 | 1 | |||||||
| FS | -.047 | -.059 | -.050 | .003 | 1 | ||||||
| Meat | .266 | .264 | -.064 | -.029 | .001 | 1 | |||||
| Diversity | -.370* | -.378* | .255 | -.388* | .037 | -.225 | 1 | ||||
| BIF | -.338* | -.370* | .234 | -.322* | -.228 | -.112 | .300 | 1 | |||
| ESCH | 338* | 319* | -.214 | .380* | -.008 | .208 | -.422** | -.459** | 1 | ||
| FAE | -.360* | -.364* | .094 | -.347* | -.092 | -.233 | .412** | .322* | -.667** | 1 | |
| RUM | -.341* | -.371* | .288 | -.315* | .087 | -.160 | .456** | .437** | -.642** | .461** | 1 |
AD, attention deficit; HD, hyperactivity disorder; PF, processed food; FS, fish and shellfish; BIF, Bifidobacterium; ESCH, Escherichia; FAE, Faecalibacterium; RUM, Ruminococcus. Correlation analysis was performed using the Pearson correlation coefficient (*p < 0.05 or **p < 0.01)
Fecal short-chain fatty acids
The fecal SCFAs of the participants were measured by gas chromatography. No significant decrease in the concentration of propionate was noted between the control and experimental groups (Table 6). However, the concentration of fecal butyrate was markedly (p < 0.05) lower in the processed food group than in the healthy group, and the ADHD group showed significantly (p < 0.05) lower concentrations of fecal acetate and butyrate than those of the control group. The analysis of correlation among ADHD score, dietary patterns, and SCFA concentration in feces is shown in Table 7. The processed dietary pattern was negatively (p < 0.05) correlated with the concentration of butyrate, and ADHD score was markedly (p < 0.05) associated with a decrease in the concentration of butyrate, compared to the control group.
Table 6. Concentrations of short-chain fatty acids in the feces of Korean elementary school students according to their dietary patterns and ADHD scores.
| Fatty acids (mmol/g) | Dietary patterns | ADHD scores | ||||||
|---|---|---|---|---|---|---|---|---|
| Healthy | Processed food | Fish and shellfish | Meat | p-value | Control | ADHD | p-value | |
| Acetic acid | 6.44±0.151) | 6.05±0.17 | 6.21±0.15 | 5.89±0.16 | NS2) | 6.34±0.09 | 5.73±0.07* | < .054) |
| Propionic acid | 4.44±0.20 | 4.25±0.12 | 4.45±0.08 | 4.37±0.12 | NS | 4.42±0.08 | 4.27±0.11 | NS5) |
| Butyric acid | 3.91±0.05a | 3.42±0.07b | 3.87±0.10a | 3.67±0.09ab | < .053) | 3.96±0.03 | 3.60±0.6* | < .05 |
1) Values are represented as the mean ± SEM
2) NS, There were no significant difference by one-way ANOVA test
3) abc Groups with different letters in the same row are significantly different by one-way ANOVA with Duncan’s multiple range test
4) The statistical significance between the control and ADHD groups was analyzed by the Student’s t-test (*p < 0.05)
5) NS, there were no significant differences by Student’s t-test
Table 7. Correlation analysis among the dietary patterns, ADHD scores, and the concentrations of short-chain fatty acids.
| AD score | HD score | Healthy | PF | FS | Meat | AA | PA | BA | |
|---|---|---|---|---|---|---|---|---|---|
| AD score | 1 | ||||||||
| HD score | .975 | 1 | |||||||
| Healthy | -.309 | -.314 | 1 | ||||||
| PF | .387* | .372* | -.046 | 1 | |||||
| FS | -.047 | -.059 | -.050 | .003 | 1 | ||||
| Meat | .266 | .264 | -.064 | -.029 | .001 | 1 | |||
| AA | -.287 | -.274 | .268 | -.185 | -.204 | .020 | 1 | ||
| PA | -.104 | -.110 | -.035 | -.065 | .008 | .177 | .308 | 1 | |
| BA | -.368* | -.346* | .286 | -.365* | -.114 | -.031 | .556** | .314 | 1 |
AD, attention deficit; HD, hyperactivity disorder; PF, processed food; FS, fish and shellfish; AA, acetic acid; PA, propionic acid; BA, butyric acid. Correlation analysis was performed using the Pearson correlation coefficient (*p < 0.05 or **p < 0.01)
Discussion
ADHD Rating Scale (ARS)-VI is a tool developed to screen ADHD for children and adolescents aged 5 to 18 [31]. The ARS-VI was consisted of nine question related to the attention deficit and hyperactivity disorders, respectively. Each question had a four-point answer format (0 = never, 1 = sometimes, 2 = often, 3 = very often). We evaluated ADHD scores of elementary school students using K-ARS, which is the Korean version of ARS-VI. The K-ARS have been generally using in research related to ADHD in Korea to screen children’s ADHD, and both attention deficit and hyperactivity disorders were also found a good internal consistency. In this study, we classified the dietary patterns of participants into four categories using a dish-based semi-quantitative FFQ and Can Pro 4.0 (The Korean Nutrition Society) database. Four dietary patterns were found good validation, and explained 44.56% of the variance in food intake. A validation study on our dietary patterns can be also found elsewhere [27, 28]. Therefore, in this study, we guess that measurements for ADHD and dietary patterns are valuable when compared to other studies. However, there is a limitation in that the ADHD score was assessed by their parents, the correlation between ADHD symptoms and dietary patterns can be biased to invalid as participants’ parents may underestimate ADHD symptoms.
In this study, participants who consume probiotics, antibiotics, and health supplements were excluded from the survey, and we investigated the basic characteristics of participants, such as sex, age, BMI, parental smoking, parental income. The basic characteristics of participants did not significantly relate to the dietary patterns and ADHD scores. However, the covariates/confounders may not be sufficiently considered, resulting in bias of the research results.
Dietary intake plays an important role in the modulation of gut microbiota and brain neurotransmission. Mental disorders are regulated by the function of the brain and an imbalance of the gut microbiota composition is likely to increase the incidence of mental disorders, such as ADHD [29]. Some studies have indicated that dietary patterns are associated with ADHD symptoms [26] and we identified four major dietary patterns (healthy, processed food, fish and shellfish, and meat), explaining 44.65% of the variation in this study. This value was similar to that reported in other studies [26, 28, 32]. Healthy, shellfish, and meat dietary patterns were not statistically correlated with ADHD scores. However, processed food dietary patterns, which are characterized by high intake of fats, sweets, and fast foods, were positively correlated with the increase in ADHD score. Fast foods and sweet dietary patterns have been reported to be associated with an exacerbation of ADHD symptoms owing to the high intake of saturated fat and refined sugar [25, 32] and we found that saturated fat was positively correlated to processed food dietary patterns as well as ADHD scores. A diet high in saturated fat may reduce the relative abundance of Alistipes strains, which are known to produce butyrate [33], and enhance the growth of harmful bacteria, such as the Enterobacter strains [34]. In addition, Zhang and Yang [35] reported that a high-saturated fat diet can decrease gut microbiota diversity, resulting in gut microbiota dysbiosis. Processed food dietary patterns can induce higher intake of saturated fat, which contributes to gut microbiota dysbiosis; these alterations may aggravate ADHD symptoms.
The composition of the gut microbiota plays an important role in the maintenance of health by regulating metabolites, such as fecal SCFAs, which can protect the host from external pathogens and potentially harmful bacteria residing in the intestine [36]. The results of qPCR revealed that the processed food dietary pattern was markedly associated with an increased number of Enterobacter strains, whereas the ratio of Lactobacillus to Enterobacter strains was significantly lower than that of the healthy group. Moreover, the number of harmful bacteria, such as the Clostridium and Enterobacter strains, was considerably higher, whereas the ratio of Lactobacillus to Enterobacter strains was significantly lower in the ADHD group than in the control group. The ratio of Lactobacillus to Enterobacter strains is known as an index of a healthy intestine, and a high index indicates improvement in intestinal protection against opportunistic pathogens [37]. A variety of studies have shown that the relative abundance of harmful bacterial strains was higher in children with psychological problems than in healthy children [38–40].
Heat map analysis of similar clusters analyzed using the CLcommunity program showed that the dietary intake was closely associated with the alteration of the gut microbiota composition, and these changes were further correlated with the ADHD score. The gut microbiota profile of the processed food and ADHD groups mostly belonged to distinct clusters, unlike that of the control group. The relative abundance of Bifidobacterium, Faecalibacterium, and Ruminococcus strains, which are closely associated with ADHD, were significantly lower in the processed food group and ADHD group than in the control group. Beneficial bacteria, such as the Bifidobacterium strains, modulate the balance of gut microbiota composition and interfere with the toxicity of pathogenic strains and may have beneficial effects on psychological health [41, 42]. It was also reported that the relative abundance of Faecalibacterium and Ruminococcus strains was markedly reduced in children with ADHD [43]. Moreover, Faecalibacterium strains can induce the production of SCFAs [44], which contribute to the modulation of the intestinal environment to facilitate the growth of beneficial bacteria, such as the Bifidobacterium strains [22]. This can contribute to the prevention and improvement of disease through the balanced composition of the gut microbiota. The processed food and ADHD groups had significantly reduced α-diversity of fecal microbiota compared to that of the control group, and the α-diversity of gut microbiota composition tended to decrease in children with ADHD [45].
The gut microbiota can synthesize neurotransmitters, such as dopamine [46], which can regulate the attention, cognition, memory, learning, and emotional behavior, and Ayano [16] reported that the reduction of dopamine in the brain was associated with the exacerbation of ADHD symptoms. The balance of gut microbiota composition plays an important role in inducing the synthesis of dopamine, whereas the collapse of a well-balanced gut microbiota may interfere with the production of dopamine [47].
The concentration of fecal SCFAs was altered depending on the dietary pattern or ADHD score. No significant difference in propionate concentration of feces was noted between the control and experimental groups; however, the processed food group and the ADHD group showed a marked decrease in the concentration of acetate or butyrate compared to that in the control group. Fecal SCFAs, such as acetate and butyrate, can regulate intestinal homeostasis and have a direct effect on the gut microbiota composition [21]. Several studies have shown that fecal SCFAs inhibit the growth of pathogenic bacteria, such as the Salmonella, E. coli, and Clostridium strains [48, 49]. In addition, butyrate plays an important role in the modulation of neuronal excitability and ENS activity [50], and Dalile et al. [51] reported that fecal SCFAs play a key role in the interaction of microbiota-gut-brain. Fecal SCFAs are recognized by receptors, such as enteroendocrine and enterocyte cells, and they can modulate brain function through the synthesis of neurotransmitters [47]. The combination of fecal SCFAs and receptors stimulates signaling to the brain through the vagal pathway by inducing the secretion of neurotransmitters, such as serotonin, which is known to be reduced in patients with ADHD [17]. Through which fecal SCFAs may be able to affect cognition and emotional functions of the brain of patients with ADHD.
Our study several limitations should be considered. First, the number of samples is insufficient, and there is a limitation in that the evaluation of ADHD was conducted by their parent-assessment, not by a doctor’s diagnosis. The association between ADHD symptoms and dietary patterns can be biased to invalid as participants may underestimate ADHD symptoms. Second, there is a limitation in that bias may occur in the research results because covariates for the characteristics of the study participants are not sufficiently considered. Future studies need to increase the number of samples and analyse the stronger association between ADHD and dietary patterns using other methods of evaluating ADHD.
In summary, we analyzed the dietary patterns and ADHD scores of 40 school-age children using FFQ or K-ARS. The feces of the participants were collected to investigate the composition of the gut microbiota and SCFAs. The dietary patterns of candidates were divided into four groups: healthy, processed food, fish and shellfish, and meat. The participants were also separated into two groups according to their ADHD scores: control or ADHD group. The ADHD score of the processed food group was significantly higher than that of the healthy group. The abundance of harmful bacteria, such as Enterobacteria, E. coli, and Clostridium, was considerably higher, while that of beneficial bacteria, such as the Bifidobacterium and Ruminococcus strains, was remarkably lower in the processed food, meat, and ADHD groups than the control group. Therefore, the finding that the processed food and ADHD groups showed remarkably reduced α-diversity of gut microbiota and significantly reduced concentrations of acetic acid or butyric acid in the feces compared to the control group. Since our study has limitations according to the number of samples and the ADHD assessment method, it is necessary to conduct research through connection with hospitals in the future to derive more meaningful results.
Acknowledgments
We appreciate to the elementary school students and their parents who participated in this study.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was supported by National Research Foundation of Korea (2016-R1D1A1B03936129). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016; 387: 1240–1250. doi: 10.1016/S0140-6736(15)00238-X [DOI] [PubMed] [Google Scholar]
- 2.Larsson H, Ryden E, Boman M, Langstrom N, Lichtenstein P, Landen M. Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br J Psychiatry. 2013; 203: 103–106. doi: 10.1192/bjp.bp.112.120808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reimherr FW, Marchant BK, Gift TE, Steans TA. ADHD and anxiety: clinical significance and treatment implications. Curr Psychiatry Rep. 2017; 9: 109. doi: 10.1007/s11920-017-0859-6 [DOI] [PubMed] [Google Scholar]
- 4.Delibas DH, Erdogan E, Gulseren S. Evaluation of clinical and suicidal behavior characteristics among urban, Turkish middle-age depressive patients with comorbid attention deficit hyperactivity disorder. Indian J Psychiatry. 2019; 61: 612–617. doi: 10.4103/psychiatry.IndianJPsychiatry_448_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marangoni C, De Chiara L, Faedda GL. Bipolar disorder and ADHD: comorbidity and diagnostic distinctions. Curr Psychiatry Rep. 2015; 17: 604. doi: 10.1007/s11920-015-0604-y [DOI] [PubMed] [Google Scholar]
- 6.Pickard JM, Zeng MY, Caruso R, Núñez G. Gut microbiota: role in pathogen colonization, immune responses, and inflammatory disease. Immunol Rev. 2017; 279: 70–89. doi: 10.1111/imr.12567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyedi M, Gholami F, Samadi M, Djalali M, Effatpanah M, Yekaninejad MS, et al. The effect of vitamin D3 supplementation on serum BDNF, dopamine, and serotonin in children with attention-deficit/hyperactivity disorder. CNS Neurol Disord Drug Targets. 2019; 18: 496–501. doi: 10.2174/1871527318666190703103709 [DOI] [PubMed] [Google Scholar]
- 8.Patterson E, Ryan PM, Cryan JF, Dinan TG, Ross RP, Fitzgerald GF, et al. Gut microbiota, obesity and diabetes. Postgrad Med J. 2016; 92: 286–300. doi: 10.1136/postgradmedj-2015-133285 [DOI] [PubMed] [Google Scholar]
- 9.Das B, Nair GB. Homeostasis and dysbiosis of the gut microbiome in health and disease. J Biosci. 2019; 44: 117. doi: 10.1007/s12038-019-9926-y [DOI] [PubMed] [Google Scholar]
- 10.Fung TC. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol Dis. 2019; 136: 104714. doi: 10.1016/j.nbd.2019.104714 [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Cao S, Zhang X. Modulation of gut microbiota-brain axis by probiotics, prebiotics, and diet. J Agric Food Chem. 2015; 63: 7885–7895. doi: 10.1021/acs.jafc.5b02404 [DOI] [PubMed] [Google Scholar]
- 12.Morrison DJ, Preston T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 2016; 7: 189–200. doi: 10.1080/19490976.2015.1134082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nerurkar A. "The gut-brain axis: how to manage pain caused by this cross-talk": an overview of the symposium. Glob Adv Health Med. 2015; 4: 61–64. doi: 10.7453/gahmj.2015.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghoshal UC. Gut microbiota-brain axis modulation by a healthier microbiological microenvironment: facts and fictions. J Neurogastroenterol Motil. 2018; 24: 4–6. doi: 10.5056/jnm17150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Crescenzo F, Cortese S, Adamo N, Janiri L. Pharmacological and non-pharmacological treatment of adults with ADHD: a meta-review. Evid Based Ment Health. 2017; 20: 4–11. doi: 10.1136/eb-2016-102415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayano G. Dopamine: receptors, functions, synthesis, pathways, locations and mental disorders: review of literatures. J Ment Disord Treat. 2016; 2: 2. doi: 10.4172/2471271X.1000120 [DOI] [Google Scholar]
- 17.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol. 2020; 11: 25. doi: 10.3389/fendo.2020.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aarts E, Ederveen THA, Naaijen J, Zwiers MP, Boekhorst J, Timmerman HM, et al. Gut microbiome in ADHD and its relation to neural reward anticipation. PLos ONE. 2017; 12: e0183509. doi: 10.1371/journal.pone.0183509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010; 107: 14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bibbo S, Ianiro G, Giorgio V, Scaldaferri F, Masucci L, Gasbarrini A, et al. The role of diet on gut microbiota composition. Eur Rev Med Pharmacol Sci. 2016; 20: 4742–4749. [PubMed] [Google Scholar]
- 21.Feng W, Ao H, Peng C. Gut microbiota, short-chain fatty acids, and herbal medicines. Front Pharmacol. 2018; 9: 1354. doi: 10.3389/fphar.2018.01354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simeng L, Enyao L, Zhenyu S, Dongjun F, Guiqin D, Miaomiao J, et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Scientific Reports. 2019; 9: 287. doi: 10.1038/s41598-018-36430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maslowski KM, Mackay CR.Diet, gut microbiota and immune responses. Nat Immunol. 2011; 12: 5–9. doi: 10.1038/ni0111-5 [DOI] [PubMed] [Google Scholar]
- 24.Ronald D, Hills J, Benjamin AP, Hillary RM, Cody AB, Steven CS, et al. Gut microbiome: Profound implications for diet and disease. Nutrients. 2019; 11: 1613. doi: 10.3390/nu11071613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millichap JG, Yee MM. The diet factor in attention-deficit/hyperactivity disorder. Pediatrics. 2012; 129: 330–337. doi: 10.1542/peds.2011-2199 [DOI] [PubMed] [Google Scholar]
- 26.Yan S, Cao H, Gu C, Ni L, Tao H, Shao H, et al. Dietary patterns are associated with attention-deficit/hyperactivity disorder (ADHD) symptoms among preschoolers in mainland China. Eur J Clin Nutr. 2018; 72: 1517–1523. doi: 10.1038/s41430-018-0131-0 [DOI] [PubMed] [Google Scholar]
- 27.An MJ, An HJ, Hwang HJ, Kwon HJ, Ha MN, Hong YC, et al. Dietary factors associated with attention deficit hyperactivity disorder (ADHD) in school-aged children. Korean J Community Nutr. 2018; 23: 397–410. doi: 10.5720/kjcn.2018.23.5.397 [DOI] [Google Scholar]
- 28.Woo HD, Kim DW, Hong YS, Kim YM, Seo JH, Choe BM, et al. Dietary patterns in children with attention deficit/hyperactivity disorder (ADHD). Nutrients. 2014; 6: 1539–1553. doi: 10.3390/nu6041539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SH. Current Status and Future Improvement of the Korean ADHD Rating Scale-4. J Emot Behav Disord. 2015; 31: 227–259. [Google Scholar]
- 30.Han KS, Balan P, Molist gasa F, Boland M. Green kiwifruit modulates the colonic microbiota in growing pigs. Lett Appl Microbiol. 2011; 52: 379–385. doi: 10.1111/j.1472-765X.2011.03012.x [DOI] [PubMed] [Google Scholar]
- 31.DuPaul GJ, Power TJ, McGoey KE, Ikeda MJ, Anastopoulos AD. Reliability and validity of parent and teacher ratings of attention-deficit/hyperactivity disorder symptoms. J Psychoeduc Assess. 1998; 16: 55–68. [Google Scholar]
- 32.Shan L, Xiaoli W, Feng J. Gut-brain psychology: rethinking psychology from the microbiota-gut-brain axis. Front Integr Neurosci. 2018; 12: 33. doi: 10.3389/fnint.2018.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F, Wu F, Zou S, Chen Y, Feng C, Fan G. Dietary, nutrient patterns and blood essential elements in Chinese children with ADHD. Nutrients. 2016: 8; 385. doi: 10.3390/nu8060352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep. 2016; 6: 37589. doi: 10.1038/srep37589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen D, Yang Z, Chen X, Huang Y, Yin B, Guo F, et al. Effect of Lactobacillus rhamnosus hsryfm 1301 on the gut microbiota and lipid metabolism in rats fed a high-fat diet. J Microbiol Biotechnol. 2015; 25: 687–695. doi: 10.4014/jmb.1409.09085 [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016; 22: 8905–8909. doi: 10.3748/wjg.v22.i40.8905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Paola M, Bonechi E, Provensi G, Costa A, Clarke G, Ballerini C, et al. Oleoylethanolamide treatment affects gut microbiota composition and the expression of intestinal cytokines in peyer’s patches of mice. Sci Rep. 2018; 8: 1488. doi: 10.1038/s41598-018-32925-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castillo M, Martin-Orue SM, Nofrarias M, Manzanilla EG, Gasa J. Changes in caecal microbiota and mucosal morphology of weaned pigs. Vet Microbiol. 2007; 124: 239–247. doi: 10.1016/j.vetmic.2007.04.026 [DOI] [PubMed] [Google Scholar]
- 39.Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, et al. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015; 138: 179–187. doi: 10.1016/j.physbeh.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 40.Argou-Cardozo I, Zeidan-Chulia F. Clostridium bacteria and autism spectrum conditions: a systematic review and hypothetical contribution of environmental glyphosate levels. Med Sci. 2018; 6: E29. doi: 10.3390/medsci6020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carissim C, Laudadio I, Palone F, Fulci V, Cesi V, Cardona F, et al. Unctional analysis of gut microbiota and immunoinflammation in children with autism spectrum disorders. Dig Liver Dis. 2019; 51: 1366–1374. doi: 10.1016/j.dld.2019.06.006 [DOI] [PubMed] [Google Scholar]
- 42.Servin AL. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens pathogens. FEMS Microbiol Rev 2004; 28: 405–440. doi: 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 43.Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, et al. The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: a randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutr Neurosci 2016; 19: 387–395. doi: 10.1179/1476830515Y.0000000023 [DOI] [PubMed] [Google Scholar]
- 44.Wan L, Ge WR, Zhang S, Sun YL, Wang B, Yang G. Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder. Front Neurosci. 2020; 14: 127. doi: 10.3389/fnins.2020.00127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foditsch C, Santos TMA, Teixeira AGV, Pereira RVV, Dias JM. Gaeta N, Bicalho RC. Isolation and characterization of Faecalibacterium prausnitzii from calves and piglets. PLoS One. 2014; 9: e116465. doi: 10.1371/journal.pone.0116465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prehn-Kristensen A, Zimmermann A, Tittmann L, Lieb W, Schreiber S, Baving L, Fischer A. Reduced microbiome alpha diversity in young patients with ADHD. PLoS One. 2018; 13: e0200728. doi: 10.1371/journal.pone.0200728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Tian T, Mao Q, Zou T, Zhou CJ, Xie J, et al. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl Psychiatry. 2020; 10: 350. doi: 10.1038/s41398-020-01038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun Y, O’Riordan MX. Regulation of bacterial pathogenesis by intestinal short-chain fatty acids. Adv Appl Microbiol. 2013; 85: 93–118. doi: 10.1016/B978-0-12-407672-3.00003-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fachi JL, Felipe JS, Pral LP, da Silva BK, Corra RO, de Andrade MCP, et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019; 27: 750–761. doi: 10.1016/j.celrep.2019.03.054 [DOI] [PubMed] [Google Scholar]
- 50.Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, et al. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010; 138: 1772–1782. doi: 10.1053/j.gastro.2010.01.053 [DOI] [PubMed] [Google Scholar]
- 51.Dalile B, Van Oudenhove L, Vervliet B, Verbeke K. The role of short-chain fatty acids in microbiota-gut-brain communication. Nat Rev Gastroenterol Hepatol. 2019; 16: 461–478. doi: 10.1038/s41575-019-0157-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.