Abstract
In December 2019, cases of pneumonia caused by infection with the previously unknown severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leading to coronavirus disease 2019 (COVID-19), were identified. Typical manifestations of COVID-19 are fever, cough, fatigue and dyspnoea. Initially, it was thought that the mechanism of action of SARS-CoV-2 was only associated with respiratory tract invasion, but it was later revealed that the infection might involve many other organs and systems, including the central and peripheral nervous systems. Neurological complications associated with SARS-CoV-2 infection include encephalopathy, encephalitis, meningitis, acute disseminated encephalomyelitis (ADEM), ischaemic and haemorrhagic stroke and cerebral venous sinus thrombosis. In cases of peripheral nervous system involvement, smell and taste disorders, myopathy or the signs and symptoms of Guillain‒Barré syndrome are observed. The most common early neurological complications, particularly during the first year of the epidemic, were anosmia and taste disorders, which, according to some studies, occurred in over 80 percent of patients with COVID-19. The proportion of patients with serious neurological manifestations was small compared to the global number of patients, but the numbers of SARS-CoV-2 infections and critical patients increased substantially. The experience from 2 years of the pandemic has shown that approximately 13% of infected patients suffer from severe neurological complications. The relationship between SARS-CoV-2 and the nervous system is not only a cause of neurological complications in previously healthy individuals but also directly and indirectly affects the courses of many nervous system diseases.
Keywords: SARS-CoV-2, COVID-19, Neurological complications
Introduction
Coronaviruses have been known for years as pathogens that commonly occur in humans and animals. They are responsible for approximately 10–20% of common cold and mild respiratory tract infections. At the end of 2019, a newly mutated form of coronavirus that causes severe pneumonia was identified in Wuhan, China. The disease spread rapidly, resulting in an epidemic in China and then a pandemic. In February 2020, the World Health Organization (WHO) defined ‘COVID-19’ as a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. On 11 March 2020, the WHO declared COVID-19 a pandemic. Nearly 550 million people were affected by the disease, and over 6 million people died over the next 2 years. Although the pandemic has not yet ended, it is already considered to be one of the most tragic epidemics in our history. The virus mainly attacks the respiratory system, but neurological complications following COVID-19 are common and develop in approximately 50 percent of patients [2].
Individuals with concomitant diseases (diabetes, hypertension or obesity) are at a particularly high risk of contracting the virus and suffering severe infection [3]. Genetic factors are also important. Certain patients with COVID-19 were seriously ill, while others experienced few signs or symptoms. New research has helped identify over 1000 genes related to a severe course of COVID-19. Moreover, it has been shown that severe COVID-19 is highly associated with a poor reaction of two immune cell types: natural killer (NK) cells and T lymphocytes (the ‘CD56 bright’ subtype) [4].
Due to its relationship with the nervous system, SARS-CoV-2 infection not only causes neurological complications in previously healthy individuals but also affects the courses of many nervous system diseases.
Neurological complications in COVID-19
Neurological complications in individuals with COVID-19 are common, particularly among hospitalised patients [5], who demonstrate higher rates than those with milder disease [6]. The most common neurological complications include smell and taste disorders, encephalopathy, encephalomyelitis, ischaemic stroke, intracranial haemorrhage and neuromuscular diseases [7]. The incidence of these complications is variously estimated (Table 1). Multiple mechanisms may underlie the development of neurologic manifestations of illness, including hypoxia, systemic illness, hypercoagulability, endothelial dysfunction, general critical illness, inflammatory response, and neurotropism of SARS-CoV-2. COVID-19 infection is associated with neurologic involvement in all stages; acute infection, subacute/post-infection, and growing evidence also suggests the postacute sequalae of COVID-19 during the chronic phase. The potential mechanisms of different neurological complications are shown in Table 2.
Table 1.
Prevalence of various neurological manifestations included in the systematic reviews and meta-analyses
| Meta-analysis | Studies, n | Pooled sample size, n | Symptom or disease | Pooled prevalence (95%CI), % | Symptom or disease | Pooled prevalence (95%CI), % |
|---|---|---|---|---|---|---|
| Vitalakumar et al. [8] | 240 | 190 785 | Headache | 14.6 (12.2–17.2) | Encephalitis | 0.6 (0.2–1.3) |
| Fatigue | 33.6 (29.5–37.8) | Malaise | 38.3 (24.7–52-9) | |||
| Olfactory dysfunction | 26.4 (21.8–31.3) | Confusion | 14.2 (6.9–23.5) | |||
| Gustatory dysfunction | 27.2 (22.3–32.3) | Movement disorders | 5.2 (1.7–10.4) | |||
| Vomiting | 6.7 (5.5–8.0) | Guillain–Barre syndrome | 6.9 (2.3–13.7) | |||
| Nausea | 9.8 (8.1–11.7) | Cerebrovascular diseases | 9.9 (6.8–13.4) | |||
| Dizziness | 6.7 (4.7–9.1) | Encephalopathy | 23.5 (14.3–34.1) | |||
| Myalgia | 21.4 (18.8–24.1) | Sleep disorders | 14.9 (1.9–36.8) | |||
| Neuralgia | 2.4 (0.8–4.7) | Seizure | 4.05 (2.5–5.8) | |||
| Arthralgia | 19.9 (15.3–25.0) | Altered mental status | 17.1 (12.3–22.5) | |||
| He et al. [9] | 168 | 292 693 | Myalgia | 33 (30–37 | Stroke | 12 (8–16) |
| Smell impairment | 33 (28–38) | Dizziness | 10 (8–13) | |||
| Taste dysfunction | 33 (27–39) | Vision impairment | 6 (3–9) | |||
| Altered mental status | 32 (22–43) | Intracerebral hemorrhage | 5 (3–9) | |||
| Headache | 29 (25–33) | Seizure | 4 (2–5) | |||
| Encephalopathy | 26 (16–38) | Encephalitis | 2 (1–3) | |||
| Alteration of consciousness | 13 (8–19) | Guillan-Barre Syndrome | 1 (0–3) | |||
| Vakili et al. [10] | 77 | 6 597 | Fatigue | 42.9 (36.7–49.3) | Headache | 10.1 (2.7–21) |
| Gustatory dysfunction | 35.4 (11.2–64.4) | Dizziness | 6.7 (3.7–10.5) | |||
| Anorexia | 28.9 (19.9–38.8) | Nausea | 5.9 (3.1–9.5) | |||
| Olfatory dysfunction | 25.3 (1.6–63.4) | |||||
| Premraj et al. [11] | 18 | 10 530 | Fatigue | 37 (25–28) | Anosmia | 12 (8–26) |
| Brain fog | 32 (10–54) | Dysguesia | 10 (6–14) | |||
| Memory issues | 28 (22–35) | Headache | 15 (4–26) | |||
| Attention disorder | 22 (7–36) | Anxiety | 23 (14–32) | |||
| Myalgia | 17 (9–25) | Depression | 17 (10–24) | |||
| Sleep disturbances | 31 (19–42) | |||||
| Misra et. al. [12] | 350 | 145 721 | Fatigue | 32 (30–35) | Delirium | 11 (7–16) |
| Myalgia | 20 (18–23) | Dizziness | 7 (5–8) | |||
| Taste impairment | 21 (15–29) | Alteration of consciousness | 7 (5–10) | |||
| Smell impairment | 19 (13–25) | Vision impairment | 4 (1–9) | |||
| Headache | 13 (12–15) | Agitation | 45 (3–93) | |||
| Rogers et al. [13] | 147 | 99 905 | Anosmia | 43.1 (35.2–51.3 | Headache | 20.7 (16.1–26.1) |
| Weakness | 40.0 (27.9–53.5) | Anxiety | 15.9 (5.6–37.7) | |||
| Fatigue | 37.8 (31.6–44.4) | Altered mental status | 8.2 (4.4–14.8) | |||
| Dysguesia | 37.2 (29.8–45.3) | Dizziness/vertgo | 6.4 (4.0–10.0) | |||
| Myalgia | 25.1 (19.8–31.3) | Tinnitus | 3.5 (1.7–7.4) | |||
| Depression | 23.0 (11.8–40.2) | Ischemic stroke | 1.9 (1.3–2.8) | |||
| Sleep disorder | 23.5 (12.0–40.9) | Hemorrhagic stroke | 0.4 (0.3–0.7) |
Table 2.
COVID-19 associated neurological complications and potential pathophysiology
| Clinical syndrome | Potential pathophysiology | Refs. |
|---|---|---|
| Parainfectious manifestations | ||
| Anosmia | Infection of olfactory epithelium or nerve | [14–16] |
| Ischemic stroke | Cytokine overproduction; Vascular endothelial damage, Endothelial dysfunction; Hypercoagulable state | [17–26] |
| Hemorrhagic stroke | Decrease in ACE2 levels; Blood pressure increase; Coagulopathy; CVST | [27–30] |
| Encephalopathy, encephalitis | Cytokine overproduction; Vascular endothelial damage; Direct CNS invasion; Hypoxia; Autoimmunity; Medication effects | [17, 28, 31–41] |
| Myalgia/rhabdomyolysis | Infection of muscle; Metabolic derangements; Medication effects | [15] |
| Myoclonus | Autoimmune cerebellar/brainstem damage; Hypoxia | [47] |
| Seizure | Fever; Hypoxia; Multiorgan failure; Metabolic derangements; Cytokine overproduction; Direct CNS invasion | [14, 32] |
| Headache | Hypoxia; Activation of peripheral trigeminal nerve endings; Cytokine overproduction; Direct CNS invasion; Hypercoagulable state | [44, 45] |
| Post-viral syndromes | ||
| Brain fog/Long Covid | Autoimmune; Neuroinflammation; Neurodegeneration | [42–47] |
| Guillain–Barre syndrome/polyneuropathy | Autoimmunity/Molecular mimicry | [46] |
| Depression, anxiety and sleep disorders | Cytokine overproduction/Neuroinflammation; Direct CNS invasion | [48–51] |
| Transverse myelitis | Immune cell mediated | [14–16] |
| Acute disseminated encephalomyelitis | T cell mediated | [15, 16] |
ACE2 angiotensin-converting enzyme-2; CVST cerebral venous sinus thrombosis; CNS central nervous system
Pathophysiological aspects of neurological complications following SARS-CoV-2 infection
SARS-CoV-2 virus can penetrate the central nervous system and affect both neurons and glial cells, causing neurological complications. Neurological pathologies result from different pathomechanisms. Findings indicate that SARS-CoV-2 enters host cells after binding to angiotensin-converting enzyme type 2 (ACE2) found on the cell surface of various tissues [14–16, 20, 52, 53]. By binding to ACE2 in many organs, including skeletal muscle, vascular endothelium and the nervous system, SARS-CoV-2 can enter the central nervous system, damaging blood vessels [52, 53]. Damage to the endothelium of the cerebral vessels and an increase in cerebral blood pressure can lead to rupture of blood vessels, resulting in massive intracerebral haemorrhage [27, 52]. On the other hand, accompanying COVID-19 infection, hypercoagulability and thromboembolic conditions can cause ischaemic stroke [19].
In the brain, ACE2 is mainly expressed within the brainstem, in the region responsible for cardiac and vascular function, which includes the nucleus of the solitary tract and the paraventricular nuclei [16, 20, 22].
A route of spread of infection from peripheral nerves to the central nervous system via synaptic connections has also been confirmed [54, 55]. Due to the particular structure of the olfactory nerve and olfactory fibres in the nasal cavity, the transport of SARS-CoV-2 along this nerve may be an example of the spread of infection via a neuronal route. Coronaviruses can colonise the nasal cavity and then penetrate the brain and cerebrospinal fluid via the olfactory nerve and bulb pathways, causing an inflammatory and demyelinating response [15, 16].
Other important gateways of brain infection may be the cervical and olfactory vessels of the lymphatic system for SARS-CoV-2 [56–58]. Post-mortem histological findings indicate that SARS-CoV-2 can damage the endothelium, causing lymphocytic inflammation in many internal organs such as the heart, kidneys, lungs, liver, small intestine and the lymphatic drainage system of the brain [56–58].
Another mechanism of damage is the generation of a global systemic inflammatory response (SIRS), resulting in excessive production of interleukin (IL-6, IL-12, IL-15) and tumour necrosis factor-alpha (TNF-α). This activates glial cells and induces a powerful proinflammatory state of the CNS, leading to severe hypoxia, resulting in cerebral vasodilation, cerebral oedema and ischaemia [54, 55].
Nerve cell damage during SARS-CoV-2 infection is favoured by direct infection of the central nervous system via the trans-synaptic pathway, damage to the blood–brain barrier, an increased inflammatory response, and prolonged hypoxia. In patients with severe COVID-19, nervous system involvement is more specific and includes impaired consciousness, encephalitis, cerebrovascular complications and/or muscle damage.
The risk of neuropsychiatric complications of COVID-19 combined with the risk of death was highest with the delta variant (B.1.617.2). In particular, the risk of cognitive deficits, epilepsy and ischaemic stroke increased [59, 60]. These risks were compounded by an increased risk of death (HR for the composite of death or cognitive deficit was 1.38 (95% CI 1.27–1.48), while the HR for cognitive deficit alone was 1.13 (1.02–1.26) [61]. The profile of neurological and psychiatric complications during the predominance of the alpha variant (B.1.1.7) and the omicron variant (B.11.529) was broadly similar to the pandemic period caused by the delta variant [59]. There was no difference in the risk of cognitive deficits, epilepsy, ischaemic stroke, psychotic disorders and mood disorders. Despite a similar rate of neurological complications, the reduced composite risk of hospitalisation and death after the omicron variant may reflect the attenuated course of SARS-CoV-2 infection [61].
Neurological manifestations have been reported in COVID-19 cases, even in patients with only a mild form of illness, and can persist as what is called long COVID. Researchers at the Boston University School of Medicine (USA) studied 16,225 patients from 179 hospitals in 24 different countries as part of the Society of Critical Care Medicine (SCCM) VIRUS study [62]. During the first year of the pandemic, 13% of patients presenting with COVID-19 developed serious neurological signs or symptoms. The most common manifestation at admission, with 1656 cases (10.2%), was encephalopathy, which alters brain function or structure. A further 331 patients (2.0%) experienced a stroke, 243 (1.5%) had seizures, and 73 (0.5%) had meningitis or encephalitis on admission or during their stay in the hospital [62].
Smell and taste disorders
The most common neurological complications are smell and taste disorders. In one study, anosmia and taste disorders were reported as early signs of COVID-19 that affected over 80 percent of patients [63]. A meta-analysis of 83 studies with more than 27,000 subjects found that smell dysfunction affected 48% of patients (95% CI 41.2–54.5) [64]. These symptoms may be the initial COVID-19 manifestations and may appear without nasal congestion or discharge; however, they are rarely the only COVID-19 clinical symptoms. Young individuals report anosmia more commonly than older patients. It also develops more frequently in women than in men [65]. The prognosis of smell and taste disorders associated with COVID-19 is generally favourable, as most patients report overall regression or substantial improvement within 2–3 weeks. Thus, the prognosis is probably better than in the case of other smell disorder aetiologies. However, serious and long-term deficits remain in 10% to 20% of cases [7]. There are limited evidence-based methods of anosmia treatment [12]. Fortunately, smell and taste disorders resolve spontaneously in most patients with COVID-19, and they do not require specific management. Nevertheless, when smell disorders persist for a longer time (> 4 weeks), smell training techniques may be beneficial as a therapy [66].
Headache
Headache is experienced by 12–70% of patients with COVID-19. It persisted in 3.6% of the patients 1 month after the resolution of fever and difficulty breathing [67]. Various types of headaches may appear during the disease. Headaches reported in studies on COVID-19 complications include tension-type headaches and headaches resulting from systemic viral infections that cause chronic cough or hypoxia. Migraine-type headache exacerbation also occurred [68]. It is suggested that headaches appearing from the seventh day of the onset of clinical symptoms may be associated with the cytokine storm that is typical of this infection. Unfortunately, there are no available data on specific options for COVID-19-related headache treatment. While describing the cases, experts recommend routine management typical for conditions that are not related to SARS-CoV-2 infection [68].
Acute encephalopathy and encephalitis associated with COVID-19
In most cases, encephalopathy develops in critical patients. Extremely rare, encephalopathy-related delirium may be an early or even asymptomatic manifestation [69]. Delirium developed in 55% of the subjects participating in a cohort study that involved 2088 patients with COVID-19 who had been admitted to intensive care units [69]. In another study, Shah et al. identified acute encephalopathy in 1092 (8.7%) subjects among 12,601 hospitalised patients [70]. Encephalopathy was observed in 31.8% of the 509 hospitalised patients with COVID-19 participating in another study [5]. Its aetiology is frequently multifactorial. It mainly affects older male patients with a history of neurological disorders, cancer, cerebral vasculitis, chronic kidney disease, diabetes, dyslipidaemia, cardiac failure, hypertension or smoking [5].
The manifestations of encephalopathy and encephalitis include neuropsychological disorders, agitation and delirium, motor disorders with extrapyramidal symptoms, coordination disorders, impaired consciousness, seizures and focal neurological deficits [7]. IL-2, IL-6, IL-7 and TNF-α are biomarkers identified in patients with severe COVID-19 [71].
Patients with encephalopathy usually do not present signs of encephalitis in neuroimaging scans or cerebrospinal fluid (CSF) analysis, although there are some exceptions. In the case of encephalitis, CT or MRI scans may reveal structural lesions and cerebral oedema as well as haemorrhagic, necrotic, inflammatory or demyelinating lesions. Imaging scans show heterogeneous nonvascular lesions in up to 66.7% of patients [72]. CSF analysis is recommended to exclude meningitis. Electroencephalography (EEG) should be performed as part of diffuse cerebral dysfunction monitoring and to identify subclinical seizures or epilepsy. Symptomatic treatment is intended to control general homeostasis (electrolyte levels or temperature), and neuroleptics and antiepileptic drugs are administered as needed. High doses of corticosteroids may be used for impaired consciousness, acute cognitive deficits and seizures. Moreover, plasmapheresis and/or intravenous immunoglobulin therapy are suggested. In severe cases, intensive care with intubation and assisted ventilation, preventive anticoagulation therapy and continuous neuromonitoring may be required [7].
Acute disseminated encephalomyelitis
There are reports on the rising number of patients with acute disseminated encephalomyelitis (ADEM) [7]. Head MRI with contrast is essential for identifying inflammatory lesions. A haemorrhagic component may also appear. Normal findings from CSF analysis do not exclude a diagnosis of ADEM. For treatment, a high dosage (1–2 g/24 h) of intravenous corticosteroids for 3–5 days is recommended, followed by oral tapering. Immunoglobulins are recommended (0.4 g/kg body weight) in cases of an inadequate response to corticosteroids [7].
Stroke and cerebral venous thrombosis
Stroke seems to be relatively rare in COVID-19 [73–76]. The rates of COVID-19-related ischaemic stroke among hospitalised patients were 0.4–2.7%, while the rates of intracranial haemorrhage were 0.2–0.9% [77, 78]. Cerebral venous thrombosis (CVT) has been reported among COVID-19 patients [79, 80]. Twelve patients with CVT were identified within 3 months in a retrospective study of over 13,000 patients, which corresponds to an incidence of 8.8 per 10,000 patients [81]. An estimate for CVT incidence was 0.08% (95% CI 0.01–0.5) in a systematic review involving 34,331 hospitalised patients with SARS-CoV-2 infection [82]. An incidence for CVT of 0.04% was estimated in another large study of 292,080 patients with COVID-19 during the first year of the pandemic. The number of CVT-related hospitalisations and CVT-related in-hospital mortality did not change compared to the prepandemic period [83].
Stroke most commonly develops 1 to 3 weeks after the onset of COVID-19 symptoms [81, 84–86]. COVID-19 was an independent risk factor for in-hospital stroke (odds ratio [OR] 20.9; 95% CI 10.4–42.0) in a report comparing 86 patients with COVID-19 and stroke confirmed by imaging scans with 499 matched controls with a history of stroke but no COVID-19 a year prior [85].
The mean age of COVID-19 patients with stroke seems similar to that of patients without COVID-19. The median age was 65 years in a systematic analysis of 10 studies involving 160 patients with COVID-19 and ischaemic stroke. The median age at presentation was 68 years in the US national stroke registry, consisting of 1143 patients with COVID-19 and acute stroke compared to 71 years in the case of patients without COVID-19 during the same period [87].
Neuromuscular diseases
Myalgia is common in both the mild and severe forms of COVID-19. It has been observed in 22–63% of patients [7] and occurs together with increased creatinine kinase (CK) levels in more severe cases (19 vs. 5%); muscle damage (myopathy) is also possible [65]. Severe rhabdomyolysis is a rare, late complication associated with COVID-19 [88]. It was seen in 0.2% of patients, while CK level elevations were observed in 13.7%. Generalised myasthenia with positive acetylcholine receptor antibodies was found in three Italian patients 5 to 7 days after the onset of fever from SARS-CoV-2 infection [89]. Although it is speculated that the immune response to SARS-CoV-2 could be the cause of myasthenia, an alternative explanation is that the infection is only a trigger for the appearance of signs and symptoms in patients with preexisting myasthenia.
Exacerbation of a preexisting neuromuscular disease was also described in the case of amyotrophic lateral sclerosis (ALS) [7]. It seems that patients with neuromuscular conditions are not at a significantly higher risk of SARS-CoV-2 infection [7].
Management of inflammatory/autoimmune muscular diseases, neuromuscular junction conditions and peripheral nerve disorders should follow the current guidelines. Plasmapheresis and immunoglobulin therapy are recommended. It is suggested to delay rituximab or long-term oral immunosuppressants and to rely on the patient’s clinical status and medical history [7].
Guillain‒Barré syndrome
Guillain–Barré syndrome (GBS) develops in approximately 0.4% of patients with COVID-19, typically 5–10 days after the onset of viral infection [46]. The timing of its signs and symptoms in relation to the initial manifestations of COVID-19 suggests that GBS is a parainfectious disorder [90]. Some reports have demonstrated that the signs and symptoms could progress earlier and that they are more severe than those seen in typical GBS. In one study, three out of five patients required mechanical ventilation [91]. Only 5 GBS cases were identified among approximately 1200 patients with COVID-19 admitted to 3 hospitals in northern Italy within a month [46]. Most patients with COVID-19 and GBS present with progressive limb weakness that develops within 1 to 4 days [92].
There are no separate guidelines for GBS management in COVID-19. Patients should be treated similarly to other individuals with GBS.
Post-COVID-19 neurological syndrome
Post-COVID-19 syndrome (PCS) is a group of signs and symptoms that occur following SARS-CoV-2 infection and persist even for several months. The most common manifestations are associated with the respiratory (apnoea or cough) and cardiovascular systems. However, the most uncomfortable conditions are neurological and psychiatric disorders [93–95]. Patients most commonly report the following issues:
Chronic fatigue—persistent tiredness, particularly effort-independent fatigue that does not resolve after rest and that reduces quality of life
Cognitive dysfunction (‘brain fog’)—problems with writing, reading and calculating that are unrelated to physical disability; trouble with clearly answering questions and conveying messages; problems performing multiple tasks at a time; difficulty remembering new information or determining the current date and the day of the week
Mood disorders—sadness, fear or anxiety
Sleep disorders—insomnia, hypersomnia, sleep–wake disorders or nightmares
Pain syndromes—headache, back pain, myalgia or neuropathic pain
Smell and taste disorders
The above manifestations may persist after acute SARS-CoV-2 infection or they may appear several weeks or months after the infection and continue for a few weeks to several months or longer. Their severity may vary from mild to extremely severe and may change over time. Most patients are able to perform daily tasks, but 50% of them reported that post-COVID-19 neurological manifestations worsened daily functioning at home and at work and reduced their quality of life [93, 94].
Effects of COVID-19 on patients with neurological diseases
Multiple sclerosis
Patients with multiple sclerosis (MS) are theoretically more susceptible to infections related to disease-modifying therapies (DMTs) [96, 97]. Particular DMTs pose various levels of risk according to their mechanisms of action. As a result, patients require a personalised approach when initiating new therapies and continuing treatment. This problem became very important during the COVID-19 pandemic. For drugs with various mechanisms of action regarding the immune system, the therapy’s impact on both susceptibility to SARS-CoV-2 and the course of COVID-19 should be considered [98]. Based on the patient risk/benefit analysis, the following recommendations were assigned to specific therapies: (1) low-risk therapies (glatiramer acetate, interferons, dimethyl fumarate and teriflunomide)—therapy discontinuation and delayed treatment initiation are not recommended; (2) intermediate-risk therapies (fingolimod, natalizumab, ocrelizumab and cladribine)—should be used with caution and require a personalised risk/benefit assessment and an analysis of the risk of MS exacerbation after drug withdrawal; and (3) high-risk therapies (alemtuzumab, mitoxantron and haematopoietic stem cell transplantation [HSCT])—therapy initiation is not recommended, and extreme caution should be exercised for further doses (Table 3).
Table 3.
Potential impact of MS therapies on COVID-19 clinical course and stratification risk of MS treatment (adapted and modified from: Bhise and Dhib-Jalbut [98])
| Medication | Potential benefit in COVID-19 | Potential adverse effect in COVID-19 | Risk of MS treatment | Currently receiving | New start |
|---|---|---|---|---|---|
| Interferon β | Reduced viral replication; inhibition of proinflammatory cytokines | Unknown | Lowest | Continue | Yes* |
| Glatiramer acetate | Counteract proinflammatory responses | Unknown | Lowest | Continue | Yes |
| Dimethyl fumarate | Reduced innate immune response to virus | Lymphopenia-related increased risk of infection and impaired viral clearance | Low | Continue | Yes |
| Teriflunomide | Anti-viral effect | Lymphopenia-related increased risk of infection and impaired viral clearance | Low | Continue | Yes |
| Fignolimod/siponimod/ozanimod | Lymphopenia may be beneficial for pneumonia and ARDS | Lymphopenia resulting in reduced viral clearance | Medium | Continue | Yes |
| Natalizumab | May interfere with SARS-CoV-2 host cell entry | Reduced SARS-CoV-2 clearance from the CNS and gut | Medium | Extend to 6-week intervals | Yes |
| Rituximab/okrelizumab/ofatumumab | Unknown | Increased risk of infection and impaired viral clearance | Medium–high | Extend interval based on B-cell counts | Yes |
| Cladribine | Unknown | Lymphopenia-related increased risk of infection and impaired viral clearance | High | Delay/switch | No** |
| Alemtuzumab | Unknown | Lymphopenia-related increased risk of infection and impaired viral clearance | High | Delay/switch | No |
| Hematopoietic stem cell therapy | Unknown | Unknown | High | Delay/switch | No |
ARDS acute respiratory distress syndrome, CNS central nervous system
*Yes: treatment can be initiated
**No: postpone treatment
However, current scientific reports indicate that MS itself is not a factor that increases the probable risk of COVID-19 and that it does not increase the risk of severe disease or death compared to the general population [99, 100]. Nevertheless, it should be noted that certain patients with MS may be more susceptible to severe COVID-19 [3]: individuals with MS over the age of 60; patients with progressive MS; patients with MS and more considerable disability (EDSS score 6 or higher); MS patients with obesity, diabetes or cardiovascular and pulmonary diseases; men with MS; and patients receiving certain MS immunomodulators [100].
Anti-CD20 agents were the only DMTs that increased the risk of severe disease observed in some studies [101]. The probability of hospitalisation was higher among patients receiving ocrelizumab or rituximab than among individuals receiving other disease-modifying therapies (adjusted odds ratio [aOR] 2.76; 95% CI 1.87–4.07) in an international cohort study of 1683 patients with MS [101]. Both ocrelizumab and rituximab were associated with a higher risk of admission to the intensive care unit, but they were not related to a higher risk of death.
In Sweden, where rituximab is the most frequent MS treatment strategy, Spelman et al. reported 292 cases of confirmed COVID-19, with the risk of hospitalisation reaching 23.2%. Therefore, rituximab-treated patients demonstrated higher hospitalisation rates compared to patients who were treated with combinations of all other DMTs (29.9 vs. 12.7%) [102]. Certain sites have attempted to modify their treatment strategies to reduce the risk of severe COVID-19. Some of them favoured other, highly effective therapies, such as natalizumab, instead of anti-CD20 treatment [103], while others successfully used prolonged anti-CD20 dosing intervals [104] and personalised ocrelizumab treatment according to B-cell CD19 levels.
Stroke
Most studies on stroke treatment during the pandemic revealed limited access to interventional treatment options (thrombolysis or thrombectomy) and a prolonged time of therapy initiation [105–107]. The global effects of the COVID-19 pandemic on the frequency of intravenous thrombolysis (IVT) and stroke-related hospitalisation within the 4 months of the pandemic’s peak (1 March to 30 June 2020) were assessed in comparison to the prepandemic period in a global study that included 457 sites in 70 countries [105]. During the pandemic, 11.5% fewer admissions (95% CI − 11.7 to − 11.3; p < 0.0001) due to ischaemic stroke and 13.2% fewer cases of thrombolysis (95% CI − 13.8 to − 12.7; p < 0.0001) were observed compared to the analogous prepandemic period [105]. The rate of stroke was 1.48% among 119,967 hospitalised individuals due to COVID-19. Severe coronavirus infection was reported in 3.3% (1722 out of 52,026) of all stroke-related admissions. Most authors emphasise that the COVID-19 pandemic was associated with a global decrease in stroke-related hospitalisations and rarer thrombolytic treatment or mechanical thrombectomy [105–109].
Neurodegenerative diseases
Patients with neurodegenerative diseases (Parkinson’s disease or Alzheimer’s disease) are particularly susceptible to infection. Experts recommend strict obedience of COVID-19 preventive measures, emphasising the fact that infections–especially those with fever–may affect motor activities and exacerbate orientation disorders (including delirium). When such patients require hospitalisation, the previous treatment should be modified with caution, as suddenly discontinuing dopaminergic therapy may substantially worsen the patient’s health status. Patients in care centres are particularly susceptible. The risk of SARS-CoV-2 infection was 60% higher than that in the general population in one study of 15,043 social care centre residents with dementia [110]. Patients with Parkinson’s disease demonstrated a higher risk of severe COVID-19 in another study, and the infection was associated with higher mortality [111].
Neurological complications following COVID-19 vaccination
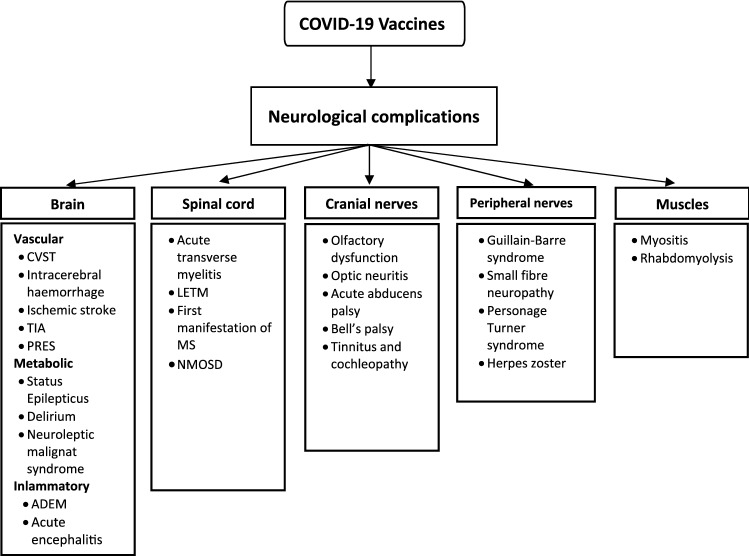
SARS-CoV-2 vaccinations are not free from side effects (Fig. 1). Usually, they are mild or moderate but can occasionally be severe. Two of these severe side effects are cerebral venous sinus thrombosis (CVST) and GBS.
Fig. 1.
Neurological complications following COVID-19 vaccination (Adapted and modified from Garg et al. [31]. ADEM acute disseminated encephalomyelitis; CVST cerebral venous sinus thrombosis; LETM longitudinally extensive transverse myelitis; MS multiple sclerosis; NMOSD neuromyelitis optica spectrum disorders; PRES posterior reversible encephalopathy syndrome; TIA transient ischemic attacks
CVST has been described after vaccination against SARS-CoV-2. The clinical characteristics of 213 postvaccination CVST cases reported to the European Medicines Agency by 8 April 2021 were published [112]. Data on adverse drug reactions after SARS-CoV-2 vaccination were obtained from the EudraVigilance database. Patients with postvaccination CVST were compared with 100 European patients with CVST from before the COVID-19 pandemic. Overall, 213 CVST cases were identified: 187 cases after vaccination with the AstraZeneca/Oxford vaccine (ChAdOx1 nCoV-19) and 26 cases after messenger RNA (mRNA) vaccination (25 cases with Pfizer/BioNTech [BNT162b2] and 1 case with Moderna [mRNA-1273]). Thrombocytopenia was reported in 107 out of 187 CVST cases (57%) in the ChAdOx1 nCoV-19 group, in none from the mRNA vaccine group and in 7 out of 100 (7%) cases in the pre-COVID-19 group. Among the 117 patients with a reported outcome in the ChAdOx1 nCoV-19 group, 44 (38%) patients died, in comparison to 2 out of 10 (20%) patients in the mRNA vaccine group and 3 out of 100 (3%) in the pre-COVID-19 group. Mortality among patients with thrombocytopenia in the ChAdOx1 nCoV-19 group was 49% (95% CI 39–60%). The authors concluded that cerebral venous sinus thrombosis occurring after ChAdOx1 nCoV-19 vaccination had a clinical profile distinct from CVST unrelated to vaccination. Only CVST after ChAdOx1 nCoV-19 vaccination was associated with thrombocytopenia [112].
Cases of GBS, which is a side effect of SARS-CoV-2 (SCoVaG) vaccination, were discussed in the literature review by Finsterer et al. [113]. Overall, 9 articles were identified describing 18 patients aged 20 to 86 years with SCoVaG. SCoVaG developed after the first vaccine dose in each patient. The AstraZeneca vaccine was administered to 14 patients, the Pfizer vaccine was used for 4 patients, and the Johnson & Johnson vaccine was administered to 1 patient. The intervals between vaccination and the onset of GBS were 3 h to 39 days. SCoVaG treatment included IVIG (n = 13), steroids (n = 3) or no therapy (n = 3). Six patients required mechanical ventilation. Only one patient recovered completely, while partial recovery was achieved in nine patients. The authors concluded that GBS may develop immediately after the first SARS-CoV-2 vaccine is administered. Although the causal relationship between SARS-CoV-2 vaccination and SCoVaG remains speculative, more evidence suggests a direct impact of the vaccine on the development of GBS [113].
Vaccines are commonly considered safe and effective, but their administration to MS patients has been controversial for a long time. Nevertheless, vaccination against the coronavirus is also recommended for individuals with MS [114]. The mRNA and vector vaccines seem to be effective and safe. To date, only a few cases of the reactivation or emergence of a demyelinating disease have been reported following vaccination with a recombinant adenovirus (Oxford/AstraZeneca) [115]. Moreover, a case was described where MS first manifested after the Pfizer/BioNTech vaccine [116]. However, a recent study of approximately 500 patients with MS demonstrated a relapse rate (approximately 2%) after administration of the Pfizer/BioNTech vaccine, which was comparable to that observed among unvaccinated individuals [117]. Therefore, despite the above rare case reports, it is mostly considered that the benefits of SARS-CoV-2 vaccines vastly outweigh the potential risks [116]. Most adverse events following vaccination are mild, and the acute relapse incidence is low [118].
Current challenges and future perspectives
The major waves of the COVID-19 epidemic appear to be behind us. We have gained a fairly good understanding of the direct effects of SARS-CoV-2 on various systems and organs and its complications. There is no doubt that the coronavirus has a powerful affinity for the nervous system. The greatest concern is now the scale of the long-term consequences of contracting COVID-19. There is growing concern that we are facing a wave of chronic illnesses about which we still know little. According to estimates, more than 100 million people may have complications [119]. Observations to date suggest that most people complain of nervous system disorders. It is estimated that the so-called neuro-COVID affects between 62 and 70% of patients [119–121]. One recently published study analysed data from almost 1.2 million patients who contracted the coronavirus between 20 January 2020 and 13 April 2022 [61]. These data were compared with data from people who had, among other things, the same vaccination status, age, and the same COVID-19 risk factors and severity but did not experience SARS-CoV-2 infection. The authors then analysed the risk of 14 psychiatric and neurological diagnoses among the participants. They compared the risk of these disorders with the control group. They also looked at how these risks differed during periods of Alpha, Delta and Omicron variant dominance. They found that symptoms of anxiety disorders were exacerbated in those who had an active SARS-CoV-2 infection; however, the risk of anxiety and depression decreased to the level of the control group over the next few months [61]. Cognitive deficits (deterioration of memory or attention), insomnia and seizures were prevalent among children after the first 6 months of infection. Among adults, there was an increased risk of brain fog, dementia, psychotic disorders and epilepsy or seizures, which was still the case at the end of the 2-year follow-up period [61]. It was also found that patients infected with the Delta variant had an increased risk of ischaemic stroke, cognitive deficits, insomnia, anxiety disorders and epilepsy compared with participants infected with the Alpha variant. Furthermore, although the Omicron variant is associated with lower mortality rates, the risk of psychiatric or neurological problems remains similar to that of Delta [61]. In particular, the high rate of dementia and seizures diagnosed after COVID-19 is of concern [61]. It seems that the greatest challenge is now to learn more about the long-term impact of COVID-19, which may allow us to develop ways to prevent late complications and help health care professionals prepare for the future.
Summary
The COVID-19 pandemic has affected the entire socioeconomic system, particularly health care, pointing out numerous systemic problems and shortcomings. It showed that the world was helpless in the face of the most dangerous challenge of the twenty-first century. Attempts to solve the issues took time and a comprehensive approach. It can be said with certainty that the pandemic generated not only health effects in the form of morbidity and death caused by the virus but also painfully affected other areas of health and health care. People undergoing oncology treatment, patients after strokes and heart attacks, and patients with diabetes and other chronic diseases had reduced access to services during the pandemic. The proportion of so-called avoidable deaths increased. Excess deaths ('excess mortality') show the hidden victims of the epidemic. This is a more comprehensive measure of the total mortality impact of the pandemic than just the number of COVID-19 deaths. It also includes COVID-19 deaths that were not properly diagnosed and reported, as well as deaths from other causes that can be attributed to the overall health system crisis.
The experience of over 2 years of fighting with the pandemic has shown that SARS-CoV-2 infection is not an ordinary respiratory tract condition. We fairly quickly discovered that COVID-19 was a multisystem and multiorgan disease. We also know that SARS-CoV-2 demonstrates tremendous neurotropic potential. The results of meta-analyses indicate that the most common complications of COVID-19 are fatigue (32–42.9%), olfactory dysfunction (12–43.1%), myalgia (20–33%), headache (10.1–29%), and dizziness (6.4–10%). In contrast, the most common serious complication is encephalopathy.
The cytokine storm, which occurs not only in the lungs but also in the central nervous system, is associated with a release of various neurotoxins responsible for neurological manifestations. Moreover, we know that SARS-CoV-2 has a special affinity with and for the processes of thrombosis and embolism. Therefore, the central nervous system, with its arteries and veins, is a good site for thrombotic lesions. As in many neurotropic viral infections, encephalitis, encephalopathy or Guillain‒Barré syndrome may develop.
Neurological manifestations in SARS-CoV-2 infection may herald the infection and occur as one of the initial signs or symptoms, such as sleep disorder, changes in sleep patterns, headache, transient ischaemic attack or limb numbness. Moreover, they may be dominant signs of severe infection with cerebral hypoxia and inflammation. Finally, they may be long-term manifestations, i.e., indicating post-COVID-19 syndrome, which most commonly occurs as chronic fatigue or ‘brain fog’. Undoubtedly, all these manifestations (neurological and neurocognitive) lead to a considerably lower quality of life as well as impaired professional, social and family-related functioning. This is a consequence of COVID-19, which we will suffer for a long time.
Abbreviations
- ACE2
Angiotensin-converting enzyme-2
- ADEM
Acute disseminated encephalomyelitis
- ALS
Amyotrophic lateral sclerosis
- aOR
Adjusted odds ratio
- ChAdOx1 nCov-19
AstraZeneca/Oxford vaccine
- BNT162b2
Pfizer/BioNTech vaccine
- CNS
Central nervous system
- COVID-19
Coronavirus disease
- CK
Creatinine kinase
- CSF
Cerebrospinal fluid
- CVST
Cerebral venous sinus thrombosis
- DMTs
Disease-modifying therapies
- EDSS
Expanded disability status scale
- EEG
Electroencephalography
- GBS
Guillain–Barré syndrome
- HSCT
Hematopoietic stem cell transplantation
- IVIG
Intravenous immune globulin
- IVT
Intravenous thrombolysis
- LETM
Longitudinally extensive transverse myelitis
- MS
Multiple sclerosis
- mRNA
Messenger RNA
- mRNA-1273
Moderna vaccine
- NMOSD
Neuromyelitis optica spectrum disorders
- NK
Natural killer cells
- PCS
Post-COVID-19 syndrome
- PRES
Posterior reversible encephalopathy syndrome
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SCoVaG
Side effect of SARS-CoV-2 vaccination
- SCCM
Society of Critical Care Medicine
- TIA
Transient ischemic attacks
- WHO
World Health Organization
Author contributions
The authors contributed equally to this work.
Funding
This study was supported by a grant from Jan Kochanowski University of Kielce, Kielce, Poland (SUPS.RN.22.034).
Declarations
Conflict of interest
The authors have no conflict of interest regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Waldemar Brola, Email: wbrola@wp.pl.
Maciej Wilski, Email: mwilski@wp.pl.
References
- 1.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman A, Niloofa R, De Zoysa IM, Cooray AD, Kariyawasam J, Seneviratne SL. Neurological manifestations in COVID-19: a narrative review. SAGE Open Med. 2020;8:2050312120957925. doi: 10.1177/2050312120957925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S, Cooper-Knock J, Weimer AK, Shi M, Kozhaya L, Unutmaz D, et al. Multiomic analysis reveals cell-type-specific molecular determinants of COVID-19 severity. Cell Syst. 2022;13(8):598–614.e6. doi: 10.1016/j.cels.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, Koralnik IJ. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. 2020;7(11):2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berlit P, Bösel J, Gahn G, Isenmann S, Meuth SG, Nolte CH, et al. Neurological manifestations of COVID-19—guideline of the German Society of Neurology. Neurol Res Pract. 2020;2(2):51. doi: 10.1186/s42466-020-00097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vitalakumar D, Sharma A, Kumar A, Flora SJS. Neurological manifestations in COVID-19 patients: a meta-analysis. ACS Chem Neurosci. 2021;12(15):2776–2797. doi: 10.1021/acschemneuro.1c00353. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Bai X, Zhu T, Huang J, Zhang H. What can the neurological manifestations of COVID-19 tell us: a meta-analysis. J Transl Med. 2021;19(1):363. doi: 10.1186/s12967-021-03039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vakili K, Fathi M, Hajiesmaeili M, Salari M, Saluja D, Tafakhori A, et al. Neurological symptoms, comorbidities, and complications of COVID-19: a literature review and meta-analysis of observational studies. Eur Neurol. 2021;84(5):307–324. doi: 10.1159/000516258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Premraj L, Kannapadi NV, Briggs J, Seal SM, Battaglini D, Fanning J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;15(434):120162. doi: 10.1016/j.jns.2022.120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Misra S, Kolappa K, Prasad M, Radhakrishnan D, Thakur KT, Solomon T, et al. Frequency of neurologic manifestations in COVID-19: a systematic review and meta-analysis. Neurology. 2021;97(23):e2269–e2281. doi: 10.1212/WNL.0000000000012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rogers JP, Watson CJ, Badenoch J, Cross B, Butler M, Song J, et al. Neurology and neuropsychiatry of COVID-19: a systematic review and meta-analysis of the early literature reveals frequent CNS manifestations and key emerging narratives. J Neurol Neurosurg Psychiatry. 2021;92(9):932–941. doi: 10.1136/jnnp-2021-326405. [DOI] [PubMed] [Google Scholar]
- 14.Majolo F, Silva GLD, Vieira L, Anli C, Timmers LFSM, Laufer S, Goettert MI. Neuropsychiatric disorders and COVID-19: what we know so far. Pharmaceuticals (Basel) 2021;14(9):933. doi: 10.3390/ph14090933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahboubi Mehrabani M, Karvandi MS, Maafi P, Doroudian M. Neurological complications associated with Covid-19; molecular mechanisms and therapeutic approaches. Rev Med Virol. 2022;9:e2334. doi: 10.1002/rmv.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenting A, Gruters A, van Os Y, Verstraeten S, Valentijn S, Ponds R, de Vugt M. COVID-19 neurological manifestations and underlying mechanisms: a scoping review. Front Psychiatry. 2020;21(11):860. doi: 10.3389/fpsyt.2020.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graham EL, Clark JR, Orban ZS, Lim PH, Szymanski AL, Taylor C, et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized Covid-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. doi: 10.1002/acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Humanitas COVID-19 task force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Yang Y, Liang X, Gao B, Liu M, Li W, Chen Z, Wang Z. COVID-19 associated ischemic stroke and hemorrhagic stroke: incidence, potential pathological mechanism, and management. Front Neurol. 2020;27(11):571996. doi: 10.3389/fneur.2020.571996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stilhano RS, Costa AJ, Nishino MS, Shams S, Bartolomeo CS, Breithaupt-Faloppa AC, et al. SARS-CoV-2 and the possible connection to ERs, ACE2, and RAGE: focus on susceptibility factors. FASEB J. 2020;34(11):14103–14119. doi: 10.1096/fj.202001394RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to severe acute respiratory syndrome coronavirus infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bukowska A, Spiller L, Wolke C, Lendeckel U, Weinert S, Hoffmann J, et al. Protective regulation of the ACE2/ACE gene expression by estrogen in human atrial tissue from elderly men. Exp Biol Med (Maywood) 2017;242(14):1412–1423. doi: 10.1177/1535370217718808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, Zhang J, Wang C, Chen X, Zhao X, Jing H, et al. COVID-19 and ischemic stroke: mechanisms of hypercoagulability (review) Int J Mol Med. 2021;47(3):21. doi: 10.3892/ijmm.2021.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esenwa C, Cheng NT, Lipsitz E, Hsu K, Zampolin R, Gersten A, et al. COVID-19-associated carotid atherothrombosis and stroke. AJNR Am J Neuroradiol. 2020;41(11):1993–1995. doi: 10.3174/ajnr.A6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Meijenfeldt FA, Havervall S, Adelmeijer J, Lundström A, Magnusson M, Mackman N, et al. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5(3):756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu TM, Seet CYH, Koh JS, Tham CH, Chiew HJ, De Leon JA, et al. Acute ischemic stroke during the convalescent phase of asymptomatic COVID-2019 infection in men. JAMA Netw Open. 2021;4(4):e217498. doi: 10.1001/jamanetworkopen.2021.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altschul DJ, Unda SR, de La Garza Ramos R, Zampolin R, Benton J, Holland R, et al. Hemorrhagic presentations of COVID-19: Risk factors for mortality. Clin Neurol Neurosurg. 2020;198:106112. [DOI] [PMC free article] [PubMed]
- 28.Luigetti M, Iorio R, Bentivoglio AR, Tricoli L, Riso V, Marotta J, et al. Assessment of neurological manifestations in hospitalized patients with COVID-19. Eur J Neurol. 2020;27(11):2322–2328. doi: 10.1111/ene.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ravindra VM, Grandhi R, Delic A, Hohmann S, Shippey E, Tirschwell D, et al. Impact of COVID-19 on the hospitalization, treatment, and outcomes of intracerebral and subarachnoid hemorrhage in the United States. PLoS ONE. 2021;16(4):e0248728. doi: 10.1371/journal.pone.0248728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. 2020;11(3):322–325. doi: 10.1007/s12975-020-00818-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID-19: a review. J Med Virol. 2021;93(1):206–222. doi: 10.1002/jmv.26207. [DOI] [PubMed] [Google Scholar]
- 32.Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12(1):69. doi: 10.1186/s13195-020-00640-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423–427. doi: 10.1002/ana.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uginet M, Breville G, Assal F, Lövblad KO, Vargas MI, Pugin J, et al. COVID-19 encephalopathy: clinical and neurobiological features. J Med Virol. 2021;93(7):4374–4381. doi: 10.1002/jmv.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yachou Y, El Idrissi A, Belapasov V, Ait BS. Neuroinvasion, neurotropic, and neuroinflammatory events of SARS-CoV-2: understanding the neurological manifestations in COVID-19 patients. Neurol Sci. 2020;41(10):2657–2669. doi: 10.1007/s10072-020-04575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fusunyan M, Praschan N, Fricchione G, Beach S. Akinetic mutism and coronavirus disease 2019: a narrative review. J Acad Consult Liaison Psychiatry. 2021;62(6):625–633. doi: 10.1016/j.jaclp.2021.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartley CM, Johns C, Ngo TT, Dandekar R, Loudermilk RL, Alvarenga BD, et al. Anti-SARS-CoV-2 and autoantibody profiles in the cerebrospinal fluid of 3 teenaged patients with COVID-19 and subacute neuropsychiatric symptoms. JAMA Neurol. 2021;78(12):1503–1509. doi: 10.1001/jamaneurol.2021.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26(2):143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis. Lancet Microbe. 2021;2(1):e13–e22. doi: 10.1016/S2666-5247(20)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hugon J, Msika EF, Queneau M, Farid K, Paquet C. Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J Neurol. 2022;269(1):44–46. doi: 10.1007/s00415-021-10655-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hugon J. Long-COVID: cognitive deficits (brain fog) and brain lesions in non-hospitalized patients. Presse Med. 2021;51(2):104090. doi: 10.1016/j.lpm.2021.104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kas A, Soret M, Pyatigoskaya N, Habert MO, Hesters A, Le Guennec L, et al. The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur J Nucl Med Mol Imaging. 2021;48(8):2543–2557. doi: 10.1007/s00259-020-05178-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolay H, Gül A, Baykan B. COVID-19 is a real headache! Headache. 2020;60(7):1415–1421. doi: 10.1111/head.13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tolebeyan AS, Zhang N, Cooper V, Kuruvilla DE. Headache in patients with severe acute respiratory syndrome coronavirus 2 infection: a narrative review. Headache. 2020;60(10):2131–2138. doi: 10.1111/head.13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keddie S, Pakpoor J, Mousele C, Pipis M, Machado PM, Foster M, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain–Barré syndrome. Brain. 2021;144(2):682–693. doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brandão PRP, Grippe TC, Pereira DA, Munhoz RP, Cardoso F. New-onset movement disorders associated with COVID-19. Tremor Other Hyperkinet Mov (NY) 2021;8(11):26. doi: 10.5334/tohm.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salari N, Khazaie H, Hosseinian-Far A, Khaledi-Paveh B, Kazeminia M, Mohammadi M, et al. The prevalence of stress, anxiety and depression within front-line healthcare workers caring for COVID-19 patients: a systematic review and meta-regression. Hum Resour Health. 2020;18(1):100. doi: 10.1186/s12960-020-00544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benedetti F, Palladini M, Paolini M, Melloni E, Vai B, De Lorenzo R, et al. Brain correlates of depression, post-traumatic distress, and inflammatory biomarkers in COVID-19 survivors: a multimodal magnetic resonance imaging study. Brain Behav Immun Health. 2021;18:100387. doi: 10.1016/j.bbih.2021.100387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abboud H, Abboud FZ, Kharbouch H, et al. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azim D, Nasim S, Kumar S, et al. Neurological consequences of 2019-nCoV infection: a comprehensive literature review. Cureus. 2020;12(6):e8790. doi: 10.7759/cureus.8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. doi: 10.1016/j.bbi.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bostancıklıoğlu M. SARS-CoV2 entry and spread in the lymphatic drainage system of the brain. Brain Behav Immun. 2020;87:122–123. doi: 10.1016/j.bbi.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed]
- 60.Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399(10335):1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, Harrison PJ. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815–827. doi: 10.1016/S2215-0366(22)00260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cervantes-Arslanian AM, Venkata C, Anand P, Burns JD, Ong CJ, LeMahieu AM, et al. Neurologic manifestations of severe acute respiratory syndrome coronavirus 2 infection in hospitalized patients during the first year of the COVID-19 pandemic. Crit Care Explor. 2022;4(4):e0686. doi: 10.1097/CCE.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID-19): a meta-analysis of 27,492 patients. Laryngoscope. 2021;131(4):865–878. doi: 10.1002/lary.29286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orsucci D, Ienco EC, Nocita G, Napolitano A, Vista M. Neurological features of COVID-19 and their treatment: a review. Drugs Context. 2020 doi: 10.7573/dic.2020-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, et al. Anosmia—a clinical review. Chem Senses. 2017;42(7):513–523. doi: 10.1093/chemse/bjx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poncet-Megemont L, Paris P, Tronchere A, Salazard JP, Pereira B, Dallel R, et al. High prevalence of headaches during Covid-19 infection: a retrospective cohort study. Headache. 2020;60(10):2578–2582. doi: 10.1111/head.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Belvis R. Headaches during COVID-19: my clinical case and review of the literature. Headache. 2020;60(7):1422–1426. doi: 10.1111/head.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, et al. COVID-19 Intensive Care International Study Group. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed]
- 70.Shah VA, Nalleballe K, Zaghlouleh ME, Onteddu S. Acute encephalopathy is associated with worse outcomes in COVID-19 patients. Brain Behav Immun Health. 2020;8:100136. doi: 10.1016/j.bbih.2020.100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaumont H, Etienne P, Roze E, Couratier C, Roger PM, Lannuzel A. Acute meningoencephalitis in a patient with COVID-19. Rev Neurol (Paris) 2020;176(6):519–521. doi: 10.1016/j.neurol.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meppiel E, Peiffer-Smadja N, Maury A, Bekri I, Delorme C, Desestret V, et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 2021;27(3):458–466. doi: 10.1016/j.cmi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the Young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bekelis K, Missios S, Ahmad J, et al. Ischemic stroke occurs less frequently in patients with COVID-19: a multicenter cross-sectional study. Stroke. 2020;51:3570. doi: 10.1161/STROKEAHA.120.031217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qureshi AI, Baskett WI, Huang W, et al. Acute ischemic stroke and COVID-19: an analysis of 27 676 patients. Stroke. 2021;52:905. doi: 10.1161/STROKEAHA.120.031786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed]
- 78.Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan. Italy Thromb Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. 2020;7(5):001691. doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tu TM, Goh C, Tan YK, Leow AS, Pang YZ, Chien J, Shafi H, Chan BP, Hui A, Koh J, Tan BY, Umapathi NT, Yeo LL. Cerebral venous thrombosis in patients with COVID-19 infection: a case series and systematic review. J Stroke Cerebrovasc Dis. 2020;29(12):105379. doi: 10.1016/j.jstrokecerebrovasdis.2020.105379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khatana SAM, Groeneveld PW. Health disparities and the coronavirus disease 2019 (COVID-19) pandemic in the USA. J Gen Intern Med. 2020;35(8):2431–2432. doi: 10.1007/s11606-020-05916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldini T, Asioli GM, Romoli M, Carvalho Dias M, Schulte EC, Hauer L, Aguiar De Sousa D, Sellner J, Zini A. Cerebral venous thrombosis and severe acute respiratory syndrome coronavirus-2 infection: a systematic review and meta-analysis. Eur J Neurol. 2021;28(10):3478–3490. 10.1111/ene.14727. [DOI] [PMC free article] [PubMed]
- 83.Nguyen TN, Qureshi MM, Klein P, Yamagami H, Abdalkader M, Mikulik R, et al. Global impact of the COVID-19 pandemic on cerebral venous thrombosis and mortality. J Stroke. 2022;24(2):256–265. doi: 10.5853/jos.2022.00752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katsoularis I, Fonseca-Rodríguez O, Farrington P, Lindmark K, Fors Connolly AM. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Katz JM, Libman RB, Wang JJ, Sanelli P, Filippi CG, Gribko M, et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51(9):e227–e231. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fridman S, Bres Bullrich M, Jimenez-Ruiz A, Costantini P, Shah P, Just C, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95(24):e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- 87.Srivastava PK, Zhang S, Xian Y, Xu H, Rutan C, Alger HM, et al. Acute ischemic stroke in patients with COVID-19: an analysis from get with the guidelines-stroke. Stroke. 2021;52(5):1826–1829. doi: 10.1161/STROKEAHA.121.034301. [DOI] [PubMed] [Google Scholar]
- 88.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis. 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Restivo DA, Centonze D, Alesina A, Marchese-Ragona R. Myasthenia gravis associated with SARS-CoV-2 infection. Ann Intern Med. 2020;173(12):1027–1028. doi: 10.7326/L20-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020;382(26):2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luijten LWG, Leonhard SE, van der Eijk AA, Doets AY, Appeltshauser L, Arends S, et al. Guillain–Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. 2021;144(11):3392–3404. doi: 10.1093/brain/awab279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Camargo-Martínez W, Lozada-Martínez I, Escobar-Collazos A, Navarro-Coronado A, Moscote-Salazar L, Pacheco-Hernández A, et al. Post-COVID 19 neurological syndrome: Implications for sequelae’s treatment. J Clin Neurosci. 2021;88:219–225. doi: 10.1016/j.jocn.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed JO, Ahmad SA, Hassan MN, Kakamad FH, Salih RQ, Abdulla BA, et al. Post COVID-19 neurological complications; a meta-analysis. Ann Med Surg (Lond) 2022;76:103440. doi: 10.1016/j.amsu.2022.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beghi E, Helbok R, Ozturk S, Karadas O, Lisnic V, Grosu O. Short- and long-term outcome and predictors in an international cohort of patients with neuro-COVID-19. Eur J Neurol. 2022;29(6):1663–1684. doi: 10.1111/ene.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giovannoni G, Hawkes C, Lechner-Scott J, Levy M, Waubant E, Gold J. The COVID-19 pandemic and the use of MS disease-modifying therapies. Mult Scler Relat Disord. 2020;39:102073. doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Czarnowska A, Kapica-Topczewska K, Zajkowska O, Adamczyk-Sowa M, Kubicka-Bączyk K, Niedziela N, et al. Symptoms after COVID-19 infection in individuals with multiple sclerosis in Poland. J Clin Med. 2021;10(22):5225. doi: 10.3390/jcm10225225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhise V, Dhib-Jalbut S. Potential risks and benefits of multiple sclerosis immune therapies in the COVID-19 era: clinical and immunological perspectives. Neurotherapeutics. 2021;18(1):244–251. doi: 10.1007/s13311-021-01008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Czarnowska A, Brola W, Zajkowska O, Rusek S, Adamczyk-Sowa M, Kubicka-Bączyk K, et al. Clinical course and outcome of SARS-CoV-2 infection in multiple sclerosis patients treated with disease-modifying therapies—the Polish experience. Neurol Neurochir Pol. 2021;55(2):212–222. doi: 10.5603/PJNNS.a2021.0031. [DOI] [PubMed] [Google Scholar]
- 100.Zabalza A, Thompson AJ, Montalban X. Two years of COVID-19 in the MS community: what have we learnt so far? Mult Scler. 2022;28(7):1005–1008. doi: 10.1177/13524585221099844. [DOI] [PubMed] [Google Scholar]
- 101.Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870–e1885. doi: 10.1212/WNL.0000000000012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spelman T, Forsberg L, McKay K, Glaser A, Hillert J. Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler. 2022;28(7):1051–1059. doi: 10.1177/13524585211026272. [DOI] [PubMed] [Google Scholar]
- 103.Cobo-Calvo A, Zabalza A, Río J, Arrambide G, Otero-Romero S, Tagliani P, et al. Impact of COVID-19 pandemic on frequency of clinical visits, performance of MRI studies, and therapeutic choices in a multiple sclerosis referral centre. J Neurol. 2022;269(4):1764–1772. doi: 10.1007/s00415-021-10958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Lierop ZY, Toorop AA, van Ballegoij WJ, Olde Dubbelink TB, Strijbis EM, de Jong BA, et al. Personalized B-cell tailored dosing of ocrelizumab in patients with multiple sclerosis during the COVID-19 pandemic. Mult Scler. 2022;28(7):1121–1125. doi: 10.1177/13524585211028833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nogueira RG, Qureshi MM, Abdalkader M, Martins SO, Yamagami H, Qiu Z, et al. Global impact of COVID-19 on stroke care and IV thrombolysis. Neurology. 2021;96(23):e2824–e2838. doi: 10.1212/WNL.0000000000011885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sobolewski P, Antecki J, Brola W, Fudala M, Bieniaszewski L, Kozera G. Systemic thrombolysis in ischaemic stroke patients with COVID-19. Acta Neurol Scand. 2022;145(1):47–52. doi: 10.1111/ane.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shokri H, Nahas NE, Basiony AE, Nguyen TN, Abdalkader M, Klein P, et al. Did COVID-19 impact stroke services? A multicenter study. Neurol Sci. 2022;43(7):4061–4068. doi: 10.1007/s10072-022-06018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Amiri HA, Razavi AS, Tabrizi N, Cheraghmakani H, Baghbanian SM, Sedaghat-Chaijan M, et al. The effects of COVID-19 on patients with acute ischemic and hemorrhagic stroke. J Stroke Cerebrovasc Dis. 2022;31(7):106512. doi: 10.1016/j.jstrokecerebrovasdis.2022.106512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nawabi NLA, Duey AH, Kilgallon JL, Jessurun C, Doucette J, Mekary RA, Aziz-Sultan MA. Effects of the COVID-19 pandemic on stroke response times: a systematic review and meta-analysis. J Neurointerv Surg. 2022;14(7):642–649. doi: 10.1136/neurintsurg-2021-018230. [DOI] [PubMed] [Google Scholar]
- 110.Bayer TA, DeVone F, McConeghy KW, Halladay CW, Quach L, Rajan A, et al. Dementia prevalence, a contextual factor associated with SARS-CoV-2 in veterans affairs community living centers. J Am Geriatr Soc. 2022 doi: 10.1111/jgs.17945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vignatelli L, Baccari F, Belotti LMB, Zenesini C, Baldin E, Calandra-Buonaura G, et al. The indirect impact of COVID-19 on major clinical outcomes of people with Parkinson’s disease or Parkinsonism: a cohort study. Front Neurol. 2022;16(13):873925. doi: 10.3389/fneur.2022.873925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krzywicka K, Heldner MR, Sánchez van Kammen M, van Haaps T, Hiltunen S, Silvis SM, et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: an analysis of cases notified to the European Medicines Agency. Eur J Neurol. 2021;28(11):3656–3662. 10.1111/ene.15029. [DOI] [PMC free article] [PubMed]
- 113.Finsterer J, Scorza FA, Scorza CA. Post SARS-CoV-2 vaccination Guillain-Barre syndrome in 19 patients. Clinics (Sao Paulo) 2021;11(76):e3286. doi: 10.6061/clinics/2021/e3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Reyes S, Cunningham AL, Kalincik T, Havrdová EK, Isobe N, Pakpoor J, et al. Update on the management of multiple sclerosis during the COVID-19 pandemic and post pandemic: an international consensus statement. J Neuroimmunol. 2021;15(357):577627. doi: 10.1016/j.jneuroim.2021.577627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed]
- 116.Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J Neurol. 2022;269(1):55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Achiron A, Mandel M, Dreyer-Alster S, Harari G, Dolev M, Menascu S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: up to 6 months cross-sectional study. J Neuroimmunol. 2021;9(361):577746. doi: 10.1016/j.jneuroim.2021.577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Czarnowska A, Tarasiuk J, Zajkowska O, Wnuk M, Marona M, Nowak K, et al. Safety of vaccines against SARS-CoV-2 among Polish patients with multiple sclerosis treated with disease-modifying therapies. Vaccines (Basel) 2022;10(5):763. doi: 10.3390/vaccines10050763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li D, Wang Q, Jia C, Lv Z, Yang J. An overview of neurological and psychiatric complications during post-COVID period: a narrative review. J Inflamm Res. 2022;26(15):4199–4215. doi: 10.2147/JIR.S375494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8(5):416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bratosiewicz-Wąsik J. Neuro-COVID-19: an insidious virus in action. Neurol Neurochir Pol. 2022;56(1):48–60. doi: 10.5603/PJNNS.a2021.0072. [DOI] [PubMed] [Google Scholar]