Abstract
Complex, high-risk percutaneous coronary intervention (HR-PCI) is increasingly being performed, often with mechanical circulatory support (MCS), though to date, there are limited randomised data on the efficacy of MCS for HR-PCI. The majority of MCS is provided by intra-aortic balloon pumps, but increasingly Impella® (Abiomed, Danvers, MA, USA) heart pumps are being used. While the Impella pumps provide greater increases in cardiac output, these devices require large bore access, which has been associated with an increased risk of bleeding and vascular complications. Decisions regarding the use of Impella are often based on risk–benefit considerations, with Impella-related bleeding risk being a major factor that can impact decisions for planned use. While bleeding risk related to large bore access is a concern, published data on the risk have been quite variable. Thus, the goal of this article is to provide a comprehensive review of reports describing bleeding and vascular complications for Impella-supported HR-PCI.
Keywords: High-risk percutaneous coronary intervention (PCI), haemodynamic support, PCI bleeding complications, mechanical circulatory support, PCI vascular complications, percutaneous left ventricular assist device (pVAD)
Percutaneous coronary intervention (PCI) has evolved over recent years, with increasingly more complex, high-risk procedures being performed, including in patients with multivessel coronary artery disease with or without left main disease, often complicated by severe left ventricular (LV) dysfunction.1 In addition, decisions about coronary bypass surgery or PCI often focus not only on coronary anatomy, but also on clinical factors including renal impairment, age and body mass index (BMI), as well as patient wishes. For many patients considered poor candidates for coronary bypass surgery due to significant comorbidities, PCI represents an appropriate alternative.
A recent analysis found that 21.5% of potential patients for coronary bypass surgery patients were deemed ineligible for surgery and underwent PCI.2 PCI for such patients (often with impaired LV function) involves an elevated risk for circulatory collapse during the procedure. Such procedures are considered high-risk PCI (HR-PCI), where protective mechanical circulatory support (MCS) may be beneficial during the PCI procedure.3,4 The intra-aortic balloon pump and the percutaneous LV assist device (pVAD) are the two most frequently used MCS devices for HR-PCI. The intra-aortic balloon pump chiefly serves to increase arterial pressures through counterpulsation, with limited improvement in cardiac output. In contrast, micro-axial rotary pumps, or pVADs, remove blood from the left ventricle and pump it into the ascending aorta, decreasing LV filling pressures and providing forward flow cardiac output.5 The Impella® 2.5 and CP heart pumps (Abiomed, Danvers, MA, USA) are the most commonly used pVADs for HR-PCI, with increasing use over the last decade.6 Though the randomised PROTECT II trial showed equivalent outcomes with Impella and intra-aortic balloon pump-supported HR-PCI at 30 days (the primary endpoint),7 there was a trend toward a late reduction in major adverse events for the Impella support group at 90 days, which attained significance when the analysis was conducted on the per-protocol population.8 Postulated benefits of pVAD haemodynamic support are to provide operators time to perform more complete revascularisation.9
Despite the potential benefits of pVAD support, bleeding and vascular complications remain a concern for elective use in HR-PCI, largely related to large-bore vascular access. Such pVAD-related vascular complications are linked with significantly higher in-hospital mortality, length of stay and cost,10 limiting the use of prophylactic pVAD circulatory support. However, the observed rate of bleeding complications with Impella use has shown significant variability in published studies, which are largely retrospective and/or observational in nature. A recent analysis by Amin et al. including cardiogenic shock, a markedly higher-risk population compared with HR-PCI, identified 2.5 times variability for Impella bleeding complications across centers.6 Thus, the true risk of bleeding and vascular complications with Impella use remains incompletely defined.
Major bleeding and vascular complications with Impella-supported high-risk percutaneous coronary intervention
To evaluate the risk of major bleeding and vascular complications in Impella-supported HR-PCI, we performed a comprehensive literature review. The literature search was conducted in the PubMed and Cochrane databases in July 2020. The full literature search strategy is available in Table 1. Our review was limited to studies reporting results of ≥20 patients,7,11–32 in an attempt to capture studies from centres with more than casual experience with large-bore access and Impella. We identified 23 such studies involving 3,466 patients undergoing elective or urgent HR-PCI, with Impella support initiated before or during PCI (Table 2).7,11–32 The dataset comprises two prospective trials (the PROTECT I feasibility trial and the randomised PROTECT II pivotal trial); 9 retrospective, multicentre studies/registries; and 11 retrospective, single-centre studies. Major bleeding complications and major vascular complications were recorded per study report, though the reports were subject to varying definitions of major bleeding or vascular complications. Nearly half of the studies (10 studies) did not directly specify a bleeding complication definition. Such variability of definitions precluded a formal meta-analysis; thus, our review is qualitative, describing the published experience of bleeding and vascular complications consistent with real-world experience.
Table 1: PubMed literature search strategy.
| Search number | Search terms | References returned |
|---|---|---|
| Search 1 | (Impella) AND (high-risk percutaneous coronary intervention) | 149 |
| Search 2 | (HRPCI) AND (Impella) | 12 |
| Search 3 | (PCI) AND (Impella) AND (elective) | 21 |
| Search 4 | (PCI) AND (Impella) AND (complication) | 95 |
| Search 5 | (Impella) AND (prophylactic) | 15 |
| Search 6 | (Impella) AND (bleeding) | 98 |
| Search 7 | (Impella) AND (PCI) AND (vascular) | 53 |
| Search 8 | (Impella) AND (PCI) AND (nonemergent) | 4 |
| Total references returned from search strategy | 443 | |
| Number of duplicates | 176 | |
| Number of unique references | 267 | |
| Number of additional unique references identified in Cochrane databasea | 20 | |
| Total references to undergo abstract review | 287 | |
The PubMed literature search strategy was run in July 2020.
aThe same strategy detailed above was run in the Cochrane database. Seventy-two references were identified, of which 30 were duplicates and 22 were duplicates to references identified in the PubMed search; a total of 20 additional unique references were therefore added from the Cochrane database search.
Table 2: Study design and patient population characteristics in studies with ≥20 patients undergoing Impella-supported high-risk percutaneous coronary intervention.
| Reference | Study design | Sample size (treatment dates) | Elective/urgent HR-PCI | Major bleeding definition* | Definition provided in Methods |
|---|---|---|---|---|---|
| Azzalini et al. (2020)16 | Retrospective, single-centre study (Germany) | 64 patients (2017–2018) | Elective or urgent (including STEMI) | 1 | N |
| Johannsen et al. (2019)27 | Retrospective, single-centre study (Germany) | 61 patients (2016–2018) | Elective or urgent (including STEMI; 46% ACS) | 2 | Y |
| Becher (2019)18 | Retrospective, single-centre study (Germany) | 54 patients (26 Impella; 2015–2016) | Elective or urgent (15% STEMI) | 3 | N |
| Riley et al. (2018)29 | Retrospective, multicentre CTO-PCI study (5 centres, USA) | 57 patients (2013–2017) | 100% elective | 2 | Y |
| Russo et al. (2019)30 | Retrospective, multicentre study (2 centres, Italy) | 37 patients (2013–2016) | 100% elective (no AMI within 24 hours) | 2 | N |
| Amponsah et al. (2017)14 | Retrospective, single-centre study (USA) | 40 patients (2013–2014) | NR | 1 | Y |
| Danek et al. (2018)22 | Retrospective, multicentre PROGRESS-CTO registry (12 centres, USA) | 1,598 patients (50 Impella 2.5/CP; 2012–2017) | 18% urgent (Impella initiated during PCI), 82% elective | 3 | Y |
| Doshi et al. (2019)24 | Retrospective, multicentre study (2 centres, USA) | 160 patients (2011–2016) | Elective or urgent (27% STEMI) | 2 | Y |
| Azzalini et al. (2020)16 | Retrospective, single-centre study (USA) | 250 patients (2009–2018) | Elective or urgent (no STEMI) | 2 | Y |
| Venugopal et al. 2015)32 | Retrospective, single-centre registry (UK) | 49 patients (45 HR-PCI; 2008–2014) | 62% urgent, 38% elective | 1 | N |
| Alasnag et al. (2011)11 | Retrospective, single-centre study (USA) | 60 patients (2008–2010) | 100% elective | 1 | N |
| Anusionwu et al. (2012)15 | Retrospective, single-centre study (USA) | 25 patients (2008–2009) | NR | 1 | N |
| Burzotta et al. (2019)19 | Retrospective, multicentre Roma-Verona registry (2 centres, Italy) | 86 patients (2007–2016) | 100% elective (no AMI within 24 hours) | 3 | Y |
| Alraies et al. (2019)12 | Retrospective, multicentre cVAD registry (USA, Canada and Europe) | 1,053 patients (2007–2015) | Elective or urgent | 3 | Y |
| Cohen et al. (2015)21 | Retrospective, multicentre USpella registry (49 centres, USA and Canada) | 637 patients (USpella patients; 2007–2013) | Elective or urgent | 3 | N |
| Cohen et al. (2015)21/O’Neill et al. (2012)7 | Multicentre PROTECT II RCT (112 centres, USA, Canada and Europe)† | 216 patients (Impella arm of PROTECT II, 2007–2010) | Elective or urgent (no recent MI) | 3 | N |
| Iliodromitis et al. (2011)26 | Retrospective, single-centre study (Germany) | 38 patients (2006–2009) | 100% urgent (unstable angina or NSTEMI) | 3 | Y |
| Ferreiro et al. (2010)25 | Retrospective, single-centre study (Spain) | 27 patients (2006–2008) | 100% elective | 3 | Y |
| Dixon et al. (2009)23 | Prospective, multicentre PROTECT I study (USA and Netherlands) | 20 patients (2006–2007) | Elective or urgent (no STEMI within 7 days or CA within 24 hours) | 1 | N |
| Kovacic et al. (2013)28 | Retrospective, single-centre study (USA) | 68 patients (36 Impella; 2005–2010) | 100% elective | 2 | Y |
| Chieffo et al. (2020)20 | Retrospective, multicentre IMP-IT registry (17 centres, Italy) | 406 patients (177 Impella HR-PCI; 2004–2018) | Elective or urgent (32% Impella placement during PCI, 0.5% after PCI) | 2 | y |
| Sjauw et al. (2009)31 | Retrospective, multicentre Europella registry (Europe) | 144 patients (2004–2007) | 100% elective (no STEMI within 48 hours) | 3 | Y |
| Baumann et al. (2020)17 | Retrospective, multicentre German Impella registry (6 centres, Germany) | 157 patients (unknown years) | Elective or ‘semi-elective’ | 1 | N |
*Major bleeding definitions in the included studies were considered to fall into three separate definition groups, identified with the numbers 1–3:
1) no major bleeding definition provided, or definition was incomplete, i.e. listing a variety of vascular complication event types and transfusion, but not specifying an interventional threshold for major bleeding
2) major bleeding defined as bleeding event requiring transfusion, surgery or any unplanned procedure
3) major bleeding defined as bleeding event requiring transfusion or surgery.
† Both Cohen (2015) and O’Neill (2012) publications are listed for the PROTECT II trial. The Cohen publication is the primary report of study outcomes; however, it did not detail bleeding and vascular complications, which were provided in the comparative analysis conducted by Cohen et al.
ACS = acute coronary syndrome; AMI = acute myocardial infarction; CA = cardiac arrest; HR-PCI = high-risk PCI; MI = myocardial infarction; NR = not reported; NSTEMI = Non-ST segment elevation myocardial infarction; PCI = percutaneous coronary intervention; STEMI = ST-segment elevation myocardial infarction.
Major bleeding
The median rate of major bleeding complications was 5.2% (range, 0–12.5%), with 22 of 23 studies contributing data. The rate of bleeding complications varied widely, likely due to variable definitions, incomplete data in some studies relative to site-specific bleeding, inclusion of non-groin-related bleeding events and occasionally the inclusion of emergent, ST-segment elevation myocardial infarction (STEMI) cases. Twelve studies (54.5%) reported major bleeding events without specifying the bleeding site.7,11,16,19–21,24–26,29–31 Five studies (22.7%) reported that no major bleeding complications occurred.14,15,18,23,32 The remaining five studies (22.7%) reported on bleeding complications and specified the bleeding site.12,17,22,27,28 Of the five studies reporting bleeding complications and bleeding site, three studies reported that 100% of major bleeding complications were access-related, with major bleeding complication rates of 2.7%, 3.3% and 6.4% reported in these three studies.17,27,28 One study reported that 80% of major bleeding complications were access-related.22 In the other study (a retrospective, gender-based analysis of 1,053 patients treated in the multicentre cVAD registry), major bleeding complications occurred in 7.0% of patients, with 90.5% of bleeding events consisting of blood loss requiring transfusion.12 The authors noted that low baseline haemoglobin levels and anaemia were mainly responsible for the elevated bleeding complication rate observed in females, as vascular complication rate was low (1.2%) and similar between genders.
Transfusion rates
Transfusion rates showed the largest variation across studies, with a median rate of 5.4% (range, 0–34.3%) and 19 studies contributing data. In some studies, the number of patients requiring transfusion exceeded the number of reported bleeding complications, which likely reflected transfusions for baseline anaemia in some cases. Transfusion rates in the four studies in which the majority of transfusions were stated as being performed in the setting of baseline anaemia ranged from 5.0% to 34.3%.12,23,26,32 Few studies included in our review reported on haemolysis (9 of 23 studies). Of these, 3 studies reported 0% haemolysis rates, 1 reported only mild haemolysis not requiring treatment, and 5 reported haemolysis occurring in one or two patients; overall haemolysis rates in studies reporting any hemolysis events ranged from 0.2% to 3.7%. Haemolysis requiring transfusion did not contribute meaningfully to overall transfusion rates in most cases (Table 3).
Table 3: Periprocedural bleeding and vascular complications in studies with ≥20 patients undergoing Impella-supported high-risk percutaneous coronary intervention.
| Reference | Major vascular complications | Major bleeding complications | Access-related complications | Transfusion only | Minor vascular/bleeding complications |
|---|---|---|---|---|---|
| Al-Rashid et al. (2020)13 | 3.1% (2/64) | NR | - | NR | NR |
| Johannsen et al. (2019)27 | 8.2% (5/61; 2 inguinal haematoma, 3 access site bleeding [1 requiring transfusion]) | 3.3% (2/61; bleeding from access site with Hgb drop ≥3 g/dl [1 requiring transfusion]) | 100% | 1.6% (1/61) | 36.1% (22/61; 95% superficial haematoma, no treatment required) |
| Becher (2019)18 | 0% (0/54) | 0% (0/26) | None | 0% (0/26) | NR |
| Riley et al. (2018)29 | 5.3% (3/57; all vascular injury requiring intervention) | 3.5% (2/57; BARC 3/5) | NR | NR | NR |
| Russo et al. (2019)30 | 2.7% (1/37; distal embolisation requiring urgent angioplasty) | 5.4% (2/37; all transfusions) | NR | 5.4% (2/37) | NR |
| Amponsah et al. (2017)14 | 0% (0/40) | 0% (0/40) | None | 0% (0/40) | 5.0% (2/40; 1 patient had Mynx rupture after Perclose, 1 had Perclose failure) |
| Danek et al. (2018)22 | NR | 10.0% (5/50; Impella 2.5: 21.1% [4/19]; Impella CP: 3.2% [1/31]) | 80% | NR | 8.0% (4/50; vascular access site bleeding; Impella 2.5: 15.8% [3/19]; Impella CP: 3.2% [1/31]) |
| Doshi et al. (2019)24 | NR | 5.0% (8/160; bleeding within 72 hours) | NR | 3.1% (5/160) | NR |
| Azzalini et al. (2020)16 | NR | 6.8% (17/250) | NR* | 11.2% (28/250) | NR |
| Venugopal et al. (2015)32 | 0% (0/45) | 0% (0/45) | None | 4.4% (2/45; transfusions to aid clinical recovery for pre-existing anaemia [no evidence of bleeding or fall in Hgb levels]) | NR |
| Alasnag et al. (2011)11 | 0% (0/60) | 10% (6/60; all transfusions) | NR | 10% (6/60) | 8.3% (5/60; groin haematomas that resolved without residual effects) |
| Anusionwu et al. (2012)15 | NR | 0% (0/25) | None | 0% (0/25) | 8.0% (2/25; groin haematoma, resolved with manual compression) |
| Burzotta et al. (2019)19 | 0% (0/86) | 4.7% (4/86; 4 BARC 3 events with 3 requiring transfusion) | NR | 3.5% (3/86) | 10.5% (9/86, 6 BARC 1 haematoma, 2 BARC 2 PTA to facilitate haemostasis, 2 minor vascular complications [1 access site vascular injury managed conservatively, 1 distal embolisation/ALI event]) |
| Alraies et al. (2019)12 | 1.2% (13/1,053; vascular complications requiring surgery) | 7.0% (74/1,053; 67 required transfusion, 7 required surgery) | Primarily anaemia related‡ | 6.4% (67/1,053)† | 6.0% (63/1,053; 40 haematoma, 23 vascular complications not requiring surgery [likely overlap between these two counts]) |
| Cohen et al. (2015)21 | 2.5% (16/637; vascular complications requiring surgery) | 11.0% (70/637; all transfusions) | NR | 11.0% (70/637)‡ | 5.2% (33/637; vascular complications not requiring surgery) |
| Cohen et al. (2015)21/O’Neill et al (2012)7 | 1.4% (3/216; vascular complications requiring surgery) | 12.5% (27/216; all transfusions) | NR | 12.5% (27/216)‡ | 9.3% (20/216; vascular complications not requiring surgery) |
| Iliodromitis et al. (2011)26 | 2.6% (1/38; pseudoaneurysm requiring prolonged hospitalisation and pressure badge) | 5.3% (2/38) | NR | 34.2% (13/38; 11 due to pre-existing anaemia for additional blood supply, 2 due to bleeding complications) | 31.6% (12/38; 6 minor bleeding, 6 femoral haematomas not requiring transfusion) |
| Ferreiro et al. (2010)25 | 3.7% (1/27; limb ischaemia requiring surgery) | 7.4% (2/27; 1 specified as intracranial bleeding 8 hours post Impella removal) | 1 not specified, 1 non-access | 3.7% (1/27)§ | 14.8% (4/27; 2 limb ischaemia managed medically and not classed as severe, 2 minor bleeding) |
| Dixon et al. (2009)23 | 0% (0/20) | 0% (0/20) | None | 10.0% (2/20; 1 due to baseline anaemia, 1 due to haematuria secondary to bladder cancer) | 40.0% (8/20; haematoma, none requiring transfusion or vascular repair) |
| Kovacic et al. (2013)28 | 8.3% (3/36; 1 pseudoaneurysm requiring thrombin injection, 1 femoral artery occlusion, treated percutaneously, 1 haematoma requiring transfusion) | 2.8% (1/36; haematoma requiring transfusion) | 100% | 2.8% (1/36) | 8.3% (3/36; 2 small haematomas and 1 large haematoma, none requiring transfusion) |
| Chieffo et al. (2020)20 | 2.8% (5/177; 5 device-related complications requiring intervention [5 limb ischaemia occurred, presumably all requiring intervention])† | 4.5% (8/177) | NR | NR | 7.9% (14/177; access site bleeding not classified as severe) |
| Sjauw et al. (2009)31 | 4.2% (6/144; spurious aneurysm, fistula) | 6.3% (9/144; 8 required transfusion, 1 required surgery) | NR | 6.3% (9/144)¶ | NR |
| Baumann et al. (2020)17 | 6.4% (10/157; 3 peripheral leg ischaemia, 2 aneurysm spurium, 2 dissection, 2 thrombus, 1 embolism) | 6.4% (10/157; access site bleeding requiring transfusion) | 100% | 6.4% (10/157) | NR |
Minor vascular/bleeding complications are per study reporting.
*Azzalini et al. reported that there was higher access-related bleeding in patients with Impella-supported HR-PCI versus those with no mechanical circulatory support; however, there was no quantification of this or description of bleeding events, so it is unknown what percentage of bleeding complications were related to access.
†The authors state that most transfusions were due to patient baseline condition/anaemia; however, this was not quantified within the reported number of bleeding complications/transfusions. Therefore, the percentage listed with major bleeding (7%; 74 patients including 67 with transfusion) includes a significant proportion with transfusion for baseline anaemia.
‡In the PROTECT II and USpella comparative study, haemolysis occurred in one patient with USpella (0.2%) and two patients with PROTECT II (0.9%). Though Cohen et al. did not report whether haemolysis events required transfusion; if all required transfusion, this would constitute 1.4% and 7.4% of all reported transfusions being performed in the setting of haemolysis.
§One patient (3.7%) experienced haemolysis requiring transfusion in this study; this constituted the only transfusion reported.
¶One patient (0.7%) experienced haemolysis requiring transfusion; this constituted 1 of 9 (11.1%) transfusions performed in the study.
ALI = acute limb ischaemia; BARC = Bleeding Academic Research Consortium; CP = Impella CP device; Hgb = haemoglobin; HR-PCI = high-risk percutaneous coronary intervention; NR = not reported; PTA = percutaneous transluminal angioplasty.
Vascular complications
Median rate of major vascular complications was 2.6% (range, 0–8.3%), with 15 of the 19 studies reporting a vascular complication rate <5%. The rate of vascular complications did not vary as widely as bleeding complication rates; this finding likely reflects the fact that major bleeding complications may stem from non-access as well as access-related causes, whereas vascular complications, by definition, are more specific.
Trends in bleeding and vascular complications over time
Bleeding complications over time demonstrated that the variability in bleeding risk seen across centres has not changed meaningfully over the last 15 years (Figure 1).7,11–16,18–32 Given the broad scope of bleeding complications (comprising both access and non-access–related bleeding during PCI), often complicated by pre-existing anaemia and the inclusion of some urgent HR-PCI patient populations, this variability is not surprising. Likewise, many series reported relatively low numbers of cases over extended time periods, suggesting that any advantages of increased operator skill, using best practices accrued through increasing experience, could not be ascertained. While we attempted to focus on higher-volume reports (≥20 patients) to provide ‘best practice’ results, eliminating ‘occasional users’, even in larger studies it is impossible to identify the extent to which specific operators or teams were involved in a series of cases, minimising our ability to assess whether any potential learning curve with the large-bore Impella procedure is driving bleeding complication rates. Thus, low yearly volumes may obscure the ability to ascertain whether improvements in outcomes over time occur with increased operator and institutional experience for large-bore access and supported HR-PCI, as has been reported for trans-aortic valve repair (TAVR).33,34 The rate of vascular complications remained consistently low over time (Figure 2).7,11–16,18–32 Transfusion rates suggest a downward trend over time (Figure 3).7,11–16,18–32
Figure 1: Major bleeding complications as reported across Impella-supported high-risk percutaneous coronary intervention studies conducted from 2004 to 2018.
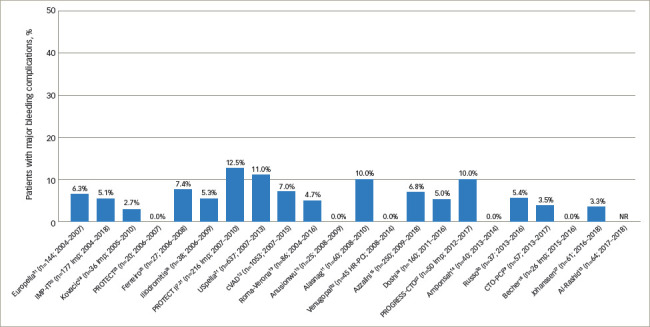
Studies are ordered from earliest year of study enrolment to latest. One study, which did not include years of enrolment (Baumann et al.17), is excluded.
NR = not reported.
Figure 2: Major vascular complications reported across Impella studies by ascending year.
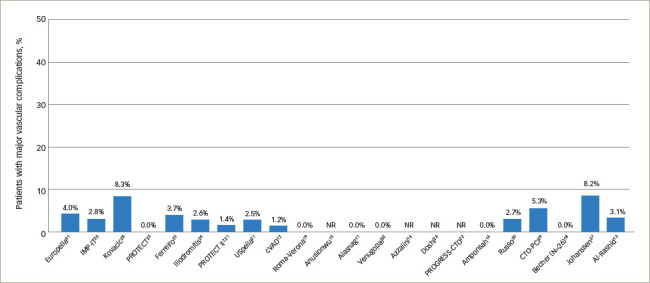
NR = not reported.
Figure 3: Rate of transfusions reported across Impella studies by ascending year.
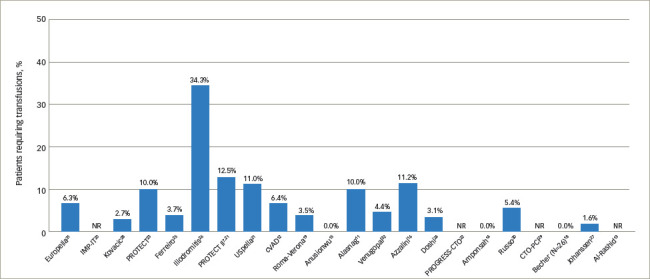
NR = not reported.
Impact of access and non-access bleeding complications on clinical outcomes
A significant limitation in published studies on HR-PCI is the relative rarity of studies identifying the specific aetiology of bleeding complications – whether access-related or gastrointestinal bleeding, etc. In a large CathPCI registry analysis of bleeding in a largely elective-urgent, not specifically high-risk PCI patient population, Rao et al. reported a similar overall bleeding rate of 5.8% and found that 32% of bleeding events had a specified site, with a further 44.6% due to haemoglobin decrease and 21.8% due to blood transfusions.35
The prognostic relationship of PCI bleeding complications with mortality varies greatly depending on the site of bleeding. A large meta-analysis of PCI studies (primarily acute myocardial infarction patient populations) specifying the site of bleeding complications found that, while both access and non-access–related bleeding complications were linked with an increased risk for mortality, the risk ratio (RR) for non-access site bleeding was considerably higher than with access site bleeding (RR 4.06 and 1.71, respectively).36 To our knowledge, there has been no comprehensive review of access versus non-access–related bleeding complications and their respective prognostic values in an exclusively elective-urgent HR-PCI population with MCS support. Our review identified the difficulties in conducting such a review from the current literature, as a majority of studies did not specify bleeding site.
The bleeding definition used also significantly impacts the prognostic value of reported bleeding events, and in our review, varied widely across published studies. Often, major bleeding definition was not specified at all. A meta-analysis of PCI studies (STEMI and elective patient populations) encompassing several different bleeding definitions found that, depending on the definition used, the mortality risk from a major bleeding event ranged from a 1.5 to 6.7 times increase.37
Factors impacting bleeding complications in Impella-supported high-risk percutaneous coronary intervention
An observed increase in femoral access complications with PCI in recent years may be due, in large part, to the swift uptake of radial access for conventional PCI procedures by many operators.38 This ‘Campeaux Paradox’ has occurred due to operators having fewer femoral access cases, with a subsequent loss of femoral skills. Though radial access for PCI has been associated with reduced complications compared with femoral access in several prospective studies,39 there will always be a requirement for femoral access in some cases with challenging anatomy, necessitating that operators maintain femoral skills, both for PCI access and for large-bore sheath procedures, such as TAVR and pVAD placement. There is also a potential learning curve for large-bore access procedures, not only on the operator level but also involving team experience with large-bore procedures and effective management of access complications. In the USpella registry, Cohen et al. identified declining transfusion rates of 12.2%, 7.4% and 6.1% in patients treated in the years 2009, 2010 and 2011, respectively.21 The authors suggested that a learning curve with large access closure techniques was potentially responsible for the decreasing transfusion rate over time.
In multiple studies identified in this review, all, or a majority of, transfusions were to aid clinical recovery due to baseline anemia.12,23,26,32 Low baseline haemoglobin levels were one of 10 variables included in a validated bleeding risk model developed by Rao et al. to delineate high bleeding risk in patients up to 72 hours after undergoing PCI.35 While some factors were related to clinical circumstances, such as acute myocardial infarction and/or shock, others were patient-related and likely risk factors related to Impella procedures including female gender, age >70 years, renal failure, diabetes and high BMI. Thus, bleeding/transfusion risk is a composite of access site and clinical patient features. Those patients deemed to be high bleeding risk merit careful consideration of whether MCS is necessary to support the HR-PCI procedure. Emerging technologies aimed to reduce vascular complications with large-bore access may ameliorate the risk of access-site bleeding and vascular complications in patients with high bleeding risk, and are discussed in a later section.
Risk–benefit assessment of elective Impella support
Considerations for MCS haemodynamic support vary by severity and complexity of anatomic coronary disease, overall patient clinical risk and baseline haemodynamic stability including overall LV function, further modulated by operator-estimated patient risk for haemodynamic collapse during PCI. While operator experience is often considered a factor, not all studies have shown an outcome benefit for high operator experience.40 Bleeding and vascular complications remain an important consideration related to MCS support for HR-PCI.
An important consideration for operators is the outcome risk when managing a patient with acute haemodynamic collapse during PCI, most often secondary to a complication. A recent cVAD registry analysis of patients undergoing HR-PCI with Impella, either pre-procedure (prophylactic) or as bailout, showed a striking risk for the bailout group.41 Specifically, those patients treated with bailout as opposed to prophylactic Impella support during HR-PCI had significantly higher in-hospital mortality (49.1% versus 4.3%).41 What remains unknown is the percentage of patients undergoing unsupported HR-PCI who ultimately experience severe haemodynamic instability requiring bailout MCS. Thus, in the absence of Level 1 evidence and specific guidelines for pVAD support during HR-PCI, variation in approaches further add to the difficulty in analysing overall procedural risk as well as bleeding risk.
Limitations of clinical dataset and future directions
In the studies we reviewed, the site of bleeding events was seldom specified. Importantly, reported bleeding complications not only reflect complications due to large-bore Impella access, but other access sites such as PCI access, in addition to gastrointestinal bleeding, transfusions to aid clinical recovery, anticoagulation practices (i.e. failure to titrate total heparin administration for the Impella purge solution), patients with high bleeding risk, or other, unspecified causes.
In recent years, there has been significant focus on developing improved techniques for access site and anticoagulation management, as well as appropriate closure techniques, all potentially limiting access-site bleeding risk. Few studies have been conducted exclusively in the last 5 years on Impella-supported HR-PCI with reported outcomes on complications observed. In fact, no studies of ≥20 patients appear to have been conducted exclusively in the last 2 years. This limits our ability to assess the impact of emerging solutions for large-bore access strategy on bleeding complication rates.
Evolving solutions
In an attempt to reduce bleeding risk, a variety of new techniques continue to be introduced. Because the number of vascular access sites required for a complex procedure increases the risk of a vascular event, a newly introduced single-access Impella technique has the potential to reduce access-site complications for Impella-supported HR-PCI by eliminating the need for a separate PCI access site.42 While an initial report on outcomes with this technique is encouraging, more data are needed to assess its safety and efficacy.42 Recent reports suggest that the use of routine ultrasound guidance for large-bore femoral access may reduce vascular and bleeding complications.43,44 Another development for large-bore procedures is the recent approval of the MANTA® VCD (Teleflex, Morrisville, NC, USA), a closure device specifically designed for 10–20 Fr large-bore procedures, though predominantly evaluated in TAVR studies published to date.45–48 While new techniques and devices are encouraging, larger studies will be required to both identify and confirm the optimal strategies to ultimately reduce bleeding and vascular complications associated with large-bore access for haemodynamic support in HR-PCI.
Conclusions
Bleeding and vascular complications adversely affect clinical outcomes after Impella-supported HR-PCI. Rates of major bleeding complications have shown wide variation over the last 15 years, underlining the importance of recognising the risks related to large-bore sheath procedures. Transfusion rates appear to have decreased over time, whereas occurrence of major vascular complications has remained relatively constant (<5% in most studies over the last 15 years). Thus, patient selection for protected HR-PCI should involve consideration of high bleeding risk factors and utilising currently accepted, patient-specific, bleeding avoidance strategies, as well as optimal vascular access management to minimise bleeding and vascular risk when Impella is used for HR-PCI.
Acknowledgments
Dana Bentley, consultant medical writer, assisted with the literature review and provided medical writing services, funded by Abiomed and in accordance with GPP3. She is now an employee of Abiomed.
Funding Statement
Support: No publication charges were associated with this article, but funding was received for medical writing support.
Footnotes
Review Process: Double-blind peer review.
Compliance with Ethics: This article involves a review of the literature and did not involve any studies with human or animal subjects performed by any of the authors.
Authorship: All named authors meet the criteria of the International Committee of Medical Journal Editors for authorship for this manuscript, take responsibility for the integrity of the work as a whole and have given final approval for the version to be published.
References
- 1.Kataruka A, Maynard CC, Kearney KE. et al. Temporal trends in percutaneous coronary intervention and coronary artery bypass grafting: insights from the Washington Cardiac Care Outcomes Assessment Program. J Am Heart Assoc. 2020;9:e015317. doi: 10.1161/JAHA.119.015317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waldo SW, Secemsky EA, O’Brien C. et al. Surgical ineligibility and mortality among patients with unprotected left main or multivessel coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2014;130:2295–301. doi: 10.1161/CIRCULATIONAHA.114.011541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bass TA. High-risk percutaneous coronary interventions in modern day clinical practice: current concepts and challenges. Circ Cardiovasc Interv. 2015;8::e003405. doi: 10.1161/CIRCINTERVENTIONS.115.003405. [DOI] [PubMed] [Google Scholar]
- 4.Myat A, Patel N, Tehrani S. et al. Percutaneous circulatory assist devices for high-risk coronary intervention. JACC Cardiovasc Interv. 2015;8:229–44. doi: 10.1016/j.jcin.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 5.Burkhoff D, Sayer G, Doshi D, Uriel N. Hemodynamics of mechanical circulatory support. J Am Coll Cardiol. 2015;66:2663–74. doi: 10.1016/j.jacc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Amin AP, Spertus JA, Curtis JP. et al. The evolving landscape of Impella use in the United States among patients undergoing percutaneous coronary intervention with mechanical circulatory support. Circulation. 2020;141:273–84. doi: 10.1161/CIRCULATIONAHA.119.044007. [DOI] [PubMed] [Google Scholar]
- 7.O’Neill WW, Kleiman NS, Moses J. et al. A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation. 2012;126:1717–27. doi: 10.1161/CIRCULATIONAHA.112.098194. [DOI] [PubMed] [Google Scholar]
- 8.Dangas GD, Kini AS, Sharma SK. et al. Impact of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump on prognostically important clinical outcomes in patients undergoing high-risk percutaneous coronary intervention (from the PROTECT II randomized trial). Am J Cardiol. 2014;113:222–8. doi: 10.1016/j.amjcard.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Vetrovec GW. Hemodynamic support devices for shock and high-risk PCI: when and which one. Curr Cardiol Rep. 2017;19:100. doi: 10.1007/s11886-017-0905-3. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Sharma A, Dalia T. et al. Vascular complications associated with percutaneous left ventricular assist device placement: A 10-year US perspective. Catheter Cardiovasc Interv. 2020;95:309–16. doi: 10.1002/ccd.28560. [DOI] [PubMed] [Google Scholar]
- 11.Alasnag MA, Gardi DO, Elder M. et al. Use of the Impella 2.5 for prophylactic circulatory support during elective high-risk percutaneous coronary intervention. Cardiovasc Revasc Med. 2011;12:299–303. doi: 10.1016/j.carrev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Alraies MC, Kaki A, Kajy M. et al. Sex-related difference in the use of percutaneous left ventricular assist device in patients undergoing complex high-risk percutaneous coronary intervention: Insight from the cVAD registry. Catheter Cardiovasc Interv. 2020;96:536–44. doi: 10.1002/ccd.28509. [DOI] [PubMed] [Google Scholar]
- 13.Al-Rashid F, Mahabadi AA, Johannsen L. et al. Impact of left-ventricular end-diastolic pressure as a predictor of periprocedural hemodynamic deterioration in patients undergoing Impella supported high-risk percutaneous coronary interventions. Int J Cardiol Heart Vasc. 2020;26:100445. doi: 10.1016/j.ijcha.2019.100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amponsah MK, Tayal R, Khakwani Z. et al. Safety and efficacy of a novel “hybrid closure” technique in large-bore arteriotomies. Int J Angiol. 2017;26:116–20. doi: 10.1055/s-0037-1598252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anusionwu O, Fischman D, Cheriyath P. The duration of Impella 2.5 circulatory support and length of hospital stay of patients undergoing high-risk percutaneous coronary interventions. Cardiol Res. 2012;3:154–7. doi: 10.4021/cr190e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azzalini L, Johal GS, Baber U. et al. Outcomes of Impella-supported high-risk nonemergent percutaneous coronary intervention in a large single-center registry. Catheter Cardiovasc Interv. 2021;97:E26–33. doi: 10.1002/ccd.28931. [DOI] [PubMed] [Google Scholar]
- 17.Baumann S, Werner N, Al-Rashid F. et al. Six months follow-up of protected high-risk percutaneous coronary intervention with the microaxial Impella pump: results from the German Impella registry. Coron Artery Dis. 2020;31:237–42. doi: 10.1097/MCA.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 18.Becher T, Baumann S, Eder F. et al. Comparison of peri and post-procedural complications in patients undergoing revascularisation of coronary artery multivessel disease by coronary artery bypass grafting or protected percutaneous coronary intervention with the Impella 2.5 device. Eur Heart J Acute Cardiovasc Care. 2019;8:360–8. doi: 10.1177/2048872617717687. [DOI] [PubMed] [Google Scholar]
- 19.Burzotta F, Russo G, Ribichini F. et al. Long-term outcomes of extent of revascularization in complex high risk and indicated patients undergoing Impella-protected percutaneous coronary intervention: report from the Roma-Verona Registry. J Interv Cardiol. 2019;2019:5243913. doi: 10.1155/2019/5243913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chieffo A, Ancona MB, Burzotta F. et al. Observational multicentre registry of patients treated with IMPella mechanical circulatory support device in ITaly: the IMP-IT registry. EuroIntervention. 2020;15:e1343–50. doi: 10.4244/EIJ-D-19-00428. [DOI] [PubMed] [Google Scholar]
- 21.Cohen MG, Matthews R, Maini B. et al. Percutaneous left ventricular assist device for high-risk percutaneous coronary interventions: real-world versus clinical trial experience. Am Heart J. 2015;170:872–9. doi: 10.1016/j.ahj.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Danek BA, Basir MB, O’Neill WW. et al. Mechanical circulatory support in chronic total occlusion percutaneous coronary intervention: insights from a multicenter U.S. registry. J Invasive Cardiol. 2018;30:81–7. [PubMed] [Google Scholar]
- 23.Dixon SR, Henriques JP, Mauri L. et al. A prospective feasibility trial investigating the use of the Impella 2.5 system in patients undergoing high-risk percutaneous coronary intervention (The PROTECT I Trial): initial U.S. experience. JACC Cardiovasc Interv. 2009;2:91–6. doi: 10.1016/j.jcin.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Doshi R, Singh A, Jauhar R, Meraj PM. Gender difference with the use of percutaneous left ventricular assist device in patients undergoing complex high-risk percutaneous coronary intervention: From pVAD Working Group. Eur Heart J Acute Cardiovasc Care. 2019;8:369–78. doi: 10.1177/2048872617745790. [DOI] [PubMed] [Google Scholar]
- 25.Ferreiro JL, Gómez-Hospital JA, Cequier Á R. et al. Use of Impella Recover LP 2.5 in elective high risk percutaneous coronary intervention. Int J Cardiol. 2010;145:235–7. doi: 10.1016/j.ijcard.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Iliodromitis KE, Kahlert P, Plicht B. et al. High-risk PCI in acute coronary syndromes with Impella LP 2.5 device support. Int J Cardiol. 2011;153:59–63. doi: 10.1016/j.ijcard.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 27.Johannsen L, Mahabadi AA, Totzeck M. et al. Access site complications following Impella-supported high-risk percutaneous coronary interventions. Sci Rep. 2019;9:17844. doi: 10.1038/s41598-019-54277-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovacic JC, Nguyen HT, Karajgikar R. et al. The Impella Recover 2.5 and TandemHeart ventricular assist devices are safe and associated with equivalent clinical outcomes in patients undergoing high-risk percutaneous coronary intervention. Catheter Cardiovasc Interv. 2013;82:E28–37. doi: 10.1002/ccd.22929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riley RF, McCabe JM, Kalra S. et al. Impella-assisted chronic total occlusion percutaneous coronary interventions: A multicenter retrospective analysis. Catheter Cardiovasc Interv. 2018;92:1261–7. doi: 10.1002/ccd.27679. [DOI] [PubMed] [Google Scholar]
- 30.Russo G, Burzotta F, D’Amario D. et al. Hemodynamics and its predictors during Impella-protected PCI in high risk patients with reduced ejection fraction. Int J Cardiol. 2019;274:221–5. doi: 10.1016/j.ijcard.2018.07.064. [DOI] [PubMed] [Google Scholar]
- 31.Sjauw KD, Konorza T, Erbel R. et al. Supported high-risk percutaneous coronary intervention with the Impella 2.5 device the Europella registry. J Am Coll Cardiol. 2009;54:2430–4. doi: 10.1016/j.jacc.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 32.Venugopal V, Spiro J, Zaphiriou A. et al. Percutaneous mechanical ventricular support in acute cardiac care: a UK quaternary centre experience using 2.5L, 3.8L and 5.0L Impella catheters. Cardiol Ther. 2015;4:47–58. doi: 10.1007/s40119-014-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo MJ, McCabe JM, Thourani VH. et al. Case volume and outcomes after tavr with balloon-expandable prostheses: insights from TVT Registry. J Am Coll Cardiol. 2019;73:427–40. doi: 10.1016/j.jacc.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Wassef AWA, Rodes-Cabau J, Liu Y. et al. The learning curve and annual procedure volume standards for optimum outcomes of transcatheter aortic valve replacement: findings from an international registry. JACC Cardiovasc Interv. 2018;11:1669–79. doi: 10.1016/j.jcin.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 35.Rao SV, McCoy LA, Spertus JA. et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovasc Interv. 2013;6:897–904. doi: 10.1016/j.jcin.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Kwok CS, Khan MA, Rao SV. et al. Access and non-access site bleeding after percutaneous coronary intervention and risk of subsequent mortality and major adverse cardiovascular events: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8:e001645. doi: 10.1161/CIRCINTERVENTIONS.114.001645. [DOI] [PubMed] [Google Scholar]
- 37.Kwok CS, Rao SV, Myint PK. et al. Major bleeding after percutaneous coronary intervention and risk of subsequent mortality: a systematic review and meta-analysis. Open Heart. 2014;1:e000021. doi: 10.1136/openhrt-2013-000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Azzalini L, Tosin K, Chabot-Blanchet M. et al. The benefits conferred by radial access for cardiac catheterization are offset by a paradoxical increase in the rate of vascular access site complications with femoral access: the Campeau Radial Paradox. JACC Cardiovasc Interv. 2015;8:1854–64. doi: 10.1016/j.jcin.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 39.Valgimigli M, Gagnor A, Calabro P. et al. Radial versus femoral access in patients with acute coronary syndromes undergoing invasive management: a randomised multicentre trial. Lancet. 2015;385:2465–76. doi: 10.1016/S0140-6736(15)60292-6. [DOI] [PubMed] [Google Scholar]
- 40.Kinnaird T, Gallagher S, Spratt JC. et al. Complex high-risk and indicated percutaneous coronary intervention for stable angina: Does operator volume influence patient outcome? Am Heart J. 2020;222:15–25. doi: 10.1016/j.ahj.2019.12.019. [DOI] [PubMed] [Google Scholar]
- 41.O’Neill W, Moses JW, Popma JJ. Outcomes of Impella use as prophylactic versus bailout strategy in patients undergoing non-emergent percutaneous coronary intervention. Catheter Cardiovasc Interv. 2020;95:S54–5. doi: 10.1002/ccd.29758. [DOI] [PubMed] [Google Scholar]
- 42.Wollmuth J, Korngold E, Croce K, Pinto DS. The Single-access for Hi-risk PCI (SHiP) technique. Catheter Cardiovasc Interv. 2020;96:114–6. doi: 10.1002/ccd.28556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Potluri SP, Hamandi M, Basra SS. et al. Comparison of frequency of vascular complications with ultrasound-guided versus fluroscopic roadmap-guided femoral arterial access in patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2020;132:93–9. doi: 10.1016/j.amjcard.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Vincent F, Spillemaeker H, Kyheng M. et al. Ultrasound guidance to reduce vascular and bleeding complications of percutaneous transfemoral transcatheter aortic valve replacement: a propensity score-matched comparison. J Am Heart Assoc. 2020;9:e014916. doi: 10.1161/JAHA.119.014916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biancari F, Romppanen H, Savontaus M. et al. MANTA versus ProGlide vascular closure devices in transfemoral transcatheter aortic valve implantation. Int J Cardiol. 2018;263:29–31. doi: 10.1016/j.ijcard.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 46.De Palma R, Settergren M, Ruck A. et al. Impact of percutaneous femoral arteriotomy closure using the MANTA(TM) device on vascular and bleeding complications after transcatheter aortic valve replacement. Catheter Cardiovasc Interv. 2018;92:954–61. doi: 10.1002/ccd.27595. [DOI] [PubMed] [Google Scholar]
- 47.Gheorghe L, Brouwer J, Mathijssen H. et al. Early outcomes after percutaneous closure of access site in transfemoral transcatheter valve implantation using the novel vascular closure device collagen plug-based MANTA. Am J Cardiol. 2019;124:1265–71. doi: 10.1016/j.amjcard.2019.07.030. [DOI] [PubMed] [Google Scholar]
- 48.Moriyama N, Lindstrom L, Laine M. Propensity-matched comparison of vascular closure devices after transcatheter aortic valve replacement using MANTA versus ProGlide. EuroIntervention. 2019;14:e1558–65. doi: 10.4244/EIJ-D-18-00769. [DOI] [PubMed] [Google Scholar]


