Abstract
Purpose:
Until recently, there has been little investigation on the effects of cochlear implantation on the transmission of acoustic stimuli through the middle-ear system. Recent studies have shown that cochlear implantation decreases low-frequency acoustic absorbance, consistent with a stiffer middle-ear system postsurgery. The objectives of this study are (a) to investigate the time course of changes in acoustic absorbance post–cochlear implantation in the implanted ear and (b) to compare changes in acoustic absorbance between implanted and nonimplanted ears over time.
Method:
Seventeen adult cochlear implant (CI) recipients within 6 months of device activation participated in this study. Wideband acoustic absorbance was measured in both ears at one to six different time points from pre-implantation up to 6-month postactivation. Analyses examined (a) changes in acoustic absorbance as compared to pre-implantation and (b) differences in acoustic absorbance between implanted and nonimplanted ears over time.
Results:
Acoustic absorbance in the implanted ear decreased postsurgery for frequencies lower than 1.5 kHz and persisted through at least 6-month postactivation. We also observed that the spectral range of decreased acoustic absorbance in the implanted ear decreased with longer time postsurgery. Differences in acoustic absorbance between implanted and nonimplanted ears occurred over a broad spectral range at the activation time point and persisted through at least 3-month postactivation, though for a narrower spectral range at the later time point.
Conclusions:
Cochlear implantation increased middle-ear stiffness as indicated by decreased acoustic absorbance of low-frequency acoustic power. The findings of this study are consistent with those of previous studies and may have important implications toward understanding spatial hearing and programming of acoustic components for CI-combined electric and binaural acoustic stimulation patients.
The middle-ear system, consisting of ossicles, ligaments, and muscles that connect the tympanic membrane to the oval window, is responsible for transmitting mechanical–acoustic sound pressure waves from the external environment to the cochlea and auditory neural pathways (e.g., Merchant & Rosowski, 2003). Wideband acoustic immittance (WAI) is a collective term used to describe measures of middle-ear and cochlear functions assessed over a broad spectral range. The most common WAI measures include acoustic absorbance and reflectance (Allen et al., 2005; Feeney et al., 2003). Acoustic absorbance represents the percentage of acoustic power that is absorbed into the middle-ear cavity, whereas acoustic reflectance represents the percentage of acoustic power that is reflected by the tympanic membrane back into the ear canal.
Recent studies have demonstrated reduced low-to-middle-frequency acoustic absorbance postsurgery in cochlear implant (CI) recipients that is consistent with changes in auditory mechanics, including a potentially stiffer middle-ear system (Merchant et al., 2020; Saoji et al., 2020; Scheperle & Hajicek, 2020). Several factors related to CI surgical procedures may contribute to a stiffer middle-ear system. Increased volume of the middle-ear cavity by introduction of the facial recess during CI surgery can cause the middle-ear transmission system to become dominated by ossicular chain stiffness (Mason, 2016; Saoji et al., 2020; Scheperle & Hajicek, 2020). Additionally, the sealing of the electrode array at the round window and the accumulation of bone dust postsurgery can lead to neo-osteogenesis, potentially increasing the stiffness of the middle-ear transmission system (Saoji et al., 2020; Scheperle & Hajicek, 2020). Increased middle-ear stiffness post–cochlear implantation may also be secondary to changes in cochlear function following surgery. The insertion of an electrode array into the cochlea may increase stiffness of the oval window membrane, in turn, increasing the stiffness of the ossicular chain and tympanic membrane. Furthermore, the development of cochlear fibrotic tissue over time post–cochlear implantation may contribute to increased middle-ear stiffness and would be consistent with decreased acoustic absorbance at longer times postsurgery as fibrosis develops.
Previous studies related to changes in acoustic absorbance post–cochlear implantation assessed acoustic absorbance at only a single point in time. In between-subjects investigations, Merchant et al. (2020) and Scheperle and Hajicek (2020) showed reduced acoustic absorbance in the implanted ears of CI recipients compared to an independent group of ears with normal hearing. It is important to note the differences in the spectral regions where reduced acoustic absorbance was observed in these studies: 708–1122 Hz at an unspecified time postsurgery (Merchant et al., 2020) versus 250–891 Hz at 3 to 25 years postsurgery (Scheperle & Hajicek, 2020). In a within-subject investigation, Saoji et al. (2020) demonstrated reduced acoustic absorbance from 600 to 1100 Hz in the implanted ears of five CI recipients between 45 and 60 days postsurgery. Investigating changes in acoustic absorbance in CI ears over several time points pre- and postsurgery will begin to address important and clinically relevant questions related to the onset, duration, and frequency-specific time course of middle-ear stiffness changes in the implanted ear postsurgery.
Additionally, many CI recipients with acoustic hearing preservation following surgery have a postoperative air–bone gap (ABG) in their behavioral hearing thresholds measured in the implanted ear(s). It is important to consider whether this postoperative conductive component and decreased acoustic absorbance in the implanted ear(s) postsurgery are due to similar underlying physiologic mechanisms. Postoperative conductive hearing loss may be due to changes in either middle-ear or cochlear physiology. To this point, Banakis Hartl et al. (2016) identified changes in the intracochlear sound pressure to air-conducted, but not bone-conducted, stimuli in human cadaveric tissue postinsertion of a CI electrode array into the cochlea. The findings of Banakis Hartl et al. (2016) suggest that postoperative ABGs may be due to a cochlear conductive component rather than to changes in middle-ear transmission, at least in human cadavers.
Previous work investigating changes in acoustic absorbance post–cochlear implantation can be further extended by comparing changes in acoustic absorbance between implanted and contralateral, nonimplanted ears for unilateral CI recipients. Unilateral changes in the middle-ear transmission of sound in an implanted ear with acoustic hearing preservation may impact binaural cue sensitivity and spatial hearing abilities when combined electric and binaural acoustic (EAS) listening is utilized. Characterizing changes in middle-ear stiffness in the implanted ear relative to the nonimplanted ear within individuals may have important implications toward understanding the binaural processing of acoustic sound in CI EAS listening.
As the number of CI EAS listeners continues to grow, it is important to understand the impact of cochlear implantation on the middle-ear transmission of low-frequency sounds where acoustic hearing is likely to be preserved and utilized with EAS technology. As such, the aims of this study were twofold. The first aim was to investigate changes in acoustic absorbance in the implanted ears of CI recipients over a time span from pre-CI baseline to 6-month postactivation. As part of this first aim, we also investigated the potential relationship between the degree of postoperative ABG and acoustic absorbance. The second aim of this study was to compare acoustic absorbance between implanted and nonimplanted ears within participants across time points. Acoustic hearing preservation was quantified for each participant, and findings were considered with particular regard for the impact of increased middle-ear stiffness post-CI in the context of EAS listening.
Method
Participant Sample
This study was approved by the Vanderbilt University Medical Center (VUMC) Institutional Review Board (No. 110550). All participants were invited to participate in this study following completion of routine clinical appointments as part of their audiologic care at VUMC and provided written informed consent acknowledging their voluntary participation in this study. All participants received monetary compensation for their time.
Twenty-two CI recipients (nine female, 13 male) participated in this study. Three participants were excluded from analyses because their WAI data suggested a poor probe fit for one or both ears (see details below) during their participation at each time point. Two additional participants were excluded because they only participated at the pre-CI baseline time point. Thus, data from 17 participants (seven female, 10 male) were included in the final analyses.
Cohorts for Implant Ear and Ear Difference Analyses
From the 17 participants who met our overall inclusion criteria, we established a cohort of 10 participants who met inclusion criteria for the implant ear analyses and a second cohort of 14 participants who met inclusion criteria for the ear difference analyses. Seven participants were excluded from analyses of acoustic absorbance changes in the implanted ear postsurgery because they did not have pre-CI baseline data. Thus, 10 participants comprised the cohort for the implant ear analyses. Two participants were excluded from ear difference analyses because they were bilateral CI recipients, and one participant was excluded due to having acoustic absorbance data for only a single ear at all time points. Thus, data for 14 participants were included in the ear difference analyses (see Table 1).
Table 1.
Demographic information for participants included at each of the six time points for this study.
| Pre-CI baseline |
Activation |
1-week postactivation |
1-month postactivation |
3-month postactivation |
6-month postactivation |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender identity | CI ear | Implant type | Include longit. | Include ear comp. | Age (years) | CI LFPTA (dB HL) | Non-CI LFPTA (dB HL) | Age (years) | CI LFPTA (dB HL) | Age (years) | CI LFPTA (dB HL) | Age (years) | CI LFPTA (dB HL) | Age (years) | CI LFPTA (dB HL) | Age (years) | CI LFPTA (dB HL) |
| S001 | Male | R | Cochlear Hybrid L24 | Yes | Yes | 72.55 | 27.00 | 25.00 | 72.80 | 68.00 | — | — | — | — | 73.04 | 83.00 | 73.30 | 73.00 |
| S002 | Male | L | Cochlear Hybrid L24 | No | Yes | — | — | — | 32.73 | 55.00 | 32.75 | 40.00 | 32.82 | 43.00 | 32.96 | 43.00 | — | — |
| S004 | Female | R | Cochlear CI422 Straight | No | Yes | — | — | — | 78.53 | 72.00 | — | — | — | — | 78.80 | ND | — | — |
| S005 | Female | L | Cochlear Hybrid L24 | No | Yes | — | — | — | 63.81 | 72.00 | 63.84 | 72.00 | — | — | — | — | 64.42 | 102.00 |
| S007 | Male | R | Cochlear CI422 Straight | Yes | Yes | 78.30 | 68.00 | 42.00 | 78.55 | 80.00 | 78.56 | 75.00 | 78.64 | 82.00 | 78.78 | 85.00 | 79.03 | 97.00 |
| S008 | Male | R | Cochlear CI422 Straight | Yes | Yes | 71.35 | 60.00 | 35.00 | 71.64 | 105+ | — | — | 71.76 | 105+ | — | — | 72.14 | 105+ |
| S009 | Female | L | AB HR90K Mid–Scala | No | Yes | — | — | — | 39.67 | 57.00 | 39.69 | 53.00 | 39.77 | 55.00 | 39.84 | 47.00 | — | — |
| S010 | Female | R | AB HR90K Mid–Scala | Yes | Yes | 63.65 | 53.00 | 57.00 | 63.76 | 68.00 | — | — | — | — | — | — | — | — |
| S011 | Male | R | Cochlear Hybrid L24 | No | Yes | — | — | — | 69.02 | 30.00 | 69.06 | 33.00 | — | — | 69.31 | 27.00 | — | — |
| S012 | Male | B (L) | MED-EL Synchrony Flex24 | Yes | No: Bilateral | 71.45 | 40.00 | NA: Bilateral | 71.57 | 72.00 | — | — | — | — | 71.82 | 67.00 | — | — |
| S013 | Male | L | Cochlear Hybrid L24 | Yes | Yes | 60.48 | 33.00 | 37.00 | 60.90 | 55.00 | 60.65 | 45.00 | — | — | 60.84 | 42.00 | 61.09 | 45.00 |
| S015 | Female | R | Cochlear CI422 Straight | Yes | Yes | 41.06 | 42.00 | 33.00 | — | — | — | — | 41.58 | 48.00 | — | — | — | — |
| S016 | Female | R | Cochlear CI422 Straight | Yes | No: WAI CI ear only | 65.53 | 27.00 | 17.00 | 65.65 | 48.00 | — | — | — | — | — | — | — | — |
| S019 | Male | R | AB HR90K Mid-Scala | Yes | Yes | 54.49 | 62.00 | 42.00 | — | — | — | — | 54.84 | 75.00 | 54.93 | 53.00 | — | — |
| S022 | Male | B (L) | MED-EL Synchrony Flex28 | Yes | No: Bilateral | 75.94 | 73.00 | NA: Bilateral | 75.98 | 105+ | — | — | 76.06 | 105+ | — | — | — | — |
| S023 | Male | R | AB HR90K Mid-Scala | No | Yes | — | — | — | — | — | 67.25 | 73.00 | — | — | 67.56 | 87.00 | — | — |
| S024 | Female | L | MED-EL Synchrony Flex28 | No | Yes | — | — | — | 72.90 | 85.00 | — | — | — | — | — | — | — | — |
| M | 65.48 | 48.00 | 36.00 | 62.52 | 69.00 | 58.83 | 56.00 | 56.50 | 73.29 | 62.79 | 59.00 | 70.00 | 84.00 | |||||
| SD | 11.25 | 16.90 | 11.99 | 13.63 | 20.56 | 16.53 | 17.42 | 19.02 | 25.76 | 15.80 | 21.93 | 7.20 | 25.37 | |||||
Note. For the two bilateral cochlear implant (CI) recipients, the test ears for the implanted ear analyses are shown in parentheses. Em dashes indicate the subject was not tested at that specific time point. Include longit. = yes/no indication of participants included in the implant ear analyses over time; Include ear comp. = yes/no indication of participants included in the ear comparison analyses over time; LFPTA = the low-frequency pure-tone average of audiometric thresholds for 0.125, 0.25, and 0.5 kHz; R = right; L = left; B = both; ND = no data; AB = Advanced Bionics; NA = not applicable; WAI = wideband acoustic immittance.
Study Timeline
Participants were tested across six potential time points—corresponding to the clinical CI follow-up schedule—with the goal of each participant completing testing for as many time points as possible. The time points were as follows: (a) pre-CI baseline, (b) CI activation, (c) 1-week postactivation, (d) 1-month postactivation, (e) 3-month postactivation, and (f) 6-month postactivation. One participant completed testing at all six time points; one participant completed testing at five time points; five participants completed testing at four time points; four participants completed testing at three time points; five participants completed testing at two time points; and only one participant completed testing for one postoperative time point (details are provided in Table 1). The age range of the participant sample tested at each time point was as follows: (a) pre-CI baseline (range: 41–78 years); (b) activation (range: 33–79 years); (c) 1-week postactivation (range: 33–79 years); (d) 1-month postactivation (range: 33–79 years); (e) 3-months postactivation (range: 33–79 years); and (f) 6-months postactivation (range: 61–79 years). Demographic data for the included participants at each time point are shown in Table 1.
The amount of time that passed between CI surgery and the date of the CI activation in this sample is an important consideration as all of the postsurgery experimental time points are referenced to the activation. A group average of 26 days (range: 3–43 days) passed between the time of the CI surgery and activation. All participants completed their study visits within ±2 weeks of the target date for the 1-week and 1-month postactivation time points and within ±5 weeks of the target date for the 3- and 6-month postactivation time points.
Baseline Audiologic Measures
Participants completed 226-Hz tympanometry and standard air- and bone-conduction audiometric testing to assess for middle-ear status and preserved acoustic hearing on the day(s) of testing. Air-conduction thresholds were measured for octave frequencies from 0.125 to 8 kHz including 3- and 6-kHz interoctaves, and bone-conduction thresholds were measured for octave frequencies from 0.25 to 4 kHz. The 0.5-kHz postoperative ABG at each postsurgical time point served as the dependent variable for ABG analyses. The 0.5-kHz frequency region was selected for the following reasons: (a) It is a spectral region where both a postoperative ABG and the effects of middle-ear stiffness are likely to occur, and (b) it is a spectral region where postoperative acoustic hearing preservation is likely to occur and contribute to EAS listening (e.g., Gifford & Stecker, 2020). The 0.25-kHz region was not assessed for ABGs in this study due to the confound of vibrotactile responses to bone-conducted stimuli at this frequency. Thus, analyses investigating the relationship between postoperative ABG and middle-ear stiffness for the 0.5-kHz spectral region demonstrate ecological validity and clinical relevance. The data for participants who had a tympanogram without a measurable peak on a given test date (Type B Jerger classification) were removed from analyses for that test date. This resulted in the exclusion of only two data points from a single participant at the activation and 1-week postactivation time points.
Acoustic hearing preservation was characterized using a low-frequency pure-tone average (LFPTA) of 0.125-, 0.25-, and 0.5-kHz air-conduction audiometric thresholds. For the purposes of this study, a postoperative LFPTA of 85 dB HL or better (lower) was considered evidence of functional acoustic hearing preservation. Acoustic hearing preservation was not included as a variable in any analyses, but it was used in the interpretation and discussion of findings.
WAI: Acoustic Absorbance
WAI recordings were used to assess acoustic absorbance. Recordings were measured at ambient pressure from 0.2 to 4 kHz elicited by 70 dB SPL chirp stimuli using the Mimosa Acoustics HearID Middle-Ear Power Analyzer hardware and software platforms coupled to an Etymotic Research ER-10C probe with a foam ear tip. The system probe was calibrated for Thevenin values in a four-chamber coupler before collecting data for each test session. This was followed by in situ calibration in the ear canal prior to collection of data in each ear. Compared to standard single-frequency tympanometry, wideband acoustic absorbance has the advantage of simultaneously assessing middle-ear and cochlear functions over a broad spectral range (Rosowski et al., 2013; Shahnaz et al., 2009; Wegner et al., 2017). Furthermore, Feeney et al. (2017) demonstrated good test–retest repeatability of wideband acoustic absorbance measures in adults, especially for frequencies between 0.7 and 3 kHz, a primary spectral range of interest in this study. All participants completed from 1 to 4 WAI recordings per ear using the same test parameters for all recordings. We completed a post hoc, laboratory-based standard quality check of all WAI recordings to assess for probe fit in the ear canal using a custom R script. The criterion used to define good probe fit was based on previous research related to characteristics of normal adult acoustic absorbance (Allen et al., 2005; Puria & Allen, 1998) and review by laboratory personnel experienced in the analysis of WAI in human adults using this experimental setup.
Good probe fit was defined as an average acoustic absorbance value between 0% and 40% for frequencies between 0.2 and 0.5 kHz. Recordings that did not meet this criterion were interpreted as having a poor probe fit and were excluded from further analyses. This decision was based on previous studies demonstrating that variability of probe placement in the ear canal contributes to high-frequency variability in WAI measures (e.g., Groon et al., 2015) and previous studies demonstrating that a small amount of low-frequency acoustic power is absorbed into the middle-ear space in a typically functioning middle-ear transmission system (Allen et al., 2005; Puria & Allen, 1998).
The number of WAI recordings per ear determined to have a good probe fit ranged from one to four among participants. WAI recordings were averaged together for further analyses. In order to maintain consistency in the number of averaged recordings among participants, we averaged only the first two recordings for participants who had three or four acceptable WAI recordings for a single ear.
Statistical Approach
Nonparametric statistics were employed due to the small sample size and inability to sufficiently determine the distribution of the data at each time point. We calculated acoustic absorbance difference scores for each participant for the 162 individual frequencies from 0.2 to 4 kHz as the dependent variable for statistical analyses. For the implant only analyses, the acoustic absorbance difference scores were calculated by subtracting the acoustic absorbance value for each postsurgery time point from the comparable acoustic absorbance value at the pre-CI baseline time point. Thus, a positive integer acoustic absorbance difference score indicated decreased acoustic absorbance postsurgery.
For the ear difference analyses, the acoustic absorbance difference scores were calculated by subtracting the implanted ear acoustic absorbance value from the nonimplanted ear acoustic absorbance value for each frequency at each time point. A positive integer acoustic absorbance difference score indicated decreased acoustic absorbance in the implanted ear. We then calculated nonparametric 95% confidence intervals for median acoustic absorbance difference scores for frequencies from 0.2 to 4 kHz for each postsurgery time point using the “groupwiseMedian” function from the “rcompanion” package in R.
A nonparametric 95% confidence interval based on the median difference scores that was positive and did not encompass zero indicated statistically significant differences in acoustic absorbance at the p < .05 significance level. Additionally, we calculated nonparametric Spearman rank-order correlation coefficients to examine the strength of the relationship between 0.5-kHz postsurgery ABG difference from baseline and the averaged wideband acoustic absorbance difference from baseline for frequencies between 0.5 and 1 kHz.
Results
Decreased Acoustic Absorbance in Implanted Ears Postsurgery
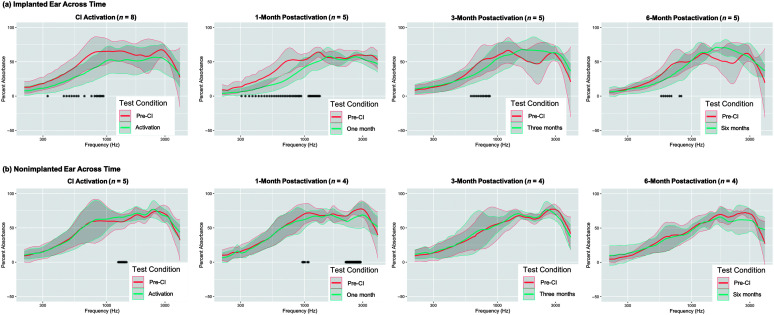
Figure 1 shows group-averaged wideband acoustic absorbance from 0.2 to 4 kHz for the pre-CI baseline and each postsurgery time point through 6-month postactivation. Data for only two participants were available at 1-week postactivation; hence, this time point was excluded from these analyses. The number of participants included at all other time points is shown in the title of each panel in Figure 1.
Figure 1.
Changes in acoustic absorbance in (a) implanted and (b) nonimplanted ears pre- and postsurgery. Line plots showing acoustic absorbance from 0.2 to 4 kHz (the spectral range investigated in this study) for ears at the pre-CI baseline time point (salmon) and at each post-CI time point (teal). The number of participants included at each time point is shown in the title of each panel. Differences in the numbers of participants between implanted and nonimplanted ears are due to the exclusion of bilateral CI recipients from nonimplanted ear analyses (n = 2 participants) and due to time limitations precluding testing of acoustic absorbance in the nonimplanted ear at the 6-month postactivation time point (n = 1 participant). Bold lines represent the group mean, and the gray shaded region represents ±1 SD. Asterisks along the x-axis indicate frequencies where statistically significant differences in acoustic absorbance were observed at the p < .05 significance level based on nonparametric 95% confidence intervals of median acoustic absorbance difference scores. CI = cochlear implant.
We identified significantly decreased acoustic absorbance in the implanted ear at all postsurgery time points, though the spectral range of decreased acoustic absorbance became narrower with longer time postactivation (see Figure 1a; CI activation: 0.328 kHz, from 0.445 to 0.586, 0.656, and 0.750 kHz and from 0.797 to 0.938 kHz; 1-month postactivation: from 0.305 to 0.938 kHz and from 1.078 to 1.336 kHz; 3-month postactivation: from 0.609 to 0.867 kHz; 6-month postactivation: from 0.563 to 0.680 kHz and from 0.797 to 0.820 kHz). We observed a trend of increased acoustic absorbance for frequencies around 2 kHz in the implanted ear at the 3- and 6-month postactivation time points (see Figure 1a). This trend did not reach statistical significance likely due to the high variability in acoustic absorbance for this frequency range among CI recipients included in this sample.
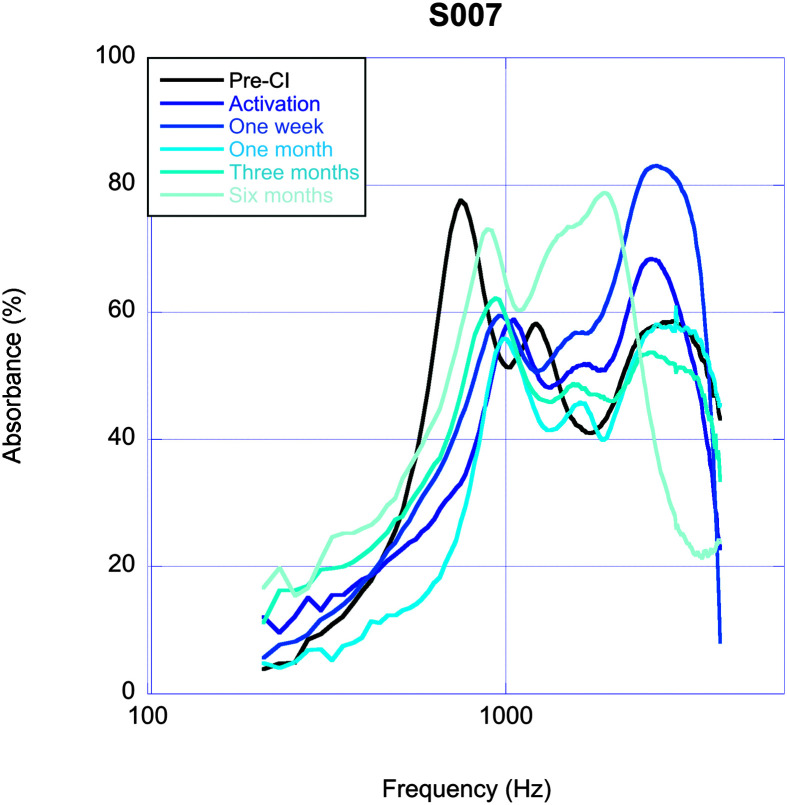
One participant (S007) completed testing for all six time points across the 6-month study period, and their data are shown in Figure 2. This participant's low-frequency acoustic absorbance decreased for all postoperative visits, and the spectral range of decreased acoustic absorbance narrowed over time, consistent with the findings noted at the group level.
Figure 2.
Changes in acoustic absorbance for one participant (S7) who completed testing for all six experimental time points across the 6-month study period. CI = cochlear implant.
Analyses in the nonimplanted ears of participants across time points demonstrated changes in postoperative acoustic absorbance that were limited to the higher frequencies (i.e., above approximately 1 kHz) and only occurred at the two earliest time points (see Figure 1b; CI activation: acoustic absorbance increased from 1.242 to 1.453 kHz; 1-month postactivation: acoustic absorbance decreased from 0.961 to 1.008 kHz, from 1.055 to 1.078 kHz, and from 2.180 to 2.883 kHz). Note that acoustic absorbance increased at the activation time point in the limited spectral range above 1 kHz and that no differences in acoustic absorbance were observed postoperatively at 3- and 6-month postactivation. The nonimplanted ear analyses demonstrate the stability of low-frequency acoustic absorbance measures over time for a spectral range where decreased postoperative acoustic absorbance occurred in implanted ears.
Relationship Between Postsurgery ABG Difference and Acoustic Absorbance Difference
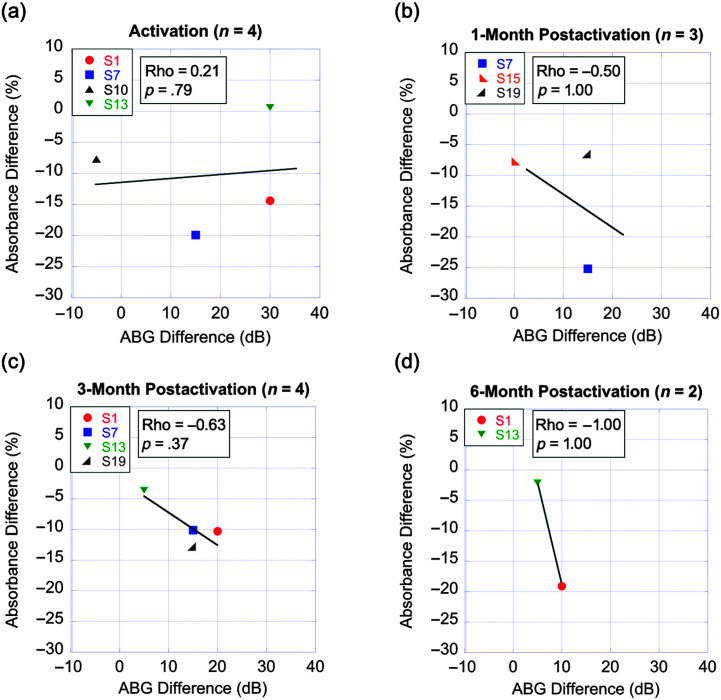
Figure 3 shows the linear relationships between 0.5-kHz ABG difference and acoustic absorbance difference for a 0.5- to 1-kHz spectral band for each time point. Difference scores were calculated by subtracting values at the baseline presurgical time point from respective values at each postsurgical time point. The numbers of participants at each time point with both air- and bone-conduction data and acoustic absorbance data are shown in the title of each panel.
Figure 3.
Relationship between postsurgery air–bone gap (ABG) difference and acoustic absorbance difference across time points. Scatter plots with linear regression lines (black) showing the linear relationship between postoperative 0.5-kHz ABG difference from baseline on the x-axis and acoustic absorbance difference from baseline for the 0.5- to 1-kHz spectral band on the y-axis across time points. The numbers of participants with air- and bone-conduction and acoustic absorbance data for each time point are shown in the title of each panel. The Spearman rank-order correlation coefficient (rho) and corresponding p value are provided in the upper corner of each panel. CI = cochlear implant.
Due to the limited number of participants with available data, these analyses serve as a preliminary description of observed trends. A trend toward a positive correlation between 0.5-kHz ABG difference and 0.5- to 1-kHz acoustic absorbance difference was observed for the activation time point only (ρ = 0.21, p > .05). Negative correlation trends were observed at all postsurgical time points from 1-month through 6-month postactivation, though these trends did not reach statistical significance likely in part due to the small sample sizes (1-month postactivation: ρ = −0.50, p > .05; 3-month postactivation: ρ = −0.63, p > .05; and 6-month postactivation: ρ = −1, p > .05). The negative correlation trends for later postsurgical time points may indicate that larger postsurgery ABGs partially contribute to decreased acoustic absorbance following cochlear implantation. Only one participant had available data for the 1-week postactivation time point precluding preliminary analyses at this time point.
Decreased Acoustic Absorbance in Implanted Compared to Nonimplanted Ears
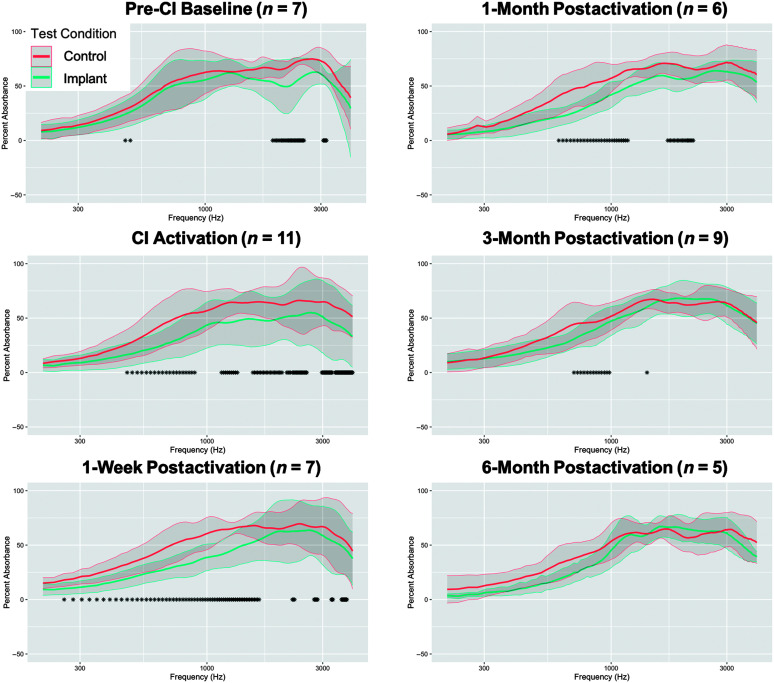
Figure 4 compares acoustic absorbance between the implanted and nonimplanted ears within participants across time points. The number of participants included at each time point is shown in the title of each panel in Figure 4. We identified decreased acoustic absorbance in the implanted compared to the nonimplanted ears for the CI activation (from 0.469 to 0.891 kHz, from 1.148 to 1.336 kHz, from 1.547 to 2.039 kHz, from 2.133 to 2.203 kHz, from 2.250 to 2.578 kHz, from 2.977 to 3.281 kHz, and from 3.375 to 3.984 kHz), 1-week postactivation (from 0.258 to 1.641 kHz, from 2.250 to 2.297 kHz, from 2.766 to 2.859 kHz, from 3.258 to 3.305 kHz and from 3.586 to 3.773 kHz), 1-month postactivation (from 0.609 to 1.172 kHz and from 1.711 to 2.180 kHz), and 3-month postactivation (from 0.703 to 0.984 kHz and at 1.406 kHz) time points. No statistically significant differences in acoustic absorbance were found between ears at the 6-month postactivation time point, though we observed a trend for decreased acoustic absorbance in the implanted ear for frequencies below 1 kHz. Furthermore, the spectral range where differences in acoustic absorbance were observed between ears became narrower and emphasized lower frequencies with longer time postsurgery.
Figure 4.
Differences in acoustic absorbance between implanted and nonimplanted ears pre- and postsurgery. Line plots showing acoustic absorbance from 0.2 to 4 kHz for implanted (teal) compared to nonimplanted (salmon) ears across time points. The numbers of participants included at each time point are shown in the title of each panel. Bold lines represent the group mean, and the gray shaded region represents ±1 SD. Asterisks along the x-axis indicate frequencies where statistically significant differences in acoustic absorbance were observed at the p < .05 significance level based on nonparametric 95% confidence intervals of median acoustic absorbance difference scores.
Discussion
Summary of Findings and Consistency Among Studies
The goals of this study were to investigate changes in acoustic absorbance in implanted ears over time, to preliminarily investigate the relationship between postoperative ABGs and acoustic absorbance in implanted ears, and to compare acoustic absorbance between implanted and nonimplanted ears within participants over time. Consistent with recent studies (Merchant et al., 2020; Saoji et al., 2020; Scheperle & Hajicek, 2020), our analyses demonstrated reduced postoperative low-frequency acoustic absorbance that persisted through at least 6-month postactivation.
Our analyses also identified a potential frequency-specific time course related to decreased acoustic absorbance postsurgery wherein the spectral range of decreased postoperative acoustic absorbance narrowed over time and emphasized frequencies lower than 1 kHz. Saoji et al. (2020) demonstrated decreased acoustic absorbance postsurgery for frequencies between 0.6 and 1.1 kHz at 45–60 day postsurgery (compared to 0.609–0.867 kHz and 1.148–1.359 kHz at 1-month postactivation in our study). Additionally, Scheperle and Hajicek (2020) showed decreased acoustic absorbance in the implanted ears of CI recipients compared to a different group of ears with normal hearing for frequencies between 0.25 and 0.891 kHz. Participants in their study were long-term CI recipients with a duration of CI use at the time of test ranging from 3 to 25 years. The findings of our and previous studies suggest that decreased acoustic absorbance in the implanted ear postsurgery may occur over a broad spectral range at CI activation including frequencies up to 4 kHz, but that decreased acoustic absorbance persists only for frequencies below 1 kHz through at least 6-month postactivation.
Preliminary Analyses of ABG and Acoustic Absorbance Correlations
We identified negative correlation trends between postsurgery 0.5-kHz ABG difference and 0.5- to 1-kHz acoustic absorbance difference for postsurgical time points from 1-month through 6-month postactivation, though these trends did not reach statistical significance. These potential relationships may suggest that larger postoperative ABGs partially contribute to the decreased acoustic absorbance observed postsurgery in CI recipients. Thus, our preliminary analyses may support the hypothesis that postsurgery ABGs are related to decreased acoustic absorbance (i.e., middle-ear stiffness) following cochlear implantation; however, additional research is needed to draw firm conclusions.
The tympanometric results of participants included in our correlational analyses are an important consideration in the interpretation of preliminary trends. Two participants did not have tympanometric results due to time limitations associated with the clinical study design (S1 at the activation time point and S15 at the 1-month postactivation time point). All other participants included in these analyses at all time points had evidence of a mobile tympanic membrane demonstrated by middle-ear compliance greater than 0.2 mmho. Thus, it does not appear that abnormal middle-ear function contributed to postsurgical ABGs in our sample of participants. The findings of our preliminary ABG analyses should be considered with regard for the small number of participants with available data. Future studies investigating the relationship between postsurgery ABGs and acoustic absorbance with larger sample sizes at multiple time points postsurgery are warranted.
Middle-Ear Stiffness and Acoustic Hearing Preservation in CI EAS Patients
We identified significantly decreased acoustic absorbance in the implanted compared to the nonimplanted ears as early as the CI activation that persisted through the 3-month postactivation time point. Although we did not identify statistically significant differences between ears at the 6-month postactivation time point, a clear trend of decreased acoustic absorbance in the implanted ear for frequencies below 1 kHz was noted (see Figure 4). One may consider that the lack of statistically significant difference between ears may not negate a clinically significant effect.
As the prevalence of acoustic hearing preservation following CI surgery continues to increase, it is important to consider ear-specific differences in middle-ear stiffness postsurgery. For example, increased middle-ear stiffness post–cochlear implantation may change the transmission of acoustic sound in the implanted ear, which may impact binaural cue sensitivity and spatial hearing abilities in EAS listening.
Avan et al. (2000) showed that increased middle-ear stiffness via elicitation of the contralateral middle-ear muscle reflex changed the cochlear encoding of phase as measured by distortion product otoacoustic emissions (DPOAEs) in human adults with normal hearing. Middle-ear stiffness-induced DPOAE phase shifts in their study were observed for frequencies from 0.6 to 1.5 kHz with a maximum 30° phase shift at 0.9 kHz. Frequencies lower than 0.6 kHz were not tested by Avan et al. Furthermore, using modeling techniques, Avan et al. confirmed that the DPOAE phase shifts observed in their study were at least in part due to middle-ear stiffness-induced changes in the forward transmission of acoustic energy. The findings of Avan et al. (2000) suggest that increased middle-ear stiffness may alter the acoustic properties of sound that reach the cochlea for a frequency range where acoustic hearing may be preserved in CI recipients. This is especially relevant for unilateral CI recipients where increased middle-ear stiffness may only occur for the implanted ear, as suggested by the findings of our ear difference analyses. Here, it is important to consider that the reduced acoustic absorbance postsurgery observed in CI recipients in this, and previous studies may be due to changes in either middle-ear or cochlear function. If due to changes in cochlear function, the findings of Avan et al. (2000) may have limited generalizability toward understanding reduced acoustic absorbance post–cochlear implantation. Thus, future investigations that determine the origin of reduced acoustic absorbance in CI recipients are warranted.
Binaural processing and spatial hearing abilities require the accurate encoding of interaural timing difference (ITD), interaural phase difference (IPD), and interaural level difference (ILD) cues (e.g., Yost, 1974, 2000). ITD cues contribute to binaural hearing primarily for frequencies of 1.5 kHz and lower, whereas ILD cues contribute to binaural hearing primarily for frequencies higher than 1.5 kHz (e.g., Yost, 2000). Most unilateral CI recipients do not have access to ILD cues due to lack of high-frequency audibility in the nonimplanted ear (e.g., Gifford & Dorman, 2019). However, CI recipients with acoustic hearing preservation may have access to ITD and IPD cues in the lower frequencies where binaural acoustic hearing is available. Indeed, a number of studies have shown that CI recipients with acoustic hearing preservation have ecologically relevant ITD sensitivity in the low-frequency region (Gifford et al., 2013, 2014; Gifford & Stecker, 2020; Körtje et al., 2020) and that behavioral ITD thresholds were significantly correlated with horizontal-plane localization (Gifford et al., 2014) and speech recognition in diffuse noise (Gifford et al., 2013, 2014; Gifford & Stecker, 2020).
Hearing preservation and subsequent use of EAS technology affords listeners significant benefits for speech recognition in complex noise (Dunn et al., 2010; Gifford et al., 2013, 2017; Plant & Babic, 2016; Rader et al., 2013) and reverberation (Gifford et al., 2013) and provides significant benefit for localization (Dunn et al., 2010; Gifford et al., 2014; Plant & Babic, 2016). Because changes in middle-ear stiffness could impact acoustic transmission in the implanted ear and potentially disrupt one's encoding of ITDs and IPDs, there is a great need to define the impact of cochlear implantation on the middle-ear system.
When considered together, previous studies support the fact that middle-ear stiffness increases in an ear with a CI (Merchant et al., 2020; Saoji et al., 2020; Scheperle & Hajicek, 2020). This unilateral change in postoperative middle-ear stiffness may change the encoding of phase (Avan et al., 2000; Büki et al., 2000; Sun, 2008) in the CI ear, resulting in larger postoperative IPDs and ITDs. Larger IPDs and ITDs may contribute to poor spatial hearing abilities (Yost, 1974, 2000) and may contribute to the variability in measurable ITD thresholds (e.g., Gifford et al., 2014; Gifford & Stecker, 2020) resulting in variable localization performance in CI EAS listeners (Dunn et al., 2010; Gifford et al., 2014; Körtje et al., 2020; Moteki et al., 2015; Plant & Babic, 2016). As such, future longitudinal investigation is warranted in larger samples to fully understand the scientific and clinical impact of cochlear implantation on middle-ear stiffness and the potential impact on binaural cue sensitivity for CI recipients with bilateral acoustic hearing.
Study Limitations
The clinical nature of this study resulted in missing data points and small sample sizes at each experimental time point. CI appointments within the first 6 months of receiving the device require approximately 1–2 hr. Some participants did not have time to complete additional experimental testing following their clinical appointments, especially at all six time points. Additionally, as expected in clinical populations, some of the included study participants had rescheduled or chosen to eliminate their postactivation clinic visits that impacted data collection for this study. Thus, the findings of this study should be considered with regard for the small sample sizes available at each time point.
Additionally, measures of acoustic absorbance are sensitive to changes in middle-ear and cochlear functions. This study was not designed to differentiate between middle-ear and cochlear origins of decreased acoustic absorbance post–cochlear implantation. Future studies investigating this important topic are warranted.
Considerations for Equity, Diversity, and Inclusion
Participant demographic characteristics such as race and ethnicity, age, and sex assignment at birth potentially impact many aspects of the auditory system and are important considerations toward increasing equity, inclusion, and diversity in scientific investigations. This study could not sufficiently investigate the effects of participant race and ethnicity, age, or sex assignment at birth due to the limited number of participants at each time point. Future studies employing a longitudinal design with a sufficient sample size to include demographic characteristics as fixed factors in a linear mixed-effects model are an important future priority for work related to changes in acoustic absorbance post–cochlear implantation.
Conclusions
The findings of this study are in accordance with recent studies demonstrating that cochlear implantation decreases low-frequency acoustic absorbance consistent with a stiffer middle-ear transmission system postsurgery. This study extends prior knowledge by demonstrating that decreased acoustic absorbance occurred as early as the CI activation time point and persisted until at least the 6-month postactivation time point. We also identified decreased acoustic absorbance in the implanted compared to the nonimplanted ear that persisted through at least 3-month postactivation. Increased middle-ear stiffness postsurgery may alter the encoding of low-frequency IPD and ITD cues in the implanted ear and may contribute to the variability observed in localization performance of CI EAS patients. Finally, it is important to note that this study highlights the scientific and clinical value of WAI for assessing middle-ear and cochlear functions. WAI measures are quick and easy to administer, provide a wealth of information related to various aspects of middle-ear and cochlear function across a broad spectral range, and enhance the assessment of middle-ear and cochlear functions for various clinical populations and research applications.
Author Contributions
Jordan M. Racca: Conceptualization (Supporting), Data curation (Equal), Formal Analysis (Lead), Investigation (Equal), Methodology (Lead), Resources (Equal), Writing – original draft (Equal). Laura L. Jones: Data curation (Equal), Investigation (Equal), Writing – review & editing (Supporting). Robert T. Dwyer: Data curation (Equal), Investigation (Equal), Writing – review & editing (Supporting). Mary Ferguson: Data curation (Equal), Investigation (Equal), Writing – review & editing (Supporting). Linsey Sunderhaus: Data curation (Equal), Investigation (Equal), Writing – review & editing (Supporting). Linda J. Hood: Conceptualization (Equal), Methodology (Equal), Resources (Equal), Supervision (Equal), Writing – original draft (Equal). René H. Gifford: Conceptualization (Equal), Data curation (Equal), Investigation (Equal), Methodology (Equal), Supervision (Equal), Writing – original draft (Equal).
Acknowledgments
This study was funded, in part, by the National Institute on Deafness and Other Communication Disorders (R01 DC009404; PI: R.H. Gifford) and research resources from the Human Auditory Physiology Laboratory (PI: L.J. Hood). The authors would like to thank Lauren Roberts for her contributions to data collection during this study.
Funding Statement
This study was funded, in part, by the National Institute on Deafness and Other Communication Disorders (R01 DC009404; PI: R.H. Gifford) and research resources from the Human Auditory Physiology Laboratory (PI: L.J. Hood).
References
- Allen, J. B. , Jeng, P. S. , & Levitt, H. (2005). Evaluation of human middle ear function via an acoustic power assessment. Journal of Rehabilitation Research & Development, 42(4s), 63–78. https://doi.org/10.1682/JRRD.2005.04.0064 [DOI] [PubMed] [Google Scholar]
- Avan, P. , Büki, B. , Maat, B. , Dordain, M. , & Wit, H. P. (2000). Middle ear influence on otoacoustic emissions. I: Noninvasive investigation of the human transmission apparatus and comparison with model results. Hearing Research, 140(1–2), 189–201. https://doi.org/10.1016/S0378-5955(99)00201-4 [DOI] [PubMed] [Google Scholar]
- Banakis Hartl, R. M. , Mattingly, J. K. , Greene, N. T. , Jenkins, H. A. , Cass, S. P. , & Tollin, D. J. (2016). A preliminary investigation of the air-bone gap: Changes in intracochlear sound pressure with air-and bone-conducted stimuli after cochlear implantation. Otology & Neurotology, 37(9), 1291. https://doi.org/10.1097/MAO.0000000000001184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büki, B. , Wit, H. P. , & Avan, P. (2000). Olivocochlear efferent vs. middle-ear contributions to the alteration of otoacoustic emissions by contralateral noise. Brain Research, 852(1), 140–150. https://doi.org/10.1016/S0006-8993(99)02227-1 [DOI] [PubMed] [Google Scholar]
- Dunn, C. C. , Perreau, A. , Gantz, B. , & Tyler, R. S. (2010). Benefits of localization and speech perception with multiple noise sources in listeners with a short-electrode cochlear implant. Journal of the American Academy of Audiology, 21(1), 44–51. https://doi.org/10.3766/jaaa.21.1.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney, M. P. , Grant, I. L. , & Marryott, L. P. (2003). Wideband energy reflectance measurements in adults with middle-ear disorders. Journal of Speech, Language, and Hearing Research, 46(4), 901–911. https://doi.org/10.1044/1092-4388(2003/070) [DOI] [PubMed] [Google Scholar]
- Feeney, M. P. , Keefe, D. H. , Hunter, L. L. , Fitzpatrick, D. F. , Garinis, A. C. , Putterman, D. B. , & McMillan, G. P. (2017). Normative wideband reflectance, equivalent admittance at the tympanic membrane, and acoustic stapedius reflex threshold in adults. Ear and Hearing, 38(3), e142–e160. https://doi.org/10.1097/AUD.0000000000000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , Davis, T. J. , Sunderhaus, L. W. , Menapace, C. , Buck, B. , Crosson, J. , O'Neill, L. , Beiter, A. , & Segel, P. (2017). Combined electric and acoustic stimulation with hearing preservation: Effect of cochlear implant low-frequency cutoff on speech understanding and perceived listening difficulty. Ear and Hearing, 38(5), 539–553. https://doi.org/10.1097/AUD.0000000000000418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , & Dorman, M. F. (2019). Bimodal hearing or bilateral cochlear implants? Ask the patient. Ear and Hearing, 40(3), 501–516. https://doi.org/10.1097/AUD.0000000000000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , Dorman, M. F. , Skarzynski, H. , Lorens, A. , Polak, M. , Driscoll, C. L. W. , Roland, P. , & Buchman, C. A. (2013). Cochlear implantation with hearing preservation yields significant benefit for speech recognition in complex listening environments. Ear and Hearing, 34(4), 413–425. https://doi.org/10.1097/AUD.0b013e31827e8163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , Grantham, D. W. , Sheffield, S. W. , Davis, T. J. , Dwyer, R. , & Dorman, M. F. (2014). Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hearing Research, 312, 28–37. https://doi.org/10.1016/j.heares.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford, R. H. , & Stecker, G. C. (2020). Binaural cue sensitivity in cochlear implant recipients with acoustic hearing preservation. Hearing Research, 390, 107929. https://doi.org/10.1016/j.heares.2020.107929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groon, K. A. , Rasetshwane, D. M. , Kopun, J. G. , Gorga, M. P. , & Neely, S. T. (2015). Air-leak effects on ear-canal acoustic absorbance. Ear and Hearing, 36(1), 155–163. https://doi.org/10.1097/AUD.0000000000000077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körtje, M. , Baumann, U. , Stöver, T. , & Weissgerber, T. (2020). Sensitivity to interaural time differences and localization accuracy in cochlear implant users with combined electric-acoustic stimulation. PLOS ONE, 15(10), e0241015. https://doi.org/10.1371/journal.pone.0241015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M. J. (2016). Structure and function of the mammalian middle ear. II: Inferring function from structure. Journal of Anatomy, 228(2), 300–312. https://doi.org/10.1111/joa.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant, S. N. , & Rosowski, J. J. (2003). Auditory physiology. Surgery of the Ear, 5, 59–82. [Google Scholar]
- Merchant, G. R. , Schulz, K. M. , Patterson, J. N. , Fitzpatrick, D. , & Janky, K. L. (2020). Effect of cochlear implantation on vestibular evoked myogenic potentials and wideband acoustic immittance. Ear and Hearing, 41(5), 1111–1124. https://doi.org/10.1097/AUD.0000000000000831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteki, H. , Kitoh, R. , Tsukada, K. , Iwasaki, S. , Nishio, S. Y. , & Usami, S. I. (2015). The advantages of sound localization and speech perception of bilateral electric acoustic stimulation. Acta Oto-Laryngologica, 135(2), 147–153. https://doi.org/10.3109/00016489.2014.951453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant, K. , & Babic, L. (2016). Utility of bilateral acoustic hearing in combination with electrical stimulation provided by the cochlear implant. International Journal of Audiology, 55(Suppl. 2), S31–S38. https://doi.org/10.3109/14992027.2016.1150609 [DOI] [PubMed] [Google Scholar]
- Puria, S. , & Allen, J. B. (1998). Measurements and model of the cat middle ear: Evidence of tympanic membrane acoustic delay. The Journal of the Acoustical Society of America, 104(6), 3463–3481. https://doi.org/10.1121/1.423930 [DOI] [PubMed] [Google Scholar]
- Rader, T. , Fastl, H. , & Baumann, U. (2013). Speech perception with combined electric-acoustic stimulation and bilateral cochlear implants in a multisource noise field. Ear and Hearing, 34(3), 324–332. https://doi.org/10.1097/AUD.0b013e318272f189 [DOI] [PubMed] [Google Scholar]
- Rosowski, J. J. , Stenfelt, S. , & Lilly, D. (2013). An overview of wideband immittance measurements techniques and terminology: You say absorbance, I say reflectance. Ear and Hearing, 34(1), 9S–16S. https://doi.org/10.1097/AUD.0b013e31829d5a14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji, A. A. , Shapiro, S. B. , Finley, C. C. , Koka, K. , & Cassis, A. M. (2020). Changes in wide-band tympanometry absorbance following cochlear implantation. Otology & Neurotology, 41(6), e680–e685. https://doi.org/10.1097/MAO.0000000000002625 [DOI] [PubMed] [Google Scholar]
- Scheperle, R. A. , & Hajicek, J. J. (2020). Wideband acoustic immittance in cochlear implant recipients: Reflectance and stapedial reflexes. Ear and Hearing, 41(4), 883–895. https://doi.org/10.1097/AUD.0000000000000810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahnaz, N. , Bork, K. , Polka, L. , Longridge, N. , Bell, D. , & Westerberg, B. D. (2009). Energy reflectance and tympanometry in normal and otosclerotic ears. Ear and Hearing, 30(2), 219–233. https://doi.org/10.1097/AUD.0b013e3181976a14 [DOI] [PubMed] [Google Scholar]
- Sun, X. M. (2008). Contralateral suppression of distortion product otoacoustic emissions and the middle-ear muscle reflex in human ears. Hearing Research, 237(1–2), 66–75. https://doi.org/10.1016/j.heares.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Wegner, I. , Shahnaz, N. , Grolman, W. , & Bance, M. L. (2017). Wideband acoustic immittance measurements in assessing crimping status following stapedotomy: A temporal bone study. International Journal of Audiology, 56(1), 1–7. https://doi.org/10.1080/14992027.2016.1214759 [DOI] [PubMed] [Google Scholar]
- Yost, W. A. (1974). Discriminations of interaural phase differences. The Journal of the Acoustical Society of America, 55(6), 1299–1303. https://doi.org/10.1121/1.1914701 [DOI] [PubMed] [Google Scholar]
- Yost, W. A. (2000). Fundamentals of hearing: An introduction. https://doi.org/10.1163/9789004501935