Abstract
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease that represents a prodigious challenge of diagnosis and treatment. In 2019, under the leadership of the Chinese Rheumatology Association, a multidisciplinary guideline development group was established to develop an evidence-based diagnosis and treatment guideline for patients with SLE in PR China. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to evaluate the quality of evidence and the strength of recommendations. The guideline was reported following the Reporting Items for Practice Guidelines in Healthcare (RIGHT) checklist. In this guideline, we provided recommendations for SLE classification criteria, disease activity monitoring and assessment, medication administration and considerations for SLE patients with organs and systems involved, and management of special populations such as SLE patients in the setting of pregnancy. This guideline serves as an evidence-based tool for Chinese clinicians to diagnose and treat patients with SLE.
Keywords: lupus erythematosus, systemic, guideline, GRADE
Background
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease, which is characterized by systemic multiple-organ involvement, relapses with large amount of autoantibodies. If patients with SLE do not receive prompt treatment, they would get irreversible organ damage, which will ultimately lead to death. The causes of SLE are complicated, including genetics, sex hormones, exposure to various kinds of pathogens (e.g., viral and bacterial).[1,2,3] The prevalence of SLE varies widely in different regions with a rate of 0–241/100,000. This number in mainland China is about 30–70/100,000,[4, 5] and the ratio of male to female is 1:10–12.[6,7,8] The survival rate of patients with SLE has increased due to the improvements in diagnosis and treatment. The 5-year survival rate of patients with SLE had increased from 50% to 60% in 1950s to more than 90% in 1990s and stabilized at 95% in high-income countries, at 92% in low-income and middle-income countries from 2008 to 2016.[9,10,11] SLE is no longer an acute and highly lethal disease, but a chronic and controllable disease. Clinicians and patients play important roles in the awareness and attention of the disease and in emerging and optimizing scientific diagnosis and treatment of the disease.
Well known societies such as the European League Against Rheumatism (EULAR), the British Society of Rheumatology (BSR), and the Pan American League Against Rheumatism (PANLAR) have developed their own guidelines for the diagnosis and treatment of SLE.[11,12,13,14] The Chinese Rheumatology Association also published the Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus in China in 2010.[15] These guidelines have played an important role in promoting evidence-based clinical decision-making. However, there were still problems applying these guidelines to the Chinese SLE patients: (1) the Chinese Systemic Lupus Erythematosus Treatment and Research Group (CSTAR) registry cohort studies[6, 16, 17] have shown that the incidence, clinical manifestations, and main clinical outcomes of patients with SLE in China are not completely identical to Europe and the United States; (2) the international guidelines for diagnosis and treatment of SLE did not include studies conducted in China, and thus did not appropriate for the clinical practice in China; (3) 10 years have passed since the publication of the Guideline for the Diagnosis and Treatment of Systemic Lupus Erythematosus by the Chinese Rheumatology Association. An update is now necessary. During this period, new diagnostic and therapeutic research results and new therapeutic medications have been emerging continuously. The concept, methods, and techniques for the diagnosis of SLE are constantly developing and updating, which make the previous Chinese guidelines inappropriate for the current SLE diagnosis and treatment in China. In view of this, with the methods and procedure in accordance with evidence-based clinical practice guidelines, based on the newest research evidences and Chinese clinical practice, Chinese Rheumatology Association, National Clinical Research Center for Dermatologic and Immunologic Diseases, and CSTAR worked together to establish “2020 Chinese Guidelines for Diagnosis and Treatment of Systemic Lupus Erythematosus” (hereinafter referred to as this guideline).
Guideline Development Methods
Guideline Initiator: This guideline was jointly initiated by Chinese Rheumatology Association, National Clinical Research Center for Dermatologic and Immunologic Diseases, and CSTAR. This work was started on March 21, 2019 and finalized on January 21, 2020.
Guideline Working Group: A multidisciplinary working group was established, which included experts in rheumatology, nephrology, dermatology, obstetrics, radiology, and evidence-based medicine. Retrieval and evaluation of the evidence was performed by the Center for Evidence-based Medicine of Lanzhou University/Chinese GRADE Center. All members of the Working Group had no direct conflict of interest with this guideline and signed a non-conflict of interest form.
Guideline Registration and Writing: The guideline was registered at the International Practice Guidelines Registry Platform, http://www.guidelines-registry.org (Registration number IPGRP-2019CN022). The content of this Guideline was designed and developed in accordance with the World Health Organization Guideline Development Manual issued by the World Health Organization in 2014,[18] the Basic Methods and Procedures for the Development/Revision of Clinical Diagnosis and Treatment Guidelines, issued by the Chinese Medical Association in 2016.[19] This guideline also refers to the Appraisal of Guidelines for Research and Evaluation II (AGREE II) instrument[20] and Reporting Items for Practice Guidelines in Healthcare (RIGHT).[21, 22]
Guideline user and target population: This guideline is intended to advise rheumatologists, dermatologists, nephrologists, obstetricians, clinical pharmacists, imaging physicians, and professionals related to diagnose, treat, and manage patients with SLE. The recommendations of this guideline are for patients with SLE.
Selection and identification of clinical questions: By systematically reviewing the published guidelines and evaluation for SLE, and affiliating with interviews with some rheumatologists, the Working Group preliminary drafted 30 clinical questions and scenarios, followed by investigating and scoring the importance of clinical questions with online questionnaires. After 2 rounds of 83 surveys, 12 clinical questions in this guideline were finally selected.
Retrieval of evidence: The Evidence Evaluation Group deconstructed the clinical questions and outcome indicators of the final inclusion criteria according to the principle of Population, Intervention, Comparison, and Outcome (PICO). Based on the results of above deconstruction, the Evidence Evaluation Group retrieved: (1) systematic reviews, meta-analyses and network meta-analyses from MEDLINE, Cochrane Library, Epistemonikos, Chinese Biomedical Literature, Wanfang Database and China National Knowledge Infrastructure Database. The retrieved data was from the establishment of the database to September 2019; (2) UpToDate, DynaMed, MEDLINE, Chinese Biomedical Literature Database, Wanfang Database and China National Knowledge Infrastructure Database, mainly included original studies such as randomized controlled trials, cohort studies, case–control studies, case series studies and epidemiological surveys, and so on. The retrieved data was from the establishment of the database to September 2019; (3) Official websites, such as the National Institute for Health and Clinical Excellence (NICE), the Scottish Intercollegiate Guidelines Network (SIGN), the American College of Rheumatology (ACR), EULAR, and the Asia-Pacific Alliance Against Rheumatism (APLAR), as well as the MEDLINE and China National Knowledge Infrastructure Databases, the retrieved data mainly included SLE related guidelines; (4) Supplement retrieval of Google Academia and other websites.
Evidence evaluation and grading: A Measurement Tool to Assess systematic Reviews (AMSTAR)[23] was used by the Evidence Evaluation Group to evaluate the risk of bias of the included system evaluation, meta-analysis, and network meta-analysis. The methodological quality of the corresponding type of original studies was evaluated using the Cochrane Risk of Bias Assessment[24] (ROB, for randomized controlled trial Studies), Quality Assessment Tool for Diagnostic Accuracy Studies[25] (QUADAS-2, Diagnostic Accuracy Test Study), Newcastle-Ottawa Scale[26] (NOS, for observational studies) and so on; the evaluation process was performed by two people independently, and if there was any disagreement, a third party was consulted to resolve the dispute. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was used to grade evidence and recommendations,[27,28,29,30,31] as shown in Table 1.
The formation of recommendations: Based on the summary of domestic and foreign evidence provided by the Evidence Evaluation Group, taking into consideration of preferences and values of patients in China, cost and advantages/disadvantages of interventions, the Working Group proposed recommendations that are consistent with the clinical diagnosis and treatment in China. Two rounds of Delphi recommendation surveys were conducted on October 05, 2019 and November 08, 2019. In total, 98 pieces of feedback were collected. Face to face discussion and modification from November 2019 to January 2020 were conducted to reach a consensus.
Future updates of this Guideline: The recommendations of this guideline are planned to be updated within 3–5 years in accordance with the methodologies in International Guidelines.[32]
Table 1.
Grading of the quality of evidence and strength of recommendations
| Item | Contents |
|---|---|
| Grading of evidence Quality | |
| High (A) | Very confident: the observed value is close to the true value |
| Medium (B) | Moderate assurance of observed values: observations may be close to true values but may vary considerably |
| Low (C) | Limited assurance of the observed value: the observed value may differ greatly from the true value |
| Very low (D) | Almost unsure of the observed value: the observed value may differ extremely from the true value |
| Grading of recommendation strength | |
| Strong (1) | Clearly demonstrated that the intervention advantages outweigh the disadvantages or vice versa |
| Weak (2) | Uncertainty in advantages and disadvantages or evidence of comparable advantages and disadvantages regardless of quality |
The summary of the recommendations is listed in Table 2.
Table 2.
Recommendation for the diagnosis and treatment of systemic lupus erythematosus
| Recommendation 1 |
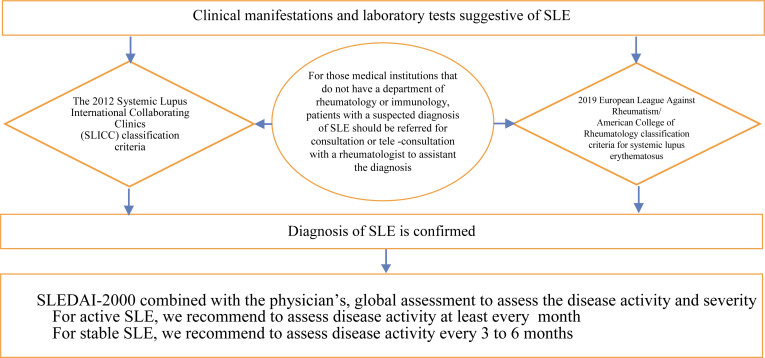
| 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for systemic lupus erythematosus or 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus to make the diagnosis of SLE in patients with suspected disease (1B). In institutions that do not have a rheumatology or immunology department, we recommend consultation with a rheumatologist or immunologist via referral/tele-consultation (2C) for patients with atypical clinical manifestations or patients who are difficult to diagnose. |
| Recommendation 2 |
| The principles of treatment of SLE is early intervention and individualized treatment to minimize disease progression, reduce organ damage, and improve prognosis (1C). The short-term goals of SLE treatment are to control disease activity and improve clinical symptoms (1C), achieve clinical remission or the lowest possible disease activity. The long-term goals are to prevent and reduce relapse, reduce adverse medication reactions, prevent and control organ damage caused by the disease, and to achieve long-term sustained remission of the disease, reduce mortality and improve quality of life (1C). |
| Recommendation 3 |
| For newly diagnosed patients with SLE and during followed-up, we recommended to use the SLE disease activity index (SLEDAI-2000) score criteria in combination with the physician's global assessment of disease activity (2C) for disease activity assessment. According to the SLEDAI-2000 score criteria, disease activity can be classified as mild activity (SLEDAI-2000 ≤ 6), moderate activity (SLEDAI-2000 7–12), and severe activity (SLEDAI-2000 > 12) (2D). For patients with active SLE, we recommended to assess the disease activity (2C) at least every month, and every 3–6months for patients with stable disease. If relapse occurs, patients should be treated as active disease (2D). |
| Recommendation 4 |
| Glucocorticoid is the basic medication for the treatment of SLE (1A). Individualized glucocorticoids regimens should be developed depending on disease activity and the type and severity of organ involvement. The lowest dose (1B) required to control the disease should be used for every patient. Low-dose glucocorticoids (≤10mg/day prednisone or equivalent) may be considered if inadequate response to hydroxychloroquine or nonsteroidal anti-inflammatory drugs in mild patients; for patients with moderately active SLE, glucocorticoids (0.5–1mg/kg/day prednisone or equivalent) combined with immunosuppressants should be considered (2C). For patients with severe active SLE, glucocorticoids (≥1mg/kg/day prednisone or equivalent) combined with immunosuppressants should be considered. The dosage of glucocorticoids should be tapered when the disease is stabilized (2C). For patients with lupus crises, pulse glucocorticoids therapy combined with immunosuppressants should be used (1B). Clinicians should pay close attention to disease activity and adjust the glucocorticoids dosage according to disease activity. Tapering of glucocorticoids or withdrawal of glucocorticoid may be considered for patients with long-term stable disease (1C). |
| Recommendation 5 |
| Long-term hydroxychloroquine is recommended as the basic treatment for patients with SLE without contraindication (1A). For patients taking hydroxychloroquine, ocular risk assessment is recommended. For high-risk patients, an annual ophthalmologic examination is recommended. Low-risk patients are advised to undergo an ophthalmologic examination (2C) annually from the fifth year of medication. |
| Recommendation 6 |
| Immunosuppressants (2B) are recommended for patients who do not respond well to glucocorticoids in combination with hydroxychloroquine or who are unable to taper the dose of glucocorticoids below the safe dose (2B). Immunosuppressants are recommended at the time of initial treatment for patients with organ involvement (2C). |
| Recommendation 7 |
| Biologics may be considered in patients who are refractory, intolerant to glucocorticoids and/or immunosuppressive therapy or relapse (2B) |
| Recommendation 8 |
| Recommendation 8.1: For patients with Type I lupus nephritis, we recommend to treat the patient based on extrarenal manifestations (2C). For patients with type II lupus nephritis, we recommend treatment with glucocorticoids and/or immunosuppressants (2C). |
| Recommendation 8.2: For patients with Type III, Type IV, and complicated Type V (Type V + Type III or Type V + Type IV) lupus nephritis, we recommend that patients should be treated with glucocorticoids combined with cyclophosphamide (1B) or mycophenolate mofetil (1B) for induction therapy and mycophenolate mofetil (1B) or azathioprine (1B) for maintenance therapy. |
| Recommendation 8.3: For patients with simple Type V lupus nephritis and renal proteinuria, we recommend patients to be treated with moderate-dose glucocorticoids in combination with mycophenolate mofetil (1B) or calcineurin inhibitors (2B) or azathioprine (2B). Angiotensin converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) is recommended to help to control blood pressure strictly (2C). |
| Recommendation 8.4: It is recommended that the diagnosis of neuropsychiatric lupus should be based on clinical manifestations, serological test results, cerebrospinal fluid tests, and neuroimaging examinations. Neuropsychiatric lupus should be carefully differentiated from the neurological presentations caused by antiphospholipid syndrome (2C). |
| Recommendation 8.5: For patients with severe neuropsychiatric lupus, glucocorticoid pulse therapy (2B) is recommended as the first-line therapy, and cyclophosphamide (2B) may be combined if the response is unsatisfactory. |
| Recommendation 8.6: For patients with thrombocytopenia or autoimmune hemolytic anemia, glucocorticoids (2D) or intravenous immunoglobulin (2D) should be used for the treatment; and immunosuppressive medications (2D) may be added for those patients who do not respond well. Rituximab (2C) may be considered for patients with life-threatening hematologic involvement. |
| Recommendation 9 |
| Patients who present with severe or refractory SLE, plasma exchange or immunoadsorption (2C) can be considered. For patients who present with refractory SLE or infection, intravenous immunoglobulin (2D) may be considered in addition to the standard treatment. |
| Recommendation 10 |
| Infection is the first cause of death in patients with SLE. The potential risk of infection must be assessed in a timely manner throughout the treatment of SLE (1B). |
| Recommendation 11 |
| For women with SLE at childbearing age, pregnancy may be considered if the disease activity is stable for at least 6months without vital organ damage, and the discontinuation of potentially teratogenic medications for a sufficiently safe period of time (2B). If pregnancy is planned, counseling with Rheumatology and Obstetrics specialists and general assessment must be conducted before the start of planning for pregnancy (1B). For pregnant patients, disease activity and fetal growth must be closely monitored (1C). If there is no contraindication, hydroxychloroquine (1B) is recommended throughout pregnancy. For patients who are currently pregnant and have active disease, glucocorticoid and azathioprine can be used to control the disease (2C). |
| Recommendation 12 |
| Lifestyle modifications is an important component in the management of SLE. We recommend that patients with SLE should follow the following principles: (1) avoid exposure to some hazardous substances; (2) avoid sun exposure; (3) moderate exercise; (4) seeking for psychological support, if necessary; (5) quit smoking; (6) vitamin D supplement (1C). |
Recommendation 1: 2012 Systemic Lupus International Collaborating Clinics (SLICC) classification criteria for systemic lupus erythematosus or 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus to make the diagnosis of SLE in patients with suspected disease (1B). In institutions that do not have a rheumatology or immunology department, we recommend consultation with a rheumatologist or immunologist via referral/tele-consultation (2C) for patients with atypical clinical manifestations or patients who are difficult to diagnose.
In order to improve the sensitivity and specificity of the classification for SLE, a set of classification criteria for SLE were jointly developed by EULAR and ACR in 2019 based on the classification criteria for SLE developed in 1997. This classification criteria includes 1 criterion for inclusion, 10 aspects, and 18 criteria. Infection, malignant tumor, medications, and other causes must be excluded before applying each criterion. Patients who met a certain criterion in the history should also be scored and take the highest weight score of each aspect into the total scores. A total score ≥ 10 can be classified as SLE.[33] Validation cohort studies showed that the sensitivity of the SLE classification criteria of 2019 EULAR/ACR, 2012 SLICC, and 1997 ACR were 96%, 97%, and 83%, respectively, and the specificity was 93%, 84%, and 93%, respectively. Sensitivity and specificity of the 2019 EULAR/ACR classification criteria for SLE were appropriate; diagnostic accuracy studies for the new criteria showed that in adult SLE patients, the sensitivity of the SLE classification criteria of 2019 EULAR/ACR (initial draft), 2012 SLICC, and 1997 ACR were 93%, 100%, and 83%, respectively, and the specificity was 73%, 75%, and 82%, respectively. The 2012 SLICC classification criteria are shown to be appropriate in adult patients with SLE.[33,34] Based on the results of the above studies, we recommend the use of these two classification criteria for patients with SLE in China. However, only the SLE classification criteria of 1997 ACR was validated in the Chinese population with SLE, the results showed that the criteria had good applicability for Chinese patients with SLE.[35,36] Thus, in the future, it is necessary to validate the applicability of 2012 SLICC and 2019 EULAR/ACR classification criteria for Chinese SLE patients.
A cross-section study showed that only 23% of 71 patients with SLE diagnosed by primary health care physicians actually could meet the 1997 ACR classification criteria for SLE (meeting ≥4 criteria), whereas among the 249 patients with SLE diagnosed by rheumatologist, 79% of patients could meet the 1997 ACR classification criteria for SLE (meeting ≥4 criteria).[37] Obviously, the participation of rheumatologists in the diagnosis of patients with SLE is helpful to improve the accuracy of diagnosis.
Recommendation 2: The principles of the treatment of SLE is early intervention and individualized treatment to minimize disease progression, reduce organ damage, and improve prognosis (1C). The short-term goals of SLE treatment are to control disease activity and improve clinical symptoms (1C), achieve clinical remission or the lowest possible disease activity. The long-term goals are to prevent and reduce relapse, reduce adverse medication reactions, prevent and control organ damage caused by the disease, and to achieve long-term sustained remission of the disease, reduce mortality and improve quality of life (1C).
Studies have shown that early high disease activity increases the risk of organ damage and death, and that early diagnosis and treatment are beneficial for controlling disease and improving patient outcomes.[38] At present, the treatment of SLE includes glucocorticoids (hereinafter referred to as glucocorticoids), antimalarials, immunosuppressants, biologics, and other medications. Different kinds of drugs have great differences in efficacy and adverse reactions, individualized treatment strategy according to the specific situation of patients should be considered.[12, 13, 15]
The definition of disease remission in SLE remains to be determined. The current consensus is that SLE should be controlled in the ideal state of clinical remission. If clinical remission could not be achieved, the disease activity should be controlled to the lowest possible disease activity.[39] Approximately 25% of patients with ≤4 years duration of SLE achieved clinical remission with treatment, and 45% had organ damage. Relapse is common in patients with SLE, and the risk of relapse is 60% in 4 years of disease duration.[40] Relapse is a marker of significantly increase of disease activity, also a major cause of organ damage and adverse prognosis. High-risk factors for relapse include younger age at disease onset, sustained clinical disease activity, and serological active disease. After achieving remission or minimal disease activity, treatment strategies usually need to be adjusted to prevent and reduce relapse. As shown in a cohort study, compared with the patients with SLE whose disease activity could not be controlled effectively, patients who could achieve disease remission (HR = 0.60, 95% CI 0.43–0.85) and low disease activity (HR = 0.66, 95% CI 0.48–0.93) have reduced new damage and better prognosis.[41] In general, the long-term goal of SLE treatment is to prevent and reduce relapse, decrease organ damage, reduce mortality, improve survival rate and quality of life.
Recommendation 3: For newly diagnosed patients with SLE and during follow-up, we recommend to use the SLE disease activity index (SLEDAI-2000) score criteria in combination with the physician's global assessment of disease activity (2C) for disease activity assessment. According to the SLEDAI-2000 score criteria, disease activity can be classified to mild activity (SLEDAI-2000 ≤6), moderate activity (SLEDAI-2000 7–12), and severe activity (SLEDAI-2000 >12) (2D). For patients with active SLE, we recommend to assess the disease activity (2C) at least every month, and every 3–6 months for patients with stable disease. If relapse occurs, patients should be treated as active disease (2D).
There are 7 SLE disease activity assessment tools[42,43,44,45,46,47,48,49] available. Each of the tools requires physician to comprehensively assess individual patient's disease history based on physical examination and laboratory test results. There are several factors that influence the choice of assessment tools: the physician's personal preferences and knowledge, assessment tools available (whether the tool needs to use a computer, cost of tests), the time needed to complete the assessment.[50] Currently, SLEDAI-2000 and BILAG-2004 are commonly used to assess disease activity in clinical practice in PR China. The scores of SLEDAI-2000 were 0–105, and the results of BILAG-2004 were classified into 5 categories: A, B, C, D, and E. SLEDAI-2000 should be preferentially selected because of the shorter assessment time compared with BILAG-2004, and simplified Mexican version eliminated immunological testing, which made the assessment easier.[51]
There are some criteria for grading disease activity based on SLEDAI-2000, and the main four disease activity grading criteria are discussed in this guideline.[12, 13, 15, 52] We recommend to use the criteria proposed by EULAR, that is, SLEDAI-2000 ≤ 6 for mild activity, 7–12 for moderate activity, and SLEDAI-2000 > 12 for severe activity.[12] Since high SLEDAI-2000 score predicted increased risk of organ damage (HR = 1.18, 95% CI 1.02–1.37) and increased risk of death (HR = 1.14, 95% CI 1.02–1.22),[38] regular monitoring of disease activity and organ damage in patients with SLE is required. Due to limitations of the assessment of disease activity based on SLEDAI-2000 and BILAG-2004, the physician global assessment (PGA) is also required. Involving clinical and other manifestations of SLE, PGA could improve the accuracy of the assessment.
Currently, no evidence indicates the frequency of clinical monitoring in patients with SLE. The UK SLE Guidelines recommend that disease activity should be assessed at least every 1–3 months for patients with active disease, whereas the Spanish SLE Guidelines recommend that disease activity should be assessed at least every 3–4 months for 1 year. For patients with SLE in a stable disease or with low disease activity, both the UK and Spanish SLE Guidelines recommended that they should be assessed every 6–12 months.[13,53] We recommend that disease activity should be assessed at least once per month in patients with active SLE (consensus: 83.33%) and every 3–6 months for patients with a stable disease (consensus: 94.44%). The SDI (SLICC damage index) is the only internationally recognized and validated assessment tool for organ damage in SLE. This tool scores 12 organ systems independently, is an effective tool for evaluating organ damage in clinical practice and provides a basis for better judging the prognosis of patients with SLE.[54] In addition, the frequency of clinical monitoring should be adjusted according to the progression of the disease and intensity of treatment. If relapse, the disease must be treated as an active disease, and the disease activity must be assessed at least once every month until the disease becomes stable.
We recommend using Chinese Rheumatism Data Center (CRDC) chronic disease management platform for follow-up of patients with SLE[55] in China.
Recommendation 4: Glucocorticoid is the basic medication for the treatment of SLE (1A). Individualized glucocorticoids regimens should be developed depending on disease activity and the type and severity of organ involvement. The lowest dose (1B) required to control the disease should be used for every patient. Low-dose glucocorticoids (≤ 10 mg/day prednisone or equivalent) may be considered if inadequate response to hydroxychloroquine or nonsteroidal anti-inflammatory drugs in mild patients; for patients with moderately active SLE, glucocorticoids (0.5 to 1 mg-kg-d prednisone or equivalent) combined with immunosuppressants should be considered (2C). For patients with severe active SLE, glucocorticoids (≥1 mg-kg-d prednisone or equivalent) combined with immunosuppressants should be considered. The dosage of glucocorticoids should be tapered when the disease is stabilized (2C). For patients with lupus crises, pulse glucocorticoids therapy combined with immunosuppressants should be used (1B). Clinicians should pay close attention to disease activity and adjust the glucocorticoids dosage according to disease activity. Tapering of glucocorticoids or withdrawal of glucocorticoid may be considered for patients with long-term stable disease (1C).
Glucocorticoid plays a crucial role in the treatment of SLE and is the most commonly used medication for the remission induction treatment of SLE, and also the basic medication for the control of disease activity. Glucocorticoid is recommended by international guidelines for the treatment of SLE.[12, 13, 15, 53, 56, 57]
For patients with SLE, individualized glucocorticoids therapy should be established according to disease activity, type and severity of organ involvement. The dosage should be adjusted according to disease activity, duration of medication use, and adverse reactions.
Patients with mildly active SLE generally do not require glucocorticoids therapy. When hydroxychloroquine or nonsteroidal anti-inflammatory medications could not adequately control the disease, low-dose glucocorticoids (prednisone ≤ 10 mg/day or equivalent) may be considered to help control disease activity.[12, 13, 15, 53, 56, 57]
For patients with moderate active SLE, moderate doses of prednisone (0.5 to 1 mg kg−1 d−1), or equivalent doses are recommended. In patients whose disease failed to under control by glucocorticoid alone, immunosuppressants may be used to reduce the cumulative dose of glucocorticoids and the risk of long-term adverse reactions.[13, 15, 56]
For patients with severe active SLE, 1 mg kg–1 d–1 prednisone or equivalent in combination with immunosuppressants are recommended, with glucocorticoid dose adjustments after disease stabilization.[15,56] In patients with severe SLE, pulse glucocorticoids therapy may be used if necessary.[12,53,57]
Glucocorticoids pulse therapy combined with immunosuppressive agents are recommended for SLE patients with lupus crisis. Glucocorticoids pulse therapy is intravenous methylprednisolone 500–1,000 mg d−1, usually over 3 consecutive days as a course of treatment. This regimen could be repeated in 5–30 days interval if necessary. After pulse therapy, prednisone 0.5–1 mg kg−1 d−1 or equivalent should be taken orally, usually for 4–8 weeks, but the duration of treatment depends on the patient's condition.[15,56] Pulse therapy resulted in rapid disease control without significant increased adverse reactions compared with conventional doses of glucocorticoids therapy.[58]
The time of glucocorticoid tapering and discontinuation depends on disease activity and the side effects. Abrupt discontinuation of glucocorticoid should be avoided. For patients with stable disease, the dose tapering must be started as soon as possible, and must be gradual and slow to avoid disease relapse.[59]
More than 30% of patients who take glucocorticoids may have adverse reactions. The most common recent adverse reactions are GI discomfort, agitation, palpitations and insomnia. Long-term adverse reactions include secondary infection, osteoporotic fracture and so on.[60] Because adverse effects of glucocorticoids are dose dependent, clinicians should use the lowest dose needed for disease control and should also avoid the risk of underuse or misuse. Previous studies have shown that patients with SLE receiving prednisone > 7.5 mg d−1 are more likely to develop glucocorticoids-related cardiovascular disease (including myocardial infarction, heart failure and cerebrovascular disease), kidney disease, musculoskeletal disease. But treatment with ≤ 7.5 mg d−1 prednisone was not associated with cumulative impairment in patients with SLE.[61, 62] Tapering and discontinuation of glucocorticoids may be considered in patients with long-term stable disease. Equivalent doses of commonly used glucocorticoids are shown in Table 3.[63]
Table 3.
Equivalent dose of commonly used glucocorticoids
| Drug category | Drug name | Equivalent dose (mg) |
|---|---|---|
| Short-term effects | Hydrocortisone | 20 |
| Cortisone | 25 | |
| Prednisone | 5 | |
| Intermediate-term effects | Prednisolone | 5 |
| Methylprednisolone | 4 | |
| Long-term effects | Triamcinolone acetonide | 4 |
| Betamethasone | 0.60 | |
| Dexamethasone | 0.75 |
Recommendation 5: Long-term hydroxychloroquine is recommended as the basic treatment for patients with SLE without contraindication (1A). For patients taking hydroxychloroquine, ocular risk assessment is recommended. For high-risk patients, an annual ophthalmologic examination is recommended. Low-risk patients are advised to undergo an ophthalmologic examination (2C) annually from the fifth year of medication.
Long-term use of hydroxychloroquine in patients with SLE reduces disease activity, the risk of organ damage, thrombosis, and improves lipid profile and survival rate.[64,65,66,67] Hydroxychloroquine-induced retinopathy is observed 5 years after hydroxychloroquine administration. High-risk populations (long-term use and/or use of high-dose hydroxychloroquine, concomitant hepatic and renal disease, concomitant use of tamoxifen, history of retinal or macular disease and advanced age) should have ophthalmologic examinations before and after treatment annually.[68,69,70] Patients taking hydroxychloroquine without high-risk factors should undergo basic and annual ophthalmologic examinations 5 years later after taking the medicine to monitor drug-induced retinopathy.[71,72]
Recommendation 6: Immunosuppressants (2B) are recommended for patients who do not respond well to glucocorticoids in combination with hydroxychloroquine or who are unable to taper the dose of glucocorticoids below the safe dose (2B). Immunosuppressants are recommended at the time of initial treatment for patient with organ involvement (2C).
The use of immunosuppressants reduces the cumulative dose of glucocorticoids and prevents disease relapse.[31] In patients with refractory (poor response to conventional therapy) or relapsed SLE, immunosuppressants can reduce glucocorticoids dosage, control disease activity and improve clinical remission rates.[73,74]
Initial treatment of lupus nephritis (induction remission), the combination of immunosuppressants significantly increases the clinical remission rate and reduces the rate of treatment failure compared with glucocorticoids monotherapy; therefore, the addition of immunosuppressants[75, 76] in patients with SLE with organ involvement should be considered at the time of initial treatment, and should choose appropriate immunosuppressants according to clinical manifestations, fertility requirements, drug safety and cost (Table 4).[77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]
Table 4.
Indications, advantages and common and significant adverse reactions of different immunosuppressants
| Immunosuppressants | Main applicable populations | Advantages | Common adverse reactions |
|---|---|---|---|
| Mycophenolate mofetil | Patients with moderate to severe SLE[74] | In patients with moderate to severe lupus nephritis, mycophenolate mofetil is an effective treatment in the induction and maintenance phases, and in reducing the relapse rate.[77] | The most common adverse reactions are gastrointestinal discomfort. Some patients develop infections, myelosuppression and liver damage,[78]and due to teratogenicity, pregnancy can only be attempted at least 6weeks after discontinuation.[79] |
| Cyclophosphamide | Moderate to severe lupus nephritis, neuropsychiatric lupus and SLE with immune thrombocytopenia[75, 80, 97] | For induction and maintenance treatment. It is effective in patients with moderate to severe lupus nephritis, and is an effective immunosuppressive agent for the treatment of neuropsychiatric and hematological disease.[75, 80] | Common adverse reactions are gastrointestinal discomfort, nausea, vomiting. Liver damage and myelosuppression are the main adverse reactions. Long-term high-dose use will increase the risk of tumors. Since it has gonadotoxicity and teratogenicity, it is recommended to discontinue at least 3months before pregnancy[81, 82, 97] |
| Leflunomide | Proliferative lupus nephritis[83, 84] | It is effective and well tolerated in some proliferative lupus nephritis patients[84] | Leflunomide causes liver damage, hypertension, leukopenia, infection and some other complications. Because of teratogenicity, it is recommended that pregnancy should only be attempted for 6months after drug withdrawal.[85, 86] |
| Methotrexate | Patients with mild to moderate SLE without renal involvement[97] | It has good efficacy in improving skin, arthritis and overall condition in patients with SLE.[87, 97] | The most common adverse reactions are gastrointestinal discomfort, such as nausea, vomiting. Hematological adverse reactions such as anemia, leukopenia and liver damage are common. Discontinuation of this medication 1–3months before pregnancy is recommended given its teratogenicity.[86,87,88] |
| Tacrolimus | Proliferative lupus nephritis, refractory lupus nephritis and SLE with immune thrombocytopenia[77, 89,90,91] | It is effective in the induction and maintenance treatment of lupus nephritis. It can also reduce relapse.[77]It can be used for the treatment of refractory lupus nephritis, especially in those with proteinuria.[89, 90]Compared with other immunosuppressants or glucocorticoids, it has low risk of causing serious infections.[92] | Common adverse reactions are gastrointestinal discomfort, renal and liver function impairment. The dosage of tacrolimus must be reduced in patients with impaired hepatic function and renal dysfunction. Blood glucose and blood pressure should be monitored during administration.[93] |
| Cyclosporine | Lupus nephritis and SLE with immune thrombocytopenia[94,95,96] | Cyclosporine in combination with other immunosuppressants may be used to treat lupus nephritis that does not respond to standard therapy, it is effective in some patients with hematological involvement.[94, 95] | The main adverse reactions are renal impairment, elevation of blood pressure and increased risk of infection.[95] |
| Azathioprine | Patients with moderate SLE[79] | Maintenance treatment for SLE. It has low risk for severe infection. It is safe during pregnancy.[79] | The main adverse reactions are myelosuppression and liver damage.[79]Testing for thiopurine methyltransferase activity is required. |
SLE, Systemic lupus erythematosus.
Recommendation 7: Biologics may be considered in patients who are refractory, intolerant to glucocorticoids and/or immunosuppressive therapy (2B) or relapsed.
For patients with refractory (poor response to conventional treatment) or relapsed SLE, the use of biologics significantly increases the complete and partial remission rates and reduces disease activity, relapse rate and glucocorticoids dosage.[98,99,100,101] Although many biological agents have been tried in the treatment of SLE and have achieved certain clinical efficacies, only Belimumab was approved by the United States Food and Drug Administration (FDA) and the China Food and Drug Administration (CFDA) for the treatment of SLE currently. However, the efficacy and safety of Belimumab in the treatment of Chinese patients with SLE need to be further validated. The advantages and common adverse reactions of different types of biologics are shown in Table 5.
Table 5.
The advantages and significant adverse reactions of biologics in the treatment of systemic lupus erythematosus
| Biologics | Approved by FDA and CFDA | Advantages | Significant adverse reactions |
|---|---|---|---|
| Belimumab | Yes | It can improve the patients’ serological indexes, reduce the risk of severe relapse and the dosage of glucocorticoids. It could be considered for patients who are poorly. controlled with current conventional therapy. | Common adverse reactions are infections, headache, and nausea |
| Rituximab | No | For patients with refractory lupus nephritis and hematologic involvement, the disease can be controlled, and the dosage of glucocorticoids can be reduced.[98, 101] | Common adverse reactions include infections and infusion reactions.[98] |
FDA, Food and Drug Administration; CFDA, China Food and Drug Administration.
Recommendation 8.1: For patients with Type I lupus nephritis, we recommend to treat the patient based on extrarenal manifestations (2C). For patients with Type II lupus nephritis, we recommend treatment with glucocorticoids and/or immunosuppressants (2C).
Recommendation 8.2: For patients with Type III, Type IV and complicated Type V (Type V + Type III or Type V + Type IV) lupus nephritis, we recommend that patients should be treated with glucocorticoids combined with cyclophosphamide (1B) or mycophenolate mofetil (1B) for induction therapy and mycophenolate mofetil (1B) or azathioprine (1B) for maintenance therapy.
Recommendation 8.3: For patients with simple Type V lupus nephritis and renal proteinuria, we recommend patients to be treated with medium-dose glucocorticoids in combination with mycophenolate mofetil (1B) or calcineurin inhibitors (2B) or azathioprine (2B). Angiotensin converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB) is recommended to help control blood pressure strictly (2C).
Recommendation 8.4: It is recommended that the diagnosis of neuropsychiatric lupus should be based on clinical manifestations, serological test results, cerebrospinal fluid tests and neuroimaging examinations. Neuropsychiatric lupus should be carefully differentiated from the neurological presentations caused by antiphospholipid syndrome (2C).
Recommendation 8.5: For patients with severe neuropsychiatric lupus, glucocorticoid pulse therapy (2B) is recommended as first-line therapy, and cyclophosphamide (2B) may be combined if the response is unsatisfactory.
Recommendation 8.6: For patients with thrombocytopenia or autoimmune hemolytic anemia, glucocorticoids (2D) or intravenous immunoglobulin (2D) should be used for the treatment; and immunosuppressive medications (2D) may be added to those patients who do not respond well. Rituximab (2C) may be considered for patients with life-threatening hematologic involvement.
Indications of renal biopsy and pathological classification of lupus nephritis should be conducted according to the latest guidelines and criteria to provide guidance for subsequent treatment.[102,103,104] For patients with Type II lupus nephritis who are at risk of histological transformation,[105, 106] particularly for patients who do not respond well to initial therapy, treatment with glucocorticoids and/or immunosuppressants are recommended. One network meta-analysis showed that mycophenolate mofetil was comparable to intravenous cyclophosphamide in patients with Type III/IV/V + III/V + IV lupus nephritis undergoing induction therapy. The complete remission rate was similar to intravenous cyclophosphamide (OR = 1.44, 95% CI 1.00–2.06), and that of calcineurin inhibitors (OR = 1.74, 95% CI 1.09–2.79). Mycophenolate mofetil was associated with a lower risk of relapse compared with azathioprine in patients with Type III/IV/V + III/V + IV lupus nephritis undergoing maintenance therapy (OR = 0.53, 95% CI 0.31–0.90). There was no significant difference in the risk of relapse between calcineurin inhibitors (OR = 0.64, 95% CI 0.22–1.88) and cyclophosphamide (OR = 1.68, 95% CI 0.51–5.51).[75] However, another meta-analysis showed that there were no statistically significant differences in mortality, incidence of end-stage renal disease, nor disease relapse between mycophenolate mofetil and azathioprine during the maintenance treatment period.[107]
One network meta-analysis showed that glucocorticoids in combination with mycophenolate mofetil and calcineurin inhibitors were more effective than glucocorticoids monotherapy in inducing remission of Type V lupus nephritis (most with renal proteinuria). The effect of azathioprine combined with glucocorticoids was not significantly different from glucocorticoids monotherapy.[76] Patients with simple Type V lupus nephritis without renal proteinuria have a good prognosis and only require ACEI/ARB for blood pressure control without immunosuppressive therapy.[108]
At present, there are no well-accepted diagnostic criteria and specific laboratory indicators for neuropsychiatric lupus, so the diagnosis of neuropsychiatric lupus is basically exclusive. For patients with diffused neuropsychiatric lupus, CSF antibodies and anti-ribosomal P antibodies are helpful. For patients with focal neuropsychiatric lupus, antiphospholipid antibodies or abnormal brain magnetic resonance imaging (MRI) findings are helpful for diagnosis.[109] MRI abnormalities (including brain atrophy, T1- and T2-weighted lesions, etc.) are more common in patients with neuropsychiatric lupus and related to specific manifestations of neuropsychiatric lupus, so MRI is an effective imaging examination for the diagnosis of neuropsychiatric lupus.[110]
For severe neuropsychiatric lupus patients, high-dose methylprednisolone pulse therapy combined with intravenous cyclophosphamide can improve their psychiatric symptoms, and the efficacy is superior to methylprednisolone pulse therapy alone, with a total improvement rates of 94.7% and 46.2%, respectively.
The initial remission rate of glucocorticoids therapy in SLE with severe autoimmune hemolytic anemia can reach 96%, the effectiveness rate of treatment of SLE related immune thrombocytopenia can reach 80%. Treatment with intravenous immunoglobulins and glucocorticoids in combination with immunosuppressants can improve hematologic symptoms in SLE patients with autoimmune hemolytic anemia.[111,112,113] For SLE patients with severe refractory thrombocytopenia, treatment with low-dose rituximab (100 mg intravenously weekly for 4 times) has an 80% response rate, which improved patient outcomes effectively. Rituximab is an effective treatment in patients with life-threatening acute hemolytic anemia.[114,115,116]
Recommendation 9: Patients with severe or refractory SLE, plasma exchange or immunoadsorption (2C) can be considered. For patients who present with refractory SLE or infection, intravenous immunoglobulin (2D) may be considered in addition to the standard treatment.
In 2018, an epidemiological survey of blood purification treatment of severe pediatric SLE was conducted in 22 hospitals by the Blood Purification Committee of the Pediatrician Association, Chinese Medical Doctor Association. The survey results showed that plasma exchange and DNA immunoadsorption could improve the clinical symptoms of severe pediatric SLE patients, with the improvement rates of 87.3% and 87.8%, respectively.[117] Plasma exchange and immunoadsorption can improve clinical symptoms in the short term in severe or refractory SLE patients, but cannot improve their final outcomes. Therefore, Plasma exchange and immunoadsorption can be used as adjunct treatments.[118, 119]
Intravenous immunoglobulins may improve clinical outcomes in patients with refractory SLE or infection, but the quality of evidence is very low.[120, 121]
Tripterygium wilfordii is an effective treatment of SLE. However, more attention must be paid to its reproductive toxicity. The reported incidence rate of reproductive toxicity is 17.9% (95% CI 0.14–0.22).[122, 123]
Recommendation 10: Infection is the leading cause of death in patients with SLE. The potential risk of infection must be assessed in a timely manner throughout the treatment of SLE (1B).
The proportion of patients with SLE dying from infection is increasing year by year in China, and infection has become the leading cause of death in patients with SLE in China. The mortality of infection is exceeding 50%[124] in SLE patients. Inappropriate use of glucocorticoids (OR = 3.05, 95% CI 1.15–8.07) and immunosuppressants (OR = 2.01, 95% CI 1.21–3.32), high SLEDAI (OR = 0.44, 95% CI 0.32–0.59), multiple organs involvement (OR = 2.53, 95% CI 1.87–3.42), and younger age at disease onset (OR = 2.09, 95% CI 1.50–2.91) are major risk factors for infection in SLE patients.[92,125] Serum high-sensitive C-reactive protein above 50 mg/L, procalcitonin above 0.5 μg/L and lymphocyte count ≤ 1.0 × 109/L suggests an increased risk of infection (HR = 4.7, 95% CI 1.6–13.7).[126,127,128] The clinical manifestations of patients should be assessed timely to identify potential infection.
Recommendation 11: For women with SLE at childbearing age, pregnancy may be considered if the disease activity is stable for at least 6 months without vital organ damage, and the discontinuation of potentially teratogenic medications for a sufficiently safe period of time (2B). If pregnancy is planned, counseling with Rheumatology and Obstetrics specialists and general assessment must be conducted before the start of planning for pregnancy (1B). For pregnant patients, disease activity and fetal growth must be closely monitored (1C). If there is no contraindication, hydroxychloroquine (1B) is recommended throughout pregnancy. If patients who are currently pregnant and have active disease, glucocorticoid and azathioprine can be used to control the disease (2C).
In order to reduce the complications of pregnancy and improve maternal and fetal outcomes in women with SLE, adequate preparation must be made before conception, and disease activity must be closely monitored during pregnancy. Women with significant impaired organ function and/or with severe organ damage should be informed of pregnancy-related risks.[129,130] Compared with patients with inactive lupus nephritis, patients with active lupus nephritis have significantly increased risk for poor maternal outcomes (OR = 2.04, 95% CI 1.21–3.45), pre-eclampsia or eclampsia (OR = 2.62, 95% CI 1.36–5.05), fetal loss (OR = 4.90, 95% CI 1.54–15.59), preterm delivery (OR = 4.26, 95% CI 2.19–8.31).[131] Patients with persistent remission for more than 6 months, proteinuria < 0.5 g/d, no renal failure, and cytotoxic drug withdrawal for > 1 year before pregnancy, have better pregnancy outcome including full term delivery rate (76.47% vs. 23.08%), live birth rate (80.39% vs. 30.77%), the risk of pregnancy-induced hypertension (17.65% vs. 23.08%), and preeclampsia or eclampsia (9.80% vs. 15.38%) when compared with patients with active disease in the preceding 6 months of pregnancy.[132]
Preconception counselling is essential for successful pregnancy, and planned pregnancy can significantly reduce the risk of disease relapse and adverse maternal and fetal outcomes during pregnancy compared with accidental pregnancy.[133,134] Active disease itself could significantly increase the risk of adverse pregnancy outcomes.[135] so the disease must be strictly controlled before pregnancy. A multidisciplinary team (including rheumatologists and obstetricians at least) must assess patients before pregnancy and decide whether the patient is suitable for pregnancy. Patient's disease activity must be strictly monitored throughout pregnancy in order to achieve the goal of improving maternal and fetal outcomes.[136,137] Multidisciplinary management of pregnant patients with SLE by rheumatologists and obstetricians can be referred to the Recommendations for Perinatal Management of Chinese Patients with Systemic Lupus Erythematosus.[138]
Strict surveillance of disease activity and fetal growth during pregnancy are essential for better maternal and fetal outcomes. Risk factors associated with complications during pregnancy, such as antiphospholipid syndrome, must be screened and tested before pregnancy. Patients must be strictly monitored during pregnancy for disease activity, placental function and fetal growth.[137,138,139,140,141]
Hydroxychloroquine may reduce the rate of preterm birth, disease relapse, disease activity and risk of adverse fetal outcomes in pregnant women with SLE. Continuous hydroxychloroquine therapy may reduce disease relapse during pregnancy and postpartum. If there is no contra-indication, we recommend continuing HCQ treatment throughout pregnancy. For active SLE patients during pregnancy, glucocorticoid and hydroxychloroquine in addition to immunosuppressants available during pregnancy may be considered for disease control.[142,143,144] The use of azathioprine during pregnancy does not cause fetal teratogenesis and may reduce the risk of disease relapse and improve fetal outcome.[145] Hydroxychloroquine, glucocorticoid, azathioprine, cyclosporine A and tacrolimus may be used to prevent or control SLE relapse during pregnancy, but mycophenolate mofetil, cyclophosphamide, leflunomide and methotrexate should be avoided.[86]
Recommendation 12: Lifestyle modifications is an important component in the management of SLE. We recommend that patients with SLE should follow the following principles: (1) avoid exposure to some hazardous substances; (2) avoid sun exposure; (3) moderate exercise; (4) seeking for psychological support if necessary; (5) quit smoking; (6) vitamin D supplement (1C).
Skin is one of the most frequently involved organs in SLE and is one of the main organ systems that perceive changes in the external environment. Some cosmetics may contain substances that may induce lupus erythematosus or aggravate the disease.[146, 147] In addition, patients with SLE should avoid exposure to hair dyes, eyebrow tattoos etc.[148, 149] Ultraviolet radiation can induce SLE and sun protection (e.g., sun cream) can protect patients from UV-induced skin irritation, reduce skin inflammation[150,151,152] and disease relapse. Patients receiving exercise intervention can reduce depression (SMD = −0.40, 95% CI −0.71 to −0.09) and fatigue (MD = −0.52, 95% CI −0.91 to −0.13).[153, 154] Psychological intervention can reduce anxiety (SMD = −0.95, 95% CI −1.57 to −0.34), mental stress (SMD = −0.63, 95% CI −1.02 to −0.23) and depression (SMD = −1.14, 95% CI −1.84 to −0.44), which is helpful for disease activity control (SMD = −0.34, 95% CI −0.57 to −0.11).[151] Patients who smoke have an increased risk of morbidity (OR = 1.49, 95% CI 1.06 to 2.08) and a higher SLEDAI (15.6 ±7.8 vs. 9.0 ±5.8) than patients who do not smoke.[155, 156] Osteoporosis is a major co-morbidity in patients with SLE. Serum vitamin D level is significantly lower in patients with SLE than in healthy subjects, and vitamin D supplementation reduces the inflammation and disease activity in patients with SLE.[157, 158]
A diagram of the diagnosis and treatment pathway for systemic lupus erythematosus (SLE) is shown in Figure 1.
Figure 1.
The diagnosis and treatment algorithm for systemic lupus erythematosus (SLE).
Principal Expert: Xiaofeng Zeng
Principal Methodologist: Yaolong Chen
Member of the Working Group of 2020 Chinese Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus (sorted by Chinese Pinyin surname): Jinwei Chen (Department of Rheumatology, Xiangya Second Hospital, Central South University); Yaolong Chen (Lanzhou University Evidence-based Medicine Center/GRADE China Center); Xinwang Duan (Department of Rheumatology and Immunology, Second Affiliated Hospital of Nanchang University); Lan He (Department of Rheumatology, First Affiliated Hospital of Xi’an Jiaotong University); Cibo Huang (Department of Rheumatology, Beijing Hospital); Hongzhong Jin (Department of Dermatology, Peking Union Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences); Junqiang Lei (Department of Radiology, the First Hospital of Lanzhou University); Caifeng Li (Department of Rheumatology, Beijing Children's Hospital Affiliated to Capital Medical University); Mengtao Li (Department of Rheumatology, Peking Union Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences); Xiaofeng Li (Department of Rheumatology, Second Hospital of Shanxi Medical University); Xiaomei Li (Department of Rheumatology and Immunology, Anmycin Provincial Hospital); Xiaoxia Li (Department of Rheumatology, Xuanwu Hospital, Capital Medical University); Jin Lin (Department of Rheumatology and Immunology, First Affiliated Hospital of Zhejiang University Medical College); Dongzhou Liu (Department of Rheumatology, Shenzhen People's Hospital); Shengyun Liu (Department of Rheumatology, First Affiliated Hospital of Zhengzhou University); Yi Liu (Department of Rheumatology and Immunology, West China Hospital, Sichuan University); Liangjing Lyu (Department of Rheumatology and Immunology, Renji Hospital Affiliated to Shanghai Jiaotong University School of Medicine); Yijun Song (Department of Gynecology and Obstetrics, Peking Union Medical College, Peking Union Medical College, Chinese Academy of Medical Sciences); Yin Su (Department of Rheumatology, Peking University People's Hospital); Lingyun Sun (Department of Rheumatology and Immunology, Drum Tower Hospital, Nanjing University School of Medicine); Xinping Tian (Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences); Qian Gong (Department of Rheumatology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences); Zhenbiao Wu (Department of Rheumatology and Immunology, Xijing Hospital, Air Force Military Medical University); Lijun Wu (Department of Rheumatology, Xinjiang Uygur Autonomous Region People's Hospital); Weiguo Xiao (Department of Rheumatology, First Affiliated Hospital of China Medical University); Huji Xu (Department of Rheumatology and Immunology, Shanghai Changzheng Hospital, the Second Military Medical University); Jian Xu (Department of Rheumatic Immunology, First Affiliated Hospital of Kunming Medical University); Xunde Yang (Department of Rheumatology, Ruijin Hospital, Shanghai Jiaotong University School of Medicine); Min Yang (Department of Rheumatology, Southern Hospital, Southern Medical University); Xiaofeng Zeng (Department of Rheumatology, Peking Union Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences); Feng Zhan (Department of Rheumatology, Hainan People's Hospital); Miaojia Zhang (Department of Rheumatology and Immunology, Jiangsu Provincial People's Hospital); Wen Zhang (Department of Rheumatology, Peking Union Medical College, Peking Union Medical College, Chinese Academy of Medical Sciences); Xiao Zhang (Guangdong Provincial General Hospital); Zhiyi Zhang (Department of Rheumatology, First Affiliated Hospital Affiliated to Harbin Medical University); Zhuoli Zhang (Department of Rheumatology, Peking University First Hospital); Cheng Zhao (Department of Rheumatology and Immunology, First Affiliated Hospital of Guangxi Medical University); Dongbao Zhao (Department of Rheumatology, First Affiliated Hospital of Naval Military Medical University); Jiuliang Zhao (Department of Rheumatology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences); Yan Zhao (Department of Rheumatology and Immunology, Peking Union Medical College, Peking Union Medical College, Chinese Academy of Medical Sciences); Yi Zheng (Department of Rheumatology, Beijing Chaoyang Hospital, Capital Medical University); Xiaoxia Zuo (Department of Rheumatology, Xiangya Hospital, Central South University).
Member of Evidence Evaluation Team
Qi Zhou (Lanzhou University College of First Clinical Medicine, Lanzhou University); Zijun Gong (Lanzhou University School of Basic Medicine); Qianling Shi (Lanzhou University School of Clinical Medicine); Jianjian Gong (Lanzhou University School of Public Health, Lanzhou University); Yanfang Ma (Center for Evidence-based Medicine, Lanzhou University); Xufei Luo (Lanzhou University School of Public Health, Lanzhou University).
Footnotes
Conflict of Interest
All authors declared that there was no conflict of interest.
References
- [1].Durcan L, O’Dwyer T, Petri M. Management Strategies and Future Directions for Systemic Lupus Erytthematosus in Adults. Lancet. 2019;393(10188):2332–2343. doi: 10.1016/S0140-6736(19)30237-5. [DOI] [PubMed] [Google Scholar]
- [2].Fava A, Petri M. Systemic Lupus Erythematosus: Diagnosis and Clinical Management. J Autoimmun. 2019;96:1–13. doi: 10.1016/j.jaut.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lisnevskaia L, Murphy G, Isenberg D. Systemic Lupus Erythematosus. Lancet. 2014;384(9957):1878–1888. doi: 10.1016/S0140-6736(14)60128-8. [DOI] [PubMed] [Google Scholar]
- [4].Rees F, Doherty M, Grainge MJ. et al. The Worldwide Incidence and Prevalence of Systemic Lupus Erythematosus: A Systematic Review of Epidemiological Studies. Rheumatology. 2017;56(11):1945–1961. doi: 10.1093/rheumatology/kex260. [DOI] [PubMed] [Google Scholar]
- [5].Zeng QY, Chen R, Darmawan J. et al. Rheumatic Diseases in China. Arthritis Res Ther. 2008;10:R17–R27. doi: 10.1186/ar2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li M, Zhang W, Leng X. et al. Chinese SLE Treatment and Research Croup (CSTAR) Registry: I. Major Clinical Characteristics of Chinese Patients With Systemic Lupus Erythematosus. Lupus. 2013;22(11):1192–1199. doi: 10.1177/0961203313499086. [DOI] [PubMed] [Google Scholar]
- [7].Zhang S, Su J, Li X. et al. Chinese SLE Treatment and Research Group (CSTAR) Registry: V. Gender Impact on Chinese Patients With Systemic Lupus Erythematosus. Lupus. 2015;24(12):1–9. doi: 10.1177/0961203315585813. [DOI] [PubMed] [Google Scholar]
- [8].Wang Z, Li M, Zhao J. et al. 220 Clinical Characteristics and Remission of Patients With Systemic Lupus Erythematosus in China: Results From SLE Treatment and Research Group (CSTAR) Registry With a Real-Time Collecting System. Lupus Sci Med. 2019;6(Suppl 1):A164. doi: 10.1136/lupus-2019-lsm.220. [DOI] [Google Scholar]
- [9].Merrell M, Shulman LE. Determination of Prognosis in Chronic Disease, Illustrated By Systemic Lupus Erythematosus. J Chronic Dis. 1955;1(1):1–32. doi: 10.1016/0021-9681(55)90018-7. [DOI] [PubMed] [Google Scholar]
- [10].Borchers AT, Keen CL, Shoenfeld Y. et al. Surviving the Butterfly and the Wolf: Mortality Trends in Systemic Lupus Erythematosus. Autoimmun Rev. 2004;3(6):423–453. doi: 10.1016/j.autrev.2004.04.002. [DOI] [PubMed] [Google Scholar]
- [11].Tektonidou MG, Lewandowski LB, Hu J. et al. Survival in Adults and Children With Systemic Lupus Erythematosus: A Systematic Review and Bayesian Meta-Analysis of Studies From 1950 to 2016. Ann Rheum Dis. 2017;76(12):2009–2016. doi: 10.1136/annrheumdis-2017-211663. [DOI] [PubMed] [Google Scholar]
- [12].Fanouriakis A, Kostopoulou M, Alunno A. et al. 2019 Update of the EULAR Recommendations for the Management of Systemic Lupus Erythematosus. Ann Rheum Dis. 2019;78(6):736–745. doi: 10.1136/annrheumdis-2019-215089. [DOI] [PubMed] [Google Scholar]
- [13].Gordon C, Amissah-Arthur MB, Gayed M. et al. The British Society for Rheumatology Guideline for the Management of Systemic Lupus Erythematosus in Adults. Rheumatology (Oxford) 2017;57(1):e1–e45. doi: 10.1093/rheumatology/kex286. [DOI] [PubMed] [Google Scholar]
- [14].Pons-Estel BA, Bonfa E, Soriano ER. First Latin American Clinical Practice Guidelines for the Treatment of Systemic Lupus Erythematosus: Latin American Group for the Study of Lupus (GLADEL)-Pan-American League of Associations of Rheumatology (PANLAR) Ann Rheum Dis. 2018;77:1549–1557. doi: 10.1136/rmdopen-2018-000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chinese Rheumatology Association. Guidelines for the Diagnosis and Treatment of Systemic Lupus Erythematosus. Chinese J Rheumatol. 2010;14(5):342–346. doi: 10.3760/cma.j.issn.1007-7480.2010.05.016. [DOI] [Google Scholar]
- [16].Wu C, Li C, Wu Q. et al. Chinese Systemic Lupus Erythematosus Treatment and Research Group Registry IX: Clinical Features and Survival of Childhood-Onset Systemic Lupus Erythematosus in China. Chin Med J. 2017;130(11):1276–1282. doi: 10.4103/0366-6999.206346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wang Ziqian. Long-term Prognosis in Chinese Patients With Systemic Lupus Erythematosus–A CSTAR Cohort Study. Beijing: Beijing Union Medical College; 2016. [Google Scholar]
- [18].World Health Organization. WHO Handbook for Guideline Development. 2nd ed. Vienna: World Health Organization; 2014. [Google Scholar]
- [19].Jiang Zhuming, Zhan Siyan, Jia Xiaowei. et al. Basic Methods and Procedures for Develop/Revising the Clinical Diagnosis and Treatment Guidelines. Nat Med J China. 2016;96(4):250–253. doi: 10.3760/cma.j.isn.0376-2491.2016.04.004. [DOI] [Google Scholar]
- [20].Brouwers MC, Kho ME, Browman GP. et al. AGREE II: Advancing Guideline Development, Reporting and Evaluation in Health Care. CMAJ. 2010;182(18):E839–E842. doi: 10.1503/cmaj.090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen Y, Yang K, Marušic A. et al. A Reporting Tool for Practice Guidelines in Health Care: the RIGHT Statement. Ann Intern Med. 2017;166(2):128–132. doi: 10.1016/j.zefq.2017.10.008. [DOI] [PubMed] [Google Scholar]
- [22].Chen YL, Wang XQ, Wang Q. et al. Follow the Reporting Guidance to Improve the Quality of Guidelines Report. Chinese J Int Med. 2018;57(3):168–170. doi: 10.3760/cma.j.isn.0578-1426.2018.03.003. [DOI] [PubMed] [Google Scholar]
- [23].Shea BJ, Grimshaw JM, Wells GA. et al. Development of AM-STAR: A Measurement Tool to Assess the Methodological Quality of Systematic Reviews. BMC Med Res Methodol. 2007;7(2):1–7. doi: 10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Higgins JP, Altman DG, Gøtzsche PC. et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Whiting PF, Rutjes AW, Westwood ME. et al. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- [26].Wells GA, Shea BJ, O’Connell D, The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. 2019. http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pxlf . Accessed at January 19, 2020.
- [27].Guyatt G, Oxman AD, Akl EA. et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- [28].Chen Yaolong, Yao Liang, Norris S. et al. Application of GRADE in Systematic Reviews: Necessity, Frequently-Asked Questions and Concerns. Chinese J Evidence-Based Med. 2013;13(12):1401–1404. doi: 10.7507/1672-2531.20130240. [DOI] [Google Scholar]
- [29].Yao Liang, Chen Yaolong, Du Liang. et al. Application of GRADE in Systematic Reviews of Diagnostic Accuracy Tests: A Case Analysis. Chinese J Evidence-Based Med. 2014;14(11):1407–1412. doi: 10.7507/1672-2531.20140226. [DOI] [Google Scholar]
- [30].Chen Yaolong, Yao Liang, Du Liang. et al. Rationales, Methods, Challenges and Development Tendency of Using GRADE in Systematic Reviews of Diagnostic Accuracy Tests. Chinese J Evidence-Based Med. 2014;14(11):1402–1406. doi: 10.7507/1672-2531.20140225. [DOI] [Google Scholar]
- [31].Yang Nan, Xiao Shujun, Zhou Qi. et al. An Introduction of Principles and Methods of Applying GRADE to Network Meta-analysis. Chinese J Evidence-Based Med. 2016;16(5):598–603. doi: 10.7507/1672-2531.20160092. [DOI] [Google Scholar]
- [32].Vernooij RW, Alonso-Coello P, Brouwers M. et al. Reporting Items for Updated Clinical Guidelines: Checklist for the Reporting of Updated Guidelines (CheckUp) PLoS Med. 2017;14(1):e1002207. doi: 10.1371/journal.pmed.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Aringer M, Costenbader K, Daikh D. et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Ann Rheum Dis. 2019;78(9):1151–1159. doi: 10.1136/annrheumdis-2018-214819. [DOI] [PubMed] [Google Scholar]
- [34].Dahlström Ö, Sjöwall C. The Diagnostic Accuracies of the 2012 SLICC Criteria and the Proposed EULAR/ACR Criteria for Systemic Lupus Erythematosus Classification Are Comparable. Lupus. 2019;28(6):778–782. doi: 10.1177/0961203319846388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zheng Yiqing. Comparison of ACR Classification Criteria for Systemic Lupus Erythematosus. Fuzhou: Fujian Medical University; 2013. [Google Scholar]
- [36].Guo Yufan, Chen Zhiwei. Preliminary verification of 2009 ACR-SLE Classification and Diagnostic Criteria. J Suzhou Univ (Medical Edition) 2012;(2):123–124. doi: CNKI: SUN: SYXU.0.2012-02-031. [Google Scholar]
- [37].McDougall JA, Helmick CG, Lim SS. et al. Differences in the Diagnosis and Management of Systemic Lupus Erythematosus by Primary Care and Specialist Providers in the American Indian/Alaska Native population. Lupus. 2018;27(7):1169–1176. doi: 10.1177/0961203318763529. [DOI] [PubMed] [Google Scholar]
- [38].Keeling SO, Vandermeer B, Medina J. et al. Measuring Disease Activity and Damage With Validated Metrics: A Systematic Review on Mortality and Damage in Systemic Lupus Erythematosus. J Rheumatol. 2018;45(10):1448–1461. doi: 10.3899/jrheum.171310. [DOI] [PubMed] [Google Scholar]
- [39].Van Vollenhoven RF, Mosca M, Bertsias G. et al. Treat-to-Target in Systemic Lupus Erythematosus: Recommendations From an International Task Force. Ann Rheum Dis. 2014;73(6):958–967. doi: 10.1136/annrheumdis-2013-205139. [DOI] [PubMed] [Google Scholar]
- [40].Nossent J, Kiss E, Rozman B. et al. Disease Activity and Damage Accrual During the Early Disease Course in a Multinational Inception Cohort of Patients With Systemic Lupus Erythematosus. Lupus. 2010;19(8):949–956. doi: 10.1177/0961203310366572. [DOI] [PubMed] [Google Scholar]
- [41].Ugarte-Gil MF, Wojdyla D, Pons-Estel GJ. et al. Remission and Low Disease Activity Status (LDAS) Protect Lupus Patients From Damage Occurrence: Data From a Multiethnic, Multinational Latin American Lupus Cohort (GLADEL) Ann Rheum Dis. 2017;76(12):2071–2074. doi: 10.1136/annrheumdis-2017-211814. [DOI] [PubMed] [Google Scholar]
- [42].Mikdashi J, Nived O. Measuring Disease Activity in Adults With Systemic Lupus Erythematosus: The Challenges of Administrative Burden and Responsiveness to Patient Concerns in Clinical Research. Arthritis Res Ther. 2015;17(1):183. doi: 10.1186/s13075-015-0702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bombardier C, Gladman DD, Urowitz MB. et al. Derivation of the SLEDAI. A Disease Activity Index for Lupus Patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- [44].Petri M, Buyon J, Skovron M. et al. Reliability of SELENA SLEDAI and Relapse as Clinical Trial Outcome Measures. Arthritis Rheum. 1998;41(9):S218. [Google Scholar]
- [45].Gladman DD, Ibanez D, Urowitz MB. Systemic Lupus Erythematosus Disease Activity Index 2000. J Rheumatol. 2002;29(2):288–291. doi: 10.1097/00124743-200202000-00018. [DOI] [PubMed] [Google Scholar]
- [46].Symmons DP, Coppock JS, Bacon PA. et al. Development and Assessment of a Computerized Index of Clinical Disease Activity in Systemic Lupus Erythematosus. Members of the British Isles Lupus Assessment Group (BILAG) Q J Med. 1988;69(259):927–937. [PubMed] [Google Scholar]
- [47].Isenberg DA, Rahman A, Allen E. et al. BILAG 2004. Development and Initial Validation of an Updated Version of the British Isles Lupus Assessment Group's Disease Activity Index for Patients With Systemic Lupus Erythematosus. Rheumatology (Oxford) 2005;44(7):902–906. doi: 10.1093/rheumatology/keh624. [DOI] [PubMed] [Google Scholar]
- [48].Liang MH, Socher SA, Larson MG. et al. Reliability and Validity of Six Systems for the Clinical Assessment of Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheum. 1989;32(9):1107–1118. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- [49].Bae SC, Koh HK, Chang DK. et al. Reliability and Validity of Systemic Lupus Activity Measure-Revised (Slam-R) for Measuring Clinical Disease Activity in Systemic Lupus Erythematosus. Lupus. 2001;10(6):405–409. doi: 10.1191/096120301678646146. [DOI] [PubMed] [Google Scholar]
- [50].Keeling SO, Alabduiubalnabi Z, Avina-Zubieta A. et al. Canadian Rheumatology Association Recommendations for the Assessment and Monitoring of Systemic Lupus Erythematosus. J Rheumatol. 2018;45(10):1426–1439. doi: 10.3899/jrheum.171459. [DOI] [PubMed] [Google Scholar]
- [51].América GU, Luis MV, Gerald MG. et al. The Systemic Lupus Activity Measure-Revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a Modified SLEDAI-2K Are Adequate Instruments to Measure Disease Activity in Systemic Lupus Erythematosus. J Rheumatol. 2004;31(10):1934–1940. doi: 10.1097/01.rhu.0000141832.32720.a4. [DOI] [PubMed] [Google Scholar]
- [52].Cook RJ, Gladman DD, Pericak D. et al. Prediction of Short Term Mortality in Systemic Lupus Erythematosus With Time Dependent Measures of Disease Activity. J Rheumatol. 2000;27(8):1892–1895. doi: 10.1097/00124743-200008000-00013. [DOI] [PubMed] [Google Scholar]
- [53].Guideline Development Group of the Clinical Practice Guideline on Systemic Lupus Erythematosus. Clinical Practice Guideline on Systemic Lupus Erythematosus. Madrid(Spain): Ministry of Health, Social Services and Equality; 2015. pp. 1–434. [Google Scholar]
- [54].Dafna G, Ellen G, Charles G. et al. The Development and Initial Validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for Systemic Lupus Erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- [55].Chinese Rheumatology Association. 2016 Guidelines for the Diagnosis and Treatment of Gout in China. Chinese J Int Med. 2016;55(11):892–899. doi: 10.3760/cma.j.isn.0578-1426.2016.11.019. [DOI] [Google Scholar]
- [56].Expert Group of the Chinese Systemic Lupus Erythematosus Research Group. Expert Consensus on the Rational Use of Glucocorticoids in Patients With Systemic Lupus Erythematosus. Chinese J Int Med. 2014;53(6):502–504. doi: 10.3760/cma.j.isn.0578-1426.06.2014.023. [DOI] [Google Scholar]
- [57].Trujillo-Martín MM, de Larrinoa IRFF, Ruíz-Irastorza G. et al. Clinical Practice Guidelines for Systemic Lupus Erythematosus: Recommendations for General Clinical Management. Med Clin (Barc) 2016;146(9):413.e1–413.e14. doi: 10.1016/j.medcli.2016.01.013. [DOI] [PubMed] [Google Scholar]
- [58].Tie Ning. Analysis of the Effect of Glucocorticoid Pulse Treatment on Metabolism and Immune Function in Female Patients With Systemic Lupus Erythematosus. Chinese J Front Med Sci (Electronic Version) 2017;9(10):149–152. doi: 10.12037/YXQY.2017.10-32. [DOI] [Google Scholar]
- [59].Ning Guang. Basic Principles of Clinical Application of Glucocorticoids. Chinese J Practical Int Med. 2013;33(10):756–759. [Google Scholar]
- [60].Sarnes E, Crofford L, Watson M. et al. Incidence and US Costs of Glucocorticoid-Associated Adverse Events: A Systematic Literature Review. Clin Ther. 2011;33(10):1413–1432. doi: 10.1016/j.clinthera.2011.09.009. [DOI] [PubMed] [Google Scholar]
- [61].Ruiz-Arruza I, Ugarte A, Cabezas-Rodriguez I. et al. Glucocorticoids and Irreversible Damage in Patients With Systemic Lupus Erythematosus. Rheumatology (Oxford) 2014;53(8):1470–1476. doi: 10.1093/rheumatology/keu148. [DOI] [PubMed] [Google Scholar]
- [62].Wei L, MacDonald TM, Walker BR. Taking Glucocorticoids by Prescription is Associated With Subsequent Cardiovascular Disease. Ann Intern Med. 2004;141(10):764–770. doi: 10.7326/0003-4819-141-10-200411160-00007. [DOI] [PubMed] [Google Scholar]
- [63].ChineseAnesthesiology Association. Expert Consensus on Peri-operative Use of Adrenal Glucocorticoids (2017 Edition) J Clin Anesthesiol. 2017;33(7):712–716. [Google Scholar]
- [64].Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P. et al. Clinical Efficacy and Side Effects Of Antimalarials in Systemic Lupus Erythematosus: A Systematic Review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- [65].Fan W, Wei Z, Shiying W. et al. Protective Effects of Antimalarials in Chinese Patients With Systemic Lupus Erythematosus. Ann Rheum Dis. 2018;78:e80. doi: 10.1136/annrheumdis-2018-213819. [DOI] [PubMed] [Google Scholar]
- [66].Sankhyan P, Boonpheng B, Cook C. Hydroxychloroquine and the Risk of Thrombotic Events in Systemic Lupus Erythematosus Patients: A Systematic Review and Meta-Analysis. Arthritis Rheumatol. 2018;70(Suppl 10):2668. [Google Scholar]
- [67].Babary H, Liu X, Ayatollahi Y. et al. Favorable Effects of Hydroxychloroquine on Serum Low Density Lipid in Patients With Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Int J Rheum Dis. 2017;21(1):84–92. doi: 10.1111/1756-185X.13159. [DOI] [PubMed] [Google Scholar]
- [68].Melles RB, Marmor MF. The Risk of Toxic Retinopathy in Patients on Long-Term Hydroxychloroquine Therapy. JAMA Ophthalmol. 2014;132(12):1453. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- [69].Mukwikwi ER, Pineau CA, Vinet E. et al. Retinal Complications in Systemic Lupus Erythematosus Patients Treated With Antimalarial Drugs. J Rheumatol. 2019 doi: 10.3899/jrheum.181102. [DOI] [PubMed] [Google Scholar]
- [70].Huang Xintao, Lin Hui’e, Zheng Ruping. et al. Literature Review of Hydroxychloroquine-induced Ocular Toxicity. Chinese J Pharmacoepidemiol. 2019;28(1):61–65. doi: CNKI: SUN: YWLX.0.2019-01-015. [Google Scholar]
- [71].Marmor MF, Kellner U, Lai TY. et al. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision) Ophthalmology. 2016;123(6):1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- [72].The Royal College of Ophthalmologists (RCOphth) Hydroxychloroquine and Chloroquine Retinopathy: Recommendations on Screening. London: The Royal College of Ophthalmologists; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Choi CB, Won S, Bae SC. Outcomes of Multitarget Therapy Using Mycophenolate Mofetil and Tacrolimus for Refractory or Relapsing Lupus Nephritis. Lupus. 2018;27(6):1007–1011. doi: 10.1177/0961203318758505. [DOI] [PubMed] [Google Scholar]
- [74].Tselios K, Gladman DD, Su J. et al. Mycophenolate Mofetil in Nonrenal Manifestations of Systemic Lupus Erythematosus: An Observational Cohort Study. J Rheumatol. 2016;43(3):552. doi: 10.3899/jrheum.150779. [DOI] [PubMed] [Google Scholar]
- [75].Palmer SC, Tunnicliffe DJ, Singh-Grewal D. et al. Induction and Maintenance Immunosuppression Treatment of Proliferative Lupus Nephritis: A Network Meta-Analysis of Randomized Trials. Am J Kidney Dis. 2017;70(3):324–336. doi: 10.1053/j.ajkd.2016.12.008. [DOI] [PubMed] [Google Scholar]
- [76].Tang KT, Tseng CH, Hsieh TY. et al. Induction Therapy for Membranous Lupus Nephritis a Systematic Review and Network Meta-Analysis. Int J Rheum Dis. 2018;21(6):1163–1172. doi: 10.1111/1756-185X.13321. [DOI] [PubMed] [Google Scholar]
- [77].Lee YH, Song GG. Comparative Efficacy and Safety of Tacrolimus, Mycophenolate Mofetil, Azathioprine, and Cyclophosphamide as Maintenance Therapy for Lupus Nephritis: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Z Rheumatol. 2016;76(10):904–912. doi: 10.1007/s00393-016-0186-z. [DOI] [PubMed] [Google Scholar]
- [78].Mok CC. Mycophenolate Mofetil for Non-Renal Manifestations of Systemic Lupus Erythematosus: A Systematic Review. Scand J Rheumatol. 2007;36(5):329–337. doi: 10.1080/03009740701607042. [DOI] [PubMed] [Google Scholar]
- [79].Ordi-Ros J, Sáez-Comet L, Pérez-Conesa M. et al. Enteric-Coated Mycophenolate Sodium Versus Azathioprine in Patients With Active Systemic Lupus Erythematosus: A Randomised Clinical Trial. Ann Rheum Dis. 2017;76(9):1575–1582. doi: 10.1136/annrheumdis-2016-210882. [DOI] [PubMed] [Google Scholar]
- [80].Fernandes Moça Trevisani V, Castro AA, Ferreira Neves Neto J. et al. Cyclophosphamide Versus Methylprednisolone for Treating Neuropsychiatric Involvement in Systemic Lupus Erythematosus. Cochrane Database Syst Rev. 2013;(2):CD002265. doi: 10.1002/14651858.CD002265.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Blumenfeld Z, Mischari O, Schultz N. et al. Gonadotropin Releasing Hormone Agonists May Minimize Cyclophosphamide Associated Gonadotoxicity in SLE and Autoimmune Diseases. Semin Arthritis Rheum. 2011;41:346–352. doi: 10.1016/j.semarthrit.2011.05.008. [DOI] [PubMed] [Google Scholar]
- [82].Marder W, McCune WJ, Wang L. et al. Adjunctive GnRH-A Treatment Attenuates Depletion of Ovarian Reserve Associated With Cyclophosphamide Therapy in Premenopausal SLE Patients. Gynecol Endocrinol. 2012;28:624–627. doi: 10.3109/09513590.2011.650752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Heng C, Yuefeng R, Lin L. et al. The Efficacy and Safety of Leflunomide for the Treatment of Lupus Nephritis in Chinese Patients: Systematic Review and Meta-Analysis. PLos One. 2015;10(12):e0144548. doi: 10.1371/journal.pone.0144548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Zhang M, Qi C, Zha Y. et al. Leflunomide Versus Cyclophosphamide in the Induction Treatment of Proliferative Lupus Nephritis in Chinese Patients: A Randomized Trial. Clin Rheumatol. 2019;38(3):859–867. doi: 10.1007/s10067-018-4348-z. [DOI] [PubMed] [Google Scholar]
- [85].Wu GC, Xu XD, Huang Q. et al. Leflunomide: Friend or Foe for Systemic Lupus Erythematosus? Rheumatol Int. 2013;33(2):273–276. doi: 10.1007/s00296-012-2508-z. [DOI] [PubMed] [Google Scholar]
- [86].Andreoli L, Bertsias GK, Agmon-Levin N. et al. EULAR Recommendations for Women's Health and the Management of Family Planning, Assisted Reproduction, Pregnancy and Menopause in Patients With Systemic Lupus Erythematosus and/or Antiphospholipid Syndrome. Ann Rheum Dis. 2016;76(3):476–485. doi: 10.1136/annrheumdis-2016-209770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Sakthiswary R, Suresh E. Methotrexate in Systemic Lupus Erythematosus: A Systematic Review of Its Efficacy. Lupus. 2014;23(3):225–235. doi: 10.1177/0961203313519159. [DOI] [PubMed] [Google Scholar]
- [88].Faught LN, Greff MJ, Rieder MJ. et al. Drug-Induced Acute Kidney Injury in Children. Br J Clin Pharmacol. 2015;80(4):901–909. doi: 10.1111/bcp.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yap DY, Ma MK, Mok MM. et al. Long-Term Data on Tacrolimus Treatment in Lupus Nephritis. Rheumatology (Oxford) 2014;53(12):2232–2237. doi: 10.1093/rheumatology/keu265. [DOI] [PubMed] [Google Scholar]
- [90].Mok CC, To CH, Yu KL. et al. Combined Low-Dose Mycophenolate Mofetil and Tacrolimus for Lupus Nephritis With Suboptimal Response to Standard Therapy: A 12-Month Prospective Study. Lupus. 2013;22(11):1135–1141. doi: 10.1177/0961203313502864. [DOI] [PubMed] [Google Scholar]
- [91].Li Y, Feng X. Efficacy and Safety of Tacrolimus in Systemic Lupus Erythematosus Patients With Refractory Thrombocytopenia: A Retrospective Study. Lupus. 2018;27(1):60–65. doi: 10.1177/0961203317711011. [DOI] [PubMed] [Google Scholar]
- [92].Singh JA, Hossain A, Kotb A. et al. Risk of Serious Infections With Immunosuppressive Drugs and Glucocorticoids for Lupus Nephritis: A Systematic Review and Network Meta-Analysis. BMC Med. 2016;14(1):137. doi: 10.1186/s12916-016-0673-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Chen W, Tang X, Liu Q. et al. Short-Term Outcomes of Induction Therapy With Tacrolimus Versus Cyclophosphamide for Active Lupus Nephritis: A Multicenter Randomized Clinical Trial. Am J Kidney Dis. 2011;57(2):235–244. doi: 10.1053/j.ajkd.2010.08.036. [DOI] [PubMed] [Google Scholar]
- [94].Jesus D, Rodrigues M, Da Silva JAP. et al. Multitarget Therapy of Mycophenolate Mofetil and Cyclosporine A for Induction Treatment of Refractory Lupus Nephritis. Lupus. 2018;27(8):1358–1362. doi: 10.1177/0961203318758508. [DOI] [PubMed] [Google Scholar]
- [95].Yang TH, Wu TH, Chang YL. et al. Cyclosporine for the Treatment of Lupus Nephritis in Patients With Systemic Lupus Erythematosus. Clin Nephrol. 2018;89(4):277–285. doi: 10.5414/CN109325. [DOI] [PubMed] [Google Scholar]
- [96].Moroni G. A Randomized Pilot Trial Comparing Cyclosporine and Azathioprine for Maintenance Therapy in Diffuse Lupus Nephritis Over Four Years. Clin J Am Soc Nephrol. 2006;1(5):925–932. doi: 10.2215/CJN.02271205. [DOI] [PubMed] [Google Scholar]
- [97].Pego-Reigosa JM, Cobo-Ibáñez T, Calvo-Alén J. et al. Efficacy and Safety of Nonbiologic Immunosuppressants in the Treatment of Nonrenal Systemic Lupus Erythematosus: A Systematic Review. Arthritis Care Res. 2013;65(11):1775–1785. doi: 10.1002/acr.22035. [DOI] [PubMed] [Google Scholar]
- [98].Peterknecht E, Keasey MP, Beresford MW. The Effectiveness and Safety of Biological Therapeutics in Juvenile-Onset Systemic Lupus Erythematosus (JSLE): A Systematic Review. Lupus. 2018;27(13):2135–2145. doi: 10.1177/0961203318804879. [DOI] [PubMed] [Google Scholar]
- [99].Wei L, Liang Y, Zhao Y. et al. Efficacy and Safety of Belimumab Plus Standard Therapy in Patients With Systemic Lupus Erythematosus: A Meta-Analysis. Clin Ther. 2016;38(5):1134–1140. doi: 10.1016/j.clinthera.2016.02.022. [DOI] [PubMed] [Google Scholar]
- [100].Lee YH, Song GG. Comparative Efficacy and Safety of Intravenous or Subcutaneous Belimumab in Combination With Standard Therapy in Patients With Active Systemic Lupus Erythematosus: A Bayesian Network Meta-Analysis of Randomized Controlled Trials. Lupus. 2018;27(1):112–119. doi: 10.1177/0961203317713143. [DOI] [PubMed] [Google Scholar]
- [101].Alshaiki F, Obaid E, Almuallim A. et al. Outcomes of Rituximab Therapy in Refractory Lupus: A Meta-Analysis. Eur J Rheumatol. 2018;5(2):118–126. doi: 10.5152/eurjrheum.2018.17096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Writing Committee of Guidelines for the Diagnosis and Treatment of Lupus Nephritis in China. Guidelines for the Diagnosis and Treatment of Lupus Nephritis in China. Chinese J Med. 2019;99(11):3441–3455. doi: 10.3760/cma.j.isn.0376-2491.2019.44.001. [DOI] [Google Scholar]
- [103].Weening JJ, D’Agati VD, Schwartz MM. et al. The Classification of Glomerulonephritis in Systemic Lupus Erythematosus Revisited. Kidney Int. 2004;65(2):521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- [104].Bajema IM, Wilhelmus S, Alpers CE. et al. Revision of the International Society of Nephrology/Renal Pathology Society Classification for Lupus Nephritis: Clarification of Definitions, and Modified National Institutes of Health Activity and Chronicity Indices. Kidney Int. 2018;93(4):789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- [105].Collado MV, Dorado E, Rausch S. et al. Long-Term Outcome of Lupus Nephritis Class II in Argentine Patients. An Open Retrospective Analysis. J Clin Rheumatol. 2016;22(6):299–306. doi: 10.1097/RHU.0000000000000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lee SG, Cho YM, So MW. et al. ISN/RPS 2003 Class II Mesangial Proliferative Lupus Nephritis: A Comparison Between Cases That Progressed to Class III or IV and Cases That Did Not. Rheumatol Int. 2012;32(8):2459–2464. doi: 10.1007/s00296-011-1986-8. [DOI] [PubMed] [Google Scholar]
- [107].Deng J, Xie H, Zhu L. et al. Maintenance Therapy for Lupus Nephritis With Mycophenolate Mofetil or Azathioprine. A Meta-analysis. Clin Nephrol. 2019;91(3):172–179. doi: 10.5414/CN109450. [DOI] [PubMed] [Google Scholar]
- [108].Hladunewich MA, Troyanov S, Calafati J. et al. The Natural History of the Non-Nephrotic Membranous Nephropathy Patient. Clin J Am Soc Nephrol. 2009;4(9):1417–1422. doi: 10.2215/CJN.01330209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].West SG, Emlen W, Wener MH. et al. Neuropsychiatric Lupus Erythematosus: A 10-Year Prospective Study on the Value of Diagnostic Tests. Am J Med. 1995;99(2):153–163. doi: 10.1016/s0002-9343(99)80135-1. [DOI] [PubMed] [Google Scholar]
- [110].Ainiala H, Dastidar P, Loukkola J. et al. Cerebral MRI Abnormalities and Their Association With Neuropsychiatric Manifestations in SLE: A Population-Based Study. Scand J Rheumatol. 2005;34(5):376–382. doi: 10.1080/03009740510026643. [DOI] [PubMed] [Google Scholar]
- [111].Gomard-Mennesson E, Ruivard M, Koenig M. et al. Treatment of Isolated Severe Immune Hemolytic Anaemia Associated With Systemic Lupus Erythematosus: 26 Cases. Lupus. 2006;15(4):223–231. doi: 10.1191/0961203306lu2292oa. [DOI] [PubMed] [Google Scholar]
- [112].Arnal C, Piette JC, Léone J. et al. Treatment of Severe Immune Thrombocytopenia Associated With Systemic Lupus Erythematosus: 59 Cases. J Rheumatol. 2002;29(1):75–83. doi: 10.0000/PMID11824975. [DOI] [PubMed] [Google Scholar]
- [113].Alba P, Karim M, Hunt B. Mycophenolate Mofetil as a Treatment for Autoimmune Haemolytic Anaemia in Patients With Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Lupus. 2003;12(8):633–635. doi: 10.1191/0961203303lu419cr. [DOI] [PubMed] [Google Scholar]
- [114].Chen H, Zheng W, Su J. et al. Low-Dose Rituximab Therapy for Refractory Thrombocytopenia in Patients With Systemic Lupus Erythematosus—A Prospective Pilot Study. Rheumatology. 2011;50(9):1640–1644. doi: 10.1093/rheumatology/ker176. [DOI] [PubMed] [Google Scholar]
- [115].Abdwani R, Mani R. Anti-CD20 Monoclonal Antibody in Acute Life Threatening Haemolytic Anaemia Complicating Childhood Onset SLE. Lupus. 2009;18(5):460–464. doi: 10.1177/0961203308098360. [DOI] [PubMed] [Google Scholar]
- [116].Al-Omary HL, Alawad ZM, Bernieh B. Anti CD20 Monoclonal Antibody (Rituximab) as a Rescue Treatment in Severe and Refractory SLE. Biomed Pharmacol J. 2018;11(1):453. doi: 10.13005/bpj/1394. [DOI] [Google Scholar]
- [117].Expert Committee on Blood Purification of Pediatricians of Chinese Medical Doctor Association. Blood Purification Therapy for Childhood-Onset Severe Systemic Lupus Erythematosus: A Multi-Center Epidemiological Investigation. Chinese J Pract Pediatr. 2018;33(7):521–527. doi: 10.19538/j.ek2018070610. [DOI] [Google Scholar]
- [118].Shen Qi, Huang Xiangui, Wu Xiaoqiu. et al. Systematic Review on Plasma Exchange for Treating Proliferative Lupus Nephritis. J Pract Med. 2013;29(1):122–125. doi: 10.3969/j.issn.1006-5725.2013.01.054. [DOI] [Google Scholar]
- [119].Kronbichler A, Brezina B, Quintana LF. et al. Efficacy of Plasma Exchange and Immunoadsorption in Systemic Lupus Erythematosus and Antiphospholipid Syndrome: A Systematic Review. Autoimmun Rev. 2015;15(1):38–49. doi: 10.1016/j.autrev.2015.08.010. [DOI] [PubMed] [Google Scholar]
- [120].Francioni C, Galeazzi M, Fioravanti A. et al. Long-Term I.V. IG Treatment in Systemic Lupus Erythematosus. Clin Exp Rheumatol. 1994;12(2):163–168. [PubMed] [Google Scholar]
- [121].Nieto-Aristizábal I, Martínez T, Urbano MA. et al. Treatment With Intravenous Immunoglobulins in Systemic Lupus Erythematosus: A Single-Center Experience With 63 Patients. Lupus. 2019;28(13):1566–1570. doi: 10.1177/0961203319883680. [DOI] [PubMed] [Google Scholar]
- [122].Ye Y, Chen B, Kalish RA. et al. Tripterygium wilfordii for the Treatment of Systemic Lupus Systematosus: Meta-Analysis of Randomized Controlled Trials. Arthritis Rheumatol. 2015;67(Suppl 10):1824. [Google Scholar]
- [123].Sun Feng, Yang Xinghua, Ma Dongmei. et al. Meta-Analysis of the Incidence of Reproductive Toxicity in Triptolide Users. Pharmacovigilance in China. 2014;11(2):94–103. [Google Scholar]
- [124].Fei Y, Shi X, Gan F. et al. Death Causes and Pathogens Analysis of Systemic Lupus Erythematosus During the Past 26 Years. Clin Rheumatol. 2013;33(1):57–63. doi: 10.1007/s10067-013-2383-3. [DOI] [PubMed] [Google Scholar]
- [125].Sun Xin, Xu Lili, Deng Yanhong. et al. The Risk Factors of Infections in Patients With Systemic Lupus Erythematosus. Chin J Nurs. 2015;50(7):828–835. doi: 10.3761/j.isn.0254-1769.2015.07.013. [DOI] [Google Scholar]
- [126].Firooz N, Albert D, Wallace D. et al. High-Sensitivity C-Reactive Protein and Erythrocyte Sedimentation Rate in Systemic Lupus Erythematosus. Lupus. 2011;20(6):588–597. doi: 10.1177/0961203310393378. [DOI] [PubMed] [Google Scholar]
- [127].Serio I, Arnaud L, Mathian A. et al. Can Procalcitonin Be Used to Distinguish Between Disease Relapse and Infection in Patients With Systemic Lupus Erythematosus: A Systematic Literature Review. Clin Rheumatol. 2014;33(9):1209–1215. doi: 10.1007/s10067-014-2738-4. [DOI] [PubMed] [Google Scholar]
- [128].Ng WL, Chu CM, Wu AK. et al. Lymphopenia at Presentation Is Associated With Increased Risk of Infections in Patients With Systemic Lupus Erythematosus. QJM. 2005;99(1):37–47. doi: 10.1093/qjmed/hci155. [DOI] [PubMed] [Google Scholar]
- [129].Yang MJ, Chen CY, Chang WH. et al. Pregnancy Outcome of Systemic Lupus Erythematosus in Relation to Lupus Activity Before and During Pregnancy. J Chin Med Assoc. 2015;78(4):235–240. doi: 10.1016/j.jcma.2014.11.008. [DOI] [PubMed] [Google Scholar]
- [130].Knight CL, Nelson-Piercy C. Management of Systemic Lupus Erythematosus During Pregnancy: Challenges and Solutions. Open Access Rheuma. 2017;9:37. doi: 10.2147/OARRR.S87828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ren Shuxia, Liu Enling, Yang Yan. Meta-Analysis of Pregnancy Outcomes in Patients With Lupus Nephritis. J North China University Technol (Medical Edition) 2018;20(2):133–139. doi: 10.19539/j.cnki.2095-2694.2018.02.011. [DOI] [Google Scholar]
- [132].Chen S, Sun X, Wu B. et al. Pregnancy in Women With Systemic Lupus Erythematosus: A Retrospective Study of 83 Pregnancies at a Single Centre. Int J Environ Res Public Health. 2015;12(8):9876–9888. doi: 10.3390/ijerph120809876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wei Q, Ouyang Y, Zeng W. et al. Pregnancy Complicating Systemic Lupus Erythematosus: A Series of 86 Cases. Arch Gynecol Obstet. 2011;284(5):1067–1071. doi: 10.1007/s00404-010-1786-5. [DOI] [PubMed] [Google Scholar]
- [134].Lazzaroni MG, Dall’Ara F, Fredi M. et al. A Comprehensive Review of the Clinical Approach to Pregnancy and Systemic Lupus Erythematosus. J Autoimmun. 2016;74:106–117. doi: 10.1016/j.jaut.2016.06.016. [DOI] [PubMed] [Google Scholar]
- [135].Wu J, Ma J, Bao C. et al. Pregnancy Outcomes Among Chinese Women With and Without Systemic Lupus Erythematosus: A Retrospective Cohort Study. BMJ Openi. 2018;8(4):e020909. doi: 10.1136/bmjopen-2017-020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Borella E, Lojacono A, Gatto M. et al. Predictors of Maternal and Fetal Complications in SLE Patients: A Prospective Study. Immunol Res. 2014;60(2–3):170–176. doi: 10.1007/s12026-014-8572-6. [DOI] [PubMed] [Google Scholar]
- [137].Aly EAH, Riyad RM, Mokbel AN. Pregnancy Outcome in Patients With Systemic Lupus Erythematosus: A Single Center Study in the High Risk Pregnancy Unit. Middle East Fertility Society J. 2016;21(3):168–174. doi: 10.1016/j.mefs.2015.12.003. [DOI] [Google Scholar]
- [138].Chinese Systemic Lupus Erythematosus Treatment and Research Group, Chinese Rheumatism Data Center. Perinatal Management Recommendations for Chinese Systemic Lupus Erythematosus Patients. Chinese Med J. 2015;95(14):1056–1060. doi: 10.3760/cma.j.isn.0376-2491.2015.14.005. [DOI] [Google Scholar]
- [139].McDonald EG, Bissonette L, Ensworth S. et al. Monitoring of Systemic Lupus Erythematosus Pregnancies: A Systematic Literature Review. J Rheumatol. 2018;45(10):1477–1490. doi: 10.3899/jrheum.171023. [DOI] [PubMed] [Google Scholar]
- [140].Bundhun PK, Soogund MZS, Huang F. Impact of Systemic Lupus Erythematosus on Maternal and Fetal Outcomes Following Pregnancy: A Meta-Analysis of Studies Published Between Years 2001–2016. J Autoimmun. 2017;79:17–27. doi: 10.1016/j.jaut.2017.02.009. [DOI] [PubMed] [Google Scholar]
- [141].Yengej FAY, van Royen-Kerkhof A, Derksen RHWM. et al. The Development of Offspring From Mothers With Systemic Lupus Erythematosus. A Systematic Review. Autoimmun Rev. 2017;16(7):701–711. doi: 10.1016/j.autrev.2017.05.005. [DOI] [PubMed] [Google Scholar]
- [142].Guillotin V, Bouhet A, Barnetche T. et al. Hydroxychloroquine for the Prevention of Fetal Growth Restriction and Prematurity in Lupus Pregnancy: A Systematic Review and Meta-Analysis. Joint Bone Spine. 2018;85(6):663–668. doi: 10.1016/j.jbspin.2018.03.006. [DOI] [PubMed] [Google Scholar]
- [143].Liu Yashu, Cao Di, Fang Sheng. et al. Efficacy and Safety of Hydroxychloroquine in Treatment of Pregnancy Complicating Lupus Erythematosus: A Meta Analysis. Chongqing Med. 2016;45(13):1803–1806. doi: 10.3969/j.issn.1671-8348.2016.13.026. [DOI] [Google Scholar]
- [144].Eudy AM, Siega-Riz AM, Engel SM. et al. Effect of Pregnancy on Disease Relapses in Patients With Systemic Lupus Erythematosus. Ann Rheum Dis. 2018;77(6):855–860. doi: 10.1136/annrheumdis-2017-212535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Saavedra MÁ, Sánchez A, Morales S. et al. Azathioprine During Pregnancy in Systemic Lupus Erythematosus Patients Is Not Associated With Poor Fetal Outcome. Clin Rheumatol. 2015;34(7):1211–1216. doi: 10.1007/s10067-015-2987-x. [DOI] [PubMed] [Google Scholar]
- [146].Wallace DJ, Weisman MH. The Role of Environmental Factors in Rheumatic Diseases. B Rheum Dis. 2002;51(10):1–4. [Google Scholar]
- [147].Zandman-Goddard G, Solomon M, Rosman Z. et al. Environment and Lupus-Related Diseases. Lupus. 2012;21(3):241–250. doi: 10.1177/0961203311426568. [DOI] [PubMed] [Google Scholar]
- [148].Sun Y, Tian L. Eyebrow Threading-Induced Tumid Lupus Erythematosus. J Am Acad Dermatol. 2019;81(4):AB210. [Google Scholar]
- [149].Smyk D, Rigopoulou EI, Bizzaro N. et al. Hair Dyes as a Risk for Autoimmunity: From Systemic Lupus Erythematosus to Primary Biliary Cirrhosis. Auto Immun Highlights. 2013;4(1):1–9. doi: 10.1007/s13317-011-0027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Zahn S, Graef M, Patsinakidis N. et al. Ultraviolet Light Protection by a Sunscreen Prevents Interferon-Driven Skin Inflammation in Cutaneous Lupus Erythematosus. Exp Dermatol. 2014;23(7):516–518. doi: 10.1111/exd.12428. [DOI] [PubMed] [Google Scholar]
- [151].Kuhn A, Gensch K, Haust M. et al. Photoprotective Effects of a Broad-Spectrum Sunscreen in Ultraviolet-Induced Cutaneous Lupus Erythematosus: A Randomized, Vehicle-Controlled, Double-Blind Study. J Am Acad Dermatol. 2011;64(1):37–48. doi: 10.1016/j.jaad.2009.12.053. [DOI] [PubMed] [Google Scholar]
- [152].Abdul Kadir WD, Jamil A, Shaharir SS. et al. Photoprotection Awareness and Practices Among Patients With Systemic Lupus Erythematosus and Its Association With Disease Activity and Severity. Lupus. 2018;27(8):1287–1295. doi: 10.1177/0961203318770016. [DOI] [PubMed] [Google Scholar]
- [153].O’Dwyer T, Durcan L, Wilson F. Exercise and Physical Activity in Systemic Lupus Erythematosus: A Systematic Review With Meta-Analyses. Semin Arthritis Rheum. 2017;47(2):204–215. doi: 10.1016/j.semarthrit.2017.04.003. [DOI] [PubMed] [Google Scholar]
- [154].Wu ML, Yu KH, Tsai JC. The Effectiveness of Exercise in Adults With Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis to Guide Evidence-Based Practice. Worldviews Evid Based Nurs. 2017;14(4):306–315. doi: 10.1111/wvn.12221. [DOI] [PubMed] [Google Scholar]
- [155].Parisis D, Bernier C, Chasset F. et al. Impact of Tobacco Smoking Upon Disease Risk, Activity and Therapeutic Response in Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Autoimmun Rev. 2019;18(11) doi: 10.1016/j.autrev.2019.102393. 102393. [DOI] [PubMed] [Google Scholar]
- [156].Ghaussy NO, Sibbitt W, Bankhurst AD. et al. Cigarette Smoking and Disease Activity in Systemic Lupus Erythematosus. J Rheumatol. 2003;30(6):1215–1221. doi: 10.1016/S1297-319X(03)00040-X. [DOI] [PubMed] [Google Scholar]
- [157].Sousa JR, Rosa ÉPC, Nunes IFOC. et al. Effect of Vitamin D Supplementation on Patients With Systemic Lupus Erythematosus: A Systematic Review. Rev Bras Reumatol Engl Ed. 2017;57(5):466–471. doi: 10.1016/j.rbre.2017.08.001. [DOI] [PubMed] [Google Scholar]
- [158].de Medeiros MCS, Medeiros J, de Medeiros HJ. et al. Dietary Intervention and Health in Patients With Systemic Lupus Erythematosus: A Systematic Review of the Evidence. Crit Rev Food Sci Nutr. 2019;59(16):2666–2673. doi: 10.1080/10408398.2018.1463966. [DOI] [PubMed] [Google Scholar]
- [159].Zhang J, Wei W, Wang C. Effects of Psychological Interventions for Patients With Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Lupus. 2012;21(10):1077–1087. doi: 10.1177/0961203312447667. [DOI] [PubMed] [Google Scholar]