Abstract
Rheumatoid arthritis (RA) remains a chronic debilitating disease with a significant negative societal impact, despite the expanding landscape of treatment options. This condition is often preceded by a phase of systemic autoimmunity with circulating autoantibodies, elevated pro-inflammatory cytokines, or subtle structural changes. The capability of identifying individuals in the preclinical phase of RA disease makes a “preventive window of opportunity” possible. Much recent work has focused on the role of imaging modalities including ultrasound (US), magnetic resonance imaging (MRI), and high-resolution peripheral quantitative computer tomography (HR-pQCT) in identifying at-risk individuals with or without early joint symptoms for the development of inflammatory arthritis. This article will review the evidence and discuss the challenges as well as opportunities of proactive risk assessment by imaging in RA.
Keywords: rheumatoid arthritis, ultrasound, magnetic resonance imaging, high-resolution peripheral quantitative computer tomography
Introduction
Rheumatoid arthritis (RA) is a common chronic systemic inflammatory condition characterized by persistent synovitis and bone erosions. The uncontrolled disease can lead to joint destruction, functional disability, decreased quality of life, cardiopulmonary complications, and a shortened lifespan.[1, 2, 3, 4, 5, 6] The outcomes of patients with RA have been revolutionized by early diagnosis and aggressive treatment strategy based on the treat-to-target approach.[7, 8] However, RA remains a lifelong incurable disease associated with the burden of long-term therapy and debilitating disease flares for most patients. It also carries substantial socioeconomic costs.[9] Currently, therapy aims to achieve clinical remission.[10] With the development of effective targeted therapies, future ambitions will be either to prevent RA or to achieve drug-free remission, effectively a cure. All these are only possible if we can identify the robust predictors of progressive disease in at-risk individuals and intervene early.
Synovitis and bone loss are the hallmarks of RA. They are crucial in the pathogenesis, diagnosis, and prognosis of the disease. It is traditionally believed that synovitis promotes the release of pro-inflammatory cytokines, which subsequently activate osteoclasts and enhance bone resorption at vulnerable anatomical sites leading to bone loss and thus joint damage.[11] This concept has been challenged by recent findings that bone changes or tendinitis could occur very early in the course of RA, even in the preclinical phases of the disease. All these abnormalities can now be detected by sensitive imaging techniques, namely, ultrasound (US), magnetic resonance imaging (MRI), and high-resolution peripheral quantitative CT (HR-pQCT). US can be regarded as an extension of the clinical examination in real-time, whereas the primary advantage of MRI is the possibility to visualize bone marrow abnormality. They both have no ionizing radiation and can be used during pregnancy. While MRI is limited by its long examination time and high cost, the main drawback of US is its operator dependency.[12] HR-pQCT is a novel three-dimensional (3D) imaging technique for detailed bone microstructure analysis. With an isotropic voxel size of 61 or 82 mm, it is capable of offering high-resolution imaging (100 or 142 mm, respectively) at the peripheral sites.[13] It was originally designed to assess volumetric bone mineral density (vBMD) and microarchitectural abnormalities in the distal tibia and radius. In the past decade, HR-pQCT has been increasingly applied to study local anabolic (e.g., osteophytes and enthesiophytes) and catabolic (e.g., erosions) bone changes and joint space parameters, mainly in the metacarpophalangeal (MCP) joints in patients with arthritis. In patients with RA, it exhibited higher sensitivity compared with other imaging modalities and has been regarded as the gold standard for detecting bone erosions (Figure 1).[14] The juxta- and intraarticular vBMD and microarchitectural abnormalities in RA can also be ascertained by HR-pQCT.[15, 16] Unfortunately, only extremities can be scanned at the moment, due to the limitation of the gantry size.
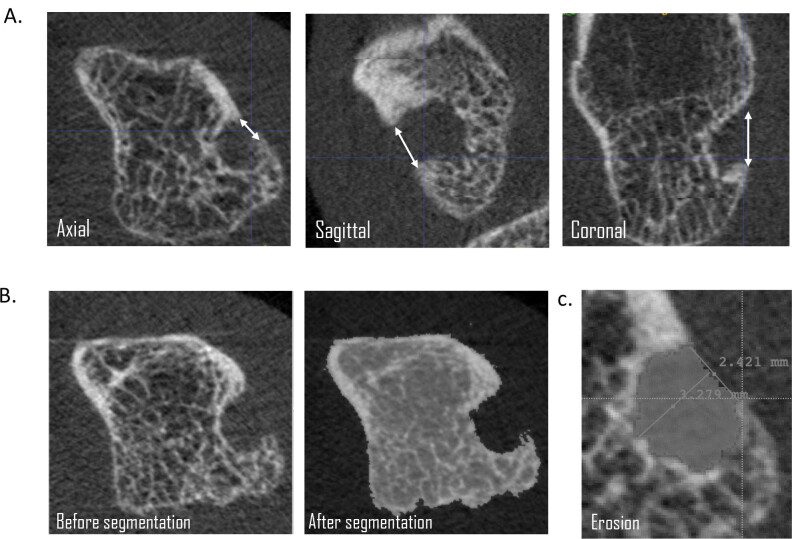
Figure 1.
Example of erosion identification and quantification on HR-pQCT. (A) Identifying erosion in the axial, sagittal, and coronal planes; (B) Example of segmentation of remaining bone; and (C) Erosion area quantified. HR-pQCT, high-resolution peripheral quantitative computer tomography.
The potential to identify the subclinical features, which are predictors for the future development of RA by imaging, raises the opportunity to prevent disease development or progression in these individuals. Studying these early structural changes could also improve our understanding of the pathogenic mechanism of inflammatory arthritis. In this review, we aim to summarize and discuss the recent literature, covering the use of US, MRI, and HR-pQCT in predicting the development of RA in at-risk individuals.
Methods
Articles included in this review were searched using the PubMed platform. Full-text English-language article searches were conducted using combinations of items, including “ultrasound,” “MRI,” “Magnetic resonance imaging,” “computed tomography,” “high-resolution peripheral quantitative CT,” “rheumatoid arthritis,” “predict,” and “prediction.” The search results were supplemented by reference citations from notable reviews on this topic. The search strategy was done till 31 August 2020. A narrative review of findings from the literature search was performed without any statistical analysis. Table 1 summarizes the sample size, follow-up duration, patient characteristics, and main results of the studies identified.
Table 1.
Summary of evidence on various imaging modalities in predicting the development of inflammatory arthritis
| First author | Sample size | Duration of follow-up | Subjects recruited | Main results | |
|---|---|---|---|---|---|
| US | Nam JL18 | 136 | Median 18.3 months | Musculoskeletal symptoms, ACPA positive, no clinical synovitis | Doppler signal and erosion over hand and foot joints associated with development of inflammatory arthritis |
| Van Beers-Tas MH19 | 163 | Median 12 months | Arthralgia, RF or ACPA positive, no clinical arthritis | Synovial thickening of hand joints associated with development of clinical arthritis | |
| Filer A20 | 58 | 18 months | Clinical synovitis at least one joint, symptom duration </= 3 months | Synovial thickening of wrists and MCPJ, and power Doppler signal of MTPJ predictive of RA | |
| Sahbudin I21 | 107 | 18 months | Clinical synovitis at least one joint, symptom duration </= 3 months | Tenosynovitis of digit flexor predictive of RA | |
| Zufferey P22 | 80 | Mean 18 months | Polyarthralgia, no RF or ACPA, no clinical synovitis | Synovial thickening of hands, elbows and knees predictive of RA | |
| Di Matteo A23 | 419 | Median 41.4 months | Musculoskeletal symptoms, ACPA positive, no clinical synovitis | Bone erosion in > 1 hand or foot joints, and bone erosion with synovitis in foot joints predictive of inflammatory arthritis | |
| MRI | Tamai M28 | 129 | 12 months | Undifferentiated arthritis | Synovitis and bone marrow edema or erosion over hand joints in conjunction of autoantibodies predictive of RA |
| Ji L29 | 31 | Median 15 months | Undifferentiated arthritis | Synovitis and bone erosion in writs associated with the development of RA | |
| Van Steenbergen HW30 | 150 | Median 6.3 months | Arthralgia of small joints <1 year, no clinical arthritis, suspected to progress to RA by rheumatologists | MRI inflammation score (sum of synovitis, bone marrow edema and tenosynovitis) over hands and feet predictive of inflammatory arthritis | |
| Wouters F32 | 490 | Progressors: median 1.2 months, Non-progressors: median 8.6 months | Arthralgia of small joints <1 year, no clinical arthritis, suspected to progress to RA by rheumatologists | Bone erosion in hands and feet associated with development of inflammatory arthritis, but not after adjustments for age and MRI inflammation | |
| HR-pQCT | Kleyer A35 | 15 patients vs 15 controls | Cross-sectional study | Patients: ACPA positive, no signs of arthritis Controls: ACPA negative, healthy |
Reduced bone mineral density and worse bone micro-architecture over metacarpal heads in patients |
| Keller KK37 | 22 patients vs 23 controls | 12 months | Patients: arthralgia, ACPA positive, no rheumatic disease Controls: ACPA negative, healthy |
Increased number and size of erosion over metacarpal heads in patients | |
| Simon D38 | 74 | 30 months | ACPA or anti-MCV positive, no signs of joint swelling | Cortical micro-channels over metacarpal heads associated with development of RA |
US: ultrasound, ACPA: anti-cyclic citrullinated peptide antibodies, RF: rheumatoid factor, MCPJ: metacarpophalangeal joint, MTPJ: metatarsophalangeal joint, RA: rheumatoid arthritis, MRI: magnetic resonance imaging, HR-pQCT: high-resolution peripheral quantitative CT, MCV: mutated citrullinated vimentin
Ultrasound
US can sensitively detect RA changes such as early bone erosions, subclinical synovitis (manifested as synovial thickening and/or abnormal power Doppler signal), and tenosynovitis.[17] In a cohort of 136 anti-cyclic citrullinated peptide antibody (ACPA)-positive individuals with musculoskeletal symptoms but no clinical synovitis, the presence of intraarticular power Doppler signal and erosion on US over any of the 32 joints (wrists, MCP joints, proximal interphalangeal joints, and metatarsophalangeal [MTP] joints) was strongly (both P < 0.001) associated with the development of inflammatory arthritis after a median follow-up of 18.3 months.[18] In another seropositive arthralgia cohort (n = 163) with a median follow-up of 12 months, baseline synovial thickening was detected in 30% of subjects in at least one joint (bilateral wrists, MCP joints 2/3, proximal interphalangeal 2/3, and MTP joints 2/3/5), and its occurrence over the finger joints was associated with the development of inflammatory arthritis.[19] Besides, synovial thickening over hand joints on US demonstrated added value to the prediction rule score based on clinical parameters alone, especially in the intermediate and high-risk groups. However, power Doppler signal was rarely identified (4%) and bone erosion was not mentioned in this study. In a study of 58 patients with very early onset (</= 3 months) synovitis, baseline US-defined synovial thickening over wrists and MCP joints, and Doppler signals over MTP joints were independent predictors for the development of RA after 18 months.[20] US changes (synovial thickening, power Doppler positivity, and erosion) in large joints and proximal interphalangeal joints as well as erosions had poor predictive value. In another US study (n = 107) of a similar population of patients with early synovitis not yet fulfilling the classification criteria and same follow-up duration, tenosynovitis over the digit flexor provided independent predictive value for the development of RA on top of the presence of ACPA and US-defined synovitis.[21] In a cohort of 80 arthralgic patients without any autoantibodies, US-detected synovial thickening was also shown to be the only predictor of evolution to RA, among other clinical variables including inflammatory markers, after a mean follow-up of 18 months.[22] Of note, a recent study in ACPA-positive patients with arthralgia but no clinical synovitis revealed that bone erosion on US was predictive of the development into inflammatory arthritis.[23] This is by far the largest study (n = 400) with the longest follow-up duration (median 41.4 months). The most intriguing findings were that the prevalence of bone erosion was significantly higher in the 5th MTP joints than in the MCP joints, and the presence of bone erosion in more than one joint was the strongest imaging predictor (odd ratio = 10.6) for the development of inflammatory arthritis. To conclude, US-defined synovial thickening, power Doppler signal, tenosynovitis, and bone erosion over peripheral joints appear to have predictive value for inflammatory arthritis.
On the contrary, there are some important considerations to be borne in mind before indiscriminate use of US in at-risk populations. First, the subclinical inflammation detectable by US might be a late feature in the development of inflammatory arthritis. Serial US assessments in a cohort of ACPA-positive at-risk individuals showed that synovial thickening or Doppler signal developed just directly before the occurrence of clinical synovitis.[24] It was hypothesized that there was a late increase in inflammatory burden before the development of arthritis as a result of a “second hit” immunogenic trigger in the at-risk individuals after a period of stability. The narrow window between the detection of US abnormalities and clinical arthritis might not allow any meaningful intervention. Second, US acquisition protocol, definitions of pathology, and scoring systems varied among studies and centers. Therefore, unified internationally recognized scoring systems should be used, such as the one endorsed by the European League Against Rheumatism (EULAR)/Outcome Measures in Rheumatology Clinical Trials (OMERACT).[25, 26] Lastly, it is also not clear which and how many joints need to be imaged for optimum predictive accuracy. Comprehensive US protocols which include most joints could take up to 60 min and may not be practical in most clinical settings.[20]
MRI
MRI can detect subclinical inflammation and bone erosion, which are indicative of RA.[27] In an early study of 129 patients with undifferentiated arthritis as determined by rheumatologists, contrast MRI-proven synovitis and bone edema or erosion over hand joints in conjunction with autoantibodies were found to be useful in predicting progression to RA at 1 year.[28] The positive predictive value of bone edema plus ACPA positivity was 100%. In a smaller study (n = 31) on a similar population of undifferentiated arthritis with a median follow-up of 15 months, wrist synovitis and erosions were associated with the final diagnosis of RA.[29] In another study of 150 patients with recent-onset arthralgia clinically suspected to progress to RA over time as judged by rheumatologists (clinically suspect arthralgia), MRI inflammation over hands and feet as reflected by synovitis, bone marrow edema, and tenosynovitis was independently associated with arthritis development after a median follow-up of 6.3 months.[30] It was subsequently found that adding feet to hands MRI did not increase the accuracy of predicting arthritis development in patients with arthralgia.[31] Compared with subclinical inflammation, the clinical value of MRI-detected bone erosions might be more doubtful. In a large cohort of patients with joint pain but no clinically overt arthritis (n = 490), although MRI erosion scores were higher in ACPA-positive than negative patients and were correlated with subclinical inflammation, they were deemed not independently predictive of inflammatory arthritis development.[32] Erosion scores were associated with arthritis development, but not after adjustments for age and subclinical inflammation. In sum, synovitis, tenosynovitis, and bone marrow edema over the hands detected by MRI could predict the development of inflammatory arthritis.
Due to the relatively long scanning time, limited access, and lack of specificity, the use of MRI is generally recommended only in difficult patient cases at least for the management of early arthritis.[33] It is noteworthy that the commonly used MRI scoring system, OMERACT RA magnetic resonance imaging scoring (RAMRIS) system, was not developed for diagnostic purposes, but for outcome measures in clinical trials.[34]
HR-pQCT
HR-pQCT studies on individuals with or without joint symptoms before the diagnosis of RA are scanty. In a cross-sectional study, asymptomatic ACPA-positive individuals (n = 15) had reduced bone mineral density and worsened microarchitecture over the metacarpal heads compared with ACPA-negative healthy controls (n = 15) on HR-pQCT.[35] Although no major difference between the two groups regarding the number and size of bone erosions could be shown, intraarticular bone loss appeared to occur in the preclinical phases of RA as reflected by the impaired microarchitecture in the ACPA-positive individuals. In a longitudinal case-control study, although the baseline number and size of erosions over metacarpal heads on HR-pQCT in ACPA-positive patients with arthralgia (n = 29) were similar to the healthy controls (n = 29), both parameters worsened only in the patient group after 1 year.[36, 37] Out of the 22 patients with long-term follow-up, 10 developed RA (RA progressors) after 1 year. In the latest study, 74 autoantibodies-positive subjects without clinically obvious joint swelling were followed for 30 months.[38] It was found that RA progressors had significantly more cortical microchannels, defined as channels connecting the periosteal to the endosteal region in the bare area of the joint, and lower bone volumes over the metacarpal heads. These observations raise the possibility that inflammatory lesions in RA might affect the bone marrow first rather than the synovial membrane. It is thus probable that detection of subtle bone changes might offer the best chance of identifying individuals at risk of developing RA. Unfortunately, HR-pQCT is only available in some research centers. Besides, due to the very high resolution, reading and interpretation of a large number of images can be labor-intensive. It is also difficult to distinguish minor abnormalities and physiological changes, for example, small erosions and vascular channels. Adoption of automatic techniques or machine learning approaches should be the future direction.
Comparison of Different Imaging Modalities
There is only one study using two imaging modalities to predict the development of RA. Kleyer et al reported the baseline HR-pQCT/MRI findings of the hands and clinical follow-up results of 20 ACPA-positive asymptomatic subjects.[39] Although they were more likely to have erosions both on HR-pQCT and MRI when compared with controls who were ACPA-negative, only tenosynovitis on MRI was associated with later development of RA. It might be possible that the assessment of MTP joints is the more sensitive site for detecting preclinical bone erosions as shown in the recent large US study mentioned above.[23] Unfortunately, HR-pQCT study focusing on bone erosions in the feet of patients with RA or at-risk individuals is not identified in the literature. A comparison of the advantages and disadvantages of the three imaging modalities is shown in Table 2.
Table 2.
Comparison of ultrasound (US), magnetic resonance imaging (MRI) and high-resolution peripheral quantitative CT (HR-pQCT): advantages and disadvantages
| US | MRI | HR-pQCT | |
|---|---|---|---|
| Advantages | Can visualizes structures in real-time | Can visualizes bone marrow edema | Very high resolution (<142 μm) |
| No ionizing radiation | No ionizing radiation | High sensitivity for bone changes | |
| Relative accessible and inexpensive | Can be used in pregnancy | Can assess bone density and micro-architectural changes | |
| Patient friendly | Comparison of sequential images relatively easy | Comparison of sequential images relatively easy | |
| Can be used in pregnancy | Short scan time (2.8 minutes to acquire an axial 9.02 mm section) | ||
| No contrast agent required | |||
| Disadvantages | Operator dependent | Long examination time | Radiation involved (up to 24μSv, which is 1/5 of a conventional chest X-ray) |
| Cannot penetrate bone | Relatively higher cost and lower availability | Limited availability | |
| Poor resolution for deep seated joints | Potential adverse events when administration of contrast agent | Cannot visualize soft tissue structures | |
| Presence of contra-indications, eg: claustrophobia, certain metallic implants, contrast agent allergy | Cannot assess joints proximal to elbows and knees | ||
| Limited field of view (e.g. metacarpophalangeal joints 2–4 only) | |||
| Contra-indicated in pregnancy |
Future Perspectives
A major recent advance in RA research has been the better understanding of the preclinical phase of the disease. This refers to a period where patients are “at-risk” of developing RA but have no clinical synovitis. The potential to identify these patients opens up a window of opportunity to prevent disease progression or even development. To this end, clinical trials have shown that immunomodulatory therapy in patients with undifferentiated arthritis is effective in delaying or preventing progression to classifiable RA.[40, 41] In seropositive arthralgic subjects who had no baseline clinical synovitis, the use of rituximab has also been shown to delay the onset of arthritis.[42] Detection of early inflammatory or structural changes by the imaging modalities discussed above could better identify at-risk individuals. Bone abnormalities over the MTP5 joints might be of particular future research interest. Although often clinically overlooked, MTP5 joint has been found to erode more and earlier compared with the joints of the hands on radiographs.[43] US-detected bone erosions over the MTP5 joint were also noted to be both specific and sensitive for RA.[44] A high-resolution imaging examining this area for changes could be used to risk-stratify individuals presented with joint symptoms. 3D US technology is reported to be more sensitive than conventional US, while the second-generation HR-pQCT can offer even higher image resolution and allow feet scanning.[45] The ability to identify the earliest abnormalities is of paramount importance for the implementation of any prompt, appropriate, and cost-effective targeted treatments aiming at preventing joint damage or even RA disease from occurring. Further detailed imaging may also be provocative for mechanistic researches in RA to better understand how systemic autoimmunity ultimately translates into an inflammatory joint disease. With the ever-advancing musculoskeletal imaging technology and targeted pharmacological treatments, the two “holy grails” of RA management—disease prevention and cure—may not be out-of-reach.
Footnotes
Conflict of Interest
None Declared.
References
- [1].Young A, Dixey J, Cox N. et al. How does Functional Disability in Early Rheumatoid Arthritis (RA) Affect Patients and their Lives? Results of 5 Years of Follow-Up in 732 Patients from the Early RA Study (ERAS) Rheumatology. 2000;39:60311. doi: 10.1093/rheumatology/39.6.603. [DOI] [PubMed] [Google Scholar]
- [2].Toussirot E. Predictive Factors for Disability as Evaluated by the Health Assessment Questionnaire in Rheumatoid Arthritis: A Literature Review. Inflamm Allergy Drug Targets. 2010;9:519. doi: 10.2174/187152810791292926. [DOI] [PubMed] [Google Scholar]
- [3].Kiltz U, van der Heijde D. Health-Related Quality of Life in Patients with Rheumatoid Arthritis and in Patients with Ankylosing Spondylitis. Clin Exp Rheumatol. 2009;27:S10811. [PubMed] [Google Scholar]
- [4].John H, Kitas G, Toms T. et al. Cardiovascular Co-Morbidity in Early Rheumatoid Arthritis. Best Pract Res Clin Rheumatol. 2009;23:7182. doi: 10.1016/j.berh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- [5].Wang D, Zang J, Lau J. et al. Mechanisms of Lung Disease Development in Rheumatoid Arthritis. Nat Rev Rheumatol. 2019;15:581–596. doi: 10.1038/s41584-019-0275-x. [DOI] [PubMed] [Google Scholar]
- [6].Zochling J, Braun J. Mortality in Rheumatoid Arthritis and Ankylosing Spondylitis. Clin Exp Rheumatol. 2009;27:S12730. [PubMed] [Google Scholar]
- [7].Nell VP, Machold KP, Eberl G. et al. Benefit of Very Early Referral and Very Early Therapy with Disease-Modifying Anti-Rheumatic Drugs in Patients with Early Rheumatoid Arthritis. Rheumatology. 2004;43:906–914. doi: 10.1093/rheumatology/keh199. [DOI] [PubMed] [Google Scholar]
- [8].Klarenbeek NB, Güler-Yüksel M, van der Kooij SM. et al. The Impact of Four Dynamic, Goal-Steered Treatment Strategies on the 5-Year Outcomes of Rheumatoid Arthritis Patients in the Best Study. Ann Rheum Dis. 2011;70:1039–1046. doi: 10.1136/ard.2010.141234. [DOI] [PubMed] [Google Scholar]
- [9].Zhu TY, Li EK, Tam LS. Societal Costs of Rheumatoid Arthritis in Hong Kong: A Prevalence-Based Cost-of-Illness Study. Rheumatology. 2011;50:1293–1301. doi: 10.1093/rheumatology/ker014. [DOI] [PubMed] [Google Scholar]
- [10].Smolen JS, Landewe RBM, Bijlsma JWJ. et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2019 Update. Ann Rheum Dis. 2020;79:685–699. doi: 10.1136/annrheumdis-2019-216655. [DOI] [PubMed] [Google Scholar]
- [11].Scherer HU, Haupl T, Burmester GR. The Etiology of Rheumatoid Arthritis. J Autoimmun. 2020;110:102400. doi: 10.1016/j.jaut.2019.102400. [DOI] [PubMed] [Google Scholar]
- [12].Carstensen SMD, Terslev L, Jensen MP. et al. Future Use of Musculoskeletal Ultrasonography and Magnetic Resonance Imaging in Rheumatoid Arthritis. Curr Opin Rheumatol. 2020;32:264–272. doi: 10.1097/BOR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- [13].Klose-Jensen R, Tse JJ, Keller KK. et al. High-Resolution Peripheral Quantitative Computed Tomography for Bone Evaluation in Inflammatory Rheumatic Disease. Front Med (Lausanne) 2020;7:337. doi: 10.3389/fmed.2020.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Figueiredo CP, Perez MO, Sales LP. et al. HR pQCT In Vivo Imaging of Periarticular Bone Changes in Chronic Inflammatory Diseases: Data from Acquisition to Impact on Treatment Indications. Modern Rheumatol. 2020 doi: 10.1080/14397595.2020.1804669. [DOI] [PubMed] [Google Scholar]
- [15].Zhu TY, Griffith JF, Qin L. et al. Structure and Strength of the Distal Radius in Female Patients with Rheumatoid Arthritis: A Case-Control Study. J Bone Miner Res. 2013;28:794–806. doi: 10.1002/jbmr.1793. [DOI] [PubMed] [Google Scholar]
- [16].Zhu TY, Griffith JF, Qin L. et al. Alterations of Bone Density, Microstructure, and Strength of the Distal Radius in Male Patients with Rheumatoid Arthritis: A Case-Control Study with HR-pQCT. J Bone Miner Res. 2014;29:2118–2129. doi: 10.1002/jbmr.2221. [DOI] [PubMed] [Google Scholar]
- [17].Wakefield RJ, Balint PV, Szkudlarek M. et al. Musculoskeletal Ultrasound Including Definitions for Ultrasonographic Pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- [18].Nam JL, Hensor EM, Hunt L. et al. Ultrasound Findings Predict Progression to Inflammatory Arthritis in Anti-CCP Antibody-Positive Patients without Clinical Synovitis. Ann Rheum Dis. 2016;75:2060–2067. doi: 10.1136/annrheumdis-2015-208235. [DOI] [PubMed] [Google Scholar]
- [19].Van Beers-Tas MH, Blanken AB, Nielen MMJ. et al. The Value of Joint Ultrasonography in Predicting Arthritis in Seropositive Patients with Arthralgia: A Prospective Cohort Study. Arthritis Res Ther. 2018;19:279. doi: 10.1186/s13075-018-1767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Filer A, de Pablo P, Allen G. et al. Utility of Ultrasound Joint Counts in the Prediction of Rheumatoid Arthritis in Patients with Very Early Synovitis. Ann Rheum Dis. 2011;70:500–507. doi: 10.1136/ard.2010.131573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sahbudin I, Pickup L, Nightingale P. et al. The Role of Ultrasound-Defined Tenosynovitis and Synovitis in the Prediction of Rheumatoid Arthritis Development. Rheumatology. 2018;57:1243–1252. doi: 10.1093/rheumatology/key025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zufferey P, Rebella C, Benaima C. et al. Ultrasound can be Useful to PRedict an Evolution Towards Rheumatoid Arthritis in Patients with Inflammatory Polyarthralgia without Anticitrullinated Antibodies. Joint Bone Spine. 2017;84:299–303. doi: 10.1016/j.jbspin.2016.05.011. [DOI] [PubMed] [Google Scholar]
- [23].Di Matteo A, Mankia K, Duquenne L. et al. Ultrasound Erosions in the Feet Best Predict Progression to Inflammatory Arthritis in Anti-CCP Positive at-Risk Individuals without Clinical Synovitis. Ann Rheum Dis. 2020;79:901–907. doi: 10.1136/annrheumdis-2020-217215. [DOI] [PubMed] [Google Scholar]
- [24].Pentony P, Mankia K, Hensor EM. et al. SAT0107 Sequential Ultrasound Shows A Late Increase in Inflammatory Burden in Anti-CCP Positive Patients with Non-Specific Musculoskeletal Symptoms Just Before Progression to Inflammatory Arthritis. Ann Rheum Dis. 2018;77(Suppl 2):916. [Google Scholar]
- [25].D’Agostino MA, Terslev L, Aegerter P. et al. Scoring Ultrasound Synovitis in Rheumatoid Arthritis: A EULAR-OMERACT Ultrasound Taskforce-Part 1: Definition and Development of A Standardised, Consensus-Based Scoring System. RMD Open. 2017;3:e000428. doi: 10.1136/rmdopen-2016-000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Terslev L, Naredo E, Aegerter P. et al. Scoring Ultrasound Synovitis in Rheumatoid Arthritis: A EULAR-OMERACT Ultrasound Taskforce-Part 2: Reliability and Application to Multiple Joints of A Standardised Consensusbased Scoring System. RMD Open. 2017;3:e000427. doi: 10.1136/rmdopen-2016-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Østergaard M, Pedersen SJ, Døhn UM. Imaging in Rheumatoid Arthritis: Status and Recent Advances for Magnetic Resonance Imaging, Ultrasonography, Computed Tomography and Conventional Radiography. Clin Rheumatol. 2008;22:1019–1044. doi: 10.1016/j.berh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- [28].Tamai M, Kawakami A, Uetani M. et al. A Prediction Rule for Disease Outcome in Patients with Undifferentiated Arthritis Using Magnetic Resonance Imaging of the Wrists and Finger Joints and Serologic Autoantibodies. Arthritis Care Res. 2009;61:772–778. doi: 10.1002/art.24711. [DOI] [PubMed] [Google Scholar]
- [29].Ji L, Li G, Xu Y. et al. Early Prediction of Rheumatoid Arthritis by Magnetic Resonance Imaging in the Absence of Anti-Cyclic Citrullinated Peptide Antibodies and Radiographic Erosions in Undifferentiated Inflammatory Arthritis Patients: A Prospective Study. Int J Rheum Dis. 2015;18:859–865. doi: 10.1111/1756-185X.12420. [DOI] [PubMed] [Google Scholar]
- [30].Van Steenbergen HW, Mangnus L, Reijnierse M. et al. Clinical Factors, Anticitrullinated Peptide Antibodies and MRI-Detected Subclinical Inflammation in Relation to Progression from Clinically Suspect Arthralgia to Arthritis. Ann Rheum Dis. 2016;75:1824–1830. doi: 10.1136/annrheumdis-2015-208138. [DOI] [PubMed] [Google Scholar]
- [31].Boer AC, Wouters F, Dakkak YJ. et al. Improving the Feasibility of MRI in Clinically Suspect Arthralgia for Prediction of Rheumatoid Arthritis by Omitting Scanning of the Feet. Rheumatology. 2020;59:1247–1252. doi: 10.1093/rheumatology/kez436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wouters F, Matthijssen XME, Boeters DM. et al. Do Magnetic Resonance Imaging-Detected Erosions Predict Progression to Rheumatoid Arthritis in Patients Presenting with Clinically Suspect Arthralgia? A Longitudinal Study. Scand J Rheumatol. 2020;49:461–467. doi: 10.1080/03009742.2020.1737221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Combe B, Landewe R, Daien CI. et al. 2016 Update of the EULAR Recommendations for the Management of Early Arthritis. Ann Rheum Dis. 2017;76:948–959. doi: 10.1136/annrheumdis-2016-210602. [DOI] [PubMed] [Google Scholar]
- [34].Østergaard M, Peterfy C, Conaghan P. et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies. Core set of MRI Acquisitions, Joint Pathology Definitions, and the OMERACT RA-MRI Scoring System. J Rheumatol. 2003;30:1385–1386. [PubMed] [Google Scholar]
- [35].Kleyer A, Finzel S, Rech J. et al. Bone Loss Before the Clinical Onset of Rheumatoid Arthritis in Subjects with Anticitrullinated Protein Antibodies. Ann Rheum Dis. 2014;73:854–860. doi: 10.1136/annrheumdis-2012-202958. [DOI] [PubMed] [Google Scholar]
- [36].Keller KK, Thomsen JS, Stengaard-Pedersen K. et al. Local Bone Loss in Patients with Anti-Citrullinated Peptide Antibody and Arthralgia, Evaluated with High-Resolution Peripheral Quantitative Computed Tomography. Scand J Rheumatol. 2018;47:110–116. doi: 10.1080/03009742.2017.1333629. [DOI] [PubMed] [Google Scholar]
- [37].Keller KK, Thomsen JS, Stengaard-Pedersen K. et al. One-Year Progression of Erosive Disease in Patients with Anti-Citrullinated Peptide Antibodies and Arthralgia. Joint Bone Spine. 2020;87:181–183. doi: 10.1016/j.jbspin.2019.09.006. [DOI] [PubMed] [Google Scholar]
- [38].Simon D, Kleyer A, Bui CD. et al. Micro-Structural Bone Changes are Associated with Broad-Spectrum Autoimmunity and Predict the Onset of Rheumatoid Arthritis. Arthrit Rheumatol. 2020 doi: 10.1002/art.41229. [DOI] [PubMed] [Google Scholar]
- [39].Kleyer A, Krieter M, Oliveira I. et al. High Prevalence of Tenosynovial Inflammation Before Onset of Rheumatoid Arthritis and its Link to Progression to RA — A Combined MRI/CT Study. Semin Arthritis and Rheum. 2016;46:143–150. doi: 10.1016/j.semarthrit.2016.05.002. [DOI] [PubMed] [Google Scholar]
- [40].Emery P, Durez P, Dougados M. et al. Impact of Tcell Costimulation Modulation in Patients with Undifferentiated Inflammatory Arthritis or Very Early Rheumatoid Arthritis: A Clinical and Imaging Study of Abatacept (the ADJUST Trial) Ann Rheum Dis. 2010;69:510–516. doi: 10.1136/ard.2009.119016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Burgers LE, Allaart CF, Huizinga TWJ. et al. Brief Report: Clinical Trials Aiming to Prevent Rheumatoid Arthritis Cannot Detect Prevention without Adequate Risk Stratification: A Trial of Methotrexate Versus Placebo in Undifferentiated Arthritis as an Example. Arthrit Rheumatol. 2017;69:926–931. doi: 10.1002/art.40062. [DOI] [PubMed] [Google Scholar]
- [42].Gerlag DM, Safy M, Maijer KI. et al. Effects of B-Cell Directed Therapy on the Preclinical Stage of Rheumatoid Arthritis: The PRAIRI Study. Ann Rheum Dis. 2019;78:179–185. doi: 10.1136/annrheumdis-2017-212763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hulsmans HM, Jacobs JW, van der Heijde DM. et al. The Course of Radiologic Damage During the First Six Years of Rheumatoid Arthritis. Arthrit Rheumatol. 2000;43:1927–1940. doi: 10.1002/1529-0131(200009)43:9<1927::AID-ANR3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- [44].Zayat AS, Ellegaard K, Conaghan PG. et al. The Specificity of Ultrasound-Detected Bone Erosions for Rheumatoid Arthritis. Ann Rheum Dis. 2015;74:897–903. doi: 10.1136/annrheumdis-2013-204864. [DOI] [PubMed] [Google Scholar]
- [45].Lai KL, Chen DY, Chen YH. et al. Assessment of Wrist Joint Inflammation in Patients with Rheumatoid Arthritis by Quantitative Two- and Three-Dimensional Power Doppler Ultrasonography. Clin Exp Rheumatol. 2014;32:674–679. [PubMed] [Google Scholar]