Abstract
We recently reported that poly lactic-co-glycolic acid (PLGA) nanoparticles (NPs) loaded with interleukin (IL)-2 and targeted to T cells inhibited the development of lupus-like disease in BDF1 mice by inducing functional T regulatory cells (Tregs). Here we show that the protection from disease and the extended survival of BDF1 mice provided by IL-2-loaded NPs targeted to T cells is not only due to an induction of Tregs but also contributed by an inhibition of T follicular helper (TFH) cells. These results identify a dual protective activity of IL-2 in the control of lupus autoimmunity, namely the inhibition of effector TFH cells, in addition to the previously known induction of Tregs. This newly recognized activity of IL-2 delivered by NPs can help better explain the beneficial effects of low-dose IL-2 immunotherapy in systemic lupus erythematosus (SLE), and might be considered as a new strategy to slow disease progression and improve outcomes in lupus patients.
Keywords: autoimmunity, systemic lupus erythematosus, T follicular helper (TFH) cells, T cells
Introduction
Antibodies to self-antigens (autoantibodies) are occasionally found in healthy individuals[1] but in systemic lupus erythematosus (SLE), they represent disease hallmarks.[2] When auto-antibodies form immune complexes with their target cognate antigen(s), they often become entrapped and/or precipitate in tissues, causing local inflammation that over time can lead to organ damage. The ensuing clinical manifestations seen in SLE patients depend on the extent of compromised organ function, and can evolve from mild to severe to fatal complications.[3]
Because of the central role of autoantibodies in the immunopathogenesis of SLE, multiple approaches have tried to reduce their production and/or limit their pathogenic activity. Such strategies have included neutralization,[4] immune switching,[5, 6] and the suppression of B cell production of immunoglobulins – either directly or indirectly,[7] or via the blockade of T cell help to B cells.[8, 9]
We recently reported that the production of autoantibodies in BDF1 lupus mice could be suppressed by the administration of poly lactic-co-glycolic acid (PLGA) nanoparticles (NPs) loaded with interleukin (IL)-2 and TGF-β targeted to T cells.[9] The induction of tolerogenic immune responses by those NPs resulted in improved renal function and increased survival of BDF1 lupus mice, and associated with an in vivo induction of functional T regulatory cells (Tregs) that suppressed production of autoantibodies from B cells.[10] Subsequent analyses identified a critical role of IL-2 in the NP-mediated protection from SLE,[11, 12] recognizing a central role of this cytokine in the protection of lupus mice from disease[13] – in line with past investigations that had highlighted the benefits of administering low doses of IL-2 to SLE patients.[14]
Although the general interpretation of the findings of the studies that employ IL-2 is that the alleviation of the immune disturbances could be secondary to a restoration of the deficient levels of IL-2 in SLE,[15, 16] this explanation does not contemplate the fact that IL-2 has pleiotropic effects on multiple cells and immune functions.
To further delineate the mechanisms by which the delivery of IL-2 by NPs to T cells can protect from SLE, we studied the effects of NPs encapsulating IL-2 on multiple immune cell populations that are involved in the production of autoantibodies (i.e., key culprits in the pathogenesis of SLE).
Among the T cells that respond to IL-2 because of their expression of the IL-2 receptor, an interesting target was represented by the T follicular helper (TFH) cells, which promote the formation of germinal center (GC)s and the production of autoantibodies, and are abnormally expanded in lupus mice and patients. With a focus on the analysis of the targeted effects of IL-2 on the number and function of TFH cells in BDF1 lupus mice, the results reported herein identify a new modality that could maximize benefits and avoid possible unwanted consequences of IL-2 administration in lupus settings.
Materials and Methods
PLGA Nanoparticles (NPs)
PLGA NPs were prepared as described before[10] and characterized for physical properties according to standard procedures for encapsulation metrics and release kinetics.[11] The dynamic light scattering indicated that the NPs had a mean ± SD hydrodynamic diameter of 245 ± 2 nm with a low polydispersity index and a relatively tight size distribution. The encapsulation of IL-2 in the NPs was measured by ELISA after NPs were disrupted using DMSO, and standard curves were generated using known cytokine concentrations. The mean content ± SD of IL-2 in the NPs was 1.9 ± 0.1 ng IL-2/mg NP. For T cell targeting, NPs were diluted in PBS and incubated for 10 min with 2 mg biotinylated anti-CD3 (clone 17A2) or anti-CXCR5 (clone SPRCL5) antibodies (ThermoFisher Scientific, Waltham, MA, USA)/mg NP prior to use.
Mice
(C57BL/6 × DBA/2)F1 (B6D2F1/J, hereafter called BDF1) mice and DBA/2 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in pathogen-free facilities in microisolator cages at the University of California Los Angeles (UCLA). Only female mice were used. Mice had unrestricted access to autoclaved food and sterile water and were used according to protocols that had been approved by the Animal Research Committee of UCLA. Lupus-like disease was induced at 8 weeks of age, according to standard protocols, by transferring 1 × 108 DBA/2 splenocytes into BDF1 mice.[17] In the recipient mice, the recognition of the host’s major histocompatibility complex (MHC) (named H2 in mice) leads to lymphoid hyperplasia and elevated production of anti–double-stranded DNA antibodies that induce immune-complex glomerulonephritis.[18]
After the transfer of DBA/2 splenocytes for the induction of SLE in recipient animals, individual BDF1 mice were given intraperitoneal (i.p.) injections of vehicle (as control) or 1 mg PLGA NPs loaded with IL-2 and decorated with anti-CD3 or anti-CXCR5 antibodies (clone SPRCL5) to evaluate the effects of local delivery of IL-2 to T cells and TFH cells. The protocol of NPs administration was the following: day 0, day 3, day 6, day 9, day 12, and day 19.[10] Uncoated NPs, with or without encapsulated IL-2, were used as controls. Blood was obtained via retroorbital bleeding and used for immune cell phenotyping or for serum monitoring of antibody titers by ELISA. Proteinuria was measured using Albustix strips (Siemens Diagnostics, Irvington, NJ, USA).
Cell preparation
Peripheral blood mononuclear cells (PBMCs) were purified from heparinized venous blood. Spleens were explanted at sacrifice of the mice and single-cell suspensions were prepared according to standard protocols.[19]
Flow cytometry
Flow cytometry analyses evaluated the possibility of phenotypic changes after administration of NPs. Following red blood cell lysis with ammonium chloride buffer, PBMCs or splenocytes were used fresh for flow cytometry. After Fc blocking, combinations of anti-mouse antibodies were used for cell staining using standard procedures. The combinations of FITC-, PE-, PerCP- or APC-conjugated anti-mouse monoclonal antibodies included anti-CD4 (clone RM4-4), anti-PD-1 (clone RMP1-30), anti-CXCR5 (clone SPRCL5), anti-CD45R (clone RA3-6B2), anti-CD19 (clone 1D3), anti-CD21 (clone 4E3), anti-CD23 (clone B3B4), anti-CD24 (clone M1/69), anti-CD27 (clone LG.7F9), anti-CD40 (clone 5C3), anti-CD80 (clone MEM-233), anti-CD43 (clone eBioR2/60), anti-H2Kb (clone AF6-88.5.5.3), anti-H2Kd (clone 34-1-2S), anti-IgM (clone II/41), anti-IgD (clone 11-26c), or isotype controls (all from ThermoFisher Scientific). Cells were acquired on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and data were analyzed using FlowJo software (BD, Franklin Lakes, NJ, USA).
ELISA
To study possible changes in Ig levels after treatment with NPs, serum IgA, IgM, and IgG and the IgG subclasses IgG1, IgG2a, and IgG3 were measured using commercial kits (Alpha Diagnostic Intl., San Antonio, TX, USA).
Statistical analyses
Statistical analyses were done using GraphPad Prism software version 5.0. Parametric testing used the Student’s t test. Differences in animal Kaplan–Meier survival curves were analyzed by the log-rank test. P values <0.05 were considered significant.
RESULTS
Treatment of BDF1 lupus mice with IL-2-loaded NPs targeted to T cells alters the distribution of B cell subsets
Our original focus on the effects of treatment of BDF1 lupus mice with IL-2-loaded NPs had been on T cells and NK cells because NPs targeted those cells via the coated antibodies. Those studies did not investigate the effects of the NPs on B cells mainly because NPs had not targeted directly those cells, yet this ancillary information could help understand how NPs affect the humoral response – which has a central role in the pathogenesis and clinical manifestations of SLE. Therefore, we measured the frequency of major splenic B cell subpopulations in BDF1 lupus mice treated with IL-2-loaded NPs that had been targeted specifically to T cells (due to coating with anti-CD3 antibodies).
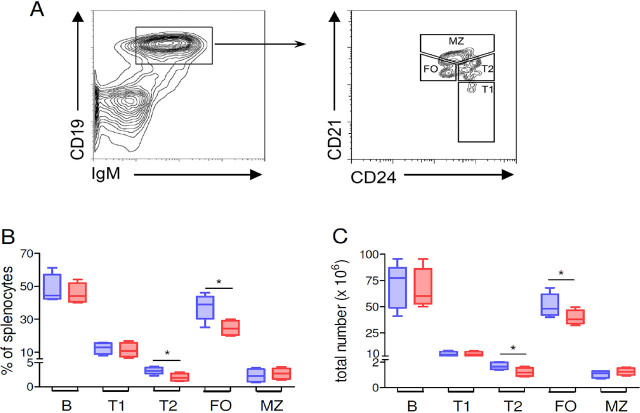
The percentages and absolute counts of multiple CD19+ B cell subpopulations were significantly different between BDF1 mice treated with T-cell-targeted NPs and controls that had received untargeted NPs (Figure 1). Specifically, there was an altered distribution in the frequency and total numbers of immature CD24+IgM+CD21int/lo transitional 2 (T2) cells and mature follicular B cells, being both those cell subsets decreased in mice treated with T-cell-targeted, IL-2-loaded NPs as compared to mice that had received untargeted NPs (Figure 1B, C). An apparent reduction in CD24+IgM+CD21− transitional 1 (T1) cells and increased CD24+IgM+CD21hi marginal zone (MZ) B cells did not reach statistical significance (Figure 1B, C). Comparable results were obtained by phenotyping T1 cells as CD24+IgM+IgDlo, T2 cells as CD24+IgM+IgD+, and MZ B cells as CD21hiCD23loIgMhi (not shown).
Figure 1.
Treatment of BDF1 lupus mice with T cell-targeted NPs loaded with IL-2 associates with changes in the frequency of different B cell subsets. (A) Gating strategy for the identification of B cell subsets by flow cytometry. Costaining of CD19+ B cells with additional markers allowed the identification of transitional 1 (T1) B cells as CD24+IgM+CD21−, transitional 2 (T2) B cells as CD24+IgM+CD21int/lo, follicular (FO) B cells as CD24loIgMloCD21int, and marginal zone (MZ) B cells as CD24+IgM+CD21hi. Percentages (B) and absolute numbers (C) of splenic B cell subsets from BDF1 lupus mice treated with T cell-targeted NPs loaded with IL-2 (red boxes) or non-targeted unloaded NPs (blue boxes). Data are at week 2 after treatment with NPs; n = 4–8 mice per group; *P < 0.04. NPs, nanoparticles.
The finding that the elevated frequency of immature T2 cells in SLE was reduced by the treatment with IL-2-loaded NPs has relevance when considering the causal involvement of transitional B cells in the early loss of B cell tolerance.[20]
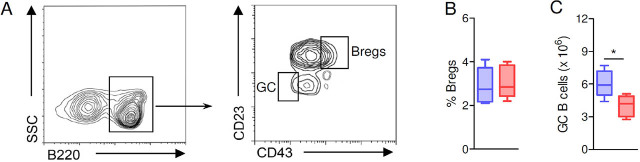
After showing that IL-2-loaded NPs targeted to CD3+ T cells had imparted changes on B cells, we wondered whether the NPs had also modulated the frequency of B cell subsets with an immunosuppressive activity—namely, the B regulatory cells (Bregs) that inhibit proinflammatory responses.[21, 22] NP-treated mice and controls had similar numbers of splenic B220+CD23+CD43+ Bregs (Figure 2A, B), suggesting uninfluential effects of the NPs on Bregs, as also confirmed by analogous counts of splenic B220+CD21+IgM+CD24hi Bregs in mice treated with IL-2, T-cell-targeted NPs, or in mice treated with untargeted NPs (1.26 ± 0.32 × 106 vs. 1.33 ± 0.26 × 106, respectively). Interestingly, these phenotypic investigations that indicated inconsequential effects of the NPs on Bregs unveiled in the meantime a reduction in CD43− B cells in NP-treated mice. In particular, the count of germinal center (GC) B220+CD43−IgM− B cells[22] showed reduced numbers of these cells in mice treated with IL-2-loaded, T-cell-targeted NPs as compared to controls (Figure 2C).
Figure 2.
Treatment of BDF1 lupus mice with IL-2-loaded NPs targeted to CD3+ T cells does not affect the pool of Bregs. (A) Gating strategy to identify splenic B220+CD23+CD43+ Bregs, and B220+CD23−CD43− germinal center (GC) B cells. B-C. BDF1 lupus mice treated IL-2-loaded, T cell-targeted NPs (red boxes) had similar frequencies of splenic Bregs (B) but reduced numbers of GC B cells (IgM−) as compared to control mice treated with non-targeted unloaded NPs (blue boxes) at week 2 post-treatment (C). n = 5–6 mice per group; *P < 0.04. Bregs, B regulatory cells; NPs, nanoparticles.
IL-2-loaded NPs targeted to T cells inhibit TFH cells
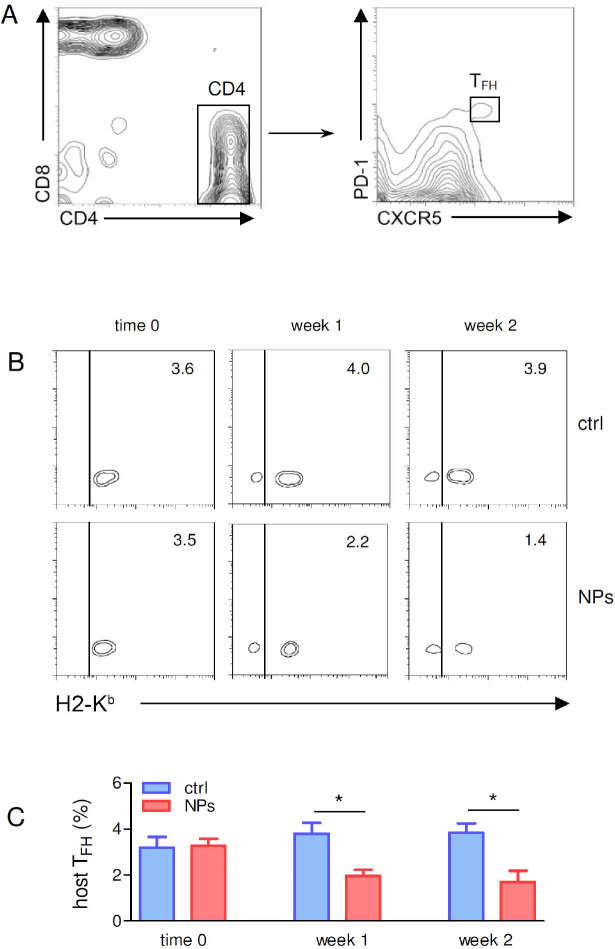
Since B cells that enter the GC reaction have been activated by cognate interactions with TFH cells and TFH cells are critical players of imprinting the B cell fate upon cell-to-cell contact, we studied the effects of IL-2-loaded NPs on TFH cells. The first step was to discriminate between the possibilities that our observations had to be ascribed to a modulation of host TFH cells (Figure 3A) or that they were rather secondary to effects on the DBA/2 TFH cells transferred to BDF1 mice for the induction of SLE (see Materials and Methods). This discrimination is possible because host (BDF1) and donor (DBA/2) cells can be sorted on the basis of H2 differences, i.e., DBA/2 donor H2d cells that induce disease in BDF1 hosts cannot stain for H2b,[11] so a staining for H2b is due to host (BDF1)-derived B cells.
Figure 3.
Treatment of BDF1 lupus mice with IL-2-loaded NPs targeted to CD3+ T cells associates with a reduced frequency of host-derived TFH cells. (A) Gating strategy for the identification of (CXCR5+PD-1+) TFH cells from the T cells. (B, C) Reduced frequency of host (H2Kb+) TFH cells in spleens from mice treated with IL-2-loaded NPs targeted to CD3+ T cells (NPs) as compared to mice treated with untargeted NPs (ctrl). Monitoring by flow cytometry was done by costaining with H2 marker to allow discrimination between (CXCR5+PD-1+ pre-gated) TFH cells from DBA/2 donors (H2Kb−) vs. BDF1 recipient hosts (H2Kb+). Representative (B) and cumulative (C) results from 4 mice per group; *P < 0.05. NPs, nanoparticles; TFH, T follicular helper.
Ex vivo monitoring by flow cytometry of the 2 H2 haplotypes on TFH cells indicated that the administration of T-cell-targeted NPs loaded with IL-2 associated with reduced numbers of H2b (host) TFH cells (Figure 3B, C). There was neither an increase in circulating H2b cells nor an increase in H2d donor TFH cells in BDF1 mice that did not receive NPs, suggesting that the reduced frequency of B cells in NP-treated BDF1 lupus mice had been the result of a decrease in host-derived TFH cells. Thus, the modulating effects of IL-2-loaded NPs on TFH cells in BDF1 lupus mice directly affected host cells.
Together, the results shown so far indicated that the treatment of BDF1 lupus mice with IL-2-loaded NPs targeted to T cells resulted in an altered distribution of B cell subsets that associated with a modulation of host TFH cells.
Treatment of BDF1 mice with NPs loaded with IL-2 and targeted to TFH cells reduces antibody production and lupus disease manifestations
Given the critical role of TFH cells in the B cell response (i.e., production of antibodies) and given the finding that NPs encapsulating IL-2 influenced host TFH cells (Figure 3), we targeted the delivery of IL-2 directly to TFH cells (rather than to all T cell as before, when using anti-CD3 antibody-coated NPs). This was done by coating IL-2-loaded NPs with anti-CXCR5 antibodies.
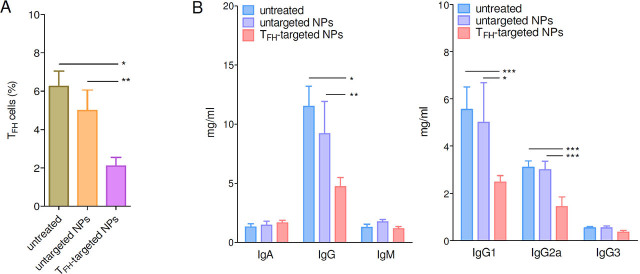
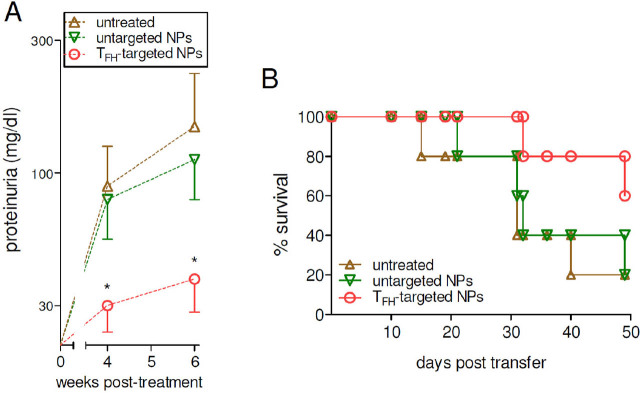
Treatment of lupus mice with IL-2-loaded NPs targeted to TFH cells resulted in a reduced frequency of TFH cells as compared to mice that had received untargeted NPs (Figure 4A). Importantly, the reduced frequency of TFH cells in mice treated with IL-2-loaded, CXCR5-targeted NPs correlated with a reduced antibody production and altered IgG subclasses profiles (Figure 4B), i.e., the serum IgG, and IgG1 and IgG2a subclasses were all significantly lower in NP-treated BDF1 lupus mice as compared to controls (Figure 4B). Since lupus mice have glomerular deposition of IgG and IgG2a,[23] the reduction of these antibodies in BDF1 lupus mice receiving NPs supported a disease-protective role. This possibility was confirmed by the finding of reduced renal disease manifestations (proteinuria, Figure 5A) and extended survival (Figure 5B) in comparison to control mice that had received no NPs or untargeted NPs.
Figure 4.
TFH (CXCR5+ cell)-targeted NPs loaded with IL-2 limit the expansion of TFH cells in BDF1 lupus mice and reduce antibody production. (A) TFH cells (gated as in Figure 3A) at week 2 after treatment. BDF1 lupus mice were either left untreated or treated with untargeted NPs as controls, or received TFH-targeted NPs (IL-2-loaded). n = 8–11 mice per group; *P < 0.003; **P < 0.04. (B) Reduced antibody production in BDF1 lupus mice that received TFH-targeted NPs loaded with IL-2. Data are at week 4 after treatment. n = 4–6 per group; *P < 0.03; **P < 0.04; **P < 0.02. NPs, nanoparticles; TFH, T follicular helper.
Figure 5.
Reduced lupus disease manifestations in BDF1 mice treated with TFH-targeted NPs. Proteinuria (A) and survival (B) were monitored in treated mice and controls at the times indicated on the x-axes. n = 5 per group; *P < 0.05 vs. untargeted NPs. NPs, nanoparticles; TFH, T follicular helper cells.
Discussion
We report that the treatment of BDF1 lupus mice with NPs loaded with IL-2 and targeted to TFH cells reduces autoantibody production and disease manifestations in lupus mice, extending their survival.
These results further our past work that had ascribed the therapeutic benefits of NP-mediated delivery of IL-2 to an expansion of Tregs,[10] identifying now TFH cells as a possibly equally important target. The inhibition of TFH cells can be particularly relevant to SLE because these cells support the maturation of B cells that produce antibodies in secondary lymphoid organs,[24] and we found that NP-mediated delivery of IL-2 inhibited antibody production sustained by TFH cells. Differential effects were seen for different Ig classes and subclasses, being the IgG and its IgG1 and IgG2a subclasses downregulated.
A brief digression is needed. IgG1 underscore Th2 responses, while IgG2a indicate a Th1 profile,[25] and Th1 responses (which are critically involved in the generation of autoantibodies with more pathogenic potential in SLE) promote a switch to IgG3.[25] Also, the different IgG subclasses in SLE associate with differences in pathogenic potential. For example, in lupus mice, autoantibodies of the IgG2a and IgG3 subclasses are considered as highly pathogenic and associate with lupus nephritis.[26] Our findings that lupus mice that received IL-2-loaded NPs targeted to TFH cells had reduced IgG1 and IgG2a responses (Figure 4) suggest a protection from pathogenic events that was confirmed by the extended survival and reduced disease manifestations (Figure 5) in TFH-inhibited mice. A possible explanation for the limited effects on IgG3 levels could be that the reduced Th1 response (as manifested by reduced IgG2a) had hampered the switch to this subclass. This possibility will be addressed in future studies. Apart from these considerations, the current study provides new information that has relevant immunotherapeutic potential. We had previously shown that the delivery of IL-2 to T cells via NPs to BDF1 lupus mice associated with an expansion of functional Tregs in vitro and in vivo.[9, 11] Here we found that the distribution of antibody-producing B cells in BDF1 lupus mice had also been altered by the treatment with IL-2-loaded NPs targeted to T cells. This apparently untargeted effect of the NPs was explained by changes in the activity of TFH cells, which were inhibited by IL-2. These new results lead to conclude that the beneficial effects of IL-2 delivered to T cells in SLE include the inhibition of pro-pathogenic TFH cells, in addition to the known promotion of immunoregulatory Tregs.[12] Both effects contribute, in a complementary fashion, to a readjustment of the dysfunctional immune response that characterizes the disease.
TFH cells are key contributors to the overproduction of pathogenic autoantibodies and tissue damage in SLE, being necessary for B cell help in T cell-dependent immune responses and GC formation.[27] TFH cells are considered interesting immunotherapeutic targets in SLE[28] because their abnormally elevated numbers in SLE promote and sustain the breakdown of B cell tolerance, fueling production of autoantibodies.[29, 30] However, despite the observation that the inhibition of TFH cells in lupus mice decreases production of autoantibodies and reduces lupus nephritis,[31] a translational approach toward clinical settings has been delayed by difficulties in finding modalities to selectively target TFH cells with no side effects. We show here that it is possible to inhibit TFH cells in lupus mice by targeting these cells with IL-2 delivered by NPs, extending the findings of an IL-2-mediated suppression of TFH cells in infection.[32]
IL-2 has a critical role in immunity, and its levels are dramatically reduced in SLE.[33] Clinical trials have shown significant benefits in lupus patients treated with low-dose IL-2, garnering much enthusiasm.[14] Although the advantages deriving from this treatment have been ascribed to an improved function of otherwise hypofunctional Tregs,[14] the changes in Treg cell numbers in those trials were generally transient and dropped to placebo control levels quickly after the last cycle of IL-2 administration.[14, 34] Moreover, low-dose IL-2-treated patients displayed improved clinical outcomes for weeks after the last cycle of IL-2 administration, without concomitant changes in the frequency of Tregs over time.[14, 34] Together, the finding of only transient changes in Tregs frequency and the presence of enduring therapeutic effects after discontinuation of IL-2 administration suggest the likelihood of additional mechanisms—concomitant and/or prolonged after the Tregs effects—to explain the long-lasting immune suppression after the end of treatment with low-dose IL-2. In view of our current data, in addition to the Tregs, it would be interesting to see whether—in SLE patients treated with low-dose IL-2—there is a downregulation of TFH cells that drive B cell differentiation and autoantibody production.
We acknowledge that a limitation of our study is the lack of investigations on the effects of NP-derived IL-2 on additional immune cell populations such as Th17 cells, CD3+CD4−CD8− double-negative T cells,[13, 35] and T follicular regulatory (TFR) cells.[36] Future studies will evaluate how NPs influence those immune cells including the TFR/TFH ratio[37, 38] and long-term B cell memory (although we did not see changes in short-term development of B220lo/+CD27mid/+CD40+CD80+ memory B cells, not shown). Other investigations could also include analyses of the metabolic control of immune cell signaling and effector programs - cell metabolism being a key player in the post-transcriptional mechanisms of TFH cell differentiation and humoral immunity.[39]
To summarize, we report a new NP-based approach to reduce TFH cell-mediated promotion of pathogenic humoral responses in SLE. The use of NPs is gaining much traction as a tool that allows the delivery of minute yet consequential amounts of payload to selected target cells in many diseases. There is a significant advantage in delivering small quantities of cargo locally, for in situ actions on a specific target to modulate cell differentiation, proliferation, and function. For example, the dosage of IL-2 packaged in our targeted NPs was about 1000-fold less than what is given systemically in typical low-dose IL-2 protocols (i.e., in mice, our total dose of IL-2 is 5 ng vs. 7 μg used for low-dose IL-2). Using NPs to deliver small amounts of the same drugs or other therapeutic molecules that would be given systemically minimizes side effects, making off-target reactions virtually negligible, overall avoiding risks of unwanted consequences.[16] In our case, the microscale delivery of IL-2 to TFH cells via NPs had no measurable side effects in treated mice (normal complete blood count and chemistry metabolic panel, not shown) and efficiently inhibited autoantibody production, reduced lupus disease manifestations, and improved outcomes with resulting extended mice survival. This encourages new investigations on the possible translatability to lupus patients.
Footnotes
Ethics Approval
All procedures performed in the mouse studies were in accordance with the institutional guidelines for the care and use of animals.
Conflict of Interest
Antonio La Cava is a Co-Editor-in-Chief of the journal. The article was subject to the journal's standard procedures, with rigorous peer-review handled independently by unrelated reviewers. David Horwitz is the founder of General Nanotherapeutics, LLC and has a financial interest in the Company. The research was conducted in the absence of any commercial or financial aspects that could be construed as a potential conflict of interest.
References
- [1].Mannoor K, Xu Y, Chen C. Natural Autoantibodies and Associated B Cells in Immunity and Autoimmunity. Autoimmunity. 2013;46:138–147. doi: 10.3109/08916934.2012.748753. [DOI] [PubMed] [Google Scholar]
- [2].La Cava A. Petrelli CT. New Research on Autoantibodies. New York: Nova Science Publishers; 2008. Antibodies to DNA in Systemic Lupus Erythematosus: A Review and Update; pp. 61–86. [Google Scholar]
- [3].La Cava A. Tsokos GC. Systemic Lupus Erythematosus. Basic, Applied and Clinical Aspects. 2nd Edn. Waltham: Academic Press; 2020. Overview of the Pathogenesis of Systemic Lupus Erythematosus; pp. 69–75. [Google Scholar]
- [4].He M, Cheng KF, VanPatten S. et al. A Structural Investigation of FISLE-412, A Peptidomimetic Compound Derived from Saquinavir That Targets Lupus Autoantibodies. Bioorg Med Chem Lett. 2017;27:4725–4729. doi: 10.1016/j.bmcl.2017.08.070. [DOI] [PubMed] [Google Scholar]
- [5].Wallace DJ. Clinical and Pharmacological Experience with LJP-394. Exp Opin Invest Drugs. 2001;10:111–117. doi: 10.1517/13543784.10.1.111. [DOI] [PubMed] [Google Scholar]
- [6].Cai C, La Cava A. Mimicking Self-Antigens with Synthetic Peptides in Systemic Autoimmune Rheumatic Diseases. Curr Clin Pharmacol. 2009;4:142–147. doi: 10.2174/157488409788184936. [DOI] [PubMed] [Google Scholar]
- [7].La Cava A. Targeting the BLyS-APRIL Signaling Pathway in SLE. Clin Immunol. 2013;148:322–327. doi: 10.1016/j.clim.2012.11.010. [DOI] [PubMed] [Google Scholar]
- [8].Mok MY. The Immunological Basis of B-Cell Therapy in Systemic Lupus Erythematosus. Int J Rheum Dis. 2010;13:3–11. doi: 10.1111/j.1756-185X.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- [9].Furie R, Nicholls K, Cheng TT. et al. Efficacy and Safety of Abatacept in Lupus Nephritis: A Twelve-Month, Randomized, Double-Blind Study. Arthritis Rheumatol. 2014;66:379–389. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- [10].Horwitz DA, Bickerton S, Koss M. et al. Suppression of Murine Lupus by CD4+ and CD8+ Treg Cells Induced by T Cell-Targeted Nanoparticles Loaded with Interleukin-2 and Transforming Growth Factor β. Arthritis Rheumatol. 2019;71:632–640. doi: 10.1002/art.40773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Horwitz DA, Liu A, Bickerton S. et al. Anti-CD2 Antibody-Coated Nanoparticles Containing IL-2 Induce NK Cells That Protect Lupus Mice via a TGF-β-Dependent Mechanism. Front Immunol. 2020;11:583338. doi: 10.3389/fimmu.2020.583338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giang S, Horwitz DA, Bickerton S. et al. Nanoparticles Engineered as Artificial Antigen-Presenting Cells Induce Human CD4+ and CD8+ Tregs That Are Functional in Humanized Mice. Front Immunol. 2021;12:628059. doi: 10.3389/fimmu.2021.628059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Katsiari CG, Tsokos GC. Transcriptional Repression of Interleukin-2 in Human Systemic Lupus Erythematosus. Autoimmun Rev. 2006;5:118–121. doi: 10.1016/j.autrev.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [14].He J, Zhang X, Wei Y. et al. Low-Dose Interleukin-2 Treatment Selectively Modulates CD4+ T Cell Subsets in Patients with Systemic Lupus Erythematosus. Nat Med. 2016;22:991–993. doi: 10.1038/nm.4148. [DOI] [PubMed] [Google Scholar]
- [15].von Spee-Mayer C, Siegert E, Abdirama D. et al. Low-Dose Interleukin-2 Selectively Corrects Regulatory T Cell Defects in Patients with Systemic Lupus Erythematosus. Ann Rheum Dis. 2016;75:1407–1415. doi: 10.1136/annrheumdis-2015-207776. [DOI] [PubMed] [Google Scholar]
- [16].Horwitz DA, Bickerton S, La Cava A. Strategies to Use Nanoparticles to Generate CD4 and CD8 Regulatory T Cells for the Treatment of SLE and Other Autoimmune Diseases. Front Immunol. 2021;12:681062. doi: 10.3389/fimmu.2021.681062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Soloviova K, Puliaiev M, Foster A. et al. The Parent-into-F1 Murine Model in the Study of Lupus-Like Autoimmunity and CD8 Cytotoxic T Lymphocyte Function. Methods Mol Biol. 2012;900:253–270. doi: 10.1007/978-1-60761-720-4_12. [DOI] [PubMed] [Google Scholar]
- [18].Iikuni N, La Cava A. La Cava A. Recent Research Developments in Rheumatology. Trivandrum: Research Signpost; 2009. Murine Models of Lupus for the Study of Human SLE; pp. 55–78. [Google Scholar]
- [19].Lim JF, Berger H, Su I. Isolation and Activation of Murine Lymphocytes. J Vis Exp. 2016;116:54596. doi: 10.3791/54596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vossenkämper A, Lutalo PM, Spencer J. Transitional B Cells in Systemic Lupus Erythematosus and Sjogren’s Syndrome: Clinical Implications and Effects of B Cell-Targeted Therapies. Clin. Exp. Immunol. 2012;167:7–14. doi: 10.1111/j.1365-2249.2011.04460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mauri C, Blair PA. Regulatory B Cells in Autoimmunity: Developments and Controversies. Nat Rev Rheumatol. 2010;6:636–643. doi: 10.1038/nrrheum.2010.140. [DOI] [PubMed] [Google Scholar]
- [22].Chen X, Cai C, Xu D. et al. Human Mesenchymal Stem Cell-Treated Regulatory CD23+CD43+ B Cells Alleviate Intestinal Inflammation. Theranostics. 2019;9:4633–4647. doi: 10.7150/thno.32260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Via CS, Rus V, Gately MK. et al. IL-12 stimulates the development of acute graft-versus-host disease in mice that normally would develop chronic, autoimmune graft-versus-host disease. J Immunol. 1994;153:40404–4047. [PubMed] [Google Scholar]
- [24].Vinuesa CG, Linterman MA, Yu D. et al. Follicular Helper T Cells. Annu Rev Immunol. 2016;34:335–368. doi: 10.1146/annurev-immunol-041015-055605. [DOI] [PubMed] [Google Scholar]
- [25].Stevens TL, Bossie A, Sanders VM. et al. Regulation of Antibody Isotype Secretion by Subsets of Antigen-Specific Helper T Cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- [26].Baudino L, Azeredo da Silveira S, Nakata M, Izui S. Molecular and Cellular Basis for Pathogenicity of Autoantibodies: Lessons from Murine Monoclonal Autoantibodies. Springer Semin Immunopathol. 2006;28:175–184. doi: 10.1007/s00281-006-0037-0. [DOI] [PubMed] [Google Scholar]
- [27].Adachi Y, Onodera T, Yamada Y. et al. Distinct Germinal Center Selection at Local Sites Shapes Memory B Cell Response to Viral Escape. J Exp Med. 2015;212:1709–1723. doi: 10.1084/jem.20142284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dong W, Zhu P, Wang Y. et al. Follicular Helper T Cells in Systemic Lupus Erythematosus: A Potential Therapeutic Target. Autoimmun Rev. 2011;10:299–304. doi: 10.1016/j.autrev.2010.11.004. [DOI] [PubMed] [Google Scholar]
- [29].Kim CH, Rott LS, Clark-Lewis I. et al. Subspecialization of CXCR5+ T Cells: B Helper Activity is Focused in a Germinal Center-Localized Subset of CXCR5+ T Cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vinuesa CG, Sanz I, Cook MC. Dysregulation of Germinal Centres in Autoimmune Disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- [31].Hu Y-L, Metz DP, Chung J. et al. B7RP-1 Blockade Ameliorates Autoimmunity through Regulation of Follicular Helper T Cells. J Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- [32].Ballesteros-Tato A, Leon B, Graf BA. et al. Interleukin-2 Inhibits Germinal Center Formation by Limiting T Follicular Helper Cell Differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Solomou EE, Juang YT, Gourley MF. et al. Molecular Basis of Deficient IL-2 Production in T Cells from Patients with Systemic Lupus Erythematosus. J Immunol. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- [34].He J, Zhang R, Shao M. et al. Efficacy and Safety of Low-Dose IL-2 in the Treatment of Systemic Lupus Erythematosus: A Randomised, Double-Blind, Placebo-Controlled Trial. Ann Rheum Dis. 2020;79:141–149. doi: 10.1136/annrheumdis-2019-215396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mizui M, Koga T, Lieberman LA. et al. IL-2 Protects Lupus-Prone Mice from Multiple End-Organ Damage by Limiting CD4−CD8− IL-17-Producing T Cells. J Immunol. 2014;193:2168–2177. doi: 10.4049/jimmunol.1400977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].La Cava A. Survive to Fight: Effector Treg Cells in Systemic Lupus Erythematosus. Arthritis Rheumatol. 2016;68:1327–1329. doi: 10.1002/art.39616. [DOI] [PubMed] [Google Scholar]
- [37].Liang K, He J, Wei Y. et al. Sustained Low-Dose Interleukin-2 Therapy Alleviates Pathogenic Autoimmunity via Elevating the Tfr/Tfh Ratio in Lupus. Clin Transl Immunol. 2021;10:e1293. doi: 10.1002/cti2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hao H, Nakayamada S, Yamagata K. et al. Conversion of T Follicular Helper Cells to T Follicular Regulatory Cells by Interleukin-2 through Transcriptional Regulation in Systemic Lupus Erythematosus. Arthritis Rheum. 2021;73:132–142. doi: 10.1002/art.41457. [DOI] [PubMed] [Google Scholar]
- [39].Fu G, Guy CS, Chapman NM. et al. Metabolic Control of TFH Cells and Humoral Immunity by Phosphatidylethanolamine. Nature. 2021;595:724–729. doi: 10.1038/s41586-021-03692-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Horwitz DA, Fahmy TM, Piccirillo CA. et al. Rebalancing Immune Homeostasis to Treat Autoimmune Diseases. Trends Immunol. 2019;40:888–908. doi: 10.1016/j.it.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]