Highlights
-
•
10.7% of MOUD patients in US substance use treatment programs were not represented.
Keywords: Trial representation, External validity, Generalizability, MOUD, Clinical Trials
Abstract
Background
The extent to which clinical trials of medications for opioid use disorder (MOUD) are representative or not is unknown. Some patient characteristics modify MOUD effectiveness; if these same characteristics differ in distribution between the trial population and usual-care population, this could contribute to lack of generalizability—a discrepancy between trial and usual-care effectiveness. Our objective was to identify interpretable, multidimensional subgroups who were prescribed MOUD in substance use treatment programs in the US but who were not represented or under-represented by clinical trial participants.
Methods
This was a secondary descriptive analysis of trial and real-world data. The trial data included twenty-seven US opioid treatment programs in the National Drug Abuse Treatment Clinical Trials Network, N=2,199 patients. The real-world data included US substance use treatment programs that receive public funding, N=740,015 patients. We characterized real-world patient populations who were non-represented and under-represented in the trial data in terms of sociodemographic and clinical characteristics that could modify MOUD effectiveness.
Results
We found that 10.7% of MOUD patients in TEDS-A were not represented in the three clinical trials. As expected, pregnant MOUD patients (n=19,490) were not represented. Excluding pregnancy, education and marital status from the characteristics, 2.6% of MOUD patients were not represented. Patients aged 65 years and older (n=11,204), and those 50-64 years who identified as other (non-White, non-Black, and non-Hispanic) race/ethnicity or multi-racial (n=7,281) were under-represented.
Conclusions
Quantifying and characterizing non- or under-represented subgroups in trials can provide the data necessary to improve representation in future trials and address research-to-practice gaps.
1. Introduction
Opioid use disorder (OUD) is a leading cause of US morbidity and mortality (Degenhardt, Bucello, Mathers, Briegleb, Ali, Hickman, McLaren, 2011, Hser, Mooney, Saxon, Miotto, Bell, Zhu, Liang, Huang, 2017, Centers for Disease Control and Prevention, Centers for Medicare & Medicaid Services), and has accelerated during the COVID-19 pandemic Soares III et al. (2021); Centers for Disease Control and Prevention (2020). Medications for opioid use disorder (MOUD)—methadone, buprenorphine and extended-release naltrexone—are the most effective tools for improving outcomes and preventing overdose among persons with OUD (Connery, 2015, Lee, Nunes Jr, Novo, Bachrach, Bailey, Bhatt, Farkas, Fishman, Gauthier, Hodgkins, et al., 2018, National Academies of Sciences, Engineering, and Medicine and others, 2019), evaluated using randomized controlled trials. However, MOUD effectiveness (e.g., in terms of treatment retention) is typically worse in real-world settings outside of clinical trials Hser et al. (2014); Morgan et al. (2018); Samples et al. (2018), which raises concerns about lack of generalizability of MOUD trial outcomes to real-world populations.
Lack of generalizability—specifically, the discrepancy between trial evidence for MOUD efficacy and the effectiveness in real-world, usual-care settings—may be attributable to at least two factors: 1) differences between clinical trial populations and real-world care-seeking populations if treatment effects are modified (increased/decreased) by factors that also relate to trial participation, and 2) differences in how treatment is delivered that also modify treatment effects. We focus on the first factor in this paper.
Those enrolled in MOUD clinical trials may not be representative of those generally seeking MOUD treatment. Lack of representation in terms sociodemographic and clinical characteristics has been shown for substance use disorder trials, generally (including multiple trials for drug- and/or alcohol-use disorder treatments grouped together and cannabis use disorder trials separately) Blanco et al. (2017); Okuda et al. (2010); Susukida et al. (2016), but the extent to which MOUD trials are representative or not is unknown. There is evidence that some patient characteristics modify MOUD effectiveness (e.g., homeless status, cocaine use disorder, race/ethnicity, years using opioids) Nunes Jr et al. (2021); Rudolph et al. (2021), so if these same characteristics differ in distribution between the trial participants and real-world population, this could contribute to the discrepancy between trial and real-world effectiveness.
Prior studies quantifying lack of representation in trials have typically been limited to considering one individual-level characteristic at a time Blanco et al. (2017); Susukida et al. (2016)—for example, identifying lack of representation based only on age. However, individuals are necessarily multidimensional, and incorporating multiple factors and relevant interactions by considering covariates jointly is needed to provide a complete picture of who is being left out of trials VanderWeele et al. (2019).
Our objective was to identify interpretable, multidimensional subgroups who were prescribed MOUD in substance use treatment programs in the US but who were not represented or were under-represented by clinical trial participants. To do so, we used data from 1) the Treatment Episode Data Set: Admissions (TEDS-A) for the US real-world data set (comprising inpatient, outpatient, and residential treatment at programs receiving federal funding) and 2) three harmonized MOUD comparative effectiveness trials that were part of the NIDA Clinical Trials Network (CTN).
2. Methods
2.1. Data and Sample.
We combined trial data (three MOUD trials, described further below, N=2,199) and real-world population data (TEDS-A data, described further below, N=740,015) across common covariates.
2.1.1. Trial data
We used data across three large randomized trials for OUD treatment that were part of the NIDA CTN: CTN0027 Potter et al. (2013); Saxon et al. (2013), which randomized 1,269 participants, mainly heroin users, to either BUP-NX or methadone, conducted 2006-2010; CTN0051 Lee et al. (2018), which randomized 570 participants to either XR-NTX or BUP-NX, conducted 2014-2017; and Phase 2 of CTN0030 Weiss et al. (2010), which randomized 360 participants, exclusively prescription opioid users, to BUP-NX and standard medical management vs. BUP-NX and medical management plus individual drug counseling, conducted 2006-2009.
All three trials enrolled adult participants over age 18 who met DSM-IV-TR criteria for opioid dependence or DSM-5 diagnosis of OUD. There was no upper age limit for enrollment. Consistent with the goals of the CTN Tai et al. (2011), the trials were broadly inclusive to treatment-seeking OUD patients and were conducted in community-based treatment programs across the US. Individuals were excluded if they had major medical and unstable psychiatric co-morbidities or were pregnant or planning to get pregnant. CTN0027 and CTN0051 included patients currently using all types of opioids, predominantly heroin users, while CTN0030 was restricted to those currently using prescription opioids and not currently using heroin. CTN0027 included patients who presented for treatment at methadone clinics, CTN0030 included patients who presented for office-based treatment, and CTN0051 included patients who presented for short-term inpatient treatment.
2.1.2. Population data
For the real-world population data, we used the TEDS-A data set, 2015-2017, which includes individuals who received treatment at substance use treatment programs (inpatient, outpatient, and residential programs) that received public funding, managed by the Substance Abuse and Mental Health Services Administration. TEDS-A does not include data from Oregon for any of the years, Georgia for 2016-2017, and South Carolina for 2015. We included TEDS-A patients: i) for whom MOUD was part of their treatment plan, and ii) who either had a DSM-IV-TR diagnosis of opioid dependence or opioid abuse or were entering treatment with heroin use, non-prescription methadone use, or other/synthetic opioid use reported at admission.
2.2. Measures.
Patient characteristics. We considered sociodemographic and clinical characteristics that were measured in both the trial and TEDS-A data and that could potentially act as modifiers of MOUD effectiveness, based on theory and previous analyses Lee et al. (2018); Nunes Jr et al. (2021): sex (male/female), age (in categories: <18, 18-34, 35-49, 50-64, >64), race/ethnicity (considered as a combined variable: non-Hispanic/Latinx white, non-Hispanic/Latinx Black, Hispanic/Latinx, non-Hispanic/Latinx other (including multiracial)), educational attainment (12 years of schooling, high school graduate, at least some college), marital status (currently married, previously married, never married), currently pregnant (yes/no), history of injection drug use, and past 30-day drug use: amphetamines, cannabis, and benzodiazepines.
2.3. Statistical analysis.
We provide a more technical and precise description of the statistical analysis in Section A of the Supplementary Materials. We first stacked the trial and TEDS-A data together, including an indicator of trial membership (yes/no) and a vector of baseline individual-level demographic and clinical covariates listed in Patient Characteristics subsection.
We were interested in characterizing those who were treated with MOUD in TEDS-A but who were not represented in any of the three trials. We describe the largest subgroups in TEDS-A who were indicated as treated with MOUD but were not represented in any of the trials. We also calculated the proportion of those treated with MOUD in TEDS-A who were not represented.
We then fit a simple classification tree Breiman et al. (1984); Therneau et al. (2015) to summarize the non-represented subgroups in terms of combinations of covariate values. A classification tree is well-suited to summarize non-represented subgroups multi-dimensionally, because each split in the tree represents an interaction between dichotomous variables, and these interactions represent relevant intersections of demographic and clinical characteristics. We used 10-fold cross-validation in fitting the tree and used a threshold complexity parameter of 0.03 to mitigate risk of over-fitting.
Nearly all of the covariates had missingness (Table A1 in the Supplementary Materials). We used multiple imputation by chained equations Buuren and Groothuis-Oudshoorn (2011) to address missing data in the covariates, resulting in 5 imputed datasets.
For each imputed dataset, we characterized the subset not represented across any of the imputations for all TEDS-A MOUD individuals. We identified this subset using all covariates listed in the Patient Characteristics subsection. In a secondary analysis, we identified this subset excluding pregnancy, as there has been at least one trial comparing buprenorphine to methadone treatment in pregnant women Jones et al. (2010), with another CTN trial ongoing Winhusen et al. (2020). In another secondary analysis, we identified the non-represented subset further excluding educational attainment and marital status characteristics, due to their large amount of missing data. We then fit the simple classification tree described above for the subset identified in the primary analysis and the subset identified without pregnancy in the secondary analysis.
R (version 4.0.4) was used for all analyses R Core Team (2020). Code to replicate the analyses is available: https://github.com/kararudolph/code-for-papers/blob/master/CTNunderrep.R.
3. Results
We found that 10.7% of individuals who were treated with MOUD in substance use treatment programs in the US were not represented in the three comparative effectiveness trials we considered (not represented across any of the imputed datasets). In Table 1 we show the 10 most common non-represented subgroups and the number of TEDS-A individuals in each subgroup. In the top portion of Table 1 we show the subgroups characterized in terms of all characteristics.
Table 1.
Largest subgroups receiving MOUD treatment in TEDS-A who are not represented in 3 MOUD trials. Abbreviations: Pop. = Population, Ethn. = Ethnicity, Preg. = Pregnancy, Amphet. = Amphetamines, Benzo. = Benzodiazepines, NH = non-Hispanic, Multi = Multiple races, HS = high school, Prev. = Previous.
| Past 30-day use of |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N of TEDS-A MOUD Pop. | Sex | Age | Race/ Ethn. | Preg. | IV drug | Amphet. | Benzo. | Cannabis | Education | Married |
| Primary analysis with all characteristics | ||||||||||
| 2917 | Men | 50-64 | NH White | No | No | No | No | No | HS | Never |
| 2181 | Women | 18-34 | NH White | Yes | No | No | No | Yes | HS | Never |
| 1669 | Women | 50-64 | NH White | No | No | No | No | No | HS | Never |
| 1408 | Women | 18-34 | NH White | Yes | No | No | No | No | HS | Never |
| 1322 | Women | 50-64 | NH Black | No | No | No | No | No | HS | Prev. |
| 1271 | Women | 18-34 | NH White | Yes | No | No | No | Yes | <HS | Never |
| 1052 | Women | 18-34 | NH White | Yes | No | No | No | Yes | >HS | Never |
| 965 | Women | 18-34 | NH Other or Multi | No | No | No | No | No | HS | Never |
| 948 | Women | 18-34 | NH White | No | No | Yes | No | Yes | <HS | Never |
| 904 | Women | 50-64 | NH Black | No | No | No | No | No | <HS | Prev. |
| Secondary analysis without pregnancy status | ||||||||||
| 2917 | Men | 50-64 | NH White | No | No | No | No | HS | Never | |
| 1673 | Women | 50-64 | NH White | No | No | No | No | HS | Never | |
| 1328 | Women | 50-64 | NH Black | No | No | No | No | HS | Prev | |
| 1124 | Women | 18-34 | NH Other or Multi | No | No | No | No | HS | Never | |
| 1051 | Women | 18-34 | NH White | No | Yes | No | Yes | <HS | Never | |
| 907 | Women | 50-64 | NH Black | No | No | No | No | <HS | Prev | |
| 807 | Women | 50-64 | NH White | No | No | No | No | <HS | Never | |
| 742 | Men | 50-64 | NH White | No | No | No | No | >HS | Prev | |
| 614 | Women | 50-64 | Hispanic/ Latinx | No | No | No | Yes | <HS | Never | |
| 590 | Women | 18-34 | NH Black | No | No | No | Yes | HS | Never | |
| Secondary analysis without pregnancy, education, and marital status | ||||||||||
| 1755 | Men | NH Black | No | No | No | No | ||||
| 1573 | Women | 18-34 | NH Black | No | No | No | Yes | |||
| 1279 | Women | 50-64 | NH Other or Multi | No | No | No | No | |||
| 1062 | Men | NH White | No | No | No | No | ||||
| 906 | Men | Hispanic/ Latinx | No | No | No | Yes | ||||
| 862 | Women | 50-64 | NH Other or Multi | No | No | No | Yes | |||
| 738 | Women | NH Black | No | No | No | No | ||||
| 609 | Men | 50-64 | Hispanic/ Latinx | No | No | Yes | No | |||
| 585 | Women | 50-64 | NH Black | No | No | Yes | No | |||
| 475 | Men | 35-49 | NH Other or Multi | Yes | No | No | Yes | |||
In the middle portion of Table 1, we exclude pregnancy status from the characteristics, because trials have been and are currently being conducted among pregnant women Jones et al. (2010); Winhusen et al. (2020). Excluding pregnancy, non-represented subgroups comprised 8.3% of individuals treated with MOUD in substance use treatment programs in the US.
In the bottom portion of Table 1, we further exclude educational attainment and marital status from the characteristics (in addition to pregnancy), because these variables had a large amount of missing data. If we exclude these variables, then the smaller set of non-represented subgroups comprised 2.6% of individuals treated with MOUD in substance use treatment programs in the US.
Table 1 describes subgroups in TEDS-A where there are no trial participants. However, if there is just one trial participant or a few trial participants for a given subgroup, that subgroup may not be well-represented enough for the purposes of later generalizing estimates from the trial to the target population. We discuss this further in the Discussion section. We therefore repeat our analyses and include results of under-represented groups where there are <5 participants representing that subgroup in the trials, shown in Table 2.
Table 2.
Largest subgroups receiving MOUD treatment in TEDS-A who are under-represented in 3 MOUD trials (i.e., <5 trial participants representing the subgroup). Abbreviations: Pop. = Population, Ethn. = Ethnicity, Preg. = Pregnancy, Amphet. = Amphetamines, Benzo. = Benzodiazepines, NH = non-Hispanic, Multi = Multiple races, HS = high school, Prev. = Previous.
| Past 30-day use of |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N of TEDS-A MOUD Pop. | Sex | Age | Race/ Ethn. | Preg. | IV drug | Amphet. | Benzo. | Cannabis | Education | Married |
| Primary analysis with all characteristics | ||||||||||
| 6230 | Men | 50-64 | NH Black | No | No | No | No | No | HS | Never |
| 4678 | Men | 50-64 | NH Black | No | No | No | No | No | <HS | Never |
| 4647 | Women | 50-64 | NH Black | No | No | No | No | No | HS | Never |
| 4044 | Men | 35-49 | NH Black | No | No | No | No | No | <HS | Never |
| 3795 | Women | 35-49 | NH Black | No | No | No | No | No | HS | Never |
| 3627 | Women | 50-64 | NH Black | No | No | No | No | No | <HS | Never |
| 3589 | Women | 35-49 | NH Black | No | No | No | No | No | <HS | Never |
| 3555 | Men | 18-34 | Hispanic/ Latinx | No | No | No | No | No | HS | Never |
| 3318 | Men | 35-49 | NH White | No | No | No | No | No | <HS | Never |
| 3234 | Women | 35-49 | NH White | No | Yes | No | No | No | <HS | Never |
| Secondary analysis without pregnancy status | ||||||||||
| 6230 | Men | 50-64 | NH Black | No | No | No | No | HS | Never | |
| 4678 | Men | 50-64 | NH Black | No | No | No | No | <HS | Never | |
| 4664 | Women | 50-64 | NH Black | No | No | No | No | HS | Never | |
| 4044 | Men | 35-49 | NH Black | No | No | No | No | <HS | Never | |
| 3864 | Women | 35-49 | NH Black | No | No | No | No | HS | Never | |
| 3661 | Women | 35-49 | NH Black | No | No | No | No | <HS | Never | |
| 3649 | Women | 50-64 | NH Black | No | No | No | No | <HS | Never | |
| 3555 | Men | 18-34 | Hispanic/ Latinx | No | No | No | No | HS | Never | |
| 3327 | Women | 35-49 | NH White | Yes | No | No | No | <HS | Never | |
| 3318 | Men | 35-49 | NH White | No | No | No | No | <HS | Never | |
| Secondary analysis without pregnancy, education, and marital status | ||||||||||
| 4167 | Women | 18-34 | NH Black | No | No | No | No | |||
| 2322 | Men | 35-49 | NH Other or Multi | No | No | No | No | |||
| 2220 | Men | 50-64 | NH Other or Multi | No | No | No | No | |||
| 2079 | Women | 50-64 | Hispanic/ Latinx | No | No | No | No | |||
| 1769 | Men | 18-34 | Hispanic/ Latinx | Yes | Yes | No | No | |||
| 1759 | Men | NH Black | Yes | No | No | No | ||||
| 1755 | Men | NH Black | No | No | No | No | ||||
| 1573 | Women | 18-34 | NH Black | Yes | No | No | No | |||
| 1396 | Men | NH White | Yes | No | No | No | ||||
| 1279 | Women | 50-64 | NH Other or Multi | No | No | No | No | |||
Excluding pregnancy, under-represented subgroups comprise 44% of the TEDS-A treatment population (including pregnancy, under-represented subgroups comprise 45%). Further excluding educational attainment and marital status, under-represented subgroups comprise 9% of the TEDS-A treatment population. Comparing Table 2 with Table 1, the number of individuals in the treatment population who fall into under-represented subgroups is much higher than those in strictly non-represented subgroups. In addition, even when pregnancy is included, none of the largest under-represented subgroups include pregnant individuals. Under-represented subgroups are dominated by non-Hispanic Black individuals who are older, never married, have lower-levels of education, and report no past-30 day use of amphetamines, benzodiazapines, nor cannabis.
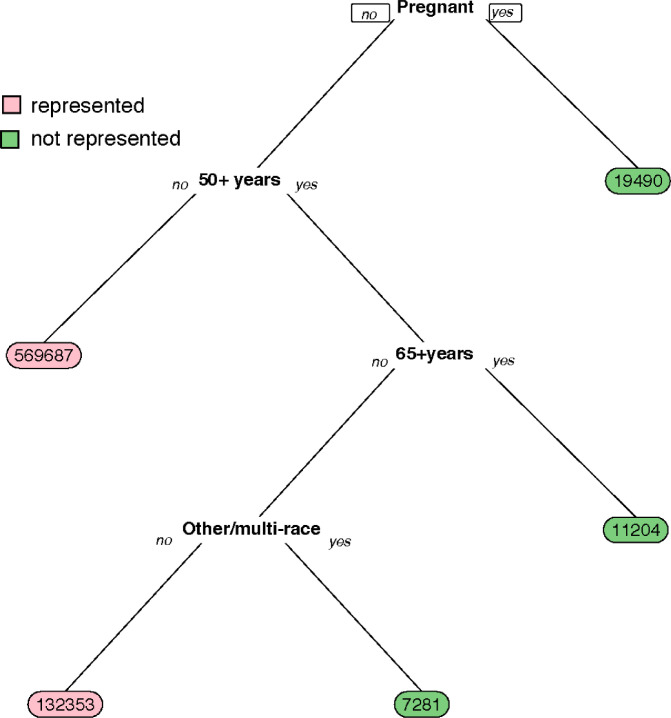
We used a classification tree to summarize commonalities across subgroups in TEDS-A who were not represented in the clinical trials. We fit a tree for each imputed data set. We show the tree from the third imputed dataset in Fig. 1. Trees of all other imputed datasets were subsets of this tree. As expected, all pregnant MOUD patients in TEDS-A (n=19,490) were not represented in any of the clinical trials. In addition, patients aged 65 years and older were under-represented (n=11,204), as well as those aged 50-64 years who identified as Other (non-White, non-Black, and non-Hispanic) race/ethnicity or multi-racial (n=7,281). The classification tree excluding pregnancy included the same splits with the exception of top split of pregnancy status (Fig. A1 in the Supplementary Materials).
Fig. 1.
Classification tree of patients receiving MOUD treatment in TEDS-A who are not represented in MOUD trials. Numbers at the end of each branch indicate the number of TEDS-A patients in the represented subgroup (pink) or non-represented subgroup (green). For example, reading from the top, pregnant TEDS-A patients (”Pregnant, yes”) were not represented (n=19,490). Patients who were not pregnant (”Pregnant, no”) and years (”50+years, yes” and ”65+years, yes”) were also not represented (n=11,204). Lastly, patients who were not pregnant (”Pregnant, no”) and were 50-64 years old (”50+years, yes” and ”65+years, no”) and Other/Multi-race (”Other/multi-race, yes”) were also not represented (n=7,281).
Although our primary goal was to identify multi-dimensional subgroups in the TEDS-A data who were non-represented or under-represented in the trials, this subgroup identification does not readily translate to a summary measure of how similar the trial data are to the population data. One recommended summary measure involves comparing the distributions of the estimated sample propensity score Tipton (2014) between the trials and TEDS-A population. In this case, the sample propensity score would be the predicted probability of being in the trials vs. TEDS-A population given covariates. The degree to which those distributions differ represents a summary of the degree of dissimilarity between the trials and population. We include these distributions in Fig. A2 of the Supplementary Materials, using the first imputed dataset and not including pregnancy status, and see generally similar distributions Tipton (2014). proposed a single-number generalizability index that essentially summarizes the difference between these two distributions, and ranges from a value of 0, meaning the trial and population are distinct, to 1, meaning the trial is a random sample of the population. The generalizability index for these data, using the first imputed dataset and not including pregnancy status, was calculated to be 0.85, indicating the trial sample is highly similar to the TEDS-A population.
4. Discussion
We quantified the number and proportion of patients treated with MOUD at substance use treatment centers across the US 2015-2017 who were not represented in three large comparative effectiveness trials for MOUD, conducted 2006-2017. We estimated that 10.7% of all MOUD patients were not represented by trials based on intersections of 10 patient characteristics, translating to 79,182 patients being treated who were not represented. Pregnant women were excluded from trials, but over 19,000 were treated with MOUD in a 3 year period, representing the largest non-represented subgroup.
Aside from pregnant women, we found that older adults ( 65 years) and middle-to-older-aged adults (50-64 years) were under-represented in the MOUD trials. Unlike pregnancy, older age was not a reason for exclusion in any of the three trials. Older MOUD patients are an increasingly relevant subpopulation, as the number of older adults entering MOUD treatment tripled 2007-2017, and the number of older adults with opioid-related emergency department visits quadrupled over this same period Carter et al. (2019); Lynch et al. (2021). This subgroup is more likely to have multiple comorbidities (e.g., cardiovascular disease, dementia) Rosen et al. (2008); Zullo et al. (2020), take medications that may interact with treatment, and metabolize treatment differently than younger patients Le Couteur et al. (2012).
We also found that MOUD patients who identified as ``other” race/ethnicity or multiracial and were middle-to-older-aged were also under-represented in the trial population. MOUD patients in TEDS-A who identified as a single, listed race other than white or Black, were most likely to identify as American Indian, Alaska Native, or Native Hawaiian (AI/AN/NH, 70%). Patients identifying as AI/AN/NH are often not included in MOUD research Lillie et al. (2021), despite having very high overdose mortality rates—second only to white patients—and these already-high rates are likely substantially underestimated Joshi et al. (2018); Scholl et al. (2019); Wilson et al. (2020). Although the included trials were conducted by CTN nodes that had coverage in Alaska, Hawaii, Arizona, New Mexico, and the Dakotas, we do not know how many of the participants identifying as “Other race” would have identified as AI/AN/NH.
Although not included in Fig. 1, many of the non-represented subgroups identified in Table 1 were women. Women comprised a majority of non-represented subgroups even when pregnancy status was excluded. This is perhaps not surprising, as women have been historically underrepresented in clinical trials across disciplines Samaei et al. (2022). MOUD patients who are women may present with several unique challenges that influence their treatment experience and probability of having a successful treatment Huhn and Dunn (2020). For example, many women (25-60%) enter MOUD treatment with a history of physical or sexual interpersonal violence (IPV), including during childhood—3-5 times the rate in the general community Brewer et al. (1998); Caetano et al. (2001); Chermack et al. (2000); De Dios et al. (2014); El-Bassel, Gilbert, Schilling, Wada, 2000, El-Bassel, Gilbert, Wu, Go, Hill, 2005; Evans et al. (2020); Santo Jr et al. (2021); Straus and Gelles (2017); Tjaden and Thoennes (1998). In fact, evidence suggests that a history of IPV increases a woman’s risk of misusing opioids Williams et al. (2021), possibly in part to cope with stress Back et al. (2011). Women entering MOUD treatment are also more likely than men to have co-morbid depression, anxiety, and/or suicidal behaviors Campbell et al. (2018); Rhee et al. (2020); Vigna-Taglianti et al. (2016), as well as co-morbid pain conditions Koons et al. (2018). Lastly, mothers entering treatment face a particularly acute form of stigma and fear of losing custody of their children Sanmartin, Ali, Lynch, 2019, Sanmartin, Ali, Lynch, Aktas, 2020; Scott et al. (2019). These challenges may present barriers to treatment initiation as well as to clinical trial participation. Recognition of these challenges can help providers facilitate delivery of comprehensive treatment that includes treatment of co-occurring psychiatric disorders, and gender-specific, trauma sensitive treatment that takes into account relationships with children and partners Barbosa-Leiker et al. (2021).
Although our primary analysis identified non-represented subgroups, it is also important to identify under-represented subgroups. To our knowledge, there is currently no proposed guideline or approach for quantifying how many units are “enough” to constitute representation, although a related topic has been discussed in the propensity score literature, seeking to quantify how many units are necessary to provide support for a contrast of interest Stürmer et al. (2010). This issue is related to practical violations of the positivity assumption in observational studies Petersen et al. (2010), and we discuss its implications and ramifications next. Future work could further examine these issues to identify best practices for identifying subgroups with poor support and mitigating estimator sensitivity and instability.
Generally, representation in clinical trials matters when there exists treatment effect heterogeneity by certain patient characteristics, meaning that the degree to which a treatment is effective, or the degree to which one treatment is more or less effective than another, differs across values of a particular patient characteristic. If treatment effects differ by certain patient characteristics and if patients with those characteristics are not well-represented in trials, then the external validity/ generalizability of the trial(s) will be threatened in at least two ways. First, treatment effectiveness of non-represented subgroups will be unknown and unable to be learned, as trial data for such patients will not exist. This means that providers treating these individuals will need to assume that treatment will function similarly for excluded patients as for included patients. Second, overall treatment effectiveness estimated from the trials (e.g., the population average effect) may not generalize to overall effectiveness in the real-world population, even after accounting for differences in the distribution of patient characteristics between trials and the usual-care population. This is because such generalization from the trial to population would rely on extrapolation for under-represented or non-represented subgroups. If the model used for extrapolation is incorrect, the resulting generalized estimates could be biased.
To our knowledge, this study is the first to quantify and characterize who is under-represented in MOUD clinical trials. Others have quantified under-representation in other types of clinical trials, but generally in terms of one patient characteristic at a time (e.g., age, race). For example, older adults are commonly under-represented in trials, including trials for cancer treatments, and even COVD-19 vaccines (where <10% of participants are years, and <1% are years), despite older adults being disproportionately more likely receive such vaccines and treatments Hutchins et al. (1999); van Marum (2020); Veronese et al. (2021); White et al. (2019). Racial/ethnic minorities are also frequently under-represented in clinical trials Betancourt and King (2003); Cavazzoni et al. (2021); FDA (2020); Sedano et al. (2022), including trials for treatment of diseases that disproportionately affect racial/ethnic minorities, like lupus Rivers et al. (2013); Williams et al. (2022). Although we know of no research assessing under-representation of MOUD trials specifically, others have identified patient characteristics (though not considered jointly) that are under-represented in substance use disorder trials in general. These include under-representation of individuals with lower educational attainment Blanco et al. (2017); Susukida et al. (2016), less than full-time employment Susukida et al. (2016), non-white race Blanco et al. (2017), who are currently married Blanco et al. (2017), with more prior treatments Susukida et al. (2016), and fewer comorbid drug use disorders Blanco et al. (2017). We also found that some racial/ethnic subgroups were under-represented in combination with some age subgroups, and also found under-representation of those with lower educational attainment, but did not find evidence of under-representation of the other previously identified characteristics.
We caution that our findings should not be interpreted as definitive in terms of MOUD trial representation. We only included three MOUD trials that were part of the NIDA CTN. MOUD treatment effectiveness is based on the results of dozens of trials Mattick, Breen, Kimber, Davoli, 2009, Mattick, Breen, Kimber, Davoli, 2014. However, the three trials we did include represent three of the largest MOUD trials conducted recently in the US, and form the basis for current clinical decision-making. In the future, it would be of interest to harmonize additional trials, particularly those targeting previously excluded groups, like pregnant women, as in the ongoing trial CTN0080 Winhusen et al. (2020).
In addition, the TEDS-A population we included—those for whom MOUD was part of their treatment plan and who had either 1) a diagnosis of opioid dependence or abuse or 2) reported current illicit opioid use at admission to the treatment program—is an imperfect approximation of the US MOUD population. For example, it is possible that those included in our TEDS-A population did not actually receive MOUD, despite it being part of their treatment plan. Also, those receiving MOUD treatment from an entity that receives no public funding would not be included.
We used the TEDS-A population data from years 2015 to 2017, which aligns with one of the trials (CTN0051) but not with the other two, which were conducted 2006-2010. If a less diverse population presented for MOUD 2006-2010, it is possible that the trials would have appeared more representative. That said, these older trials continue to inform current clinical decision-making for MOUD, so the degree to which they represent the contemporary population of those being treated for MOUD remains relevant.
As discussed above, lack of representation in clinical trials is of consequence only in cases where the non- or under-represented characteristics are also modifiers of treatment effectiveness. In terms of MOUD effectiveness and comparative effectiveness, there is evidence of treatment effect heterogeneity by the following patient characteristics: age, sex, race/ethnicity, co-morbid substance use disorders, homeless status, previous treatment, and duration of opioid use Nunes Jr et al. (2021); Olfson et al. (2020); Rudolph et al. (2021); Samples et al. (2018). Thus, it is plausible that the non-represented and under-represented subgroups we identified here are relevant in terms of limiting generalizability/external validity. However, we lacked information (from TEDS-A, the trials, or both) on several of the patient characteristics previously determined to be MOUD effect modifiers, including co-morbid substance use disorders, homeless status, previous treatment, and duration of opioid use. Consequently, we could not assess representation in terms of these potentially relevant characteristics, possibly resulting in an “optimistic” overestimate of the extent to which the trials represented the TEDS-A MOUD population.
Broadly, understanding who is and is not represented in clinical trials is fundamental for optimizing the generalizability and practical relevance of future clinical trials, including those for OUD treatment. By characterizing subgroups of individuals who initiate treatment with MOUD but who are not represented or are under-represented in MOUD trials, we hope to support better accountability for representation and inform targeting for future MOUD trial recruitment. Ultimately, quantifying and characterizing non- or under-represented subgroups in trials can provide the data necessary to improve equity and address research-to-practice gaps.
Credit Author Statment
Contributors: KER designed the study, led the analysis, drafted the paper, and revised the paper. MR contributed to the data, the data analysis, and revising the paper. SXL, JR, and EVN contributed to the data and revising the paper.
Declaration of Competing Interest
R has received medication for research studies from Alkermes. EVN has received medication for research studies from Alkermes/Cephalon, Duramed Pharmaceuticals, and Reckitt-Benckiser. The remaining authors have no conflicts of interest to report.
Acknowledgements
This work was supported by the National Institute on Drug Abuse (R00DA042127; PI Rudolph).
Footnotes
Supplementary material associated with this article can be found, in the online version, at 10.1016/j.dadr.2022.100084
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- Back S.E., Lawson K.M., Singleton L.M., Brady K.T. Characteristics and correlates of men and women with prescription opioid dependence. Addictive Behaviors. 2011;36(8):829–834. doi: 10.1016/j.addbeh.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Leiker C., Campbell A.N., McHugh R.K., Guille C., Greenfield S.F. Opioid use disorder in women and the implications for treatment. Psychiatric Research and Clinical Practice. 2021;3(1):3–11. doi: 10.1176/appi.prcp.20190051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt J.R., King R.K. Unequal treatment: the institute of medicine report and its public health implications. Public Health Reports. 2003;118(4):287. doi: 10.1016/S0033-3549(04)50252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C., Campbell A.N., Wall M.M., Olfson M., Wang S., Nunes E.V. Towards national estimates of treatment effectiveness for substance use. Journal of Clinical Psychiatry. 2017;78(1):e64. doi: 10.4088/JCP.15m10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman L., Friedman J., Stone C.J., Olshen R.A. CRC press; 1984. Classification and regression trees. [Google Scholar]
- Brewer D.D., Fleming C.B., Haggerty K.P., Catalano R.F. Drug use predictors of partner violence in opiate-dependent women. Violence and Victims. 1998;13(2):107–115. [PubMed] [Google Scholar]
- Buuren S., Groothuis-Oudshoorn K. mice: Multivariate imputation by chained equations in r. Journal of Statistical Software. 2011;45(3) [Google Scholar]
- Caetano R., Nelson S., Cunradi C. Intimate partner violence, dependence symptoms and social consequences from drinking among white, black and hispanic couples in the united states. American Journal on Addictions. 2001;10:s60–s69. doi: 10.1080/10550490150504146. [DOI] [PubMed] [Google Scholar]
- Campbell A.N., Barbosa-Leiker C., Hatch-Maillette M., Mennenga S.E., Pavlicova M., Scodes J., Saraiya T., Mitchell S.G., Rotrosen J., Novo P., et al. Gender differences in demographic and clinical characteristics of patients with opioid use disorder entering a comparative effectiveness medication trial. American Journal on Addictions. 2018;27(6):465–470. doi: 10.1111/ajad.12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M.W., Yang B.K., Davenport M., Kabel A. Increasing rates of opioid misuse among older adults visiting emergency departments. Innovation in Aging. 2019;3(1):igz002. doi: 10.1093/geroni/igz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavazzoni P., Anagnostiadis E., Lolic M. 2020 drug trials snapshots summary report. Food and Drug Administration. 2021 [Google Scholar]; https://www.fda.gov/media/145718/download
- Chermack S.T., Fuller B.E., Blow F.C. Predictors of expressed partner and non-partner violence among patients in substance abuse treatment. Drug and Alcohol Dependence. 2000;58(1-2):43–54. doi: 10.1016/s0376-8716(99)00067-8. [DOI] [PubMed] [Google Scholar]
- Connery H.S. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harvard Review of Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075. [DOI] [PubMed] [Google Scholar]
- De Dios M.A., Anderson B.J., Caviness C.M., Stein M. Intimate partner violence among individuals in methadone maintenance treatment. Substance Abuse. 2014;35(2):190–193. doi: 10.1080/08897077.2013.835764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L., Bucello C., Mathers B., Briegleb C., Ali H., Hickman M., McLaren J. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106(1):32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- El-Bassel N., Gilbert L., Schilling R., Wada T. Drug abuse and partner violence among women in methadone treatment. Journal of Family Violence. 2000;15(3):209–228. doi: 10.1007/s10896-008-9183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bassel N., Gilbert L., Wu E., Go H., Hill J. Hiv and intimate partner violence among methadone-maintained women in new york city. Social Science & Medicine. 2005;61(1):171–183. doi: 10.1016/j.socscimed.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Evans E.A., Goff S.L., Upchurch D.M., Grella C.E. Childhood adversity and mental health comorbidity in men and women with opioid use disorders. Addictive Behaviors. 2020;102:106149. doi: 10.1016/j.addbeh.2019.106149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2020. 2015-2019 drug trials snapshots summary report: Five-year summary and analysis of clinical trial participation and demographics, https://www.fda.gov/media/143592/download.
- Hser Y.-I., Mooney L.J., Saxon A.J., Miotto K., Bell D.S., Zhu Y., Liang D., Huang D. High mortality among patients with opioid use disorder in a large healthcare system. Journal of Addiction Medicine. 2017;11(4) doi: 10.1097/ADM.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]; 315–9
- Hser Y.-I., Saxon A.J., Huang D., Hasson A., Thomas C., Hillhouse M., Jacobs P., Teruya C., McLaughlin P., Wiest K., et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhn A.S., Dunn K.E. Challenges for women entering treatment for opioid use disorder. Current Psychiatry Reports. 2020;22(12):1–10. doi: 10.1007/s11920-020-01201-z. [DOI] [PubMed] [Google Scholar]
- Hutchins L.F., Unger J.M., Crowley J.J., Coltman Jr C.A., Albain K.S. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. New England Journal of Medicine. 1999;341(27):2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- Jones H.E., Kaltenbach K., Heil S.H., Stine S.M., Coyle M.G., Arria A.M., O’grady K.E., Selby P., Martin P.R., Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine. 2010;363(24):2320–2331. doi: 10.1056/NEJMoa1005359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S., Weiser T., Warren-Mears V. Drug, opioid-involved, and heroin-involved overdose deaths among american indians and alaska natives-washington, 1999–2015. Morbidity and Mortality Weekly Report. 2018;67(50):1384. doi: 10.15585/mmwr.mm6750a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koons A.L., Greenberg M.R., Cannon R.D., Beauchamp G.A. Women and the experience of pain and opioid use disorder: a literature-based commentary. Clinical Therapeutics. 2018;40(2):190–196. doi: 10.1016/j.clinthera.2017.12.016. [DOI] [PubMed] [Google Scholar]
- Le Couteur D.G., McLachlan A.J., de Cabo R. Aging, drugs, and drug metabolism. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2012;67(2):137–139. doi: 10.1093/gerona/glr084. [DOI] [PubMed] [Google Scholar]
- Lee J.D., Nunes Jr E.V., Novo P., Bachrach K., Bailey G.L., Bhatt S., Farkas S., Fishman M., Gauthier P., Hodgkins C.C., et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (x: Bot): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–318. doi: 10.1016/S0140-6736(17)32812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie K.M., Shaw J., Jansen K.J., Garrison M.M. Buprenorphine/naloxone for opioid use disorder among alaska native and american indian people. Journal of Addiction Medicine. 2021;15(4):297–302. doi: 10.1097/ADM.0000000000000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A., Arndt S., Acion L. Late-and typical-onset heroin use among older adults seeking treatment for opioid use disorder. American Journal of Geriatric Psychiatry. 2021;29(5):417–425. doi: 10.1016/j.jagp.2020.12.005. [DOI] [PubMed] [Google Scholar]
- van Marum R.J. Underrepresentation of the elderly in clinical trials, time for action. British Journal of Clinical Pharmacology. 2020;86(10):2014. doi: 10.1111/bcp.14539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick R.P., Breen C., Kimber J., Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database of Systematic Reviews. 2009;3:CD002209. doi: 10.1002/14651858.CD002209. [DOI] [PubMed] [Google Scholar]
- Mattick R.P., Breen C., Kimber J., Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub2. [DOI] [PubMed] [Google Scholar]
- Morgan J.R., Schackman B.R., Leff J.A., Linas B.P., Walley A.Y. Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a united states commercially insured population. Journal of Substance Abuse Treatment. 2018;85:90–96. doi: 10.1016/j.jsat.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of SciencesEngineering, and Medicine and others . National Academies Press; 2019. Medications for opioid use disorder save lives. [PubMed] [Google Scholar]
- Nunes Jr E.V., Scodes J.M., Pavlicova M., Lee J.D., Novo P., Campbell A.N., Rotrosen J. Sublingual buprenorphine-naloxone compared with injection naltrexone for opioid use disorder: Potential utility of patient characteristics in guiding choice of treatment. American Journal of Psychiatry. 2021;178(7) doi: 10.1176/appi.ajp.2020.20060816. [DOI] [PMC free article] [PubMed] [Google Scholar]; 660–71
- Okuda M., Hasin D.S., Olfson M., Khan S.S., Nunes E.V., Montoya I., Liu S.-M., Grant B.F., Blanco C. Generalizability of clinical trials for cannabis dependence to community samples. Drug and Alcohol Dependence. 2010;111(1-2):177–181. doi: 10.1016/j.drugalcdep.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M., Zhang V., Schoenbaum M., King M. Buprenorphine treatment by primary care providers, psychiatrists, addiction specialists, and others: Trends in buprenorphine treatment by prescriber specialty-primary care providers, psychiatrists, and addiction medicine specialists. Health Affairs. 2020;39(6):984–992. doi: 10.1377/hlthaff.2019.01622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.L., Porter K.E., Gruber S., Wang Y., van der Laan M.J. Diagnosing and responding to violations in the positivity assumption. Statistical methods in medical research. 2010 doi: 10.1177/0962280210386207. [DOI] [PMC free article] [PubMed] [Google Scholar]; 0962280210386207
- Potter J.S., Marino E.N., Hillhouse M.P., Nielsen S., Wiest K., Canamar C.P., Martin J.A., Ang A., Baker R., Saxon A.J., et al. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: findings from starting treatment with agonist replacement therapies (start) Journal of Studies on Alcohol and Drugs. 2013;74(4):605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team, 2020. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/.
- Rhee T.G., Peltier M.R., Sofuoglu M., Rosenheck R.A. Do sex differences among adults with opioid use disorder reflect sex-specific vulnerabilities? a study of behavioral health comorbidities, pain, and quality of life. Journal of Addiction Medicine. 2020;14(6):502–509. doi: 10.1097/ADM.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers D., August E.M., Sehovic I., Green B.L., Quinn G.P. A systematic review of the factors influencing african americans’ participation in cancer clinical trials. Contemporary Clinical Trials. 2013;35(2):13–32. doi: 10.1016/j.cct.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Rosen D., Smith M.L., Reynolds III C.F. The prevalence of mental and physical health disorders among older methadone patients. American Journal of Geriatric Psychiatry. 2008;16(6):488–497. doi: 10.1097/JGP.0b013e31816ff35a. [DOI] [PubMed] [Google Scholar]
- Rudolph K.E., Díaz I., Luo S.X., Rotrosen J., Nunes E.V. Optimizing opioid use disorder treatment with naltrexone or buprenorphine. Drug and Alcohol Dependence. 2021:109031. doi: 10.1016/j.drugalcdep.2021.109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaei M., McGregor A.J., Jenkins M.R. Inclusion of women in fda-regulated premarket clinical trials: A call for innovative and recommended action. Contemporary Clinical Trials. 2022;116:106708. doi: 10.1016/j.cct.2022.106708. [DOI] [PubMed] [Google Scholar]
- Samples H., Williams A.R., Olfson M., Crystal S. Risk factors for discontinuation of buprenorphine treatment for opioid use disorders in a multi-state sample of medicaid enrollees. Journal of Substance Abuse Treatment. 2018;95:9–17. doi: 10.1016/j.jsat.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin M.X., Ali M.M., Lynch S. Foster care admissions and state-level criminal justice-focused prenatal substance use policies. Children and Youth Services Review. 2019;102:102–107. [Google Scholar]
- Sanmartin M.X., Ali M.M., Lynch S., Aktas A. Association between state-level criminal justice–focused prenatal substance use policies in the us and substance use–related foster care admissions and family reunification. JAMA Pediatrics. 2020;174(8):782–788. doi: 10.1001/jamapediatrics.2020.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo Jr T., Campbell G., Gisev N., Tran L.T., Colledge S., Di Tanna G.L., Degenhardt L. Prevalence of childhood maltreatment among people with opioid use disorder: A systematic review and meta-analysis. Drug and Alcohol Dependence. 2021;219:108459. doi: 10.1016/j.drugalcdep.2020.108459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A.J., Ling W., Hillhouse M., Thomas C., Hasson A., Ang A., Doraimani G., Tasissa G., Lokhnygina Y., Leimberger J., et al. Buprenorphine/naloxone and methadone effects on laboratory indices of liver health: a randomized trial. Drug and Alcohol Dependence. 2013;128(1-2):71–76. doi: 10.1016/j.drugalcdep.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl L., Seth P., Kariisa M., Wilson N., Baldwin G. Drug and opioid-involved overdose deaths–united states, 2013–2017. Morbidity and Mortality Weekly Report. 2019;67(51-52):1419. doi: 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L.F., Stone C., Duwve J. Policy perceptions of us state public health and child and family services regarding maternal opioid use and neonatal exposure. Archives of Psychiatric Nursing. 2019;33(5):22–30. doi: 10.1016/j.apnu.2019.04.002. [DOI] [PubMed] [Google Scholar]
- Sedano R., Hogan M., Mcdonald C., Aswani-Omprakash T., Ma C., Jairath V. Underrepresentation of minorities and underreporting of race and ethnicity in crohn’s disease clinical trials. Gastroenterology. 2022;162(1):338–340. doi: 10.1053/j.gastro.2021.09.054. [DOI] [PubMed] [Google Scholar]
- Soares III W.E., Melnick E.R., Nath B., D’Onofrio G., Paek H., Skains R.M., Walter L.A., Casey M.F., Napoli A., Hoppe J.A., et al. Emergency department visits for nonfatal opioid overdose during the covid-19 pandemic across 6 us healthcare systems. Annals of Emergency Medicine. 2021;79(2) doi: 10.1016/j.annemergmed.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; 158–67
- Straus M.A., Gelles R.J. Physical violence in American families. Routledge; 2017. How violent are american families? estimates from the national family violence resurvey and other studies; pp. 95–112. [Google Scholar]
- Stürmer T., Rothman K.J., Avorn J., Glynn R.J. Treatment effects in the presence of unmeasured confounding: dealing with observations in the tails of the propensity score distribution–a simulation study. American journal of epidemiology. 2010;172(7):843–854. doi: 10.1093/aje/kwq198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susukida R., Crum R.M., Stuart E.A., Ebnesajjad C., Mojtabai R. Assessing sample representativeness in randomized controlled trials: application to the national institute of drug abuse clinical trials network. Addiction. 2016;111(7):1226–1234. doi: 10.1111/add.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai B., Sparenborg S., Liu D., Straus M. The national drug abuse treatment clinical trials network: forging a partnership between research knowledge and community practice. Substance Abuse and Rehabilitation. 2011;2:21–28. doi: 10.2147/SAR.S16756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2020. https://www.cdc.gov/drugoverdose/data/analysis.html (accessed July 11, 2020). Opioid Data Analysis and Resources
- Centers for Disease Control and Prevention, 2020. Overdose Deaths Accelerating During COVID-19. Accessed: 2021-02-03 https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html, (accessed Feb 2, 2021).
- Centers for Medicare & Medicaid Services, 2020. https://www.cms.gov/About-CMS/Agency-Information/Emergency/EPRO/Current-Emergencies/Ongoing-emergencies (accessed July 11, 2020). Ongoing emergencies & disasters.
- Therneau, T., Atkinson, B., Ripley, B., Ripley, M. B., 2015. Package ‘rpart’. Available online: cran.ma.ic.ac.uk/web/packages/rpart/rpart.pdf (accessed on 20 April 2016).
- Tipton E. How generalizable is your experiment? an index for comparing experimental samples and populations. Journal of Educational and Behavioral Statistics. 2014;39(6):478–501. [Google Scholar]
- Tjaden, P., Thoennes, N., 1998. Prevalence, incidence, and consequences of violence against women: Findings from the national violence against women survey. research in brief, https://eric.ed.gov/?id=ED434980 accessed Mar 30, 2022.
- VanderWeele T.J., Luedtke A.R., van der Laan M.J., Kessler R.C. Selecting optimal subgroups for treatment using many covariates. Epidemiology (Cambridge, Mass.) 2019;30(3) doi: 10.1097/EDE.0000000000000991. [DOI] [PMC free article] [PubMed] [Google Scholar]; 334–41
- Veronese N., Petrovic M., Benetos A., Denkinger M., Gudmundsson A., Knol W., Marking C., Soulis G., Maggi S., Cherubini A., et al. Underrepresentation of older adults in clinical trials on covid-19 vaccines: a systematic review. Ageing Research Reviews. 2021;71:101455. doi: 10.1016/j.arr.2021.101455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigna-Taglianti F.D., Burroni P., Mathis F., Versino E., Beccaria F., Rotelli M., Garneri M., Picciolini A., Bargagli A.M., Group V.S. Gender differences in heroin addiction and treatment: results from the vedette cohort. Substance Use & Misuse. 2016;51(3):295–309. doi: 10.3109/10826084.2015.1108339. [DOI] [PubMed] [Google Scholar]
- Weiss R.D., Potter J.S., Provost S.E., Huang Z., Jacobs P., Hasson A., Lindblad R., Connery H.S., Prather K., Ling W. A multi-site, two-phase, prescription opioid addiction treatment study (poats): rationale, design, and methodology. Contemporary Clinical Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.N., Dotan E., Catalano P.J., Cardin D.B., Berlin J.D. Advanced pancreatic cancer clinical trials: The continued underrepresentation of older patients. Journal of Geriatric Oncology. 2019;10(4):540–546. doi: 10.1016/j.jgo.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.N., Dall’Era M., Lim S.S., Feldman C.H., Arntsen K.A., Blazer A.D., Goode T., Merrill J.T., Sheikh S., Stevens A.M., et al. Increasing ancestral diversity in systemic lupus erythematosus clinical studies. Arthritis Care & Research. 2022;74(3) doi: 10.1002/acr.24474. [DOI] [PMC free article] [PubMed] [Google Scholar]; 420–42
- Williams J.R., Girdler S., Williams W., Cromeens M.G. The effects of co-occurring interpersonal trauma and gender on opioid use and misuse. Journal of Interpersonal Violence. 2021;36(23-24):NP13185–NP13205. doi: 10.1177/0886260519900309. [DOI] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith IV H., Davis N.L. Drug and opioid-involved overdose deaths–united states, 2017–2018. Morbidity and Mortality Weekly Report. 2020;69(11):290. doi: 10.15585/mmwr.mm6911a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhusen T., Lofwall M., Jones H.E., Wilder C., Lindblad R., Schiff D.M., Wexelblatt S., Merhar S., Murphy S.M., Greenfield S.F., et al. Medication treatment for opioid use disorder in expectant mothers (moms): Design considerations for a pragmatic randomized trial comparing extended-release and daily buprenorphine formulations. Contemporary Clinical Trials. 2020;93:106014. doi: 10.1016/j.cct.2020.106014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo A.R., Moyo P., Jutkowitz E., Zhang W., Thomas K.S. Opioid use disorder among hospitalized older adults: Prevalence, characteristics, and discharge status. Journal of the American Medical Directors Association. 2020;21(4):557–559. doi: 10.1016/j.jamda.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/