Abstract
Background:
Small cell neuroendocrine (NE) carcinomas of the prostate classically lose androgen receptor (AR) expression, may harbor loss of the RB1, TP53 and PTEN tumor suppressor genes, and are associated with a poor prognosis. However usual-type adenocarcinomas may also contain areas of NE differentiation, and in this context the molecular features and biological significance are less certain.
Methods:
We examined the molecular phenotype and oncologic outcomes of primary prostate adenocarcinomas with ≥5% NE differentiation (≥5% chromogranin A-positive NE cells in any given tumor spot on tissue microarray) using 3 independent study sets: a set of tumors with Paneth cell-like NE differentiation (n=26), a retrospective case-cohort of intermediate- and high-risk patients enriched for adverse outcomes (n = 267), and primary tumors from a retrospective series of men with eventual castration-resistant metastatic prostate cancer (CRPC) treated with abiraterone or enzalutamide (n=55).
Results:
Benign NE cells expressed significantly lower quantified AR levels compared to paired benign luminal cells (p<0.001). Similarly, Paneth-like NE carcinoma cells or carcinoma cells expressing chromogranin A expressed significantly lower quantified AR levels than paired non-NE carcinoma cells (p<0.001). Quantified ERG protein expression, was also lower in chromogranin A-labeled adenocarcinoma cells compared to unlabeled cells (p<0.001) and tumors with NE differentiation showed lower gene expression scores for AR activity compared to those without. Despite evidence of lower AR signaling, adenocarcinomas with NE differentiation did not differ by prevalence of TP53 missense mutations, or PTEN or RB1 loss, compared to those without NE differentiation. Finally, NE differentiation was not associated with time to metastasis in intermediate- and high-risk patients (p=0.6 on multivariate analysis), nor with progression-free survival in CRPC patients treated with abiraterone or enzalutamide (p=0.9).
Conclusion:
NE differentiation in usual-type primary prostate adenocarcinoma is a molecularly and clinically distinct form of lineage plasticity from that occurring in small cell NE carcinoma.
Keywords: Prostate adenocarcinoma, neuroendocrine differentiation, androgen receptor, Paneth cells
Introduction
Neuroendocrine (NE) cells occur scattered within the epithelium of normal prostate glands, where they secrete peptide hormones and affect target cells by autocrine, paracrine and endocrine function (1, 2). In addition to normal NE cells, a wide spectrum of NE differentiation may be seen in both primary and metastatic prostate carcinoma (3, 4). Perhaps the best studied form of NE differentiation in prostate cancer - prostatic small cell NE carcinoma - is a highly aggressive tumor that may occur in the primary setting or develop as a secondary resistance mechanism to therapies targeting the androgen signaling axis (3, 4). Such tumors classically lose androgen receptor (AR) expression and gain expression of neuroendocrine markers such as chromogranin A and synaptophysin (5, 6). These tumors are thought to arise from epithelial cells via lineage plasticity facilitated by key underlying molecular alterations, including loss of the RB1, TP53 and PTEN tumor suppressor genes (7–11). Consistent with this model of transdifferentiation, tumors with hybrid features between small cell NE carcinoma and high-grade adenocarcinoma are increasingly recognized, particularly in the post-treatment setting where they may also be associated with a poor prognosis (3, 12).
Importantly, however, a similar form of lineage plasticity is commonly seen in untreated usual-type prostatic adenocarcinomas, where neuroendocrine markers are expressed in 10–100% of cases, depending on the cohort examined and the methodology used (3, 4, 13, 14). Neuroendocrine cells within usual-type adenocarcinomas are frequently impossible to recognize morphologically without immunohistochemical stains for chromogranin A, synaptophysin or N-CAM, or they may take the form of the more easily identified Paneth cell-like neuroendocrine cells, which have prominent eosinophilic cytoplasmic granules similar to their intestinal counterparts (15, 16). In either case, the biological and clinical significance of this lineage plasticity within otherwise usual-type prostatic adenocarcinomas remains unclear. Most contemporary studies in surgical cohorts and conservatively managed patients have found that NE differentiation in usual-type prostatic adenocarcinomas is not independently associated with adverse clinical outcomes, though it may be more common in high grade disease (16–18). However, a minority of studies have suggested that this may represent a form of lineage plasticity on the spectrum of that described in small cell carcinoma (8, 9) or hybrid NE tumors. Like prostatic small cell NE carcinoma, NE differentiation in adenocarcinomas is more prevalent after androgen deprivation and in metastatic castration resistant prostate cancer (mCRPC) (19). This raises the question of whether extensive NE differentiation within usual-type primary tumors could be a harbinger of future castration resistance and poor prognosis.
To better characterize the biology and clinical significance of NE lineage plasticity within usual-type prostatic adenocarcinomas, we examined the molecular phenotype and oncologic outcomes of these tumors in several large contemporary cohorts.
Materials and Methods
Patients and tissue samples:
With Johns Hopkins institutional review board-approval conducted under a waiver of consent, three patient sets were included in this study. 1) The first set included 26 cases with morphologically identified Paneth cell-like neuroendocrine differentiation (both radical prostatectomies and transrectal core-needle biopsies on standard histologic slides), including cases from Johns Hopkins Hospital, the Johns Hopkins uropathology consultation service and a previously published Weill Cornell Medicine cohort (20). 2) The second set included primary tumors at radical prostatectomy on a tissue microarray (TMA) constructed from a previously described retrospective case-cohort for metastatic progression, including 267 intermediate- or high-risk patients (predominantly European-American) who underwent radical prostatectomy between 1992 and 2010 and received no additional treatment until the time of metastasis (Intermediate/High Risk cohort) (21, 22). 3) The third set included primary tumor tissues on TMA from a previously described cohort of patients who received abiraterone or enzalutamide treatment for castration-resistant prostate cancer between 2010 and 2015 at Johns Hopkins and were followed for progression-free survival after these therapies (Abiraterone/Enzalutamide cohort) (23). All TMAs described above included three to four individual 0.6 mm punches of the dominant tumor nodule from each case (slightly more than 1 mm2 of tissue for analysis).
Immunohistochemistry (IHC):
Previously described and genetically validated IHC protocols for p53 (BP53–11, Roche/Ventana Medical Systems, Tucson, AZ) (22), PTEN (D4.3, Cell Signaling Technology) (24–26), ERG (Clone EPR3864; Roche/Ventana Medical Systems) (27) and Cyclin D1 (SP4-R, Roche/Ventana Medical Systems) (28) were performed on the Ventana Benchmark autostainer. Chromogranin A (LK2H10, Roche/Ventana Medical Systems, Tucson, AZ), INSM1 (A8, Santa Cruz Biotechnology, Dallas, TX) and AR (androgen receptor) (SP107, CellMarque/Millipore/Sigma, Rocklin, CA) IHC were also performed on the Ventana Benchmark autostainer.
Immunohistochemistry scoring:
Each TMA spot containing tumor cells was visually assessed for the percent of adenocarcinoma cells with chromogranin A IHC signal by a urologic pathologist (TLL and HBK). A spot was considered to have significant neuroendocrine differentiation if ≥5% of the cells were visually chromogranin A positive in at least one TMA spot and was not considered to have significant neuroendocrine differentiation otherwise. This cutoff was based on prior studies (17, 18) and was further validated by gene expression profiling as discussed in the Results below. Each TMA spot containing tumor cells was visually dichotomously scored for presence or absence of nuclear p53 and ERG or cytoplasmic PTEN staining signal using genetically validated and previously reported scoring systems (22–26, 29). As previously validated for assessment of RB1 functional status, cyclin D1 loss was also dichotomously scored (28).
Immunofluorescence (IF):
To quantify AR expression level in NE cells and surrounding non-NE cells, we performed dual IF with AR (Cell Signaling Technology, D6F11, XP®, 1:100 dilution) and neuroendocrine marker INSM1 (Clone A-8, Santa Cruz Biotechnology, Dallas, TX; 1:100 dilution) for Paneth cell-like cases, or with chromogranin A (Santa Cruz SC-393941 1:100 dilution) for benign prostate tissue and usual-type prostate cancer cases with NE differentiation. The 13 benign prostate tissues included in these experiments were stained on TMA sections selected from radical prostatectomy specimens. The 10 Paneth cell-like NE prostate carcinoma cases were stained on standard histologic sections and included biopsies and radical prostatectomy samples. Usual-type prostate cancer cases with NE differentiation were selected for AR quantification from two radical prostatectomy cohorts described above, the Intermediate/High Risk cohort (7 cases on standard histologic sections) and the Abiraterone/Enzalutamide cohort (20 cases on TMA spots) if they contained ≥5% NE cells based on chromogranin A immunohistochemistry (described above) and had available tissue for analysis. After standard deparaffinization and antigen retrieval, the slides were first incubated with primary antibody (AR and chromogranin A or AR and INSM1) overnight at 4°C and then were incubated with secondary antibodies (Alexafluor-488 or Alexafluor-594 conjugated, anti- Rabbit or anti-Mouse IgG, Thermo Fisher Scientific) at a dilution of 1:200 for 1 hour at room temperature. Subsequently they were dehydrated in graded ethanol and mounted with ProLong Gold Antifade with DAPI (Thermo Fisher Scientific).
To quantify and compare the ERG protein expression between NE cells and surrounding non-NE cells, we performed dual immunofluorescence with ERG (Clone EPR3864; Roche/Ventana Medical Systems) and chromogranin A (Santa Cruz SC-393941, 1:100 dilution) in usual-type prostate cancer cases with NE differentiation and ERG protein expression. 15 usual-type prostate cancer cases with ≥5% NE differentiation (by chromogranin A) and ERG expression by previous IHC were selected for ERG IF quantification from two radical prostatectomy cohorts described above, the Intermediate/High Risk cohort (7 cases on standard histologic sections) and the Abiraterone/Enzalutamide cohort (8 cases on TMA spots). A similar IF protocol to the one described above for AR and chromogranin A was followed.
Immunofluorescence quantification:
Images were analyzed using ImageJ. For each stained section, a region of interest was manually drawn around chromogranin A- or INSM1-labeled NE cells, and a threshold was applied to remove background noise. For benign NE cells, quantification was done on TMA spots, with a median of 1 region per spot analyzed (range: 1–2 regions). For adenocarcinoma cells with Paneth cell-like NE differentiation, 10 cases were scored on standard histologic slides with median of 8 regions analyzed (range: 4–13 regions). For adenocarcinoma cells with immunohistochemically-detected NE differentiation, 20 samples were scored on TMA spots with median of 1 region per spot analyzed (range: 1–7 regions) and 7 cases were scored on standard histologic slides with median of 26 regions analyzed (range: 8–52 regions). Analogous regions of interest were manually drawn around surrounding luminal tumor or benign cells for comparison to NE cells. The mean fluorescence intensity of AR within the regions of interest was measured by multiplying the mean fluorescence density by the area of the region of interest.
We similarly quantified the ERG protein expression among neuroendocrine and non-neuroendocrine prostate adenocarcinoma cells. For tumors from the Intermediate-High Risk cohort, 7 cases were scored on standard histologic slides with median of 5 regions analyzed (range: 2–22 regions) and for tumors from the Abiraterone/Enzalutamide cohort, 8 samples were scored on TMA spots with median of 1.5 regions per spot analyzed (range: 1–4 regions). A similar ERG quantification protocol to the one described above for AR was followed.
Gene expression analysis:
Messenger RNA expression profiling for the Intermediate/High-Risk cohort was previously reported on the Affymetrix 1.0ST microarray platform using punches from formalin-fixed paraffin-embedded tumors (21) (GSE79957). Data were available for 94% (252/267) of tumors with IHC staining results.
Statistical Analysis:
Paired, two sided T-tests were used to compare AR expression levels in NE and non-NE cells. For comparison of cases with and without significant NE differentiation, Wilcoxon test was used for continuous variables (age and gene expression scores) and Chi-squared test or Fisher’s Exact test was used for all other categorical variables. For each TMA set, tests were 2-sided with a p-value of 0.05 deemed statistically significant.
Results
AR expression.
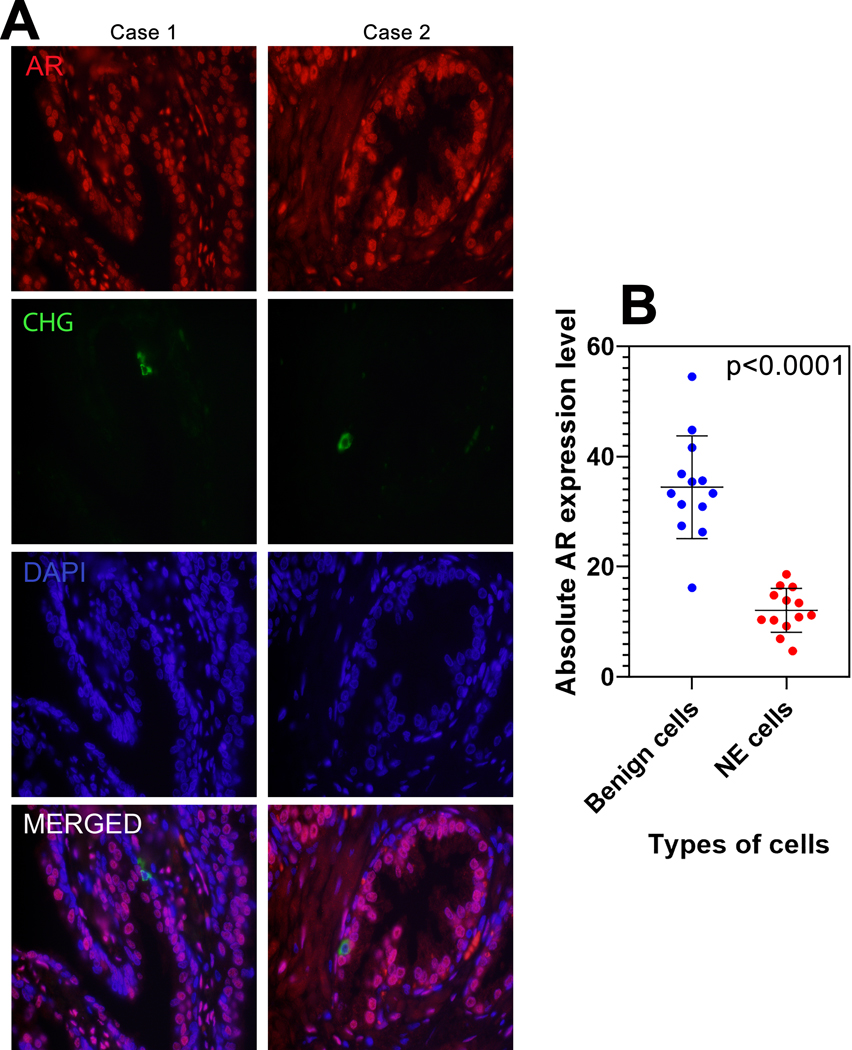
Several previous studies have examined AR levels in benign prostatic NE cells and in carcinomas with NE differentiation and most have reported expression of AR to be negative in the benign NE cells and low or variable in the malignant cells (30–33). However, to our knowledge, no prior studies have quantified AR expression in prostatic NE cells. To begin to address this question, we used double-labeling by immunofluorescence to identify chromogranin A-expressing NE cells in benign prostatic tissue and quantified AR expression in these cells relative to their non-chromogranin A labeled luminal epithelial counterparts. Quantified median AR expression in benign NE cells was significantly lower than that in benign prostatic luminal cells (11.0 vs 33.3 A.U.; p<0.0001) (Figure 1).
Figure 1: AR expression in benign NE cells.
(A) Representative AR and chromogranin A immunostaining in formalin fixed paraffin embedded benign prostate glands. The histologic sections were subjected to indirect immunofluorescence with anti-AR (red) and anti-chromogranin (green) antibodies to detect neuroendocrine cells and quantify AR expression. Images reduced from 630X. (B) Absolute androgen receptor (AR) expression level comparison between benign luminal and benign neuroendocrine cells. p-value by paired T-test.
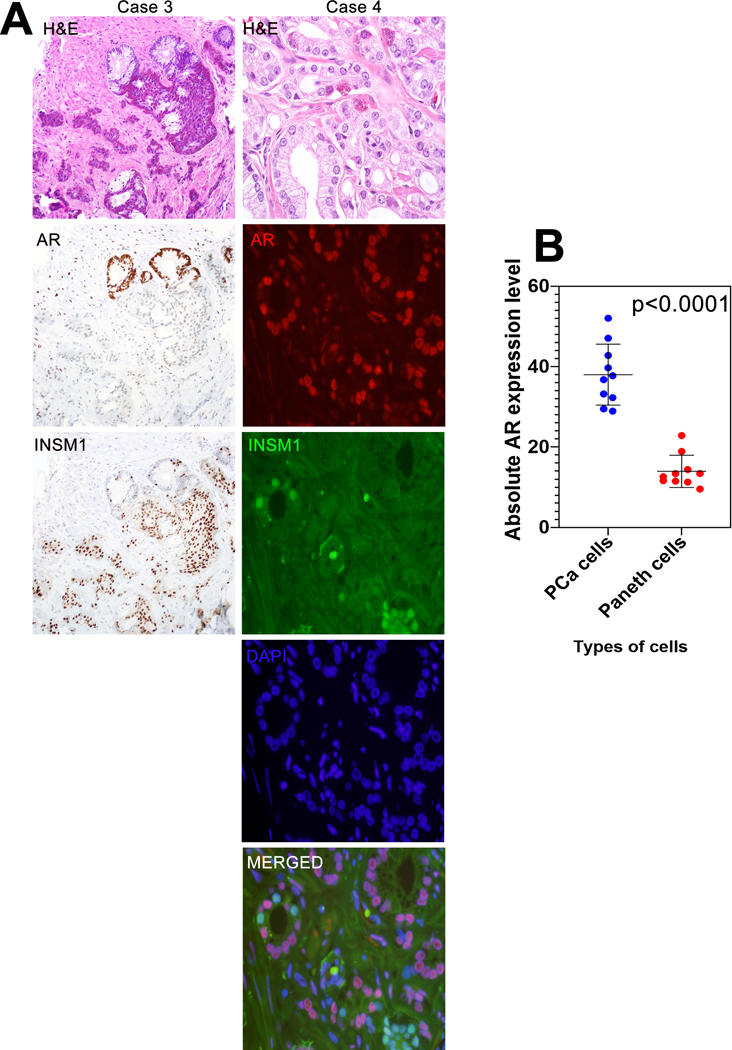
We next performed a similar experiment using prostatic adenocarcinoma with Paneth cell-like NE differentiation, comparing nuclear AR expression in Paneth cell-like tumor cells labeled by INSM1 and non-NE carcinoma cells that were INSM1-negative. The clinical-pathologic features of these cases are included in Table 1. We used INSM1 labelling in this case to identify NE cells because the large number of NE granules in Paneth cell-like tumor cells frequently obscured the nucleus for AR detection when labeled with chromogranin A (Figure 2A). Using dual labeling by immunofluorescence, quantified median AR expression was significantly lower in Paneth cell-like tumor cells compared to non-NE carcinoma cells (13.0 vs. 37.2 A.U.; p<0.0001) (Figure 2B).
Table 1.
Clinical-pathologic and molecular characteristics of prostate adenocarcinomas with Paneth cell-like NE differentiation
| Gleason grade groups, N (%) | |
| 1 | 7 (27) |
| 2 | 9 (35) |
| 3 | 7 (27) |
| 4 | 2 (8) |
| PIN-like ductal | 1 (4) |
| Tumor stage, N (%) | |
| T2 | 11 (69) |
| T3a | 5 (31) |
| T3b | - |
| ERG, N (%) | |
| Negative | 17 (77) |
| Positive | 5 (23) |
| PTEN, N (%) | |
| Intact | 16 (62) |
| Loss | 10 (38) |
| p53, N (%) | |
| Nuclear accumulation absent | 22 (88) |
| Nuclear accumulation present | 3 (12) |
| Cyclin D1, N (%) | |
| Intact | 23 (100) |
| Loss | 0 (0) |
Figure 2: AR expression in Paneth cell-like carcinoma cells.
(A) Representative H&E, AR and INSM1 immunostaining (Case 3) in formalin fixed and paraffin embedded primary prostate tumors with Paneth cell-like differentiation. INSM1 labeling clearly identified Paneth cell-like tumor cells by immunohistochemical labeling and AR expression in an adjacent section showed decreased AR expression when compared to non-NE carcinoma cells. Case 4 shows representative H&E, with dual immunofluorescence for AR (red) and INSM1 (green). Images reduced from 200X (IHC) and 630X (Immunofluorescence). (B) Absolute androgen receptor (AR) expression level comparison between Paneth-like cells and prostate adenocarcinoma (PCa) cells. p-value by paired T-test.
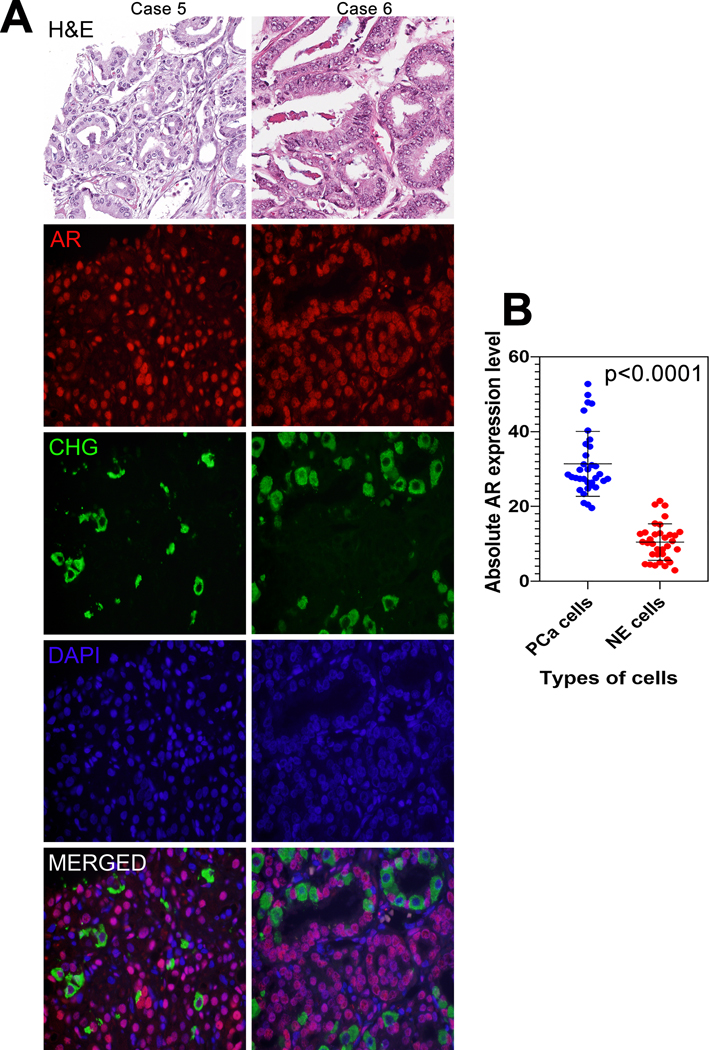
Finally, we examined AR expression in usual-type prostatic adenocarcinoma with significant NE differentiation, labeling the NE carcinoma cells with chromogranin A and comparing them to adenocarcinoma cells that did not express chromogranin A (Figure 3A). Quantified median AR expression level in NE carcinoma cells was significantly lower than that in non-NE carcinoma cells (10.4 vs. 28.2 A.U., p<0.0001) (Figure 3B).
Figure 3: AR expression in NE carcinoma cells.
(A) Representative H&E with AR (red) and chromogranin A (green) dual immunofluorescence in formalin fixed and paraffin embedded usual-type primary prostate tumors with significant NE differentiation. Images reduced from 630X. (B) Absolute androgen receptor (AR) expression level comparison between neuroendocrine (NE) cells and prostate adenocarcinoma (PCa) cells. p-value by paired T-test.
AR signaling.
To more quantitatively assess downstream AR signaling in NE cells by immunohistochemistry, we examined ERG protein expression. ERG gene rearrangements occur in approximately half of prostatic adenocarcinomas, and may themselves be mediated in part by androgen signaling (34, 35). Once an ERG rearrangement occurs, ERG expression is typically controlled by the androgen-regulated promoter of the 5’ ERG fusion partner gene. We and others have previously shown that tumors without AR expression (such as prostatic small cell NE carcinoma), or prostate adenocarcinomas exposed to previous androgen deprivation therapy (ADT), have low or undetectable ERG expression even in the presence of an ERG gene rearrangement (6, 36). Thus, in the context of ERG gene rearrangement, ERG protein expression can serve as a readout of AR signaling activity.
We first examined the prevalence of ERG protein expression within prostatic adenocarcinomas with NE differentiation as a group. Surprisingly, a majority of the tumors with Paneth cell-like differentiation were ERG-negative (homogenously negative including both Paneth cells and usual adenocarcinoma cells) and only 23% (5/22) of tumors with Paneth-like cells were ERG-positive (Table 1), despite the fact that Paneth-like cells only comprise a small proportion of tumor cellularity. This rate is lower than that seen in prostate acinar adenocarcinomas, where typically half of all cases are ERG-positive. Next, we sought to examine the frequency of ERG expression within prostatic adenocarcinomas with immunohistochemically-detected NE differentiation using two large primary tumor cohorts, described in the Methods section and in Tables 2 and 3. Based on prior studies (17, 18), we defined significant NE differentiation within these adenocarcinomas as the presence of ≥5% chromogranin A-positive NE cells in any given tumor spot on tissue microarray. This cutoff was further validated by gene expression analyses described below. We compared these tumors to those with fewer than 5% chromogranin A-positive NE cells. In the Intermediate/High-Risk cohort, tumors with significant NE differentiation comprised 9% (23/267) of patients, while in the Abiraterone/Enzalutamide cohort, these were identified in 29% (16/55) of patients. There were no significant differences in clinical-pathologic parameters (including race, Gleason score, pathologic stage or margin status) stratified by presence or absence of NE differentiation in either cohort (Tables 2 and 3). Overall, ERG-positive tumors were found in 40% (105/265) of the Intermediate/High-Risk cohort and 45% (25/55) of the Abiraterone/Enzalutamide cohort however there was no significant difference in frequency of ERG-positive tumors between those with and without NE differentiation in either cohort (Tables 2 and 3).
Table 2.
Clinical-pathologic and molecular characteristics of prostate adenocarcinomas from Intermediate/High-risk cohort stratified by NE differentiation statusa
| Significant NE differentiation absent (n=244) | Significant NE differentiation present (n=23) | p valueb | |
|---|---|---|---|
| Median Age, Years | 61 | 58 | 0.3 |
| Race, N (%) | |||
| White | 220 (90.2) | 21 (91.3) | 0.9 |
| Non-White | 24 (9.8) | 2 (8.7) | |
| Gleason grade groups, N (%) | |||
| 1 | 1 (0.4) | 0 (0.0) | 0.9 |
| 2 | 101 (41.4) | 11 (47.8) | |
| 3 | 50 (20.5) | 5 (21.7) | |
| 4 | 28 (11.5) | 2 (8.7) | |
| 5 | 61 (25.0) | 5 (21.7) | |
| Stage, N (%) | |||
| T2 | 74 (30.3) | 3 (13.0) | 0.05 |
| T3a | 122 (50.0) | 22 (47.8) | |
| T3b | 45 (18.4) | 9 (39.1) | |
| Margin, N (%) | |||
| Negative | 182 (74.6) | 16 (69.6) | 0.5 |
| Positive | 59 (24.2) | 7 (30.4) | |
| ERG, N (%) | |||
| Negative | 149 (61.1) | 11 (47.8) | 0.2 |
| Positive | 93 (38.1) | 12 (52.2) | |
| PTEN, N (%) | |||
| Intact | 165 (67.6) | 14 (60.9) | 0.5 |
| Loss | 79 (32.4) | 9 (39.1) | |
| p53, N (%) | |||
| Nuclear accumulation absent | 228 (93.4) | 22 (95.6) | 0.9 |
| Nuclear accumulation present | 16 (6.6) | 1 (4.4) | |
| Cyclin D1, N (%) | |||
| Intact | 267 (100) | 267 (100) | 1.0 |
| Loss | 0 (0) | 0 (0) |
Significant NE differentiation defined as the presence of ≥5% chromogranin-positive NE cells in any given tumor spot on tissue microarray.
Wilcoxon test was used for age. Chi-squared test or Fisher’s Exact test was used for the categorical variables.
Table 3.
Clinical-pathologic and molecular characteristics of prostate adenocarcinomas from Abiraterone/Enzalutamide cohort stratified by NE differentiation statusa
| Significant NE differentiation absent (n=39) | Significant NE differentiation present (n=16) | p valueb | |
|---|---|---|---|
| Median age, Years | 59 | 61 | 0.3 |
| Race, N (%) | |||
| White | 33 (86.9) | 15 (93.8) | 0.5 |
| Non-White | 5 (13.1) | 1 (6.2) | |
| Gleason grade groups, N (%) | |||
| 1 | 1 (2.6) | 0 (00 | 0.4 |
| 2 | 9 (23.0) | 5 (31.2) | |
| 3 | 11 (28.2) | 4 (25) | |
| 4 | 6 (15.4) | 0 (0) | |
| 5 | 12 (30.8) | 7 (43.8) | |
| Stage b , N (%) | |||
| T2 | 2/38 (5.3) | 3 (18.75) | 0.4 |
| T3a | 15 (39.5) | 4 (25) | |
| T3b | 10 (26.3) |
5 (31.25) |
|
| N1 | 11 (28.9) | 4 (25.0) | |
| Margin, N (%) | |||
| Negative | 16 (41.0) | 10 (62.5) | 0.1 |
| Positive | 23 (59.0) | 6 (37.5) | |
| ERG, N (%) | |||
| Negative | 21 (53.8) | 9 (56.2) | 0.9 |
| Positive | 18 (46.2) | 7 (43.8) | |
| PTEN, N (%) | |||
| Intact | 19 (48.7) | 8 (50) | 0.9 |
| Loss | 20 (51.3) | 8 (50) | |
| P53, N (%) | |||
| Nuclear accumulation absent | 30 (77.0) | 11 (68.7) | 0.5 |
| Nuclear accumulation present | 9 (23.0) | 5 (31.3) | |
| Cyclin D1, N (%) | |||
| Intact | 39 (100) | 15 (93.8) | 0.3 |
| Loss | 0 (0) | 1 (6.2) |
Significant NE differentiation defined as the presence of ≥5% chromogranin-positive NE cells in any given tumor spot on tissue microarray.
Wilcoxon test was used for age. Chi-squared test or Fisher’s Exact test was used for the categorical variables.
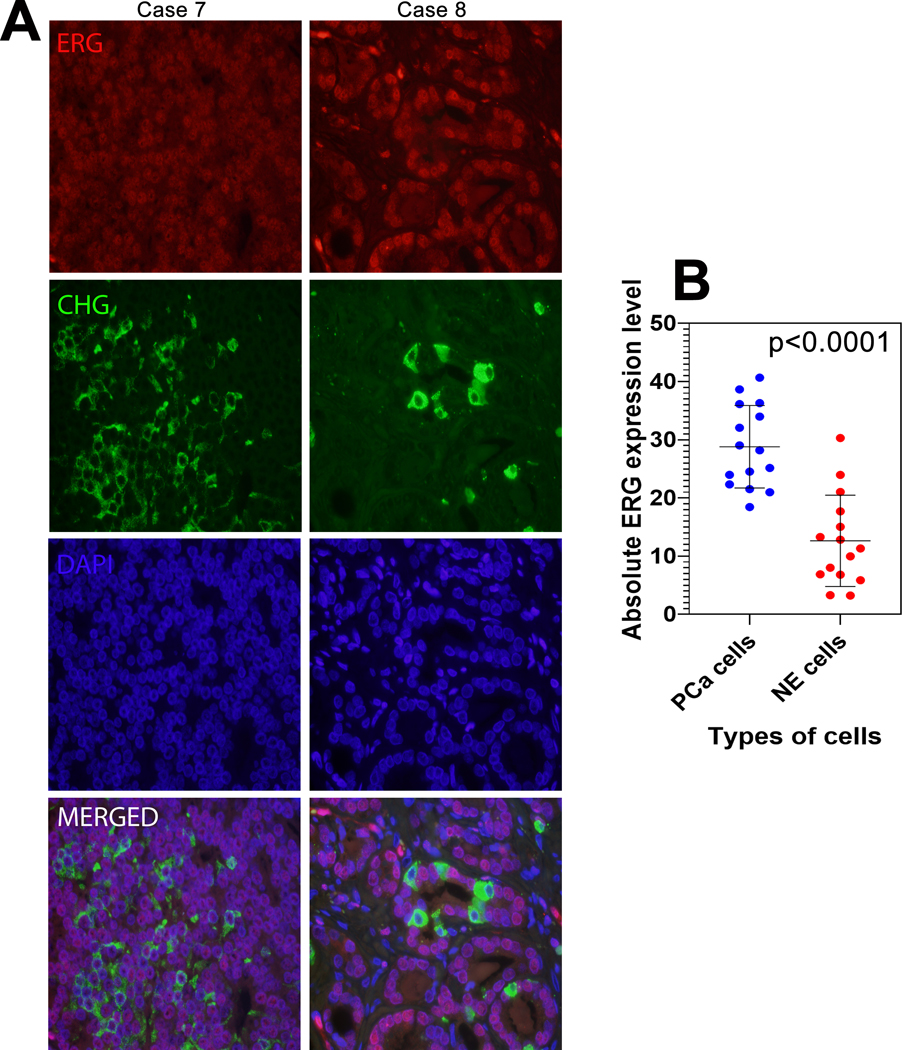
Next, we quantified ERG protein expression among NE and non-NE adenocarcinoma cells from 15 ERG-positive tumors with significant NE differentiation. Quantified median ERG expression was significantly lower in chromogranin A-labeled adenocarcinoma cells compared to paired unlabeled cells overall (11.3 vs. 28.2 A.U.; p<0.0001) (Figure 4).
Figure 4: ERG expression in NE carcinoma cells.
(A) Representative ERG (red) and chromogranin A (green) immunofluorescence in formalin fixed and paraffin embedded usual-type primary prostate tumors with significant NE differentiation. (B) Absolute ERG expression level comparison between neuroendocrine (NE) cells and prostate adenocarcinoma (PCa) cells. p-value by paired T-test.
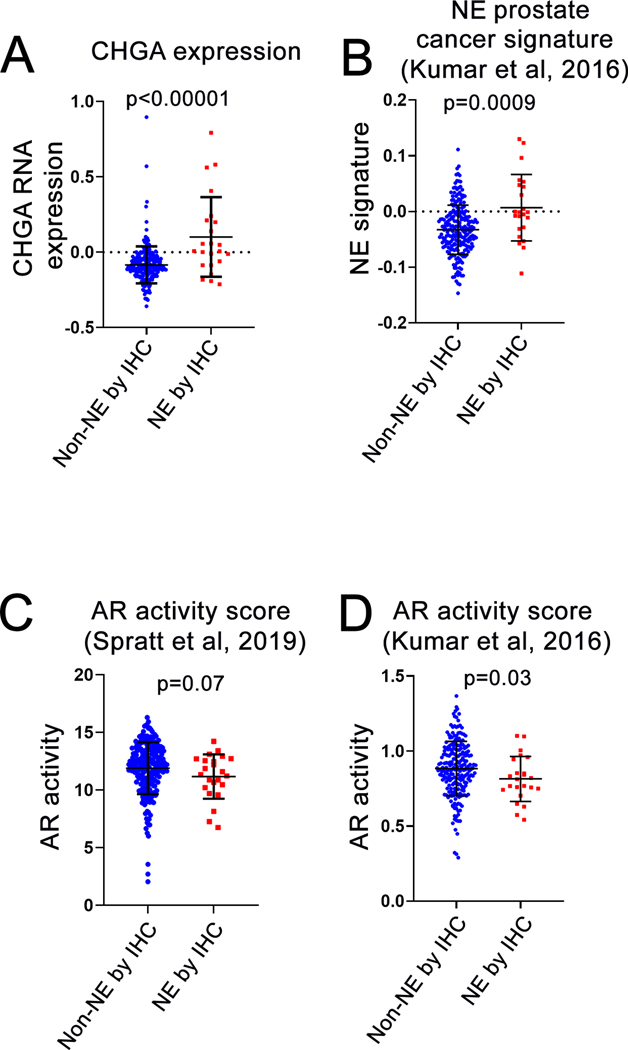
The lower level of both AR and ERG expression among NE adenocarcinoma cells compared to non-NE adenocarcinoma cells suggested that downstream AR signaling may be lower specifically within NE tumor cells. However the NE cells comprise only a small fraction of the total tumor cell population in each case. To determine whether AR signaling levels are lower overall in tumors with significant NE differentiation compared to those without, we queried previously published bulk tumor gene expression data for AR activity and neuroendocrine markers in the Intermediate/High Risk cohort (21). We first validated our TMA-based IHC scoring cutoff for NE differentiation (≥5% cells with chromogranin A protein expression) using gene expression data. As expected, CHGA gene expression was significantly higher in tumors with ≥5% NE differentiation by chromogranin A IHC compared to those without (median 0.008 vs −0.093, p<0.00001). Similarly, a previously published prostatic small cell carcinoma gene expression signature (37) was also significantly higher in tumors with ≥5% NE differentiation by IHC (median score −0.001 vs −0.033, p=0.0009) (Figure 5A and B). Next, we examined two different validated AR activity (AR-A) scores in this cohort which summarize gene expression levels across sets of canonical AR transcriptional target genes (37–39). Using one such score called AR-A (38, 39), tumors with significant NE differentiation defined by IHC had somewhat lower AR-A scores overall compared to those without (median AR-A score of 11.3 vs 12.2, p=0.07). Similar results were observed using another validated AR activity gene expression signature (37) (median AR activity score of 0.81 vs 0.87, p=0.03) (Figure 5C and D). Taken together, we conclude that individual NE cells in prostatic adenocarcinoma demonstrate significantly lower AR and downstream AR target protein expression compared to non-NE cells, and AR activity is modestly suppressed when measured in bulk RNA extracted from tumors with ≥5% NE differentiation.
Figure 5: NE and androgen receptor activity gene expression in carcinomas with and without significant NE differentiation.
Comparison of tumors with and without significant neuroendocrine (NE) differentiation (as defined by a 5% cutoff for chromogranin A staining) in Intermediate/High Risk cohort (21) for (A) CHGA gene expression, (B) NE prostate cancer signature activity (37), (C) AR activity score (AR-A) (38, 39) and (D) AR activity (37). p-values by Wilcoxon test.
Prevalence of PTEN, TP53 and RB1 inactivation by validated immunohistochemistry assays in prostatic adenocarcinomas with NE differentiation.
Previous studies have demonstrated that tumors with Paneth cell-like NE differentiation may harbor AURKA gene amplifications, a molecular alteration seen in small cell NE carcinoma (20, 40). This raises the question of whether extensive NE differentiation within prostatic adenocarcinomas is associated with other molecular alterations seen in small cell NE carcinoma, such as PTEN, TP53 and RB1 tumor suppressor inactivation. Using genetically validated immunohistochemistry assays (22, 25, 28), we first examined the prevalence of loss of these tumor suppressors in tumors with Paneth cell-like differentiation. Overall, 38% (10/26) of these tumors showed PTEN loss, 12% (3/25) had nuclear p53 accumulation signifying underlying inactivating TP53 missense mutations, and no cases (0/23) had cyclin D1 loss, a sensitive surrogate for underlying RB1 loss or dysfunction in prostatic small cell carcinoma (28) (Table 1). p53 immunohistochemistry data for the Intermediate/High-Risk cohort (22, 41) and PTEN and p53 immunohistochemistry data in the Abiraterone/Enzalutamide cohorts (23) were previously reported. Here, we added PTEN IHC for the Intermediate/High-risk cohort and cyclin D1 IHC for both cohorts and examined these data stratified by presence or absence of NE differentiation. We found no significant differences between prevalence of PTEN loss, nuclear p53 over-expression or cyclin D1 loss among adenocarcinomas with and without significant NE differentiation (Tables 2 and 3). Consistent with our prior observations that RB1 inactivation is extremely rare in primary prostate adenocarcinoma (7, 28), no cases in the Intermediate/High-risk cohort and only one case in the Abiraterone/Enzalutamide cohort had cyclin D1 loss. The case with cyclin D1 loss was an unusual adenocarcinoma noted to have hybrid features with small cell NE carcinoma.
Oncologic outcomes in prostatic adenocarcinomas with NE differentiation.
Finally, we examined clinical outcomes in the Intermediate/High-risk and Abiraterone/Enzalutamide cohorts stratified by presence or absence of significant NE differentiation. In the Intermediate/High-risk cohort, there was no association of NE differentiation with prostate cancer metastasis after radical prostatectomy, either on univariable or multivariable analysis (Table 4). Similarly, Kaplan-Meier analysis of progression-free or overall survival among men with surgically treated and subsequently metastatic prostate cancer treated with abiraterone or enzalutamide did not show any significant association of NE differentiation in the primary tumor with response to these therapies (Supplementary Figure S1).
Table 4.
Hazard Ratio (HR) of Prostate Cancer Metastasis in Intermediate/High-risk Cohort
| Univariable Analysis | Multivariable Analysisa | |||||
|---|---|---|---|---|---|---|
| Significant NE differentiation | N (Case/Control) | HR (95% CI) | p value | HR (95% CI) | p value | |
| Absent | 15/188 | 1.00 [Reference] | 1.00 [Reference] | |||
| Present | 12/107 | 1.23 (0.66–2.20) | 0.5 | 0.78 (0.34–1.81) | 0.6 | |
Adjusted for age, PSA, race, Gleason score, stage and surgical margin status.
Discussion
Our study is among the first to quantify androgen receptor (AR) expression and to molecularly characterize NE cells occurring in usual-type prostatic adenocarcinoma particularly with respect to p53, PTEN and Rb1 status. We find that NE cells have significantly reduced AR expression compared to surrounding benign luminal cells or non-NE adenocarcinoma cells. This attenuated AR expression is also reflected in the decrease in androgen-dependent downstream targets, such as ERG in tumors with ERG gene rearrangements. Consistent with our quantitative findings, previous qualitative studies have also suggested that NE adenocarcinoma cells may have lower expression of downstream AR signaling targets such as PSA (33). We found that while AR signaling was decreased on a cellular level in NE cells in untreated prostate cancers, it was only modestly decreased at the tumor-level using AR gene expression activity scores in tumors with significant numbers of NE cells by IHC. Interestingly, tumors with Paneth cell-like differentiation also appear less likely to have ERG gene rearrangements (which may themselves be AR regulated (35)) than most usual-type adenocarcinomas, though these data require validation in a larger cohort. Yet, whether AR suppression is a cause or an effect of NE differentiation in prostate cancer remains unclear. Numerous studies have shown that NE differentiation is more common after androgen deprivation therapy (ADT) (19, 42, 43), and untreated tumors with low AR activity by gene expression are enriched for NE gene expression (39). Though some mechanisms linking AR signaling inhibition to molecular drivers of NE differentiation have been proposed in vitro (44–46), it remains unclear whether AR inhibition is necessary or sufficient to initiate NE differentiation in vivo.
Despite the observed decrease in AR expression and AR signaling in tumors with NE differentiation, we demonstrate that they are not significantly different from tumors without NE differentiation in terms of the frequency of common molecular alterations such as PTEN, TP53 and RB1 loss. Though recent data suggest that TP53 and RB1 co-loss facilitate lineage plasticity in prostate cancer and are associated with aggressive disease (8, 9), our findings indicate that NE differentiation in usual-type adenocarcinomas may be independent of these tumor suppressors. These results are largely concordant with outcome data presented in this study as well as previous publications. Though some older studies have reported that NE differentiation is associated with adverse outcomes (47–49), other studies have found a limited or no prognostic association (50–54). Of interest, we did find that NE differentiation was more prevalent in the Abiraterone/Enzalutamide cohort (where all patients subsequently developed metastatic prostate cancer) compared to the Intermediate/High-risk cohort (where only a third of patients had metastases). Nonetheless, we observed that NE differentiation is not associated with overall or progression-free survival and time to metastasis in surgically treated prostate cancer cohort, consistent with our findings that p53/PTEN/Rb1 tumor suppressor loss is also not enriched among cases with NE differentiation. To our knowledge, our study is the first to test whether the extent of NE differentiation in the primary tumor is associated with subsequent response to second-generation AR signaling antagonists (abiraterone and enzalutamide). In light of these findings, the fact that NE differentiation is more prevalent in metastatic castration-resistant prostate cancer (mCRPC) may simply reflect the direct or indirect effects of AR signaling modulation on NE transdifferentiation (19, 42) rather than an enrichment of NE differentiation in aggressive tumor subsets (19).
There are some limitations of the current study. First, the outcomes analyses were performed using TMAs and NE cells can be very heterogeneously localized. Thus, there may be a bias with tumor sampling which may not reflect the true extent of NE differentiation in usual-type prostate adenocarcinoma. Nonetheless, we were able to show that NE gene expression and NE gene expression signatures were significantly higher in tumors with NE differentiation by IHC. Since different tumor samples were used for IHC scoring and gene expression analyses, these data suggest that our sampling method does capture tumor heterogeneity to some extent. Second, we identified NE cells in prostate adenocarcinoma by chromogranin A immunostaining alone rather than with several NE markers. Chromogranin A is the most specific and well-studied marker of NE differentiation in prostate cancer to date (3) and we further validated it by showing that chromogranin A expression by IHC correlates with a gene expression signature of prostatic small cell carcinoma. However, newer NE markers such as INSM1 have performed superiorly compared to chromogranin A in other organ systems (55, 56). A recent study compared expression of NE markers in prostate cancer and demonstrated that INSM1 was a more sensitive and specific marker for detecting prostatic small cell carcinoma compared with chromogranin A (57). Finally, we used a visually scored 5% cutoff to identify significant NE differentiation within prostatic adenocarcinomas, rather than using a digitally quantified continuous scale for this marker. Given previous studies and the aforementioned issues of heterogeneity with detecting these markers on TMAs, we felt that additional quantification was not likely to add significantly to the current analyses and we further validated this cut-off with gene expression analyses herein.
Conclusions
In conclusion, we quantitatively demonstrate that benign and malignant NE cells in the prostate have significantly lower AR expression and downstream AR signaling compared to luminal benign and malignant non-NE prostate cells. This difference at the cellular level is paralleled by a more modest decrease in overall AR signaling among prostate tumors with NE differentiation compared to those without. Despite this similarity to small cell carcinoma in terms of decreased AR signaling, prostate adenocarcinomas with NE differentiation are not enriched for other molecular alterations seen in small cell carcinomas, such as RB1, TP53 or PTEN tumor suppressor gene loss, and are not associated with adverse oncologic outcomes in diverse patient cohorts. Thus, these prostate cancers should not be grouped with those showing small cell NE histology.
Supplementary Material
Supplementary Figure S1: Progression-free survival and overall survival after enzalutamide or abiraterone treatment of metastatic prostate cancer, stratified by presence (CHG 1) or absence (CHG 0) of significant NE differentiation (≥5% Chromogranin A positive cells on IHC).
Financial Support:
This work was supported in part by the NIH/NCI Prostate SPORE P50CA58236; and the NCI Cancer Center Support Grant 5P30CA006973–52.
Footnotes
Data accessibility: The raw data for mRNA expression profiling reported herein is deposited in the GEO database under accession number GSE79957.
Disclosure/Conflict of Interest: TLL has received research support from Ventana Medical Systems and GenomeDx Biosciences for other studies. ESA and BLM discloses no relevant financial conflicts of interest.
References:
- 1.Di Sant’Agnese PA, Cockett AT. The prostatic endocrine-paracrine (neuroendocrine) regulatory system and neuroendocrine differentiation in prostatic carcinoma: a review and future directions in basic research. The Journal of urology 1994;152:1927–31. [DOI] [PubMed] [Google Scholar]
- 2.Fetissof F, Dubois MP, Arbeille-Brassart B, et al. Endocrine cells in the prostate gland, urothelium and Brenner tumors. Immunohistological and ultrastructural studies. Virchows Arch B Cell Pathol Incl Mol Pathol 1983;42:53–64. [DOI] [PubMed] [Google Scholar]
- 3.Epstein JI, Amin MB, Beltran H, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38:756–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fine SW. Neuroendocrine tumors of the prostate. Mod Pathol 2018;31:S122–32. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Epstein JI. Small cell carcinoma of the prostate. A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol 2008;32:65–71. [DOI] [PubMed] [Google Scholar]
- 6.Lotan TL, Gupta NS, Wang W, et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod Pathol 2011;24:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan HL, Sood A, Rahimi HA, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 2014;20:890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mu P, Zhang Z, Benelli M, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017;355:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ku SY, Rosario S, Wang Y, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tzelepi V, Zhang J, Lu JF, et al. Modeling a lethal prostate cancer variant with small-cell carcinoma features. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18:666–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aparicio AM, Shen L, Tapia EL, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin Cancer Res 2016;22:1520–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 2018;36:2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mucci NR, Akdas G, Manely S, Rubin MA. Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression. Human pathology 2000;31:406–14. [DOI] [PubMed] [Google Scholar]
- 14.di Sant’Agnese PA. Neuroendocrine differentiation in human prostatic carcinoma. Human pathology 1992;23:287–96. [DOI] [PubMed] [Google Scholar]
- 15.Adlakha H, Bostwick DG. Paneth cell-like change in prostatic adenocarcinoma represents neuroendocrine differentiation: report of 30 cases. Hum Pathol 1994;25:135–9. [DOI] [PubMed] [Google Scholar]
- 16.Tamas EF, Epstein JI. Prognostic significance of paneth cell-like neuroendocrine differentiation in adenocarcinoma of the prostate. Am J Surg Pathol 2006;30:980–5. [DOI] [PubMed] [Google Scholar]
- 17.Jeetle SS, Fisher G, Yang ZH, et al. Neuroendocrine differentiation does not have independent prognostic value in conservatively treated prostate cancer. Virchows Arch 2012;461:103–7. [DOI] [PubMed] [Google Scholar]
- 18.Cindolo L, Cantile M, Franco R, et al. Parallel determination of NeuroD1, chromogranin-A, KI67 and androgen receptor expression in surgically treated prostate cancers. International braz j urol : official journal of the Brazilian Society of Urology 2011;37:57–66. [DOI] [PubMed] [Google Scholar]
- 19.Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol 2004;45:586–92; discussion 92. [DOI] [PubMed] [Google Scholar]
- 20.Park K, Chen Z, MacDonald TY, et al. Prostate cancer with Paneth cell-like neuroendocrine differentiation has recognizable histomorphology and harbors AURKA gene amplification. Hum Pathol 2014;45:2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross AE, Johnson MH, Yousefi K, et al. Tissue-based Genomics Augments Post-prostatectomy Risk Stratification in a Natural History Cohort of Intermediate- and High-Risk Men. Eur Urol 2016;69:157–65. [DOI] [PubMed] [Google Scholar]
- 22.Guedes LB, Almutairi F, Haffner MC, et al. Analytic, Preanalytic, and Clinical Validation of p53 IHC for Detection of TP53 Missense Mutation in Prostate Cancer. Clin Cancer Res 2017;23:4693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maughan BL, Guedes LB, Boucher K, et al. p53 status in the primary tumor predicts efficacy of subsequent abiraterone and enzalutamide in castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 2018;21:260–8. [DOI] [PubMed] [Google Scholar]
- 24.Lotan TL, Gurel B, Sutcliffe S, et al. PTEN protein loss by immunostaining: analytic validation and prognostic indicator for a high risk surgical cohort of prostate cancer patients. Clin Cancer Res 2011;17:6563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotan TL, Wei W, Ludkovski O, et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod Pathol 2016;29:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotan TL, Heumann A, Rico SD, et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget 2017;8:65566–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morais CL, Herawi M, Toubaji A, et al. PTEN loss and ERG protein expression are infrequent in prostatic ductal adenocarcinomas and concurrent acinar carcinomas. Prostate 2015;75:1610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai H, Morais CL, Alshalalfa M, et al. Cyclin D1 Loss Distinguishes Prostatic Small-Cell Carcinoma from Most Prostatic Adenocarcinomas. Clin Cancer Res 2015;21:5619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaux A, Albadine R, Toubaji A, et al. Immunohistochemistry for ERG expression as a surrogate for TMPRSS2-ERG fusion detection in prostatic adenocarcinomas. Am J Surg Pathol 2011;35:1014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krijnen JL, Janssen PJ, Ruizeveld de Winter JA, et al. Do neuroendocrine cells in human prostate cancer express androgen receptor? Histochemistry 1993;100:393–8. [DOI] [PubMed] [Google Scholar]
- 31.Bonkhoff H, Stein U, Remberger K. Androgen receptor status in endocrine-paracrine cell types of the normal, hyperplastic, and neoplastic human prostate. Virchows Arch A Pathol Anat Histopathol 1993;423:291–4. [DOI] [PubMed] [Google Scholar]
- 32.Nakada SY, di Sant’Agnese PA, Moynes RA, et al. The androgen receptor status of neuroendocrine cells in human benign and malignant prostatic tissue. Cancer Res 1993;53:1967–70. [PubMed] [Google Scholar]
- 33.Huang J, Yao JL, di Sant’Agnese PA, et al. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate 2006;66:1399–406. [DOI] [PubMed] [Google Scholar]
- 34.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644–8. [DOI] [PubMed] [Google Scholar]
- 35.Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet 2010;42:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Udager AM, Shi Y, Tomlins SA, et al. Frequent Discordance Between ERG Gene Rearrangement and ERG Protein Expression in a Rapid Autopsy Cohort of Patients With Lethal, Metastatic, Castration-Resistant Prostate Cancer. Prostate 2014;74:1199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Coleman I, Morrissey C, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med 2016;22:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faisal FA, Sundi D, Tosoian JJ, et al. Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur Urol 2016;70:14–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spratt DE, Alshalalfa M, Fishbane N, et al. Transcriptomic Heterogeneity of Androgen Receptor Activity Defines a de novo low AR-Active Subclass in Treatment Naive Primary Prostate Cancer. Clin Cancer Res 2019;25:6721–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosquera JM, Beltran H, Park K, et al. Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia (New York, NY) 2013;15:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaur HB, Lu J, Guedes LB, et al. TP53 missense mutation is associated with increased tumor-infiltrating T cells in primary prostate cancer. Hum Pathol 2019;87:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology 1998;51:585–9. [DOI] [PubMed] [Google Scholar]
- 43.Ito T, Yamamoto S, Ohno Y, et al. Up-regulation of neuroendocrine differentiation in prostate cancer after androgen deprivation therapy, degree and androgen independence. Oncology reports 2001;8:1221–4. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Zheng D, Zhou T, et al. Androgen deprivation promotes neuroendocrine differentiation and angiogenesis through CREB-EZH2-TSP1 pathway in prostate cancers. Nat Commun 2018;9:4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abida W, Bryce AH, Balar AV, et al. TRITON2: An international, multicenter, open-label, phase II study of the PARP inhibitor rucaparib in patients with metastatic castration-resistant prostate cancer (mCRPC) associated with homologous recombination deficiency (HRD). Journal of Clinical Oncology 2018;36:TPS388-TPS. [Google Scholar]
- 46.Bishop JL, Thaper D, Vahid S, et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov 2017;7:54–71. [DOI] [PubMed] [Google Scholar]
- 47.Theodorescu D, Broder SR, Boyd JC, Mills SE, Frierson HF, Jr. Cathepsin D and chromogranin A as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer 1997;80:2109–19. [PubMed] [Google Scholar]
- 48.Cohen RJ, Glezerson G, Haffejee Z. Neuro-endocrine cells--a new prognostic parameter in prostate cancer. Br J Urol 1991;68:258–62. [DOI] [PubMed] [Google Scholar]
- 49.Bostwick DG, Qian J, Pacelli A, et al. Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol 2002;168:1204–11. [DOI] [PubMed] [Google Scholar]
- 50.Noordzij MA, van der Kwast TH, van Steenbrugge GJ, Hop WJ, Schroder FH. The prognostic influence of neuroendocrine cells in prostate cancer: results of a long-term follow-up study with patients treated by radical prostatectomy. International journal of cancer 1995;62:252–8. [DOI] [PubMed] [Google Scholar]
- 51.Cohen MK, Arber DA, Coffield KS, et al. Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer 1994;74:1899–903. [DOI] [PubMed] [Google Scholar]
- 52.Casella R, Bubendorf L, Sauter G, et al. Focal neuroendocrine differentiation lacks prognostic significance in prostate core needle biopsies. J Urol 1998;160:406–10. [PubMed] [Google Scholar]
- 53.Abrahamsson PA, Cockett AT, di Sant’Agnese PA. Prognostic significance of neuroendocrine differentiation in clinically localized prostatic carcinoma. The Prostate Supplement 1998;8:37–42. [PubMed] [Google Scholar]
- 54.Niederst MJ, Sequist LV, Poirier JT, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rooper LM, Bishop JA, Westra WH. INSM1 is a Sensitive and Specific Marker of Neuroendocrine Differentiation in Head and Neck Tumors. Am J Surg Pathol 2018;42:665–71. [DOI] [PubMed] [Google Scholar]
- 56.Rooper LM, Sharma R, Li QK, Illei PB, Westra WH. INSM1 Demonstrates Superior Performance to the Individual and Combined Use of Synaptophysin, Chromogranin and CD56 for Diagnosing Neuroendocrine Tumors of the Thoracic Cavity. Am J Surg Pathol 2017;41:1561–9. [DOI] [PubMed] [Google Scholar]
- 57.Xin Z, Zhang Y, Jiang Z, et al. Insulinoma-associated protein 1 is a novel sensitive and specific marker for small cell carcinoma of the prostate. Hum Pathol 2018;79:151–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1: Progression-free survival and overall survival after enzalutamide or abiraterone treatment of metastatic prostate cancer, stratified by presence (CHG 1) or absence (CHG 0) of significant NE differentiation (≥5% Chromogranin A positive cells on IHC).