Abstract
Chronic pain coexists with disability, anxiety, depression, and sleep disturbances, which are factors of pain chronicity in the fear-avoidance model. Self-efficacy for managing pain plays a protective role against pain chronicity. For chronic pain sufferers, social support from caregivers is important. However, such caregivers face enormous physical and mental burdens. This study aimed to assess how self-efficacy and factors related to the fear-avoidance model affect caregiver burden. Participants were 135 chronic pain patients and their caregivers who visited our outpatient pain special clinic. In clinical assessments, numeric rating scale (NRS), pain catastrophizing scale (PCS), hospital anxiety and depression scale (HADS), Athens insomnia scale (AIS), pain disability assessment scale (PDAS), pain self-efficacy questionnaire (PSEQ) for the patients and Zarit Burden Interview (ZBI) for their caregivers were evaluated. Participants were divided into 2 groups (L group ZBI < 24 points and H group ZBI ≥ 24 points) and compared. Regression analyses were conducted to identify factors correlated with the ZBI scores. Compared to L group, H group showed significantly higher NRS and HADs depression scores, and lower PSEQ scores. In univariate regression analysis, ZBI scores were significantly correlated with NRS, PCS, HADS anxiety, HADS depression, PDAS and PSEQ. Multiple linear regression analysis revealed that ZBI scores were significantly correlated with PSEQ. The caregivers who perceived high caregiver burden had significantly higher patients’ pain intensity, depression, and lower self-efficacy than those who perceived low caregiver burden. Caregiver burden correlated with the pain intensity, pain catastrophizing, anxiety, depression, disability, and self-efficacy of chronic pain patients. Among these factors, self-efficacy was the most negatively correlated with caregiver burden. Treatments focused on increasing self-efficacy for managing pain have the potential to reduce caregiver burden.
Keywords: caregiver burden, chronic pain, fear-avoidance model, self-efficacy, Zarit Burden Interview
1. Introduction
Chronic pain is defined as pain lasting beyond normal tissue-healing time, which is generally considered to be 12 weeks. It is a major health care problem, with a weighted mean prevalence of 20% in adults.[1,2] The World Health Organization has recognized that chronic pain is a public health problem that occurs throughout the world. It has assessed growing evidence and determined that the prevalence of chronic pain in the general population is high internationally.[3] Chronic pain coexists with disability, anxiety, depression, and sleep disturbances,[4] which are considered factors of pain chronicity in the fear-avoidance model.[5,6] In this model, the patient’s perception of the pain experience is catastrophic, resulting in anxiety, depression, and insomnia that disturb daily activities, further exacerbating the pain.[7] On the other hand, self-efficacy for managing pain plays a protective role against pain chronicity factors.[8,9]
While caregiver support of chronic pain patients is important because of patients’ decreased activities of daily living,[10] caregivers who provide substantial daily support often face considerable physical and mental burdens. Although a previous study revealed that depression, insomnia, and disability were related to the caregiver burden for such patients,[11] evidence regarding relationships between caregiver burden, patient self-efficacy, and the factors of pain chronicity in chronic pain patients is still lacking. We hypothesized that in chronic pain patients, factors of pain chronicity and low self-efficacy would be associated with increased caregiver burden through an increased need for support of daily activity. Therefore, the objective of this study is to investigate the relationships among the fear-avoidance model factors, self-efficacy of the patients, and caregiver’s burden in cases of chronic pain patients.
2. Methods
2.1. Participants
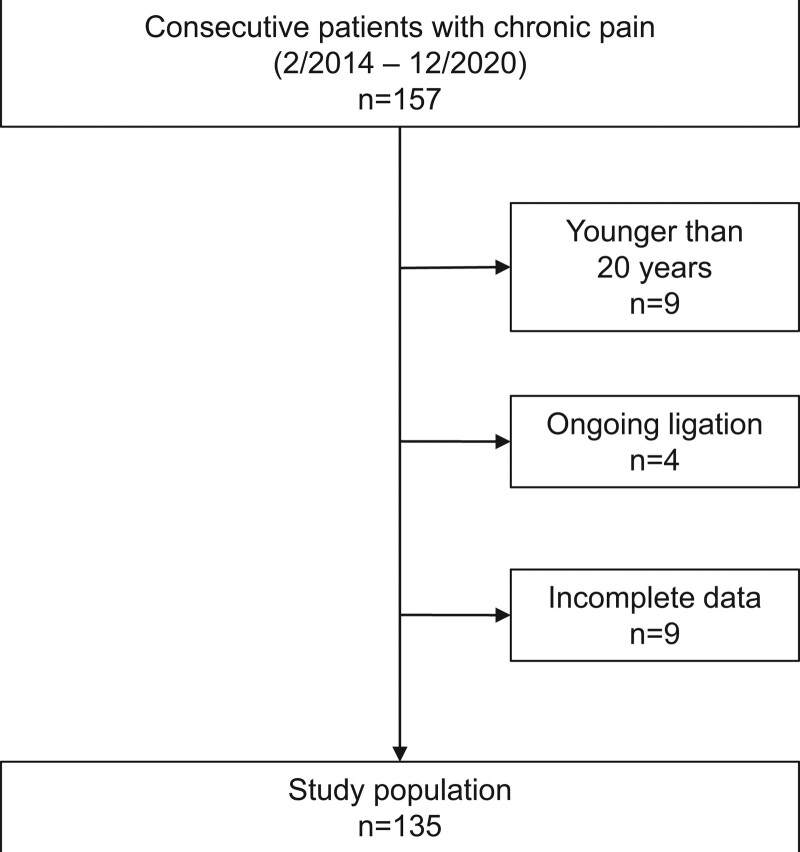
This study was conducted at the Okayama university hospital. Patients aged over 20 years with non-cancer chronic pain lasting for longer than 3 months who had visited our outpatient specialty pain clinic with their caregivers were included in this study. Participants included 135 patients (48 men, 87 women) and their attendants who had visited the clinic between February 2014 and December 2020 (Fig. 1). The exclusion criteria for participation were as follows: ongoing litigation, delirium, dementia, and other conditions that made completing questionnaires difficult. At the first outpatient clinic visit, we distributed pain-related assessment questionnaires to patients. Then, patients nominated caregivers who mainly took care of the patients’ daily activity and care-burden assessment questionnaires were obtained from caregivers. We obtained approval from the institutional review board of Okayama university hospital (approval number 1508-014) and written informed consent from participants and conducted the study in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Figure 1.
Flowchart showing study population and patient recruitment.
2.2. Main measurements
2.2.1. Assessment of caregiver burden.
The Zarit Burden Interview (ZBI) schedule was originally developed to assess caregivers’ subjective burden levels when caring for those with dementia. It is composed of 22 items that measure the impact of caregiving on caregivers’ physical and emotional health, social activities, and financial status. We scored each item from 0 (never) to 4 (nearly always), with total scores ranging from 0 to 88 and higher scores indicating a greater caregiver burden.[12] The ZBI is considered a generic measure of caregiver burden; its Japanese version has a high test-retest reliability and internal consistency.[13,14] A previous report showed that a ZBI cutoff score of 24 accurately identified 72% of caregivers with probable depression.[15]
2.3. Evaluation of patients’ pain-related factors
2.3.1. Pain intensity.
The Numeric Rating Scale (NRS) evaluates patients’ pain intensity. NRS scores range from 0 to 10, with 0 representing no pain and 10 representing the worst pain imaginable.[16] The average pain intensity in the past 1 week was used in this study.
2.3.2. Pain catastrophizing.
The Pain Catastrophizing Scale (PCS) is a 13-item questionnaire used to measure patients’ pain catastrophizing, with items assessing rumination, magnification, and helplessness. The scores can range from 0 to 52. Each item is rated on a 5-point scale, with 0 to denote “not at all” and 4 denoting “all the time.” A higher score indicates a greater degree of pain catastrophizing.[17]
2.3.3. Anxiety and depression.
The Hospital Anxiety and Depression Scale (HADS) was used to assess patients’ anxiety and depression. It is composed of a 7-item depression scale and a 7-item anxiety scale. Each item is scored from 0 to 3, and scores range from 0 to 21 on each scale. A higher score indicates more severe depression and/or anxiety.[18]
2.3.4. Insomnia.
The Athens Insomnia Scale (AIS) was used to assess patients’ insomnia. It is composed of 8 questions; each scored from 0 (no problem) to 3 (very serious problem). The total score, ranging from 0 to 24, is the sum of all the scores, with higher scores indicating more severe insomnia.[19]
2.3.5. Disability due to pain.
The Pain Disability Assessment Scale (PDAS) was used to assess to what extent pain interfered with patients’ activities of daily living during the previous week.[20] It is composed of 20 items; each item is rated on a 4-point scale, with 0 denoting that “pain did not interfere with this activity” to 3 being “pain interfered with this activity.” The PDAS scores range from 0 to 60, with a higher score indicating greater pain-related interference.
2.3.6. Self-efficacy.
The Pain Self-Efficacy Questionnaire (PSEQ) was used to assess patients’ self-efficacy for managing pain. It is composed of 10 items scored from 0 (not at all confident) to 6 (completely confident); total scores range from 0 to 60. A higher score indicates greater self-efficacy and functioning despite pain.[21]
2.4. Statistical analysis
Descriptive statistics are presented as mean ± standard deviations for continuous variables and as numbers and percentages for categorical variables. The normality of the variables was evaluated using the Shapiro–Wilk test. Based on a previous report,[15] participants were divided into 2 groups with a ZBI cutoff score of 24 points (L group < 24 points and H group ≥ 24 points), and the measured variables were compared between the 2 groups using the Mann–Whitney U test. Subsequently, we analyzed correlations between the ZBI scores and each measured variable using univariate linear regression analyses. In a further analysis, we performed multiple linear regression to determine factors associated with the ZBI, and a standardized partial regression coefficient was calculated. The explanatory variables included NRS, PCS, HADS anxiety, HADS depression, AIS, PDAS, and PSEQ, and the covariates were age and gender. For conducting the statistical analyses, we used EZR software (Saitama Medical Center Jichi Medical University, Tochigi, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing). Results were considered significant at a level of P < .05. To determine the number of test samples for multiple linear regression analysis, a prior sample size calculation was performed with effect size of 0.2, α error of 0.05 and (1-β) of 0.95 using G*power software version 3.1.9.7.[22] This resulted in a required sample size of 117.
3. Results
The characteristics of patients and caregivers are shown in Table 1. The mean age of patients was 60.8 (standard deviation:19.0) years. The numbers of patients with pain at various sites were 32 (23.7%) in the cranio-cervical region, 23 (17.0%) in the upper limb, 70 (51.9%) in the trunk, and 65 (48.2%) in the lower limb. The relationships between chronic pain patients and their caregivers were as follows: for 64 (47.4%) patients the caregiver was a spouse; for 36 (26.7%) patients, he or she was a child; for 24 (17.8%) patients, he or she was a parent; and for 11 (8.2%) patients, he or she was “others.” Among all patients, 101 (74.8%) lived in the same household as their caregiver. The caregivers’ ZBI scores and the chronic pain patients’ NRS, PCS, HADS anxiety, HADS depression, AIS, PDAS, and PSEQ scores are shown in Table 2. The patient/and caregiver relationship and them living in the same household were not significantly correlated to the ZBI scores as shown in Table 3. The measured variables of L group (n = 101, 74.8%) and H group (n = 34, 25.2%) are presented in Table 4. Compared to L group, H group showed significantly higher NRS and HADs depression scores and lower PSEQ scores.
Table 1.
Participants’ characteristics.
| Number of patients (n = 135) | |
|---|---|
| Patient background | |
| Age (yrs) | 60.8 ± 19.0 |
| Gender (male/female) | 48 (35.6%)/87 (64.4%) |
| Pain site | |
| Cranio-cervical | 32 (23.7%) |
| Upper limb | 23 (17.0%) |
| Trunk | 70 (51.9%) |
| Lower limb | 65 (48.2%) |
| Caregiver relation with patient | |
| Spouse | 64 (47.4%) |
| Child | 36 (26.7%) |
| Parent | 24 (17.8%) |
| Others | 11 (8.2%) |
| Caregiver and patient living together | 101 (74.8%) |
Data are expressed as mean ± standard deviations for continuous variables and as numbers (proportions) for categorical variables.
Table 2.
Measured parameters of the participants.
| Variables | |
|---|---|
| ZBI | 18.0 ± 15.0 |
| NRS | 6.2 ± 2.1 |
| PCS | 38.1 ± 9.0 |
| HADS anxiety | 8.9 ± 4.2 |
| HADS depression | 9.7 ± 4.6 |
| AIS | 9.5 ± 5.2 |
| PDAS | 29.5 ± 13.4 |
| PSEQ | 20.1 ± 13.6 |
Data are expressed as mean ± standard deviations.
AIS = Athens insomnia scale, HADS = hospital anxiety and depression scale, NRS = numeric rating scale, PCS = pain catastrophizing scale, PDAS = pain disability assessment scale, PSEQ = pain self-efficacy questionnaire, ZBI = Zarit burden interview.
Table 3.
Zarit Burden Interview for caregivers and caregiver status.
| Variables | ZBI | P value |
|---|---|---|
| Caregiver relation with patient | ||
| Spouse | 18.8 ± 15.5 | .109 |
| Child | 20.8 ± 15.5 | |
| Parent | 15.42 ± 14.0 | |
| Others | 9.2 ± 8.0 | |
| Caregiver and patient living together | ||
| Living together | 15.5 ± 14.6 | .185 |
| Not living together | 18.8 ± 15.1 |
Data are expressed as mean ± standard deviations for continuous variables. The P value for caregiver relation was calculated by one-way analysis of variance and post hoc Bonferroni test. The P value for living together was calculated using the Mann–Whitney U test.
ZBI = Zarit Burden Interview.
Table 4.
The measured variables of L group and H group.
| Variables | ZBI | P value | |
|---|---|---|---|
| L group, ZBI < 24 (n = 101, 74.8%) | H group, ZBI ≥ 24 (n = 34, 25.2%) | ||
| ZBI | 10.7 ± 6.8 | 39.4 ± 11.6 | .000** |
| NRS | 6.0 ± 2.1 | 6.8 ± 1.8 | .042* |
| PCS | 37.3 ± 9.5 | 40.6 ± 6.9 | .077 |
| HADS anxiety | 8.5 ± 4.3 | 9.9 ± 3.9 | .089 |
| HADS depression | 9.3 ± 4.5 | 11.0 ± 4.6 | .044 |
| AIS | 9.1 ± 4.9 | 10.6 ± 5.9 | .208 |
| PDAS | 28.5 ± 13.0 | 32.5 ± 14.2 | .14 |
| PSEQ | 21.6 ± 13.5 | 15.6 ± 12.9 | .027* |
Data are expressed as mean ± standard deviations.
AIS = Athens insomnia scale, HADS = hospital anxiety and depression scale, NRS = numeric rating scale, PCS = pain catastrophizing scale, PDAS = pain disability assessment scale, PSEQ = pain self-efficacy questionnaire, ZBI = Zarit burden interview.
P values were calculated using the Mann–Whitney U test. Asterisks indicate statistical significance:
P < .05,
P < .01.
The results of the univariate analysis of the ZBI scores and the measured variables are shown in Table 5. The ZBI scores were significantly correlated with NRS, PCS, HADS anxiety, HADS depression, PDAS, and inversely correlated with PSEQ scores. Next, we performed multiple linear regression analysis to investigate the relationship between ZBI scores and the other variables. We found that ZBI scores were only significantly negatively correlated with PSEQ as seen in Table 6.
Table 5.
Univariate regression analysis and correlation coefficients for Zarit Burden Interview for caregivers and measured variables.
| Variables | Regression coefficient | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| NRS | 1.271 | 0.044 | 2.498 | .042* |
| PCS | 0.312 | 0.032 | 0.592 | .029* |
| HADS anxiety | 0.633 | 0.039 | 1.228 | .037* |
| HADS depression | 0.829 | 0.284 | 1.373 | .003** |
| AIS | 0.409 | -0.081 | 0.899 | .101 |
| PDAS | 0.264 | 0.077 | 0.450 | .006** |
| PSEQ | -0.359 | -0.538 | -0.181 | .000** |
AIS = Athens insomnia scale, CI = confidence interval, HADS = hospital anxiety and depression scale, NRS = numeric rating scale, PCS = pain catastrophizing scale, PDAS = pain disability assessment scale, PSEQ = pain self-efficacy questionnaire.
Asterisks indicate statistical significance:
P < .05,
P < .01.
Table 6.
Multiple linear regression analysis and standardized regression coefficients associated with the Zarit Burden Interview for caregivers.
| Variables | Standardized regression coefficient | 95% CI | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| NRS | -0.054 | -0.142 | 0.251 | .584 |
| PCS | -0.006 | -0.224 | 0.212 | .958 |
| HADS anxiety | 0.010 | -0.256 | 0.236 | .937 |
| HADS depression | 0.141 | -0.093 | 0.374 | .236 |
| AIS | -0.024 | -0.222 | 0.174 | .809 |
| PDAS | 1.120 | -0.072 | 0.297 | .232 |
| PSEQ | -0.222 | -0.412 | -0.025 | .028* |
| Constant term | 0.000 | -0.163 | 0.163 | 1.000 |
AIS = Athens insomnia scale, CI = confidence interval, HADS = hospital anxiety and depression scale, NRS = numeric rating scale, PCS = pain catastrophizing scale, PDAS = pain disability assessment scale, PSEQ = pain self-efficacy questionnaire.
Asterisks indicate statistical significance:
P < .05.
4. Discussion
The caregivers who perceived high caregiver burden had significantly higher patients’ pain intensity, depression, and lower self-efficacy than those who perceived low caregiver burden. The use of univariate analyses in this study indicated that caregiver burden was correlated with pain intensity, pain catastrophizing, anxiety, depression, disability, and self-efficacy. However, the multiple linear regression analysis revealed that, among these factors, only self-efficacy was significantly correlated with caregiver burden.
Caregiver burden has been reported among caregivers of elderly dementia patients,[23,24] with both caregivers’ mental and physical problems being reported. As far as mental problems are concerned, depression and anxiety symptoms have been reported among dementia caregivers.[25] A longitudinal study found that among caregivers without these conditions at baseline, 37% and 55% developed major depressive disorder and anxiety disorder, respectively, after a 24-month interval.[26] In terms of physical problems, caregivers are at higher risk of cardiovascular diseases, especially hypertension, which is mediated by a chronic inflammatory response.[27,28] On the one hand, a longitudinal study reported that dementia patients’ neuropsychiatric symptoms, function, and overall health were factors that increased caregiver burden.[29] On the other hand, self-efficacy was reported as a protective factor among caregivers of patients with chronic diseases, such as Alzheimer’s disease,[30] cancer,[31] or chronic obstructive pulmonary disease.[32] Our results support this evidence, suggesting that for caregivers of those with chronic pain, patients’ pain intensity, pain catastrophizing, anxiety, depression, and disability were positively correlated with caregiver burden, while self-efficacy was negatively (inversely) correlated. One reason for this finding may be that self-efficacy in this study was related to patients coping more effectively with their pain. As such, patients with high self-efficacy may have been able to perform activities of daily living independently even though they were experiencing pain, resulting in lower caregiver burden.
This study’s univariate regression analysis revealed that multiple pain chronicity factors in patients were correlated with the caregiver burden. These results indicate that multifaceted evaluations, including assessments of psychological factors, are necessary for patients with chronic pain.[33] Moreover, our results showed that a range of patient factors were correlated with caregiver burden, indicating that patient factors affect both the patients and their caregivers. While a previous study suggested that chronic pain patients’ depression, disability, and insomnia were correlated with caregiver burden,[11] our study did not find that insomnia was correlated with it. A reason for this may be that our study included some caregivers (25%) who did not live with patients but assisted them with activities of daily living. In all likelihood, patients’ insomnia would not have affected these caregivers, and thus, insomnia would not have been correlated with caregiver burden. However, our study did show that patients’ depression and disability were correlated with caregiver burden, a result similar to that of the previous study.[11]
Cognitive-behavioral therapy has been shown to improve the self-efficacy and chronic pain of patients.[34] Therefore, it is an important treatment for these patients that can also influence caregiver burden. A therapy that would improve chronic pain patients’ self-efficacy would also enable them to perform their activities of daily living better, even in pain. It should reduce caregiver burden. One practical implication of our study’s findings, then, is that cognitive-behavioral therapy targeting self-efficacy in chronic pain patients has the potential to reduce caregiver burden.
A limitation of this study is that it was cross-sectional, and hence, treatment effects could not be evaluated. Additionally, our results indicated that about one-fourth of the caregivers of chronic pain patients perceived high caregiver burden with probable risk of depression, based on a ZBI cutoff score from a previous report.[15] However, caregivers were only evaluated using the ZBI, which prevented us from assessing their emotional factors, such as depression or anxiety, and results may vary in cohorts of participants with higher caregiver burdens. Additionally, as this study included non-cancer chronic pain patients, results may vary for other types of patients, such as chronic cancer pain patients.
Based on these limitations, longitudinal studies are needed to explore factors that influence caregiver burden after cognitive-behavioral therapy with chronic pain patients and caregivers.
In conclusion, this study demonstrated that caregiver burden was correlated with pain intensity, pain catastrophizing, anxiety, depression, disability in chronic pain patients and self-efficacy. Among these factors, self-efficacy was the most highly but inversely correlated with caregiver burden. A cognitive-behavioral treatment targeting self-efficacy in chronic pain patients has the potential to reduce caregiver burden.
Acknowledgments
The authors are grateful to all the study participants and would like to thank the Okayama University Hospital Locomotive Pain Center personnel for their help with data collection. This study is granted by Grant of Japan Orthopaedics and Traumatology Research Foundation No. 388.
Author contributions
Conceptualization: Hironori Tsuji, Tomoko Tetsunaga, Yoshiaki Oda.
Data curation: Hironori Tsuji, Tomoko Tetsunaga, Shinichiro Takao.
Formal analysis: Hironori Tsuji.
Investigation: Hironori Tsuji.
Methodology: Hironori Tsuji, Yoshiaki Oda.
Project administration: Hironori Tsuji, Tomoko Tetsunaga.
Software: Hironori Tsuji.
Supervision: Tomoko Tetsunaga, Haruo Misawa, Keiichiro Nishida, Toshifumi Ozaki.
Validation: Hironori Tsuji, Tomoko Tetsunaga, Haruo Misawa, Yoshiaki Oda.
Visualization: Hironori Tsuji, Tomoko Tetsunaga, Yoshiaki Oda.
Writing – original draft: Hironori Tsuji.
Writing – review & editing: Tomoko Tetsunaga, Haruo Misawa, Keiichiro Nishida, Toshifumi Ozaki.
Abbreviations:
- AIS =
- Athens insomnia scale
- HADS =
- hospital anxiety and depression scale
- NRS =
- numeric rating scale
- PCS =
- pain catastrophizing scale
- PDAS =
- pain disability assessment scale
- PSEQ =
- pain self-efficacy questionnaire
- ZBI =
- Zarit burden interview
This study was supported by JSPS KAKENHI Grant Number 17K16691 and Grant of Japan Orthopaedics and Traumatology Research Foundation No. 388.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Tsuji H, Tetsunaga T, Tetsunaga T, Misawa H, Oda Y, Takao S, Nishida K, Ozaki T. Factors influencing caregiver burden in chronic pain patients: A retrospective study. Medicine 2022;101:39(e30802).
Contributor Information
Hironori Tsuji, Email: me15055@s.okadai.jp.
Tomonori Tetsunaga, Email: tomonoritetsunaga@okayama-u.ac.jp.
Haruo Misawa, Email: misharuo@gmail.com.
Yoshiaki Oda, Email: odaaaaaaamn@yahoo.co.jp.
Shinichiro Takao, Email: mechamecha_su_te_ki@yahoo.co.jp.
Keiichiro Nishida, Email: knishida@md.okayama-u.ac.jp.
Toshifumi Ozaki, Email: tozaki@md.okayama-u.ac.jp.
References
- [1].Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- [2].Patel AS, Farquharson R, Carroll D, et al. The impact and burden of chronic pain in the workplace: a qualitative systematic review. Pain Pract. 2012;12:578–89. [DOI] [PubMed] [Google Scholar]
- [3].Eccleston C, Palermo TM, Williams AC, et al. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2014;5:CD003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;4:CD011279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–32. [DOI] [PubMed] [Google Scholar]
- [6].Asmundson GJ, Norton PJ, Norton GR. Beyond pain: the role of fear and avoidance in chronicity. Clin Psychol Rev. 1999;19:97–119. [DOI] [PubMed] [Google Scholar]
- [7].Leeuw M, Goossens ME, Linton SJ, et al. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30:77–94. [DOI] [PubMed] [Google Scholar]
- [8].Tsuji H, Tetsunaga T, Tetsunaga T, et al. The factors driving self-efficacy in intractable chronic pain patients: a retrospective study. J Orthop Surg Res. 2019;14:473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Martinez-Calderon J, Zamora-Campos C, Navarro-Ledesma S, et al. The role of self-efficacy on the prognosis of chronic musculoskeletal pain: a systematic review. J Pain. 2018;19:10–34. [DOI] [PubMed] [Google Scholar]
- [10].Leadley RM, Armstrong N, Reid KJ, et al. Healthy aging in relation to chronic pain and quality of life in Europe. Pain Pract. 2014;14:547–58. [DOI] [PubMed] [Google Scholar]
- [11].Yamaguchi M, Yamada K, Iseki M, et al. Insomnia and caregiver burden in chronic pain patients: a cross-sectional clinical study. PLoS One. 2020;15:e0230933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Anthony-Bergstone CR, Zarit SH, Gatz M. Symptoms of psychological distress among caregivers of dementia patients. Psychol Aging. 1988;3:245–8. [DOI] [PubMed] [Google Scholar]
- [13].Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649–55. [DOI] [PubMed] [Google Scholar]
- [14].Arai Y, Kudo K, Hosokawa T, et al. Reliability and validity of the Japanese version of the Zarit Caregiver Burden interview. Psychiatry Clin Neurosci. 1997;51:281–7. [DOI] [PubMed] [Google Scholar]
- [15].Schreiner AS, Morimoto T, Arai Y, et al. Assessing family caregiver’s mental health using a statistically derived cut-off score for the Zarit Burden interview. Aging Ment Health. 2006;10:107–11. [DOI] [PubMed] [Google Scholar]
- [16].Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–26. [DOI] [PubMed] [Google Scholar]
- [17].Osman A, Barrios FX, Gutierrez PM, et al. The pain catastrophizing scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–65. [DOI] [PubMed] [Google Scholar]
- [18].Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [19].Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48:555–60. [DOI] [PubMed] [Google Scholar]
- [20].Yamashiro K, Arimura T, Iwaki R, et al. A multidimensional measure of pain interference: reliability and validity of the pain disability assessment scale. Clin J Pain. 2011;27:338–43. [DOI] [PubMed] [Google Scholar]
- [21].Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11:153–63. [DOI] [PubMed] [Google Scholar]
- [22].Faul F, Erdfelder E, Lang AG, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [23].Chiao CY, Wu HS, Hsiao CY. Caregiver burden for informal caregivers of patients with dementia: a systematic review. Int Nurs Rev. 2015;62:340–50. [DOI] [PubMed] [Google Scholar]
- [24].Zhao Y, Feng H, Hu M, et al. Web-based interventions to improve mental health in home caregivers of people with dementia: meta-analysis. J Med Internet Res. 2019;21:e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc. 2015;16:1034–41. [DOI] [PubMed] [Google Scholar]
- [26].Joling KJ, van Marwijk HW, Veldhuijzen AE, et al. The two-year incidence of depression and anxiety disorders in spousal caregivers of persons with dementia: who is at the greatest risk? Am J Geriatr Psychiatry. 2015;23:293–303. [DOI] [PubMed] [Google Scholar]
- [27].Roepke SK, Allison M, Von Känel R, et al. Relationship between chronic stress and carotid intima-media thickness (IMT) in elderly Alzheimer’s disease caregivers. Stress. 2012;15:121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Richardson TJ, Lee SJ, Berg-Weger M, et al. Caregiver health: health of caregivers of Alzheimer’s and other dementia patients. Curr Psychiatry Rep. 2013;15:367. [DOI] [PubMed] [Google Scholar]
- [29].Connors MH, Seeher K, Teixeira-Pinto A, et al. Dementia and caregiver burden: a three-year longitudinal study. Int J Geriatr Psychiatry. 2020;35:250–8. [DOI] [PubMed] [Google Scholar]
- [30].Gallagher D, Ni Mhaolain A, Crosby L, et al. Self-efficacy for managing dementia may protect against burden and depression in Alzheimer’s caregivers. Aging Ment Health. 2011;15:663–70. [DOI] [PubMed] [Google Scholar]
- [31].Johansen S, Cvancarova M, Ruland C. The effect of cancer patients’ and their family caregivers’ physical and emotional symptoms on caregiver burden. Cancer Nurs. 2018;41:91–9. [DOI] [PubMed] [Google Scholar]
- [32].Kar S, Zengin N. The relation between self-efficacy in patients with chronic obstructive pulmonary disease and caregiver burden. Scand J Caring Sci. 2020;34:754–61. [DOI] [PubMed] [Google Scholar]
- [33].Nicolson SE, Caplan JP, Williams DE, et al. Comorbid pain, depression, and anxiety: multifaceted pathology allows for multifaceted treatment. Harv Rev Psychiatry. 2009;17:407–20. [DOI] [PubMed] [Google Scholar]
- [34].Turner JA, Anderson ML, Balderson BH, et al. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain. 2016;157:2434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]