Abstract
Malignant pleural mesothelioma (MPM) is an aggressive tumor with a poor prognosis. In our study, we aimed to investigate the specific clinical, laboratory, and radiological features of the tumor and the prognostic effect of SUVmax (maximum standardized uptake values) according to PET/CT (positron emission tomography). Demographic, therapeutic, clinical, and survival information of patients diagnosed with histologically-validated pleural mesothelioma in our hospital between January 2010 to December 2019 will be retrospectively scanned from the hospital records. A total of 116 patients, 61 men (52.6%), and 55 women (47.4%), were analyzed. Thirty five patients (30.2%) were over the age of 65. Percentage of patients over 65 years of age, neutrophil count, and PET SUV Max values, asbestos exposure and pleural thickening rate were significantly higher in the deceased patients’ group than in the living patients’ group (P = .042, P = .039, P = .002, P = .004, P = .037). T stage (tumor stage), N stage (lymph nodes stage), metastasis stage, and Grade distribution were significantly higher in the deceased patients’ group than in the living patients’ group (P < .000, P < .000, P = .003, P < .000). The rates of chemotherapy and surgical treatment, right lung location, and epithelioid pathology were significantly lower in the deceased patients’ group compared to the living patients’ group (P = .016, P = .030, P = .018, P = .008). The mean follow-up time was 13 months. Key determinants of survival in MPM include age, male gender, neutrophil increase, pleural thickening, high PET SUV max values, stage, histological type, asbestos exposure, and treatment regimen.
Keywords: asbest, malignant pleural mesothelioma, PET CT, prognostic factors, survival, systemic immune-inflammation index
1. Introduction
Malignant pleural mesothelioma (MPM) is a considerably aggressive tumor linked with environmental asbestos exposure.[1] MPM most commonly originates from the parietal pleura[2] occurs most often in the male sex and between the ages of 60 and 80.[3]
MPM has varied histologic types namely epithelioid, sarcomatoid, and biphasic.[4] Chronic inflammation, which is a consequence of asbestos exposure, plays a critical part in the pathogenesis of MPM. Inflammatory markers; neutrophil lymphocyte ratio (NLR), and platelet lymphocyte ratio (PLR) are valuable in determining prognosis.[5] Positron emission tomography (PET) high maximum standardized uptake values (SUVmax [maximum standardized uptake values]) were found to be adverse prognostic factors.[6] It has been accepted that factors such as male gender, old age, and sarcomatoid type reflect a more aggressive disease course in MPM.[7] Female patients have a better life expectancy than male ones.[8] With an overall survival less than 12 months, the disease is highly resistant to chemotherapy and radiotherapy.[9] Platinum-based chemotherapy offers the highest tumor response rates[10] and multimodal treatments combining have gained importance. Treatment methods such as immunotherapy, immune checkpoint inhibitor treatments, new targeted immune treatments, and tumor treating fields are also being developed.[11] Epithelioid type and an early-stage disease show a solid survival chance following a surgical treatment.[12] In addition to its high aggressiveness, the mean overall survival (OS) time of MPM (regardless of tumor stage) is 9 to 17 months (averagely 12 months).[13]
2. Materials and Methods
2.1. Selection of patients
The age, sex, smoking history, asbestos exposure, basic laboratory parameters, inflammation markers, tumor location, bilateral pleural plaque presence, histologic subtype, stage, PET SUVmax, surgery type and chemotherapy, and survival information (OS) of patients diagnosed with histologically-validated pleural mesothelioma in Ankara Ataturk Chest Diseases and Thoracic Surgery Training and Research Hospital between January 2010 and December 2019 were retrospectively scanned (OS was defined as the time from diagnosis to death or to the latest follow-up). The presence of benign mesothelial tumor and the tumor sites located outside the pleura were set as the exclusion criteria.
NLR was established by dividing the absolute neutrophil count by the absolute lymphocyte count. PLR was established by dividing the absolute thrombocyte count by the absolute lymphocyte count. LMR (lymphocyte to monocyte ratio) was established by dividing the absolute lymphocyte count by the absolute monocyte count.
18F-FDG PET-CT (PET/CT) was performed prior to surgery or chemotherapy in all patients for the sake of preliminary analysis. The stages of the disease were categorized according to the eighth edition of tumor-lymph nodes-metastases (TNM [tumor-lymph nodes-metastases]) classification of MPM by Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC).[14] Extrapleural pneumonectomy (EPP), pleurectomy/decortication or partial pleurectomy was performed on resectable MPM patients who can tolerate aggressive surgeries. Neo adjuvant or adjuvant chemotherapy which includes pemetrexed and cisplatin or carboplatin was performed in four to six cycles in combination with surgery. For patients not suitable for surgery, palliative chemotherapy together with pemetrexed and cisplatin or carboplatin was performed instead. Chemotherapy cycles were repeated at 21-day intervals.
The requirement for informed consent from the patients was waived off due to the retrospective nature of the study. Confidentiality of patient data was maintained throughout the study.
2.2. Statistical method
Mean, standard deviation, median, minimum, maximum value frequency, and percentage were used for descriptive statistics. The distribution of variables was checked with Kolmogorov–Smirnov test. Independent Samples t test and Mann–Whitney U test were used for the comparison of quantitative data. Chi-Square test was used for the comparison of the qualitative data. Cox regression was used in the survival analysis. SPSS 27.0 was used for statistical analyses.
3. Results
One hundred sixteen patients were analyzed in total out of which 61 were male (52.6%) and 55 were female (47.4%). Eighty one patients (69.8%) were under the age of 65 and 35 patients (30.2%) were over the age of 65. 41.4% of the patients (n = 48) were smokers. Laboratory data belonging to the patients are shown below (Table 1).
Table 1.
Laboratory data of patients.
| Min–Max | Median | Mean ± SD/n% | |||
|---|---|---|---|---|---|
| Age | >65 | 35 | 30.2% | ||
| <65 | 81 | 69.8% | |||
| Gender | Female | 55 | 47.4% | ||
| Male | 61 | 52.6% | |||
| Smoke | 48 | 41.4% | |||
| Lymphocyte | 0.4–5.4 | 1.8 | 1.9 ± 0.8 | ||
| Monocyte | 0.0–1.6 | 0.5 | 0.6 ± 0.3 | ||
| Neutrophil | 2.4–35.0 | 5.5 | 6.1 ± 3.5 | ||
| HGB | 8.5–17.3 | 13.3 | 13.2 ± 2.0 | ||
| HCT | 26.0–52.6 | 39.8 | 39.9 ± 5.7 | ||
| MCV | 62.1–98.6 | 86.4 | 85.6 ± 6.8 | ||
| RDW | 11.8–24.2 | 14.7 | 14.9 ± 2.1 | ||
| PLT | 82.0–950.0 | 306.0 | 331.5 ± 121.3 | ||
| MPV | 0.0–17.5 | 7.8 | 8.0 ± 1.8 | ||
| NLR | 0.9–22.2 | 3.0 | 3.5 ± 2.5 | ||
| PLR | 36.6–877.5 | 164.5 | 200.0 ± 121.2 | ||
| LMR | 0.4–10.5 | 3.0 | 3.6 ± 2.0 | ||
HCT = hematocrit, HGB = hemoglobin, LMR = lymphocyte to monocyte ratio, MCV = mean corpuscular volume, Min–Max = minimum–maximum, MPV = mean thrombocyte volume, NLR = neutrophil to lymphocyte ratio, PLR = platelet to lymphocyte ratio, PLT = platelets, RDW = red cell distribution width.
The patients were interviewed. It was questioned whether they were exposed to environmental asbestos. The answers given by the patients to the questions were accepted. Environmental asbestos exposure was detected in 61.2% of the patients. As for histologic MPM types, 82.8% of the patients had epithelial, 13.8% had biphasic, and 3.4% had sarcomatoid type MPM. Percentages for the primary sites of the disease were 37.1% right, 61.2% left, and 1.7% bilateral. Mean follow-up time was 13 months. Data regarding the staging rates, diagnosis methods, pleural thickening ratios, treatment methods, and mortality rates of the patients are shown below (Table 2).
Table 2.
Tumor characteristics.
| Min–Max | Median | Mean ± SD/n% | |||
|---|---|---|---|---|---|
| Follow-up time | 1.0–108.0 | 13.0 | 19.0 ± 18.6 | ||
| Asbestos | 71 | 61.2% | |||
| PET CT SUV Max | 1.5–21.1 | 7.6 | 8.0 ± 4.0 | ||
| TM side | Right | 43 | 37.1% | ||
| Left | 71 | 61.2% | |||
| Bilateral | 2 | 1.7% | |||
| Bilateral pleural plaque | 5 | 4.3% | |||
| T stage | I | 77 | 66.4% | ||
| II | 19 | 16.4% | |||
| III | 11 | 9.5% | |||
| IV | 9 | 7.8% | |||
| N stage | 0 | 13 | 11.2% | ||
| I | 55 | 47.4% | |||
| II | 33 | 28.4% | |||
| III | 15 | 12.9% | |||
| M stage | 0 | 109 | 94.0% | ||
| I | 7 | 6.0% | |||
| Grade | I | 1311.2% | |||
| II | 47 | 40.5% | |||
| III | 3025.9% | ||||
| IV | 2622.4% | ||||
| Pathology | Epithelioid | 96 | 82.8% | ||
| Sarcomatous | 4 | 3.4% | |||
| Biphasic | 16 | 13.8% | |||
| Diagnosis | Pleura biopsy | 110 | 94.8% | ||
| Thoracoscopy | 3 | 2.6% | |||
| TTNAB | 3 | 2.6% | |||
| Pleural thickening | >1 cm | 60 | 51.7% | ||
| <1 cm | 56 | 48.3% | |||
| Chemotherapy | 96 | 82.8% | |||
| Radiotherapy | 15 | 12.9% | |||
| Surgical treatment | 18 | 15.5% | |||
| Surgical method | |||||
| Extrapleural pneumonectomy | 316.7% | ||||
| Decortication | 1161.1% | ||||
| Partial pleurectomy | 2 11.1% | ||||
| No comment | 2 | 11.1% | |||
| Pleurodesis | 5244.8% | ||||
| Mortality | (+) | 53 | 45.7% | ||
| (–) | 63 | 54.3% | |||
M stage = metastasis stage, Min–Max = minimum–maximum, N stage = lymph nodes stage, PET CT = positron emission tomography, SUVmax = maximum standardized uptake values, TM = tumor, T stage = tumor stage.
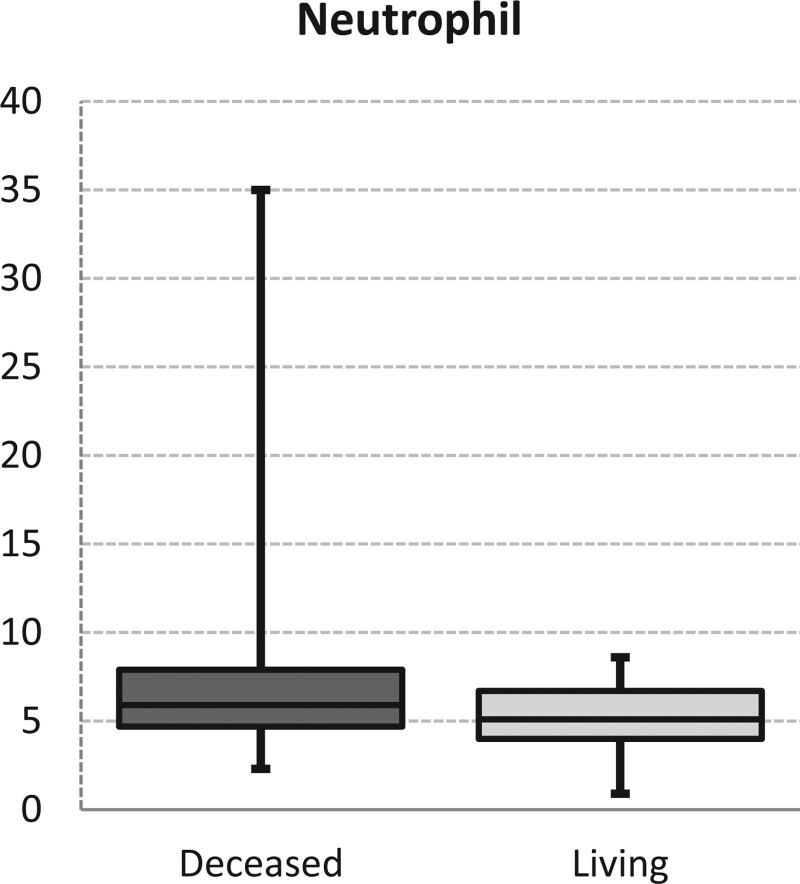
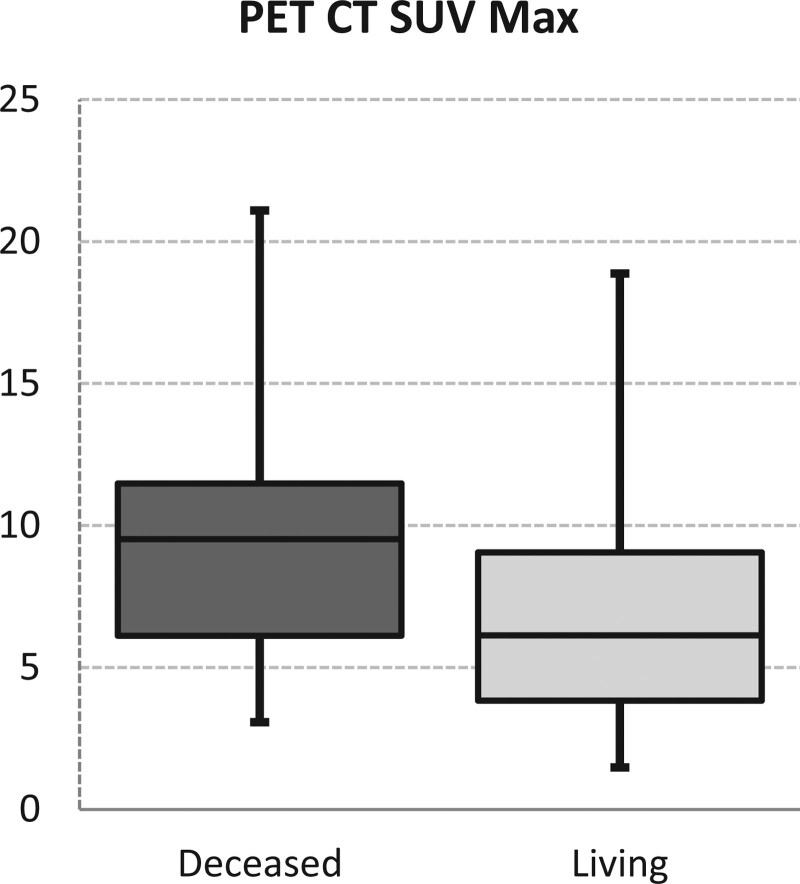
In the deceased patients’ group, the percentage of patients over the age of 65 was significantly higher than in the living patients’ group (P = .042). Regarding sex distribution and smoking rates, no significant difference was observed between the deceased patients’ group and living patients’ group (P > .05). Also, no significant difference was seen in lymphocyte, monocyte, neutrophil, hemoglobin, hematocrit, MCV (mean corpuscular volume), RDW (red cell distribution width), PLT (platelets), MPV (mean thrombocyte volume), NLR, PLR and LMR values between both groups (P > .05). Neutrophil values were significantly higher in the deceased patients’ group than in the living patients’ group (P = .039 Fig. 1). In the mortality group, PET SUV Max values were significantly higher than in the living patients’ group (P = .002 Fig. 2) (Table 3).
Figure 1.
Neutrophil levels.
Figure 2.
PET CT SUV Max levels. PET CT = positron emission tomography; SUVmax = maximum standardized uptake values.
Table 3.
Group characteristics comparison data.
| Deceased | Living | P | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD/n% | Median | Mean ± SD/n% | Median | ||||||
| Age | >65 | 21 | 39.6% | 14 | 22.2% | .042 | ‡ | ||
| <65 | 32 | 60.4% | 49 | 77.8% | |||||
| Gender | Female | 26 | 49.1% | 29 | 46.0% | .745 | ‡ | ||
| Male | 27 | 50.9% | 34 | 54.0% | |||||
| Smoke | 25 | 47.2% | 23 | 36.5% | .245 | ‡ | |||
| Lymphocyte | 2.0 ± 0.9 | 1.8 | 1.9 ± 0.7 | 1.9 | .883 | † | |||
| Monocyte | 0.6 ± 0.3 | 0.5 | 0.6 ± 0.2 | 0.5 | .243 | † | |||
| Neutrophil | 7.0 ± 4.7 | 5.9 | 5.4 ± 1.5 | 5.1 | .039 | † | |||
| HGB | 12.8 ± 1.8 | 12.8 | 13.5 ± 2.1 | 13.6 | .054 | * | |||
| HCT | 38.9 ± 5.1 | 39.1 | 40.7 ± 6.1 | 40.7 | .058 | † | |||
| MCV | 85.3 ± 5.5 | 85.5 | 85.8 ± 7.7 | 86.8 | .265 | † | |||
| RDW | 14.9 ± 1.8 | 14.8 | 14.9 ± 2.3 | 14.5 | .559 | † | |||
| PLT | 334.2 ± 127.5 | 303.0 | 329.2 ± 116.7 | 314.0 | .901 | † | |||
| MPV | 7.8 ± 1.6 | 7.8 | 8.1 ± 2.0 | 7.8 | .805 | † | |||
| NLR | 3.8 ± 3.1 | 3.1 | 3.3 ± 1.9 | 2.7 | .224 | † | |||
| PLR | 206.6 ± 146.8 | 161.7 | 194.5 ± 95.5 | 165.9 | .940 | † | |||
| LMR | 3.2 ± 1.6 | 2.8 | 3.8 ± 2.2 | 3.2 | .272 | † | |||
| PET CT SUV Max | 9.2 ± 3.6 | 9.5 | 7.0 ± 3.9 | 6.1 | .002 | * | |||
Bold and italic indicate significant values: P < .05.
HCT = hematocrit, HGB = hemoglobin, LMR = lymphocyte to monocyte ratio, MCV = mean corpuscular volume, MPV = mean thrombocyte volume, NLR = neutrophil to lymphocyte ratio, PET CT = positron emission tomography, PLR = platelet to lymphocyte ratio, PLT = platelets, RDW = red cell distribution width, SUVmax = maximum standardized uptake values.
t test.
Mann–Whitney U test.
Chi-square test.
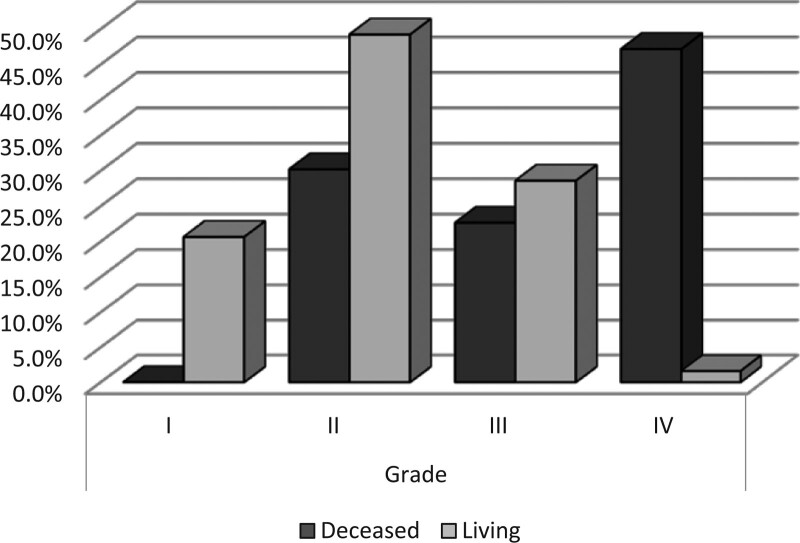
Asbestos exposure rate was significantly higher in the deceased patients’ group than in the living patients’ group (P = .004). The ratio of tumor presence on the right side was significantly lower in the deceased patients’ group (P = .018). Left side presence and bilateral presence ratios show no significant difference between the deceased patients’ group and living patients’ group (P > .05). Bilateral pleural plaque ratios show no significant difference between the deceased patients’ group and living patients’ group (P > .05). T stage (tumor stage) distribution was significantly higher in the deceased patients’ group (P < .000). N stage (lymph nodes stage) distribution was significantly higher in the deceased patients’ group (P < .000). metastasis stage (M stage) distribution was significantly higher in the deceased patients’ group (P = .003). Grade distribution was significantly higher in the deceased patients’ group (P < .000 Fig. 3) (Table 4).
Figure 3.
Grade levels.
Table 4.
Deceased group data.
| Deceased | Median | P | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Asbestos | 40 | 75.5% | 31 | 49.2% | .004 * | |
| Right | 1324.5% | 30 | 47.6% | .018 * | ||
| TM side | Left | 38 | 71.7% | 33 | 52.4% | .053* |
| Bilateral | 23.8% | 00.0% | .206* | |||
| Bilateral pleural plaque | 35.7% | 2 | 3.2% | .511* | ||
| T stage | I | 27 | 50.9% | 50 | 79.4% | .000 * |
| II | 8 | 15.1% | 11 | 17.5% | ||
| III | 9 | 17.0% | 2 | 3.2% | ||
| IV | 9 | 17.0% | 0 | 0.0% | ||
| N stage | 0 | 0 | 0.0% | 13 | 20.6% | .000 * |
| I | 22 | 41.5% | 33 | 52.4% | ||
| II | 17 | 32.1% | 16 | 25.4% | ||
| III | 14 | 26.4% | 1 | 1.6% | ||
| M stage | 0 | 46 | 86.8% | 63 | 100.0% | .003 * |
| I | 7 | 13.2% | 0 | 0.0% | ||
| Grade | I | 0 | 0.0% | 13 | 20.6% | .000 * |
| II | 16 | 30.2% | 31 | 49.2% | ||
| III | 12 | 22.6% | 18 | 28.6% | ||
| IV | 25 | 47.2% | 1 | 1.6% | ||
| Pathology | Epithelioid | 38 | 71.7% | 58 | 92.1% | .008 * |
| Sarcomatous | 4 | 7.5% | 0 | 0.0% | .087* | |
| Biphasic | 11 | 20.8% | 5 | 7.9% | .847* | |
| Diagnosis | Pleura biopsy | 50 | 94.3% | 60 | 95.2% | .839* |
| Thoracoscopy | 1 | 1.9% | 2 | 3.2% | 1.000* | |
| TTNAB | 2 | 3.8% | 1 | 1.6% | .591* | |
| Pleural thickening | >1 cm | 33 | 62.3% | 27 | 42.9% | .037 * |
| <1 cm | 20 | 37.7% | 36 | 57.1% | ||
| Chemotherapy | 39 | 73.6% | 57 | 90.5% | .016 * | |
| Radiotherapy | 5 | 9.4% | 10 | 15.9% | .303* | |
| Surgical treatment | 4 | 7.5% | 14 | 22.2% | .030 * | |
| Pleurodesis | 27 | 50.9% | 25 | 39.7% | .224* | |
Bold and italic indicate significant values: P < .05.
M stage = metastasis stage, N stage = lymph nodes stage, TM = tumor, T stage = tumor stage.
Chi-square test.
In the mortality group, epithelioid pathology ratio was significantly lower than in the living patients’ group (P = .008). Biphasic pathology ratios and diagnosis distribution show no significant difference between both groups (P > .05). Pleural thickening ratio was significantly higher in the mortality group (P = .037) (Table 4).
Chemotherapy rate was significantly lower in the deceased patients’ group (P = .016). Radiotherapy rates show no significant difference between the deceased patients’ group and living patients’ group (P > .05). In the deceased patients’ group, surgical treatment ratio was significantly lower than in the living patients’ group (P = .030) (Tablo 4).
3.1. Univariate analysis
Factors such as age, neutrophil, PET SUVmax, asbestos, T stage, N stage, grade, pathology, pleural thickening, chemotherapy, and surgical treatment have shown significant efficacy in predicting the mortality in univariate model (P < .05) (Table 5).
Table 5.
Univariate and multivariate analysis.
| Univariate model | Multivariate model | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age | 0.435 | 0.194–0.978 | .044 | |||
| Neutrophil | 0.787 | 0.651–0.952 | .013 | |||
| PET CT SUV Max | 0.856 | 0.771–0.950 | .003 | |||
| ASBEST | 0.315 | 0.142–0.699 | .004 | |||
| T stage | 0.358 | 0.211–0.607 | .000 | |||
| N stage | 0.281 | 0.160–0.495 | .000 | |||
| Grade | 0.226 | 0.129–0.395 | .000 | 0.226 | 0.129–0.395 | .000 |
| Pathology | 0.489 | 0.274–0.873 | .016 | |||
| Pleural Thickening | 2200 | 1043–4642 | .038 | |||
| Chemotherapy | 3410 | 1206–9643 | .021 | |||
| Surgical treatment | 3500 | 1076–11,386 | .037 | |||
Logistic regression (forward LR).
Bold and italic indicate significant values: P < .05.
CI = confidence interval, N stage = lymph nodes stage, OR = odds ratio, PET CT = positron emission tomography, SUVmax = maximum standardized uptake values, T stage = tumor stage.
3.2. Multivariate analysis
Grade has shown significant efficacy in predicting the mortality in reduced multivariate model (P < .05) (Table 5).
4. Discussion
MPM is a tumor with an aggressive course and is only partially responsive to conventional treatments. Due to its aggressive course, identifying decisive prognostic factors is important and thus is pursued. This study was carried out on a Turkish patient group with the aim of exploring potential prognostic factors including demographic and clinicopathologic features, basic laboratory parameters, inflammatory markers, tumor location, histologic subtype, stage, PET SUVmax, surgery type and chemotherapy, and survival information.
MPM is generally caused by professional or environmental exposure to asbestos. In Turkey, environmental exposure to asbestos and erionite initially begins with birth in an environment containing asbestos.[15] Consequently, MPM is diagnosed at younger ages, and the both sexes are affected equally. Environmental asbestos exposure was present in 61.2% of the patients. Male to female ratio showed similarities, and most of the patients were under the age of 65 (69.8%). In our study, asbestos exposure rate was significantly higher in the deceased patients’ group than in the living patients’ group (P < .05). Primary disease site percentages were 37.1% right side, 61.2% left side, and 1.7% bilateral. In our study, the rate of tumor presence on the right side was significantly lower in the deceased patients’ group than in the living patients’ group (P < .05).
Even though the age factor was a topic of controversy in identifying the prognosis in mesothelioma patients, a recent study has shown that the risk of death due to MPM increases with the increasing age.[16] In our study, the percentage of patients over the age of 65 was significantly higher in the deceased patients’ group than in the living patients’ group (P < .05). Also, similar to prior studies, no significant relationship between smoking and prognosis was found.[15]
As for histologic MPM types, 82.8% of the patients had epithelial, 13.8% had biphasic, and 3.4% had sarcomatoid type MPM. Non-epithelioid histology is generally considered as an adverse prognostic factor.[8] Similarly, in our study, epithelioid pathology rate was significantly lower in the deceased patients’ group than in the living patients’ group (P < .05).
Pleural thickening over 1 cm by lung CT scan is a specific MPM symptom.[17] In our study, 51% of the patients had pleural thickening over 1 cm in width. An increased thickening on the pleural region increases the tumor load on the same region. In short, patients with thicker pleurea have worsened survival. In our study, pleural thickening rate was significantly higher in the deceased patients’ group than in the living patients’ group (P < .05).
With the wide spread use of PET/CT in the oncology field, it has become an invaluable imaging technique for identifying and staging of MPM and estimating its prognosis. PET/CT accurately diagnoses the MPM, and it estimates survival and recurrence of the disease. Higher SUVmax levels were found to be associated with a lower survival.[6] Similarly to our study, Flores et al[18] have demonstrated that high PET SUVmax levels are correlated with an adverse prognosis. In our study, PET SUV Max values were significantly higher in the deceased patients’ group than in the living patients’ group (P < .05).
Inflammation is an important component of the tumor microenvironment[19] and plays a significant part in the development of MPM. White blood cell (WBC), neutrophil, lymphocyte, and NLR are systemic inflammatory markers. Systemic inflammatory response is vital in cancer progression.[20] Inflammation plays a role from the diagnosis to the advanced stages of cancer. The severity of this inflammation can be an identifying factor for the prognosis. High neutrophil counts provide the tumor with a suitable environment for growth, thus reflecting tumor progression, and weak response.[21] Numerous studies have demonstrated the role of inflammatory parameters such as NLR and PLR in MPM prognosis.[6] In our study, neutrophil values were significantly higher in the deceased patients’ group up than in the living patients’ group (P < .05).
Additionally, chronic inflammation related to asbestos exposure is reported to have a critical role in the development and advancement of MPM and found to be a prognostic factor in foreseeing OS.[22] In our study, asbestos exposure ratio was significantly higher in the deceased patients’ group than in the living patients’ group (P < .05). There are various staging systems for showing the prognostic importance of tumor stages in survival of MPM patients. The eighth edition of UICC/AJCC staging system for MPM has been recently published.[16] Tumour stage and histology are the most often studied tumor related prognostic factors. This proposed staging system has been accepted as the international MPM staging system by UICC (Union for International Cancer Control) and AJCC (American Joint Commission on Cancer) in their latest staging guidelines.[23] Rusch et al[24] reported in an international database analysis that the T, N and M stages considerably affect the survival. In this study, we have identified the advanced tumor stage to be a poor prognostic factor. T stage, N stage, M stage and grade was significantly higher in the deceased patients’ group than in the living patients’ group (P < .05).
MPM generally has an adverse prognosis, and its median survival time is 8 to 12 months.[25] Surgery, radiotherapy and neoadjuvant or adjuvant chemotherapy together with multimodal treatment is the only way to long survival for selected patients with suitable prognostic factors. With this method, the mean survival is raised up to 20 to 29 months.[26] In our study, mean follow-up was 13 months.
Among chemotherapeutic agents, pemetrexed and a platinum-based regimen have been recommended as a first-line treatment due to their proven capabilities in increasing the survival rate.[27] If and when the disease advances, a second-line treatment with a single-agent medication containing pemetrexed (if not used in the first-line), gemsitabin or vinorelbine[28] can be proposed. In the deceased patients’ group, chemotherapy ratio was significantly lower than in the living patients’ group (P < .05).
Surgical methods are pivotal for improving the clinical results, and such methods (e.g., extraplural pneumonectomy [EPP] or pleurectomy/decortication) must be picked according to the patient’s status.[29] In the deceased patients’ group, surgical treatment ratio was significantly lower than in the living patients’ group (P < .05). In our study, no relationship between the pleurodesis presence and survival was found. Multimodality therapy is recommended for good-performing patients with early-stage disease.
To estimate the prognosis of MPM, parameters whose measurements are beneficial, feasible and affordable must be defined. MPM generally has a poor prognosis. The best-known clinical prognostic scoring systems for MPM are developed by European Organisation for Research and Treatment of Cancer (EORTC) and Cancer and Leukaemia Group B (CALGB).[8,29] We have found and validated that bad performance, male sex, non-epithelioid histology, high WBC count, and low hemoglobin levels are independent adverse prognostic factors in MPM. Similar to our results, the age,[29] male sex,[30] advanced stage and non-epithelioid histology factors are found to be MPM-related prognostic factors in other studies as well.
Factors such as age, neutrophil, PET CT SUVmax, asbestos, T stage, N stage, grade, pathology, pleural thickening, chemotherapy, and surgical treatment have shown significant efficacy in predicting the mortality in univariate model (P < .05). Grade has shown significant efficacy in predicting the mortality in reduced multivariate model (P < .05).
This is a single-center research project. Our study had a number of flaws, including a retrospective design and a small sample size. Our study’s strongest feature is that it uses real-world data.
5. Conclusions
MPM is still a disease with an adverse prognosis. In this study, we have discussed certain parameters that might have an impact on the MPM prognosis. Among the key survival determinants are male sex, increasing neutrophil count, pleural thickening, high PET SUV max values, stage, histologic type, asbestos exposure, and treatment regimens. Understanding the importance of these determinants on the prognosis of MPM will benefit the development of targeted therapies. Therefore, we believe further studies on larger samples are needed to thoroughly study the prognostic determinants.
Author contributions
Conceptualization: Filiz Cimen.
Data curation: Filiz Cimen, Sevim Düzgün, Yetkin Agackiran.
Formal analysis: Aysegül Senturk, Filiz Cimen, Sevim Düzgün, Yetkin Agackiran.
Funding acquisition: Filiz Cimen.
Investigation: Filiz Cimen, Melike Aloglu, Sevim Düzgün, Yetkin Agackiran.
Methodology: Aysegül Senturk, Filiz Cimen, Yetkin Agackiran.
Project administration: Filiz Cimen, Sevim Düzgün, Sükran Atikcan, Yetkin Agackiran.
Resources: Filiz Cimen, Sükran Atikcan.
Software: Aysegül Senturk, Filiz Cimen, Melike Aloglu.
Supervision: Filiz Cimen.
Validation: Filiz Cimen.
Visualization: Filiz Cimen.
Writing – original draft: Filiz Cimen.
Writing – review & editing: Filiz Cimen, Melike Aloglu.
Abbreviations:
- AJCC =
- American Joint Committee on Cancer
- MPM =
- malignant pleural mesothelioma
- N stage =
- lymph nodes stage
- NLR =
- neutrophil to lymphocyte ratio
- PET CT =
- positron emission tomography
- PLR =
- platelet to lymphocyte ratio
- SUVmax =
- maximum standardized uptake values
- T stage =
- tumor stage
- UICC =
- Union for International Cancer Control
All data generated or analyzed during this study are included in this published article.
All support for this study came from institutional and departmental resources.
The authors have no conflicts of interest to disclose.
Ankara Atatürk Chest Diseases and Thoracic Surgery Training and Research Hospital Medical Specialization Hospital gave their approval for this study (Date: 09.01.2020, Decision No: 658).
How to cite this article: Cimen F, Agackiran Y, Düzgün S, Aloglu M, Senturk A, Atikcan S. Factors affecting the life expectancy in malignant pleural mesothelioma: Our 10 years of studies and experience. Medicine 2022;101:39(e30711).
Contributor Information
Yetkin Agackiran, Email: yetkinagackiran@gmail.com.
Sevim Düzgün, Email: sevimduzgun@yahoo.com.tr.
Melike Aloglu, Email: drmelikeb@gmail.com.
Aysegül Senturk, Email: ayseguldr8@gmail.com.
Sükran Atikcan, Email: sukranatikcan@yahoo.com.
References
- [1].Meniawy TM, Creaney J, Lake RA, et al. Existing models, but not neutrophil-to-lymphocyte ratio, are prognostic in malignant mesothelioma. Br J Cancer. 2013;109:1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Raj V, Kirke R, Bankart MJ, et al. Multidetector CT imaging of pleura: comparison of two contrast infusion protocols. Br J Radiol. 2011;84:796–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rusch VW. Diffuse malignant mesothelioma. Shields TW, LoCicero J, III, Reed CE, Feins RH. (eds). In: General thoracic surgery. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins, 2009;847–59. [Google Scholar]
- [4].Aisner DL, Bauman J, Chirieac LRD, et al. NCCN Guidelines Version 2.2017 Panel Members Malignant Pleural Mesothelioma NCCN Guidelines Panel Disclosures-Farber/Brigham and Women’s Cancer Center. 2017.
- [5].Pinato DJ, Mauri FA, Ramakrishnan R, et al. Inflammation-based prognostic indices in malignant pleural mesothelioma. J Thorac Oncol. 2012;7:587–94. [DOI] [PubMed] [Google Scholar]
- [6].Sharif S, Zahid I, Routledge T, et al. Does positron emission tomography offer prognostic information in malignant pleural mesothelioma? Interact Cardiovasc Thorac Surg. 2011;12:806–11. [DOI] [PubMed] [Google Scholar]
- [7].Borasio P, Berruti A, Bille A, et al. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33:307–13. [DOI] [PubMed] [Google Scholar]
- [8].Edwards JG, Abrams KR, Leverment JN, et al. Prognostic factors for malignant mesothelioma in 142 patients: validation of CALGB and EORTC prognostic scoring systems. Thorax. 2000;55:731–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nowak AK, Alannah J, Calvin S. Management of advanced pleural mesothelioma—at the crossroads. J Oncol Pract. 2022;18:116–24. [DOI] [PubMed] [Google Scholar]
- [10].Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. [DOI] [PubMed] [Google Scholar]
- [11].Davis A, Ke H, Kao S, et al. An update on emerging therapeutic options for malignant pleural mesothelioma. Lung Cancer: Targets Ther. 2022;13:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Meyerhoff RR, Yang CF, Speicher PJ, et al. Impact of mesothelioma histologic subtype on outcomes in the surveillance, epidemiology, and end results database. J Surg Res. 2015;196:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tsao AS, Wistuba I, Roth JA, et al. Malignant pleural mesothelioma. J Clin Oncol. 2009;27:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lim C, Seok H, Hyun S, et al. Evaluation of a diagnostic 18F-FDG PET/CT strategy for differentiating benign from malignant retroperitoneal soft-tissue masses. Clin Radiol. 2019;74:207–15. [DOI] [PubMed] [Google Scholar]
- [15].Tanrikulu AC, Abakay A, Kaplan MA, et al. A clinical, radiographic and laboratory evaluation of prognostic factors in 363 patients with malignant pleural mesothelioma. Respiration. 2010;80:480–7. [DOI] [PubMed] [Google Scholar]
- [16].Gunatilake S, Lodge D, Neville D, et al. “Predicting survival in malignant pleural mesothelioma using routine clinical and laboratory characteristics”. BMJ Open Respir Res. 2021;8:e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis. 2008;3:34–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Flores RM, Akhurst T, Gonen M, et al. Positron emission tomography predicts survival in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2006;132:763–68. [DOI] [PubMed] [Google Scholar]
- [19].Grosse-Steffen T, Giese T, Giese N, et al. Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clin Dev Immunol. 2012;2012:720768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33:79–84. [DOI] [PubMed] [Google Scholar]
- [21].Donskov F, Bennedsgaard KM, Hokland M, et al. Leukocyte orchestration in blood and tumour tissue following interleukin-2 based immunotherapy in metastatic renal cell carcinoma. Cancer Immunol Immunother. 2004;53:729–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kao SC, Pavlakis N, Harvie R, et al. High blood neutrophil-tolymphocyte ratio is an indicator of poor prognosis in malignant mesothelioma patients undergoing systemic therapy. Clin Cancer Res. 2010;16:5805–13. [DOI] [PubMed] [Google Scholar]
- [23].Edge S, Byrd DR, Compton CC, et al. American joint committee on cancer. AJCC Cancer Staging Handbook. 7th ed. New York: Springer, 2010. [Google Scholar]
- [24].Rusch VW, Giroux D, Kennedy C, et al. Initial analysis of the international association for the study of lung cancer mesothelioma database. J Thorac Oncol. 2012;7:1631–9. [DOI] [PubMed] [Google Scholar]
- [25].Brims FJH, Maskell NA. Prognostic factors for malignant pleural mesothelioma. Curr Respir Care Rep. 2013;2:100–8. [Google Scholar]
- [26].Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol. 2015;10:267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ceresoli G, Grosso F, Zucali P, et al. Prognostic factors in elderly patients with malignant pleural mesothelioma: results of a multicenter survey. Br J Cancer. 2014;111:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zauderer MG, Kass SL, Woo K, et al. Vinorelbina and gemcitabine as second—or third-line therapy for malignant pleural mesothelioma. Lung Cancer. 2014;84:271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wolf AS, Flores RM. Current treatment of mesothelioma: extrapleural pneumonectomy versus pleurectomy/decortication. Thorac Surg Clin. 2016;26:359–75. [DOI] [PubMed] [Google Scholar]
- [30].Montanaro F, Rosato R, Gangemi M, et al. Survival of pleural malignant mesothelioma in Italy: a population-based study. Int J Cancer. 2009;124:201–7. [DOI] [PubMed] [Google Scholar]