Abstract
A new insertion element of 1,449 bp with 25-bp perfect terminal repeats, designated IS1409, was identified in the chromosome of 4-chlorobenzoate-degrading Arthrobacter sp. strain TM1 NCIB12013. Upon insertion, IS1409 causes a target duplication of 8 bp. IS1409 carries only a single open reading frame of 435 codons encoding the transposase TnpA. Both TnpA and the overall organization of IS1409 are highly similar to those of some related insertion elements of the ISL3 group (J. Mahillon and M. Chandler, Microbiol. Mol. Biol. Rev. 62:725–774, 1998). IS1409 was also found in other 4-chlorobenzoate-degrading Arthrobacter strains and Micrococcus luteus. Based on IS1409, a series of transposons carrying resistance genes for chloramphenicol and gentamicin were constructed. These transposons were used to demonstrate transposition events in vivo and to mutagenize Arthrobacter sp. strains.
Insertion elements (IS elements) are small mobile non-self-replicating DNA regions specifying only the gene(s) required for their transposition (16). According to the phylogenetic relationship of the transposases and some features involved in the transposition (e.g., the inverted repeats which constitute the ends of most elements, as well as the recognition sites of the transposase and the target duplication caused by most elements), they can be grouped in different families (16). At least for some IS elements, a target-sequence-dependent site preference has been demonstrated, while members of other families apparently transpose in a random fashion. They are widespread in bacteria and contribute to the plasticity of bacterial genomes due to their transposition ability and to their role as target sites for homologous recombination, which can give rise to deletions, inversions, or more complex rearrangements (20). Some composite transposons derived from IS elements, in addition to the genes required for transposition, harbor genes encoding resistances to antibiotics or degradative pathways.
Arthrobacter, a very common soil bacterium, and related genera like Micrococcus form one of the major branches of the actinomycetes, the Micrococcaceae (30). Some Arthrobacter strains are known to degrade aromatic compounds as well as chlorinated aromatic compounds such as 4-chlorobenzoate (4-CBA). Hydrolytic dehalogenation of 4-CBA by Arthrobacter sp. strain SU DSM20407 requires three genes organized in an operon which maps on plasmid pASU1 (25). The same set of genes is also present in Arthrobacter sp. strain TM1 (17). In the course of cloning and sequencing of these genes (unpublished data), we identified an IS element upstream of the dehalogenase operon in Arthrobacter sp. strain TM1. So far, only one other IS element in Arthrobacter and the Micrococcaceae, IS1473 from Arthrobacter nicotinovorans (which belongs to the IS3 family), has been described (19). A growing number of genes specifying degradation of aromatic compounds are known to be located on or associated with transposable elements (for recent reviews see references 31 and 41), with the best known examples being the very large catabolic transposons Tn4651 and Tn4653 on the TOL plasmids of pseudomonads. Thus, it seems possible that the genes for the hydrolytic dehalogenation of 4-CBA are located on a transposon.
In order to demonstrate that IS1409 is a functional transposable element, a series of transposons were constructed based on a slightly modified IS1409 and carrying antibiotic resistance genes. These transposons are the first which can be used for transposon mutagenesis in Arthrobacter.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture media, and antibiotics.
Bacterial strains and plasmids used in this study are listed in Table 1. 4-CBA-degrading strains were grown at 28°C in a minimal medium as described by Seiler (27) at pH 8.0, supplemented with 1 g of 4-CBA/liter as the only carbon and energy source and 0.2 ml of trace element solution/liter (9). All other actinomycetes were grown in NBYE medium (8 g of nutrient broth and 5 g of yeast extract/liter; pH 7.5) at 28°C. Escherichia coli strains were grown in TBY (10 g of tryptone, 5 g of yeast extract, and 5 g of NaCl/liter; pH 7.5) at 37°C. For growing recombinant E. coli strains harboring pUC vector derivatives, ampicillin (150 μg/ml) was added to the medium. For Arthrobacter spp., chloramphenicol (10 μg/ml) or gentamicin (40 μg/ml) was added to the medium after electroporation of pKGT452 derivatives.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant genotype and/or phenotype | Reference or sourcea |
|---|---|---|
| Strains | ||
| Arthrobacter sp. strain SU (DSM20407) | pASU1; 4-CBA+ | 21 |
| Arthrobacter sp. strain SUX | 4-CBA; plasmid-free curing derivative of strain SU | 25 |
| Arthrobacter sp. strain TM1 (NCIB12013) | 4-CBA+ | 17 |
| Arthrobacter oxidans CBS2 | pAO2; 4-CBA+ | 14 |
| Arthrobacter nicotinovorans DSM420 | pAO1; IS1473 | DSM |
| Micrococcus luteus | R. Bergmann, University of Hamburg, Hamburg, Germany | |
| Aureobacterium sp. strain RH025 | 4-FBA+b | J. Eberspächer, University of Hohenheim, Hohenheim, Germany |
| Mycobacterium (Rhodococcus) chlorophenolicus PCPI (DSM43286) | PCP+b | DSM |
| Nocardia sp. strain NCIB10503 | Biphenyl+ | 29 |
| Corynebacterium glutamicum ATCC 13022 | ISCg1 | 10 |
| Clavibacter michiganensis subsp. michiganensis NCPPB382 | NCPPB | |
| Clavibacter michiganensis subsp. sepedonicus NCPPB299 | NCPPB | |
| Clavibacter michiganensis subsp. insidiosum NCPPB1099 | NCPPB | |
| E. coli JM109 | recA1 endA1 thi hsdR17 supE44 relA1 Δ(lac-proAB) gyrA96 F′(traD36 proAB+ lacIq lacZΔM15) | 42 |
| Plasmids | ||
| pUC13 | Apr; cloning vector | 39 |
| pUC18 | Apr; cloning vector | 42 |
| pJE258 | Mini-F cosmid; Cmr Tcr | 7 |
| pEZ1 | pJE258::25-kb Sau3A fragment from strain TM1; 4-CBA+ | 26 |
| pKGT21 | pUC13::1-kb SstI fragment of pEZ1 | This work |
| pKGT451 | pUC13::2.3-kb EcoRV/NruI fragment of pKGT45; IS1409 | This work |
| pKGT452 | pUC13::modified IS1409; see text | This work |
| pKGT452Cα | Tn1409Cα; tnpA and cmx in different orientations | This work |
| pKGT452Cβ | Tn1409Cβ; tnpA and cmx in the same orientation | This work |
| pKGT452Cα | Tn1409Cα; tnpA and aacC1 in different orientations | This work |
| pKGT452Cβ | Tn1409Cβ; tnpA and aacC1 in the same orientations | This work |
| pKGT1000 | pUC18::4.8-kb SphI fragment of mutant A1 | This work |
| pKGT1010 | pUC18::5.0-kb SphI fragment of mutant A27 | This work |
DSM, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany; NCPPB, National Collection of Plant Pathogenic Bacteria, Harpenden, Herts United Kingdom.
4-FBA, 4-fluorobenzoate; PCP, pentachlorophenol.
DNA isolation, manipulation, and transfer.
Plasmid DNA of E. coli was prepared by the alkaline lysis method (22). Plasmid DNA used for sequencing and electroporation was isolated and purified with Qiagen columns as specified by the manufacturer (Qiagen, Hilden, Germany). The endogenous plasmids of Arthrobacter oxidans and Arthrobacter sp. strain SU were isolated from 1-liter cultures grown overnight in half-strength NBYE containing 1 g of 4-CBA/liter by the method of Kieser (13) using 40°C as the lysis temperature. Preparation of total DNA for hybridization was done as described by Hopwood et al. (9). For total DNA preparation, all strains were grown in rich medium overnight. DNA restriction, ligation, and transformation were carried out by standard procedures (22). Isolation of restriction fragments from agarose gels was done with the JETSORB kit as specified by the manufacturer (Genomed, Bad Oeynhausen, Germany).
Electroporation.
Cultures (100 ml) were grown in NBYE to an optical density at 580 nm of about 0.5. The cells were harvested and resuspended in 10 ml of ice-cold deionized water containing 10% glycerol. Lysozyme (100 μl; 4 mg/ml) was added, and the cells were incubated at 28°C for 30 min. After harvesting, cells were washed twice with deionized water–10% (vol/vol) glycerol and then resuspended in 1 to 5 ml of deionized water–10% (vol/vol) glycerol. For electroporation, 1 μg of DNA was added to 200 μl of pretreated cells. Electroporation was performed with a Bio-Rad gene pulser apparatus applying the following parameters: capacity, 25 μF; voltage, 2.5 kV/cm; resistance, 600 to 800 Ω, giving a pulse length of 10 to 13 ms. Cells were mixed immediately with 0.8 ml of SB medium (10 g of tryptone/liter, 5 g of yeast/liter, 4 g of NaCl/liter, 0.5 M sorbitol, 20 mM MgCl2, 20 mM CaCl2 [pH 7.5]) and incubated for 2 h at 28°C before plating on appropriate selective media. Afterwards colonies were restreaked on NBYE plates containing the appropriate antibiotics.
Southern blot analysis.
The internal 1-kb SstI fragment of the tnpA gene from pKGT21 was labeled using the random primed DNA labeling kit (Boehringer Mannheim, Mannheim, Germany) and used as probe for the transposase gene of IS1409. A 2.2-kb SphI/BglII fragment of pKGT452Cβ was used to detect the cmx gene. pUC13 DNA labeled with digoxigenin-11-dUTP by nick translation (22) was used as a probe for integration of the transposon delivery vector. Digested chromosomal and plasmid DNA samples were separated on 0.7 to 1.0% agarose gels and transferred to nylon membranes (Hybond [Amersham, Little Chalfont, United Kingdom] or Nytrans [Schleicher & Schuell, Dassel, Germany]) using a vacuum blotter (Vacugene; Pharmacia). Hybridizations were carried out at 68°C overnight in a buffer containing 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.02% sodium dodecyl sulfate, 0.1% Na laurylsarcosyl, and 2% blocking reagent (Boehringer Mannheim). The nylon membrane was washed twice with 0.1× SSC–0.1% sodium dodecyl sulfate at 68°C for 15 min.
DNA sequencing and sequence analysis.
For DNA sequencing, the dideoxy chain termination method (24) was used with [α-32P]dATP (3,000 Ci/mmol) employing Sequenase 2.0 or Taqquence sequencing kits from U.S. Biochemicals (Cleveland, Ohio) as specified by the manufacturer. For sequence determination, either subclones of pKGT45 were constructed or primer walking with synthetic oligonucleotides (obtained from TIB Molbio, Berlin, Germany, or from Birsner and Grob-Biotech GmbH, Denzlingen, Germany) was conducted. Oligonucleotides ISC2 (5′-GGA ACC TCA CCA ACT ACA TAG C-3′) and ISN2 (5′-CAT GCA GTT GCG CCC ACT ACA C-3′) were used to determine the sequence of insertion sites in Tn1409 mutants. The oligonucleotides IS1 to IS6 were used to introduce two base exchanges into the 5′ end of IS1409 in the construction of pKGT452 (IS1, 5′-AAT TCC GGT ACC ATG GCT CTT CAG AAT TGA GGG TGT-3′; IS2, 5′-AGT GGG CGC AAC TGC ATG CAG CGC CGA GGG CTA GCG GCG T-3′; IS3, 5′-GAT TCA GAC AAG GTG AGG GCC TCG GGA GAG AAT CAG ATT GTC TA-3′; IS4, 5′-GAT CTA GAC AAT CTG ATT CTC TCC CGA GGC CCT CAC CT-3′; IS5, 5′-TGT CTG AAT CAC GCC GCT AGC CCT CGG CGC TGC ATG CAG T-3′; IS6, 5′-TGC GCC CAC TAC ACC CTC AAT TCT GAA GAG CCA TGG TAC CGG-3′).
Sequence assembly and sequence analysis were done with the Microgenie (Beckman, Palo Alto, Calif.) and PCGENE (Intelligenetics, Mountain View, Calif.) programs. The BLAST search programs (1) were used to compare the DNA and deduced protein sequences with database entries at the server of the National Center of Biotechnology Information.
Nucleotide sequence accession number.
The sequence reported here has been deposited in GenBank under accession no. AF042490.
RESULTS
Nucleotide sequence of IS1409.
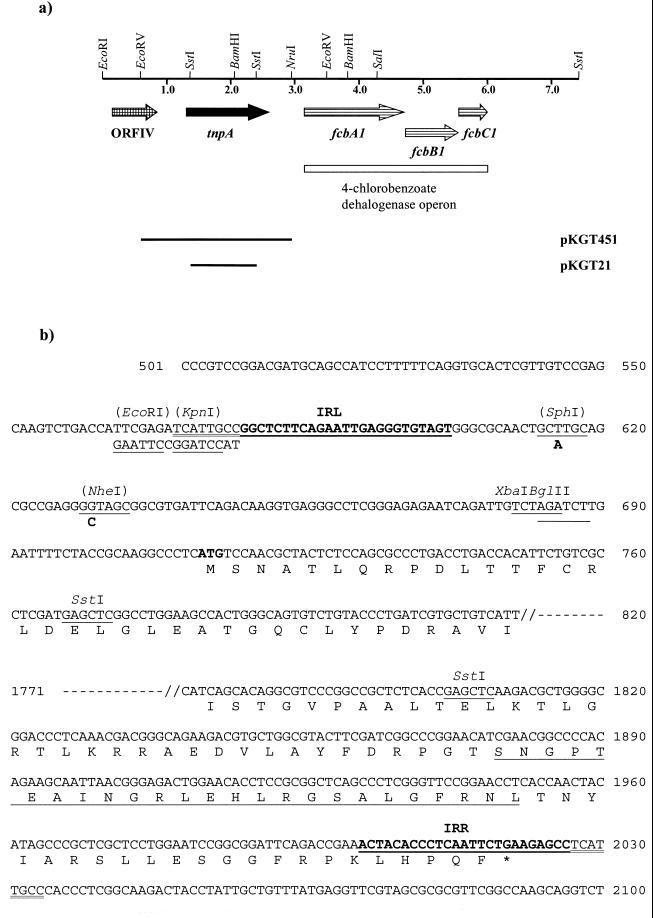
During the analysis of the nucleotide sequence of the chromosomally borne 4-CBA dehalogenase operon of Arthrobacter sp. strain TM1 (NCIB12013) on a 25-kb Sau3A fragment inserted into the cosmid pJE258 (pEZ1) (26), an open reading frame (ORF) with significant identities to those encoding transposases was detected. A 2.35-kb EcoRV/NruI fragment of pEZ1 was subcloned into the SmaI site of pUC13, resulting in plasmid pKGT451 (Fig. 1a). The nucleotide sequence of both strands of pKGT451 was completely determined. The nucleotide sequence and the deduced amino acid sequence are presented in Fig. 1b. The sequence surrounding the transposase gene was found to fulfill the criteria required for an IS element. On both sides of this inserted element a target sequence duplication of 8 nucleotides (nt) was found and confirmed by comparison with the homologous sequence on plasmid pASU1 of Arthrobacter sp. strain SU (25), which lacks the IS element. IS1409 is 1,449 bp long and has perfect terminal inverted repeats of 25 bp. The GC content of the IS element is 61.8%, which is in agreement with the GC content of the Arthrobacter host. IS1409 is inserted about 500 bp upstream of the 4-CBA dehalogenase operon.
FIG. 1.
(A) Physical map of the chromosomal region encoding the 4-CBA dehalogenase genes of Arthrobacter sp. strain TM1. Arrows show directions of transcription. Bars indicate regions cloned into plasmids used in this study. (B) Part of the nucleotide sequence of the 2.35-kb EcoRV/NruI insert of pKGT451. Relevant restriction sites are underlined and labeled; sites present only in pKGT452 are in parentheses. As the 5′ end of pKGT451 up to the BglII site was exchanged by synthetic oligonucleotides, pKGT452 starts with an EcoRI site 47 and 62 nt upstream of nucleotide exchanges introduced in pKGT451, leading to the unique sites for SphI and NheI in pKGT452. The 8-bp target duplication is double underlined. The 25-bp perfect inverted repeats (IRL and IRR) of IS1409 are underlined and boldface. The highly conserved C-terminal region of TnpA, which is characteristic of the ISL3 family, is underlined.
Only one ORF of 1,308 bp, spanning almost the entire element, was found. It starts with an ATG codon 110 nt downstream of the left inverted repeat and ends with a TGA stop codon inside the right inverted repeat. The ORF encodes the putative transposase TnpA of IS1409, a protein of 435 amino acids (48.9 kDa). It is preceded by a putative ribosome binding site 5 bp upstream of the ATG start codon.
Homology of TnpA from IS1409 to transposases of other IS elements.
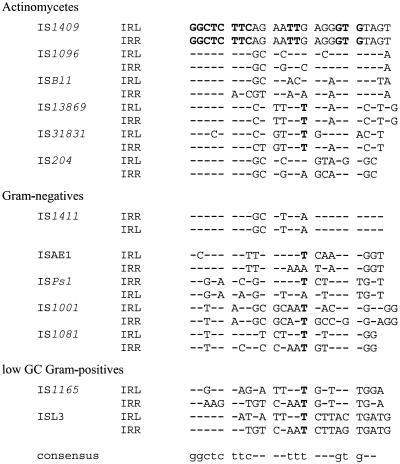
Database searches with the deduced TnpA sequence revealed a number of highly similar transposases of bacterial IS elements, as shown in Table 2. The most closely related transposases are found in actinomycetes, with the exception of IS1411 from a phenol-degrading Pseudomonas putida strain (12). For IS1096, IS31831, and IS1411 there is very high identity at the DNA level, with 72, 60, and 57% identities, respectively, observed over nearly the whole length of the transposase gene (data not shown). The inverted repeats of these IS elements are also highly conserved (Fig. 2). Based on the homology to these transposases, the 8-nt duplication, and the inverted repeats of 25 bp, IS1409 is classified as a member of the ISL3 family (16).
TABLE 2.
Comparison of IS1409 with some insertion sequences of the ISL3 family
| IS element | Origin | Accession no. | Length of TnpA (aa) | Identity/similarity (%) | Length of IRc (bp) | Target duplication (bp) | Reference |
|---|---|---|---|---|---|---|---|
| IS1409 | Arthrobacter sp. strain TM1 | AF042490 | 435 | 25 | 8 | This work | |
| IS1096a | Mycobacterium smegmatis | M76495 | 413 | 77/87 | 25 | 8 | 3 |
| ISB11 | Brevibacterium linens | AF052055 | 436 | 69/79 | 25 | 8 | Unpublished data |
| IS1411 | Pseudomonas putida | M57500 | 433 | 60/76 | 24 | 8 | 12 |
| IS13869 | Brevibacterium lactofermentum | Z66534 | 436 | 42/57 | 26 | 8 | 4 |
| IS31831 | Corynebacterium glutamicum | D17429 | 436 | 42/56 | 24 | 8 | 36 |
| IS2001 | Bifidobacterium lactis | AJ243948 | 442 | 43/55 | 26 | ?d | Unpublished data |
| IS204 | Nocardia asteroides | U10634 | 377b | 30/47 | 23 | 8 | 43 |
| ISPs1 | Pseudomonas stutzeri | AJ012352 | 425 | 27/48 | 24 | 8 | 2 |
| ISAe1 | Alcaligenes eutrophus | M86608 | 408 | 27/41 | 24 | 8 | 15 |
| IS1165 | Leuconosioc mesenteroides | X62617 | 412 | 23/38 | 24 | 8 or 3 | 11 |
| IS1001 | Bordetella parapertussis | X66858 | 406 | 22/37 | 28 | 6 or 8 | 34 |
| ISL3 | Lactobacillus delbrueckii | X79114 | 434 | 20/37 | 38 | 8 | 8 |
| IS1181 | Staphylococcus aureus | L14544 | 439 | 19/38 | 23 | 8 | 6 |
IS1096 is the only one of this group of IS elements which additionally contains a putative resolvase gene
IS204 may contain a frameshift sequencing error or represent a nonfunctional IS element, as the conserved C-terminal domain of the transposase displayed in Fig. 1 occurs in a different reading frame from the tnpA gene.
IR, inverted repeat.
?, data not available.
FIG. 2.
Sequence alignment of the terminal inverted repeats (IRL and IRR) of some members of the ISL3 family. Nucleotides identical to those in the inverted repeats of IS1409 are shown as dashes.
Distribution of IS1409 among various bacterial strains.
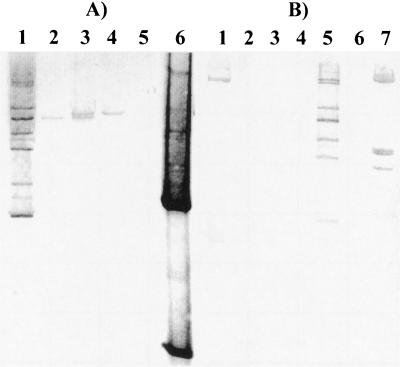
Hybridization experiments with an internal 1-kb SstI fragment of IS1409 as a probe against total or plasmid DNA of various Arthrobacter strains were conducted under stringent conditions. For this, DNA was digested with BglII, which does not cut inside the tnpA gene (Fig. 3). In Arthrobacter sp. strain TM1, at least eight copies of IS1409 were found, displaying different signal intensities. The 4-CBA degrading Arthrobacter sp. strain SU carries two copies, while only one copy could be identified for the plasmid-cured derivative Arthrobacter sp. strain SUX (Fig. 3), indicating that in these strains one copy of IS1409 is carried by plasmid pASU1. A. oxidans CBS2 DNA gave only one and Micrococcus luteus DNA gave two positive signals under stringent conditions. IS1409 was not identified in any of the other actinomycetes tested (Table 1 and Fig. 3) under stringent conditions (data not shown for all strains).
FIG. 3.
Southern blot of total DNA of various actinomycete strains digested with BglII and hybridized with a digoxigenin-labeled internal 1-kb SstI fragment of tnpA. (A) Lane 1, Arthrobacter sp. strain TM1; lane 2, A. oxidans; lane 3, Arthrobacter sp. strain SU; lane 4, Arthrobacter sp. strain SUX; lane 5, A. nicotinovorans DSM420; lane 6, SstI-hydrolyzed pKGT21. (B) Lane 1, M. luteus; lanes 2 to 4, Clavibacter michiganensis subsp. michiganensis, sepedonicus, and insidiosum; lane 5, Arthrobacter sp. strain TM1; lane 6, A. nicotinovorans DSM420; lane 7, digoxigenin-labeled λEcoRI/HindIII.
Construction of resistance transposons from IS1409.
To prove that IS1409 located on pKGT451 is a functional IS element, derivatives carrying resistance genes were constructed. As the nucleotide sequence of pKGT451 revealed that there are no unique restriction sites present between the left inverted repeat and the start of the putative transposase gene, the 120 bp at the 5′ end of IS1409 up to the BglII site was replaced by six overlapping synthetic oligonucleotides (IS1 through IS6) which carried two nucleotide exchanges so that unique SphI and NheI sites were created. The oligonucleotides were annealed and inserted into EcoRI/BglII-linearized pKGT451, resulting in pKGT452 (Fig. 1b). The changes in the sequence were confirmed by restriction analysis and nucleotide sequence determination.
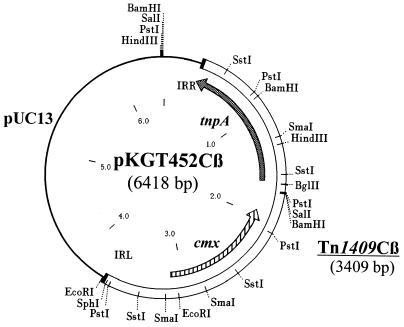
Then, the chloramphenicol resistance gene cmx, encoding an exporter protein of Tn5564 of Corynebacterium striatum (32), and the gentamicin acetyltransferase gene aacC1 from Tn1696 (40) were inserted into the NheI site of pKGT452. Both orientations were obtained after ligation as confirmed by restriction analysis and partial sequencing. The resulting plasmids were designated pKGT452Cα and -β (Fig. 4) and pKGT452Gα and -β, carrying the transposons Tn1409Cα and -β and Tn1409Gα and -β, respectively, depending on the orientation of the resistance cassette relative to the tnpA gene. The size of these transposons is about 3.4 kb.
FIG. 4.
Physical map of pKGT452Cβ. There are no recognition sites for EcoRV and StuI in pKGT452Cβ. The SphI site is located 11 bp inside Tn1409Cβ.
Transposon mutagenesis of Arthrobacter sp.
A mutagenesis was conducted with Arthrobacter sp. strains SU and SUX by electroporation using covalently closed circular plasmid DNA of pKGT452Cα and -β and pKGT452Gα and -β isolated from E. coli JM109. Since pUC13 is not able to replicate in Arthrobacter, all resistant clones should arise by transposition or recombination with endogenous copies of IS1409. The average rates of antibiotic-resistant clones obtained ranged from 2 × 103 to 1 × 104/μg of DNA in independent experiments. For both pKGT452Cβ and pKGT452Gβ, which contain the resistance gene and the tnpA gene oriented in the same direction, about twice as many antibiotic-resistant clones were obtained as for the corresponding plasmids where transposase and resistance genes were transcribed in opposite directions. No significant difference in the number of resistant clones was found between pKGT452C and pKGT452G.
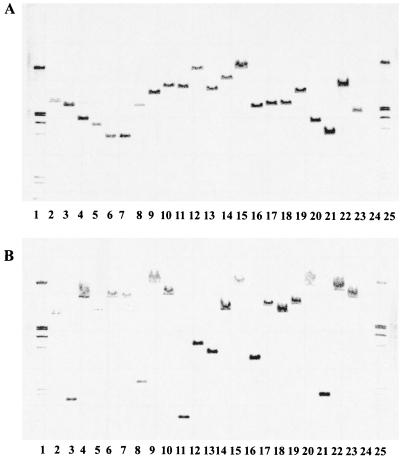
To distinguish between transposition and recombination, chloramphenicol-resistant clones were chosen at random for hybridization. Total DNA was hydrolyzed with BglII or SphI, both of which leave the tnpA gene intact, so that hybridization signals corresponding to sizes larger than 1.4 and 3.4 kb were expected with the tnpA probe. With the exception of two clones which gave an identical signal in both digestions, all clones produced signals of different sizes larger than 1.4 or 3.4 kb (Fig. 5). Thus, Tn1409 seems to transpose in a random fashion. Surprisingly, we observed that in the course of the experiments a copy of the endogenous IS1409 in strains ASU and ASUX was lost, as indicated by a missing band (compare Fig. 3, lanes 3 and 4, with Fig. 5, lanes 2 and 24). Only 1 out of 20 clones probed gave a signal after hybridization with a digoxigenin-labeled pUC13 probe (data not shown), indicating that integration of the complete pKGT452Cβ by either homologous recombination or cointegrate formation had occurred. In about 10% of the transposon mutants of strain ASU the signal of the endogenous copy of IS1409 was missing, indicating a recombination event or a more complex rearrangement between the introduced transposon and the endogenous IS1409 (data not shown). However, this does not impair the usefulness of Tn1409 for transposon mutagenesis, especially when a strain which does not carry IS1409 is used.
FIG. 5.
Southern hybridization of various chloramphenicol-resistant Arthrobacter sp. mutants generated with pKGT452Cβ. Total DNA was digested with SphI (A) or BglII (B) and probed with the internal 1-kb SstI fragment of tnpA. As no SphI and BglII site occurs in the tnpA gene, one signal with a minimal size of 3.4 or 1.4 kb (SphI/BglII) is expected after insertion of Tn1409Cβ. Lane 1, digoxigenin-labeled λEcoRI/HindIII; lane 2 Arthrobacter sp. strain SU; lanes 3 to 23, Arthrobacter sp. strain SUX mutants D1 to D21; lane 24, Arthrobacter sp. strain SUX; lane 25, digoxigenin-labeled λEcoRI/HindIII. Mutants D4 and D5 (lanes 6 and 7) seem to be identical. All other mutants display different hybridization patterns, indicating a random insertion.
Sequence analysis of insertion sites of Tn1409 in Arthrobacter.
Total DNA of some mutants generated with Tn1409Cβ was isolated and digested with SphI (one site in Tn1409Cβ 11 bp downstream of the left inverted repeat), which leaves the cmx gene intact. The digested DNA was hybridized with digoxigenin-labeled probes for tnpA (a 1-kb SstI fragment from pKGT21), the cmx gene (a 2.2-kb SphI/BglII fragment from pKGT452Cβ), and pUC13. The insertion sites from two mutants (A1 and A27) which did not hybridize with pUC13 were subsequently isolated as SphI fragments giving a signal with the cmx probe.
From total DNA of mutant A27, the region containing a 5.0-kb SphI band was cut from the agarose gel, purified, cloned into SphI-linearized pUC18, and used to transform E. coli JM109. Colonies resistant to both chloramphenicol and ampicillin were obtained, and one of them was designated pKGT1010 and chosen for sequencing. The sequence obtained was compared to the GenBank database. These data revealed the insertion of Tn1409Cβ 59 bp upstream of a gene homologous to branched-chain α-keto acid dehydrogenases (5, 28) in mutant A27. From another mutant Arthrobacter strain, SU A1, the hybridizing 4.8-kb SphI fragment was cloned in an analogous procedure (pKGT1000). Preliminary single-stranded sequence determination revealed the insertion of Tn1409Cβ into an ORF with homology to the membrane component of ABC transporters involved in iron uptake (23). However, growth of mutant A1 on iron-limited media was not impaired (data not shown). In addition, downstream a small ORF without homologs in the database followed by a third ORF designated tdk, similar to thymidine kinase genes (33), was found. The putative target duplication in these mutants confirmed the apparent random transposition, as no preference for a specific insertion site was detected.
DISCUSSION
In this report we describe a novel IS element, IS1409, found upstream of the 4-CBA dehalogenase operon of Arthrobacter sp. strain TM1. This IS element was found only in members of the Micrococcaceae, in 4-CBA-degrading Arthrobacter strains, and in M. luteus. IS1409 displays typical features of members of the ISL3 family, with the highest similarity to IS elements from other actinomycetes. The high extent of identity to IS1411 from P. putida may indicate horizontal transfer of this element from gram-positive to gram-negative bacteria.
The mode of transposition, whether replicative or conservative, for the members of the ISL3 family is still unknown. The putative resolvase gene occurring in IS1096 of Mycobacterium smegmatis is not necessary for transposition (18). For IS31831 and ISPs1, the formation of excised transposon circles was reported (2, 38). Integration of the delivery plasmid at low frequency, which was also observed for IS31831 (37), could arise either by recombination or cointegrate formation.
The insertion sites of IS1409 and Tn1409 analyzed so far do not indicate a preference for a specific sequence for insertion. However, both ends of the 8-nt target duplication are rich in GC with an AT-rich central region, for example, TCATTGCCC (endogenous copy), GCCAAAAC (mutant A1), and ACGAAAGT (mutant A27). For the transposons derived from IS1096 and IS31831 (18, 37), the same pattern was reported for the insertion sites. This may often lead to insertions within promoter regions in these high-GC gram-positive bacteria; for example, this may have occurred in mutant A27. Such integrations resulting in gene activation and/or inactivation have also been described for pseudomonads (e.g., for IS1411 and ISPs1) (2, 12).
IS1409 was used to construct antibiotic resistance gene transposons after the exchange of two nucleotides in the upstream region of the tnpA gene. The transposition rates of about 103 transpositions/μg of DNA used in electroporation of Arthrobacter are in the same range as those obtained with transposons constructed from IS1096 (3) and IS31831 (37). Since transposition rates strictly depend on the efficiency of transformation, transposon mutagenesis requires optimization of the conditions for electroporation. Also, the extent of methylation of the DNA used may be important (35).
Although the strains used in transposition experiments possess endogenous copies of IS1409, in most cases a transposition of Tn1409 occurred. Only once did we observe integration of the complete delivery plasmid, and in two cases we found indications of rearrangements as the endogenous IS1409 was lost. Both phenomena could arise either by recombination or during transposition.
The construction of transposon Tn1409, which has a high transposition rate and shows no obvious preference for specific insertion sites, now provides a system for transposon mutagenesis for Arthrobacter which may be very useful for genetic investigations in this important actinomycete.
ACKNOWLEDGMENTS
We thank our colleagues J. Eberspächer (University of Stuttgart, Stuttgart, Germany), J. Kalinowski and A. Tauch (University of Bielefeld, Bielefeld, Germany), F. Lingens (University of Stuttgart), and R. Müller (University of Hamburg, Hamburg, Germany) for supplying strains and plasmids. We are also indebted to E.-M. Zellermann for excellent technical assistance.
This work was supported by the Deutsche Forschungsgemeinschaft Ei 140/11-2.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolognese F, di Lecce C, Galli E, Barbieri P. Activation and inactivation of Pseudomonas stutzeri methylbenzene catabolism pathways mediated by a transposable element. Appl Environ Microbiol. 1999;65:1876–1882. doi: 10.1128/aem.65.5.1876-1882.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cirillo J F, Barletta R G, Bloom B R, Jacobs W R., Jr A novel transposon trap for mycobacteria: isolation and characterization of IS1096. J Bacteriol. 1991;173:7772–7780. doi: 10.1128/jb.173.24.7772-7780.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Correia A, Pisabarro A, Castro J M, Martin J F. Cloning and characterization of an IS-like element present in the genome of Brevibacterium lactofermentum ATCC 13869. Gene. 1996;170:91–94. doi: 10.1016/0378-1119(95)00866-7. [DOI] [PubMed] [Google Scholar]
- 5.Denoya C D, Fedechko R W, Hafner E W, McArthur H A, Morgenstern M R, Skinner D D, Stutzman-Engwall K, Wax R G, Wernau W C. A second branched-chain alpha-keto acid dehydrogenase gene cluster (bkdFGH) from Streptomyces avermitilis: its relationship to avermectin biosynthesis and the construction of a bkdF mutant suitable for the production of novel antiparasitic avermectins. J Bacteriol. 1995;177:3504–3511. doi: 10.1128/jb.177.12.3504-3511.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derbise A, Dyke K G H, El Solh N. Isolation and characterization of IS1181, an insertion element from Staphylococcus aureus. Plasmid. 1994;31:251–264. doi: 10.1006/plas.1994.1027. [DOI] [PubMed] [Google Scholar]
- 7.Eichenlaub R. Development of plasmids and cloning procedures. In: Becker Y, editor. Recombinant DNA research and viruses. Boston, Mass: Martinus Nijhoff Publishing; 1985. pp. 39–57. [Google Scholar]
- 8.Germond J-E, Lapierre L, Delley M, Mollet B. A new mobile genetic element in Lactobacillus delbrueckii subsp. bulgaricus. Mol Gen Genet. 1995;248:407–416. doi: 10.1007/BF02191640. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulations of Streptomyces. A laboratory manual. Norwich, United Kingdom: John Innes Foundation; 1985. [Google Scholar]
- 10.Jäger W, Schäfer A, Kalinowski J, Pühler A. Isolation of insertion elements from Gram-positive Brevibacterium, Corynebacterium, and Rhodococcus strains using the Bacillus subtilis sacB gene as a positive selection marker. FEMS Microbiol Lett. 1995;126:1–6. doi: 10.1111/j.1574-6968.1995.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 11.Johansen E, Kibenich A. Isolation and characterization of IS1165, an insertion sequence of Leuconostoc mesenteroides subsp. cremoris and other lactic acid bacteria. Plasmid. 1992;27:200–206. doi: 10.1016/0147-619x(92)90022-3. [DOI] [PubMed] [Google Scholar]
- 12.Kallastu A, Horak R, Kivisaar M. Identification and characterization of IS1411, a new insertion sequence which causes transcriptional activation of the phenol degradation genes in Pseudomonas putida. J Bacteriol. 1998;180:5306–5312. doi: 10.1128/jb.180.20.5306-5312.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieser T. Factors affecting the isolation of cccDNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12:19–36. doi: 10.1016/0147-619x(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 14.Klages U, Lingens F. Degradation of 4-chlorobenzoic acid by a Nocardia species. FEMS Lett. 1979;10:213–215. [Google Scholar]
- 15.Kung S-S, Chen J, Chow W-Y. Molecular and genetic characterization of an Alcaligenes eutrophus insertion element. J Bacteriol. 1992;174:8023–8029. doi: 10.1128/jb.174.24.8023-8029.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marks T S, Smith A R W, Quirk A V. Degradation of 4-chlorobenzoic acid by Arthrobacter sp. Appl Environ Microbiol. 1984;48:1020–1025. doi: 10.1128/aem.48.5.1020-1025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam R A, Weisbrod T R, Martin J, Scuderi J D, Brown A M, Cirillo J D, Bloom B R, Jacobs W R., Jr In vivo growth characteristics of leucine and methionine auxotrophic mutants of Mycobacterium bovis BCG generated by transposon mutagenesis. Infect Immun. 1995;63:1004–1012. doi: 10.1128/iai.63.3.1004-1012.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menendez C, Igloi G L, Brandsch R. IS1473, a putative insertion sequence identified in the plasmid pAO1 from Arthrobacter nicotinovorans: isolation, characterization, and distribution among Arthrobacter species. Plasmid. 1997;37:35–41. doi: 10.1006/plas.1996.1272. [DOI] [PubMed] [Google Scholar]
- 20.Olasz F, Stalder R, Arber W. Formation of tandem repeat (IS30)2 and its role in IS30-mediated transpositional DNA rearrangements. Mol Gen Genet. 1993;239:177–187. doi: 10.1007/BF00281616. [DOI] [PubMed] [Google Scholar]
- 21.Ruisinger S, Klages L, Lingens F. Abbau der 4-Chlorbenzoesäure durch eine Arthrobacter-Species. Arch Microbiol. 1976;110:253–256. doi: 10.1007/BF00690235. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 23.Sanders J D, Cope L D, Hansen E J. Identification of a locus involved in the utilization of iron by Haemophilus influenzae. Infect Immun. 1994;62:4515–4525. doi: 10.1128/iai.62.10.4515-4525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitz A, Gartemann K-H, Fiedler J, Grund E, Eichenlaub R. Cloning and sequence analysis of genes for dehalogenation of 4-chlorobenzoate from Arthrobacter sp. strain SU. Appl Environ Microbiol. 1992;58:4068–4071. doi: 10.1128/aem.58.12.4068-4071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz A, Gartemann K-H, Fiedler J, Grund E, Denecke B, Eichenlaub R. Degradation of polycyclic and halogenated aromatic compounds by Rhodococcus and Arthrobacter species. Biotekhnologiya. 1995;N7-8/95:150–154. [Google Scholar]
- 27.Seiler H. Identification key for coryneform bacteria derived by numerical taxonomic studies. J Gen Microbiol. 1983;129:1433–1471. doi: 10.1099/00221287-129-5-1433. [DOI] [PubMed] [Google Scholar]
- 28.Skinner D D, Morgenstern M R, Fedechko R W, Denoya C D. Cloning and sequencing of a cluster of genes encoding branched-chain alpha-keto acid dehydrogenase from Streptomyces avermitilis and the production of a functional E1[αβ] component in Escherichia coli. J Bacteriol. 1995;177:183–190. doi: 10.1128/jb.177.1.183-190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith M R, Ratledge C. Catabolism of biphenyl by Pseudomonas sp. NCIB10643 and Nocardia sp. NCIB10503. Appl Microbiol Biotechnol. 1989;30:395–401. [Google Scholar]
- 30.Stackebrandt E, Rainey F A, Ward-Rainey N L. Proposal for a new hierarchic classification system. Actinobacteria classis nov. Int J Syst Bacteriol. 1997;47:479–491. [Google Scholar]
- 31.Tan H-M. Bacterial catabolic transposons. Appl Microbiol Biotechnol. 1999;51:1–12. doi: 10.1007/s002530051356. [DOI] [PubMed] [Google Scholar]
- 32.Tauch A, Zheng Z, Pühler A, Kalinowski J. The Corynebacterium striatum resistance transposon Tn5564: genetic organization and transposition in Corynebacterium glutamicum. Plasmid. 1998;40:126–139. doi: 10.1006/plas.1998.1362. [DOI] [PubMed] [Google Scholar]
- 33.Valerie K, Stevens J, Lynch M, Henderson E E, de Riel J K. Nucleotide sequence and analysis of the 58.3 to 65.5-kb early region of bacteriophage T4. Nucleic Acids Res. 1986;14:8637–8654. doi: 10.1093/nar/14.21.8637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van der Zee A, Agterberg C, van Agterveld M, Peeters M, Mooi F R. Characterization of IS1001, an insertion element of Bordetella parapertussis. J Bacteriol. 1993;175:141–147. doi: 10.1128/jb.175.1.141-147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vertes A A, Inui M, Kobayashi M, Kurusu Y, Yukawa H. Presence of mrr- and mcr-like restriction systems in coryneform bacteria. Res Microbiol. 1993;144:181–185. doi: 10.1016/0923-2508(93)90043-2. [DOI] [PubMed] [Google Scholar]
- 36.Vertes A A, Inui M, Kobayashi M, Kurusu Y, Yukawa H. Isolation and characterization of IS31831, a transposable element from Corynebacterium glutamicum. Mol Microbiol. 1994;11:739–746. doi: 10.1111/j.1365-2958.1994.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 37.Vertes A A, Asai Y, Inui M, Kobayashi M, Kurusu Y, Yukawa H. Transposon mutagenesis of coryneform bacteria. Mol Gen Genet. 1994;245:397–405. doi: 10.1007/BF00302251. [DOI] [PubMed] [Google Scholar]
- 38.Vertes A A, Asai Y, Inui M, Kobayashi M, Yukawa H. The corynebacterial insertion sequence IS31831 promotes the formation of excised transposon fragment. Biotechnol Lett. 1995;17:1143–1148. [Google Scholar]
- 39.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 40.Wohlleben W, Arnold W, Bissonette L, Pelletier A, Tanguay A, Roy P H, Gamboa G C, Barry G F, Aubert E, Davies J, Kagan S A. On the evolution of Tn21-like multiresistance transposons: sequence analysis of the gene (aacC1) for gentamicin acetyltransferase (AAC(3)-I), another member of the Tn21-based expression cassette. Mol Gen Genet. 1989;217:202–208. doi: 10.1007/BF02464882. [DOI] [PubMed] [Google Scholar]
- 41.Wyndham R C, Cashore A E, Nakatsu C H, Peel M C. Catabolic transposons. Biodegradation. 1994;5:323–342. doi: 10.1007/BF00696468. [DOI] [PubMed] [Google Scholar]
- 42.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 43.Yao W, Yang Y, Chiao J. IS204: an insertion sequence from Nocardia asteroides (mexicana) YP21. Plasmid. 1994;32:262–269. doi: 10.1006/plas.1994.1065. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Hui F M, Morrison D A. Characterization of IS1167, a new insertion sequence in Streptococcus pneumoniae. Plasmid. 1995;33:127–138. doi: 10.1006/plas.1995.1014. [DOI] [PubMed] [Google Scholar]