Abstract
Purpose
Individuals with fibromyalgia and obesity experience significant impairment in physical functioning. Pain catastrophizing, kinesiophobia, and pain acceptance have all been identified as important factors associated with the level of disability. The objective of this study was to evaluate the role of pain catastrophizing, kinesiophobia, and pain acceptance as mediators of the association between perceived pain severity and physical functioning in individuals with fibromyalgia and obesity.
Patients and Methods
In this cross-sectional study, 165 women with fibromyalgia and obesity completed self-report questionnaires of perceived pain severity (ie, Numeric Pain Rating Scale), pain catastrophizing (ie, Pain Catastrophizing Scale), kinesiophobia (ie Tampa Scale of Kinesiophobia), pain acceptance (ie, Chronic Pain Acceptance Questionnaire), and perceived physical functioning (ie, Physical Functioning subscale of the Fibromyalgia Impact Questionnaire). In addition, a performance-based test (ie, 6-minute walking test) was conducted to assess objective physical functioning. Two multiple mediation analyses were performed.
Results
Pain acceptance and kinesiophobia mediated the relationship between pain severity and self-reported physical functioning. Pain catastrophizing and kinesiophobia mediated the relationship between pain severity and performance-based functioning.
Conclusion
Pain acceptance, kinesiophobia, and pain catastrophizing should be addressed in rehabilitative intervention to improve physical functioning. Interestingly, the subjective and objective aspects of physical functioning are influenced by different factors. Therefore, interventions for women with fibromyalgia and obesity should focus on factors related to both subjective and performance-based physical functioning.
Keywords: chronic pain, fear-avoidance model, fibromyalgia, functioning, obesity, psychological flexibility model
Plain Language Summary
Our work highlights how psychological factors (ie, pain catastrophizing, kinesiophobia, and pain acceptance) explain the association between pain severity and physical functioning. Interestingly, the relationship between pain intensity and different aspects of physical functioning (ie, self-reported and performance-based physical functioning) is associated with different psychological factors. Pain acceptance and kinesiophobia explain the relationship between pain severity and self-reported physical functioning (ie, what I think I can do). Whereas, pain catastrophizing and kinesiophobia explain the relationship between pain severity and performance-based functioning (ie, what I can actually do). Our results could inform the development of interventions aimed at improving physical functioning both in its self-reported and in its performance-based dimension.
Introduction
Fibromyalgia (FM) is a chronic pain syndrome characterized by widespread and persistent pain, fatigue, poor sleep quality, cognitive problems, and mood disturbances.1 FM has a negative impact on the quality of life2 and severely impairs physical functioning.3 The etiology of FM is unclear and multifaceted, and it appears that a complex intertwining of genetic, biological, and psychological factors plays a role in its development and maintenance.4
Impaired physical functioning is one of the most challenging consequences of FM, especially when there is comorbid obesity.5 Obesity has a high prevalence in FM5–7 and further contributes to functional disability.5,8,9 Higher levels of BMI are associated with increased pain intensity, decreased quality of life, and higher levels of disability in patients with FM.5 Notably, pain and fatigue associated with FM can lead to sedentary behavior, physical inactivity, and weight gain, which can, in turn, exacerbate both conditions.10
Impairments in physical functioning are associated with the severity of perceived pain;11–13 however, evidence showed that reducing pain intensity does not result in a proportional improvement in physical functioning.14–17 Inconsistencies in the relationship between pain severity and physical functioning suggest that this association may be mediated by other factors.18 The biopsychosocial approach has emphasized the role of psychological factors in the development and maintenance of chronic pain and associated disabilities.19 Indeed, cognitive, affective, and behavioral factors contribute to pain severity and disability in chronic pain conditions,20–24 including FM.25,26
The Fear Avoidance Model22 and the Psychological Flexibility Model27,28 are two of the most well-known models that describe the progression of transient pain into persistent pain and explain why chronic pain occurs only in a subset of individuals who have experienced acute pain. According to the Fear Avoidance Model, pain catastrophizing and kinesiophobia are two cognitive-affective pain responses that contribute to the development of chronic pain and associated disability.23,24,26,29,30 This model posits that individuals who cognitively evaluate pain as catastrophic and experience pain-related fear may engage in avoidance of movement and activities associated with pain.31,32 Avoidance behaviors eventually contribute to physical impairments, increased disability and pain chronification.33,34
Pain catastrophizing is defined as an exaggerated and negative appraisal of pain that occurs in response to actual or anticipated pain experiences.35,36 People who are prone to pain catastrophization magnify the threat value of pain, ruminate about pain episodes, and feel helpless. Kinesiophobia, on the other hand, refers to an excessive, irrational, and debilitating fear of physical movement and activity resulting from a feeling of vulnerability due to a painful injury or re-injury.37 According to the Psychological Flexibility Model,38 pain acceptance is defined as the willingness to live with pain without trying to reduce, avoid, or change it,27 meaning that individuals are willing to engage in valued activities and focus on personal goals and values despite the presence of chronic pain. Importantly, these two models posit that the psychological response to pain determines, at least in part, the level of disability, not just the intensity of pain. Thus, pain catastrophizing and kinesiophobia create a negative trajectory that leads to disability, whereas pain acceptance creates a positive trajectory that leads to improved functioning despite pain.39,40
Pain catastrophizing and kinesiophobia have been consistently reported in individuals with FM25,26,41–43 and have been associated with both higher pain intensity and reduced physical functioning in FM26,43 and other pain conditions.24,25,30,44–46 Whereas, lower pain acceptance is associated with increased pain intensity, higher levels of disability, and poorer physical functioning.47 These findings suggest that people with higher levels of pain severity may experience higher levels of pain catastrophization and kinesiophobia, as well as reduced acceptance, resulting in avoidance of movement and activities.48
Evidence reported higher levels of pain catastrophizing in individuals with severe obesity and osteoarthritis compared with individuals with less severe degrees of obesity and overweight.49 Similarly, kinesiophobia levels were found to be higher in patients with chronic low back and knee pain-related diagnoses with obesity compared to those without.50,51 With respect to pain acceptance in chronic pain conditions with comorbid obesity, the evidence is sparse. To our knowledge, there is only a recent study conducted by our group that found an association between lower levels of pain acceptance and poorer physical functioning in patients with FM and obesity.25 In sum, individuals with chronic pain and obesity who exhibit higher levels of pain catastrophizing, kinesiophobia, and lower levels of pain acceptance in response to pain, may reduce physical activity and movement. This might lead to physical deconditioning and disuse, which contribute to increased pain, greater disability, and potentially additional weight gain10 perpetuating the vicious cycle.
Studies regarding the physical consequences of chronic pain and the impact of psychological factors on disability are numerous and have provided important insights in the past decades.52 However, most studies on physical functioning in individuals with chronic pain, including FM, have focused on self-reported (ie, subjective perception) measures. Even though this is an important aspect to consider, it appears that there might be a mismatch between self-reported and performance-based physical functioning (eg, six-minute walking test). Indeed, it has been suggested that these two types of measures provide different and complementary information.53
The novelty of the present study is that, following the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMPAACT) guidelines,54 physical functioning is assessed by taking into account both self-reported and performance-based measures. We aimed to investigate psychological mediators in the pain-to-physical functioning relationship using both measures of physical status, in order to develop tailored treatments according to the outcome targeted (ie, self-reported or performance-based physical functioning).
To our knowledge, this is the first study to explore the mediating effects of multiple psychological factors, such as pain catastrophizing, kinesiophobia, and pain acceptance, on the association between pain severity and both self-reported and performance-based physical functioning in individuals with FM and obesity. Based on previous results on patients with chronic low back pain,24 heterogeneous chronic pain,55,56 and FM,26 we hypothesized that pain catastrophizing, kinesiophobia, and pain acceptance would mediate the relationship between pain severity and physical functioning. The mediators’ differential contributions to self-reported and performance-based physical functioning will be evaluated in an exploratory manner because there is insufficient evidence to support any hypothesis.
Materials and Methods
A cross-sectional study was performed. We consecutively recruited, by convenience sampling, 165 participants at the beginning of a one-month hospitalization for physical rehabilitation and weight loss. The recruitment at the Istituto Auxologico Italiano began in January 2019 and ended in October 2019. Data were collected during the first week of diagnostic assessment, before the start of any physical therapy and nutritional intervention. The study was conducted according to the guidelines of the Declaration of Helsinki of 1975, as revised in 1983, and approved by the Ethics Committee of Istituto Auxologico Italiano. The present study is a secondary data analysis of this data collection that resulted in a published article.25
Participants were eligible if they met the following criteria: (a) had previously been diagnosed with FM by a rheumatologist;1 (b) met the American College of Rheumatology (ACR) Research Criteria,57,58 as measured by the Fibromyalgia Survey Questionnaire;59 (c) were between the ages of 18 and 65; and (d) could sign an informed consent form. Patients were excluded if they (a) had psychiatric disorders with psychotic symptoms; (b) had a diagnosed personality disorder; (c) had previously received psychological intervention for FM management; (d) or had comorbid acute or chronic pain conditions other than FM.
Measures
Participants completed a self-report form with sociodemographic information including age, weight (in kilograms), and height (in centimeters) that were used to calculate the Body Mass Index (BMI; kg/m2).
Predictor
The Numeric Pain Rating Scale (NPRS) was used to assess pain severity. This is a validated and widely used measure of pain severity in chronic pain conditions. It is composed of an 11-point scale where 0 = “no pain” and 10 = “worst possible pain”.60
Mediators
The Pain Catastrophizing Scale (PCS)35 is a measure of catastrophic thinking about pain. It includes 13 items on a five-point Likert scale, with responses ranging from 0 = “not at all” to 4 = “all the time”. The total score ranges from 0 to 52, with higher scores corresponding to higher levels of pain catastrophizing.61 The Italian version61 has psychometric properties similar to the original version. Internal consistency was excellent in the current study (Cronbach’s α = 0.90).
The Chronic Pain Acceptance Questionnaire (CPAQ) is a self-report measure of pain acceptance.38,62,63 It consists of 20 items on a 7-point Likert scale, from 0= “never true” to 6= “always true”. The maximum total score is 120, with higher scores corresponding to higher pain acceptance. The Italian version of the questionnaire64 has psychometric properties consistent with the original English version. In the present study, the internal consistency of this measure was excellent (Cronbach’s α = 0.89).
The Italian version65 of the Tampa Scale of Kinesiophobia (TSK)65 was used to assess kinesiophobia. The TSK consists of 13 items ranging from “strongly disagree” to “strongly agree”, on a 4-point Likert scale.66 The total score ranges from 13 to 52, and higher scores indicate higher levels of kinesiophobia.65 The Italian version of the TSK shows a good factorial structure and acceptable psychometric properties.65 In the current study, the internal consistency of this measure was excellent (Cronbach’s α = 0.90).
Outcomes
The FIQR is a 21-item measure that uses a numerical rating scale of 0 to 10, with 10 corresponding to higher levels of disability. All questions refer to the functioning during the previous seven days. The FIQR evaluates three domains: (a) “physical function”, (b) “overall impact”, and (c) “symptoms”. The total score measures the impact of FM on overall functioning, with higher total scores indicating a greater impact of the disease and poorer functioning. Given the purpose of the current study, the Physical Functioning subscale was used to assess self-reported disability (PF-FIQR). The PF-FIQR includes nine items that assess the degree of physical impairment. This scale has previously been used in research.67–70 The physical functioning subscale of the FIQR has a score range of 0 to 30, with higher scores indicating poorer physical functioning. The Italian version of the FIQR71 has good psychometric properties in line with the original version.72 In the current study, the internal consistency of the scale was good (Cronbach’s α = 0.82).
The 6-minute walking test (6MWT) is a performance-based measure of physical functioning. The 6MWT has been used in research with FM patients68,70,73 with good applicability and reliability.68,74 The participant is required to walk for six minutes along a rectangular course of 45.7 m. The walking distance is measured in meters. Higher scores indicate better physical functioning. The distance walked during 6MWT appears to reflect the ability to perform daily living activities75,76 and was found to be shorter in female FM patients compared to healthy women.77 Furthermore, the 6MWT has good reproducibility and is recommended for evaluating walking ability in individuals with obesity.78
Statistical Analysis
Descriptive statistics, including ranges, means, and standard deviations are presented in Table 1. According to Fritz and Mackinnon,79 our sample size is sufficient for performing a mediation analysis with small-to-medium (0.26) a path and b paths with a 0.80 power. Preliminary analyses were conducted to check multicollinearity using Pearson’s correlations.
Table 1.
Means and Standard Deviations of the Sociodemographic and Clinical Measures
| Sociodemographic Factors | N=165 | Mean ± SD | |
|---|---|---|---|
| Age in years (mean± SD) | 43.8±7.26 | ||
| Body Mass Index (mean± SD) | 44.4±7.22 | ||
| Pain duration in years (mean± SD) | 7.13±2.71 | ||
| Current opioid use (%) | 20% | ||
| Clinical factors | Theoretical range | Actual range | Mean ± SD |
| Numeric Pain Rating Scale | 0–10 | 3–10 | 5.71±1.59 |
| Pain Catastrophizing Scale | 0–52 | 0–44 | 26.9±10.7 |
| Chronic Pain Acceptance Questionnaire | 0–120 | 21–74 | 51.5±11.5 |
| Tampa Scale of Kinesiophobia | 13–52 | 23–52 | 38.8±9.05 |
| Physical functioning subscale | 0–30 | 9–29 | 17.8±4.75 |
| 6-Minute Walking Test | NA | 201–402 | 304±59.8 |
Pearson’s correlation coefficients were also used to assess potential confounders. The level of significance of Pearson’s correlations was adjusted for multiple comparisons with Bonferroni correction (ie, α ≤ 0.05/10= p ≤ 0.005). To control for confounding variables, sociodemographic and clinical characteristics that showed significant associations with the outcome variable in bivariate analyses were planned to be included in the multiple mediation analysis.
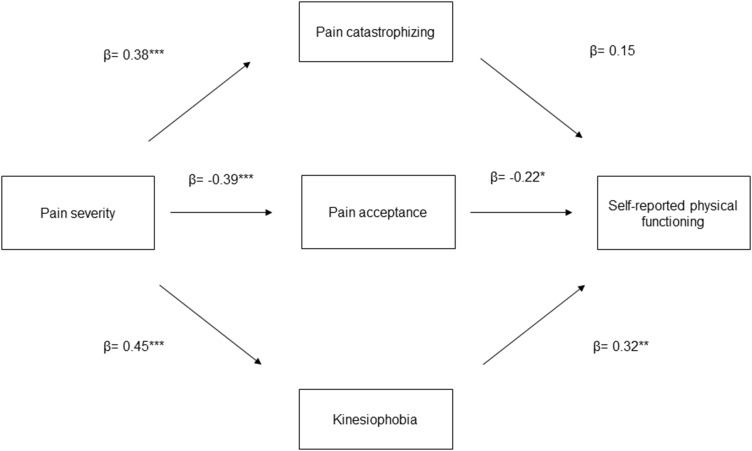
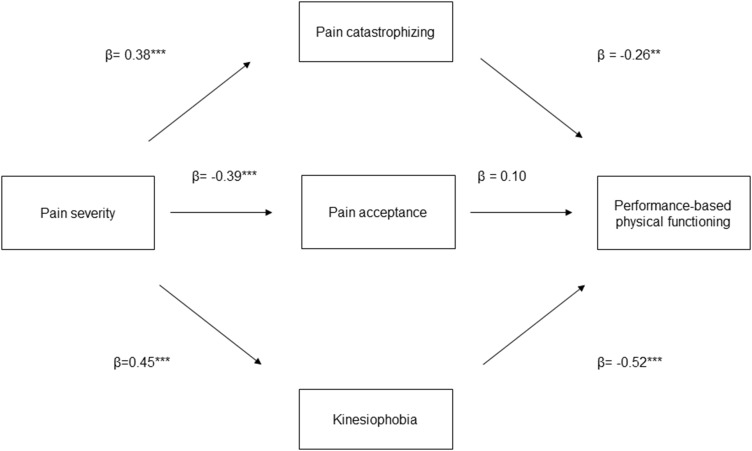
We performed two multiple mediation models. In the first model (see Figure 1), we evaluated pain catastrophizing, pain acceptance, and kinesiophobia as mediators (ie, M) of the relationship between pain severity (ie, X) and self-reported physical functioning (ie, Y). In the second model (see Figure 2), we evaluated pain catastrophizing, pain acceptance, and kinesiophobia as mediators of the relationship between pain severity and performance-based physical functioning. In the mediation analyses, we calculated direct, indirect, and total effects.80 The effect of X (ie, pain severity) on Y (ie, physical functioning) is referred to as the direct effect (c’ path). The effect of X on Y via M (ie, pain catastrophizing, pain acceptance, kinesiophobia) is referred to as the indirect effect (ab path). The path a represents the effect of X on M, whereas path b is the effect of M on Y when controlling for the effect of X. Lastly, we determined the total effect of X on Y (c path), which is the sum of direct and indirect effects. The paths were quantified with both unstandardized (B) and standardized (β) regression coefficients. To test the significance of indirect effects, bias-corrected bootstrap confidence intervals (BC-CI) were calculated following the procedures recommended by Preacher and Hayes.80 The bootstrap estimates were based on 5000 bootstrap samples.81 A 95% CI was used. Significant effects are those in which the confidence interval does not contain zero. Mediation analyses were conducted using the PROCESS macro for SPSS version 27.0.82,83
Figure 1.
Conceptual diagram of the first mediation model. *p<0.05, **p<0.01, ***p<0.001.
Figure 2.
Conceptual diagram of the second mediation model. **p<0.01, ***p<0.001.
Results
Participants’ Characteristics
Overall, 165 individuals were included in this study. Table 1 shows the demographic and clinical characteristics of the participants, as well as the means, standard deviations, and ranges for the main measures.
Pearson Correlation Analyses
Correlation analyses were performed for all study variables (see Table 2). Correlation coefficients were interpreted according to Cohen84 (0.10=small; 0.30=medium; 0.50=large). Pain catastrophizing showed positive and moderate correlations with pain severity (r= 0.38, p<0.001), self-reported disability (r=0.44, p<0.001), and kinesiophobia (r=0.55, p<0.001), as well as negative and strong correlations with pain acceptance (r=−.52, p<0.001) and negative strong correlations with performance-based physical functioning (r=−.57, p<0.001). Kinesiophobia showed a moderate and positive correlation with pain intensity (r= 0.46, p<0.001) and a positive and strong correlation with self-reported physical functioning (r=0.54, p<0.001). On the contrary, kinesiophobia was negatively and strongly correlated with pain acceptance (r=−.64, p<0.001) and performance based-physical functioning (r=−.70, p<0.001). Pain acceptance was positively and moderately correlated with performance-based physical functioning (r=0.54, p<0.001), while negatively and moderately correlated with pain severity (r=−.39, p<0.001) and self-reported disability (r=−.50, p<0.001). Because none of the demographic and clinical variables (ie, BMI, pain duration, opioid use) were significantly correlated with outcomes (see Table 2), no covariates were included in the model.
Table 2.
Bonferroni Adjusted Correlations Matrix for Study Variables
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Age | – | ||||||||
| 2. BMI | 0.03 | – | |||||||
| 3. Pain duration | 0.03 | 0.03 | – | ||||||
| 4. Opioid use | 0.17 | 0.10 | −0.14 | – | |||||
| 5. NPRS | −0.01 | 0.21 | −0.08 | 0.03 | – | ||||
| 6. PCS | 0.09 | 0.20 | 0.05 | −0.05 | 0.38* | – | |||
| 7. CPAQ | −0.07 | −0.19 | −0.07 | 0.01 | −0.39* | −0.52* | – | ||
| 8. TSK | 0.12 | 0.26* | 0.09 | 0.06 | 0.46* | 0.55* | −0.64* | – | |
| 9. PF-FIQR | 0.06 | 0.031 | −0.03 | 0.04 | 0.35* | 0.44* | −0.50* | 0.54* | - |
| 10. 6MWT | −0.87 | −0.16 | −0.02 | −0.12 | −0.40 | −0.57* | 0.54* | −0.70* | −0.54* |
Note: *Bonferroni adjusted 2-sided significance level of α ≤ 0.05/10= p ≤ 0.005.
Abbreviations: BMI, Body Mass Index; NPRS, Numeric Pain Rating Scale; PCS, Pain Catastrophizing Scale; CPAQ, Chronic Pain Acceptance Questionnaire; TSK, Tampa Scale of Kinesiophobia; PF-FIQ, Physical Functioning subscale of the Fibromyalgia Impact Questionnaire; 6MWT, 6 Minute Walking Test.
Mediation Analyses
Pain catastrophizing, pain acceptance, and kinesiophobia were tested in two multiple mediation models to explore their overall indirect effect and their contributions to the relationship between pain severity and self-reported and performance-based physical functioning. The results are presented in Table 3. Regarding self-reported physical functioning, there is a significant indirect effect of pain severity through pain acceptance (B=0.24, SE=0.12, LLCI=0.05, ULCI=0.53) and kinesiophobia (B=0.40, SE=0.14, LLCI=0.15, ULCI=0.69) and a significant total effect of pain severity through mediators (B=1.05, SE=0.22, LLCI=0.62, ULCI=1.47). However, the direct effect was not significant (B = 0.24, SE = 0.20, LLCI = −0.12, ULCI = 0.64), indicating full mediation.
Table 3.
Mediation Estimates of Pain Severity on Self-Reported Physical and Performanace Based Functioning Through Pain Catastrophizing, Pain Acceptance, and Kinesiophobia
| Indirect effect of pain severity on self-reported physical functioning via the mediators | ||||||
|---|---|---|---|---|---|---|
| B | SE | LLCI | ULCI | β | ||
| Pain catastrophizing | 0.16 | 0.12 | −0.03 | 0.43 | 0.06 | |
| Pain acceptance | 0.24 | 0.12 | 0.05 | 0.53 | 0.08 | |
| Kinesiophobia | 0.40 | 0.14 | 0.15 | 0.69 | 0.14 | |
| Direct effect of pain severity on self-reported physical functioning | ||||||
| B | SE | LLCI | ULCI | β | ||
| 0.24 | 0.20 | −0.12 | 0.64 | 0.09 | ||
| Total effect of pain severity on self-reported physical functioning via mediators | ||||||
| B | SE | LLCI | ULCI | β | ||
| 1.05 | 0.22 | 0.62 | 1.47 | 0.35 | ||
| Indirect effect of pain severity on performance-based physical functioning via the mediators | ||||||
| B | SE | LLCI | ULCI | β | ||
| Pain catastrophizing | −3.33 | 1.38 | −6.73 | −1.18 | −0.09 | |
| Pain acceptance | −1.40 | 1.12 | −4.03 | 0.51 | −0.04 | |
| Kinesiophobia | −8.26 | 1.50 | −11.46 | −5.58 | −0.24 | |
| Direct effect of pain severity on performance-based physical functioning | ||||||
| B | SE | LLCI | ULCI | β | ||
| −1.85 | 2.25 | −6.12 | 2.75 | −0.05 | ||
| Total effect of pain severity on performance-based physical functioning via mediators | ||||||
| B | SE | LLCI | ULCI | β | ||
| −14.84 | 2.69 | −20.13 | −9.56 | −0.40 | ||
Abbreviations: B, unstandardized beta; SE, standard error; LLCI, 95% lower limit confidence interval; ULCI, 95% upper limit confidence interval; β, standardized beta.
In relation to performance-based functioning, we found a significant indirect effect of pain severity via pain catastrophizing (B=−3.33, SE=1.38, LLCI=−6.73, ULCI=−1.18) and kinesiophobia (B=−8.26, SE=1.50, LLCI=−11.46, ULCI=−5.58) and a significant total effect of pain severity via the mediators (B=−14.85, SE=2.69, LLCI=−20.13, ULCI=−9.56). The direct effect was non-significant (B=−1.85, SE= 2.25, LLCI=−6.12, ULCI= 2.75), indicating full mediation.
Discussion
The goal of our study was to explore the mediating roles of pain catastrophizing, kinesiophobia, and pain acceptance in the relationship between pain severity and both self-reported and performance-based physical functioning. According to our results: i) pain acceptance and kinesiophobia mediated the relationship between pain severity and self-reported physical functioning; b) pain catastrophizing and kinesiophobia mediated the relationship between pain severity and performance-based functioning; c) kinesiophobia had a greater mediating effect on both self-reported and performance-based functioning compared with the other mediators. Overall, these findings support the idea that pain severity impacts the physical functioning of people with FM and obesity via psychological factors. Importantly, the relationship between pain severity, self-reported and performance-based physical functioning was mediated by different factors.
Concerning self-reported functioning, our results are consistent with previous findings in individuals with chronic low-back pain and obesity, in which kinesiophobia mediated the relationship between pain severity and disability.24 They are also consistent with the results of other cross-sectional studies that explored this relationship in individuals with chronic low-back pain16,85 and whiplash injury.86 Our results regarding pain acceptance are also in line with previous evidence. For example, pain acceptance significantly mediated the relationship between pain severity and self-reported performance of valued activities in individuals with rheumatoid arthritis.87 However, in another study, pain acceptance did not result as a significant mediator of the relationship between pain severity and self-reported functioning in a sample of individuals with FM.41 However, these differences could be explained by the different outcomes measured. Indeed, in this previous study, the outcome was the impact of FM (ie, the total score of the Fibromyalgia Impact Questionnaire). Differently, we used the self-reported physical functioning subscale of the Fibromyalgia Impact Questionnaire. Our results suggest that kinesiophobia and acceptance might explain the influence of pain severity on self-reported disability. Therefore, experiencing a higher level of pain might lead to greater fear of movement and lower acceptance of the pain experience, resulting in greater self-reported disability. Interestingly, the full mediation indicates that kinesiophobia and pain acceptance account for all the observed relationship between pain severity and self-reported physical functioning.
Pain catastrophizing did not result as a significant mediator of the relationship between pain severity and self-reported functioning. This finding is surprising because pain catastrophizing is one of the most consistent predictors of self-reported disability in chronic pain, and previous evidence confirmed its mediating role.26 However, in a study conducted by Lami et al, the authors did not find a significant mediating effect of catastrophizing in the relationship between pain severity and self-reported FM impact.41 Previous results in individuals with chronic low back pain and obesity found that kinesiophobia, but not pain catastrophizing, was associated with self-reported disability.30,88 Also, it is important to note that most research did not include individuals with comorbid obesity and, importantly, did not account for the contribution of other significant additional mediators.26,89,90 Finally, because psychosocial factors tend to be intercorrelated, predictive models in chronic pain should account for this shared variance between predictors to develop parsimonious models of pain and disability.91 The fact that the present study included three important psychological predictors of disability might explain why not all of them contributed unique variance to physical performance.
The relationship between pain severity and performance-based physical functioning was fully mediated by kinesiophobia and pain catastrophizing, but not by pain acceptance. Thus, higher pain severity negatively impacts physical performance through higher levels of catastrophizing and kinesiophobia. These results support the idea that, during the 6-minute walking test, the psychological evaluation and response to potential harm and pain worsening due to movement might play a prominent role. Pain is a prominent and attention-demanding signal that prompts protective action to avoid or minimize its impact.92 Thus, pain catastrophizing and kinesiophobia might serve a protective function against pain and its worsening by encouraging avoidance and restriction of movement. It is possible that a repertoire of protective cognitive/affective responses, including pain catastrophizing and kinesiophobia, leads to safety-seeking behavior such as avoidance of activities, as suggested in the Fear Avoidance Model.93 These protective behaviors are functional in the case of acute pain, but paradoxically increase disability in chronic pain by avoiding confrontation with the pain.
Kinesiophobia was the only significant mediator of the relationship between pain severity and both self-reported and performance-based disability. Another interesting finding was that kinesiophobia had a greater weight than other psychological factors in both models. Obesity might play a central role in the explanation of these results. Indeed, this condition imposes additional limitations on movements, such as respiratory difficulty, discomfort, and skin friction.94 Thus, individuals with FM and obesity may develop a greater fear of movement.43
However, targeting fear of movement in this population might have a dual health benefit. Indeed, physical activity improves physical functioning in chronic pain;95,96 and it is an essential component of weight loss programs.97 Reducing kinesiophobia may improve adherence to physical activity regimens (which are often inadequate in both patients with chronic pain98 and obesity),99,100 with positive effects in both conditions.
Overall, it appears that pain catastrophizing, kinesiophobia, and pain acceptance should be key therapeutic targets in the interdisciplinary treatment of people with FM and obesity to improve self-reported and performance-based physical functioning. The impact of pain severity on physical functioning may be minimized by reducing pain catastrophizing and kinesiophobia and increasing pain acceptance.
An important additional finding was that our results supported the full mediation of psychological factors, which suggests that the selected mediators were relevant. While acknowledging this, it is important to note that the list of potential mediators is far from complete. We did not measure the level of depressive symptomatology. Indeed, depression has a significant impact on physical performance.101 Additionally, depression and pain catastrophizing are two constructs that are frequently overlapped and associated.44,102 Kinesiophobia103 and acceptance102 also seem to be associated with depression. The inclusion of depression in future mediation models could result in interesting novel findings.
Our results might have several clinical implications. Interventions aimed at improving physical functioning should include not only pain reduction interventions but also interventions aimed at modifying cognitive and affective responses to pain. Furthermore, our findings support the idea that different aspects of physical functioning are influenced by different factors. Therefore, clinicians should prioritize the factors related to the specific aspects of physical functioning targeted in the intervention. Kinesiophobia might be relevant for both objective and subjective physical performance, while acceptance and catastrophizing would be more relevant for self-report and performance-based physical functioning, respectively.
A strength of the present study is the combination of factors from two different models of pain, namely, the Fear Avoidance Model (ie, kinesiophobia and pain catastrophizing) and the Psychological Flexibility Model (ie, pain acceptance). According to our findings, combining the two models could be beneficial because factors from both the Fear Avoidance Model and the Psychological Flexibility Model contributed independently to the mediation model. Interventions that combine therapeutic strategies from Cognitive Behavioral Therapy and Acceptance and Commitment Therapy may be a valuable option for patients with obesity and FM.41,104
Several limitations must be discussed. The cross-sectional design of the study prevents us from drawing causality conclusions. Furthermore, some potentially confounding factors (eg, anxiety and depression) were not assessed. The use of a single measurement may not be ideal in chronic pain conditions such as fibromyalgia, in which pain has a wide variability within and between days. An ecological momentary assessment design could provide reliable data.105
Conclusion
Pain acceptance and kinesiophobia appear to be significant mediators of the relationship between pain severity and self-reported physical functioning. On the other hand, pain catastrophizing and kinesiophobia had a mediating effect on the relationship between pain severity and performance-based functioning. Thus, the relationship between pain severity and different aspects of physical functioning appears to be mediated by different psychological factors. Psychological interventions targeting these factors should be included in multidisciplinary rehabilitation programs for people with FM and obesity. By reducing pain catastrophizing and kinesiophobia and improving pain acceptance, the effect of pain severity on physical functioning could be mitigated.
Funding Statement
Research funded by the Italian Ministry of Health.
Abbreviations
6MWT, Six Minute Walking Test; ACR, American College of Rheumatology; BC-CI, Bias Corrected Confidence Interval; CPAQ, Chronic Pain Acceptance Questionnaire; FIQR, Fibromyalgia Impact Questionnaire Revised; FM, Fibromyalgia; IMMPACT, Initiative on Methods, Measurement and Pain Assessment in Clinical Trials; NPRS, Numeric Pain Rating Scale; PCS, Pain Catastrophizing Scale; PF-FIQR, Physical Functioning subscale of Fibromyalgia Impact Questionnaire Revised; TSK, Tampa Scale of Kinesiophobia.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Giuseppe Plazzi reports personal fees from Jazz Pharmaceuticals, Takeda, Idorsia, and Bioprojet, outside the submitted work. The authors report no other potential conflicts of interest in this work.
References
- 1.Wolfe F, Clauw DJ, Fitzcharles M-A, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi: 10.1016/j.semarthrit.2016.08.012 [DOI] [PubMed] [Google Scholar]
- 2.Verbunt JA, Pernot DH, Smeets RJ. Disability and quality of life in patients with fibromyalgia. Health Qual Life Outcomes. 2008;6(8). doi: 10.1186/1477-7525-6-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamundí A, Miranda JGV, França LGS, de Santana CN, Montoya P. Altered functional performance in patients with fibromyalgia. Front Hum Neurosci. 2017;11:1–9. doi: 10.3389/fnhum.2017.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stisi S, Cazzola M, Buskila D, et al. Etiopathogenetic mechanisms of fibromyalgia syndrome. Reumatismo. 2011;60(1s):25–35. doi: 10.4081/reumatismo.2008.1s.25 [DOI] [PubMed] [Google Scholar]
- 5.Okifuji A, Donaldson GW, Barck L, Fine PG. Relationship between fibromyalgia and obesity in pain, function, mood, and sleep. J Pain. 2010;11(12):1329–1337. doi: 10.1016/j.jpain.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent A, Clauw D, Oh TH, Hipple MO, Toussaint LL. Decreased physical activity attributable to higher body mass index influences fibromyalgia symptoms. PM R. 2014;6(9):802–807. doi: 10.1016/j.pmrj.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2596 people with fibromyalgia. BMC Musculoskelet Disord. 2007;8:1–11. doi: 10.1186/1471-2474-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aparicio VA, Ortega FB, Carbonell-Baeza A, Camiletti D, Ruiz JR, Delgado-Fernández M. Relationship of weight status with mental and physical health in female fibromyalgia patients. Obes Facts. 2011;4(6):443–448. doi: 10.1159/000335293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim CH, Luedtke CA, Vincent A, Thompson JM, Oh TH. Association of body mass index with symptom severity and quality of life in patients with fibromyalgia. Arthritis Care Res. 2012;64(2):222–228. doi: 10.1002/acr.20653 [DOI] [PubMed] [Google Scholar]
- 10.Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399–408. doi: 10.2147/JPR.S55598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52(1):296–303. doi: 10.1002/art.20779 [DOI] [PubMed] [Google Scholar]
- 12.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Care Res. 2008;59(7):961–967. doi: 10.1002/art.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoy D, March L, Brooks P, et al. The global burden of low back pain: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73(6):968–974. doi: 10.1136/annrheumdis-2013-204428 [DOI] [PubMed] [Google Scholar]
- 14.Suso-Ribera C, Camacho-Guerrero L, Osma J, Suso-Vergara S, Gallardo-Pujol D. A reduction in pain intensity is more strongly associated with improved physical functioning in frustration tolerant individuals: a longitudinal moderation study in chronic pain patients. Front Psychol. 2019;10:1–12. doi: 10.3389/fpsyg.2019.00907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gauthier N, Thibault P, Sullivan MJL. Individual and relational correlates of pain-related empathic accuracy in spouses of chronic pain patients. Clin J Pain. 2008;24(8):669–677. doi: 10.1097/AJP.0b013e318173c28f [DOI] [PubMed] [Google Scholar]
- 16.Costa LDCM, Maher CG, McAuley JH, Hancock MJ, Smeets RJEM. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15(2):213–219. doi: 10.1016/j.ejpain.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 17.Bendayan R, Ramírez-Maestre C, Ferrer E, López A, Esteve R. From acute to chronic back pain: using linear mixed models to explore changes in pain intensity, disability, and depression. Scand J Pain. 2017;16:45–51. doi: 10.1016/j.sjpain.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 18.Steiner JL, Bigatti SM, Slaven JEAD. The complex relationship between pain intensity and physical functioning in fibromyalgia: the mediating role of depression. J Appl Biobehav Res. 2017;22(4):e12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatchel RJ, Maddrey AM. The biopsychosocial perspective of pain. In: Raczynski JM, Leviton LC, editors. Healthcare Psychology Handbook. American Psychological Association Press; 2004. [Google Scholar]
- 20.Keefe FJ, Rumble ME, Scipio CD, Giordano LA, Perri LCM. Psychological aspects of persistent pain: current state of the science. J Pain. 2004;5(4):195–211. doi: 10.1016/j.jpain.2004.02.576 [DOI] [PubMed] [Google Scholar]
- 21.Goossens EJB, Linton SJ, Crombez G, Leeuw M, Boersma K, Vlaeyen JWS. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007;30(1):77–94. doi: 10.1007/s10865-006-9085-0 [DOI] [PubMed] [Google Scholar]
- 22.Crombez G, Eccleston C, Damme S, Van Vlaeyen JWS, Karoly P. Fear-avoidance model of chronic pain. Clin J Pain. 2012;28(6):475–483. [DOI] [PubMed] [Google Scholar]
- 23.Giusti EM, Manna C, Varallo G, et al. The predictive role of executive functions and psychological factors on chronic pain after orthopaedic surgery: a longitudinal cohort study. Brain Sci. 2020;10(10):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varallo G, Scarpina F, Giusti EM, et al. Does kinesiophobia mediate the relationship between pain intensity and disability in individuals with chronic low-back pain and obesity? Brain Sci. 2021;11:684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varallo G, Scarpina F, Giusti EM, et al. The role of pain catastrophizing and pain acceptance in performance-based and self-reported physical functioning in individuals with fibromyalgia and obesity. J Pers Med. 2021;11(8):1–16. doi: 10.3390/jpm11080810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paschali M, Lazaridou A, Paschalis T, Napadow V, Edwards RR. Modifiable psychological factors affecting functioning in fibromyalgia. J Clin Med. 2021;10(4):803. doi: 10.3390/jcm10040803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain. 2014;15(3):221–234. doi: 10.1016/j.jpain.2013.10.014 [DOI] [PubMed] [Google Scholar]
- 28.Thompson M, McCracken LM. Acceptance and related processes in adjustment to chronic pain. Curr Pain Headache Rep. 2011;15(2):144–151. doi: 10.1007/s11916-010-0170-2 [DOI] [PubMed] [Google Scholar]
- 29.Suso-Ribera C, García-Palacios A, Botella C, Ribera-Canudas MV. Pain catastrophizing and its relationship with health outcomes: does pain intensity matter? Pain Res Manag. 2017;2017. doi: 10.1155/2017/9762864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Varallo G, Giusti EM, Scarpina F, Cattivelli R, Capodaglio P, Castelnuovo G. The association of kinesiophobia and pain catastrophizing with pain-related disability and pain intensity in obesity and chronic lower-back pain. Brain Sci. 2020;11(1):11. doi: 10.3390/brainsci11010011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Picavet HSJ, Vlaeyen JWS, Schouten JSAG, Catastrophizing P. Kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156(11):1028–1034. doi: 10.1093/aje/kwf136 [DOI] [PubMed] [Google Scholar]
- 32.Lundberg M, Frennered K, Hägg O, Styf J. The impact of fear-avoidance model variables on disability in patients with specific or nonspecific chronic low back pain. Spine. 2011;36(19):1547–1553. doi: 10.1097/BRS.0b013e3181f61660 [DOI] [PubMed] [Google Scholar]
- 33.Zale EL, Ditre JW. Pain-related fear, disability, and the fear-avoidance model of chronic pain. Curr Opin Psychol. 2015;5:24–30. doi: 10.1016/j.copsyc.2015.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatchel RJ, Neblett R, Kishino N, Ray CT. Fear-avoidance beliefs and chronic pain. J Orthop Sports Phys Ther. 2016;46(2):38–43. doi: 10.2519/jospt.2016.0601 [DOI] [PubMed] [Google Scholar]
- 35.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
- 36.Quartana PJ, Campbell CM, Edwards R. Pain catastrophizing: a critical review. Expert Rev Neurother. 2010;9(5):745–758. doi: 10.1586/ERN.09.34.Pain [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swinkels RAHM, Verbeek ALM, Vlaeyen JWS, et al. Psychometric properties of the Tampa Scale for kinesiophobia and the fear-avoidance beliefs questionnaire in acute low back pain. Man Ther. 2003;8:29–36. doi: 10.1054/math.2002.0484 [DOI] [PubMed] [Google Scholar]
- 38.Vowles KE, McCracken LM. Acceptance and values-based action in chronic pain: a study of treatment effectiveness and process. J Consult Clin Psychol. 2008;76(3):397–407. doi: 10.1037/0022-006X.76.3.397 [DOI] [PubMed] [Google Scholar]
- 39.Ravn SL, Vang ML, Vaegter HB, Andersen TE. Pain-related acceptance as a mediator in the fear avoidance model of chronic pain: a preliminary study. Pain Med. 2018;19(9):1764–1771. doi: 10.1093/pm/pnx223 [DOI] [PubMed] [Google Scholar]
- 40.Sturgeon JA, Zautra AJ. Psychological Resilience, Pain Catastrophizing, and Positive Emotions. Perspectives on comprehensive modeling of individual pain adaptation. Curr Pain Headache Rep. 2013;17(3). doi: 10.1007/s11916-012-0317-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lami MJ, Martínez MP, Miró E, Sánchez AI, Guzmán MA. Catastrophizing, acceptance, and coping as mediators between pain and emotional distress and disability in fibromyalgia. J Clin Psychol Med Settings. 2018;25(1):80–92. doi: 10.1007/s10880-018-9543-1 [DOI] [PubMed] [Google Scholar]
- 42.Koçyiğit BF, Akaltun MS. Kinesiophobia levels in fibromyalgia syndrome and the relationship between pain, disease activity, depression. Arch Rheumatol. 2020;35(2):214–219. doi: 10.46497/ArchRheumatol.2020.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leon-Llamas JL, Murillo-Garcia A, Villafaina S, Domínguez-Muñoz FJ, Morenas J, Gusi N. Relationship between kinesiophobia and mobility, impact of the disease, and fear of falling in women with and without fibromyalgia: a cross-sectional study. Int J Environ Res Public Heal. 2022;19:8257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards RR, Calahan C, Mensing G, Smith M, Haythornthwaite JA. Pain, catastrophizing, and depression in the rheumatic diseases. Nat Rev Rheumatol. 2011;7(4):216–224. doi: 10.1038/nrrheum.2011.2 [DOI] [PubMed] [Google Scholar]
- 45.Larice S, Ghiggia A, Di Tella M, et al. Pain appraisal and quality of life in 108 outpatients with rheumatoid arthritis. Scand J Psychol. 2020;61(2):271–280. doi: 10.1111/sjop.12592 [DOI] [PubMed] [Google Scholar]
- 46.Edwards RR, Bingham CO, Bathon J, Haythornthwaite JA. Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis Care Res. 2006;55(2):325–332. doi: 10.1002/art.21865 [DOI] [PubMed] [Google Scholar]
- 47.Rodero B, Casanueva B, Luciano JV, Gili M, Serrano-Blanco A, García-Campayo J. Relationship between behavioural coping strategies and acceptance in patients with fibromyalgia syndrome: elucidating targets of interventions. BMC Musculoskelet Disord. 2011;12. doi: 10.1186/1471-2474-12-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroska EB. A meta-analysis of fear-avoidance and pain intensity: the paradox of chronic pain. Scand J Pain. 2016;13:43–58. doi: 10.1016/j.sjpain.2016.06.011 [DOI] [PubMed] [Google Scholar]
- 49.Somers TJ, Keefe FJ, Carson JW, et al. Pain catastrophizing in borderline morbidly obese and morbidly obese individuals with osteoarthritic knee pain. Pain Res Manag. 2008;13(5):401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vincent HK, Omli MR, Day T, Hodges M, Vincent KR, George SZ. Fear of movement, quality of life, and self-reported disability in obese patients with chronic lumbar pain. Pain Med. 2011;12(1):154–164. [DOI] [PubMed] [Google Scholar]
- 51.Vincent HK, Lamb KM, Day TI, Tillman SM, Vincent KR, George SZ. Morbid obesity is associated with fear of movement and lower quality of life in patients with knee pain-related diagnoses. PMRJ. 2010;2(8):713–722. doi: 10.1016/j.pmrj.2010.04.027 [DOI] [PubMed] [Google Scholar]
- 52.Jensen MP, Turk DC. Contributions of psychology to the understanding and treatment of people with chronic pain. Am Psychol. 2014;69(2):105–118. doi: 10.1037/a0035641 [DOI] [PubMed] [Google Scholar]
- 53.Greenberg J, Mace RA, Popok PJ, et al. Psychosocial correlates of objective, performance-based, and patient-reported physical function among patients with heterogeneous chronic pain. J Pain Res. 2020;13:2255–2265. doi: 10.2147/JPR.S266455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. doi: 10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 55.Esteve R, Ramírez-Maestre C, López-Marínez AE. Adjustment to chronic pain: the role of pain acceptance, coping strategies, and pain-related cognitions. Ann Behav Med. 2007;33(2):179–188. [DOI] [PubMed] [Google Scholar]
- 56.Martinez-Calderon J, Flores-Cortes M, Clavero-Cano S, et al. The role of positive psychological factors in the association between pain intensity and pain interference in individuals with chronic musculoskeletal pain: a cross-sectional study. J Clin Med. 2020;9(10):3252. doi: 10.3390/jcm9103252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594 [DOI] [PubMed] [Google Scholar]
- 58.Wolfe F. Fibromyalgia research criteria. J Rheumatol. 2014;41(1):187. doi: 10.3899/jrheum.131224 [DOI] [PubMed] [Google Scholar]
- 59.Varallo G, Ghiggia A, Arreghini M, Capodaglio P, Manzoni GM. The reliability and agreement of the fibromyalgia survey questionnaire in an Italian sample of obese patients. Front Psychol. 2020;12:2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ritter PL, González VM, Laurent DD, Lorig KR. Measurement of pain using the visual numeric scale. J Rheumatol. 2006;33(3):574–580. [PubMed] [Google Scholar]
- 61.Monticone M, Baiardi P, Ferrari S, Rocca B, Vanti C. Development of the Italian version of the Pain Catastrophising Scale (PCS-I): cross-cultural adaptation, factor analysis, reliability, validity and sensitivity to change. Spine. 2012;35(12):1241–1246. doi: 10.1007/s11136-011-0007-4 [DOI] [PubMed] [Google Scholar]
- 62.McCracken LM, Yang SY. The role of values in a contextual cognitive-behavioral approach to chronic pain. Pain. 2006;123(1–2):137–145. doi: 10.1016/j.pain.2006.02.021 [DOI] [PubMed] [Google Scholar]
- 63.Mccracken LM, Vowles KE, Eccleston C. Acceptance of chronic pain: component analysis and a revised assessment method. Pain. 2004;107:159–166. doi: 10.1016/j.pain.2003.10.012 [DOI] [PubMed] [Google Scholar]
- 64.Bernini O, Pennato T, Cosci F, Berrocal C. The psychometric properties of the chronic pain acceptance questionnaire in Italian patients with chronic pain. J Health Psychol. 2010;15(8):1236–1245. doi: 10.1177/1359105310365576 [DOI] [PubMed] [Google Scholar]
- 65.Monticone M, Giorgi I, Baiardi P, Barbieri M, Rocca B, Bonezzi C. Development of the Italian version of the Tampa Scale of Kinesiophobia (TSK-I): cross-cultural adaptation, factor analysis, reliability, and validity. Spine. 2010;35(12):1241–1246. [DOI] [PubMed] [Google Scholar]
- 66.Woby SR, Roach NK, Urmston M, Watson PJ. Psychometric properties of the TSK-11: a shortened version of the Tampa Scale for Kinesiophobia. Pain. 2005;117:137–144. doi: 10.1016/j.pain.2005.05.029 [DOI] [PubMed] [Google Scholar]
- 67.Estévez-López F, Álvarez-Gallardo IC, Segura-Jiménez V, et al. The discordance between subjectively and objectively measured physical function in women with fibromyalgia: association with catastrophizing and self-efficacy cognitions. The al-Ándalus project. Disabil Rehabil. 2018;40(3):329–337. doi: 10.1080/09638288.2016.1258737 [DOI] [PubMed] [Google Scholar]
- 68.Mannerkorpi K, Svantesson UBC. Relationships between performance-based tests and patients’ ratings of activity limitations, self-efficacy, and pain in fibromyalgia. Arch Phys Med Rehabil. 2006;87(2):259–264. doi: 10.1016/j.apmr.2005.10.013 [DOI] [PubMed] [Google Scholar]
- 69.Ayán C, Martín V, Alonso-Cortés B, Alvarez MJ, Valencia M BM. Relationship between aerobic fitness and quality of life in female fibromyalgia patients. Clin Rehabil. 2007;21(12):1109–1113. [DOI] [PubMed] [Google Scholar]
- 70.Carbonell-Baeza A, Ruiz JR, Aparicio VA, Ortega FB. The 6-minute walk test in female fibromyalgia patients: relationship with tenderness, symptomatology, quality of life, and coping strategies. Pain Manag Nurs. 2013;14(4):193–199. doi: 10.1016/j.pmn.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 71.Salaffi F, Franchignoni F, Giordano A, et al. Psychometric characteristics of the Italian version of the revised fibromyalgia impact questionnaire using classical test theory and rasch analysis. Clin Exp Rheumatol. 2013;31(6):S41e9. [PubMed] [Google Scholar]
- 72.Bennett RM, Friend R, Jones KD, Ward R, Han BK, Ross RL. The revised fibromyalgia impact questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther. 2009;11(4):1–14. doi: 10.1186/ar2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carbonell-Baeza A, Aparicio VA, Ortega FB, Cuevas AM, Alvarez IC, Ruiz JR. Does a 3-month multidisciplinary intervention improve pain, body composition and physical fitness in women with fibromyalgia? Br J Sport Med. 2011;45(15):1189–1195. [DOI] [PubMed] [Google Scholar]
- 74.Pankoff B, Overend T, Lucy DWK. Validity and responsiveness of the 6 minute walk test for people with fibromyalgia. J Rheumatol. 2000;27(11):2666–2670. [PubMed] [Google Scholar]
- 75.Du H, Newton PJ, Salamonson Y, Carrieri-Kohlman VLDP. A review of the six-minute walk test: its implication as a self-administered assessment tool. Eur J Cardiovasc Nurs. 2009;8(1):232–234. doi: 10.1016/j.ejcnurse.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 76.ATS. Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. [DOI] [PubMed] [Google Scholar]
- 77.Jones J, Rutledge DN, Jones KD, Matallana LRD. Self-assessed physical function levels of women with fibromyalgia: a national survey. Womens Heal Issues. 2008;18(5):406–412. doi: 10.1016/j.whi.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 78.Larsson UERS. The six-minute walk test in outpatients with obesity: reproducibility and known group validity. Physiother Res Int. 2008;13(2):84–93. [DOI] [PubMed] [Google Scholar]
- 79.Fritz MS, MacKinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolin JH, Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. J Educ Meas. 2014;51(3):335–337. doi: 10.1111/jedm.12050 [DOI] [Google Scholar]
- 81.Hayes AF. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monogr. 2009;76(4):408–420. doi: 10.1080/03637750903310360 [DOI] [Google Scholar]
- 82.IBM Corp. IBM SPSS Statistics for Windows, Version 27.0; 2020.
- 83.Hayes AF. Introduction to Mediation, Moderation and Conditional Process Analysis. New York: Guiford Press; 2013. [Google Scholar]
- 84.Cohen J. Set correlation and contingency tables. Appl Psychol Meas. 1988;12(4):425–434. doi: 10.1177/014662168801200410 [DOI] [Google Scholar]
- 85.Marshall PWM, Schabrun S, Knox MF. Physical activity and the mediating effect of fear, depression, anxiety, and catastrophizing on pain related disability in people with chronic low back pain. PLoS One. 2017;12(7):1–15. doi: 10.1371/journal.pone.0180788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kamper SJ, Maher CG, Menezes Costa LDC, McAuley JH, Hush JM, Sterling M. Does fear of movement mediate the relationship between pain intensity and disability in patients following whiplash injury? A prospective longitudinal study. Pain. 2012;153(1):113–119. doi: 10.1016/j.pain.2011.09.023 [DOI] [PubMed] [Google Scholar]
- 87.Ahlstrand I, Vaz S, Falkmer T, Thyberg IBM. Self-efficacy and pain acceptance as mediators of the relationship between pain and performance of valued life activities in women and men with rheumatoid arthritis. Clin Rehabil. 2017;31(6):824–834. [DOI] [PubMed] [Google Scholar]
- 88.Vincent HK, Seay AN, Montero C, Conrad BP, Hurley RW, Vincent K. Kinesiophobia and Fear Avoidance Beliefs in Overweight Older Adults with chronic low back pain, relationship to walking endurance: part II. Am J Phys Med Rehabil. 2014;92(5):439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim HJ, Park HJH. The mediating role of pain catastrophizing on the association between depression and pain severity and interference among elderly asian immigrants with chronic pain. J Pain Res. 2021;14:737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mun CJ, Okun MA, Karoly P. Trait mindfulness and catastrophizing as mediators of the association between pain severity and pain-related impairment. Pers Individ Dif. 2014;66:68–73. doi: 10.1016/J.PAID.2014.03.016 [DOI] [Google Scholar]
- 91.Martínez-Borba V, Ripoll-Server P, Yakobov ES. Predicting the physical and mental health status of individuals with chronic musculoskeletal pain from a biopsychosocial perspective: a multivariate approach. Clin J Pain. 2021;37(3):211–218. [DOI] [PubMed] [Google Scholar]
- 92.Moore DJ, Keogh E, Eccleston C, et al. The interruptive effect of pain on attention The interruptive effect of pain on attention. Quaterly J Exp Psychol. 2012;65(3):37–41. [DOI] [PubMed] [Google Scholar]
- 93.Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain. 2016;157(8):1588–1589. [DOI] [PubMed] [Google Scholar]
- 94.van der Hulst M, Vollenbroek-Hutten MM, Rietman JS, Schaake L, Groothuis-Oudshoorn KG, Hermens H. Back muscle activation patterns in chronic low back pain during walking: a “Guarding” hypothesis. Clin J Pain. 2010;26(1):30–37. [DOI] [PubMed] [Google Scholar]
- 95.Ambrose KR, Golightly YM. Physical exercise as non-pharmacological treatment of chronic pain: why and when. Best Pract Res Clin Rheumatol. 2015;29(1):120–130. doi: 10.1016/j.berh.2015.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Geneen LJ, Moore RA, Clarke C, Martin D, Colvin LA, Smith BH. Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;4(4). doi: 10.1002/14651858.CD011279.pub3.www.cochranelibrary.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006;(4). doi: 10.1002/14651858.CD003817.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peek K, Sanson-Fisher R, Mackenzie L, Carey M. Interventions to aid patient adherence to physiotherapist prescribed self-management strategies: a systematic review. Physiother. 2016;102(2):127–135. doi: 10.1016/j.physio.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 99.Dalle Grave R, Calugi S, Centis E, El Ghoch M, Marchesini G. Cognitive-behavioral strategies to increase the adherence to exercise in the management of obesity. J Obes. 2011;2011. doi: 10.1155/2011/348293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ekkekakis P, Lind E. Exercise does not feel the same when you are overweight: the impact of self-selected and imposed intensity on affect and exertion. Int J Obes. 2006;30(4):652–660. doi: 10.1038/sj.ijo.0803052 [DOI] [PubMed] [Google Scholar]
- 101.Alschuler KN, Theisen-Goodvich ME, Haig AJ, Geisser ME. A comparison of the relationship between depression, perceived disability, and physical performance in persons with chronic pain. Eur J Pain. 2008;12(6):757–764. doi: 10.1016/j.ejpain.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 102.Weiss KE, Hahn A, Wallace DP, Biggs B, Bruce BK, Harrison TE. Acceptance of pain: associations with depression, catastrophizing, and functional disability among children and adolescents in an interdisciplinary chronic pain rehabilitation program. J Pediatr Psychol. 2013;38(7):756–765. doi: 10.1093/jpepsy/jst028 [DOI] [PubMed] [Google Scholar]
- 103.Maclachlan LR, Matthews M, Hodges PW, Collins NJ, Vicenzino B. The psychological features of patellofemoral pain: a cross-sectional study. Scand J Pain. 2018;18(2):261–271. doi: 10.1515/sjpain-2018-0025 [DOI] [PubMed] [Google Scholar]
- 104.Cattivelli R, Guerrini Usubini A, Manzoni GM, et al.; ACTonFood. ACTonFood. Acceptance and Commitment Therapy-Based Group Treatment Compared to Cognitive Behavioral Therapy-Based Group Treatment for Weight Loss Maintenance: an Individually Randomized Group Treatment Trial. Int J Environ Res Public Health. 2021;18(18):9558. doi: 10.3390/ijerph18189558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Suso-Ribera C, Castilla D, Zaragozá I, Ribera-Canudas MV, Botella C, García-Palacios A. Validity, reliability, feasibility, and usefulness of pain monitor: a multidimensional smartphone app for daily monitoring of adults with heterogenous chronic pain. Clin J Pain. 2018;34(10):900–908. doi: 10.1097/AJP.0000000000000618 [DOI] [PubMed] [Google Scholar]