Abstract
Background
A few years after the publication of the British guidelines, national recommendations were published by the Swedish Medical Products Agency in October 2012, promoting the cessation of antibiotic prophylaxis in dentistry for the prevention of infective endocarditis (IE). The aim of this study was to evaluate whether the incidence of oral streptococcal IE increased among high-risk individuals after October 2012.
Methods
This nationwide cohort study included all adult individuals (>17 years) living in Sweden from January 2008 to January 2018, with a diagnose code or surgical procedure code indicating high risk of IE. Cox proportional hazard models were performed to calculate adjusted ratios of oral streptococcal IE before and after October 2012 between high-risk individuals and references.
Results
This study found no increased incidence of oral streptococcal IE among high-risk individuals during the 5 years after the cessation, compared with before. Hazard rate ratios were 15.4 (95% confidence interval [CI]: 8.3–28.5) before and 20.7 (95% CI: 10.0–42.7) after October 2012 for prevalent high-risk individuals. Corresponding ratios for incident high-risk individuals were 66.8 (95% CI: 28.7–155.6) and 44.6 (95% CI: 22.9–86.9). Point estimates for interaction with time period were 1.4 (95% CI: .6–3.5) and 0.8 (95% CI: .5–1.3) for prevalent and incident high-risk individuals, respectively.
Conclusion
The results suggest that the current Swedish recommendation not to administer antibiotic prophylaxis for the prevention of IE in dentistry has not led to an increased incidence of oral streptococcal IE among high-risk individuals.
Keywords: prophylactic antibiotics, dentistry, infective endocarditis, viridans group streptococci
The findings of this cohort study suggest no increased incidence of oral streptococcal infective endocarditis among high-risk individuals in Sweden since the recommended cessation of antibiotic prophylaxis in dentistry for the prevention of endocarditis in October 2012.
Individuals with prior infective endocarditis (IE), prosthetic heart valve, or a cyanotic congenital heart disease (CHD) are at increased risk of developing IE [1–4]. The incidence of IE among the overall population has been reported to be 2–7 cases per 100 000 individuals per year [5–7]. Among high-risk groups, however, incidence rates of IE are 100-fold compared with the general population [1–4]. In Sweden, with a population of approximately 10 million individuals, there are approximately 600 cases of IE per year, of which 25%–30% are caused by oral viridans group streptococci (VGS-IE) [7]. Current American and European guidelines state that high-risk individuals should receive antibiotic prophylaxis (AP) before invasive dental procedures to reduce the risk of post procedural VGS-IE, despite a lack of evidence to support the efficacy of the prophylaxis. The United Kingdom and Sweden are the only countries to have abandoned AP to individuals considered to be at high risk for developing IE.
In England, cessation of AP was recommended by the National Institute for Health and Care Excellence in 2008. After the cessation, Dayer et al found an increased incidence of IE among high-risk individuals, and a 78.6% fall in AP prescribing [8]. The authors were unable to study the incidence of VGS-IE. Although the National Institute for Health and Care Excellence revised its guidance in 2015, the decision not to routinely recommend AP remains [9].
In Sweden, AP is generally prescribed by the dentist, and the dentist is ultimately responsible for the decision to administer or not to administer AP before dental procedures [10]. National recommendations published in October 2012 state that the use of prophylactic antibiotics for the prevention of IE in dentistry is no longer recommended [10]. An addendum published in March 2016 specifies that although AP is not routinely recommended, it may be considered if advised by the patient’s physician. In such cases, the physician is responsible for notifying the dentist [11]. Previously, 2 g of amoxicillin orally was recommended to be taken 1 hour before invasive dental procedures to patients with valve prosthesis, complicated heart valve disease, or previous endocarditis. The number of prescriptions of amoxicillin by Swedish dentists decreased by 20% in 2013 compared with 2012 [12], and by 22.2% from 2013 to 2017 [13]. Amoxicillin is still recommended as prophylaxis for some oral surgical procedures because the infection risk is considered very high, regardless of the health status of a patient. Therefore, a 100% reduction cannot be expected. The Swedish national register of IE (SRIE) contains detailed data on cases of IE in Sweden reported by all 30 departments of infectious disease (ID) in Swedish hospitals since 1995. The SRIE enables the study of VGS-IE among high-risk individuals at a time when AP was and was not recommended for this patient group. Considering the severity of IE, it is of great importance to investigate the effect of refraining from AP to high-risk individuals.
The aim of this cohort study was to evaluate whether the incidence of VGS-IE increased among high-risk individuals after the recommended cessation of AP in Swedish dentistry and determine the incidence of IE among the 3 high-risk groups.
MATERIALS AND METHODS
Study Design
The present study was a nationwide cohort study conducted using Swedish health registers. Record linkage was achieved because of the Swedish national registration number assigned to every citizen has been used since 1947 [14]. The study is reported according to Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Study Population
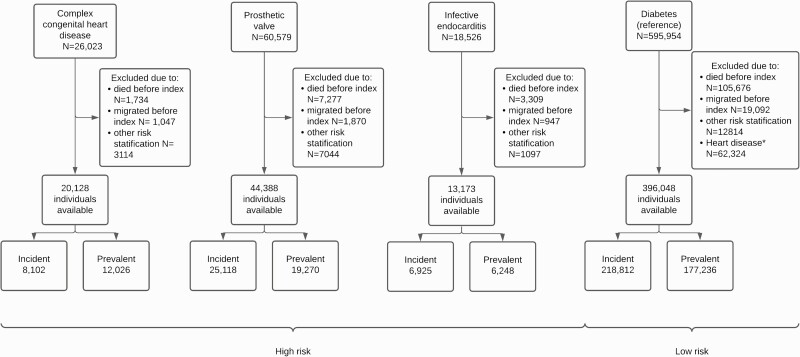
The study population consisted of two cohorts, one at high risk of IE because of prior IE, a prosthetic heart valve, or cyanotic congenital heart disease, and a reference cohort at low risk of IE not eligible for AP. Table 1 and Table 2 describe the high-risk cohort. The low-risk cohort was included to calculate and compare hazard rate ratios (HRRs) for IE before and after the change in recommendations. Figure 1 is a flow chart describing the inclusion of individuals in the study.
Table 1.
Prevalent Cohort
| High Risk | Reference | |
|---|---|---|
| N = | 36,140 | 176,884 |
| Age at index (%) | ||
| <30 | 5966 (17) | 14,930 (8) |
| 30–60 | 8941 (25) | 47,008 (27) |
| 60–80 | 13,156 (36) | 78,550 (44) |
| 80+ | 8077 (22) | 36,396 (21) |
| Sex (%) | ||
| Female | 15,592 (43) | 83,409 (47) |
| Educational attainment (%) | ||
| Missing/not completed compulsory education | 1267 (4) | 5136 (3) |
| Compulsory/upper secondary | 13,129 (36) | 72,492 (41) |
| Postsecondary | 13,680 (38) | 68,054 (38) |
| Postgraduate education | 8064 (22) | 31,202 (18) |
| Comorbidities (%) | ||
| Congenital heart diseasea | 11,738 (32) | 0 (0) |
| Pacemaker | 6441 (18) | 0 (0) |
| Rheumatic fever | 269 (1) | 0 (0) |
| Diabetes | 4512 (12) | 176,884 (100) |
| Drug use | 337 (1) | 0 (0) |
| Intravenous catheter/hemodialysis | 224 (1) | 0 (0) |
| Heart transplant | 498 (1) | 0 (0) |
Characteristics of the prevalent cohorts. Comorbidities were identified in the Swedish National Patient Register and the Swedish Medical Birth Register, since 1964 and 1973, respectively.
Congenital heart disease (International Classification of Diseases Q20–Q28) not classified as cyanotic congenital heart disease (Q20.0–Q20.4, Q21.2–Q21.4, Q21.8, Q26.2).
Table 2.
Incident Cohort
| High Risk | Reference | |
|---|---|---|
| N = | 38,791 | 218,642 |
| Age at index (%) | ||
| <30 | 7445 (19) | 6418 (3) |
| 30–60 | 5530 (14) | 47,443 (22) |
| 60–80 | 17,714 (46) | 116,355 (53) |
| 80+ | 8102 (21) | 48,426 (22) |
| Sex (%) | ||
| Female | 15,226 (39) | 99,746 (46) |
| Educational attainment (%) | ||
| Missing/not completed compulsory education | 1174 (3%) | 4859 (2) |
| Compulsory/upper secondary | 12,777 (33%) | 88,959 (41) |
| Postsecondary | 16,000 (41%) | 88,657 (41) |
| Postgraduate education | 8840 (23%) | 36,167 (17) |
| Comorbidities (%) | ||
| Congenital heart diseasea | 4277 (11) | 0 (0) |
| Pacemaker | 7557 (19) | 0 (0) |
| Rheumatic fever | 71 (0) | 0 (0) |
| Diabetes | 4523 (12) | 218,642 (100) |
| Drug use | 656 (2) | 0 (0) |
| Intravenous catheter/hemodialysis | 600 (2%) | 0 (0%) |
| Heart transplant | 111 (0%) | 0 (0%) |
Characteristics of the incident cohorts. Comorbidities were identified in the Swedish National Patient Register and the Swedish Medical Birth Register, since 1964 and 1973, respectively.
Congenital heart disease (International Classification of Diseases Q20–Q28) not classified as cyanotic congenital heart disease (Q20.0–Q20.4, Q21.2–Q21.4, Q21.8, Q26.2).
Figure 1.
Flow chart describing of the inclusion of individuals. Those who underwent heart valve surgery before or during infective endocarditis (IE) hospitalization were included in the prior IE group. Individuals diagnosed with cyanotic congenital heart disease (CHD) after implantation of a prosthetic heart valve, or after hospitalization because of IE, were categorized as patients with a prosthetic heart valve or as patients with prior IE. ∗The reference cohort consisted of individuals with no previous heart disease. Index = start of follow-up.
The high-risk cohort consisted of adult individuals (>17 years of age) living in Sweden during the 10-year study period (January 2008–January 2018), with a main or secondary International Classification of Diseases (ICD) diagnosis code or a surgical procedure code indicating high risk of IE (Table 3). To identify high-risk individuals, data were collected on all inpatient episodes with the relevant ICD and surgical procedure codes (Table 3) in the National Patient Register (NPR) and the Swedish Medical Birth Register (MBR) since 1964 and 1973, respectively. Children and adolescents younger than age 17 years were not included, as they are treated at pediatric clinics not reporting to the SRIE.
Table 3.
Codes for Inclusion of High-risk Individuals
| Endocarditis—ICD-10 codes |
|---|
| I33, I339, I389 |
| Cyanotic congenital heart disease—ICD-10 codes |
| Q200, Q201, Q202, Q203, Q204, Q212, Q213, Q214, Q218, Q262 |
| Prosthetic heart valve ICD-10 codes |
| Z952, Z953, Z954 |
| Prosthetic heart valve—surgical procedure codes |
| FAA10, FCA60, FCA70, FCA80, FCC70, FCC76, FCD00, FDC10, FGA96, FGE00, FGE10, FGE20, FGE96, FHB80, FHF00, FJF00, FJF10, FJF12, FJF20, FJF96, FKD00, FKD10, FKD20, FKD96, FMD00, FMD10, FMD12, FMD13, FMD20, FMD30, FMD33, FMD40, FMD96 |
Codes for the inclusion of high-risk individuals. Corresponding codes for earlier episodes of disease classified according to ICD-7-9 were identified using translator tables produced by the National Board of Health and Welfare.
Abbreviation: ICD, International Classification of Diseases.
The reference cohort consisted of adult low-risk individuals with diabetes, and no medical history of heart disease, hemodialysis, pacemaker, heart transplant, rheumatic fever, or drug use (Z86.4, Z72.2, R78.1–R78.6) before study entry according to NPR or MBR records. The reference cohort was selected to represent individuals with relatively low risk of IE not affected by the change in recommendations of AP.
Stratification
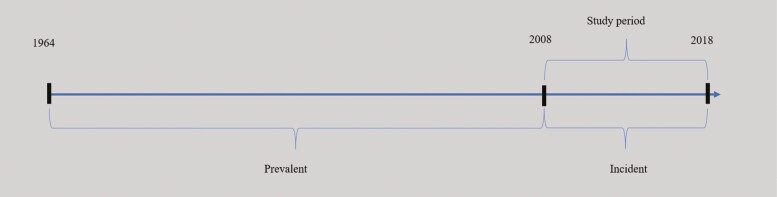
As illustrated in Figure 2, high-risk individuals were divided into 1 prevalent and 1 incident cohort analyzed separately to avoid survivorship bias. Previous research indicates a relatively high mortality rate among high-risk individuals [1, 15], as well as a relatively high risk of IE during the first few years after hospitalization from IE or replacement of a heart valve [1, 16, 17]. It was hypothesized that the incidence of IE was lower among high-risk individuals who survived until the start of follow-up in 2008 (prevalent high-risk individuals) than among those who became high-risk individuals during the follow-up (incident high-risk individuals). Analyzing these cohorts together could lead to biased estimates of the incidence of IE. Stratification was conducted by date of first hospital admission with risk factor, so that those admitted between 1964 and 2008, deemed at high risk of survivorship bias, were assigned to the prevalent cohort, and those admitted from January 2008 onward were assigned to the incident cohort.
Figure 2.
All high-risk individuals available in Swedish medical records were included since the start of the National Patient Register in 1964. High-risk individuals were assigned to the prevalent cohort if the date of first diagnosis of a risk factor for IE was before 2008, and to the incident cohort if they became risk individuals during the study period. This stratification was carried out to avoid survivorship bias. Individuals in the reference group, consisting of diabetics, were assigned to a prevalent and incident cohort in a similar way, based on the date of diagnosis.
SOURCES OF DATA
National Patient Register
Identification of risk individuals was performed using the NPR (Table 3). The register was also used to gather information on history of comorbidities among the cohorts adjusted for in the analyses (Tables 1 and 2). The Swedish NPR contains individual data from all hospitals, and more than 99% of the discharges in somatic care are covered by the register [18]. Surgical procedure codes for replacement of heart valves (Table 3) were used to identify individuals with prosthetic valves. In Sweden, ICD-10 has been used to classify episodes of disease since 1997. Corresponding historic classifications for disease (ICD-7-9) were used for identification of earlier episodes in the NPR.
Medical Birth Register
The Swedish MBR was founded in 1973 and contains information from medical records in prenatal, delivery, and neonatal care. It is compulsory for every health care provider to report to the register, and information is missing in only 0.5% of births [19]. In addition to the NPR, this register was used to gather data on congenital heart disease in the cohorts (Table 3).
Swedish National Register of Infective Endocarditis
In 1995, the Swedish Society for Infectious Diseases introduced a Swedish national registry of IE. All 30 departments of ID in Sweden have participated in the registry since its inception. These ID departments have regional responsibility for the care of patients with severe infections; patients requiring acute surgery for IE are, in most cases, treated in ID departments during the pre- and/or postoperative period. All cases are reported on a standardized questionnaire at the time of discharge and a second questionnaire after follow-up (mean: 3 months after treatment). Data regarding risk factors, presence of prosthetic valve, type of prosthetic valve, and presence of other implantable cardiac devices are collected. The origin of the etiologic agent is verified using, for example, blood cultures, cultures from valves during surgery, and 16S RNA sequencing from tissue samples of valves.
Total Population Register
Data on sex, age, educational attainment, and date of death was obtained from the total population register held by Statistics Sweden, the government agency responsible for developing, producing, and disseminating official statistics and other government statistics in Sweden.
Statistical Analysis
Among the cohorts, all cases of IE were identified that fulfilled the criteria for definite or possible IE according to the modified Duke criteria [20], with hospital admission dates during the study period. Cases of IE within 3 months after the date of hospitalization because of heart valve replacement or IE were not included in the analysis. This 90-day grace period was set to ensure follow-up occurred during stable conditions and to reduce the risk of counting the same case of IE twice [18]. VGS-IE was considered of oral origin and thus of relevance regarding the cessation of AP during invasive dental procedures. The follow-up time was split into 2 periods: before and after October 2012. We calculated the adjusted HRRs between the high-risk cohorts and the reference cohorts (Table 4).
Table 4.
Crude Incidence Rates, Adjusted Hazard Rate Ratio of Infective Endocarditis Among the Cohorts Before and After October 2012
| n/PY (100 000) | Crude IR/100 000 PYs (95% CI) |
Adjusteda HRR (95% CI) |
Interaction termb (95% CI) | |
|---|---|---|---|---|
| Total IE | ||||
| Prevalent | ||||
| January 2008–October 2012 | ||||
| Reference | 80/6.8274 | 11.7 (9.4–14.6) | 1 | |
| High risk | 216/1.4845 | 145.5 (127.3–166.3) | 12.7 (9.7–16.7) | |
| CHD | 8/0.5320 | 15.0 (7.5–30.0) | 1.8 (0.8–4.2) | |
| Prosthetic valve | 118/0.7348 | 160.6 (134.1–192.4) | 10.6 (7.9–14.3) | |
| Previous IE | 90/0.2188 | 411.4 (334.6–505.8) | 29 (21.0–40.0) | |
| November 2012–January 2018 | ||||
| Reference | 72/5.2091 | 13.8 (11.0–17.4) | 1 | |
| High risk | 179/1.2042 | 148.7 (128.4–172.1) | 10.2 (7.6–13.7) | 0.8 (0.6–1.2) |
| CHD | 7/0.5003 | 14.0 (6.7–29.4) | 1.4 (0.6–3.2) | 0.7 (0.3–2.2) |
| Prosthetic valve | 96/0.5249 | 182.9 (149.7–223.4) | 8.9 (6.4–12.3) | 0.8 (0.5–1.3) |
| Previous IE | 76/0.1803 | 421.6 (336.7–527.9) | 21.8 (15.4–31.0) | 0.8 (0.5–1.2) |
| Incident | ||||
| January 2008–October 2012 | ||||
| Reference | 72/1.5487 | 46.5 (36.9–58.6) | 1 | |
| High risk | 244/0.2600 | 938.6 (828.0–1100) | 32.1 (23.2–44.4) | |
| CHD | 5/0.0710 | 70.4 (29.3–169.1) | 1.3 (0.5–3.1) | |
| Prosthetic valve | 155/0.1560 | 993.4 (848.8–1200) | 30.0 (21.4–42.3) | |
| Previous IE | 85/0.0328 | 2600 (2100–3200) | 69.0 (47.7–99.6) | |
| November 2012–January 2018 | ||||
| Reference | 72/1.8043 | 39.9 (31.7–50.2732) | 1 | |
| High risk | 272/0.3084 | 882.0 (783.1–993.3) | 26.1 (19.5–34.9) | 0.8 (0.5–1.3) |
| CHD | 0/0.5393 | … | … | … |
| Prosthetic valve | 164/0.2081 | 788.0 (676.1–918.2) | 23.0 (16.8–31.4) | 0.8 (0.5–1.2) |
| Previous IE | 113/0.0510 | 2200 (1800–2700) | 56.3 (40.9–77.6) | 0.8 (0.5–1.3) |
| VGS-IE | ||||
| Prevalent | ||||
| January 2008–October 2012 | ||||
| Reference | 14/6.8275 | 2.1 (1.2–3.5) | 1 | |
| High risk | 50/1.4893 | 33.6 (25.4–44.3) | 15.4 (8.3 - 28.5) | |
| CHD | 4/0.5322 | 7.5 (2.8–20.0) | 3.7 (0.9–14.7) | |
| Prosthetic valve | 32/0.7369 | 43.4 (30.7–61.4) | 15.2 (7.9–29.1) | |
| Previous IE | 14/0.2205 | 63.5 (37.6–110) | 26.3 (12.0–57.3) | |
| November 2012–January 2018 | ||||
| Reference | 9/5.2116 | 1.7 (0.9–3.3) | 1 | |
| High risk | 49/1.2099 | 40.5 (30.6–53.6) | 20.7 (10.0–42.7) | 1.4 (0.6–3.5) |
| CHD | 3/0.5006 | 6.0 (1.9–18.6) | 3.3 (0.7–15.2) | 0.9 (0.2–5.0) |
| Prosthetic valve | 23/0.5262 | 43.7 (29.0–65.8) | 16.0 (7.3–34.9) | 1.0 (0.4–2.8) |
| Previous IE | 23/0.1828 | 130 (83.6–1.90) | 54.1 (24.5–119.5) | 2.2 (0.8–6.1) |
| Incident | ||||
| January 2008–October 2012 | ||||
| Reference | 10/1.5488 | 6.5 (3.5–12.0) | 1 | |
| High risk | 62/0.2602 | 240 (190–310) | 66.8 (28.7–155.6) | |
| CHD | 0/0.0710 | … | … | |
| Prosthetic valve | 38/0.1561 | 240 (180–330) | 55.9 (23.2–134.4) | |
| Previous IE | 24/0.0329 | 730 (490–1100) | 157.9 (64.6–385.6) | |
| November 2012–January 2018 | ||||
| Reference | 12/1.8053 | 6.7 (3.8–11.7) | 1 | |
| High risk | 76/0.3090 | 250 (200–310) | 44.6 (22.9–86.9) | 0.8 (0.5–1.3) |
| CHD | 0/0.0539 | … | … | … |
| Prosthetic valve | 51/0.2083 | 240 (190–320) | 41.9 (21.0–83.6) | 0.8 (0.3–2.2) |
| Previous IE | 24/0.0510 | 470 (320–700) | 75.5 (35.9–158.9) | 0.5 (0.2–1.6) |
Abbreviations: CI, confidence interval; CHD, congenital heart disease; HRR, hazard rate ratio; IE, infective endocarditis; IR, incidence rate; PY, person-years; VGS-IE, viridans group streptococci infective endocarditis.
Hazard rate ratios were adjusted for age at inclusion, sex, educational attainment, history of congenital heart disease, pacemaker, rheumatic fever, hemodialysis, implantable cardioverter defibrillator, stent, cardiomyopathy, and heart transplant.
Point estimates; an interaction term was introduced between time periods (before/after October 2012) and high risk in the regression analysis to determine any effect of time period on the incidence of IE. The HRRs of the prevalent cohorts were also adjusted for time with risk factor.
Sensitivity Analysis
The main results are based on the SRIE. As a completing analysis, the NPR was used to calculate the total incidence of IE among the cohorts (data not included in the study).
In the main analysis, the 3 risk groups (CHD, prosthetic heart valve, and previous IE) were merged into 1 high-risk group (Figure 1). In an additional analysis, however, the risk of IE was evaluated in each risk group separately to detect any increased risk that may have been masked by the grouping. For this analysis, some individuals could overlap between groups (Figure 1). No individual was simultaneously included in more than 1 risk group. Those who underwent valve surgery following IE hospitalization were assigned to the prior IE group. Those who had CHD and underwent valve replacement were assigned to the CHD group until the date of surgery; thereafter, they were assigned to the prosthetic heart valve group.
For the prevalent cohort, the Cox proportional hazard model was adjusted for age at index, sex, educational attainment, time with risk factor, history of CHD, pacemaker, rheumatic fever, hemodialysis, implantable cardioverter defibrillator, stent, cardiomyopathy, and heart transplant. Individuals were followed until date of death, emigration, positive outcome, or until the end of the study period in January 2018, whichever occurred first. For the incident cohort, the Cox proportional hazard model was adjusted for age at index, sex, educational attainment, history of CHD, pacemaker, rheumatic fever, hemodialysis, implantable cardioverter defibrillator, stent, cardiomyopathy, and heart transplant. All individuals were followed until date of death, emigration, positive outcome, or a maximum of 2 years of follow up, whichever occurred first. The 2-year limit was set for 2 reasons. First, the risk of IE seems to be relatively high during the first 2 years after heart valve replacement or hospitalization because of IE and decrease after [1, 16]. Therefore, by studying this timeframe, the aim was to detect any increase in VGS-IE at the time when risk individuals are most vulnerable. Second, such a decreasing risk of IE over time causes bias, leading to an underestimation of the incidence after October 2012 in the high-risk cohort, if no restriction of the follow up time is made.
The main parameter of interest was the interaction term between high risk and time period. P < .05 was considered statistically significant. For completeness, the same analysis was conducted of the total incidence of IE, calculated using the SRIE and NPR. The statistical analysis was carried out using Stata/IC 15 (StataCorp 2017, Stata Statistical Software: Release 15; College Station, TX: StataCorp LLC).
Ethics
This study was approved by the Regional Ethical Review Board, Karolinska Institutet, Stockholm, Sweden (ref: 2018/370-31) before onset of the study.
An ethical permit and approval for the study was also obtained from the register board of the SRIE before the collection of data.
Informed consent was waived because the study does not pose any risk of harm to the study persons; no identifying information is published as results are presented on group level only.
Patient and Public Involvement
The patients and public were not involved in the preparation of the study design, dissemination of results, or evaluation of this study.
RESULTS
As shown by Figure 1, the cohorts consisted of 76 762 high-risk individuals, and 396 048 low-risk individuals. Table 1 presents the baseline characteristics of the prevalent cohort and Table 2 of the incident cohort. The prevalent cohort comprised 36 158 high-risk individuals, of whom 20 569 (57%) were male. At the end of the 10-year study period, 395 (1.1%) had developed IE at a mean follow-up of 7.5 years. The incident cohort comprised 36 363 high-risk individuals, of whom 21 983 (61%) were male. Following the study subjects for a maximum of 2 years, with a mean follow-up of 1.6 years, 516 (1.4%) had developed IE at the end of the study period.
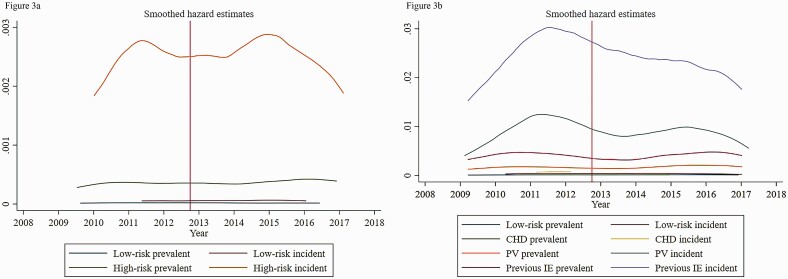
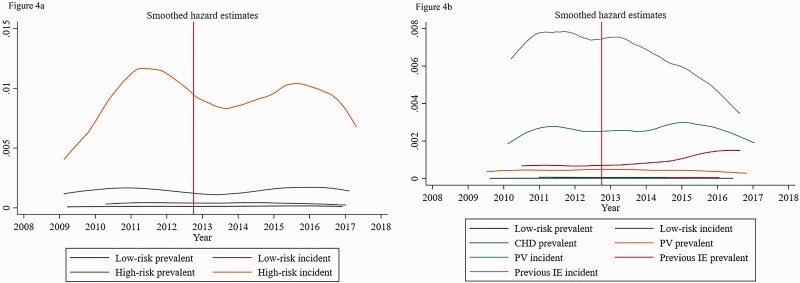
The crude incidence of VGS-IE increased among prevalent high-risk individuals, from 33.6 (95% confidence interval [CI], 25.4–44.3) cases per 100 000 individuals per year before October 2012, to 40.5 (95% CI: 30.6–53.6) after October 2012 (Table 4). Among incident high-risk individuals, there was also an increase in the incidence, from 240 (190–310) before, to 250 (200–310) after. For completeness, Figures 3 and 4 show nonparametric smoothed hazard estimates for IE and VGS-IE across the study period. Adjusted HRRs increased from 15.4 (8.3–28.5) to 20.7 (10.0–42.7) among the prevalent high-risk individuals and decreased from 66.8 (28.7–155.6) to 44.6 (22.9–86.9) among incident high-risk individuals.
Figure 3.
(A) Smoothed hazard estimates of IE among the cohorts during the study period. (B) The same data, stratified by risk group. CHD, cyanotic congenital heart disease; IE, infective endocarditis; PV, prosthetic heart valve.
Figure 4.
(A) Smoothed hazard estimates of oral streptococcal IE (VGS-IE) among the cohorts during the study period. (B) The same data, stratified by risk group. CHD, cyanotic congenital heart disease; IE, infective endocarditis; PV, prosthetic heart valve.
Overall, stratification of high-risk individuals by risk group showed that the incidence of IE was lowest among those with CHD, higher among those with prosthetic valve and highest among those with previous IE (Table 4).
For VGS-IE, no statistically significant interaction was found between time period and high risk at the 95% CI level. Point estimates for the interaction term: 1.4 (95% CI: .6–3.5) and 0.8 (95% CI: .5–1.3) for the prevalent and incident cohort, respectively (Table 4).
Sensitivity Analysis
Analyzing each risk group separately did not change the direction of these results. Using the NPR to calculate the total incidence of IE, no statistically significant increase was found in the prevalent or incident high-risk cohort. Adjusted HRR for the prevalent cohort was 13.6 (95% CI: 11.2–16.4) before and 11.0 (95% CI: 9.0–13.5) after October 2012 and interaction term between period and risk group = .8 (0.6–1.1). Adjusted HRR for the incident cohort was 20.9 (95% CI: 17.0–25.6) before and 23.9 (95% CI: 19.4–29.3) after, with an interaction term of 1.1 (95% CI: .9–1.5).
DISCUSSION
The aim of the study was to evaluate whether there was any increase in cases of VGS-IE among high-risk individuals after the recommended cessation of AP for preventing IE in Swedish dentistry. The results imply no increase in the incidence of VGS-IE among prevalent or new high-risk individuals after the recommended cessation of AP in dentistry for the prevention of IE, despite a 40% reduction of amoxicillin prescriptions among Swedish dentists.
Notably, our main results were calculated using the SRIE, rather than the NPR. For completeness, however, we also performed the analyses of the total IE incidence using the NPR and did not find any statistically significant increase after October 2012.
The incidence of VGS-IE found in our study was similar to that found by Tubiana et al [4], who studied the incidence of VGS-IE among individuals with prosthetic heart valves in France, where AP is still recommended for high-risk individuals. With a median follow-up of 1.7 years, they found the incidence to be 92/100 000 person-years (95% CI: 82.5–106.6), similar to our results for this subset of risk individuals, at 89/100 000 person-years (95% CI: 76–105).
Although our results seem reassuring for Swedish policy makers, any extrapolation of them depends on the assumption that Swedish dentists have adhered to the recommendation not to prescribe AP to high-risk individuals. In Sweden, AP is in general prescribed by the dentist, who is ultimately responsible for the decision to administer or not to administer AP before dental procedures [10]. In the Swedish national recommendations, there are remaining indications for amoxicillin prophylaxis other than prevention of IE, such as such as prevention of osteoradionecrosis and before specific oral surgical procedures. Therefore, the overall 40% reduction of amoxicillin prescriptions among Swedish dentists showed by national administrative data may indicate adherence to guidelines, although this was not evaluated in each specific case.
During the study period, in March 2016, a revision of the recommendations for AP in Swedish dentistry was made, stating that although the recommendation not to administer AP in dentistry to high-risk individuals for the prevention of IE remained, AP could be considered if recommended by the patient’s physician [11]. It is unclear to what extent, if any, this has affected the prescription of AP among dentists. Another limitation is that the frequency and nature of dental procedures carried out among the cohorts is unknown. Differences in exposure to dental treatment among the cohorts from, for example, oral status could skew the results. Such information bias should be nondifferential, such as tooth extractions, oral surgery, and subgingival scaling are dental treatments indicated by national Swedish guidelines to treat oral disease, and indications for these treatments have not changed during the study period. Furthermore, such differences may, at least in part, be considered as the results were adjusted for age and educational attainment. Nevertheless, future studies should include detailed data on the frequency and nature of invasive dental procedures among the high-risk individuals. This study adds to the existing evidence indicating that AP in dentistry for the prevention of IE may be discontinued. In the future, internationally congruent guidelines similar to the Swedish recommendations could reduce antibiotic use and thereby the development of antibiotic resistant bacteria.
CONCLUSIONS
The findings of this study suggest that the current Swedish recommendation not to administer antibiotic prophylaxis for the prevention of IE in dentistry has not led to an increased incidence of VGS-IE among high-risk individuals.
Notes
Availability of data. The study was conducted using national Swedish registries. The data that support the findings of this study are available from the SRIE, Statistics Sweden and the Swedish National Board of Health and Welfare but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Financial support. This work was supported by funding from the board of doctoral education at Karolinska Institutet, the Public Health Agency of Sweden, Folktandvården Stockholm AB, Steering Group for Collaborative Odontological Research at Karolinska Institutet and Stockholm City County, and the Swedish Dental Association.
Contributor Information
Niko Vähäsarja, Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden; Department of Oral and Maxillofacial Surgery, Eastmaninstitutet, Folktandvården Stockholms Län AB, Folktandvården Eastmaninstitutet, Stockholm, Sweden.
Bodil Lund, Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden; Medical Unit for Reconstructive Plastic- and Craniofacial Surgery, Karolinska University Hospital, Stockholm, Sweden.
Anders Ternhag, Department of Medicine Solna, Division of Infectious Diseases, Karolinska Institutet, Stockholm, Sweden.
Bengt Götrick, Department of Oral Diagnostics Faculty of Odontology, Malmö University, Malmö, Sweden.
Lars Olaison, Department of Infectious Diseases, Institute of Biomedicine, Sahlgrenska University Hospital, Göteborg, Sweden.
Margareta Hultin, Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden.
Anna Warnqvist, Division of Biostatistics, Institute of Environmental Medicine, Karolinska Institutet, Solna, Sweden.
Carina Krüger Weiner, Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden; Department of Oral and Maxillofacial Surgery, Eastmaninstitutet, Folktandvården Stockholms Län AB, Folktandvården Eastmaninstitutet, Stockholm, Sweden.
Aron Naimi-Akbar, Division of Oral Diagnostics and Rehabilitation, Department of Dental Medicine, Karolinska Institutet, Huddinge, Sweden; Department of Oral and Maxillofacial Surgery, Eastmaninstitutet, Folktandvården Stockholms Län AB, Folktandvården Eastmaninstitutet, Stockholm, Sweden; Health Technology Assessment-Odontology (HTA-O), Faculty of Odontology, Malmö University, Malmö, Sweden.
References
- 1. Østergaard L, Valeur N, Ihlemann N, et al. Incidence of infective endocarditis among patients considered at high risk. Eur Heart J 2018; 39:623–9. [DOI] [PubMed] [Google Scholar]
- 2. Steckelberg JM, Wilson WR.. Risk factors for infective endocarditis. Infect Dis Clin North Am 1993; 7:9–19. [PubMed] [Google Scholar]
- 3. Gersony WM, Hayes CJ, Driscoll DJ, et al. Bacterial endocarditis in patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation 1993; 87:I121–6. [PubMed] [Google Scholar]
- 4. Tubiana S, Blotière PO, Hoen B, et al. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: nationwide population based cohort and a case crossover study. BMJ 2017; 358:j3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Que YA, Moreillon P.. Infective endocarditis. Nat Rev Cardiol 2011; 8:322–36. [DOI] [PubMed] [Google Scholar]
- 6. Tleyjeh IM, Abdel-Latif A, Rahbi H, et al. A systematic review of population-based studies of infective endocarditis. Chest 2007; 132:1025–35. [DOI] [PubMed] [Google Scholar]
- 7. Vahasarja N, Lund B, Anders T, et al. Incidence of infective endocarditis caused by viridans group streptococci in Sweden – effect of cessation of antibiotic prophylaxis in dentistry for risk individuals. J Oral Microbiol 2020; 12:1768342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dayer MJ, Jones S, Prendergast B, et al. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time-series analysis. Lancet 2015; 385:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thornhill MH, Dayer M, Lockhart PB, et al. Guidelines on prophylaxis to prevent infective endocarditis. Br Dent J 2016; 220:51–6. [DOI] [PubMed] [Google Scholar]
- 10. Läkemedelsverket SMPA, (MPA). Indikationer för antibiotikaprofylax i tandvården – ny rekommendation. Information från Läkemedelsverket 2012; 5:22–35. [Google Scholar]
- 11. Läkemedelsverket MPA. Antibiotikaprofylax för att förebygga endokardit i samband med odontologiska ingrepp 2016. https://lakemedelsverket.se/Alla-nyheter/NYHETER-2016/Antibiotikaprofylax-for-att-forebygga-endokardit-i-samband-med-odontologiska-ingrepp/. Accessed 19 December 2016.
- 12. Folkhälsomyndigheten PHAoS. Tandläkares antibiotikaförskrivning i öppenvård till och med mars 2019 2019. Available from: https://www.folkhalsomyndigheten.se/contentassets/3609491f2c594228b098329bd2f176a5/tandvard-antibiotikaforskrivning-mars2019.pdf. Accessed 30 April 2019.
- 13. Swedres-Svarm. Consumption of antibiotics and occurrence of resistance in Sweden. In: Erika Olsson and Olov Aspevall, Sweden PHAo, Oskar Nilsson and Märit Pringle, et al., eds. A report on Swedish Antibiotic Utilisation and Resistance in Human Medicine (Swedres) and Swedish Veterinary Antibiotic Resistance Monitoring (Svarm), 2017.
- 14. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 2009; 24:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ternhag A, Cederström A, Törner A, et al. A nationwide cohort study of mortality risk and long-term prognosis in infective endocarditis in Sweden. PLoS One 2013; 8:e67519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glaser N, Jackson V, Holzmann MJ, et al. Prosthetic valve endocarditis after surgical aortic valve replacement. Circulation 2017; 136:329–31. [DOI] [PubMed] [Google Scholar]
- 17. Bjursten H, Rasmussen M, Nozohoor S, et al. Infective endocarditis after transcatheter aortic valve implantation: a nationwide study. Eur Heart J 2019; 40:3263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Welfare CfE-NBoHa. The Swedish medical birth register—a summary of content and quality. Online 2003; 112:3–33. [Google Scholar]
- 20. Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]