Abstract
Objectives
Potential interactions between feminizing hormone therapy (FHT) and pre-exposure prophylaxis (PrEP) may be a barrier to PrEP use among transgender women (TGW). We aimed to assess the impact of FHT on PrEP plasma pharmacokinetics (PK) among TGW.
Methods
This was a PK substudy of the effects of FHT on tenofovir disoproxil fumarate/emtricitabine nested to a trans-specific PrEP demonstration study (NCT03220152). Participants were assigned to receive PrEP only (noFHT) or standardized FHT (sFHT; oestradiol valerate 2–6 mg plus spironolactone 100–300 mg) plus PrEP for 12 weeks, after which they could start any FHT (aFHT). Short- and long-term PK assessment occurred at Weeks 12 and 30–48, respectively (plasma samples prior and 0.5, 1, 2, 4, 6, 8 and 24 h after dose). Non-compartmental PK parameters of tenofovir and emtricitabine were compared as geometric mean ratios (GMRs) between noFHT and PrEP and FHT (sFHT at short-term PK; aFHT at long-term PK) participants.
Results
No differences in tenofovir and emtricitabine plasma PK parameters were observed between the short-term PK of noFHT (n = 12) and sFHT participants (n = 18), except for emtricitabine Cmax [GMR: 1.15 (95% CI: 1.01–1.32)], or between noFHT short-term PK and aFHT long-term PK (n = 13). Most participants were on oestradiol valerate 2 mg at the short-term PK (56%) and 4 mg at the long-term PK (54%). Median (IQR) oestradiol levels were 56.8 (43.2–65.4) pg/mL at short-term PK (sFHT) and 44.8 (24.70–57.30) pg/mL at long-term PK (aFHT). No participants in this analysis seroconverted during the study.
Conclusions
Our results indicate no interaction of FHT on tenofovir levels, further supporting PrEP use among TGW using FHT.
Introduction
HIV infection disproportionately affects transgender women (TGW), who show approximately 50-fold increased odds of having HIV globally compared with adults of reproductive age in general.1 In Rio de Janeiro, the city with the second largest number of HIV/AIDS cases in Brazil, 31.2% of 345 TGW enrolled in a study during 2015–16 were living with HIV.2 Moreover, 43.7% of TGW with newly diagnosed infections had a negative HIV test result in the previous 12 months, underscoring the need for access to effective prevention strategies in this population. In a cross-sectional study conducted during 2018–20 in Rio de Janeiro, Brazil, the estimated annualized HIV incidence rate in TGW based on a limiting-antigen avidity assay was 9.16% (95% CI: 4.05–17.32).3
Pre-exposure prophylaxis (PrEP) using daily oral tenofovir disoproxil fumarate 300 mg combined with emtricitabine 200 mg has proven to be an efficacious and safe strategy to prevent HIV in people at higher risk for HIV infection.4,5 However, PrEP efficacy is based on optimal adherence, which is a challenge for many populations, including TGW.6 The PrEP Brasil study showed a downward trend over time in tenofovir diphosphate (tenofovir-DP) levels measured by dried-blood spot (DBS) among TGW.4 Results from the PrEParadas study, the first trans-specific PrEP demonstration study in Latin America, which enrolled 130 TGW at high risk for HIV infection in Rio de Janeiro, Brazil, showed decreasing adherence over time up to Week 48.7
Part of the standard of care for TGW, the goal of feminizing hormone therapy (FHT) is to induce secondary female sex characteristics while reducing male sex characteristics by using a combination of an oestrogen (e.g. oestradiol valerate) and an anti-androgen (e.g. spironolactone).8,9 Some studies suggest that FHT reduces the efficacy of PrEP among TGW, which would limit the benefit of PrEP for this population.10,11 Other studies indicated that seroconversions among TGW were probably related to adherence issues and different baseline characteristics rather than to potential interactions between FHT and tenofovir disoproxil fumarate/emtricitabine.12,13 Given the mixed results shown by studies to date, the clinical significance of drug interactions between tenofovir disoproxil fumarate/emtricitabine and FHT among TGW remains unclear.14 We aimed to evaluate the impact of FHT on tenofovir disoproxil fumarate/emtricitabine pharmacokinetics (PK) among TGW in the context of the PrEParadas demonstration study.7
Methods
Study design and participants
This was a drug–drug interaction (DDI) substudy on daily oral PrEP with tenofovir disoproxil fumarate/emtricitabine (300/200 mg) and FHT nested to PrEParadas, a 48 week PrEP trans-specific demonstration study conducted in Rio de Janeiro, Brazil between August 2017 and January 2020. PrEParadas study procedures have been described elsewhere.7 Briefly, eligibility criteria were: (1) male sex assigned at birth; (2) self-identification as TGW or any gender identity of the feminine spectrum; (3) age ≥18 years; (4) living in Rio de Janeiro or its metropolitan area; (5) HIV-negative status at screening and enrolment (baseline visit); and (6) engaging in HIV high-risk behaviour [at least one of the following: condomless anal sex in the last 6 months; sexually transmitted infection (STI) diagnosis in the last 12 months; transactional sex in the last 6 months; current sexual partner known to be living with HIV, regardless of HIV viral load]. During the screening visit, a subset of PrEParadas participants were invited to participate in the DDI substudy. Participants using any medication known to interact with at least one of the study drugs, whose estimated creatinine clearance (CLCR) was <60 mL/min, or who had previous transfeminine bottom surgery were not enrolled.
Study procedures
The Evandro Chagas National Institute of Infectious Diseases (INI)-FIOCRUZ Institutional Review Board reviewed and approved this project. The PrEParadas study is registered with Clinicaltrials.gov (NCT03220152). All participants provided written consent before any study procedures. All participants had to be off FHT for at least 15 (oral regimens) or 45 (injectable regimens) days before screening. Participants were assigned to receive PrEP only (noFHT), or PrEP plus standardized FHT (sFHT: oestradiol valerate 2–6 mg plus spironolactone 100–300 mg) at the screening visit, according to the participant’s willingness to use FHT for 12 weeks. All participants initiated PrEP at the baseline visit and those willing to use hormones initiated FHT at screening. The study endocrinologist prescribed FHT and could adjust doses according to each participant’s goals and self-satisfaction, according to international guidelines.15,16 After 12 weeks, participants from both groups could use any oestradiol-containing FHT. Participants underwent an intensive PK evaluation at the Week 12 visit to assess short-term DDI of FHT on PrEP. Subsequently, participants who decided to start or maintain any oestradiol-containing FHT (aFHT) were invited for an intensive PK evaluation between Weeks 30 and 48 to assess long-term DDI.
We evaluated participants’ weight, BMI, ALT and AST at baseline and both intensive PK evaluation visits: short-term (Week 12) and long-term (between Weeks 30 and 48). Serum creatinine (SCr), estimated CLCR and estimated glomerular filtration rate (eGFR) were measured at baseline, Week 4 and the long-term intensive PK visit. eGFR was calculated using the modification of diet in renal disease study (MDRD) equation. Serum total testosterone, prolactin, sex hormone-binding globulin (SHBG) and oestradiol levels were measured at baseline and at the short-term PK visit (oestradiol was also measured at the Week 4 visit). At the long-term PK visit, testosterone, oestradiol and prolactin were also evaluated. All hormonal levels were evaluated at pre-dose sampling (C24). We used the physiological female levels (100–200 pg/mL) as reference for oestradiol levels.8
During intensive PK visits, blood samples were collected in a fasted state prior to directly observed dosing administration of PrEP and FHT, and after 0.5, 1, 2, 4, 6, 8 and 24 h. Thirty minutes after the drug intake, we offered a standard breakfast. All participants were contacted by a study team member the week prior to each intensive PK visit. In addition, they received text-message reminders 7 days and the day before the PK visit to reinforce adherence. We rescheduled the PK visit in case of any self-reported missing dose in the previous 7 days. We evaluated adherence by DBS levels [tenofovir-DP and emtricitabine triphosphate (emtricitabine-TP)] at PK visits. We estimated adherence using tenofovir-DP levels as previously described: low (<350 fmol/punch, suggestive of <2 doses of PrEP per week), medium (350–699 fmol/punch, suggestive of 2–3 doses of PrEP per week) and high (≥700 fmol/punch, suggestive of ≥4 doses of PrEP per week).17
Laboratory analysis
After collection, blood samples were immediately centrifuged to perform plasma separation and stored at −80°C in cryotubes. We determined tenofovir and emtricitabine concentrations in plasma samples by LC-MS/MS at the University of Colorado Antiviral Pharmacology Laboratory (Denver, CO, USA) with standard procedures.18 The lower limit of quantification of tenofovir and emtricitabine was 10 ng/mL. We also used an LC-MS/MS assay for tenofovir-DP and emtricitabine-TP quantification of DBS samples.17,19 Serum total testosterone, prolactin, SHBG and oestradiol levels were determined by chemiluminescence immunoassay (ADVIA Centauro; Siemens).
Data analysis
We used non-compartmental analysis (Pharsight WinNonlin version 7.6, Certara) to estimate tenofovir and emtricitabine PK parameters, such as AUC0–24, Cmax, Cmin, CL/F, V/F and t½. We excluded participants: (1) with low adherence (undetectable emtricitabine-TP DBS levels or tenofovir-DP DBS levels consistent with <2 doses per week); (2) who did not attend successive study visits; (3) who had blood collection difficulties; (4) who had taken medication prohibited by the study protocol; or (5) who had taken PrEP or FHT before the direct observed dosing at the PK visit. We used median (IQR) or frequency (percentage) to describe participants’ characteristics. Non-compartmental PK parameters were summarized as geometric means with 95% CI. We initially compared PK parameters (tenofovir and emtricitabine) at the short-term PK visit using independent two-sample t-test after log transformation. In addition, we compared PK parameters (tenofovir and emtricitabine) of noFHT participants at the short-term PK visit versus aFHT participants at the long-term PK visit. Wilcoxon rank sum test was used to compare hormonal levels between noFHT versus sFHT participants, and also between noFHT participants at the short-term PK visit versus aFHT participants at the long-term PK visit. We initially aimed to include 24 participants in each group to observe a difference of at least 20% between PrEP and PrEP + FHT, with 80% power, considering a non-paired analysis and 5% two-sided alpha error. Sample size at short-term PK evaluation provided 80% power to detect a difference of at least 26% and 17% on tenofovir and emtricitabine plasma geometric mean AUC0–24, respectively, at a significance level of 0.05. Sample size at long-term PK evaluation enabled detection of a difference of at least 31% and 26% on tenofovir and emtricitabine plasma geometric mean AUC0–24, respectively. We used Spearman rank correlation to evaluate whether tenofovir or emtricitabine AUC0–24 had potential correlations with hormone levels, BMI, AST, ALT and age at both PK assessments, and CLCR at the long-term PK assessment. We used R software version 4.0.5 to perform all statistical analyses, which considered a two-tailed 5% significance level.
Results
Study population
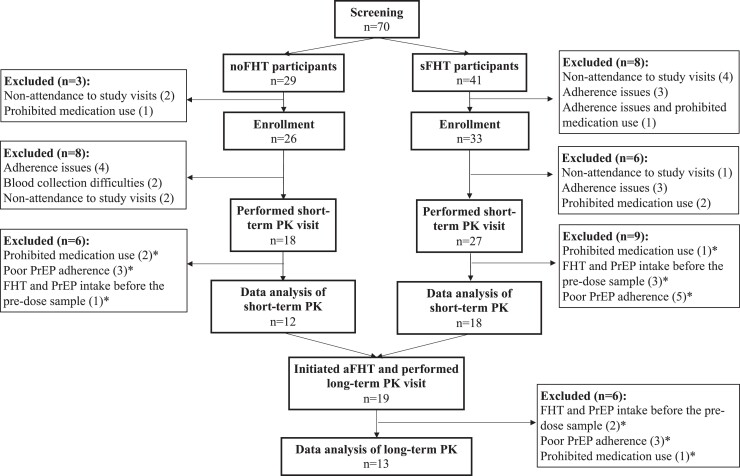
Overall, 59 participants were enrolled in the DDI study: 45 (76.3%) underwent the short-term PK assessment (noFHT: 18; sFHT: 27) and 30 (50.8%) were included in the short-term PK analysis (noFHT: 12; sFHT: 18) (Figure 1). At the short-term PK assessment, noFHT participants were older than sFHT participants [median (IQR) 30.5 years (26.8–39.3) and 26.0 years (23.0–27.8), respectively; P = 0.03] (Table 1). Among the 30 participants included in the short-term PK analysis, 27 (90%) had DBS levels consistent with high adherence [noFHT participants: 9/12 (75%); sFHT participants: 18/18 (100%)]. The levels of three noFHT participants [3/12 (25%)] were consistent with medium adherence. Among the 30 participants included in the short-term PK analysis, 19 (63.3%) initiated any oestradiol-based FHT and performed the long-term PK visit; 13 of them were included in the long-term PK analysis. All of them had DBS levels suggestive of high adherence. After 48 weeks of follow-up, no DDI substudy participant had seroconverted to HIV.
Figure 1.
Study flow chart of participants of the PrEParadas DDI substudy. * excluded during data analysis.
Table 1.
Characteristics of TGW on PrEP only (noFHT) and on PrEP plus standardized FHT (sFHT) of the PrEParadas DDI substudy
| Characteristics | noFHTa (n = 12) |
sFHTb (n = 18) |
P valuec |
|---|---|---|---|
| Age (years), median (IQR) | 30.5 (26.8–39.3) | 26.0 (23.0–27.8) | 0.03 |
| Weight (kg), median (IQR) | |||
| Baseline | 71.9 (69.5–87.0) | 67.2 (56.2–82.3) | 0.13 |
| Short-term PK | 71.6 (68.5–86.7) | 66.0 (55.9–83.7) | 0.14 |
| BMI (kg/m2), median (IQR) | |||
| Baseline | 25.7 (25.0–28.6) | 22.7 (20.1–27.7) | 0.15 |
| Short-term PK | 25.4 (24.8–28.8) | 22.5 (20.0–28.2) | 0.18 |
| Race, n (%) | |||
| Black | 1 (8) | 4 (22) | — |
| Pardo | 9 (75) | 9 (50) | — |
| White | 2 (17) | 4 (22) | — |
| Other | 0 (0) | 1 (6) | — |
| Condomless anal sex in last 6 months | 10 (83) | 16 (89) | — |
| HIV-positive partner | 1 (8) | 1 (6) | — |
| Transactional sex | 6 (50) | 6 (33) | — |
| AST (U/L), median (IQR) | |||
| Baseline | 23.5 (21.8–25.3) | 23.0 (19.0–28.0) | 0.93 |
| Short-term PK | 23.0 (22.0–28.5) | 21.5 (19.0–25.8) | 0.26 |
| ALT (U/L), median (IQR) | |||
| Baseline | 26.5 (22.6–36.8) | 30.0 (25.0–45.5) | 0.40 |
| Short-term PK | 28.5 (26.8–31.8) | 231.5 (26.0–41.8) | 0.31 |
| SCr (mg/dL), median (IQR) | |||
| Baseline | 0.86 (0.79–0.93) | 0.76 (0.70–0.88) | 0.08 |
| Short-term PK | 0.85 (0.82–1.00) | 0.85 (0.75–0.95) | 0.45 |
| eGFRd (mL/min/1.73 m2), median (IQR) | |||
| Baseline | 102.0 (94.5–108.5) | 122.0 (105.0–134.0) | 0.01 |
| W4 | 97.0 (90.3–109.0) | 107.0 (96.0–129.0) | 0.15 |
| CLCRe (mL/min), median (IQR) | |||
| Baseline | 122.3 (119.6–168.5) | 138.5 (119.2–163.7) | 0.63 |
| W4 | 123.3 (110.6–151.1) | 129.2 (110.5–146.9) | 0.98 |
Bold type indicates statistical significance.
noFHT, only on PrEP at baseline and short-term PK; n = 12 (baseline and short-term PK).
sFHT, on standardized hormones at baseline and on PrEP + standardized hormones at short-term PK; n = 18 (baseline and short-term PK).
Wilcoxon rank sum test comparing noFHT versus sFHT participants.
eGFR using MDRD equation (using assigned sex at birth).
Estimated using the Cockcroft–Gault equation (using assigned sex at birth).
FHT use and hormone levels
At the short-term PK visit, most sFHT participants were on daily oestradiol valerate 2 mg (10/18; 56%); oestradiol valerate dosages of the remaining participants were 4 mg (7/18; 39%) and 6 mg (1/18; 6%). Median (IQR) oestradiol dosage was 2 (2–4) mg. Spironolactone dosages were, respectively, 100 and 200 mg among 56% (10/18) and 44% (8/18) of sFHT participants at the short-term PK visit. In the long-term PK visit, all aFHT participants (n = 13) were on oestradiol valerate at the following dosages: 2 mg (5/13; 38%), 4 mg (7/13; 54%) and 6 mg (1/13; 8%). Median (IQR) oestradiol dosage was 4 (2–4) mg. Most aFHT participants (10/13; 79%) maintained spironolactone at the long-term PK visit at the following dosages: 100 mg (5/13; 38%), 200 mg (4/13; 31%) and 300 mg (1/13; 8%); 46% of participants (6/13) were using cyproterone acetate (25 mg: 3/6; 50 mg: 3/6).
At the Week 4 visit, oestradiol levels were significantly higher among sFHT participants compared with noFHT participants (Table 2). Only 11% (2/18) of sFHT participants at the short-term PK visit and 7% (1/13) of aFHT participants at the long-term PK evaluation had oestradiol levels in the physiological female range. aFHT participants at the long-term PK evaluation had significantly lower testosterone levels compared with noFHT participants at the short-term PK visit [median (IQR) 61.1 (25.2–145.9) and 424.1 (315.4–682.1) ng/dL, respectively; P value = 0.001]. Higher prolactin levels were observed among aFHT participants at the long-term PK evaluation compared with noFHT participants at the short-term PK visit [median (IQR) 13.0 (8.6–18.6) versus 7.6 (6.7–10.0) ng/mL, respectively; P value = 0.03]. Oestradiol levels of aFHT participants did not differ from levels observed among noFHT participants at the short-term PK visit [median (IQR) 44.8 (24.70–57.30) versus 44.3 (32.8–48.6) pg/mL, respectively; P value = 0.89].
Table 2.
Hormonal levels of participants of the PrEParadas DDI substudy during the short-term assessment according to FHT use
| Hormone | noFHTa (n = 12) |
sFHTb (n = 18) |
P valuec |
|---|---|---|---|
| Baselined | |||
| Oestradiol (pg/mL) | 43.0 (37.7–47.2) | 39.4 (33.8–56.8) | 1 |
| Testosterone (ng/dL) | 421.6 (260.5–543.5) | 507.6 (84.4–594.5) | 1 |
| Prolactin (ng/mL) | 6.25 (5.12–8.07) | 7.85 (5.55–12.33) | 0.09 |
| SHBG (nmol/L) | 48.5 (41.91–57.0) | 65.8 (51.1–92.4) | 0.02 |
| Week 4 | |||
| Oestradiol (pg/mL) | 38.9 (34.8–44.5) | 46.0 (39.8–69.6) | 0.03 |
| Week 12 | |||
| Oestradiol (pg/mL) | 44.3 (32.8–48.6) | 56.8 (43.2–65.4) | 0.05 |
| Testosterone (ng/dL) | 424.1 (315.4–682.1) | 363.1 (152.5–617.7) | 0.42 |
| Prolactin (ng/mL) | 7.55 (6.68–10.00) | 9.40 (6.98–11.78) | 0.32 |
| SHBG (nmol/L) | 41.6 (31.1–65.6) | 85.4 (54.8–140.0) | 0.003 |
Bold type indicates statistical significance.
On PrEP only, no FHT at baseline and short-term PK.
On PrEP plus FHT on short-term PK.
Wilcoxon rank sum test comparing noFHT versus sFHT participants.
noFHT and sFHT participants evaluated at enrolment and screening visits, respectively.
Tenofovir and emtricitabine PK
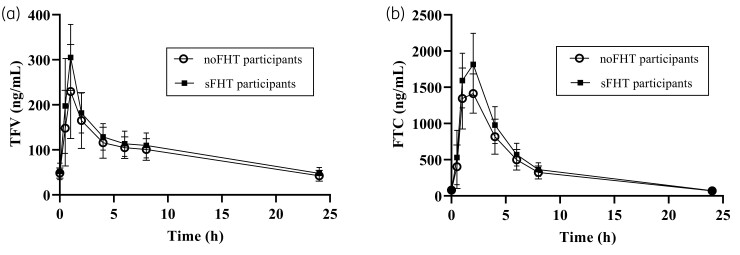
Tenofovir and emtricitabine mean concentration–time profiles measured at the short-term PK assessment are presented in Figure 2. There were no differences between non-compartmental tenofovir and emtricitabine PK parameters when PrEP and standardized FHT were co-administered (sFHT, short-term PK; Table 3), except for emtricitabine Cmax (P = 0.04).
Figure 2.
Concentration versus time at short-term PK assessment of noFHT and sFHT participants. Plasma tenofovir (TFV) and emtricitabine (FTC) at pre-dose and 0.5, 1, 2, 4, 6, 8 and 24 h in TGW are shown in (a) and (b), respectively, for noFHT participants (no FHT, PrEP only; circles, n = 12) and sFHT participants (PrEP plus standardized FHT; squares, n = 18). Data are means with error bars indicating SDs.
Table 3.
Tenofovir (TFV) and emtricitabine (FTC) non-compartmental PK parameters of participants of the PrEParadas DDI substudy according to FHT use and period of evaluation
| Parameter | Short-term PK | Long-term PK | |||||
|---|---|---|---|---|---|---|---|
| noFHTa (n = 12) GM (95% CI) |
sFHTb (n = 18) GM (95% CI) |
GMRc (95% CI) |
P valued | aFHTe (n = 13) GM (95% CI) |
GMRf (95% CI) |
P valueg | |
| TFV AUC0–24 (ng·h/mL) | 2136.27 (1831.41–2491.87) | 2392.38 (2109.75–2712.87) | 1.12 (0.92–1.37) | 0.25 | 2160.80 (1858.14–2512.74) | 1.01(0.82–1.24) | 0.91 |
| TFV Cmax (ng/mL) | 247.86(208.92–294.05) | 299.60(260.58–344.46) | 1.21(0.97-1.51) | 0.09 | 269.15(229.50–315.65) | 1.09(0.85–1.38) | 0.49 |
| TFV Tmax (h) | 0.94(0.76–1.17) | 0.89(0.75–1.06) | 0.94(0.72–1.24) | 0.67 | 1.00(0.74–1.34) | 1.06(0.71–1.57) | 0.76 |
| TFV CL/F (L/h) | 140.43(120.39–163.81) | 125.40(110.58–142.20) | 0.89(0.73–1.09) | 0.25 | 138.84(119.39–161.45) | 0.99(0.80-1.22) | 0.91 |
| TFV V/F (L) | 2778.20(2309.96–3341.36) | 2503.86(2153.58 - 2911.12) | 0.90(0.71–1.14) | 0.38 | 2872.17(2398.88–3438.85) | 1.03(0.78–1.36) | 0.80 |
| TFV Cmin (ng/mL) | 39.41(33.44–46.44) | 43.13(37.72–49.32) | 1.09(0.89–1.35) | 0.39 | 40.70(34.08–48.60) | 1.03(0.84–1.27) | 0.75 |
| TFV lambda (h−1) | 0.05(0.045–0.057) | 0.05(0.046–0.056) | 1.00(0.86–1.17) | 0.97 | 0.049(0.044–0.055) | 0.98(0.82–1.17) | 0.82 |
| FTC AUC0–24 (ng·h/mL) | 9693.51(8761.14–10725.11) | 10980.78(10110.49–11925.99) | 1.13(0.99–1.29) | 0.06 | 9512.94(8200.43–11035.53) | 0.98(0.82–1.17) | 0.83 |
| FTC Cmax (ng/mL) | 1656.00(1489.67–1840.90) | 1911.02(1752.79–2083.54) | 1.15(1.01–1.32) | 0.04 | 1848.62(1642.59–2080.49) | 1.12(0.96–1.30) | 0.15 |
| FTC Tmax (h) | 1.50(1.22–1.84) | 1.59(1.34–1.87) | 1.06(0.81–1.38) | 0.66 | 1.30(1.06–1.61) | 0.87(0.65–1.17) | 0.34 |
| FTC CL/F (L/h) | 20.63(18.23–22.83) | 18.21(16.77–19.78) | 0.88(0.77–1.01) | 0.06 | 21.02(18.12–24.39) | 1.02(0.86–1.21) | 0.83 |
| FTC V/F (L) | 192.30(163.26–226.51) | 167.55(146.58–191.51) | 0.87(0.71–1.08) | 0.19 | 205.78(163.45–259.07) | 1.07(0.81–1.42) | 0.63 |
| FTC Cmin (ng/mL) | 63.39(53.20–75.52) | 66.41(57.56–76.62) | 1.05(0.84–1.31) | 0.68 | 60.97(49.66–74.85) | 0.96(0.76–1.22) | 0.74 |
| FTC lambda (h−1) | 0.11(0.10–0.12) | 0.11(0.10–0.12) | 1.01(0.89–1.14) | 0.86 | 0.10(0.09–0.12) | 0.96(0.82–1.10) | 0.44 |
GM, geometric mean; GMR, geometric mean ratio. Bold type indicates statistical significance.
On PrEP only (n = 12).
On PrEP plus standardized FHT (n = 18).
sFHT (short-term PK)/noFHT (short-term PK) GMR.
t-test comparing noFHT versus sFHT participants.
On PrEP plus any oestradiol-based FHT (n = 13).
aFHT (long-term PK)/noFHT (short-term PK) GMR.
t-test comparing noFHT (short-term PK) versus aFHT (long-term PK) participants.
Tenofovir and emtricitabine PK parameters of aFHT participants (long-term PK evaluation) did not differ from the short-term assessment of noFHT participants (Table 3). Tenofovir and emtricitabine AUC0–24 were not correlated to hormonal levels, age or CLCR (P > 0.05). BMI had a negative correlation with tenofovir AUC0–24 (rho = −0.49; P < 0.001).
Discussion
Our results suggest that PK parameters of tenofovir and emtricitabine for daily oral PrEP are not significantly affected by oestradiol-based FHT. Previous studies had assessed this interaction via PK evaluations; however, results were based on short-term follow-up: one study from Thailand evaluated after 8 weeks (iFact, n = 20);10 two studies from the USA evaluated after 7 days (n = 8)20 and 14 days (n = 15).21 To our knowledge, this is the first trans-specific PK study to evaluate the impact of both standardized and aFHT on PrEP among participants at a higher risk for HIV that includes longer-term follow-up (i.e. beyond 12 weeks). The PrEParadas study showed a high retention rate and decreasing adherence levels over time, particularly among those with higher social vulnerability.7
Our analysis did not detect differences between tenofovir and emtricitabine PK parameters between sFHT participants and noFHT participants, except for emtricitabine Cmax. Moreover, no differences were detected between the short-term assessment of noFHT participants and aFHT participants in a long-term follow-up.
Recent short-term PK studies evaluated DDI between FHT and tenofovir disoproxil fumarate/emtricitabine. A study from Thailand revealed decreased tenofovir AUC0–24 and concentration at 24 h after the dose (C24) (12% and 18%, respectively) among TGW on tenofovir disoproxil fumarate/emtricitabine and FHT (oestradiol valerate and cyproterone acetate) after 8 weeks.10 The authors suggested an association between lower tenofovir exposure and potentially increased oral clearance related to FHT. A recent PK study conducted in Baltimore, USA reported lower tenofovir C24 and AUC0–24 [32% (P = 0.01) and 27% (P = 0.07), respectively] and 38% higher (P = 0.07) tenofovir CL/F among TGW (n = 8) on tenofovir disoproxil fumarate/emtricitabine and aFHT as compared with cisgender men (n = 8) on tenofovir disoproxil fumarate/emtricitabine after 7 days.20 The same study also showed that TGW had 32% lower emtricitabine C24 (P = 0.04), 24% lower emtricitabine AUC0–24 (P = 0.03) and 31% higher emtricitabine CL/F (P = 0.03). Tenofovir-DP and emtricitabine-TP levels in PBMCs and colon tissue were similar among cisgender men and TGW, although the authors noted that this result may be due to the small sample size and assay variability. A study from Nebraska, USA reported 24% and 14% lower tenofovir and emtricitabine AUC0–24, respectively, among TGW (n = 15) on 17β-oestradiol plus spironolactone after 14 days of PrEP when compared with historical controls (cisgender men and women).21 Tenofovir exposure decrease was lower when restricting the analysis to participants with a BMI of <30 kg/m2. The Discover study reported that cisgender men and TGW on FHT plus PrEP had clinically comparable levels of tenofovir-DP and emtricitabine-TP in PBMC samples.22 Among people living with HIV, TGW using tenofovir disoproxil fumarate/emtricitabine-based ART and FHT presented rectal levels of tenofovir-DP:deoxyATP ratio 7-fold lower than cisgender participants. The rectal levels of tenofovir-DP:deoxyATP ratio were also inversely correlated with female sex hormones, suggesting a negative impact of FHT on PrEP efficacy, independently of tenofovir plasma levels.11
Interestingly, tenofovir and emtricitabine exposures (AUC0–24) were lower in our study population, regardless of FHT use, than in controls from previous studies from the USA. Median (IQR) tenofovir and emtricitabine were, respectively, 3430 ng·h/mL (2720–3650) and 12 700 ng·h/mL (11 110–13 640) among cisgender men from Baltimore, USA,20 and historical controls of cisgender men from Denver and San Francisco, USA were 2650 ng·h/mL (1900–3800) and 11 000 ng·h/mL (8800–13 100).23 Previous PK studies had evaluated US20,21 and Thai10 populations. Tenofovir and emtricitabine PK present high variability (10%–30%) and may be influenced by several factors, including race.24 Brazil is a multi-ethnic country with the sixth largest population globally and its diversity limits the evaluation of possible correlations between race and PrEP exposure, as well as the comparison between current results and other studies enrolling populations of different ancestry.25 However, it is noteworthy that median emtricitabine and tenofovir AUC0–24 in short- and long-term assessments were within the range described in previous controlled studies (8000–16 484 ng·h/mL for emtricitabine and 2000–3000 ng·h/mL for tenofovir).24,26
Our study has some important strengths toward understanding DDIs between PrEP and FHT among TGW. We enrolled TGW at high risk of HIV infection who would also most benefit from PrEP. Furthermore, we closely monitored FHT use until Week 12 to avoid interference of concomitant drugs with PrEP PK. In addition, we evaluated any oestradiol-based FHT in a long-term follow-up.
This study has several limitations. As we did not evaluate tenofovir-DP and emtricitabine-TP in PBMCs or rectal tissue CD4+ cells, the evaluation of tenofovir disoproxil fumarate/emtricitabine efficacy is beyond the scope of the current analysis. We did not perform directly observed therapy throughout the study follow-up. However, we indirectly estimated adherence based on tenofovir-DP and emtricitabine-TP in DBS and excluded participants with levels consistent with low adherence. The parallel study design without randomization could contribute to data variability regarding tenofovir and emtricitabine PK. We did not have long-term data on TGW on PrEP only. As many TGW consider FHT an important component of their medical transition,27 it would be neither ethical nor feasible to ask them to remain off FHT for a long period of time. FHT dosages were adjusted according to participants’ goals. Despite the option of increasing the oestradiol valerate dosage, the majority of participants remained on their initially prescribed dosage along the study, though only a small percentage of them had achieved oestradiol levels within the adult female range. This was also observed in other DDI studies on FHT and PrEP.10,21 Our study evaluated only one oestrogen-based FHT regimen in a short period and diverse regimens in a long period. As such, current results may not be extrapolated to other FHT regimens and/or dosages. Finally, PK studies usually comprise small samples among specific groups, and results cannot be generalized to the whole population.
PrEP and FHT are important biomedical approaches that may be lifesaving for TGW. Our results contribute to a growing body of evidence supporting the concomitant use of FHT and PrEP among TGW. PrEP may and should be offered to all TGW engaging in HIV high-risk behaviour, regardless of their hormone use. Future studies should focus on examining factors that impact PrEP retention, adherence and persistence in order to improve the benefit of PrEP for TGW around the world.
Acknowledgements
Beatriz Grinsztejn acknowledges funding from the Brazilian Research Council (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES) and Scientific Development and Research Funding Agency of the State of Rio de Janeiro. We acknowledge Ruth Khalili Friedman, Maria Regina Cotrim and Bushman Lane for their support.
Contributor Information
Vitória Berg Cattani, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Emilia Moreira Jalil, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Leonardo Eksterman, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Thiago Torres, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Sandra Wagner Cardoso, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Cristiane R V Castro, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Laylla Monteiro, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Erin Wilson, University of California, San Francisco, USA.
Lane Bushman, University of Colorado, Denver, USA.
Peter Anderson, University of Colorado, Denver, USA.
Valdilea Gonçalves Veloso, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Beatriz Grinsztejn, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil.
Rita Estrela, Evandro Chagas National Institute of Infectious Diseases INI Fiocruz, Rio de Janeiro, Brazil; Faculty of Pharmacy, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil.
PrEParadas study team:
Isabele Moura, Daniel M McMahon Waite, Desirée Vieira, José Roberto Granjeiro, Josias Freitas, Toni Santos, Nilo Fernandes, Sandro Nazer, Luana M S Marins, Valéria R T Ribeiro, Robson P N Silva, Giovanna G Costa, Ana Carolina Vieira, Renata A Bastos, Aline Alves, Tania Krstic, Ana Cristina G Ferreira, Monica Derrico, Luciana Kamel, Cristina M Jalil, Eduardo Carvalheira Netto, Marcos Davi G de Sousa, Pedro Leite, Kim Geraldo Mattos, Jessica Bezerra Felix, Tamires Vilela Baião, Gisele Hottz, Natália Gomes Maia, Tamiris Paixão da Silva, Michelle Ramos, and Porto Tiago
Members of the PrEParadas study team
Isabele Moura, Daniel M. McMahon Waite, Desirée Vieira, José Roberto Granjeiro, Josias Freitas, Toni Santos, Nilo Fernandes, Sandro Nazer, Luana M. S. Marins, Valéria R. T. Ribeiro, Robson P. N. Silva, Giovanna G. Costa, Ana Carolina Vieira, Renata A. Bastos, Aline Alves, Tania Krstic, Ana Cristina G. Ferreira, Monica Derrico, Luciana Kamel, Cristina M. Jalil, Eduardo Carvalheira Netto, Marcos Davi G. de Sousa, Pedro Leite, Kim Geraldo Mattos, Jessica Bezerra Felix, Tamires Vilela Baião, Gisele Hottz, Natália Gomes Maia, Tamiris Paixão da Silva, Michelle Ramos and Tiago Porto.
Funding
This work was supported by the Brazilian Ministry of Health (Brasília, Brazil; #01/2013 BRA/K57) and Secretaria de Vigilancia em Saúde (SVS; #281/2013) and partially supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES - Finance Code 001. Gilead Sciences donated the study drug and covered costs related to drug concentration assessment, but had no role in study design, collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the manuscript for publication.
Transparency declarations
Peter Anderson has received consulting fees from Gilead, Merck and ViiV, and research funding paid to his institution from Gilead. All other authors declare no competing interests.
Author contributions
Emilia Moreira Jalil, Valdilea Gonçalves Veloso, Beatriz Grinsztejn and Rita Estrela conceived the study and interpreted the findings. Emilia Moreira Jalil, Beatriz Grinsztejn, Rita Estrela and Vitória Berg Cattani drafted the manuscript. Vitória Berg Cattani did the statistical analyses with aid from Emilia Moreira Jalil and Rita Estrela. Thiago Torres, Sandra Wagner Cardoso, Leonardo Eksterman, Cristiane R.V. Castro and Laylla Monteiro helped with data acquisition, interpretation of the findings, and drafting the manuscript. Valdilea Gonçalves Veloso, Peter Anderson, Lane Bushman and Erin Wilson were involved in revising the manuscript for important intellectual content. All authors read and approved the final manuscript.
References
- 1. Baral SD, Poteat T, Stromdahl Set al. . Worldwide burden of HIV in transgender women: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13: 214–22. [DOI] [PubMed] [Google Scholar]
- 2. Grinsztejn B, Jalil EM, Monteiro Let al. . Unveiling of HIV dynamics among transgender women: a respondent-driven sampling study in Rio de Janeiro, Brazil. Lancet HIV 2017; 4: e169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Teixeira SLM, Jalil CM, Jalil EMet al. . Evidence of an untamed HIV epidemic among MSM and TGW in Rio de Janeiro, Brazil: a 2018 to 2020 cross-sectional study using recent infection testing. J Int AIDS Soc 2021; 24: e25743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grinsztejn B, Hoagland B, Moreira RIet al. . Retention, engagement, and adherence to pre-exposure prophylaxis for men who have sex with men and transgender women in PrEP Brasil: 48 week results of a demonstration study. Lancet HIV 2018; 5: e136–45. [DOI] [PubMed] [Google Scholar]
- 5. Fonner VA, Dalglish SL, Kennedy CEet al. . Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016; 30: 1973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rowniak S, Ong-Flaherty C, Selix Net al. . Attitudes, beliefs, and barriers to PrEP among trans men. AIDS Educ Prev 2017; 29: 302–14. [DOI] [PubMed] [Google Scholar]
- 7. Jalil EM, Torres TS, Luz PMet al. . Low PrEP adherence despite high retention among transgender women in Brazil: the PrEParadas study. J Int AIDS Soc 2022; 25: e25896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hembree WC, Cohen-Kettenis PT, Gooren Let al. . Endocrine treatment of gender- dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2017; 102: 3869–903. [DOI] [PubMed] [Google Scholar]
- 9. Coleman E, Bockting W, Botzer Met al. . Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgend 2012; 13: 165–232. [Google Scholar]
- 10. Hiransuthikul A, Janamnuaysook R, Himmad Ket al. . Drug-drug interactions between feminizing hormone therapy and pre-exposure prophylaxis among transgender women: the iFACT study. J Int AIDS Soc 2019; 22: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cottrell ML, Prince HMA, Schauer APet al. . Decreased tenofovir diphosphate concentrations in a transgender female cohort: implications for HIV pre-exposure prophylaxis PrEP. Clin Infect Dis 2019; 69: 2201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mehrotra ML, Westreich D, McMahan VMet al. . Baseline characteristics explain differences in effectiveness of randomization to daily oral TDF/FTC PrEP between transgender women and cisgender men who have sex with men in the iPrEx trial [Letter to the Editor]. J Acquir Immune Defic Syndr 2019; 81: e94–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deutsch MB, Glidden DV, Sevelius Jet al. . HIV pre-exposure prophylaxis in transgender women- a subgroup analysis of the iPrEx trial. Lancet HIV 2015; 2: e512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poteat TC, Radix A. HIV antiretroviral treatment and pre-exposure prophylaxis in transgender individuals. Drugs 2020; 80: 965–72. [DOI] [PubMed] [Google Scholar]
- 15. World Professional Association for Transgender Health (WPATH) . Standards of Care for the Health of Transsexual, Transgender, and Gender Nonconforming People. Version 7. 2012. https://www.wpath.org/media/cms/Documents/SOC%20v7/SOC%20V7_English2012.pdf.
- 16. University of California San Francisco, Center of Excellence for Transgender Health . Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People. Second edition. 2016. https://transcare.ucsf.edu/sites/transcare.ucsf.edu/files/Transgender-PGACG-6-17-16.pdf.
- 17. Anderson PL, Liu AY, Castillo-Mancilla JRet al. . Intracellular tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy. Antimicrob Agents Chemother 2017; 62: e01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Delahunty T, Bushman L, Robbins Bet al. . The simultaneous assay of tenofovir and emtricitabine in plasma using LC/MS/MS and isotopically labeled internal standards. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877: 1907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng J-H, Rower C, McAllister Ket al. . Application of an intracellular assay for determination of tenofovir-diphosphate and emtricitabine-triphosphate from erythrocytes using dried blood spots. J Pharm Biomed Anal 2016; 122: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shieh E, Marzinke MA, Fuchs EJet al. . Transgender women on oral HIV pre-exposure prophylaxis have significantly lower tenofovir and emtricitabine concentrations when taking oestrogen when compared to cisgender men. J Int AIDS Soc 2019; 22: e25405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cirrincione LR, Podany AT, Havens JPet al. . Plasma and intracellular pharmacokinetics of tenofovir disoproxil fumarate and emtricitabine in transgender women receiving feminizing hormone therapy. J Antimicrob Chemother 2020; 75: 1242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cespedes MS, Majeed SR, Prins Met al. . Discover: no effect of hormones on F/TAF or F/TDF PK, efficacy & safety in transwomen. Conference on Retroviruses and Opportunistic Infections Boston, MA, USA, 2020. Abstract 1020. [Google Scholar]
- 23. Blum MR, Chittick GE, Begley JAet al. . Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 2007; 47: 751–9. [DOI] [PubMed] [Google Scholar]
- 24. Yager JL, Anderson PL. Pharmacology and drug interactions with HIV PrEP in transgender persons receiving gender affirming hormone therapy. Expert Opin Drug Metab Toxicol 2020; 16: 463–74. [DOI] [PubMed] [Google Scholar]
- 25. Suarez-Kurtz G. Pharmacogenetics in the Brazilian population. Front Pharmacol 2010; 1: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Truvada (emtricitabine and tenofovir disoproxil fumarate) [package insert]. Gilead Sciences, Inc. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021752s035lbl.pdf.
- 27. Ferreira ACG, Coelho LE, Jalil EMet al. . Transcendendo: a cohort study of HIV-infected and uninfected transgender women in Rio de Janeiro. Brazil. Transgender Health 2019; 4: 107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]