Abstract
Idiopathic Parkinson’s disease (PD) may take decades to develop, during which many risk or protective factors may come into play to initiate the pathogenesis or modify its progression to clinical PD. The lack of understanding of this prodromal phase of PD and the factors involved has been a major hurdle in the study of PD etiology and preventive strategies. Although still controversial, the Braak and dual-hit hypotheses that PD may start peripherally in the olfactory structures and/or the gut provides a theoretical platform to identify the triggers and modifiers of PD prodromal development and progression. This is particularly true for the search of environmental causes of PD as the olfactory structures and gut are the major human mucosal interfaces with the environment. In this review, we lay out our personal views about how the Braak and dual-hit hypotheses may help us search for the environmental triggers and modifiers for PD, summarize available experimental and epidemiological evidence, and discuss research gaps and strategies.
Keywords: Parkinson disease, environmental risk factors, olfactory impairment, microbiota, gut-brain axis
Introduction
Idiopathic Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease, affecting 1–2% of the older adults. Multiple recent reports show that PD emerges as the fastest growing neurological disease in terms of both prevalence and death (Darweesh et al., 2018; GBD Parkinson’s Disease Collaborators, 2018), which cannot be solely explained by population aging. There is preliminary albeit inconsistent evidence that the incidence of PD might have been increasing over the past decades (Savica et al., 2017). Despite this increasing burden of PD, research on the environmental triggers and modifiers for PD development has been stagnant. While a portion of the PD cases have a clear genetic component, most late-onset PD are likely the results of environmental contributions and their interactions with genetic susceptibility. The search for environmental causes of PD has been hampered by the current lack of understanding of the disease’s natural history. In particular, by the time of PD diagnosis with bothering cardinal motor signs, the disease might have undergone decades of “insidious” prodromal development, during which many factors may come into play to initiate pathology or modify its progression. While some experimental models (e.g., Thy1-aSyn mouse) recapitulates key pathogeneses (e.g., alpha-synuclein accumulation and progressive changes in dopamine release and striatal content) and phenotypical features of prodromal PD (Chesselet et al., 2012), animal models and human studies are yet to fully unveil these complex, decades-long processes. To some extent, the lack of understanding of this “black box” of PD prodromal development has led to the “blind men and an elephant” dilemma in the research of PD etiology. Consequently, even the most robust epidemiological findings on non-genetic risk factors for PD (e.g., smoking or coffee drinking) are subject to alternative explanations such as reverse causation or unmeasured confounding (Ritz and Rhodes, 2010). On the other hand, the prolonged prodromal development of PD may also present an unprecedented opportunity to understand PD etiology and disease prevention. For example, by identifying factors that contribute to various stages of PD prodromal development, we may be able to eventually reveal the sequence of events in PD development and their causes, fundamentally improving understanding of PD etiology and developing preventive strategies.
The scientific premises of such research endeavors however critically depend on a better understanding of the stages of PD prodromal development and the identification of readily assessable staging markers. To this end, the Braak hypothesis (Braak et al., 2006) presents the exact hypothetical framework that dissects the steps of PD prodromal development, which posits that the PD pathogenesis may first initiate in the olfactory structures and the gut enteric nerves, years if not decades, before spreading to the substantia nigra where dopaminergic neuron death occurs and PD motor dysfunction arises. Although the hypothesis itself is still somewhat controversial as clinical PD is heterogenous and contradictory pathological findings exist, it raises the intriguing possibility that, at least in some PD patients, the pathogenesis may start in the peripheral system, and some of the long-observed nonmotor symptoms in PD are likely integral part of the disease’s natural history and prodromal development. In support, accumulating evidence shows that olfactory impairment (Chen et al., 2017; Ross et al., 2008), REM sleep behavior disorder (RBD) (Postuma et al., 2009; Postuma et al., 2015), and constipation (Abbott et al., 2001; Gao et al., 2011; Savica et al., 2009) strongly predict future risk of PD, and they may have developed years or decades before PD clinical diagnosis. Most symptoms, with the exception of polysomnography-confirmed RBD, lack clinical specificity to PD. However, research on these prodromal symptoms, coupled with studies on other PD biomarkers (e.g., neuroimaging or blood, saliva or skin-biopsy based), has opened the window to characterize at-risk populations for PD, define PD prodromal phenotype, and identify potential causes and preventive measures to slow down or stop its progression to overt clinical PD.
More importantly, these advances may enable us to entertain the fundamental question of PD etiology and prevention - when, where, and how does PD pathogenesis start, develop and progress? Figure 1 presents a hypothetical scenario (Chen, 2018; Chen and Ritz, 2018). At birth or young age, the person may have some of the genetic variants or nongenetic events (e.g., head injury) that put them at a higher risk of PD. With occupational uses of certain pesticides or organic solvents, PD pathogenesis may begin to develop at mid-adulthood. This process may be insidious initially, but over time as the pathogenesis progresses, non-specific symptoms (e.g., poor olfaction, constipation, sleep problems) or even the specific RBD symptoms may develop. Occupational hazards continue, and additional environmental factors may come into play (e.g., stopped smoking, or another head injury). Eventually, the more notable cardinal motor signs develop, leading to a PD diagnosis later in life. It is important to note that this process is slowly progressive and may take decades, and its clinical presentations and sequential orders are likely heterogeneous. While there are still substantial challenges to adequately define prodromal PD, by assessing these early nonmotor and motor symptoms as intermediate phenotypes, we may be able to bring new insights into this “black-box” of PD prodromal development by identifying factors that initiate PD pathogenesis, lead to its prodromal phenotypes, or modify progression to clinical PD.
Figure 1: A Systematic Life-long Approach to Study Environmental Triggers and Modifiers of PD.
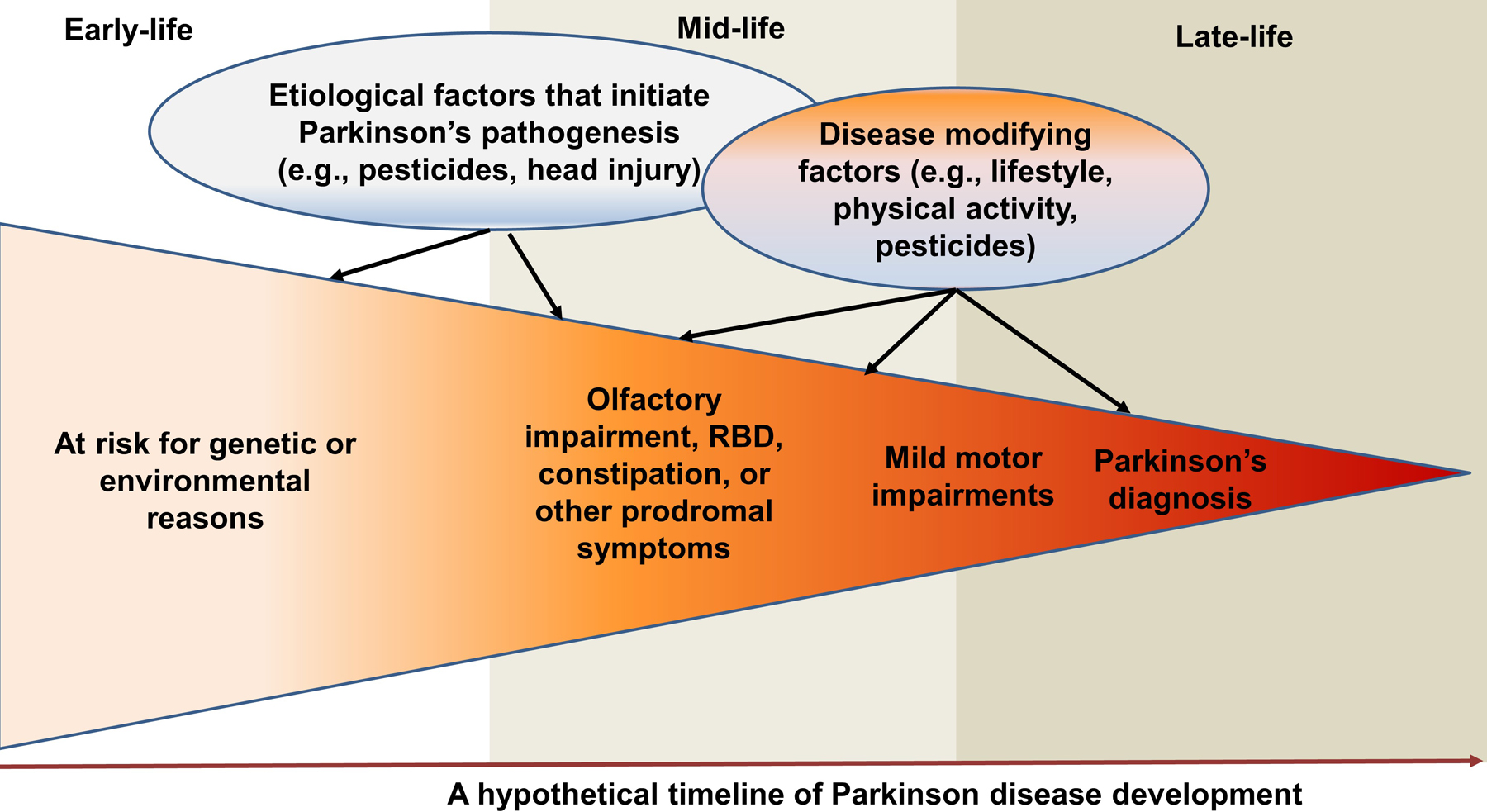
Late-onset sporadic PD takes decades to develop and has a prolonged prodromal stage. By assessing PD prodromal symptoms as intermediate phenotypes, we may be able to bring new insights into the “black-box” of PD etiology by identifying factors that trigger PD pathogenesis, lead to its prodromal phenotypes, or modify its phenotypical conversion to clinical PD at various stages of disease development. Modified from J Parkinsons Dis. Chen & Ritz, “The Search for Environmental Causes of Parkinson’s Disease: Moving Forward” 8 (2018) S9–S17. Copyright (2018), with permission from IOS Press. The original publication is available at IOS Press through http://dx.doi.org/10.3233/JPD-181493.
The dual-hit hypothesis further speculates that environmental toxins such as pesticides, air pollutants, metals or virus, may enter the body via the nasal cavity or the gut, initiate PD pathogenesis, gain access to the brain via the olfactory pathway or the vagus nerve, and eventually lead to clinical PD (Figure 2) (Hawkes et al., 2007; Hawkes et al., 2009). Notably, the nasal cavity and gut are the two anatomic sites where the human mucosal surfaces directly interact with the environment, where inflammation commonly occurs, and where paths to the brain are well established. Although the PD dual-hit hypothesis itself is yet to be systematically examined, if proven true, it will have profound implications for PD prevention and treatment as these environmental factors are modifiable. In recent reviews (Chen, 2018; Chen and Ritz, 2018), we have speculated how this prodromal framework may help advance the understanding of environmental causes for PD, major research directions, and challenges. In this review, we focus on summarizing available epidemiological and experimental evidence for this PD dual-hit hypothesis. The potential roles of the gut-brain axis and gut microbiota in PD etiology have stimulated substantial research interests and have been the topic of extensive recent reviews (Elfil et al., 2020; Killinger and Labrie, 2019; Lionnet et al., 2018; Scheperjans et al., 2018; Sharabi et al., 2021; Travagli et al., 2020), we hereby first discussed in detail the olfactory pathway and its environmental relevance in the context of PD etiological research.
Figure 2: The Environmental Dual-Hits that May Trigger PD Pathogenesis.
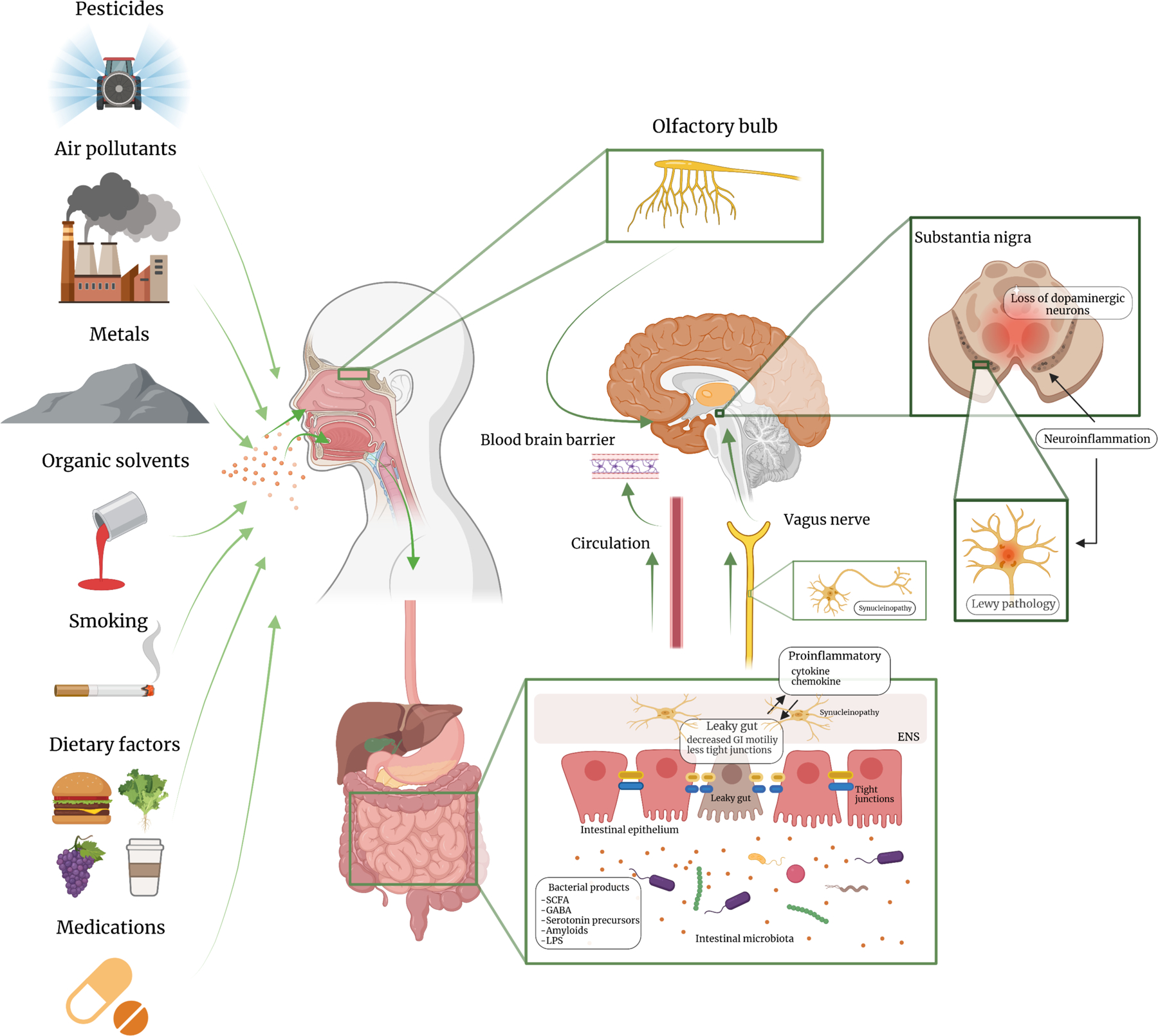
Environmental exposures may enter the body via the nose or the mouth, which may initiate PD pathogenesis in susceptible individuals via mechanisms such as inflammation or microbiota dysbiosis; over years, the pathology may progress to the central olfactory structures and/or the lower brain stem, and eventually lead to dopaminergic neuron loss in the substantia nigra. [Created with BioRender.com] Part of the figure was modified from Ambrosini et al. Frontiers in Aging Neurosciecne (2019), Volume 11, Article 130 doi: 10.3389/fnagi.2019.00130
The olfactory pathway
In the Braak staging paradigm, the olfactory bulb is one of the sites where PD pathogenesis may start. While poor olfaction has long been recognized as a prodromal symptom of PD (Ross et al., 2008), it is difficult to estimate its timeline leading to PD clinical onset. Nonetheless, population-based cohort studies have shown that poor olfaction strongly predicts PD risk with up to 10 years of follow-up (Chen et al., 2017; Mahlknecht et al., 2018). Further, in a reconstructed timeline based on data from 154 idiopathic RBD patients, poor olfaction appeared to be the first symptom of clinical synucleinopathy, starting >20 years before phenotypical conversion to PD or Lewy body dementia (Fereshtehnejad et al., 2019). In comparison, constipation began to develop ~10 years before the phenotypical conversion and motor signs ~5 years before.
The causes for poor olfaction in older adults also remain elusive. Except for age, male sex, and a few acquired causes (Doty, 2009), the roles of environmental exposures in age-related olfactory impairment are largely unknown (Doty, 2015). We hereby critically evaluate the rationale and empirical evidence for key environmental exposures in relation to poor olfaction and their potential relevance to PD, focusing on pesticides, air pollutants, cigarette smoking, heavy metals and organic solvents (Table 1). These airborne environmental exposures have been studied in relation to PD risk and, at least theoretically, they can gain access to the brain via the nasal and olfactory pathway bypassing the blood-brain barrier (Maher et al., 2016; Prediger et al., 2011; Sasajima et al., 2015).
Table 1.
Summary of epidemiological evidence a on selected potential airborne environmental “risk factors” for PD in relation to olfaction
| Study design | Study setting and participants | Exposure assessment | Outcome assessment | Primary findings | Comments | |
|---|---|---|---|---|---|---|
| Pesticides | Cross-sectional, case-control, & longitudinal studies | Mostly occupational setting (e.g., farmers or farm workers vs. others); a few population-based studies; mostly working age population or older; primarily men | Self-reported pesticide exposures; farmer as a surrogate for pesticide exposure; biospecimen concentration (e.g., urinary sample) | Sniffin-Stick odor identification and threshold tests; self-reported olfactory impairment | Mostly positive associations (OR: 1.02–1.49), but data are not entirely consistent, especially on which olfaction domain was impaired. | Exposure assessment errors; self-reported olfaction not accurate; no data on whether the impairment was transitory, progressive or permanent (i.e., age related decline) |
| Air pollutants | Cross-sectional, & case-control studies | Population-based studies; wide age range (i.e., young, middle, & old age); both men and women | Ecological (e.g., residents of Mexico city vs. another city); criteria air pollutants (e.g., NO2 or PM2.5) | Sniffin-Stick odor identification and threshold tests; clinical anosmia based on ICD codes | Mostly positive associations (OR: 1.20–1.73), but some null association findings | No longitudinal studies; unsure if the impairment was transitory or age-related; possible effect modification by age |
| Cigarette smoking | Cross-sectional, & longitudinal studies | Population-based studies; PD patients vs. others; wide age range, including young, middle, and old age, both men and women | Self-reported smoking history (e.g., never, past, current), pack-years, years of smoking, smoking cessation | Sniffin-Stick odor threshold and odor identification; UPSIT; MODSIT; SDOIT; self-reported OI; B-SIT; SOIT; Connecticut Chemosensory Clinical Research Center test; other non-commercial tests (e.g., identification of series dilution of common odors) | Mostly positive associations (OR: 1.36–2.43) especially for current smoking, but not entirely consistent. In PD patients, smoking was not positively associated with poor olfaction | Unsure if the impairment was transitory or age-related; analyses limited to PD may lead to a biased association of smoking with poor olfaction |
| Heavy metals | Cross-sectional studies | Predominantly occupational settings (e.g., welders vs. others); a few population-based studies; mostly working age population or older, occasionally children | Surrogate (e.g., welder); biospecimen concentrations (blood, urine, hair, fingernail, bone); most studied metals include manganese, cadmium, chromium and lead | Olfactory acuity by olfactometer; Sniffin-Stick odor threshold and odor identification; UPSIT; self-reported olfaction other non-commercial tests (e.g., identification of series dilution of common odors) | Manganese: inconsistent - positive, negative or null associations; cadmium, & lead: inconsistent - positive or null associations; Chromium: positive associations, but data are limited | Small sample sizes, cross-sectional, and occupational setting; unsure if the impairment was transitory (due to occupational hazards) or age-related. |
| Organic solvents | Cross-sectional studies | Occupational settings (e.g., workers in paint manufacturing facilities, printing workers, or medical laboratory employee vs others); working age; men and women | Surrogate (e.g., job-title based exposure assessment) | Self-reported olfaction; Sniffin-Stick odor threshold and odor identification; UPSIT | Mostly positive associations, but some show null associations. There is a potential effect modification by smoking | Small sample sizes, cross-sectional, and occupational setting; unsure if the impairment was transitory (due to occupational hazards) or age-related. |
Abbreviations: OR: odds ratio; UPSIT: University of Pennsylvania Smell Identification Test; MODSIT: Modular Smell Identification Test; SDOIT: The San Diego Odor Identification Test; B-SIT: Brief Smell Identification Test; SOIT: Scandinavian Odor Identification Test
See references and citations in the text due to space limit.
Epidemiological evidence
Overwhelming epidemiological and experimental evidence supports roles of pesticides in PD development, albeit data on specific pesticides, detrimental exposure levels, windows of exposure, and routes of entry are still limited. One can, however, reasonably hypothesize that pesticides may trigger PD pathogenesis through the nasal olfactory pathway. When airborne, pesticides may damage the olfactory epithelium, impairing nerve function, inducing local acute or chronic inflammation, and disrupting the xenobiotic function, immune protection, and microbiome of the olfactory mucosa (Doty, 2015). Pesticides may find their way to the brain via olfactory structures, and in susceptible individuals, may initiate or exacerbate the abnormal aggregation of α-synuclein (Kumar et al., 2016; Maturana et al., 2015; Rey et al., 2016; Rey et al., 2018; Rokad et al., 2017). The nasal mucosa, at the interface between the body and the environment, once damaged, may have diminished mechanical and/or immune protection and thus impart a higher susceptibility to future virus invasions or environmental neurotoxins that lead to PD. Alternatively, pesticides may also enter the body via the digestive tract and initiate synucleinopathy in the gut, which may later spread to the brain (Braak et al., 2006). Over time, these mechanisms may individually or synergistically contribute to age-related poor olfaction and neurodegeneration.
Despite such plausible mechanisms, there are few empirical studies of pesticides and olfaction in humans. A case-report clearly documented progressive and irreversible loss of the sense of smell in an Italian physician following acute indoor exposure to high levels of a pyrethrin-based insecticide (Gobba and Abbacchini, 2012). Several other studies (Ahman et al., 2001; Holmstrom et al., 2008; Quandt et al., 2017; Quandt et al., 2016) reported that farmers or farm workers were more likely to report poor olfaction or fail a smell test than the comparison group; however, these studies are mostly cross-sectional and did not directly examine pesticide and poor olfaction. We examined both occupational pesticide uses and high pesticide exposure events (HPEE, e.g., major accidental spills) in relation to self-reported poor olfaction ~20 years later among farmers in the Agricultural Health Study (Shrestha et al., 2019; Shrestha et al., 2021). In the analysis of occupation data from 20,409 farmers, we found modest associations with self-reported poor olfaction for 20 out of the 50 specific pesticides (odds ratios between 1.11 and 1.33) (Shrestha et al., 2021). In another analysis of 11,232 farmers (Shrestha et al., 2019), a history of HPEE was associated with a 49% higher odds of reporting poor olfaction ~20 years later, and statistically significant associations were found for two organochlorine insecticides (DDT and lindane) and four herbicides (alachlor, metolachlor, 2,4-D, and pendimethalin). These preliminary data, once confirmed, may suggest that these pesticides contribute to the early stages of PD development. To our knowledge, no epidemiological study has examined whether pesticides modify the phenotypical conversion from poor olfaction to clinical PD.
Many of the same biological rationales for linking pesticides to poor olfaction and/or PD can be easily extended to air pollutants. For example, the fine particulate matter (PM2.5) may induce α-synuclein pathology in the olfactory bulb via mechanisms of neuroinflammation and oxidative stress (Block et al., 2012), and alternatively, they may also gain access to the human body via the gastrointestinal tract, for example, by nasal dripping or saliva swallowing as the dual-hit hypothesis has suggested (Hawkes et al., 2009). Unlike pesticides, the epidemiological evidence on major air pollutants and PD risk has been inconsistent (Jo et al., 2021; Liu et al., 2016; Palacios et al., 2017; Ritz et al., 2016; Toro et al., 2019; Willis et al., 2010). For example, in the most recent comprehensive analysis of the Korean National Health Insurance data, the only statistical difference in PD risk was found when comparing the highest vs. lowest exposure categories of nitrogen dioxide (NO2), whereas no associations were found of PD with other major criteria pollutants (i.e., PM2.5, PM10, ozone, or carbon monoxide) (Jo et al., 2021).
Data on air pollutants and poor olfaction are also limited. Earlier studies are mostly ecological in design and reported worse olfaction in residents of highly polluted cities such as Mexico City as compared to their counterparts in surrounding areas of cleaner air (Calderon-Garciduenas et al., 2010; Guarneros et al., 2009; Hudson et al., 2006; Sorokowska et al., 2015; Sorokowska et al., 2013). The association of ambient air pollutants with olfaction was also examined in two large cross-sectional studies (Adams et al., 2016; Ajmani et al., 2016) and a case-control study (Zhang et al., 2021). In a US population, positive associations with poor olfaction were found for both exposures to PM2.5 (Ajmani et al., 2016) and NO2 (Adams et al., 2016); however, the association with PM2.5 was limited to younger participants ages 57–64 and participants from the Northeast regions. In a recent case-control study, PM2.5 exposure was associated with the odds of having anosmia (Zhang et al., 2021). Therefore, the evidence on air pollution and olfactory impairment is preliminary but suggestive.
Unlike pesticides or air pollutants, cigarette smoking was inversely associated with the risk of PD (Chen et al., 2010). This relationship is inarguably the best-established epidemiological observation for PD, albeit its causality is still debatable. If this association is indeed causal that smoking reduces the risk of developing PD, one would logically expect smoking may protect against poor olfaction which is a prodromal marker of PD. The overall evidence to date however suggests the opposite. Most available studies are cross-sectional in design and found current smokers had a higher odds of having poor olfaction, as summarized in a 2017 meta-analysis(Ajmani et al., 2017). This observation is further supported by two recent large cohort analyses, both of which found current smokers are about 2–3 times more likely to develop poor olfaction (Palmquist et al., 2020; Schubert et al., 2021). Further, several recent studies specifically investigated this association in PD patients and found that PD smokers had better olfaction than PD nonsmokers (Lucassen et al., 2014; Sharer et al., 2015). This type of analysis however can be misleading in the causal inference of smoking and olfaction in prodromal PD due to potential collider bias (Hernan et al., 2004). As such, the evidence is not consistent with the possibility that smoking protects against hyposmia. Interestingly, a few studies also examined the association of smoking with RBD, the other most prominent prodromal symptom of PD. In these studies, cigarette smoking is associated modestly with a higher risk of RBD (Postuma et al., 2012; Shrestha et al., 2018) but not with its phenotypical conversion to PD or other types of clinical synucleinopathy (Postuma et al., 2015). Therefore, data to date do not reconcile with the strong inverse association of smoking with PD. While they do not necessarily rule out a causal relationship that cigarette smoking reduces PD risk for several reasons (Chen, 2018), they offer no support that smoking is protective in the early stage of PD development.
Finally, the Braak and dual-hit hypotheses also provide a platform to systematically examine the roles of several other environmental exposures such as heavy metals (e.g., manganese) (Mezzaroba et al., 2019) and organic solvents (e.g. trichloroethylene ) (Lock et al., 2013) in relation to olfactory impairment in the context of PD prodromal development. Available studies were often conducted in occupational settings, participants were often young, and it was unclear whether the olfaction deficit was transitory or permanent. Data are not entirely consistent. Several studies reported that occupational exposures to cadmium, lead, and chromium were associated with poor olfaction (Adams and Crabtree, 1961; Casjens et al., 2018; Grashow et al., 2015; Kitamura et al., 2003; Liu et al., 1985; Mascagni et al., 2003; Potts, 1965; Sulkowski et al., 2000). In contrast, a couple of studies reported that occupational exposure to manganese or higher blood manganese level was associated with better olfaction performance (Antunes et al., 2007; Casjens et al., 2017; Lucchini et al., 1997), whereas children from areas with high manganese exposures performed worse on olfactory identification than their peers from low exposure areas (Guarneros et al., 2020). Studies on solvent exposures were predominantly published before 2010, and most found workers with occupational exposures to solvents or related chemicals were more likely to report poor olfaction or perform worse on the olfaction identification or threshold tests than their counterparts without such exposures (Ahlström et al., 1986; Baelum et al., 1982; Bolla et al., 1995; Holmström et al., 1995; Lee et al., 2018; Lucchini et al., 1997; Sandmark et al., 1989; Schwartz et al., 1989; Schwartz et al., 1990; Yu et al., 2004; Zibrowski and Robertson, 2006). Therefore, the overall literature appears to support associations of occupational exposures to certain heavy metals and solvents with poor olfaction, but the relevance of these associations to PD development is yet to be assessed.
Experimental evidence
Compared to epidemiological data, experimental studies have more directly examined the Braak and dual hit hypotheses in the context of PD research, as summarized below. It is important to note that although there is experimental evidence for self-propagating alpha-synuclein assemblies spread from the gut or the olfactory bulb to the brain, most evidence is based on studies of alpha-synuclein preformed fibril (PFFs) injections (Challis et al., 2020; Kim et al., 2019; Van Den Berge et al., 2021), and thus the factors that may trigger the de novo formation of “spread-competent” alpha-synuclein assemblies remain unknown. Furthermore, it is still unclear if this spreading process is driven by a prion-like or cell-autonomous mechanism. Therefore, the environmental “triggers” considered here should be viewed in the context of the experimental setting and the observed phenotype, which often lacks the hallmark pathological α-synuclein inclusions. Nevertheless, animal studies have provided preliminary evidence that the α-synuclein pathology can spread from the olfactory bulb throughout the brain to secondary sites, and to some extent, mimic the distribution of synucleinopathy in human diseases such as PD (Rey et al., 2013; Rey et al., 2016).
Experimental studies showed that several pesticides, when administered through the nasal cavity, can damage dopamine-containing neurons in the brain and recapitulate some of the PD phenotypes (Sasajima et al., 2015; Sasajima et al., 2017; Toyoda et al., 2020; Voronkov et al., 2017). Rotenone is a pesticide that inhibits the complex I of the mitochondrial electron transport chain, which leads to the inhibition of ATP synthesis, excessive production of oxidative radicals, and the death of dopaminergic neurons. Intranasally administered rotenone to mice impairs mitral cell activity in the olfactory bulb, possibly due to the denervation of inhibitory dopaminergic inputs (Sasajima et al., 2017). Mitral cells and their projections are common Lewy pathology containing structure in PD olfactory bulb (Sengoku et al., 2008). It is possible that mitral cell dysfunction following rotenone exposure results in Lewy pathology which then could project to several brain nuclei involved in PD (Cersosimo, 2018). Chronically administered intranasal rotenone to mice may damage the nigrostriatal dopamine system, specifically axons of the dorsal striatum (Ferrante et al., 1997), although data are not consistent across studies (Rojo et al., 2007). Furthermore, structures resembling Lewy pathology have been observed in the substantia nigra of mice with chronic exposure to rotenone (Betarbet et al., 2000). Intranasally administered pesticide paraquat accumulates in structures throughout the mouse brain and results in impairment of olfactory discrimination task (Anderson et al., 2021). However, like rotenone, the PD phenotype produced by paraquat inhalation in rodents is mild (Rojo et al., 2007), and therefore it is yet to demonstrate if nasal exposure to these pesticides alone is sufficient to initiate PD pathogenesis. MPTP has a chemical structure similar to paraquat, and intranasal exposure closely recapitulates many PD phenotypes, including olfactory dysfunction, motor abnormalities, and dopaminergic neuron loss in the olfactory bulb and substantia nigra (Prediger et al., 2010; Prediger et al., 2006; Prediger et al., 2009; Rojo et al., 2006).
Metals have long been implicated in PD pathogenesis. Various metals may promote α-synuclein aggregation (Jinsmaa et al., 2014; Paik et al., 1999; Wright et al., 2009), but intranasal metal exposure has not been extensively investigated in a PD model. Nevertheless, the olfactory bulb absorbs metal fumes (Sunderman, 2001), and some (e.g., manganese, nickel, and zinc) may be further transported throughout the brain via secondary olfactory neurons (Gianutsos et al., 1997; Sunderman, 2001). Recent evidence further suggests that manganese may promote the spread of α-synuclein oligomers via exosomes (Harischandra et al., 2019). Furthermore, mice exposed to intranasal vanadium, a metal in welding fumes that has been associated with PD (Park et al., 2005; Racette et al., 2001), showed a loss of tyrosine hydroxylase positive neurons of the olfactory bulb glomerular layer (Ngwa et al., 2014). However, the Lewy pathology was not assessed in the study (Ngwa et al., 2014). As to organic solvents, although systemically administered trichloroethylene may kill midbrain dopamine neurons (Guehl et al., 1999; Keane et al., 2019) and induce α-synuclein accumulation (Liu et al., 2010), to our knowledge, its potential adverse effects via inhalation has not been directly investigated.
In summary, despite the high biological plausibility that environmental toxicants may trigger PD pathogenesis through the olfactory pathway, relevant research is still in its infancy. Preliminary data from animal models suggest that the intranasal administration of certain pesticides or heavy metals may lead to the initiation and spreading of abnormal α-synuclein and aggregation that, to some extent, recapitulate the human Lewy pathology. But conclusive systematic research is yet to be conducted. Epidemiological evidence on environmental risk factors for olfactory impairment is preliminary. To our knowledge, none were conducted in the context of PD research, and thus far has provided limited clues to environmental triggers and accelerators of PD pathogenesis in humans.
The gut-to-brain pathway
The Braak hypothesis also posits that synucleinopathy may initiate in the enteric nerves of the gut and later spread along the vagus nerve into the brain (Hawkes et al., 2009). In fact, the presence of Lewy pathology in the enteric nervous system of PD patients was reported decades earlier (Wakabayashi et al., 1988). Further, constipation has been robustly identified as an early prodromal symptom of PD (Abbott et al., 2001; Gao et al., 2011; Savica et al., 2009), albeit the strength of the association was modest as compared with that for poor olfaction or RBD. The pivotal roles of the gut-brain axis to PD pathogenesis have been the topic of extensive recent reviews (Elfil et al., 2020; Killinger and Labrie, 2019; Lionnet et al., 2018; Scheperjans et al., 2018; Sharabi et al., 2021; Travagli et al., 2020). We hereby briefly summarized the key epidemiological and experimental evidence on this topic and experimental findings on the potential roles of environmental factors.
Epidemiological evidence
Besides the well-established observation of constipation in prodromal PD, multiple lines of human empirical evidence support a critical role of the gut-brain axis in PD pathogenesis. First, in a systematic histopathology study of PD patients (n=17) and other synucleinopathies, Beach et al. reported phosphorylated alpha-synuclein throughout the gastrointestinal system (Beach et al., 2010). Interestingly, they did not find isolated synucleinopathy in the gut, challenging the gut-to-brain spreading in prodromal PD. In their recent publication with more PD patients (n=53), 81% had synucleinopathy in the stomach and 89% in the vagus nerve adjacent to the neck carotid artery, as compared with no positivity in control subjects, supporting the involvement of the gastrointestinal tract and vagus nerve in PD pathogenesis (Beach et al., 2021). Second, multiple register-based epidemiologic analyses reported a modest positive association of inflammatory bowel diseases and PD with a meta-analyzed risk ratio of 1.24 (95% confidence interval: 1.15–1.34) (Zhu et al., 2022). Interestingly, IBD patients with anti-tumor necrosis factor treatment had a 78% lower risk than those without the therapy (Peter et al., 2018). These findings are consistent with the hypothesis that chronic intestinal inflammation or a “leaky gut” may contribute to PD pathogenesis. Third, epidemiological evidence suggests that H. pylori infection is not only associated with clinical PD and its severity(Dardiotis et al., 2018), it may also play a role in developing PD. In a retrospective cohort analysis of insurance data from Taiwan, H. pylori infection was associated with a >2 fold higher PD risk (Huang et al., 2018). Fourth, several studies examined surgical removal of the appendix or tonsil, the two prominent mucosa-associated lymphatic tissues in the gastrointestinal tract, in relation to PD risk. Findings are not consistent (Marras et al., 2016; Palacios et al., 2018; Tysnes et al., 2015), but analyses of the Swedish patient registers showed a lower PD risk in people with appendectomy(Killinger et al., 2018; Liu et al., 2020). Finally, two independent studies, using data from the Swedish and Danish patient registers, reported that people with truncal vagotomy had about half of the PD risk compared to people without(Liu et al., 2017a; Svensson et al., 2015), supporting the possibility of the gut-to-brain spreading pathway via the vagus nerve. These data, taken together, lend supports to the roles of gut inflammation and gut-brain axis in the development of PD. Notably, much of the evidence was from analyses of administrative databases. While this type of data has clear strengths (e.g., large samples and comprehensive coverage), they were not collected for research purposes, have limited data on potential confounders, and identify cases from diagnostic codes (Mues et al., 2017). Therefore, caution is needed in analyzing administrative data and interpreting their results.
Experimental evidence
Animal experimental studies provided more direct support to this spreading hypothesis, demonstrating that peripheral α-synuclein aggregates can be actively transported to the brain via the vagal nerve and can initiate a PD-like phenotype. Following injection, PFFs rapidly disseminate to neural circuits (Okuzumi et al., 2018) based on connectivity and possibly α-synuclein expression patterns (Henderson et al., 2019). It appears that they are actively trafficked via axonal transport (Freundt et al., 2012; Holmqvist et al., 2014), possibly by interacting/binding with mobile membranous organelles and vesicles. PFFs injected into the gut travel along the vagal nerve to the dorsal motor nucleus (Ulusoy et al., 2017; Ulusoy et al., 2013), but other routes are also possible (Borghammer, 2021). Bidirectional spread between the central and peripheral nervous systems is also possible. A recent comprehensive analysis in rodents found gut-to-brain spread, caudal to rostral progressive α-synuclein pathology, motor/cognitive impairments, gastrointestinal dysfunction, and dopaminergic neuron loss in the midbrain (Kim et al., 2019). Convincingly, both in wide-type mice with a vagotomy and SNCA knockout mice, the phenotype following PFFs injections into the gut was abolished (Kim et al., 2019). In summary, the spread of synucleinopathy from peripheral sites to the brain is largely supported by experimental evidence. However, PFFs injected into the gut do not invariably spread to the CNS and progressively throughout the brain (Manfredsson et al., 2018), and several critical factors including dose, site of injection, and the PFF strain/species are important. To date, the evidence of “gut only” α-synuclein pathology in humans has yet to be demonstrated (Beach et al., 2010) which is required for the gut origin hypothesis of PD. However the lack of evidence could be due to the relatively low sampling of gut tissues per volume, akin to searching for a needle in a haystack.
Potential roles of environmental factors
One key implication of the gut-to-brain transmission of PD pathogenesis is the potential environmental contributions. Research on the roles of environmental toxicants and the gut-to-brain synucleinopathy spreading has been largely limited to pesticides in experimental studies. Orally administered rotenone causes apparent progressive pathology in the intermediolateral nucleus of the spinal cord and substantia nigra (Pan-Montojo et al., 2010), which can be prevented by vagotomy (Pan-Montojo et al., 2012). However, the pathology was determined by total α-synuclein protein staining, which has a low specificity for the disease process. A separate study determined that TLR4 knockout mice are somewhat protected from the inflammatory and neurotoxic effects of oral rotenone treatment (Perez-Pardo et al., 2019), suggesting a critical role of inflammation in this process. Indeed, experimental studies have found that both acute and chronic gut inflammation may modulate the expression and possibly aggregation of α-synuclein in rodents (Kishimoto et al., 2019; Prigent et al., 2019), but it is yet to be demonstrated that gut inflammation can lead to prion-like synucleinopathy. Besides rotenone, paraquat was also tested in animal models. Orally administered paraquat to mice expressing A53T α-synuclein had accelerated PSER129 pathology formation throughout the small intestine (Naudet et al., 2017). Although pesticides might promote abnormal α-synuclein aggregates in the gut, other compounds might exacerbate their toxic effects. For example, paraquat produces a mild and often poorly reproducible PD-like phenotype in mice, but small doses of paraquat taken with dietary lectins produce a phenotype with several key features of PD, including the peripheral PSER129 inclusion (Anselmi et al., 2018). Authors of this study hypothesize that lectins may promote the absorption of the orally administered paraquat and thus enhance its toxic effects (Anselmi et al., 2018).
Microbiota
In recent years, the potential role of the microbiota in PD pathogenesis and progression has gained much attention (Boertien et al., 2019; Elfil et al., 2020) as it modulates the immune system and the absorption and metabolism of nutrients, medications, and environmental toxins. Certain aspects of microbiota composition are already determined in earliest childhood, e.g., by mode of delivery, breastfeeding, and antibiotic exposure. During vaginal delivery, the first colonization of the offspring occurs through the maternal microbiota, potentially affecting the inheritance of certain vulnerabilities independently of genotype (Sandoval-Motta et al., 2017). Throughout the lifespan, the microbiota colonizing the outer and inner surfaces of our body continues to be at the interface between the environment and the human organism and potentially influences their interactions. Further, converging evidence suggests that the gut microbiota influences the development, function, and aging of the brain (Heijtz et al., 2021), although the fundamental mechanisms by which the microbiota influences PD pathogenesis remain poorly understood.
Epidemiological evidence
Many studies have linked the gut microbiota to PD and to its motor and nonmotor symptoms. Despite some variabilities in the results and adjustments for medications, comorbidities and diet, study findings suggest a shift to a potentially proinflammatory microbiome composition in PD patients (Romano et al., 2021) with increased markers of gut inflammation and permeability (Aho et al., 2021; Schwiertz et al., 2018). Other potential pathways by which microbiota may influence PD development and progression include bacterial metabolites as well as potential cross seeding between bacterial amyloid proteins and α-synuclein (Aho et al., 2021; Chen et al., 2016; Cirstea et al., 2020). The most consistent metabolic alteration so far has been reduced fecal levels of bacterially produced short-chain fatty acids (SCFAs), in particular butyrate, in PD patients (Aho et al., 2021; Unger et al., 2016). SCFAs can have a broad influence on the human host and brain through impact on (neuro)inflammation, epigenetic regulation, and other mechanisms (Silva et al., 2020).
The few longitudinal studies on microbiota in PD suggest links between gut microbiota composition and disease progression in established PD patients (Aho et al., 2019). Studies about microbiota contributions in the prodromal phase are scarce. To this end, one study found substantial overlaps in differential microbiome abundant taxa between PD patients and idiopathic RBD patients as compared with healthy controls, suggesting microbiota changes before PD clinical diagnosis (Heintz-Buschart et al., 2018). However, another study found that microbiota changes were more pronounced in established PD as compared to iRBD (Nishiwaki et al., 2020), suggesting that changes may evolve as disease progresses. A recent large study examined microbiota associations with known PD prodromal and risk markers and found differential microbiota abundance linked to constipation, possible RBD, smoking, and subthreshold parkinsonism (Heinzel et al., 2021), but not to olfactory impairment or other markers of prodromal PD.
Few human studies have examined nasal and oral microbiota in PD. Reliable sampling of the microbiota and DNA amplification from the nasal cavity has turned out to be challenging due to the low bacterial biomass encountered in nasal swabs. Therefore, there is a considerable risk of laboratory contaminants to influence the results when many amplification cycles are needed. Two recent studies(Pereira et al., 2017; Li et al., 2021) failed to find significant differential compositions of nasal microbiota between PD patients and controls based on swab sampling. The third study (Heintz-Buschart et al., 2018) used a nasal wash sampling technique and found no convincing differences of nasal microbiota across PD patients, iRBD patients and healthy controls. With respect to the oral microbiota, several studies reported significant differences between PD patients and controls. For example, the above referenced two studies (Pereira et al., 2017; Li et al., 2021) reported low abundance of Neisseria and Neisseriaceae and increased abundance in Lactobacillaceae /Lactobacillales in PD oral swab samples. A recent study (Rozas et al., 2021) further reported increased oral soft tissue abundance of Lactobacillus together with an increase of Tannerella forsythia, Prevotella intermedia, and opportunistic pathogens. Also oral hard tissue samples showed increased of opportunicstic oral pathogens in PD. Factors that significantly influenced soft tissue beta-diversity and microbiota composition included dysphagia, drooling, and salivary pH. Taken together, while studies to date failed to find convincing links of nasal microbiota to PD, changes in oral microbiota have been suggested.
Experimental evidence
The role of gut microbiota in PD has also been investigated in experimental studies. When raised germ-free or when the microbiota was eradicated by antibiotics, alpha-synuclein overexpressing mice showed little synucleinopathy, neuroinflammation, and motor symptoms (Sampson et al., 2016), suggesting gut microbiota are required for PD pathogenesis. Conversely, introducing PD-patient microbiota to these mice resulted in PD pathology and motor impairment (Sampson et al., 2016), which may be related to SCFAs produced by the microbiota. On the other hand, several studies suggest a beneficial or even neuroprotective role of SCFAs such as butyrate in PD experimental models (Liu et al., 2017b; Paiva et al., 2017)
Potential roles of environmental factors
It is also important to consider that the gut microbiota acts at the interface of the human organism with the environment, and thus may play a role in how environmental influences impact disease initiation and progression. One such influence is the regulation of gut wall permeability (Ambrosini et al., 2019). Indeed, increased gut wall permeability or “leaky gut” has been described in PD (Forsyth et al., 2011), although this finding has not always been consistent and may vary between gut segments. Such increased permeability may facilitate access of potentially harmful environmental factors to the gut tissue and systemic circulation as well as immune activation.
Gut microbiota may influence how pesticides impact PD pathogenesis. Experimental evidence suggests that oral application of rotenone affects gut permeability only if a gut microbiota is present, but not in germ-free mice (Bhattarai et al., 2021). Rotenone induces microbiota changes, and these may have proinflammatory effects that are dependent on TLR4 signaling (Perez-Pardo et al., 2019; Perez-Pardo et al., 2018). Smoking and coffee drinking, two factors associated with lower PD risk in observational studies, may influence microbiota composition (González et al., 2020; Huang and Shi, 2019), raising the intriguing possibility that their roles in PD may be modulated or mediated by the gut microbiota. Further, there is evidence that children living in rural areas are more likely to have a microbiota that is linked to a lower risk of autoimmune diseases (Kirjavainen et al., 2019), the potential relevance of this finding to PD is yet to be investigated. Finally, a recent study (Mertsalmi et al., 2020) reported a higher PD risk in people with past exposure to multiple courses of specific antibiotics treatments, in particular macrolides and lincosamides, antianaerobic, and broad-spectrum antibiotics, along with antifungal medications. Since these antibiotics have the highest impact on gut microbiota, it is likely that this finding was due to altered microbiota following the use of antibiotics (Mertsalmi et al., 2020). Interestingly, these associations were the strongest for exposures 10–15 years before PD diagnosis, consistent with the concept of a prolonged prodromal phase of PD.
Taken together, recent research not only supports a critical role of the gut microbiota in PD pathogenesis but also raises the possibility that they may modulate or mediate the effects of environmental risk factors for PD.
Research Gaps and Future Directions
In the past several decades, research into the environmental causes of PD has significantly lagged behind genetic research, owing largely to the lack of understanding of the disease’s natural history and its prolonged and complex prodromal development, as well as the lack of tools to adequately assess complex environmental exposures over an extended life period. The Braak and dual-hit hypotheses, albeit still controversial, have provided a timely theoretical framework to peek into the complexity of PD prodromal development (Braak et al., 2006; Hawkes et al., 2009). These hypotheses, coupled with empirical advances in understanding PD prodromal symptoms and markers, have opened an unprecedented window to define the environmental triggers and modifiers at various stages of PD development and to query novel etiological hypotheses of PD that have not been speculated before. These activities have been best illustrated by the preliminary inquiries of the etiological relevance of the gut-brain axis and gut microbiota in PD, as detailed above.
While promising, these lines of research are still in their infancy, with substantial research gaps and challenges ahead. First, the Braak hypothesis itself is still, to some extent, controversial (Lionnet et al., 2018). For example, neuropathological studies are yet to document standalone synucleinopathy in the gut without brain involvement (Beach et al., 2010), and the body-first vs. brain-first etiology of PD has emerged as an important topic of investigation (Borghammer et al., 2021). Researchers must be fully mindful of the etiological, pathological, and clinical complexity of PD development and presentations (Berg et al., 2021). Second, the potential roles of Lewy pathology and its spreading in PD pathogenesis and symptomatic presentation remain speculative. Although preliminary animal experimental research has provided provocative evidence supporting for the spreading of alpha-synuclein pathology induced by PFFs from olfactory and intestinal structures to the brain, but several aspects of the PFF model fall short. For example, the spreading in many instances does not seem progressive but instead occurs discretely amongst interconnected neurons, but over time the spread stops. This brings into question whether this spread is really a disease driving mechanism in PD prodromal and clinical progression (Killinger and Kordower, 2019). Further, PFF seeded Lewy pathology does not closely resemble bona fide Lewy pathology seen in the human PD brain. Many Lewy bodies in the human brain have exquisite halo structure of radiating filaments intermingled with damaged organelles, hinting at cellular regulated processes. These fine structural characteristics do not seem to be recapitulated with PFFs, or are not typically experimentally determined. Third, despite contributions from animal models of PD, it is challenging to have experimental models that recapitulate the scope of PD pathological and clinical endpoints in humans, including dopaminergic neuron loss, Lewy pathology, motor and nonmotor impairment as well as their sequential development. Fourth, roles of environmental toxins remain to be investigated in PD experimental models, at levels relevant to human health, via routes that mimic human exposure, and during various stages of prodromal development. This is particularly relevant to PD research as the disease is chronic and progressive, and the two-hit hypothesis specifically demands experimental studies via inhalation and gastrointestinal routes. Finally, humans are exposed to mixtures of chemicals, physical agents, microbial, and other environmental exposures throughout our lifetime, and new exposures emerge, yet PD research has been almost exclusively focused upon its known or suspected environmental causes (e.g., pesticides, metals, and air pollutants). Future research should capitalize on the new technology, such as large-scale screening to search for novel environmental chemicals for PD pathogenesis.
In human studies, it is also critical to target PD prodromal development, for example, by identifying triggers of PD pathogenesis and modifiers for its prodromal progression and phenotypical conversion (Chen and Ritz, 2018). Conducting such research is, however, very challenging. Although the Braak hypothesis provides a staging scheme of prodromal development, there are substantial knowledge gaps in identifying reliable markers and characterizing their sequential orders (if any) and phenotypical heterogeneity. Prodromal symptoms identified so far, except for PSG-confirmed RBD, lack specificity to PD (Chen et al., 2015). Research into the risk factors for RBD and factors that modify its phenotypical conversion is informative (Postuma et al., 2015; Postuma et al., 2012), but only about a quarter of PD patients have prodromal RBD (Chen et al., 2015). The International Movement Disorder Society recently updated its probability-based research criteria for prodromal PD, based on PD risk factors, prodromal nonmotor symptoms, subtle motor signs, neuroimaging, and other markers (Heinzel et al., 2019). It remains to see if whether and how this concept may lead to the identification and characterization of environmental contributions to prodromal PD.
Unlike the human genome, we do not have readily available tools to assess our environmental exposures, which are complex mixtures and consistently change throughout our lifetime (Patel et al., 2017). Further, it is yet to be studied whether the potential effects of exposures may be differential by exposure windows (e.g., prenatal, childhood, early, mid vs. late adulthood)(Gao et al., 2015) and stages of disease development (e.g., initiation vs. phenotypical conversion) (Postuma et al., 2015; Postuma et al., 2012). Finally, such effects are likely complex, cumulative, and interactive with other environmental and genetic factors, making them extremely difficult to assess (Chen and Ritz, 2018). Such complexity demands coordinated efforts from multiple large prospective cohorts with longitudinal and comprehensive assessments of environmental exposures, genetic, and other molecular signatures (e.g., epigenomic, metabolomic, microbiomic, and proteomic). Such studies should also collect and bank biospecimen (e.g., blood, feces, saliva, nasal mucosa) for future research needs (Marras et al., 2019). Principal investigators of these studies should work collaboratively to assess currently available resources, develop strategies to harmonize data and pool resources for PD prodromal research, and identify future research opportunities and strategies (Marras et al., 2019). This has been exemplified by the ever-growing international PD genetic research consortia (Aligning Science Across Parkinson’s (ASAP) Initiative, 2021). Pooling cohorts for non-genetic research will be much more challenging and risky (Smith-Warner et al., 2006), but its success will be extremely rewarding by identifying modifiable causes of PD to prevent or delay the disease’s clinical onset (Marras et al., 2019).
In conclusion, PD prodromal research represents an unprecedented opportunity for identifying the environmental triggers and effect modifiers of PD (Chen and Ritz, 2018). Such research, however, will be very challenging, calling for multidisciplinary efforts across biologists, epidemiologists, and clinical scientists to identify priorities, strategize approaches, and implement research activities (De Miranda et al., 2021). Further research should also take a life-long approach and leverage emerging tools to assess exposures and their biological effects to understand how PD starts and develops and how environmental factors contribute to these processes.
Acknowledgements:
This work is supported in part by the National Institute of Environmental Health Sciences (R01ES029227), the Office of the Assistant Secretary of Defense for Health Affairs through the Parkinson’s Research Program (Award No. W81XWH-17-1-0536), and the Parkinson’s Foundation (Grant No. PF-IMP-1825). Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the National Institutes of Health and the US Department of Defense. Dr. Scheperjans is supported by the Academy of Finland (295724, 310835, 342758), the Hospital District of Helsinki and Uusimaa (UAK1014004, UAK1014005, UAK1024MTM, TYH2018224, TYH2020335), The Olvi Foundation, the Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, and the Stockmann Foundation.
Footnotes
Conflict of Interest:
Dr. Scheperjans is founder and CEO of NeuroInnovation Oy and NeuroBiome Ltd., is a member of the scientific advisory board and has received consulting fees and stock options from Axial Biotherapeutics. He has patents issued (FI127671B & US10139408B2) and pending (US16/186,663 & EP3149205) that are assigned to NeuroBiome Ltd. Other authors did not report and financial conflict of interest.
Contributor Information
Honglei Chen, Epidemiology and Biostatistics, College of Human Medicine, Michigan State University, East Lansing, MI, 48824, USA
Keran Wang, Epidemiology and Biostatistics, College of Human Medicine, Michigan State University, East Lansing, MI, 48824, USA
Filip Scheperjans, Department of Neurology, Helsinki University Hospital, and Clinicum, University of Helsinki, Haartmaninkatu 4, 00290 Helsinki, Finland
Bryan Killinger, Graduate College, Rush University Medical Center, Chicago IL, 60612, USA
References:
- Abbott RD, et al. , 2001. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology 57, 456–62. [DOI] [PubMed] [Google Scholar]
- Adams DR, et al. , 2016. Nitrogen dioxide pollution exposure is associated with olfactory dysfunction in older U.S. adults. Int Forum Allergy Rhinol 6, 1245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams RG, Crabtree N, 1961. Anosmia in alkaline battery workers. British Journal of Industrial Medicine 18, 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlström R, et al. , 1986. Impaired odor perception in tank cleaners. Scandinavian Journal of Work, Environment & Health 12, 574–581. [DOI] [PubMed] [Google Scholar]
- Ahman M, et al. , 2001. Nasal symptoms and pathophysiology in farmers. Int Arch Occup Environ Health 74, 279–84. [DOI] [PubMed] [Google Scholar]
- Aho VTE, et al. , 2021. Relationships of gut microbiota, short-chain fatty acids, inflammation, and the gut barrier in Parkinson’s disease. Mol Neurodegener 16, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho VTE, et al. , 2019. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine 44, 691–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani GS, et al. , 2016. Fine particulate matter exposure and olfactory dysfunction among urban-dwelling older US adults. Environ Res 151, 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajmani GS, et al. , 2017. Smoking and olfactory dysfunction: A systematic literature review and meta-analysis. Laryngoscope 127, 1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aligning Science Across Parkinson’s (ASAP) Initiative, Global Parkinson’s Genetics Program https://www.michaeljfox.org/grant/global-parkinsons-genetics-program-gp2. 2021.
- Ambrosini YM, et al. , 2019. The Gut-Brain Axis in Neurodegenerative Diseases and Relevance of the Canine Model: A Review. Frontiers in Aging Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T, et al. , 2021. Paraquat Inhalation, a Translationally Relevant Route of Exposure: Disposition to the Brain and Male-Specific Olfactory Impairment in Mice. Toxicological Sciences: An Official Journal of the Society of Toxicology 180, 175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi L, et al. , 2018. Ingestion of subthreshold doses of environmental toxins induces ascending Parkinsonism in the rat. NPJ Parkinson’s disease 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antunes MB, et al. , 2007. San Francisco/Oakland Bay Bridge Welder Study: olfactory function. Neurology 69, 1278–1284. [DOI] [PubMed] [Google Scholar]
- Baelum J, et al. , 1982. Acute and subacute symptoms among workers in the printing industry. British Journal of Industrial Medicine 39, 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, et al. , 2021. Vagus Nerve and Stomach Synucleinopathy in Parkinson’s Disease, Incidental Lewy Body Disease, and Normal Elderly Subjects: Evidence Against the “Body-First” Hypothesis. J Parkinsons Dis 11, 1833–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach TG, et al. , 2010. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta neuropathologica 119, 689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, et al. , 2021. Prodromal Parkinson disease subtypes - key to understanding heterogeneity. Nat Rev Neurol 17, 349–361. [DOI] [PubMed] [Google Scholar]
- Betarbet R, et al. , Chronic systemic pesticide exposure reproduces features of Parkinson’s disease Vol. 3, 2000, pp. 1301–1306. [DOI] [PubMed] [Google Scholar]
- Bhattarai Y, et al. , 2021. Role of gut microbiota in regulating gastrointestinal dysfunction and motor symptoms in a mouse model of Parkinson’s disease. Gut Microbes 13, 1866974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, et al. , 2012. The outdoor air pollution and brain health workshop. Neurotoxicology 33, 972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boertien JM, et al. , 2019. Increasing Comparability and Utility of Gut Microbiome Studies in Parkinson’s Disease: A Systematic Review. J Parkinsons Dis 9, S297–S312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, et al. , 1995. Comparison of neurobehavioral function in workers exposed to a mixture of organic and inorganic lead and in workers exposed to solvents. American Journal of Industrial Medicine 27, 231–246. [DOI] [PubMed] [Google Scholar]
- Borghammer P, 2021. The α-Synuclein Origin and Connectome Model (SOC Model) of Parkinson’s Disease: Explaining Motor Asymmetry, Non-Motor Phenotypes, and Cognitive Decline. Journal of Parkinson’s Disease 11, 455–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghammer P, et al. , 2021. Neuropathological evidence of body-first vs. brain-first Lewy body disease. Neurobiol Dis [DOI] [PubMed] [Google Scholar]
- Braak H, et al. , 2006. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Movement Disorders 21, 2042–2051. [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, et al. , 2010. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Exp Toxicol Pathol 62, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casjens S, et al. , 2017. Associations between former exposure to manganese and olfaction in an elderly population: Results from the Heinz Nixdorf Recall Study. Neurotoxicology 58, 58–65. [DOI] [PubMed] [Google Scholar]
- Casjens S, et al. , 2018. Associations between blood lead, olfaction and fine-motor skills in elderly men: Results from the Heinz Nixdorf Recall Study. Neurotoxicology 68, 66–72. [DOI] [PubMed] [Google Scholar]
- Cersosimo MG, 2018. Propagation of alpha-synuclein pathology from the olfactory bulb: possible role in the pathogenesis of dementia with Lewy bodies. Cell and Tissue Research 373, 233–243. [DOI] [PubMed] [Google Scholar]
- Challis C, et al. , 2020. Gut-seeded alpha-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat Neurosci 23, 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, 2018. The Changing Landscape of Parkinson Epidemiologic Research. J Parkinsons Dis 8, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. , 2010. Smoking duration, intensity, and risk of Parkinson disease. Neurology 74, 878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Ritz B, 2018. The Search for Environmental Causes of Parkinson’s Disease: Moving Forward. J Parkinsons Dis 8, S9–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. , 2017. Olfaction and incident Parkinson disease in US white and black older adults. Neurology 89 1441–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, et al. , 2015. Meta-analyses on prevalence of selected Parkinson’s nonmotor symptoms before and after diagnosis. Translational neurodegeneration 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SG, et al. , 2016. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci Rep 6, 34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, et al. , 2012. A progressive mouse model of Parkinson’s disease: the Thy1-aSyn (“Line 61”) mice. Neurotherapeutics 9, 297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MS, et al. , 2020. Microbiota Composition and Metabolism Are Associated With Gut Function in Parkinson’s Disease. Mov Disord 35, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Dardiotis E, et al. , 2018. H. pylori and Parkinson’s disease: Meta-analyses including clinical severity. Clin Neurol Neurosurg 175, 16–24. [DOI] [PubMed] [Google Scholar]
- Darweesh SKL, et al. , 2018. Parkinson Matters. J Parkinsons Dis 8, 495–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Miranda BR, et al. , 2021. Preventing Parkinson’s Disease: An Environmental Agenda. J Parkinsons Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2009. The olfactory system and its disorders. Semin Neurol 29, 74–81. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2015. Neurotoxic exposure and impairment of the chemical senses of taste and smell. Handb Clin Neurol 131, 299–324. [DOI] [PubMed] [Google Scholar]
- Elfil M, et al. , 2020. Implications of the Gut Microbiome in Parkinson’s Disease. Mov Disord 35, 921–933. [DOI] [PubMed] [Google Scholar]
- Fereshtehnejad SM, et al. , 2019. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: a prospective study. Brain 142, 2051–2067. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, et al. , 1997. Systemic administration of rotenone produces selective damage in the striatum and globus pallidus, but not in the substantia nigra. Brain Research 753, 157–162. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, et al. , 2011. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One 6, e28032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundt EC, et al. , 2012. Neuron-to-neuron transmission of α-synuclein fibrils through axonal transport. Annals of Neurology 72, 517–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, et al. , 2015. Head injury, potential interaction with genes, and risk for Parkinson’s disease. Parkinsonism & related disorders 21, 292–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, et al. , 2011. A prospective study of bowel movement frequency and risk of Parkinson’s disease. Am J Epidemiol 174, 546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD Parkinson’s Disease Collaborators, 2018. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianutsos G, et al. , 1997. Accumulation of manganese in rat brain following intranasal administration. Fundamental and Applied Toxicology: Official Journal of the Society of Toxicology 37, 102–105. [DOI] [PubMed] [Google Scholar]
- Gobba F, Abbacchini C, 2012. Anosmia after exposure to a pyrethrin-based insecticide: a case report. Int J Occup Med Environ Health 25, 506–12. [DOI] [PubMed] [Google Scholar]
- González S, et al. , 2020. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients 12, 1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashow R, et al. , 2015. Cumulative lead exposure is associated with reduced olfactory recognition performance in elderly men: The Normative Aging Study. Neurotoxicology 49, 158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneros M, et al. , 2009. Mexico City air pollution adversely affects olfactory function and intranasal trigeminal sensitivity. Chem Senses 34, 819–26. [DOI] [PubMed] [Google Scholar]
- Guarneros M, et al. , 2020. Metal-containing Particulate Matter and Associated Reduced Olfactory Identification Ability in Children from an Area of High Atmospheric Exposure in Mexico City. Chemical Senses 45, 45–58. [DOI] [PubMed] [Google Scholar]
- Guehl D, et al. , 1999. Trichloroethylene and parkinsonism: a human and experimental observation. European Journal of Neurology 6, 609–611. [DOI] [PubMed] [Google Scholar]
- Harischandra DS, et al. , 2019. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of α-synuclein. Science Signaling 12, eaau4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, et al. , 2007. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33, 599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, et al. , 2009. Parkinson’s disease: the dual hit theory revisited. Ann N Y Acad Sci 1170, 615–22. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, et al. , 2021. Young microbiota rejuvenates the aging brain. Nature Aging 1, 625–627. [DOI] [PubMed] [Google Scholar]
- Heintz-Buschart A, et al. , 2018. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov Disord 33, 88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel S, et al. , 2021. Gut Microbiome Signatures of Risk and Prodromal Markers of Parkinson Disease. Ann Neurol [DOI] [PubMed] [Google Scholar]
- Heinzel S, et al. , 2019. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord 34, 1464–1470. [DOI] [PubMed] [Google Scholar]
- Henderson MX, et al. , 2019. Spread of α-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nature Neuroscience 22, 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernan MA, et al. , 2004. A structural approach to selection bias. Epidemiology 15, 615–25. [DOI] [PubMed] [Google Scholar]
- Holmqvist S, et al. , 2014. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathologica 128, 805–820. [DOI] [PubMed] [Google Scholar]
- Holmström M, et al. , 1995. Upper airway symptoms and function in wood surface coating industry workers. American Journal of Industrial Medicine 28, 207–220. [DOI] [PubMed] [Google Scholar]
- Holmstrom M, et al. , 2008. Nasal complaints and signs of disease in farmers--a methodological study. Acta Otolaryngol 128, 193–200. [DOI] [PubMed] [Google Scholar]
- Huang C, Shi G, 2019. Smoking and microbiome in oral, airway, gut and some systemic diseases. Journal of Translational Medicine 17, 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang HK, et al. , 2018. Helicobacter pylori infection is associated with an increased risk of Parkinson’s disease: A population-based retrospective cohort study. Parkinsonism Relat Disord 47, 26–31. [DOI] [PubMed] [Google Scholar]
- Hudson R, et al. , 2006. Effect of air pollution on olfactory function in residents of Mexico City. Chem Senses 31, 79–85. [DOI] [PubMed] [Google Scholar]
- Jinsmaa Y, et al. , 2014. Divalent metal ions enhance DOPAL-induced oligomerization of alpha-synuclein. Neuroscience Letters 569, 27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, et al. , 2021. Association of NO2 and Other Air Pollution Exposures With the Risk of Parkinson Disease. JAMA Neurol 78, 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane PC, et al. , 2019. Trichloroethylene and its metabolite TaClo lead to degeneration of substantia nigra dopaminergic neurones: Effects in wild type and human A30P mutant α-synuclein mice. Neuroscience Letters 711, 134437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger B, Labrie V, 2019. The Appendix in Parkinson’s Disease: From Vestigial Remnant to Vital Organ? J Parkinsons Dis 9, S345–S358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killinger BA, Kordower JH, 2019. Spreading of alpha-synuclein - relevant or epiphenomenon? J Neurochem 150, 605–611. [DOI] [PubMed] [Google Scholar]
- Killinger BA, et al. , 2018. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, et al. , 2019. Transneuronal Propagation of Pathologic α-Synuclein from the Gut to the Brain Models Parkinson’s Disease. Neuron 103, 627–641.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirjavainen PV, et al. , 2019. Farm-like indoor microbiota in non-farm homes protects children from asthma development. Nat Med 25, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, et al. , 2019. Chronic Mild Gut Inflammation Accelerates Brain Neuropathology and Motor Dysfunction in alpha-Synuclein Mutant Mice. Neuromolecular Med 21, 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura F, et al. , 2003. Increase of olfactory threshold in plating factory workers exposed to chromium in Korea. Industrial Health 41, 279–285. [DOI] [PubMed] [Google Scholar]
- Kumar A, et al. , 2016. Role of cytochrome c in alpha-synuclein radical formation: implications of alpha-synuclein in neuronal death in Maneb- and paraquat-induced model of Parkinson’s disease. Mol Neurodegener 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-J, et al. , 2018. Risk of olfactory dysfunction of the workers in the automobile repair, printing, shoemaking and plating industries in Korea: a cross-sectional study. BMJ open 8, e022678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. , 2021. Oral, Nasal, and Gut Microbiota in Parkinson’s Disease. Neuroscience 480, 65–78. [DOI] [PubMed] [Google Scholar]
- Lionnet A, et al. , 2018. Does Parkinson’s disease start in the gut? Acta Neuropathol 135, 1–12. [DOI] [PubMed] [Google Scholar]
- Liu B, et al. , 2017a. Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 88, 1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, et al. , 2020. Appendectomy, Tonsillectomy and Parkinson’s Disease Risk: A Swedish Register-Based Study. Front Neurol 11, 510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, et al. , 2017b. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J Neurol Sci 381, 176–181. [DOI] [PubMed] [Google Scholar]
- Liu M, et al. , 2010. Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. Journal of Neurochemistry 112, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, et al. , 2016. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ Health Perspect 124, 1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YZ, et al. , 1985. Effects of cadmium on cadmium smelter workers. Scandinavian Journal of Work, Environment & Health 11 Suppl 4, 29–32. [PubMed] [Google Scholar]
- Lock EA, et al. , 2013. Solvents and Parkinson disease: a systematic review of toxicological and epidemiological evidence. Toxicol Appl Pharmacol 266, 345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen EB, et al. , 2014. History of smoking and olfaction in Parkinson’s disease. Movement Disorders: Official Journal of the Movement Disorder Society 29, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, et al. , 1997. Motor function, olfactory threshold, and hematological indices in manganese-exposed ferroalloy workers. Environmental Research 73, 175–180. [DOI] [PubMed] [Google Scholar]
- Maher BA, et al. , 2016. Magnetite pollution nanoparticles in the human brain. Proc Natl Acad Sci U S A 113, 10797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht P, et al. , 2018. Reader response: Olfaction and incident Parkinson disease in US white and black older adults. Neurology 90, 940. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, et al. , 2018. Induction of alpha-synuclein pathology in the enteric nervous system of the rat and non-human primate results in gastrointestinal dysmotility and transient CNS pathology. Neurobiology of Disease 112, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, et al. , 2019. Environment, lifestyle, and Parkinson’s disease: Implications for prevention in the next decade. Mov Disord 34, 801–811. [DOI] [PubMed] [Google Scholar]
- Marras C, et al. , 2016. Appendectomy in mid and later life and risk of Parkinson’s disease: A population-based study. Mov Disord 31, 1243–7. [DOI] [PubMed] [Google Scholar]
- Mascagni P, et al. , 2003. Olfactory function in workers exposed to moderate airborne cadmium levels. Neurotoxicology 24, 717–724. [DOI] [PubMed] [Google Scholar]
- Maturana MG, et al. , 2015. Unveiling the role of the pesticides paraquat and rotenone on alpha-synuclein fibrillation in vitro. Neurotoxicology 46, 35–43. [DOI] [PubMed] [Google Scholar]
- Mertsalmi TH, et al. , 2020. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov Disord 35, 431–442. [DOI] [PubMed] [Google Scholar]
- Mezzaroba L, et al. , 2019. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 74, 230–241. [DOI] [PubMed] [Google Scholar]
- Mues KE, et al. , 2017. Use of the Medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol 9, 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naudet N, et al. , 2017. Oral Exposure to Paraquat Triggers Earlier Expression of Phosphorylated α-Synuclein in the Enteric Nervous System of A53T Mutant Human α-Synuclein Transgenic Mice. Journal of Neuropathology and Experimental Neurology 76, 1046–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngwa HA, et al. , 2014. Vanadium exposure induces olfactory dysfunction in an animal model of metal neurotoxicity. Neurotoxicology 43, 73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki H, et al. , 2020. Short-Chain Fatty Acid-Producing Gut Microbiota Is Decreased in Parkinson’s Disease but Not in Rapid-Eye-Movement Sleep Behavior Disorder. mSystems 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuzumi A, et al. , 2018. Rapid dissemination of alpha-synuclein seeds through neural circuits in an in-vivo prion-like seeding experiment. Acta Neuropathologica Communications 6, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SR, et al. , 1999. Copper(II)-induced self-oligomerization of alpha-synuclein. The Biochemical Journal 340 ( Pt 3), 821–828. [PMC free article] [PubMed] [Google Scholar]
- Paiva I, et al. , 2017. Sodium butyrate rescues dopaminergic cells from alpha-synuclein-induced transcriptional deregulation and DNA damage. Hum Mol Genet 26, 2231–2246. [DOI] [PubMed] [Google Scholar]
- Palacios N, et al. , 2017. Air Pollution and Risk of Parkinson’s Disease in a Large Prospective Study of Men. Environ Health Perspect 125, 087011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios N, et al. , 2018. Appendectomy and risk of Parkinson’s disease in two large prospective cohorts of men and women. Mov Disord 33, 1492–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmquist E, et al. , 2020. A Prospective Study on Risk Factors for Olfactory Dysfunction in Aging. J Gerontol A Biol Sci Med Sci 75, 603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo F, et al. , 2010. Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5, e8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Montojo F, et al. , 2012. Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2, doi: 10.1038/srep00898. Epub 2012 Nov 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park RM, et al. , 2005. Potential occupational risks for neurodegenerative diseases. Am J Ind Med 48, 63–77. [DOI] [PubMed] [Google Scholar]
- Patel CJ, et al. , 2017. Opportunities and Challenges for Environmental Exposure Assessment in Population-Based Studies. Cancer Epidemiol Biomarkers Prev 26, 1370–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PAB, et al. , 2017. Oral and nasal microbiota in Parkinson’s disease. Parkinsonism Relat Disord 38, 61–67. [DOI] [PubMed] [Google Scholar]
- Perez-Pardo P, et al. , 2019. Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut 68, 829–843. [DOI] [PubMed] [Google Scholar]
- Perez-Pardo P, et al. , 2018. Gut bacterial composition in a mouse model of Parkinson’s disease. Benef Microbes 9, 799–814. [DOI] [PubMed] [Google Scholar]
- Peter I, et al. , 2018. Anti-Tumor Necrosis Factor Therapy and Incidence of Parkinson Disease Among Patients With Inflammatory Bowel Disease. JAMA Neurol 75, 939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, et al. , 2009. Quantifying the risk of neurodegenerative disease in idiopathic REM sleep behavior disorder. Neurology 72, 1296–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, et al. , 2015. Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: A multicenter study. Annals of neurology 77, 830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, et al. , 2012. Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study. Neurology 79, 428–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts CL, 1965. CADMIUM PROTEINURIA--THE HEALTH OF BATTERY WORKERS EXPOSED TO CADMIUM OXIDE DUST. The Annals of Occupational Hygiene 8, 55–61. [DOI] [PubMed] [Google Scholar]
- Prediger RD, et al. , 2011. Intranasal Administration of Neurotoxicants in Animals: Support for the Olfactory Vector Hypothesis of Parkinson’s Disease. Neurotox Res 21, 90–116. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, et al. , 2010. Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotoxicity Research 17, 114–129. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, et al. , 2006. The risk is in the air: Intranasal administration of MPTP to rats reproducing clinical features of Parkinson’s disease. Experimental Neurology 202, 391–403. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, et al. , 2009. Risk is in the air: an intranasal MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) rat model of Parkinson’s disease. Annals of the New York Academy of Sciences 1170, 629–636. [DOI] [PubMed] [Google Scholar]
- Prigent A, et al. , 2019. Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. J Neurochem 148, 746–760. [DOI] [PubMed] [Google Scholar]
- Quandt SA, et al. , 2017. Olfactory Function in Latino Farmworkers Over 2 Years: Longitudinal Exploration of Subclinical Neurological Effects of Pesticide Exposure. J Occup Environ Med 59, 1148–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt SA, et al. , 2016. Olfactory Function in Latino Farmworkers: Subclinical Neurological Effects of Pesticide Exposure in a Vulnerable Population. J Occup Environ Med 58, 248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette BA, et al. , 2001. Welding-related parkinsonism: clinical features, treatment, and pathophysiology. Neurology 56, 8–13. [DOI] [PubMed] [Google Scholar]
- Rey NL, et al. , 2013. Transfer of human α-synuclein from the olfactory bulb to interconnected brain regions in mice. Acta Neuropathologica 126, 555–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, et al. , 2016. Widespread transneuronal propagation of alpha-synucleinopathy triggered in olfactory bulb mimics prodromal Parkinson’s disease. J Exp Med 213, 1759–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey NL, et al. , 2018. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109, 226–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, et al. , 2016. Traffic-Related Air Pollution and Parkinson’s Disease in Denmark: A Case-Control Study. Environ Health Perspect 124, 351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, Rhodes SL, 2010. After half a century of research on smoking and PD, where do we go now? Neurology 74, 870–1. [DOI] [PubMed] [Google Scholar]
- Rojo AI, et al. , 2007. Chronic inhalation of rotenone or paraquat does not induce Parkinson’s disease symptoms in mice or rats. Experimental Neurology 208, 120–126. [DOI] [PubMed] [Google Scholar]
- Rojo AI, et al. , 2006. Persistent penetration of MPTP through the nasal route induces Parkinson’s disease in mice. The European Journal of Neuroscience 24, 1874–1884. [DOI] [PubMed] [Google Scholar]
- Rokad D, et al. , 2017. Role of neurotoxicants and traumatic brain injury in alpha-synuclein protein misfolding and aggregation. Brain Res Bull 133, 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano S, et al. , 2021. Meta-analysis of the Parkinson’s disease gut microbiome suggests alterations linked to intestinal inflammation. NPJ Parkinsons Dis 7, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross GW, et al. , 2008. Association of olfactory dysfunction with risk for future Parkinson’s disease. Annals of Neurology 63, 167–173. [DOI] [PubMed] [Google Scholar]
- Rozas NS, et al. , 2021. Oral Factors That Impact the Oral Microbiota in Parkinson’s Disease. Microorganisms 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, et al. , 2016. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 167, 1469–1480.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandmark B, et al. , 1989. Olfactory function in painters exposed to organic solvents. Scandinavian Journal of Work, Environment & Health 15, 60–63. [DOI] [PubMed] [Google Scholar]
- Sandoval-Motta S, et al. , 2017. Evolving Ecosystems: Inheritance and Selection in the Light of the Microbiome. Arch Med Res 48, 780–789. [DOI] [PubMed] [Google Scholar]
- Sasajima H, et al. , 2015. Intranasal administration of rotenone in mice attenuated olfactory functions through the lesion of dopaminergic neurons in the olfactory bulb. Neurotoxicology 51, 106–15. [DOI] [PubMed] [Google Scholar]
- Sasajima H, et al. , 2017. Intranasal Administration of Rotenone to Mice Induces Dopaminergic Neurite Degeneration of Dopaminergic Neurons in the Substantia Nigra. Biol Pharm Bull 40, 108–112. [DOI] [PubMed] [Google Scholar]
- Savica R, et al. , 2009. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology 73, 1752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savica R, et al. , 2017. Time trends in the incidence of Parkinson’s disease: a 30-year study. JAMA Neurology 32, 227–34. [Google Scholar]
- Scheperjans F, et al. , 2018. The Gut and Parkinson’s Disease: Hype or Hope? J Parkinsons Dis 8, S31–S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, et al. , 2021. Exposure to Cadmium, Lead, and Tobacco Smoke and the 10-Year Cumulative Incidence of Olfactory Impairment: The Beaver Dam Offspring Study. JAMA Otolaryngol Head Neck Surg 147, 510–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, et al. , 1989. Olfactory function in chemical workers exposed to acrylate and methacrylate vapors. American Journal of Public Health 79, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz BS, et al. , 1990. Solvent-associated decrements in olfactory function in paint manufacturing workers. American Journal of Industrial Medicine 18, 697–706. [DOI] [PubMed] [Google Scholar]
- Schwiertz A, et al. , 2018. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord 50, 104–107. [DOI] [PubMed] [Google Scholar]
- Sengoku R, et al. , 2008. Incidence and extent of Lewy body-related alpha-synucleinopathy in aging human olfactory bulb. Journal of Neuropathology and Experimental Neurology 67, 1072–1083. [DOI] [PubMed] [Google Scholar]
- Sharabi Y, et al. , 2021. Parkinson’s disease outside the brain: targeting the autonomic nervous system. Lancet Neurol 20, 868–876. [DOI] [PubMed] [Google Scholar]
- Sharer JD, et al. , 2015. Olfactory dysfunction in Parkinson’s disease: Positive effect of cigarette smoking. Mov Disord 30, 859–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, et al. , 2018. Factors associated with dream enacting behaviors among US farmers. Parkinsonism Relat Disord 57, 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, et al. , 2019. High Pesticide Exposure Events and Olfactory Impairment among U.S. Farmers. Environ Health Perspect 127, 17005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, et al. , 2021. Occupational pesticide use and self-reported olfactory impairment in US farmers. Occup Environ Med 78, 179–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva YP, et al. , 2020. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne) 11, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Warner SA, et al. , 2006. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 163, 1053–64. [DOI] [PubMed] [Google Scholar]
- Sorokowska A, et al. , 2015. Determinants of human olfactory performance: a cross-cultural study. Sci Total Environ 506–507, 196–200. [DOI] [PubMed] [Google Scholar]
- Sorokowska A, et al. , 2013. Olfaction and environment: Tsimane’ of Bolivian rainforest have lower threshold of odor detection than industrialized German people. PLoS One 8, e69203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski WJ, et al. , 2000. Smell impairment in workers occupationally exposed to cadmium. Acta Oto-Laryngologica 120, 316–318. [DOI] [PubMed] [Google Scholar]
- Sunderman FW, 2001. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Annals of Clinical and Laboratory Science 31, 3–24. [PubMed] [Google Scholar]
- Svensson E, et al. , 2015. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78, 522–9. [DOI] [PubMed] [Google Scholar]
- Toro R, et al. , 2019. Parkinson’s disease and long-term exposure to outdoor air pollution: A matched case-control study in the Netherlands. Environ Int 129, 28–34. [DOI] [PubMed] [Google Scholar]
- Toyoda H, et al. , 2020. Intranasal Administration of Rotenone Reduces GABAergic Inhibition in the Mouse Insular Cortex Leading to Impairment of LTD and Conditioned Taste Aversion Memory. International Journal of Molecular Sciences 22, E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travagli RA, et al. , 2020. Parkinson disease and the gut: new insights into pathogenesis and clinical relevance. Nat Rev Gastroenterol Hepatol 17, 673–685. [DOI] [PubMed] [Google Scholar]
- Tysnes OB, et al. , 2015. Does vagotomy reduce the risk of Parkinson’s disease? Ann Neurol 78, 1011–2. [DOI] [PubMed] [Google Scholar]
- Ulusoy A, et al. , 2017. Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections. Acta Neuropathologica 133, 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A, et al. , 2013. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO molecular medicine 5, 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger MM, et al. , 2016. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32, 66–72. [DOI] [PubMed] [Google Scholar]
- Van Den Berge N, et al. , 2021. Ageing promotes pathological alpha-synuclein propagation and autonomic dysfunction in wild-type rats. Brain 144, 1853–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronkov DN, et al. , 2017. Immunomorphological Changes in the Olfactory Bulbs of Rats after Intranasal Administration of Rotenone. Bull Exp Biol Med 164, 203–206. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, et al. , 1988. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76, 217–221. [DOI] [PubMed] [Google Scholar]
- Willis AW, et al. , 2010. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol 172, 1357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JA, et al. , 2009. Unique copper-induced oligomers mediate alpha-synuclein toxicity. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 23, 2384–2393. [DOI] [PubMed] [Google Scholar]
- Yu IT-S, et al. , 2004. Occupational exposure to mixtures of organic solvents increases the risk of neurological symptoms among printing workers in Hong Kong. Journal of Occupational and Environmental Medicine 46, 323–330. [DOI] [PubMed] [Google Scholar]
- Zhang Z, et al. , 2021. Exposure to Particulate Matter Air Pollution and Anosmia. JAMA Netw Open 4, e2111606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, et al. , 2022. Association between inflammatory bowel diseases and Parkinson’s disease: systematic review and meta-analysis. Neural Regen Res 17, 344–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibrowski EM, Robertson JM, 2006. Olfactory sensitivity in medical laboratory workers occupationally exposed to organic solvent mixtures. Occupational Medicine (Oxford, England) 56, 51–54. [DOI] [PubMed] [Google Scholar]


