Graphical abstract
To the Editor
Coronavirus disease 2019 (COVID-19) is a life-threatening disease, especially for older individuals and people with multiple risk factors. Geriatric environments are therefore at high risk of COVID-19 outbreaks with increased mortality [1]. We describe an outbreak of nosocomial COVID-19 in a long-term care facility (LTCF) in Eastern France, starting 1 month after a double-dose vaccination campaign with the BNT162b2 mRNA vaccine.
The 93 residents of the LTCF included 66 females and 27 males with a median age of 88 years (range 63–99 years), none of them immunocompromised. Seventy residents (75.3%) and 38 HCWs (52.1%) were fully vaccinated with two doses of the BNT162b2 mRNA vaccine on 25th January and 15th February 2021. Among the other 23 residents (24.7%) not vaccinated at the beginning of the outbreak, 11 were not vaccinated due to the residents' decision, two received one dose of vaccine, and ten received two doses on 9th April and 30th April each during the outbreak.
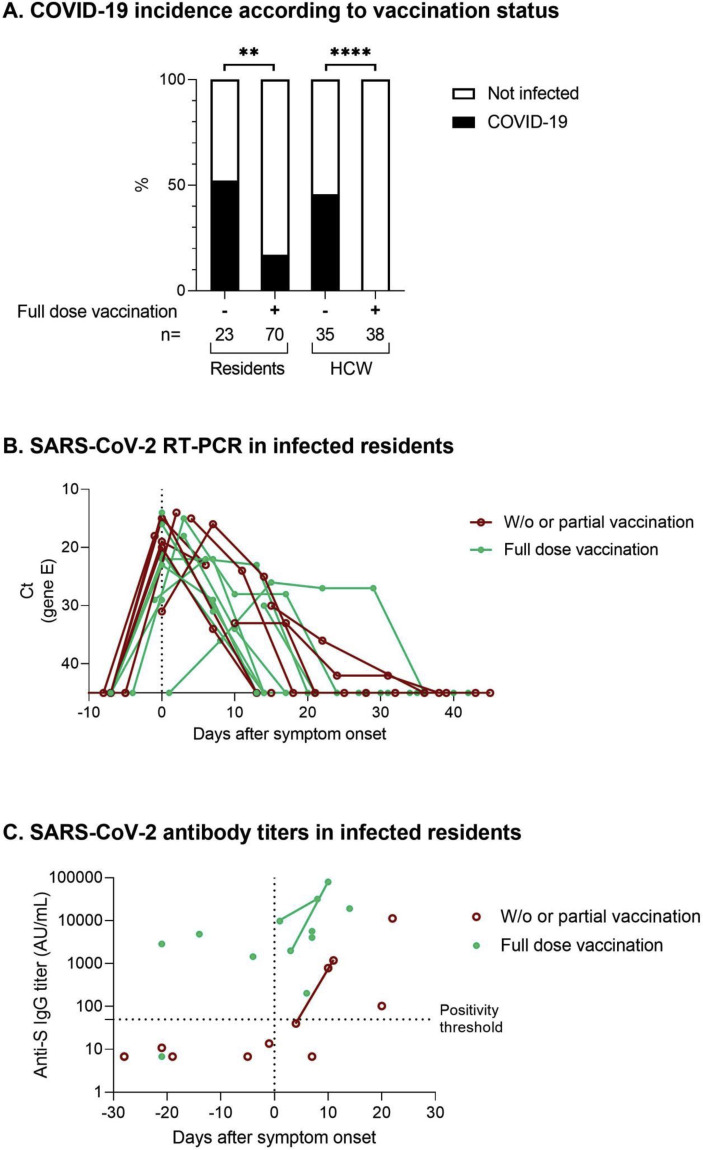
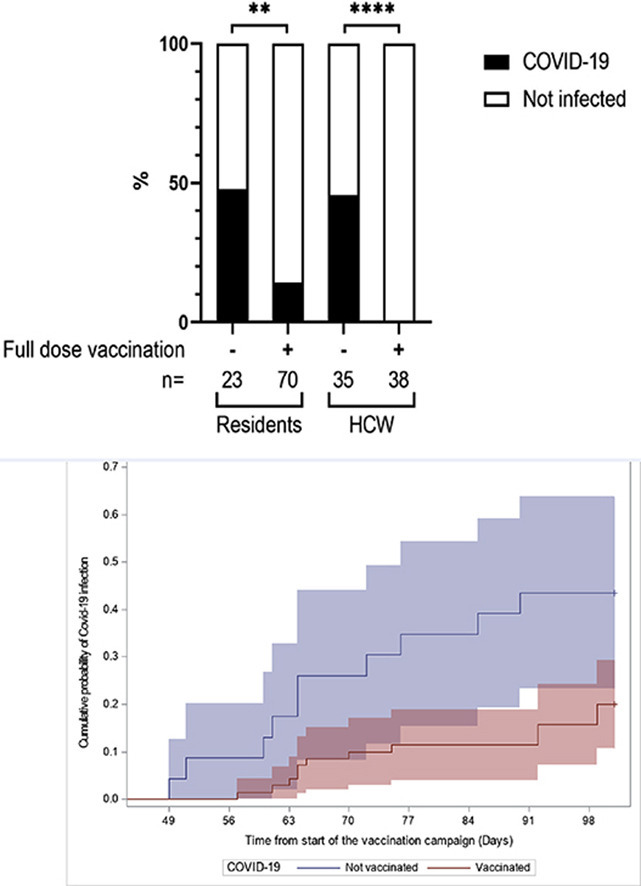
Between 15th March and 6th May 2021, 40 subjects tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection using either rapid antigen detection (n = 18) (Biosynex COVID-19 BSS IgG/IgM) and/or real-time reverse transcriptase PCR (RT-PCR) tests (Biosynex) (n = 22) on nasopharyngeal swabs. These COVID-19 cases included 24/93 residents (25.8%) and 16/73 healthcare workers (HCWs) (21.9%) (Fig. 1 ). Positive residents were older and more frequently male than their uninfected counterparts: age 91 years (range 72–99 years) and 87 years (range 63–97 years), and of these 10/24 (41.7%) and 17/69 (24.66%) were male, respectively. COVID-19 cases were more frequent among residents who were not fully vaccinated (12/23, 52.2%) than among fully vaccinated residents (12/70, 17.1%). Residents who were vaccinated versus those not fully vaccinated were asymptomatic (four versus two) or displayed mild (four versus three), moderate (four versus four) or severe (none versus three) symptoms. One unvaccinated 83-year-old' woman died 1 month after being hospitalized, and a 98-year-old fully vaccinated female resident died of sudden death.
Fig. 1.
Outbreak epidemiological and biological characterization according to vaccination status. (A) Incidence of coronavirus disease 2019 (COVID-19) among residents and healthcare workers (HCWs) in the long-term healthcare facility according to vaccination status. (B) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RT-PCR was performed on nasopharyngeal swabs at least once in 21 of the 24 SARS-CoV-2-positive residents. The cycle thresholds (Ct) corresponding to the amplification target on the envelope (E) gene are represented according to the delay after symptom onset. (C) Quantitative measurements of IgG directed against the receptor binding domain of the spike protein (S) are represented for 21 of the 24 SARS-CoV-2-positive residents who gave their consent for this analysis. All had a serology carried out on 6th April 2021, and the same analysis was conducted 1 week earlier in three COVID-19 residents (1–4 days after symptom onset). Serology results were represented according to the delay after symptom onset. The vaccination status of residents against SARS-CoV-2 before the outbreak is depicted with red circles for unvaccinated or partially vaccinated residents and green dots for those who received two doses of the vaccine.
Variant screening by RT-PCR (Seegene Inc., Seoul, Korea) and Sanger sequencing of the spike protein gene identified the SARS-CoV-2 α variant in all positive samples. Quantitative measurement of anti-spike (S) IgG (Abbott SARS-CoV-2 IgG II Quant assay) was carried out for 79 residents on 6th April, including 62 fully vaccinated residents who all except one displayed positive serology. Three vaccinated residents were infected after that date despite previous high anti-S IgG titres (1452, 4842 and 2861 AU/mL, respectively) (Fig. 1).
To assess the effectiveness of the BNT162b2 mRNA vaccine, we conducted a time-to-event analysis using a multivariate proportional hazard Cox model with vaccination status, age, and gender as covariates. The hazard of developing a SARS-CoV-2 infection was, for fully vaccinated residents, 0.32 times that of subjects who were not fully vaccinated (95%CI 0.14–0.73, p 0.006). Male residents were at significantly higher risk of developing COVID-19 (HR 2.79, 95%CI 1.18–6.59, p 0.02), as were older subjects with an HR of 1.62 for a 5-year increase in age (95%CI 1.16–2.27, p 0.005). The adjusted hazard ratio (HR) for fully versus not fully vaccinated residents was used to estimate the vaccine effectiveness ((1-HR) x100) at 68% (95%CI 27–86%).
Sixteen unvaccinated HCWs (16/35, 45.7%; median age 35 years, r 19–59) tested positive for COVID-19 with mild to moderate symptoms, while all the vaccinated HCWs were COVID-19-negative (p < 0.0001) (Fig. 1).
Our results confirm that the BNT162b2 mRNA vaccine was highly effective in preventing COVID-19 infection. Residents who were not fully vaccinated were three times more likely to develop COVID-19. The protective effect of the vaccine is also underscored by the fact that none of the vaccinated residents developed severe COVID-19, whereas this was the case for three residents who were not fully vaccinated. Most of the vaccinated residents had anti-S-IgG at the time of the outbreak. Several data on vaccination report the effectiveness of a two-dose regimen with BNT162b2 mRNA vaccine in preventing COVID-19, both in the general population and in older people, including those infected by the α variant [2]. Our study confirms the protective effect of the vaccine but underscores that elderly people, despite being vaccinated and displaying positive anti-S IgG up to 4842 AU/mL, are at greater risk of SARS-CoV-2 infection. In our cohort, the vaccine effectiveness (68%) appears lower than the 95% previously reported by Polack et al. [3], possibly related to the age of the LTCF patients reported with natural immunity which, although effective in preventing COVID-19, is lower in older people [4].
This outbreak highlights the critical importance of a high rate of vaccination of residents and HCWs.
Author contributions
MM, SC and SFK conceived the study. All authors contributed to design of the study.CK performed the statistical analysis. MM, FG, CK and SFK wrote the manuscript. All authors critically revised the manuscript and approved its final version.
Transparency declaration
The authors have no conflicts of interests to disclose. No external funding was received for this work.
Ethical statement
The informed non-opposition to the use of medical data was obtained from all residents or from relatives responsible for legal matters. This study was approved by the local ethics committee of the University of Strasbourg in France (ClinicalTrials.gov Identifier: NCT04405726).
Acknowledgements
The authors thank Lionel Barrand, Aurelie Velay, Marie-Josée Wendling and Christian Guth for their help in collecting data.
Editor: L. Leibovici
References
- 1.Hamilton W.L., Tonkin-Hill G., Smith E.R., Aggarwal D., Houldcroft C.J., Warne B., et al. Genomic epidemiology of COVID-19 in care homes in the east of England. Elife. 2021;10 doi: 10.7554/eLife.64618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA COVID-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen C.H., Michlmayr D., Gubbels S.M., Molbak K., Ethelberg S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: a population-level observational study. Lancet. 2021;397:1204–1212. doi: 10.1016/S0140-6736(21)00575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]