Abstract
Introduction
Few randomised controlled trials (RCTs) have directly compared long-acting muscarinic antagonist/long-acting β2-agonist (LAMA/LABA) dual maintenance therapies for patients with chronic obstructive pulmonary disease (COPD). This systematic literature review and network meta-analysis (NMA) compared the efficacy of umeclidinium/vilanterol (UMEC/VI) versus other dual and mono-bronchodilator therapies in symptomatic patients with COPD.
Methods
A systematic literature review (October 2015–November 2020) was performed to identify RCTs ≥ 8 weeks long in adult patients with COPD that compared LAMA/LABA combinations against any long-acting bronchodilator-containing dual therapy or monotherapy. Data extracted on changes from baseline in trough forced expiratory volume in 1 s (FEV1), St George’s Respiratory Questionnaire (SGRQ) total score, Transitional Dyspnoea Index (TDI) focal score, rescue medication use and moderate/severe exacerbation rate were analysed using an NMA in a frequentist framework. The primary comparison was at 24 weeks. Fixed effects model results are presented.
Results
The NMA included 69 full-length publications (including 10 GSK clinical study reports) reporting 49 studies. At 24 weeks, UMEC/VI provided statistically significant greater improvements in FEV1 versus all dual therapy and monotherapy comparators. UMEC/VI provided similar improvements in SGRQ total score compared with all other LAMA/LABAs, and significantly greater improvements versus UMEC 125 μg, glycopyrronium 50 μg, glycopyrronium 18 μg, tiotropium 18 μg and salmeterol 50 μg. UMEC/VI also provided significantly better outcomes versus some comparators for TDI focal score, rescue medication use, annualised moderate/severe exacerbation rate, and time to first moderate/severe exacerbation.
Conclusion
UMEC/VI provided generally better outcomes compared with LAMA or LABA monotherapies, and consistent improvements in lung function (measured by change from baseline in trough FEV1 at 24 weeks) versus dual therapies. Treatment with UMEC/VI may improve outcomes for symptomatic patients with COPD compared with alternative maintenance treatments.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02234-x.
Keywords: Dual bronchodilators, Dual inhaler therapy, COPD, LABA, LAMA, Network meta-analysis
Plain Language Summary
Bronchodilators are medicines that open the airways, allowing patients with chronic obstructive pulmonary disease (COPD) to breathe more easily. There are two different types of bronchodilators, namely long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs), which can be used on their own or combined (LAMA/LABAs). Only a few clinical trials have compared different LAMA/LABA combinations with each other, so it is unclear which LAMA/LABA combination provides the greatest benefits for patients.
In this study, we used network meta-analysis to compare a LAMA/LABA combination medicine called umeclidinium and vilanterol (UMEC/VI) with other LAMAs and LABAs used alone or in combination to treat patients with COPD. Network meta-analysis is a way of comparing two or more medicines by analysing data from many studies. We systematically searched for evidence from clinical trials in adult patients with COPD that were at least 8 weeks long and that compared LAMA/LABA combinations with a LAMA, a LABA, or another LAMA/LABA combination. We analysed data from 49 clinical trials that met these criteria.
We found that patients treated with UMEC/VI had better lung function than patients treated with alternative LAMA/LABA combinations or bronchodilators used on their own. Patients treated with UMEC/VI had better quality of life than those receiving some other treatments, but not all. All the medicines we compared had similar side effects.
Our results suggest that treating patients with COPD with UMEC/VI might improve their lung function and quality of life more than alternative bronchodilators.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02234-x.
Key Summary Points
| Why carry out this study? |
| Long-acting bronchodilators are the mainstay of maintenance therapy for patients with chronic obstructive pulmonary disease (COPD), but there is no consensus on the timing of treatment initiation with dual therapy with a long-acting muscarinic antagonist and a long-acting β2-agonist (LAMA/LABA) combination versus monotherapy with a LAMA or a LABA. |
| Several dual bronchodilator therapies are available for COPD treatment; however, only a few head-to-head randomised controlled trials have compared outcomes between dual bronchodilator therapies, and these may have been affected by differences in inhaler devices. |
| This network meta-analysis investigated the relative efficacy of umeclidinium/vilanterol (UMEC/VI) versus other dual bronchodilator combinations and LAMA and LABA monotherapies with reduced confounding due to inhaler device differences. |
| What was learned from the study? |
| UMEC/VI dual therapy provided better outcomes in terms of lung function (as measured by change from baseline in trough FEV1) compared with alternative dual therapies and monotherapies, as well as improvements in health-related quality of life, symptoms, rescue medication use, moderate/severe exacerbation rates, and time to first moderate/severe exacerbation compared with monotherapies. |
| These results suggest that treatment with UMEC/VI may improve outcomes for symptomatic patients with COPD compared with alternative dual and monotherapies. |
Introduction
Long-acting bronchodilators are the mainstay of maintenance therapy for patients with chronic obstructive pulmonary disease (COPD). However, treatment guidelines provide different recommendations on when to initiate long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) dual therapy rather than LAMA or LABA monotherapy. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report typically recommends a stepwise approach with escalation to LAMA/LABA dual therapy for patients who have persistent dyspnoea or exacerbations on LAMA or LABA monotherapy, although LAMA/LABA dual therapy can also be considered as an initial maintenance therapy option for patients with severe symptoms [1]. In contrast, the American Thoracic Society (ATS) and the UK National Institute for Health and Care Excellence (NICE) guidelines recommend initiating treatment with LAMA/LABA dual therapy for all patients with dyspnoea [2, 3].
Previous meta-analyses of clinical trial data comparing dual and mono-bronchodilator therapies have shown that treatment with LAMA/LABA combinations provide better outcomes for patients than LAMA or LABA monotherapy [4–9]. All currently available LAMA/LABA combinations provided greater improvements in lung function, health status and breathlessness compared with monotherapies [4–6, 9]. However, further evidence from large randomised controlled trials (RCTs) evaluating efficacy and safety of LAMA/LABA dual therapy versus monotherapy is now available, so there is a need for updated analyses to incorporate these findings.
The relative differences in safety and efficacy between available LAMA/LABA treatments within the dual bronchodilator class also remain unclear. There is a need to identify whether there is a difference between bronchodilators in the benefits they produce across different outcomes to help guide treatment decisions. Previous meta-analyses showed a gradient of effectiveness between LAMA/LABA fixed-dose combinations in patients with moderate-to-severe COPD based on the evidence available in 2015 [4, 6]. Although within-class RCTs comparing outcomes between LAMA/LABA dual therapies remain limited, recent evidence may help to clarify the relative benefits of different LAMA/LABA combinations.
An updated synthesis of recent and previously available evidence is therefore needed to understand the potential advantages of starting treatment with LAMA/LABA dual therapy versus LABA or LAMA monotherapy, and which treatments provide the best outcomes within the LAMA/LABA dual therapy class. The aim of this network meta-analysis (NMA) was to investigate whether treatment with umeclidinium/vilanterol (UMEC/VI) provides better outcomes than (a) monotherapy with LABA or LAMA, and (b) other dual bronchodilator combinations in symptomatic patients with moderate-to-severe COPD.
Methods
Systematic Literature Review Rationale
The systematic literature review (SLR) methodology was consistent with recommendations published in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement, as well as guidance provided by the Centre for Reviews and Dissemination and the Cochrane Collaboration [10, 11].
A previously published SLR identified publications up to October 2015 reporting RCTs in patients with COPD that compared selected maintenance therapies, including LAMA and LABA monotherapies and LAMA/LABA dual therapies, to inform an NMA [6]. An update to this search was conducted to identify publications between October 2015 and November 2020 reporting studies in which any LAMA, LABA or LABA/LAMA either as a monotherapy or as a combination with inhaled corticosteroids (ICS; dual therapy) were compared with LABA/LAMA dual therapy (fixed-dose or open combination). Studies with an ICS/LABA treatment arm were used to construct the networks, but were excluded from the comparisons.
Search Strategy
The systematic bibliographic searches were conducted between 2 October 2015 and 19 November 2020 using the Ovid Platform in the following databases: MEDLINE and MEDLINE In-Process, EMBASE, The Cochrane Library: Cochrane Database of Systematic Review (CDSR) and Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA) websites, HTA database and the National Institute for Health Research (NIHR). To complement the evidence from the bibliographic databases, a secondary systematic searches were performed in clinical trial registries including Clinicaltrials.gov (https://clinicaltrials.gov/ct2/search/advanced), the US National Institutes of Health clinical trial register; World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; http://apps.wo.int/trialsearch/AdvSearch.aspx); ISRCTN registry Clinical Trials Register (ISRCTN; http://www.controlled-trials.com/editAdvancedSearch); Klinische Prüfungen PharmNet.Bund (http://www.pharmnet-bund.de/dynamic/de/klinische-pruefungen/index.htm); the International Prospective Register of Systematic Reviews (PROSPERO; https://www.crd.york.ac.uk/prospero/#searchadvanced); National Institute for Health Research—Health Technology Assessment (NIHR HTA; http://www.nets.nihr.ac.uk/projects) and EU Clinical Trials Register (EU-CTR; www.clinicaltrialsregister.eu). The search was restricted to articles written in English or German.
Inclusion Criteria and Study Selection Process
The outcomes included in the SLR are listed in Table 1. For all outcomes evaluated at 12 and 24 weeks, an extended margin of time was permitted to strengthen the networks. If the endpoint of interest was not reported at 12 or 24 weeks, but instead between 8 and 16 weeks and 20 and 28 weeks, the proxy outcome was reported. The primary outcome was trough FEV1 at 24 weeks.
Table 1.
PICOS criteria
| Population | Adult patients with COPD |
|---|---|
| Intervention |
Dual therapies: •UMEC/VI 62.5/25 µg OD •ACL/FOR 400/12 µg or 400/6 µg •GLY/FOR 18/9.6 µg •IND/GLY 27.5/15.6 µg or 110/50 µg or 150/50 µg •TIO/OLO 2.5/5 µg or 5/5 µg •TIO 18 µg + FOR 10 µg or TIO 18 µg + FOR 12 µg •TIO 18 µg + IND 150 µg •Any other combination of a LABA and LAMA LAMA monotherapies: •UMEC 62.5 µg or 125 µg OD •ACL 400 µg BID •TIO 5 µg or 18 µg OD •GLY 15.6 µg or 18 µg or 50 µg OD LABA monotherapies: •SAL 50 µg BID •FOR 9.6 µg or 10 µg or 12 µg BID •IND 25.5 µg or 75 µg or 150 µg or 300 µg OD •OLO 5 µg or 10 µg OD |
| Comparator | Any comparison between the interventions of interest (including combination therapies) or of those interventions with placebo, as long as one arm receiving a LABA/LAMA dual therapy was included in the study |
| Outcomes |
•Trough FEV1 •Post-bronchodilator FEV1 •SGRQ total score •Proportion of patients with an improvement of at least 4 units in SGRQ total score (responder analysis) •TDI focal score •Proportion of patients with an improvement of at least 1 unit in TDI score (responder analysis) •Rescue medication use (including SABAs and ICS) •Rate of exacerbations per patient-year over the trial period across definitions •Proportion of patients experiencing at least one exacerbation (across definitions) at the end of the study •Time to first moderate/severe exacerbation |
| Study design | RCTs with a duration ≥ 8 weeks |
ACL aclidinium; BID twice daily; COPD chronic obstructive pulmonary disease; FEV1 forced expiratory volume in 1 s; FOR formoterol; ICS inhaled corticosteroid; IND indacaterol; GLY glycopyrronium; LABA long-acting β2-agonist; LAMA long-acting muscarinic antagonist; OD once daily; OLO olodaterol; PICOS population, intervention, comparator, outcomes, and study design; RCT randomised controlled trial; SABA short-acting β2-agonist; SAL salmeterol; SGRQ St George’s Respiratory Questionnaire; TDI Transitional Dyspnoea Index; TIO tiotropium; UMEC umeclidinium; VI vilanterol
All abstracts and full articles were reviewed against the eligibility criteria by two systematic reviewers; disagreements were referred to a third reviewer and an agreement was reached. A PRISMA flow diagram, indicating the numbers of studies included and excluded at each stage of the review, is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram of study selection process. CSR clinical study report, NMA network meta-analysis, SLR systematic literature review
Quality Assessment
The Cochrane Collaboration’s tool was used to assess risk of bias at study level. The scored items were extracted in the data extraction form.
Network Meta-Analysis
In traditional pairwise meta-analysis, all included studies compare the same intervention with the same comparator. NMA is an extension of this approach that includes multiple different pairwise comparisons across a range of different interventions (Supplementary Methods) [12–14]. For the current study, all analyses were conducted using a frequentist weighted regression-based approach as previously described [15]. The efficacy outcomes were lung function (trough forced expiratory volume in 1 s [FEV1]), health-related quality of life (HRQoL; St George’s Respiratory Questionnaire [SGRQ] total score), breathlessness (Transitional Dyspnoea Index [TDI] focal score), rescue medication use, moderate/severe exacerbation rate and time to first moderate/severe exacerbation. For safety outcomes, analyses were not feasible because of differences in outcome definitions. Both fixed effects (FE) and random effects (RE) models were used; in the absence of heterogeneity, results from both models were identical. Where heterogeneity was present, the RE models automatically accounted for this; RE model results are presented in the supplementary material. Networks were stratified by observation time horizon (12 and 24 weeks) depending on data availability and the primary comparison was at 24 weeks. Results from the frequentist approach are presented as point estimates with a 95% confidence interval (CI). Point estimates and 95% CIs are presented in figures and tables; only statistically significant results at 24 weeks have been reported in the text. The frequentist regression-based NMA was conducted using R v4.1.2 (www.r-project.org), using the netmeta package [16]. Further details are provided in the Supplementary Methods.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Results
Studies and Patient Characteristics
In the SLR and its update, a total of 6847 abstracts were reviewed following the removal of duplicates. Of those, 753 full length publications were assessed for inclusion and data were extracted from 83 publications and 13 GSK clinical study reports (CSRs). In total, 96 publications reporting 61 studies were included in the SLR. Following assessment of the feasibility of including studies identified in the SLR, the NMA included 69 publications (including 10 GSK CSRs) reporting 49 different studies (Fig. 1).
The characteristics of the studies included in the NMA are shown in Table 2; the characteristics of patients included in these studies are shown in Table 3.
Table 2.
Study characteristics of the trials included in the NMA
| Author, year | Study name, NCT number | Treatment | ITT/study duration, weeks | Study design | Study phase | Crossover |
|---|---|---|---|---|---|---|
| Lipworth, 2018 [30] | PINNACLE-4, NCT02343458 |
GLY/FOR (MDI) 18/9.6 GLY 18 FOR 9.6 PBO |
24 | RCT, MC, DB, PG, PC | 3 | No |
| Singh, 2015 [31] | OTEMTO 1, NCT01964352 |
TIO/OLO 5/5 TIO/OLO 2.5/5 TIO 5 PBO |
12 | RCT, MC, DB, PC | 3 | No |
| Singh, 2015 [31] | OTEMTO 2, NCT02006732 |
TIO/OLO 5/5 TIO/OLO 2.5/5 TIO 5 PBO |
12 | RCT, MC, DB, PC | 3 | No |
| Vogelmeier, 2008 [32] | NCT00134979 |
FOR 10 TIO 18 TIO 18 + FOR 10 PBO |
24 | RCT, MC, DB (TIO open label), AC | 4 | No |
| Maleki-Yazdi, 2014 [33] | ZEP117115, NCT01777334 |
UMEC/VI 62.5/25 TIO 18 |
24 | RCT, MC, DB, DD, PG | 3 | No |
| Calverley, 2018 [34] | DYNAGITO, NCT02296138 |
TIO/OLO 5/5 TIO 5 |
52 | RCT, MC, MN, DB, PG, AC | 3 | No |
| Kerwin, 2017 [35] | A2349, NCT02487446 | IND/GLY 27.5/15.6 BID | 12 | RCT, MC, DB, DD, AC, 2-period crossover | 3 | Yes, at 12 weeks |
| Kerwin, 2017 [35] | A2350, NCT02487498 |
IND/GLY 27.5/15.6 BID UMEC/VI 62.5/25 |
12 | RCT, MC, DB, DD, AC, 2-period crossover | 3 | Yes, at 12 weeks |
| Maltais, 2019 [22] | EMAX, NCT03034915 |
UMEC/VI 62.5/25 UMEC 62.5 SAL 50 |
24 | RCT, MC, MN, DB, DD, PG | 3 | No |
| Feldman, 2017 [25] | GSK 204990, NCT02799784 |
UMEC/VI 62.5/25 TIO/OLO 5/5 |
8 | RCT, MC, open label, two-period crossover study | 4 | Yes, at 8 weeks |
| Kalberg, 2016 [36] | GSK 116961, NCT02257385 |
UMEC/VI 62.5/25 TIO 18 + IND 150 |
12 | RCT, MC, DB, triple dummy | 3 | No |
| Riley, 2018 [37] | GSK 201317, NCT02275052 |
UMEC/VI 62.5/25 PBO |
12 | RCT, MC, DB, PC, crossover | 4 | Yes, at 12 weeks |
| Mahler, 2012 [38] | INTRUST-1, NCT00846586 |
TIO 18 + IND 150 TIO 18 |
12 | RCT, MC, DB, PG | 3 | No |
| Mahler, 2012 [38] | INTRUST-2, NCT00877383 |
TIO 18 + IND 150 TIO 18 |
12 | RCT, MC, DB, PG | 3 | No |
| Vincken, 2014 [39] | GLOW6, NCT01604278 |
IND 150 + GLY 50 IND 150 |
12 | RCT, MC, DB, PG | 3 | No |
| Wedzicha, 2013 [40] | SPARK, NCT01120691 |
IND/GLY 110/50 GLY 50 TIO 18 |
64 | RCT, MC, DB, PG | 3 | No |
| GSK CSRa | DB2113374, NCT01316913 |
UMEC 125 UMEC/VI 62.5/25 UMEC/VI 125/25 TIO 18 |
24 | RCT, MC, DB, DD, PG | 3 | No |
| GSK CSRa | DB2113360, NCT01316900 |
UMEC 125 UMEC/VI 62.5/25 UMEC/VI 125/25 TIO 18 |
24 | RCT, MC, DB, DD, PG | 3 | No |
| GSK CSRa | DB2113373, NCT01313650 |
UMEC 125 UMEC/VI 62.5/25 UMEC/VI 125/25 TIO 18 |
24 | RCT, MC, DB, PC | 3 | No |
| Vogelmeier, 2016 [41] | AFFIRM, NCT01908140 |
ACL/FOR 400/12 SAL/FP 50/500 |
24 | RCT, MC, DB | 3b | No |
| Wedzicha, 2016 [42] | FLAME, NCT01782326 |
IND/GLY 110/50 SAL/FF 50/500 |
52 | RCT, MC, DB | 3 | No |
| Maltais, 2019 [24] | AERISTO, NCT03162055 |
GLY/FOR 18/9.6 UMEC/VI 62.5/25 |
24 | RCT, MC, DB, double dummy | 3b | No |
| Sethi, 2019 [43] | AMPLIFY, NCT02796677 |
ACL/FOR 400/12 ACL 400 FOR 12 TIO 18 |
24 | RCT, DB, DD, PG | 3 | No |
| D'Urzo, 2014 [44] | AUGMENT, NCT01437397 |
ACL/FOR 400/12 ACL/FOR 400/6 ACL 400 FOR 12 PBO |
24 | RCT, MC, DB, PC | 3 | No |
| D'Urzo, 2017 [45]b | AUGMENT EXTENSION, NCT01572792 |
ACL/FOR 400/12 ACL/FOR 400/6 ACL 400 FOR 12 PBO |
28 | RCT, MC, DB, PC, long-term extension | 3 | No |
| Ferguson, 2016 [46] | FLIGHT3, NCT01682863 |
IND/GLY 27.5/15.6 BID IND/GLY 27.5/31.2 BID IND 75 |
52 | RCT, MC, DB | 3 | No |
| Mahler, 2015 [47] | FLIGHT1, NCT01727141 |
IND/GLY 27.5/15.6 BID IND 27.5 GLY 15.6 PBO |
12 | RCT, MC, DB, PC, PG | 3 | No |
| Mahler, 2015 [47] | FLIGHT2, NCT0171251 |
IND/GLY 27.5/15.6 BID IND 27.5 GLY 15.6 PBO |
12 | RCT, MC, DB, PC, PG | 3 | No |
| Siler, 2016 [48] | 201211, NCT02152605 |
UMEC/VI 62.5/25 PBO |
12 | RCT, MC, DB, PC | 3 | No |
| Kerwin, 2017 [49] | DB2116960, NCT01899742 | UMEC/VI 62.5/25 | 12 | RCT, MC, DB, DD | 3 | No |
| Donohue, 2016 [50] | NCT01437540 |
ACL/FOR 400/12 FOR 12 |
52 | RCT, MC, DB, AC | 3 | No |
| Martinez, 2017 [51] | PINNACLE-1, NCT01854645 |
GLY/FOR 18/9.6 GLY 18 FOR 9.6 PBO TIO 18 |
24 | RCT, MC, DB, PC | 3 | No |
| Martinez, 2017 [51] | PINNACLE-2, NCT01854658 |
GLY/FOR 18/9.6 GLY 18 FOR 9.6 PBO TIO 18 |
24 | RCT, MC, DB, PC | 3 | No |
| Bateman, 2013 [18] | SHINE, NCT01202188 | IND/GLY 110/50 | 26 | RCT, MC, DB, PG, PC, AC | 3 | No |
| Buhl, 2015 [52] | QUANTIFY, NCT01120717 |
IND/GLY 110/50 TIO 18 + FOR 12 |
26 | RCT, MC, DB, AC, PG, triple dummy | 3 | No |
| Tashkin, 2009 [53] | NR |
TIO 18 + FOR 12 TIO 18 |
12 | RCT, MC, DB (TIO open label), AC | NR | No |
| Frith, 2018 [54] | FLASH, NCT02516592 |
IND/GLY 110/50 SAL/FF 50/500 |
12 | RCT, MC, DB, DD, AC | 4 | No |
| Celli, 2014 [55] | NCT01313637 |
UMEC/VI 125/25 VI 25 UMEC 125 PBO |
24 | RCT, MC, DB, AC, PC | 3 | No |
| Singh, 2015 [56] | DB2116134, NCT01822899 |
UMEC/VI 62.5/25 SAL/FP 50/500 |
12 | RCT, MC, DB, PC | 3b | No |
| Donohue, 2015 [57] | DB2114930, NCT01817764 |
UMEC/VI 62.5/25 SAL/FP 50/250 |
12 | RCT, MC, DB, DD | 3 | No |
| Donohue, 2015 [57] | DB2114951, NCT01879410 |
UMEC/VI 62.5/25 SAL/FP 50/250 |
12 | RCT, MC, DB, DD | 3 | No |
| Vogelmeier, 2013 [58] | ILLUMINATE NCT01315249 |
IND/GLY 110/50 SAL/FF 50/500 |
26 | RCT, MC, DB, PC | 3 | No |
| Zhong, 2015 [59] | LANTERN, NCT01709903 | IND/GLY 110/50 | 26 | RCT, MC, DB, DD | 3 | No |
| Hoshino, 2015 [60] | NR |
TIO 18 + IND 150 SAL/FF 50/250 |
16 | RCT, open label | _ | No |
| Singh, 2014 [61] | ACLIFORM-COPD, NCT01462942 |
ACL/FOR 400/12 ACL/FOR 400/6 ACL 400 FOR 12 PBO |
24 | RCT, MC, DB, PG | 3 | No |
| ZuWallack, 2014 [62] | ANHELTO 1, NCT01694771 |
TIO 18 TIO 18 + OLO 5 |
12 | RCT, MC, DB, AC, PG | 3 | No |
| ZuWallack, 2014 [62] | ANHELTO 2, NCT01696058 |
TIO 18 TIO 18 + OLO 5 |
12 | RCT, MC, DB, AC, PG | 3 | No |
| Dahl, 2013 [63] | ENLIGHTEN, NCT01120717 |
IND/GLY 110/50 PBO |
52 | RCT, MC, DB, PC | 3 | No |
| Buhl, 2015b [19] | TONADO 1, NCT01431274 |
OLO 5 TIO 2.5 TIO 5 TIO/OLO 2.5/5 TIO/OLO 5/5 |
52 | RCT, MC, DB, PG | 3 | No |
| Buhl, 2015b [19] | TONADO 2, NCT01431287 |
OLO 5 TIO 2.5 TIO 5 TIO/OLO 2.5/5 TIO/OLO 5/5 |
52 | RCT, MC, DB, PG | 3 | No |
| Author, year | Simplified inclusion criteria | Primary outcomes | Permitted background treatments | Prohibited background treatments | ICS allowed |
|---|---|---|---|---|---|
| Lipworth, 2018 [30] | Age ≥ 40 years; FEV 1/FVC < 0.70 predicted and FEV1 of ≤ 80%; diagnosed with COPD; smoking history of > 10 pack-years | FEV1 | Sponsor-provided ipratropium bromide (administered four times daily) and albuterol sulfate (as rescue), ICS monotherapy | Oral β2-agonists, LABAs, cromoglycate or nedocromil inhalers, leukotriene antagonists, ketotifen (except as eye drops), and LAMAs | Yes |
| Singh, 2015 [31] | Age ≥ 40 years; moderate-to-severe COPD (GOLD stage 2–3); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; FEV1/FVC < 70% predicted; smoking history of > 10 pack-years | SGRQ | Open-label salbutamol (as rescue); continued ICS therapy if patients were on a stable dose for 6 weeks prior to screening |
LAMAs or LABAs other than study medication SAMAs were permitted only during the screening period |
Yes |
| Singh, 2015 [31] | Age ≥ 40 years; moderate-to-severe COPD (GOLD stage 2–3); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; FEV1/FVC < 70% predicted; smoking history of > 10 pack-years | SGRQ | Open-label salbutamol (as rescue); continued ICS therapy if patients were on a stable dose for 6 weeks prior to screening | LAMAs or LABAs other than study medication; SAMAs were permitted only during the screening period | Yes |
| Vogelmeier, 2008 [32] | Age ≥ 40 years at COPD onset; stable COPD; FEV1 < 70% of patient’s predicted normal value (and ≥ 1.00 L); FEV1/FVC < 70%; smoking history ≥ 10 pack-years | FEV1 | Salbutamol (as rescue), ICS monotherapy | Not specified | Yes |
| Maleki-Yazdi, 2014 [33] | Outpatient; ≥ 40 years of age; diagnosed with COPD, post-salbutamol FEV1 ≤ 70% and post-salbutamol FEV1/FVC ratio < 0.7. Smoking history ≥ 10 pack-years | FEV1 | ICS (dose ≤ 1000 μg/day of FP or equivalent), salbutamol (as rescue) | LABAs, ICS/LABA combination products, oral SABAs and LABAs, inhaled SABAs, inhaled short-acting anticholinergics, and ICS/SABA combinations | Yes |
| Calverley, 2018 [34] | Age ≥ 40 years; postbronchodilator FEV1/FVC < 0.70 and postbronchodilator FEV1 ≤ 60% predicted; history of at least one moderate or severe exacerbation in the preceding year requiring treatment with systemic corticosteroids or antibiotics or both, with or without hospitalisation; smoking history of > 10 pack-years | COPD exacerbations rate | ICS monotherapy, open-label salbutamol (as rescue) | Other short-acting β2-agonists, LAMAs, and LABAs (other than the study medication) | Yes |
| Kerwin, 2017 [35] | Age ≥ 40 years; diagnosis of COPD (according to the GOLD 2015 criteria); moderate-to-severe airflow limitation; smoking history of > 10 pack-years | FEV1 | ICS monotherapy, albuterol (as rescue) | ICS/LABA | Yes |
| Kerwin, 2017 [35] | Age ≥ 40 years; diagnosis of COPD (according to the GOLD 2015 criteria); moderate-to-severe airflow limitation; smoking history of > 10 pack-years | FEV1 | ICS monotherapy, albuterol (as rescue) | ICS/LABA | Yes |
| Maltais, 2019 [22] | Age ≥ 40 years; diagnosis of COPD (ATS/ERS definition), pre- and post-salbutamol FEV1/FVC ratio < 0.7, post-salbutamol FEV1 of ≥ 30 to ≤ 80% predicted, CAT score ≥ 10, with ≤ 1 moderate exacerbation and no severe exacerbations in the previous year; smoking history of > 10 pack-years | FEV1 | Before screening and during the 4-week run-in period, bronchodilator maintenance therapy was limited to a LAMA or LABA; as-needed salbutamol was allowed throughout all study phases | All patients were required to be ICS and ICS/LABA free for ≥ 6 weeks and LAMA/LABA free for ≥ 2 weeks prior to run-in | No |
| Feldman, 2017 [25] | Age ≥ 40 years; diagnosis of COPD (ATS/ERS definition), pre- and post-albuterol/salbutamol FEV1/FVC ratio < 0.70, post albuterol ≤ 70% and ≥ 50% predicted; mMRC grade scale score ≥ 2; smoking history of > 10 pack-years | FEV1 | Albuterol (as rescue), short-acting inhaled muscarinic antagonists, mucolytics, rhinitis medications, influenza vaccine, pneumonia vaccine, antibiotics, systemic corticosteroids, oxygen, localised corticosteroid injections, immunotherapy injections, topical or ophthalmic corticosteroids | Not specified | No |
| Kalberg, 2016 [36] | Age ≥ 40 years; established COPD (in accordance with the ATS/ERS criteria); pre- and post-bronchodilator FEV1/FVC ratio < 0.7 and a post-bronchodilator FEV1 ≤ 70% predicted; mMRC grade scale score ≥ 2; smoking history of > 10 pack-years | FEV1 | ICS monotherapy, albuterol (as rescue) | ICS/LABAs, PDE4 inhibitors, theophyllines, oral β2-agonists, LAMAs, LABAs, and LAMA/LABA combinations (other than those under study) | No |
| Riley, 2018 [37] | Age ≥ 40 years; diagnosis of COPD (according to ATS/ERS); FEV1/FVC ratio < 0.7 and a post-bronchodilator FEV1 30–70% predicted; resting FRC ≥ 120% predicted; mMRC grade scale score ≥ 2; smoking history of > 10 pack-years | Exercise endurance time | During the washout period: short-acting anticholinergic medications, salbutamol (as rescue) | Not specified | No |
| Mahler, 2012 [38] | Age ≥ 40 years; post-bronchodilator FEV1 ≤ 65% and ≥ 30%; post-bronchodilator FEV1/FVC < 70%; smoking history ≥ 10 pack-years | FEV1 |
ICS monotherapy, salbutamol/ albuterol (as rescue) |
LABAs, short-acting β2-agonists (except those prescribed in the study), theophylline, anticholinergics | Yes |
| Mahler, 2012 [38] | Age ≥ 40 years; post-bronchodilator FEV1 ≤ 65% and ≥ 30%; post-bronchodilator FEV1/FVC < 70%; smoking history ≥ 10 pack-years | FEV1 | ICS monotherapy, salbutamol/albuterol (as rescue) | LABAs, short-acting β2-agonists (except those prescribed in the study), theophylline, anticholinergics | Yes |
| Vincken, 2014 [39] | Diagnosed with moderate to severe stable COPD (stage II or III according to GOLD criteria); FEV1 ≥ 30% and/or < 80% predicted, a post-bronchodilator FEV1/FVC < 0.70; symptomatic patients (according to daily diary data); smoking history ≥ 10 pack-years | FEV1 | ICS monotherapy, salbutamol (as rescue) | Long-acting bronchodilators before starting the run-in period (≥ 7 days for LAMAs and the LABA indacaterol, and ≥ 48 h for other LABAs or ICS/LABA) | Yes |
| Wedzicha, 2013 [40] | Age ≥ 40 years; severe or very severe COPD (stage III or IV according to GOLD 2008 criteria); post-bronchodilator FEV1 < 50%; FEV1/FVC < 0.70; ≥ 1 exacerbation in the previous 12 months requiring systemic corticosteroids or antibiotics; smoking history ≥ 10 pack-years | COPD exacerbations rate | Stable dose of ICS, salbutamol (as rescue) | Long-acting bronchodilators | Yes |
| GSK CSRa | Outpatient; ≥ 40 years of age; diagnosis of COPD, post-salbutamol FEV1 ≤ 70% and post-salbutamol FEV1/FVC ratio < 0.7. Smoking history ≥ 10 pack-years | FEV1 | ICS (dose ≤ 1000 μg/day of FP or equivalent), salbutamol/albuterol (as rescue) | LABAs, oral SABAs and LABAs, inhaled SABAs, inhaled short-acting anticholinergics, and SABA/ICS combination products | Yes |
| GSK CSRa | Outpatient; ≥ 40 years of age; diagnosed with COPD, post-salbutamol FEV1 ≤ 70% and post-salbutamol FEV1/FVC ratio < 0.7. Smoking history ≥ 10 pack-years | FEV1 | ICS (dose ≤ 1000 μg/day of FP or equivalent), salbutamol/albuterol (as rescue) | LABAs, short-acting β2-agonists, short-acting anticholinergics and SABA/ICS combination products | Yes |
| GSK CSRa | Outpatient; ≥ 40 years of age; diagnosed with COPD; post-salbutamol FEV1/FVC ratio of < 0.70 and a post-salbutamol FEV1 of ≤ 70%; smoking history ≥ 10 pack-years | FEV1 | ICS (dose ≤ 1000 μg/day of FP or equivalent), salbutamol/albuterol (as rescue) | LABAs, ICS/LABA combination products, SABAs, short-acting anticholinergics, and ICS/SABA combination products | Yes |
| Vogelmeier, 2016 [41] | Age ≥ 40 years; diagnosed with moderate-to-severe COPD (stage II or III according to GOLD 2013 criteria); post-bronchodilator FEV1/FVC < 70% and FEV1 < 80% predicted; symptomatic patients; CAT score ≥ 10; smoking history ≥ 10 pack-years | FEV1 | Salbutamol (as rescue) | Use of triple therapy (LAMA/LABA/ICS) within 4 weeks of the screening visit; patients discontinued all bronchodilator and ICS medication the night before the randomisation visit (visit 2) | NR |
| Wedzicha, 2016 [42] | Age ≥ 40 years; diagnosed with stable COPD (according to GOLD 2011 criteria); post-bronchodilator FEV1/FVC < 0.70%; post-bronchodilator FEV1 ≥ 25 and < 60% predicted; symptomatic patients; documented history of at least 1 COPD exacerbation in the previous 12 months that required treatment with systemic glucocorticosteroids and/or antibiotics; mMRC grade scale score ≥ 2; smoking history ≥ 10 pack-years | COPD exacerbation rate | Open-label salbutamol (as rescue) | Pre-existing LABA, LAMA, ICS, and LABA/ICS fixed-combination therapies were discontinued prior to run-in; ICS and bronchodilators (apart from study medications) were prohibited during the run-in and treatment periods | NR |
| Maltais, 2019 [24] | Age ≥ 40 years; post-bronchodilator FEV1/FVC < 0.7 and FEV1 < 80% predicted; smoking history ≥ 10 pack-years | FEV1 | Patients prescribed only rescue medication or maintenance monotherapy before the study were required to be symptomatic (CAT score 10) at screening, whereas there was no symptom requirement at screening for patients prescribed dual maintenance therapy (ICS/LABA or LAMA/LABA) before the study | Not specified | No |
| Sethi, 2019 [43] | Age ≥ 40 years; diagnosed with moderate-to-severe COPD; post-bronchodilator FEV1/FVC < 70% and FEV1 < 80% predicted; CAT score ≥ 10; smoking history ≥ 10 pack-years | FEV1 | Inhaled, oral, or parenteral corticosteroids (dose equivalent to ≤ 10 mg/day prednisone); oxygen therapy (< 15 h/day); oral sustained-release theophylline, selective β-blocking agents (e.g. atenolol, metoprolol, nebivolol; stable administration for ≥ 2 weeks) | LABAs, LAMAs, SABAs (except albuterol/salbutamol, which were permitted as needed throughout all study periods), SAMAs (except ipratropium, during washout and screening only), methylxanthines, leukotriene modifiers, PDE4 inhibitors, or non-selective beta-blocking agents | Yes |
| D'Urzo, 2014 [44] | Age ≥ 40 years; FEV1 < 80% and ≥ 30% predicted; post-bronchodilator FEV1/FVC < 70; smoking history ≥ 10 pack-years | FEV1 | Albuterol/salbutamol (as rescue); other COPD medications such as theophylline, ICS, oral or parenteral corticosteroids (≤ 10 mg/day or 20 mg every other day of prednisone) were allowed if treatment was stable ≥ 4 weeks prior to screening | LABAs other than investigational treatment | Yes |
| D'Urzo, 2017 [45]b | Age ≥ 40 years; FEV1 < 80% and ≥ 30% predicted; post-bronchodilator FEV1/FVC < 70; smoking history ≥ 10 pack-years | Safety | Theophylline, ICS, oral or parenteral corticosteroids (≤ 10 mg/day or 20 mg every other day of prednisone), albuterol/salbutamol (as rescue) | Long-acting bronchodilators other than the investigative treatment | Yes |
| Ferguson, 2016 [46] | Age ≥ 40 years; diagnosed with stable COPD (according to GOLD 2011 criteria); moderate-to-severe airflow limitation; post-bronchodilator FEV1/FVC < 70% and FEV1 ≥ 30% and < 80% predicted; symptomatic patients (defined by mMRC grade ≥ 2); smoking history ≥ 10 pack-years | Safety and tolerability | Salbutamol/albuterol (as rescue) | Nebulized salbutamol/albuterol, non-potassium-sparing diuretics, non-selective beta-blocking agents, cardiac antiarrhythmics class Ia and III, drugs with QT prolongation potential, tricyclic antidepressants, antipsychotic agents, LAMA, ICS/LABA, SAMA, SABA, SABA/SAMA; patients receiving selective serotonin reuptake inhibitors, ICS, intranasal steroids, H1-antagonists, inactivated influenza, pneumococcal or any other inactivated vaccine were excluded unless on stable dose | No |
| Mahler, 2015 [47] | Age ≥ 40 years; stable COPD (according to GOLD 2011 guidelines); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; post-bronchodilator FEV1/FVC < 0.70 at run-in; mMRC grade ≥ 2 at run-in; smoking history ≥ 10 pack-years | FEV1 | ICS | COPD-related medications other than investigational therapy (LAMAs, SAMAs, ICS/LABA combinations, SABA, SAMA/SABA combinations, etc.); patients receiving SSRIs, ICS, intranasal steroids, H1-antagonists, or inactivated influenza, pneumococcal or any other inactivated vaccines should be excluded unless on stable dose | Yes |
| Mahler, 2015 [47] | Age ≥ 40 years; stable COPD (according to GOLD 2011 guidelines); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; post-bronchodilator FEV1/FVC < 0.70 at run-in; mMRC grade ≥ 2 at run-in; smoking history ≥ 10 pack-years | FEV1 | ICS | COPD-related medications other than investigational therapy (LAMAs, SAMAs, ICS/LABA combinations, SABA, SAMA/SABA combinations, etc.); patients receiving SSRIs, ICS, intranasal steroids, H1-antagonists, or inactivated influenza, pneumococcal or any other inactivated vaccines should be excluded unless on stable dose | Yes |
| Siler, 2016 [48] | Age ≥ 40 years; diagnosed with stable COPD; moderate-to-severe airflow limitation; pre- and post-albuterol FEV1/FVC < 0.70 and post-albuterol FEV1 ≤ 70% predicted; symptomatic patients (defined by mMRC grade ≥ 2); mMRC grade scale score ≥ 2; smoking history ≥ 10 pack-years | SGRQ | Albuterol (as rescue) | Depot corticosteroids, systemic, oral or parenteral corticosteroids, ICS/LABA combination products, ICS (dose > 1000 µg/day of FP or equivalent), initiation or discontinuation of ICS use, PDE4 inhibitors (roflumilast), LAMAs, inhaled LABAs, LAMA/LABA combination, theophyllines, oral β2-agonists, inhaled SABAs, inhaled short-acting anticholinergics, inhaled short-acting anticholinergic/SABA combination products | No |
| Kerwin, 2017 [49] | Age ≥ 40 years; diagnosed with stable COPD (according to ATS/ERS); post-bronchodilator FEV1 ≤ 70% and ≥ 50% predicted; mMRC grade scale score ≥ 1; smoking history ≥ 10 pack-year | FEV1 | Albuterol (as rescue) | Not specified | No |
| Donohue, 2016 [50] | Age ≥ 40 years; diagnosed with moderate to severe COPD; FEV1/FVC ratio < 70%; post-bronchodilator FEV1 30% and < 80% predicted; smoking history ≥ 10 pack-year | Safety | Albuterol (as rescue but not within 6 h before a visit), ICS and oral or parenteral corticosteroids at doses ≤ 10 mg/day, theophylline and H1-antihistamine (dosage was stable for ≥ 4 weeks prior to screening and throughout the trial), chronic use of oxygen therapy (up to 15 h/day provided the dosage was stable for ≥ 4 weeks prior to screening), select β1-blocking agents (atenolol, metoprolol, nebivolol; stable dosage for ≥ 2 weeks prior to screening) | Indacaterol within 15 days prior to screening or during the trial; select β1-blocking agents (atenolol, metoprolol, nebivolol) were permitted for chronic use if the dosage was stable for ≥ 2 weeks prior to screening | Yes |
| Martinez, 2017 [51] | Age ≥ 40 years; diagnosed with moderate-to-very severe COPD (according to ATS/ERS); FEV1/FVC ratio < 0.70 and FEV1 < 80% predicted; FEV1 < 30% predicted were required to have postbronchodilator FEV1 ≥ 750 mL; smoking history ≥ 10 pack-year | FEV1 | Albuterol (as rescue), oral corticosteroid (stable dose equivalent to ≤ 5 mg/day or ≤ 10 mg every other day), prednisone, ICS and/or PDE4 inhibitors; if ICS was administered as part of an FDC, this was discontinued and substituted with an ICS single agent (fluticasone, mometasone, or budesonide) at the equivalent dose | Theophylline (> 400 mg/day), leukotriene antagonists, mast-cell stabilisers, non-selective β-blockers, antiarrhythmic agents, antipsychotics and antidepressants (except selective serotonin [or serotonin–norepinephrine] reuptake inhibitors) | Yes |
| Martinez, 2017 [51] | Age ≥ 40 years; diagnosed with moderate-to-very severe COPD (according to ATS/ERS); FEV1/FVC ratio < 0.70 and FEV1 < 80% predicted; FEV1 < 30% predicted were required to have postbronchodilator FEV1 ≥ 750 mL; smoking history ≥ 10 pack-year | FEV1 | Albuterol (as rescue), oral corticosteroid (stable dose equivalent to ≤ 5 mg/day or ≤ 10 mg every other day), prednisone, ICS and/or PDE4 inhibitors; if ICS was administered as part of an FDC, this was discontinued and substituted with an ICS single agent (fluticasone, mometasone, or budesonide) at the equivalent dose | Theophylline (> 400 mg/day), leukotriene antagonists, mast-cell stabilisers, non-selective β-blockers, antiarrhythmic agents, antipsychotics and antidepressants (except selective serotonin [or serotonin–norepinephrine] reuptake inhibitors) | Yes |
| Bateman, 2013 [18] | Age ≥ 40 years; moderate or severe COPD (stage II or III according to GOLD 2008 criteria); post-bronchodilator FEV1 < 80% and ≥ 30%; post-bronchodilator FEV1/FVC < 0.70; smoking history ≥ 10 pack-years | FEV1 | Salbutamol/albuterol (as rescue), inhaled or intranasal corticosteroids (at constant doses) | LABAs, LAMAs, and ICS/LABA | Yes |
| Buhl, 2015 [52] | Age ≥ 40 years; moderate-to-severe stable COPD (GOLD stage 2–3); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; post-bronchodilator FEV1/FVC < 0.7; smoking history of ≥ 10 pack-years | SGRQ | Salbutamol (as rescue), SSRI, H1-antagonists (except mizolastine or terfenadine), inactivated influenza, pneumococcal or any other inactivated vaccine if not administered within 48 h before the study visit; patients receiving ICS at baseline continued treatment at the same or equivalent dose and regimen | Not specified | Yes |
| Tashkin, 2009 [53] | Age ≥ 40 years; post-bronchodilator FEV1 < 70% and > 30% of the predicted normal value or > 0.75 L, whichever was lesser at run-in; FEV1/FVC < 0.70 | FEV1 | Continued use of prior stable ICS regimens and systemic corticosteroids for the treatment of exacerbations was permitted throughout the study; all patients were provided with albuterol inhalers for use as rescue medication | Following screening, prohibited medications (i.e. β-agonists, β-blockers, cromolyn sodium, ipratropium bromide, leukotriene antagonists, cytotoxic agents, and theophylline) were withdrawn | Yes |
| Frith, 2018 [54] | Age ≥ 40 years; post-bronchodilator FEV1/FVC < 70%; post-bronchodilator FEV1 ≤ 70% predicted; post-bronchodilator FEV1 ≥ 30% and < 80% predicted; mMRC grade scale score ≥ 2; treated with SFC 50/500 μg BID for ≥ 3 months prior to screening; CAT score of ≥ 10; smoking history of ≥ 10 pack-years | FEV1 | Pre-randomisation maintenance therapy with salmeterol/fluticasone, SABA (salbutamol or albuterol) for use as a rescue inhaler on an ‘as-needed’ basis throughout the study | LAMA monotherapy, SABA (except when used as a rescue medication), SAMA, SABA/SAMA, LABA monotherapy, oral PDE4 inhibitor, xanthines, parenteral or oral corticosteroids (except as allowed for treating COPD exacerbations) and intramuscular depot corticosteroids | No |
| Celli, 2014 [55] | Age ≥ 40 years; diagnosed with established COPD; post-bronchodilator FEV1/FVC < 70%; post-salbutamol FEV1 ≤ 70% predicted; mMRC grade scale score ≥ 2; smoking history of ≥ 10 pack-years | FEV1 | Albuterol (as rescue) | Depot corticosteroids; systemic, oral, or parenteral corticosteroids; antibiotics (for lower respiratory tract infection); cytochrome P450 3A4 strong inhibitors; LABA/ICS combination products; ICS > 1000 µg/day; PDE4 inhibitor (e.g. roflumilast); TIO; theophyllines; oral leukotriene inhibitors; oral β2-agonists; inhaled LABA; inhaled sodium cromoglycate or nedocromil sodium; inhaled SABAs; inhaled short-acting anticholinergics; inhaled short-acting anticholinergic/short-acting β2-agonist combination products | NR |
| Singh, 2015 [56] | Age ≥ 40 years; diagnosed with established COPD; post-bronchodilator FEV1/FVC < 0.7; a post-salbutamol FEV1 ≥ 30% and < 70% predicted; mMRC grade scale score ≥ 2; smoking history of ≥ 10 pack-years | FEV1 | Salbutamol (as rescue) | Depot corticosteroids, antibiotics for lower respiratory tract infection, ICS/LABA products, PDE4 inhibitors, long-acting anticholinergics, xanthines, oral leukotriene inhibitors, nedocromil sodium, SABAs, short-acting anticholinergics, SABA combination products, herbal medications potentially containing oral or systemic steroids, any other investigational medication for COPD | NR |
| Donohue, 2015 [57] | Age ≥ 40 years; diagnosed with established COPD; post-bronchodilator FEV1 ≥ 30% and < 70% predicted; post-bronchodilator FEV1/FVC < 0.7; mMRC grade scale score ≥ 2; smoking history of ≥ 10 pack-years | FEV1 | Albuterol (as rescue) | Depot corticosteroids, systemic, oral or parenteral corticosteroids, antibiotics, cytochrome inhibitors, herbal medications potentially containing steroids, ICS, ICS/LABA, PDE4 inhibitors, olodaterol, indacaterol, inhaled long-acting anticholinergics, xanthines, oral leukotriene inhibitors, oral beta-agonists, SAL and FOR, inhaled sodium cromoglycate or nedocromil sodium, inhaled SABAs, inhaled short-acting anticholinergics, inhaled short-acting anticholinergic/SABA combination products, investigational medication | No |
| Donohue, 2015 [57] | Age ≥ 40 years; diagnosed with established COPD; post-bronchodilator FEV1 ≥ 30% and < 70% predicted; post-bronchodilator FEV1/FVC < 0.7; mMRC grade scale score ≥ 2; smoking history of ≥ 10 pack-years | FEV1 | Albuterol (as rescue) | Depot corticosteroids, systemic, oral or parenteral corticosteroids, antibiotics, cytochrome inhibitors, herbal medications potentially containing steroids, ICS, ICS/LABA, PDE4 inhibitors, olodaterol, indacaterol, inhaled long-acting anticholinergics, xanthines, oral leukotriene inhibitors, oral beta-agonists, SAL and FOR, inhaled sodium cromoglycate or nedocromil sodium, inhaled SABAs, inhaled short-acting anticholinergics, inhaled short-acting anticholinergic/SABA combination products, investigational medication | No |
| Vogelmeier, 2013 [58] | Age ≥ 40 years; diagnosed with moderate-to-severe stable COPD (stage II or stage III; according to GOLD guideline); post-bronchodilator FEV1 ≥ 40% and < 80% predicted; post-bronchodilator FEV1/FVC < 0.7; symptomatic patients (mMRC grade score 1); smoking history of ≥ 10 pack-years | FEV1 | Salbutamol (as rescue), selective serotonin reuptake inhibitors (stable treatment regimen for ≥ 1 month prior to screening visit and during the study screening normal ECG), inactivated vaccine (not administered < 48 h prior to a study visit), intranasal corticosteroids (constant doses and dose regimens for ≥ 5 days prior to screening), H1-antagonists (in constant doses and dose regimens for ≥ 5 days prior to screening) | Not specified | NR |
| Zhong, 2015 [59] | Age ≥ 40 years; diagnosed with moderate-to-severe stable COPD (stage II or stage III; according to GOLD 2010 guideline); post-bronchodilator FEV1 ≥ 30% and < 80% predicted; post-bronchodilator FEV1/FVC < 0.7; mMRC grade score ≥ 2; smoking history of ≥ 10 pack-years | FEV1 | Selective serotonin reuptake inhibitors (stable dose for ≥ 1 month prior to visit 1 and during the study), intranasal corticosteroids (stable dose for ≥ 30 days prior to visit 1), H1-antagonists (stable dose for ≥ 30 days prior to visit 1), inactivated influenza, pneumococcal or any other inactivated vaccine (not administered < 48 h prior to a study visit) | Not specified | Yes |
| Hoshino, 2015 [60] | Age ≥ 40 years; FEV1 < 80% and ≥ 30% predicted; post-bronchodilator FEV1/FVC < 70%; smoking history of ≥ 10 pack-years | Airway dimensions | Salbutamol (as rescue) | Antibiotics, systemic glucocorticosteroids | No |
| Singh, 2014 [61] | Age ≥ 40 years; FEV1 < 80% and ≥ 30% predicted; post-bronchodilator FEV1/FVC < 70%; smoking history of ≥ 10 pack-years | FEV1 | Albuterol/salbutamol (as rescue); other COPD medications, e.g. theophylline, ICS, oral or parenteral corticosteroids (≤ 10 mg/day or 20 mg every other day of prednisone) were allowed if treatment was stable ≥ 4 weeks prior to screening | LABAs other than investigational treatment | Yes, provided treatment was stable ≥ 4 weeks pre-screening |
| ZuWallack, 2014 [62] | Age ≥ 40 years; post-bronchodilator FEV1 ≥ 30% and < 80% of predicted normal; post-bronchodilator FEV1/FVC < 70% (GOLD stage 2–3); smoking history of > 10 pack-years | FEV1 | ICS, oral (≤ 10 mg prednisone per day, or equivalent) and injected steroids; cromolyn sodium/nedocromil sodium; antihistamines; antileukotrienes; methylxanthines; mucolytics; theophyllines; albuterol permitted as rescue medication only | Concurrent ICS in fixed combination with LABA, ICS/SABA combinations, SAMA/SABA combinations or PDE4 inhibitors | Yes |
| ZuWallack, 2014 [62] | Age ≥ 40 years; post-bronchodilator FEV1 ≥ 30% and < 80% of predicted normal; post-bronchodilator FEV1/FVC < 70% (GOLD stage 2–3); smoking history of > 10 pack-years | FEV1 | ICS, oral (≤ 10 mg prednisone per day, or equivalent) and injected steroids; cromolyn sodium/nedocromil sodium; antihistamines; antileukotrienes; methylxanthines; mucolytics; theophyllines; albuterol permitted as rescue medication only | Concurrent ICS in fixed combination with LABA, ICS/SABA combinations, SAMA/SABA combinations or PDE4 inhibitors | Yes |
| Dahl, 2013 [63] | Age ≥ 40 years; moderate or severe COPD (stage II or III according to GOLD 2008 criteria); post-bronchodilator FEV1 < 80% and ≥ 30%; post-bronchodilator FEV1/FVC < 0.70; smoking history ≥ 10 pack-years | Safety and tolerability | Albuterol (as rescue), ICS monotherapy | Long-acting bronchodilators (LABAs, LAMAs, theophylline) and short-acting muscarinic antagonists | Yes |
| Buhl, 2015b [19] | Outpatient; age ≥ 40 years; smoking history of > 10 pack-years; moderate-to-very-severe COPD (GOLD stage 2–4); post-bronchodilator FEV1 < 80% predicted; post-bronchodilator FEV1/FVC < 70% | FEV1 | ICS | Oxygen | Yes |
| Buhl, 2015b [19] | Outpatient; age ≥ 40 years; smoking history of > 10 pack-years; moderate-to-very-severe COPD (GOLD stage 2–4); post-bronchodilator FEV1 < 80% predicted; post-bronchodilator FEV1/FVC < 70% | FEV1 | ICS | Oxygen | Yes |
AC active-controlled, ACL aclidinium, ATS American Thoracic Society, CAT COPD Assessment Test, COPD chronic obstructive pulmonary disease, CSR clinical study report, DB double blind, DD double dummy, ECG electrocardiogram, ERS European Respiratory Society, FEV1 forced expiratory volume in 1 s, FOR formoterol fumarate, FP fluticasone propionate, FRC functional residual capacity, FVC forced vital capacity, ICS inhaled corticosteroid, GLY glycopyrronium, GOLD Global Initiative for Chronic Obstructive Lung Disease, IND indacaterol, ITT intent-to-treat, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, MC multicentred, MDI metered dose inhaler, mMRC modified Medical Research Council, NMA network meta-analysis, NR not reported, OLO olodaterol, PBO placebo, PC placebo controlled, PDE phosphodiesterase, PG parallel group, RCT randomised controlled trial, SAL salmeterol, SABA short-acting β2-agonist, SAMA short-acting muscarinic antagonist, SGRQ St George’s Respiratory Questionnaire, SSRI selective serotonin reuptake inhibitor, TIO tiotropium, UMEC umeclidinium, VI vilanterol
aCSRs are available from clinicalstudydatarequest.com
bThe extension trial AUGMENT EXTENSION was not counted as a unique trial but secondary publication to main trial
Table 3.
Baselines characteristics of patients in the studies included in the NMA
| Author & year | Treatment | ITT population, n | Male, % | Age, years | Current smokers, % | Severe or very severe COPD, % | Concomitant ICS, % | Mean/median duration of COPD, yearsa | Smoking history, pack-years | Trough mean/median pre-bronchodilator FEV1, La | Mean/median post-bronchodilator FEV1, La | Pre-bronchodilator FEV1% predicted | Post-bronchodilator FEV1% predicted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipworth, 2018 [30] | GLY/FOR (MDI) 18/9.6 | 551 | 74.0 | 64.7 | 45.7 |
Severe: 34.8 Very severe: 4.2 |
30.7 | 6.2 | 45.9 | NR | NR | NR | 53.9 |
| GLY 18 | 474 | 73.0 | 64.0 | 44.1 |
Severe: 35.4 Very severe: 3.0 |
30.2 | 6.2 | 44.8 | NR | NR | NR | 54.8 | |
| FOR 9.6 | 480 | 76.0 | 64.1 | 43.3 |
Severe: 35.6 Very severe: 2.9 |
29.6 | 6.1 | 46.9 | NR | NR | NR | 53.9 | |
| PBO | 235 | 72.8 | 63.9 | 48.1 |
Severe: 36.6 Very severe: 2.6 |
33.6 | 6.1 | 45.7 | NR | NR | NR | 54.4 | |
|
Singh, 2015 [31] (OTEMTO 1) |
TIO + OLO 5/5 | 203 | 56.2 | 64.7 | 54.7 |
GOLD 3: 36.0 GOLD 4: 0 |
41.9 | NR | NR | 1.3 | 1.5 | NR | 54.9 |
| TIO + OLO 2.5/5 | 202 | 57.4 | 64.7 | 48.5 |
GOLD 3: 34.2 GOLD 4: 0.5 |
38.6 | NR | NR | 1.3 | 1.5 | NR | 55.5 | |
| TIO 5 | 203 | 61.1 | 64.9 | 48.3 |
GOLD 3: 36.0 GOLD 4: 1.0 |
37.9 | NR | NR | 1.3 | 1.5 | NR | 54.7 | |
| PBO | 204 | 62.3 | 65.1 | 43.1 |
GOLD 3: 36.0 GOLD 4: 0.5 |
34.8 | NR | NR | 1.4 | 1.6 | NR | 56.3 | |
|
Singh, 2015 [31] (OTEMTO 2) |
TIO + OLO 5/5 | 202 | 65.8 | 65.2 | 45.5 |
GOLD 3: 38.1 GOLD 4: 0 |
35.6 | NR | NR | 1.4 | 1.6 | NR | 54.8 |
| TIO + OLO 2.5/5 | 202 | 62.4 | 64.4 | 44.6 |
GOLD 3: 34.7 GOLD 4: 1.5 |
41.1 | NR | NR | 1.3 | 1.5 | NR | 54.5 | |
| TIO 5 | 203 | 64.0 | 64.7 | 44.8 |
GOLD 3: 32.5 GOLD 4: 0 |
35.0 | NR | NR | 1.4 | 1.6 | NR | 55.9 | |
| PBO | 202 | 57.9 | 64.0 | 47.0 |
GOLD 3: 39.1 GOLD 4: 0.5 |
35.1 | NR | NR | 1.3 | 1.5 | NR | 54.3 | |
| Vogelmeier, 2008 [32] | FOR 10 | 210 | 75.7 | 61.8 | NR | NR | NR | 7 | 35.4 | 1.5 | NR | 51.6 | NR |
| TIO 18 | 221 | 79.2 | 63.4 | NR | NR | NR | 6.9 | 38.6 | 1.5 | NR | 51.6 | NR | |
| TIO 18 + FOR 10 | 207 | 79.2 | 62.6 | NR | NR | NR | 7.2 (7.0) | 37.9 | 1.5 | NR | 50.4 | NR | |
| PBO | 209 | 77.5 | 62.5 | NR | NR | NR | 6.7 | 40.1 | 1.5 | NR | 51.1 | NR | |
| Maleki-Yazdi, 2014 [33] | UMEC/VI 62.5/25 | 454 | 68.0 | 61.9 | 59.0 | 60.0 | 54.0 | NR | 44.1 | 1.3 |
Post salbutamol: 1.4 Post ipratropium: 1.5 |
NR | 46.2 |
| TIO 18 | 451 | 67.0 | 62.7 | 54.0 | 58.0 | 53.0 | NR | 44.4 | 1.3 |
Post salbutamol: 1.4 Post ipratropium: 1.5 |
NR | 46.5 | |
| Calverley, 2018 [34] | TIO/OLO 5/5 | 3939 | 71.0 | 66.5 | 36.0 |
GOLD C: 4.0 GOLD D: 40 |
ICS only: 3 LABA–ICS: 26 LAMA–ICS: 2 LAMA–LABA–ICS: 39 |
NR | 44.8 | NR | 1.2 | NR | 44.6 |
| TIO 5 | 3941 | 72.0 | 66.3 | 36.0 |
GOLD C: 4.0 GOLD D: 39 |
ICS only: 2 LABA–ICS: 26 LAMA–ICS: 2 LAMA–LABA–ICS: 40 |
NR | 44.7 | NR | 1.2 | NR | 44.5 | |
| Kerwin, 2017 [49] (A2349) | IND/GLY 27.5/15.6 BID and UMEC/VI 62.5/25 | 357 | 52.1 | 64.1 | 56.9 | GOLD 3: 35.6 | 36.1 | 8.1 | 52.3 | 1.2 | 1.5 | NR | 54.0 |
| Kerwin, 2017 [49] (A2350) | IND/GLY 27.5/15.6 BID and UMEC/VI 62.5/25 | 355 | 54.1 | 63.9 | 57.2 | GOLD 3: 37.5 | 36.1 | 8.4 | 54.0 | 1.3 | 1.6 | NR | 54.6 |
| Maltais, 2019 [22] | UMEC/VI 62.5/25 | 812 | 61.0 | 64.6 | 49 | GOLD 3: 36.0 | NR | 8.8 | 49.4 | 1.5 | 1.6 | NR | 54.9 |
| UMEC 62.5 | 804 | 59.0 | 64.9 | 49 | GOLD 3: 34.0 | NR | 7.8 | 47.6 | 1.5 | 1.6 | NR | 55.9 | |
| SAL 50 | 809 | 58.0 | 64.4 | 51 | GOLD 3: 35.0 | NR | 8.3 | 48.1 | 1.5 | 1.6 | NR | 55.6 | |
| Feldman, 2017 [25] | UMEC/VI 62.5/25 | 236 | 60.0 | 64.4 | 53 | 5.0 | 4 | NR | 50.2 | NR | 1.7 | NR | 59.6 |
| TIO/OLO 5/5 | 236 | 60.0 | 64.4 | 53 | 5.0 | 4 | NR | 50.2 | NR | 1.7 | NR | 59.6 | |
| Kalberg, 2016 [36] | UMEC/VI 62.5/25 | 482 | 74.0 | 64.0 | 41.0 | 56.0 | 56.0 | NR | 43.2 | NR | 1.4 | NR | NR |
| TIO 18 + IND 150 | 479 | 71.0 | 64.0 | 46.0 | 58.0 | 51.0 | NR | 42.3 | NR | 1.4 | NR | NR | |
| Riley, 2018 [37] | UMEC/VI 62.5/25 | 198 | 53.0 | 60.7 | 64.0 | 46.0 | 28.0 | NR | 52.2 | 1.4 | 1.5 | NR | 50.5 |
| PBO | 198 | 53.0 | 60.7 | 64.0 | 46.0 | 28.0 | NR | 52.2 | 1.4 | 1.5 | NR | 50.5 | |
| Mahler, 2012 [38] (INTRUST-1) | TIO 18 + IND 150 | 570 | 70.0 | 64.0 | 40.0 | 53.0 | 52.0 | 7.1 | 47.2 |
Pre salbutamol: 1.2 Pre ipratropium: 1.2 |
Post salbutamol: 1.3 Post ipratropium: 1.4 |
NR | 48.3 |
| TIO 18 | 561 | 67.0 | 63.4 | 36.0 | 53.0 | 52.0 | 6.6 | 47.2 |
Pre salbutamol: 1.2 Pre ipratropium: 1.2 |
Post salbutamol: 1.3 Post ipratropium: 1.4 |
NR | 48.9 | |
| Mahler, 2012 [38] (INTRUST-2) | TIO 18 + IND 150 | 572 | 63.0 | 63.1 | 38.0 | 54.0 | 57.0 | 7.3 | 46.2 |
Pre salbutamol: 1.1 Pre ipratropium: 1.2 |
Post salbutamol: 1.3 Post ipratropium: 1.3 |
NR | 48.6 |
| TIO 18 | 570 | 68.0 | 62.8 | 43.0 | 54.0 | 51.0 | 7.1 | 46.3 |
Pre salbutamol: 1.2 Pre ipratropium: 1.2 |
Post salbutamol: 1.3 Post ipratropium: 1.4 |
NR | 48.6 | |
| Vincken, 2014 [39] | IND 150 + GYL 50 | 226 | 79.6 | 63.4 | 42.5 | 38.5 | 61.1 | 7.1 | 44.5 | NR | 1.5 | 54.2 | NR |
| IND 150 | 223 | 84.2 | 64.1 | 41.6 | 33.0 | 64.3 | 7.2 | 44.4 | NR | 1.6 | 55.5 | NR | |
| Wedzicha, 2013 [40] | IND/GLY 110/50 | 729 | 76.0 | 63.1 | 38.0 |
Severe: 79.0 Very severe: 21 |
75.0 | 7.2 | 45.0 | 0.9 | 1.0 | NR | 37.0 |
| GLY 50 | 740 | 73.0 | 63.1 | 38.0 |
Severe: 79.0 Very severe: 21.0 |
75.0 | 7.1 | 44.0 | 0.9 | 1.0 | NR | 37.3 | |
| TIO 18 | 737 | 75.0 | 63.6 | 37.0 |
Severe: 79.0 Very severe: 21.0 |
76.0 | 7.2 | 47.0 | 0.9 | 1.0 | NR | 37.4 | |
| GSK CSRb (DB2113374) | UMEC 125 | 222 | 67.0 | 64.5 | 44.0 | 61.0 | 56.0 | NR | 47.6 | NR | 1.1 | NR | 46.2 |
| UMEC/VI 62.5/25 | 217 | 65.0 | 65.0 | 42.0 | 51.0 | 47.0 | NR | 47.8 | NR | 1.2 | NR | 47.7 | |
| UMEC/VI 125/25 | 215 | 69.0 | 63.8 | 45.0 | 59.0 | 53.0 | NR | 46.9 | NR | 1.2 | NR | 47.1 | |
| TIO 18 | 215 | 71.0 | 65.2 | 47.0 | 52.0 | 53.0 | NR | 54.0 | NR | 1.2 | NR | 47.4 | |
| GSK CSRb (DB2113360) | UMEC/VI 125/25 | 214 | 71.0 | 62.9 | 58.0 | 53.0 | 48.0 | NR | 43.5 | NR | 1.3 | NR | 47.2 |
| UMEC/VI 62.5/25 | 212 | 70.0 | 63.0 | 46.0 | 50.0 | 44.0 | NR | 44.8 | NR | 1.3 | NR | 48 | |
| VI 25 | 209 | 68.0 | 63.2 | 51.0 | 54.0 | 40.0 | NR | 41.6 | NR | 1.3 | NR | 47.7 | |
| TIO 18 | 208 | 67.0 | 62.6 | 48.0 | 53.0 | 45.0 | NR | 41.9 | NR | 1.3 | NR | 47.8 | |
| GSK CSRb (DB2113373) | UMEC 62.5 | 418 | 71.0 | 64.0 | 50.0 | 54.0 | 52.0 | NR | 46.8 | 1.2 | 1.3 | NR | 46.8 |
| VI 25 | 421 | 68.0 | 62.7 | 47.0 | 53.0 | 50.0 | NR | 44.7 | 1.2 | 1.4 | NR | 48.2 | |
| UMEC/VI 62.5/25 | 413 | 74.0 | 63.1 | 49.0 | 51.0 | 51.0 | NR | 46.5 | 1.3 | 1.4 | NR | 47.8 | |
| PBO | 280 | 70.0 | 62.2 | 54.0 | 58.0 | 49.0 | NR | 47.2 | 1.2 | 1.4 | NR | 46.7 | |
| Vogelmeier, 2016 [41] | ACL/FOR 400/12 | 468 | 65.7 | 63.5 | NR | GOLD D: 43.5 | 37.7 | NR | 41.6 | NR | 1.4 | NR | 53.3 |
| SAL/FP 50/500 | 466 | 64.4 | 63.3 | NR | GOLD D: 44.8 | 39.1 | NR | 42.6 | NR | 1.4 | NR | 53.2 | |
| Wedzicha, 2016 [42] | IND/GLY 110/50 | 1680 | 77.3 | 64.6 | 40.0 | 57.9 | 56.8 | 7.2 | NR | 1.0 | 1.2 | NR | 44.0 |
| SAL/FF 50/500 | 1682 | 74.8 | 64.5 | 40.0 | 58.3 | 55.8 | 7.3 | NR | 1.0 | 1.2 | NR | 44.1 | |
| Maltais 2019 [24] | GLY/FOR 18/9.6 | 552.0 | 74.3 | 64.3 | 52.7 | 53.5 | 53.3 | 8.1 | 39.5 | NR | NR | NR | 48.5 |
| UMEC/VI 62.5/25 | 552.0 | 71.0 | 63.8 | 54.3 | 52.6 | 52.7 | 8.0 | 38.7 | NR | NR | NR | 48.9 | |
| Sethi, 2019 [43] | ACL/FOR 400/12 | 314 | 61.5 | 64.4 | 52.2 | 47.5 | 33.1 | NR | 46.2 | 1.3 | NR | NR | 50.9 |
| ACL 400 | 475 | 64.0 | 64.4 | 52.2 | 51.4 | 32.4 | NR | 45.4 | 1.3 | NR | NR | 49.6 | |
| FOR 12 | 319 | 59.6 | 64.7 | 51.1 | 53.6 | 34.2 | NR | 45.2 | 1.3 | NR | NR | 49.6 | |
| TIO 18 | 475 | 58.1 | 64.0 | 52.6 | 45.7 | 29.9 | NR | 46.4 | 1.3 | NR | NR | 51.2 | |
| D'Urzo, 2014 [44] | ACL/FOR 400/12 | 335 | 50.1 | 64.2 | 51.6 | 42.4 | NR | NR | 53.3 | 1.3 | NR | NR | 53.2 |
| ACL/FOR 400/6 | 333 | 56.2 | 63.9 | 52.9 | 38.1 | NR | NR | 52.1 | 1.4 | NR | NR | 54.7 | |
| ACL 400 | 337 | 55.8 | 64.4 | 50.7 | 43.6 | NR | NR | 52.0 | 1.3 | NR | NR | 53 | |
| FOR 12 | 332 | 50.9 | 63.7 | 51.5 | 39.5 | NR | NR | 52.5 | 1.4 | NR | NR | 53.9 | |
| PBO | 332 | 52.7 | 63.5 | 50.9 | 45.2 | NR | NR | 53.3 | 1.4 | NR | NR | 52.6 | |
| D'Urzo, 2017 [45]c | ACL/FOR 400/12 | 182 | 48.4 | 63.7 | 53.8 | 44.0 | NR | NR | 53.3 | 1.3 | NR | NR | 52.1 |
| ACL/FOR 400/6 | 204 | 58.8 | 63.6 | 54.4 | 36.8 | NR | NR | 53.7 | 1.4 | NR | NR | 55.1 | |
| ACL 400 | 194 | 53.6 | 62.9 | 59.3 | 45.4 | NR | NR | 52.3 | 1.3 | NR | NR | 52.7 | |
| FOR 12 | 192 | 46.9 | 62.8 | 53.6 | 37.0 | NR | NR | 53.1 | 1.4 | NR | NR | 55.1 | |
| PBO | 146 | 55.5 | 63.2 | 52.7 | 45.2 | NR | NR | 54.5 | 1.4 | NR | NR | 53.2 | |
| Ferguson, 2016 [46] | IND/GLY 27.5/15.6 BID | 204 | 64.2 | 64.0 | 49.5 | 35.8 | 46.6 | 6.7 | NR | 1.3 | 1.5 | NR | 55 |
| IND/GLY 27.5/31.2 BID | 204 | 60.3 | 63.9 | 51.5 | 38.2 | 48.5 | 6.8 | NR | 1.2 | 1.5 | NR | 54.2 | |
| IND 75 | 207 | 72.0 | 62.8 | 51.7 | 35.7 | 48.8 | 6.6 | NR | 1.3 | 1.6 | NR | 53.9 | |
| Mahler, 2015 [47] (FLIGHT1 & FLIGHT2, pooled) | IND/GLY 27.5/15.6 BID | 508 | 63.4 | 63.4 | 50.4 | 37.8 | 45.9 | 7.1 | NR | 1.3 | 1.5 | NR | 54.9 |
| IND 27.5 | 511 | 65.8 | 63.7 | 52.1 | 39.9 | 48.9 | 7 | NR | 1.3 | 1.5 | NR | 54.4 | |
| GLY 15.6 | 511 | 63.8 | 63.4 | 52.3 | 37.4 | 42.9 | 7 | NR | 1.3 | 1.5 | NR | 54.6 | |
| PBO | 508 | 60.2 | 63.2 | 51.6 | 39.2 | 45.5 | 7.1 | NR | 1.3 | 1.5 | NR | 54.4 | |
| Siler, 2016 [48] | UMEC/VI 62.5/25 | 248 | 58.0 | 64.1 | 55.0 | GOLD D: 64 | 45.0 | NR | 38.8 | NR | NR | NR | 46.5 |
| PBO | 248 | 60.0 | 62.6 | 52.0 | GOLD D: 56 | 50.0 | NR | 38.4 | NR | NR | NR | 48.4 | |
| Kerwin, 2017 [49] | UMEC/VI 62.5/25 | 247 | 66.0 | 64.5 | 52.0 | 0.0 | NR | NR | 38.6 | NR | 1.8 | NR | 59.8 |
| TIO 18 | 247 | 65.0 | 64.3 | 48.0 | 0.0 | NR | NR | 40.4 | NR | 1.8 | NR | 59.4 | |
| Donohue, 2016 [50] | ACL/FOR 400/12 | 392 | 55.1 | 63.9 | 46.9 | 46.2 | 35.2 | NR | 50.9 | 1.3 | NR | NR | 51.8 |
| FOR 12 | 198 | 55.1 | 64.7 | 43.9 | 46.5 |
ICS: 34.3 ICS + LABA: 0.6 |
NR | 52.6 | 1.3 | NR | NR | 50.5 | |
| Martinez, 2017 [51] (PINNACLE-1) | GLY/FOR 18/9.6 | 526 | 55.1 | 62.6 | 53.4 | 46.0 | 33.7 | NR | 50.9 | NR | 1.5 | NR | 51.4 |
| GLY 18 | 451 | 56.5 | 62.9 | 54.3 | 47.0 | 35.9 | NR | 50.4 | NR | 1.5 | NR | 50.7 | |
| FOR 9.6 | 449 | 54.8 | 63.0 | 54.3 | 47.0 | 36.7 | NR | 52.9 | NR | 1.5 | NR | 51.2 | |
| PBO | 219 | 55.7 | 62.5 | 57.5 | 47.0 | 35.2 | NR | 50.8 | NR | 1.5 | NR | 50.6 | |
| TIO 18 | 451 | 59.6 | 63.0 | 52.8 | 47.0 | 36.4 | NR | 53.0 | NR | 1.5 | NR | 51.4 | |
| Martinez, 2017 [51] (PINNACLE-2) | GLY/FOR 18/9.6 | 510 | 53.3 | 62.8 | 52.5 | 47.7 | 37.6 | NR | 50.5 | NR | 1.5 | NR | 52.1 |
| GLY 18 | 439 | 55.1 | 62.8 | 51.5 | 46.2 | 39.2 | NR | 50.4 | NR | 1.5 | NR | 51.5 | |
| FOR 9.6 | 437 | 56.5 | 62.6 | 57.7 | 47.1 | 35.9 | NR | 50.6 | NR | 1.5 | NR | 51.9 | |
| PBO | 223 | 56.1 | 64.2 | 49.3 | 47.5 | 39.9 | NR | 53.2 | NR | 1.5 | NR | 52.5 | |
| Bateman, 2013 [18] | IND/GLY 110/50 | 475 | 76.4 | 64.0 | 40.5 | Severe: 34.0 | 57.0 | 6.0 | NR | 1.3 | 1.5 | NR | 55.7 |
| IND 150 | 477 | 74.4 | 63.6 | 38.7 | Severe: 38.2 | 57.0 | 6.3 | NR | 1.3 | 1.5 | NR | 54.9 | |
| GLY 50 | 475 | 77.2 | 64.3 | 40.0 | Severe: 36.6 | 58.0 | 6.5 | NR | 1.3 | 1.5 | NR | 55.1 | |
| TIO 18 | 483 | 75.0 | 63.5 | 39.4 | Severe: 38.3 | 59.0 | 6.1 | NR | 1.3 | 1.5 | NR | 55.1 | |
| PBO | 234 | 72.8 | 64.4 | 40.1 | Severe: 32.3 | 58.0 | 6.4 | NR | 1.3 | 1.5 | NR | 55.2 | |
| Buhl, 2015 [52] | IND/GLY 110/50 | 476 | 66.6 | 62.6 | 49.2 | 41.7 | 42.0 | 6.5 | 41.1 | 1.3 | 1.6 | 53.3 | NR |
|
TIO 18 + FOR 12 |
458 | 65.1 | 63.1 | 48.9 | 43 | 40.0 | 6.8 | 41.8 | 1.3 | 1.5 | 53.0 | NR | |
| Tashkin, 2009 [53] |
TIO 18 + FOR 12 |
124 | 65.0 | 63.8 | 49.0 | NR | 27.0 | NR | NR | NR | NR | NR | NR |
| TIO 18 | 131 | 68.0 | 63.9 | 46.0 | NR | 27.0 | NR | NR | NR | NR | NR | NR | |
| Frith, 2018 [54] | IND/GLY 110/50 | 248 | 88.7 | 65.0 | 36.7 | 46.0 | 100.0 | 6.4 | 44.3 | NR | NR | NR | 51.3 |
| SAL/FF 50/500 | 250 | 89.6 | 65.1 | 38.0 | 46.6 | 100.0 | 6.4 | 45.3 | NR | NR | NR | 51.7 | |
| Celli, 2014 [55] | UMEC 125 | 407 | 66.0 | 63.1 | 53.0 |
GOLD 3: 44.0 GOLD 4: 8.0 |
47.0 | NR | 44.0 | NR | NR | NR | 48.8 |
| VI 25 | 404 | 66.0 | 62.8 | 52.0 |
GOLD 3: 40.0 GOLD 4: 9.0 |
47.0 | NR | 42.8 | NR | NR | NR | 48.5 | |
| UMEC/VI 125/25 | 403 | 66.0 | 63.4 | 50.0 |
GOLD 3: 47.0 GOLD 4: 9.0 |
44.0 | NR | 45.4 | NR | NR | NR | 47.7 | |
| PBO | 275 | 64.0 | 62.2 | 52.0 |
GOLD 3: 48.0 GOLD 4: 8.0 |
50.0 | NR | 43.6 | NR | NR | NR | 47.6 | |
| Singh, 2015 [56] | UMEC/VI 62.5/25 | 358 | 73.0 | 61.8 | 57.0 | GOLD D: 46.0 | NR | 6.6 | 40.7 | 1.4 | 1.6 | NR | 50.2 |
| SAL/FP 50/500 | 358 | 71.0 | 61.4 | 61.0 | GOLD D: 44.0 | NR | 6.6 | 39.4 | 1.5 | 1.6 | NR | 51.1 | |
| Donohue, 2015 [57] (DB2114930) | UMEC/VI 62.5/25 | 353 | 72.0 | 62.5 | 45.0 | 52.0 | NR | –d | 43.2 | 1.3 | 1.4 | NR | 49.2 |
| SAL/FP 50/250 | 353 | 69.0 | 63.0 | 41.0 | 50.0 | NR | –d | 41.7 | 1.3 | 1.5 | NR | 49.6 | |
| Donohue, 2015 [57] (DB2114951) | UMEC/VI 62.5/25 | 349 | 76.0 | 63.2 | 51.0 | 50.0 | NR | –d | 43.8 | 1.3 | 1.5 | NR | 49.4 |
| SAL/FP 50/250 | 348 | 76.0 | 64.0 | 53.0 | 50.0 | NR | –d | 44.5 | 1.3 | 1.5 | NR | 49.5 | |
| Vogelmeier, 2013 [58] | IND/GLY 110/50 | 258 | 70.2 | 63.2 | 47.7 | 19.8 | 32.9 | 6.4 | 40.7 | 1.5 | 1.7 | 51.1 | 60.5 |
| SAL/FF 50/500 | 264 | 71.6 | 63.4 | 48.1 | 19.7 | 37.1 | 7.5 | 39.6 | 1.4 | 1.7 | 50.7 | 60.0 | |
| Zhong, 2015 [59] | IND/GLY 110/50 | 372 | 91.7 | 64.8 | 26.0 | 47.0 | 55.4 | 5.2 | NR | 1.3 | 1.3 | NR | 51.6 |
| SAL/FF 50/500 | 369 | 89.7 | 65.3 | 26.0 | 45.8 | 54.2 | 5.1 | NR | 1.2 | 1.3 | NR | 52.0 | |
| Hoshino, 2015 [60] | TIO 18 + IND 150 | 22 | 81.8 | 72.0 | NR | NR | NR | NR | 56.2 | 1.4 | NR | 61.9 | NR |
| SAL/FF 50/250 | 21 | 85.7 | 69.0 | NR | NR | NR | NR | 60.4 | 1.4 | NR | 60.8 | NR | |
| Singh, 2014 [61] | ACL/FOR 400/12 | 385 | 67.8 | 62.7 | 47.0 | 40.5 | 22.1 | NR | NR | 1.4 | NR | NR | 54.6 |
| ACL/FOR 400/6 | 381 | 68.0 | 62.9 | 47.8 | 39.6 | 18.9 | NR | NR | 1.4 | NR | NR | 54.1 | |
| ACL 400 | 385 | 66.5 | 63.1 | 47.3 | 40.9 | 20.5 | NR | NR | 1.4 | NR | NR | 53.6 | |
| PBO | 194 | 71.1 | 64.2 | 48.5 | 39.9 | 20.1 | NR | NR | 1.4 | NR | NR | 55.0 | |
| FOR 12 | 384 | 66.4 | 63.4 | 46.6 | 37.6 | 17.7 | NR | NR | 1.4 | NR | NR | 54.5 | |
| ZuWallack, 2014 [62] (ANHELTO 1) | TIO 18 | 565 | 50.4 | 64.8 | 52.2 | 40.2 | 37.9 | 7.9 | 52.7 | 1.3 | 1.5 | NR | 53.9 |
|
TIO 18 + OLO 5 |
567 | 49.2 | 64.3 | 49.7 | 39.5 | 35.8 | 8.5 | 54.0 | 1.2 | 1.5 | NR | 54.2 | |
| ZuWallack, 2014 [62] (ANHELTO 2) | TIO 18 | 569 | 53.3 | 63.6 | 48.2 | 44.3 | 37.8 | 7.1 | 51.4 | 1.3 | 1.4 | NR | 53.0 |
|
TIO 18 + OLO 5 |
566 | 53.9 | 64.6 | 45.8 | 40.3 | 37.6 | 8.2 | 53.9 | 1.3 | 1.5 | NR | 53.6 | |
| Dahl, 2013 [63] | IND/GLY 110/50 | 225 | 77.3 | 62.5 | 45.3 | 31.1 | 45.8 | 5.8 | 36.3 | 1.4 | 1.6 | NR | 56.4 |
| PBO | 113 | 76.1 | 62.9 | 45.1 | 19.5 | 38.9 | 5.5 | 38.1 | 1.5 | 1.7 | NR | 59.4 | |
| Buhl, 2015b [19] (TONADO 1) | OLO 5 | 528 | 73.1 | 63.7 | 37.1 | 51.3 | 47.2 | NR | NR | 1.2 | 1.4 | 49.9 | NR |
| TIO 2.5 | 525 | 74.7 | 64.2 | 41.0 | 48.6 | 46.5 | NR | NR | 1.3 | 1.4 | 50.9 | NR | |
| TIO 5 | 527 | 72.7 | 64.2 | 35.7 | 50.1 | 45.0 | NR | NR | 1.2 | 1.4 | 49.7 | NR | |
|
TIO/OLO 2.5/5 |
522 | 74.5 | 64.1 | 37.5 | 48.5 | 49.8 | NR | NR | 1.2 | 1.4 | 50.5 | NR | |
| TIO/OLO 5/5 | 522 | 73.6 | 64.8 | 36.2 | 50.6 | 51.7 | NR | NR | 1.2 | 1.3 | 49.5 | NR | |
| Buhl, 2015b [19] (TONADO 2) | OLO 5 | 510 | 74.1 | 64.7 | 35.7 | 46.0 | 50.2 | NR | NR | 1.2 | 1.4 | 50.7 | NR |
| TIO 2.5 | 507 | 71.2 | 63.9 | 34.1 | 50.7 | 45.8 | NR | NR | 1.2 | 1.4 | 49.7 | NR | |
| TIO 5 | 506 | 73.5 | 63.5 | 36.0 | 49.6 | 45.3 | NR | NR | 1.2 | 1.4 | 49.7 | NR | |
|
TIO/OLO 2.5/5 |
508 | 72.4 | 64.1 | 34.6 | 50.6 | 45.9 | NR | NR | 1.2 | 1.4 | 50.0 | NR | |
| TIO/OLO 5/5 | 507 | 68.8 | 62.7 | 41.6 | 51.8 | 46.5 | NR | NR | 1.2 | 1.4 | 49.1 | NR |
ACL aclidinium, COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, GOLD Global Initiative for Chronic Obstructive Lung Disease, ICS inhaled corticosteroid, IND indacaterol, ITT intent-to-treat, LABA long-acting β2-agonist, MDI metered dose inhaler, NMA network meta-analysis, NR not reported, OLO olodaterol, PBO placebo, SAL salmeterol, TIO tiotropium, UMEC umeclidinium, VI vilanterol
aReported data are mean or median, as reported in the corresponding study. If both the mean and median were reported, the mean is presented
bAvailable from clinicalstudydatarequest.com
cThe extension trial AUGMENT EXTENSION was not counted as a unique trial but secondary publication to main trial
dDuration of COPD reported by category (< 1 year, ≥ 1 to < 5 years, ≥ 5 to < 10 years and ≥ 10 years)
Lung Function
Trough FEV1 data were available from 22 and 44 studies at 24 and 12 weeks, respectively (Supplementary Fig. S1). Not all comparators had data available at 24 weeks, including tiotropium/olodaterol (TIO/OLO) fixed-dose and open combinations. The networks of evidence showed that the TONADO 1 and TONADO 2 studies formed a disconnected network at 24 weeks (Supplementary Fig. S1A). In the FE model at 24 weeks, change from baseline in trough FEV1 was statistically significant in favour of UMEC/VI versus all comparators, including dual therapies (Fig. 2a) and monotherapies (Fig. 2b). Mean difference in change from baseline in trough FEV1 (95% CI) versus dual therapies was 108.87 ml (79.27, 138.47) versus aclidinium/formoterol (ACL/FOR) 400/6 µg, 97.50 ml (72.89, 122.11) versus ACL/FOR 400/12 µg, 74.19 ml (55.06, 93.31) versus glycopyrronium (GLY)/FOR 18/9.6 µg, 39.43 ml (19.56, 59.30) versus indacaterol (IND)/GLY 110/50 µg, and 107.43 ml (70.18, 144.67) versus TIO 18 µg + FOR 12 µg. UMEC/VI showed clinically meaningful improvements (treatment difference ≥ 100 ml) [17] compared with ACL/FOR 400/6 µg and TIO 18 µg + FOR 12 µg dual therapies. At 12 weeks, UMEC/VI provided statistically significantly greater improvements in trough FEV1 than all dual therapies, with the exception of GLY/IND (Supplementary Fig. S2A), and provided greater improvements than all monotherapies and placebo (Supplementary Fig. S2B). All therapies were significantly more effective than placebo in increasing trough FEV1, with UMEC/VI providing the largest improvements at both time points (Supplementary Figs. S3 and S4). The RE model produced consistent results (Supplementary Tables S1 and S2).
Fig. 2.
Fixed effects model of mean difference in change from baseline in trough FEV1 of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 35.33%; Q = 44.84, p = 0.0305. ACL aclidinium, CFB change from baseline, CI confidence interval, FEV1 forced expiratory volume in 1 s, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, TIO tiotropium, UMEC umeclidinium, VI vilanterol
Health-Related Quality of Life
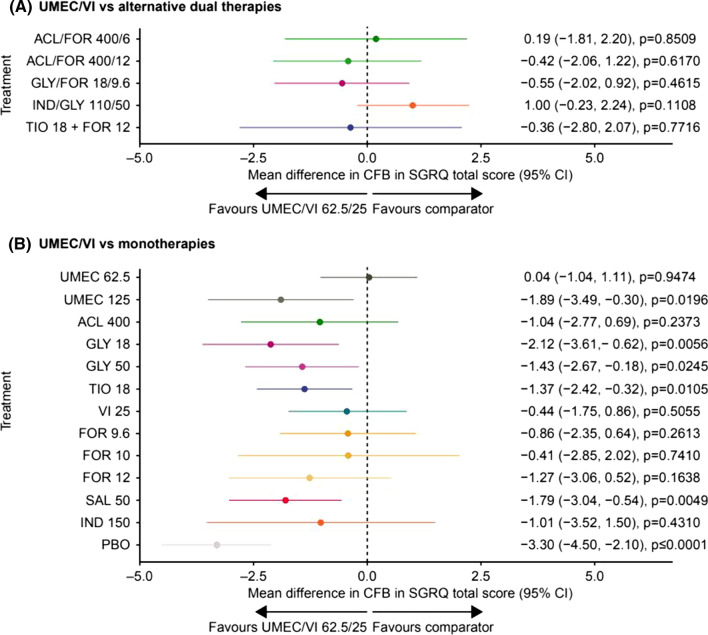
SGRQ total score was available in 17 studies at 24 weeks (Supplementary Fig. S5) and 20 studies at 12 weeks. In the FE model at 24 weeks, there was no evidence of any statistically significant differences in SGRQ total score with UMEC/VI compared with all other LAMA/LABAs (Fig. 3a), whereas UMEC/VI provided statistically significantly greater improvements versus UMEC 125 μg (mean difference in change from baseline [95% CI] − 1.89 [− 3.49, − 0.30), GLY 18 μg (− 2.12 [− 3.61, − 0.62]), GLY 50 μg (− 1.43 [− 2.67, − 0.18]), TIO 18 μg (− 1.37 [− 2.42, − 0.32]), and salmeterol (SAL) 50 μg (− 1.79 [− 3.04, − 0.54]), but not versus other monotherapies (Fig. 3b). At 12 weeks, improvements in SGRQ total score with UMEC/VI were not statistically significant compared with other LAMA/LABA combinations; statistically significant improvements were seen for IND/GLY 110/50 μg and TIO 18 µg + IND 150 µg compared with UMEC/VI (Supplementary Table S2). UMEC/VI provided statistically significantly greater improvements in SGRQ total score at 12 weeks than TIO 18 μg and SAL 50 μg, but not compared with the other monotherapies (Supplementary Table S2). In the FE model, all treatments provided statistically significant improvements in SGRQ total score versus placebo, with the exception of UMEC 125 μg at 24 and 12 weeks and SAL 50 μg and IND 150 μg at 24 weeks (Supplementary Tables S3 and S4).
Fig. 3.
Fixed effects model of mean difference in change from baseline in SGRQ total score of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 22.49%; Q = 32.25; p = 0.1508. ACL aclidinium, CFB change from baseline, CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, SGRQ St George’s Respiratory Questionnaire, TIO tiotropium, UMEC umeclidinium, VI vilanterol
SGRQ responder analyses were available in 14 and 12 studies at 24 (Supplementary Fig. S6) and 12 weeks, respectively. At 24 weeks, UMEC/VI was associated with a statistically significantly greater proportion of SGRQ responders compared with UMEC 62.5 μg (SGRQ responders odds ratio [95% CI] 1.19 [1.02, 1.40]), GLY 18 μg (1.54 [1.22, 1.95]), TIO 18 μg (1.18 [1.00, 1.39]), FOR 9.6 μg (1.36 [1.08, 1.72]) and SAL 50 μg (1.47 [1.22, 1.78]) (Supplementary Fig. S7). At 12 weeks, the odds of being a responder were significantly greater with UMEC/VI versus UMEC 62.5 μg, ACL 400 μg and SAL 50 μg (Supplementary Table S2). All treatments provided statistically significantly greater proportion of SGRQ responders compared with placebo, with the exception of GLY 18 μg at 24 weeks; ACL 400 μg and FOR 12 μg at 12 weeks; and SAL 50 μg at both time points (Supplementary Tables S3 and S4).
The RE model produced consistent results (Supplementary Tables S1–S4).
Breathlessness
Breathlessness as measured by TDI focal score was available in 14 studies at 24 weeks (Supplementary Fig. S8) and 21 studies at 12 weeks. In the FE model at 24 weeks, TDI focal score was statistically significant in favour of UMEC/VI versus GLY/FOR 18/9.6 µg (mean difference in change from baseline [95% CI] 0.33 [0.13, 0.52]), UMEC 62.5 µg (0.32 [0.08, 0.57]), UMEC 125 µg (0.55 [0.16, 0.93]), GLY 18 µg (0.68 [0.32, 1.04]), TIO 18 µg (0.34 [0.03, 0.64)], VI 25 µg (0.42 [0.13, 0.71]), FOR 9.6 µg (0.48 [0.11, 0.84]) and SAL 50 µg (0.43 [0.14, 0.72]) (Fig. 4). At 12 weeks, UMEC/VI provided statistically significantly greater improvements in TDI focal score than UMEC 62.5 µg, UMEC 125 µg, TIO 18 µg, VI 25 µg and SAL 50 µg; IND/GLY 27.5/15.6 µg, TIO/OLO 2.5/5 µg and TIO/OLO 5/5 µg provided statistically significantly greater improvements in TDI focal score than UMEC/VI (Supplementary Table S2). At both time points, all therapies provided statistically significantly greater improvements than placebo, with the exception of TIO 18 µg + FOR 12 µg at 12 weeks (Supplementary Tables S3 and S4).
Fig. 4.
Fixed effects model of mean difference in change from baseline in TDI focal score of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 0%; Q = 12.60; p = 0.4793. ACL aclidinium, CFB change from baseline, CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, TDI Transitional Dyspnoea Index, TIO tiotropium, UMEC umeclidinium, VI vilanterol
TDI responder analyses were available for 10 and 11 studies at 24 (Supplementary Fig. S9) and 12 weeks, respectively. In the FE model, the odds of being a responder were statistically significantly greater with UMEC/VI versus GLY 15.6 µg and TIO 18 µg at 12 weeks, and versus UMEC 62.5 µg, VI 25 µg and SAL 50 µg at both 24 and 12 weeks (TDI responders odds ratio at 24 weeks [95% CI]; UMEC 62.5 µg: 1.33 [1.14, 1.56]; VI 25 µg: 1.38 [1.14, 1.67]; SAL 50 µg: 1.41 [1.17, 1.70]) (Supplementary Fig. S10; Supplementary Table S2). At both time points, all therapies provided statistically significantly greater proportion of TDI responders than placebo, with the exception of TIO 18 µg + FOR 12 µg at 24 weeks (Supplementary Tables S3 and S4).
The RE model produced consistent results (Supplementary Tables S1–4).
Rescue Medication Use
Rescue medication use data were available in 14 studies at 24 weeks and 15 studies at 12 weeks. At 24 weeks, the ILLUMINATE study was disconnected from the network (Supplementary Fig. S11). In the FE model at 24 weeks, change from baseline in rescue medication use was statistically significant in favour of UMEC/VI versus ACL/FOR 400/12 µg (mean difference in change from baseline [95% CI] − 0.46 [− 0.66, − 0.25]) and all monotherapies (mean difference in change from baseline [95% CI]; UMEC 62.5 µg: − 0.33 [− 0.48, − 0.18]; UMEC 125 µg: − 0.36 [− 0.72, − 0.01]; ACL 400 µg: − 0.37 [− 0.61, − 0.12]; GLY 18 µg: − 0.58 [− 0.80, − 0.37]; GLY 50 µg: − 0.86 [− 1.24, − 0.48]; TIO 18 µg: − 0.50 [− 0.51, − 0.49]; FOR 9.6 µg: − 0.27 [− 0.49, − 0.06]; FOR 12 µg: − 0.27 [− 0.53, 0.00]; SAL 50 µg: − 0.28 [− 0.43, − 0.13]; IND 150 µg: − 0.51 [− 0.89, − 0.13]), with the exception of VI 25 µg: − 0.29 (− 0.64, 0.06) (Fig. 5). At 12 weeks, UMEC/VI provided statistically significantly greater improvements in rescue medication use than TIO/OLO 5/5 µg, UMEC 62.5 µg, TIO 18 µg and SAL 50 µg; IND/GLY 27.5/15.6 µg and TIO 18 µg + IND 150 µg provided statistically significantly greater improvements in rescue medication use than UMEC/VI (Supplementary Table S2). All treatments provided statistically significantly greater improvements in rescue medication use compared with placebo, with the exception of GLY 50 µg at 24 weeks and UMEC 125 µg at 12 weeks (Supplementary Tables S3 and S4).
Fig. 5.
Fixed effects model of mean difference in change from baseline in rescue medication use of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 49.21%; Q = 33.47; p = 0.0098. ACL aclidinium, CFB change from baseline, CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, TIO tiotropium, UMEC umeclidinium, VI vilanterol
Some differences were observed in the findings of the RE model for rescue medication use. At 24 weeks, change from baseline in rescue medication use was statistically significant in favour of UMEC/VI versus ACL/FOR 400/12 µg, ACL 400 µg, GLY 18 µg, GLY 50 µg and TIO 18 µg (Supplementary Table S1). At 12 weeks, UMEC/VI provided statistically significantly greater improvements versus TIO 18 µg only (Supplementary Table S2).
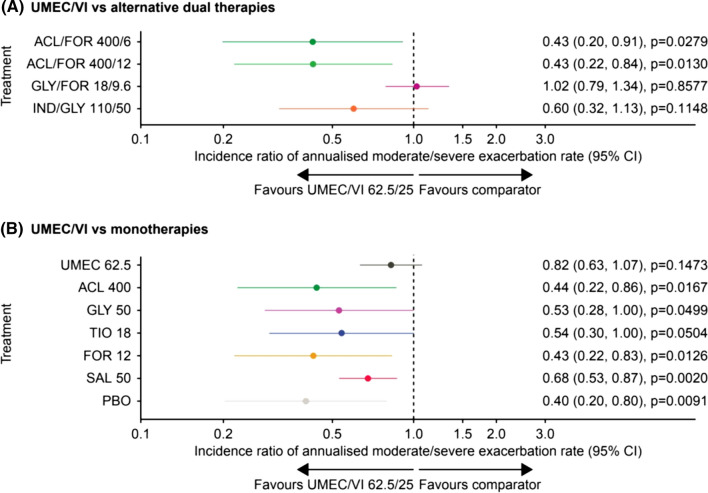
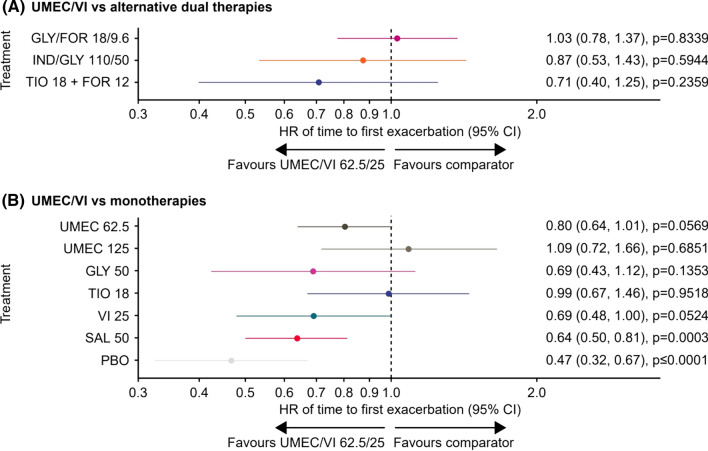
Annualised Moderate/Severe Exacerbations Rates
Moderate/severe exacerbation data were available in nine studies (Supplementary Fig. S12). In the FE model, UMEC/VI provided statistically significantly lower annualised rates of moderate/severe exacerbations versus ACL/FOR 400/6 µg (incidence rate ratio [95% CI] 0.43 [0.20, 0.91]) and ACL/FOR 400/12 µg (0.43 [0.22, 0.84]), and versus all monotherapies with the exception of UMEC 62.5 µg and TIO 18 µg (Fig. 6). Only UMEC/VI 62.5/25 µg, GLY/FOR 18/9.6 µg and IND/GLY 110/50 µg provided statistically significantly lower annualised rates of moderate/severe exacerbations versus placebo, with IND/GLY 110/50 µg providing statistically significant improvements only in the RE model (Supplementary Table S3).
Fig. 6.
Fixed effects model of incidence ratio of annualised moderate/severe exacerbations rates of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 38.86%; Q = 6.54; p = 0.1622. ACL aclidinium, CFB change from baseline, CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, IND indacaterol, PBO placebo, SAL salmeterol, TIO tiotropium, UMEC umeclidinium, VI vilanterol
Time to First Exacerbation
In total, 12 studies had data available for time to first exacerbation (Supplementary Fig. S13). In the FE model, hazard of a first exacerbation was statistically significantly lower with UMEC/VI versus SAL 50 µg (hazard ratio [95% CI] 0.64 [0.50, 0.81]) and placebo (0.47 [0.32, 0.67]) (Fig. 7), which was consistent with the RE model (Supplementary Table S1). In the FE model, among dual therapies, UMEC/VI 62.5/25 µg, IND/GLY 110/50 µg and GLY/FOR 18/9.6 µg provided statistically significantly lower hazard of a first exacerbation than placebo, with IND/GLY 110/50 µg only providing statistically significant improvements versus placebo in the RE model (Supplementary Table S3). UMEC 62.5 µg, UMEC 125 µg and TIO 18 µg monotherapies provided statistically significant reductions in hazard of a first exacerbation versus placebo; results from the RE model were similar (Supplementary Table S3).
Fig. 7.
Fixed effects model of hazard ratio of time to first exacerbation of UMEC/VI versus a dual therapy and b monotherapy at 24 weeks. Assessment of heterogeneity/inconsistency: I2 = 35.54%; Q = 10.86; p = 0.1448. CI confidence interval, FOR formoterol fumarate, FP fluticasone propionate, GLY glycopyrronium, HR hazard ratio, IND indacaterol, PBO placebo, SAL salmeterol, TIO tiotropium, UMEC umeclidinium, VI vilanterol
Safety Overview
Safety outcomes were not analysed in the NMA, but are summarised for the included studies in Supplementary Table S5. Briefly, the percentage of patients with ≥1 adverse event (AE) ranged from 21% to 94%, and the percentage with ≥1 serious AE from 0.6% to 24.0%. Pneumonia was reported in 0–16.0% of patients. In total, 0.2–33.0% of patients withdrew, and 0.5–10.6% of early withdrawals were due to AEs. On-treatment mortality was reported for 0–3.1% of patients.
Discussion
This study evaluated the comparative efficacy of UMEC/VI dual therapy versus other mono and dual therapies in symptomatic patients with COPD at 24 and 12 weeks. Treatment with dual therapy resulted in greater improvements in lung function as measured by trough FEV1 compared with LABA or LAMA monotherapy, with improvements in HRQoL, symptoms, rescue medication use and moderate/severe exacerbation rates seen in some comparisons; AEs were also summarised and showed a similar safety profile across the treatments of interest.
At both time points, all treatments provided greater improvements in lung function compared with placebo; the two UMEC formulations performed better than other monotherapies, and the twice-daily LABAs (SAL 50 µg; FOR 12 µg and FOR 9.6 µg) performed worse than other LABAs. Treatment with UMEC/VI resulted in significant improvements in lung function as measured by trough FEV1 compared with all LAMA and LABA monotherapies. These findings support existing evidence from clinical trials that dual therapy provides greater improvements than monotherapies [18–22], and suggest that starting treatment with dual therapy may improve outcomes for many symptomatic patients compared with a stepwise approach to treatment escalation [1]. The non-interventional prospective DETECT study in 3653 patients showed improvements in lung function, QoL and morning symptoms in the year following a switch from prior treatment (most commonly bronchodilator monotherapy) to fixed-dose ACL/FOR, GLY/IND or UMEC/VI [23]. Previous NMAs have also demonstrated that LAMA/LABA dual therapy is more effective than LAMA or LABA monotherapy in terms of lung function improvement [4, 5, 8]. Notably, while many trials comparing dual bronchodilator therapies with monotherapy are likely to have been affected by the confounding effects of concomitant ICS use, the EMAX trial has demonstrated that UMEC/VI provides better outcomes than UMEC and SAL in symptomatic patients at low exacerbations risk not taking concomitant ICS [22]. Taken together, this evidence suggests that dual therapy may be an appropriate initial maintenance therapy in this patient population, as recommended by the ATS and NICE guidelines [2, 3]. However, it should be noted that the present study also supported the efficacy of LAMA and LABA monotherapies compared with placebo.
At both time points, UMEC/VI demonstrated larger improvements in lung function than other dual therapies. The smallest treatment differences were seen with UMEC/VI versus IND/GLY formulations, while the other dual therapies performed similarly; clinically meaningful differences (based on a mean difference in change from baseline of ≥ 100 ml) [17] were seen with UMEC/VI versus some dual therapies at 24 weeks. Treatment effects on SGRQ total score, TDI focal score, rescue medication use and moderate/severe exacerbations were less clear. The results of this updated NMA are consistent with head-to-head trials. The AERISTO trial in 1119 symptomatic patients with moderate-to-very severe COPD found that GLY/FOR was non-inferior to UMEC/VI when comparing treatment effects on peak FEV1, but was inferior when comparing the co-primary endpoint of trough FEV1 [24]. UMEC/VI provided numerical improvements compared with GLY/FOR on symptoms outcomes (TDI focal score and COPD Assessment Test score) in the AERISTO trial, although these were not considered clinically significant [24]. Another head-to-head study showed that UMEC/VI provided statistically significant increases in lung function-related outcomes compared with TIO/OLO in 236 symptomatic patients [25]. Results for patient-reported outcomes (PRO) were less clear; while UMEC/VI provided a significantly greater reduction in rescue medication use during the study and a significant decrease in CAT score at week 4 versus TIO/OLO, there were no consistent treatment differences on exacerbations or other symptoms outcomes, although the study was not powered to demonstrate treatment effects on these measures [25].
Despite the efficacy gradient suggested by the findings of head-to-head trials, previous NMAs have shown mixed results when comparing treatments within the LAMA/LABA class. An NMA of studies in moderate-to-very severe patients with COPD found that TIO/OLO 5/5 µg was ranked highest on efficacy and cardiovascular safety when compared with alternative LAMA/LABAs, including UMEC/VI 62.5/25 µg; however, SGRQ and TDI response rates, rescue medication use, and moderate/severe exacerbations were not included in the analysis [26]. Another previous NMA including RCTs of patients with stable COPD compared six LAMA/LABA dual therapies, and reported that UMEC/VI reduced total exacerbation events compared with alternative LAMA/LABAs (with the exception of GLY/FOR) in one network, although no statistically significant differences were seen in an alternative network [19]. Similarly, the current updated NMA found that UMEC/VI reduced the rate of moderate/severe exacerbations versus two formulations of ACL/FOR, but not GLY/FOR 18/9.6 µg or IND/GLY 110/50 µg. Taken together, the evidence from head-to-head trials and NMAs generally supports a gradient of effectiveness within the LAMA/LABA class, with UMEC/VI providing greater improvements in lung function compared with other LAMA/LABAs, and improvements in other outcomes including exacerbations and PROs in some analyses. The relationship between improvement in lung function and PROs remains unclear; a pooled analysis of 23 clinical trials in COPD found a correlation between improvement in lung function and PRO improvements [27], but disparities have been noted in other studies [28, 29]. This dissociation may be related to factors such as study design, patient population, and statistical power.
This NMA is an updated extension of our original NMA comparing the efficacy of UMEC/VI with fixed-dose and open LAMA/LABA combinations, with TIO, and with placebo [6]. In the present study we have used well-established frequentist NMA approach to compare UMEC/VI with dual therapies and monotherapies, and we have incorporated a larger number of studies, with an additional five RCTs included in the updated NMA. The updated NMA also included additional outcomes such as the rate of moderate/severe exacerbations, time to first moderate/severe exacerbation, and responder analyses for symptoms and health status outcomes. Similar to our original NMA [6] and to a previous NMA that compared RCTs of patients with COPD comparing five combinations of LAMA/LABA dual therapies [4], the present study also found evidence for a potential gradient of effectiveness in treatment effects within the LAMA/LABA class. However, this updated NMA did not analyse certain outcomes relevant to patients with COPD, such as exercise capacity, because of lack of sufficient clinical trial data to construct networks.
A general strength of NMA approaches is the inclusion of a broader population than individual RCTs; specific strengths of this study include the greater number of studies available for inclusion in the NMA compared with similar previous analyses, which allowed for a better estimation of relative treatment effects. Potential general limitations of NMA approaches include a risk of unknown imbalances in effect modifiers and residual confounding bias. Only RCTs were included in this study, eliminating the risk of bias due to imbalanced effect modifiers within the trials as a result of randomisation. However, randomisation does not control for known treatment effect modifiers across studies, and unknown treatment effect modifiers could still bias the results. Another potential general limitation of NMA approaches is the risk of violation of the three assumptions of similarity, consistency and transitivity. In this study, the similarity assumption (i.e. the comparability of baseline characteristics) and consistency assumption (tested through heterogeneity statistics such as I2, the Q statistic and the corresponding p value) were deemed to hold, providing certainty that the transitivity assumption also holds. This study was also limited by the availability of data for inclusion in the NMA, which in some cases included relatively small sample sizes and sparse networks of evidence. This resulted in wide CIs which in some cases covered the decision threshold, raising the possibility that potential differences between interventions were not identified.
Conclusion
The findings of this SLR and NMA suggest that UMEC/VI provides better outcomes in terms of lung function (as measured by change from baseline in trough FEV1) with a similar safety profile compared with LAMA and LABA monotherapies and other LAMA/LABA dual therapies. UMEC/VI also provided additional benefits on HRQoL, symptoms, rescue medication use and moderate/severe exacerbations compared with some monotherapies. These results suggest that treatment with UMEC/VI dual therapy may improve outcomes for symptomatic patients with COPD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study/analysis was funded by GSK (GSK study 214886). GSK-affiliated authors had a role in study design, data analysis, data interpretation, and writing of the report, and GSK funded the journal’s Rapid Service and Open Access Fees.
Medical Writing, Editorial and Other Assistance
Editorial support (in the form of writing assistance during development of the initial draft, assembling tables and figures, collating authors comments, grammatical editing, and referencing) was provided by Maria Guillermina Casabona, PhD, and Mark Condon, DPhil, of Fishawack Indicia Ltd, UK, part of Fishawack Health, and was funded by GSK.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Author Contributions
All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. ASI, KH, AC, VT, MM and MN were involved in the conception and design of the study, the acquisition of data, and data analysis and interpretation. JA, MZ-G, YS and XD were involved in the acquisition of data and data analysis and interpretation. MD and CC were involved in the conception and design of the study, and in data analysis and interpretation. CFV and DMGH were involved in the data analysis and interpretation. All authors contributed to the writing and reviewing of the manuscript, and have approved the final manuscript.
Prior Presentation
An abstract reporting some of the data included in this manuscript was presented at the 2022 American Thoracic Society international conference.
Disclosures
Afisi S. Ismaila, Alexandrosz Czira, Maria Duarte and Chris Compton are employees of GSK and hold stock and shares at GSK. Afisi S. Ismaila is also a part-time member of the McMaster University faculty. Katrin Haeussler, Vanita Tongbram, Mia Malmenäs, Jatin Agarwal, Maria Nassin, Marija Živković-Gojović, Yunrong Shen and Xinzhe Dong are employees of ICON (or were at the time of the study), which received funding from GSK to conduct the study. Claus F. Vogelmeier has received grants from AstraZeneca, Boehringer Ingelheim, GSK, Grifols and Novartis, and has received lecturing and personal fees from AstraZeneca, Boehringer Ingelheim, Berlin Chemie/Menarini, Chiesi, CSL Behring, GSK, Grifols, MedUpdate, Novartis, Aerogen and Nuvaira. David M.G. Halpin has received personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer and Sanofi, and non-financial support from Boehringer Ingelheim and Novartis.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com. Extracted data summaries are available upon request to the authors.
Footnotes
The original version was revised due to update in figure 4.
Change history
9/2/2022
A Correction to this paper has been published: 10.1007/s12325-022-02288-x
References
- 1.Global Initiative for Chronic Obstructive Pulmonary Disease. Global strategy for prevention, diagnosis and management of COPD. 2022. https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf. Accessed December 2021.
- 2.National Institute of Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. 2018. https://www.nice.org.uk/guidance/ng115/resources/chronic-obstructive-pulmonary-disease-in-over-16s-diagnosis-and-management-pdf-66141600098245. Accessed July 2021. [PubMed]
- 3.Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201:e56–e69. doi: 10.1164/rccm.202003-0625ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149:1181–1196. doi: 10.1016/j.chest.2016.02.646. [DOI] [PubMed] [Google Scholar]
- 5.Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting beta-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71:15–25. doi: 10.1136/thoraxjnl-2014-206732. [DOI] [PubMed] [Google Scholar]
- 6.Sion KYJ, Huisman EL, Punekar YS, Naya I, Ismaila AS. A network meta-analysis of long-acting muscarinic antagonist (LAMA) and long-acting beta2-agonist (LABA) combinations in COPD. Pulm Ther. 2017;3:297–316. doi: 10.1007/s41030-017-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aziz MIA, Tan LE, Wu DB, et al. Comparative efficacy of inhaled medications (ICS/LABA, LAMA, LAMA/LABA and SAMA) for COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:3203–3231. doi: 10.2147/COPD.S173472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MK, Ray R, Foo J, Morel C, Hahn B. Systematic literature review and meta-analysis of US-approved LAMA/LABA therapies versus tiotropium in moderate-to-severe COPD. NPJ Prim Care Respir Med. 2018;28:32. doi: 10.1038/s41533-018-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi: 10.2147/COPD.S130482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Thomas J, Chandler J, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021): Cochrane; 2021. www.training.cochrane.org/handbook.
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ades AE. A chain of evidence with mixed comparisons: models for multi-parameter synthesis and consistency of evidence. Stat Med. 2003;22:2995–3016. doi: 10.1002/sim.1566. [DOI] [PubMed] [Google Scholar]
- 13.Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 15.Rücker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods. 2012;3:312–324. doi: 10.1002/jrsm.1058. [DOI] [PubMed] [Google Scholar]
- 16.Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. Package 'netmeta': network meta-analysis using frequentist methods; 2022. https://cran.r-project.org/web/packages/netmeta/netmeta.pdf. Accessed December 2021.
- 17.Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2:111–124. doi: 10.1081/COPD-200053377. [DOI] [PubMed] [Google Scholar]
- 18.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42:1484–1494. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4) Eur Respir J. 2015;45:969–979. doi: 10.1183/09031936.00136014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107:1538–1546. doi: 10.1016/j.rmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Maleki-Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short-term deterioration in maintenance-naive patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three randomized trials. Adv Ther. 2017;33:2188–2199. doi: 10.1007/s12325-016-0430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20:238. doi: 10.1186/s12931-019-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plate T, Friedrich FW, Beier J. Effectiveness and tolerability of LABA/LAMA fixed-dose combinations aclidinium/formoterol, glycopyrronium/indacaterol and umeclidinium/vilanterol in the treatment of COPD in daily practice—results of the non-interventional DETECT study. Int J Chron Obstruct Pulmon Dis. 2020;15:1335–1347. doi: 10.2147/COPD.S252354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maltais F, Ferguson GT, Feldman GJ, et al. A randomized, double-blind, double-dummy study of glycopyrrolate/formoterol fumarate metered dose inhaler relative to umeclidinium/vilanterol dry powder inhaler in COPD. Adv Ther. 2019;36:2434–2449. doi: 10.1007/s12325-019-01015-3. [DOI] [PubMed] [Google Scholar]
- 25.Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in symptomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34:2518–2533. doi: 10.1007/s12325-017-0626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rogliani P, Matera MG, Ritondo BL, et al. Efficacy and cardiovascular safety profile of dual bronchodilation therapy in chronic obstructive pulmonary disease: a bidimensional comparative analysis across fixed-dose combinations. Pulm Pharmacol Ther. 2019;59:101841. doi: 10.1016/j.pupt.2019.101841. [DOI] [PubMed] [Google Scholar]
- 27.Donohue JF, Jones PW, Bartels C, et al. Correlations between FEV1 and patient-reported outcomes: a pooled analysis of 23 clinical trials in patients with chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2018;49:11–19. doi: 10.1016/j.pupt.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Kostikas K, Aalamian-Mattheis M, Pagano VA, et al. Early changes in eDiary COPD symptoms predict clinically relevant treatment response at 12 weeks: analysis from the CRYSTAL study. COPD. 2018;15:185–191. doi: 10.1080/15412555.2018.1445213. [DOI] [PubMed] [Google Scholar]
- 29.Price D, Ostrem A, Thomas M, Welte T. Dual bronchodilation in COPD: lung function and patient-reported outcomes—a review. Int J Chron Obstruct Pulmon Dis. 2017;12:141–168. doi: 10.2147/COPD.S116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obstruct Pulmon Dis. 2018;13:2969–2984. doi: 10.2147/COPD.S171835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh D, Ferguson GT, Bolitschek J, et al. Tiotropium + olodaterol shows clinically meaningful improvements in quality of life. Respir Med. 2015;109:1312–1319. doi: 10.1016/j.rmed.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Vogelmeier C, Kardos P, Harari S, Gans SJ, Stenglein S, Thirlwell J. Formoterol mono- and combination therapy with tiotropium in patients with COPD: a 6-month study. Respir Med. 2008;102:1511–1520. doi: 10.1016/j.rmed.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Maleki-Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: results of a 24-week, randomized, controlled trial. Respir Med. 2014;108:1752–1760. doi: 10.1016/j.rmed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Calverley PMA, Anzueto AR, Carter K, et al. Tiotropium and olodaterol in the prevention of chronic obstructive pulmonary disease exacerbations (DYNAGITO): a double-blind, randomised, parallel-group, active-controlled trial. Lancet Respir Med. 2018;6:337–344. doi: 10.1016/S2213-2600(18)30102-4. [DOI] [PubMed] [Google Scholar]
- 35.Kerwin E, Ferguson GT, Sanjar S, et al. Dual bronchodilation with indacaterol maleate/glycopyrronium bromide compared with umeclidinium bromide/vilanterol in patients with moderate-to-severe COPD: results from two randomized, controlled, cross-over studies. Lung. 2017;195:739–747. doi: 10.1007/s00408-017-0055-9. [DOI] [PubMed] [Google Scholar]
- 36.Kalberg C, O'Dell D, Galkin D, Newlands A, Fahy WA. Dual bronchodilator therapy with umeclidinium/vilanterol versus tiotropium plus indacaterol in chronic obstructive pulmonary disease: a randomized controlled trial. Drugs R D. 2016;16:217–227. doi: 10.1007/s40268-016-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riley JH, Kalberg CJ, Donald A, Lipson DA, Shoaib M, Tombs L. Effects of umeclidinium/vilanterol on exercise endurance in COPD: a randomised study. ERJ Open Res. 2018;4. 10.1183/23120541.00073-2017. [DOI] [PMC free article] [PubMed]
- 38.Mahler DA, D'Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012;67:781–788. doi: 10.1136/thoraxjnl-2011-201140. [DOI] [PubMed] [Google Scholar]
- 39.Vincken W, Aumann J, Chen H, Henley M, McBryan D, Goyal P. Efficacy and safety of coadministration of once-daily indacaterol and glycopyrronium versus indacaterol alone in COPD patients: the GLOW6 study. Int J Chron Obstruct Pulmon Dis. 2014;9:215–228. doi: 10.2147/COPD.S51592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 41.Vogelmeier C, Paggiaro PL, Dorca J, et al. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48:1030–1039. doi: 10.1183/13993003.00216-2016. [DOI] [PubMed] [Google Scholar]
- 42.Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 43.Sethi S, Kerwin E, Watz H, et al. AMPLIFY: a randomized, phase III study evaluating the efficacy and safety of aclidinium/formoterol vs monocomponents and tiotropium in patients with moderate-to-very severe symptomatic COPD. Int J Chron Obstruct Pulmon Dis. 2019;14:667–682. doi: 10.2147/COPD.S189138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Urzo AD, Rennard SI, Kerwin EM, et al. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123. doi: 10.1186/s12931-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D'Urzo A, Rennard S, Kerwin E, et al. A randomised double-blind, placebo-controlled, long-term extension study of the efficacy, safety and tolerability of fixed-dose combinations of aclidinium/formoterol or monotherapy in the treatment of chronic obstructive pulmonary disease. Respir Med. 2017;125:39–48. doi: 10.1016/j.rmed.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson GT, Taylor AF, Thach C, et al. Long-term maintenance bronchodilation with indacaterol/glycopyrrolate versus indacaterol in moderate-to-severe COPD patients: the FLIGHT 3 study. Chronic Obstr Pulm Dis. 2016;3:716–728. doi: 10.15326/jcopdf.3.4.2016.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192:1068–1079. doi: 10.1164/rccm.201505-1048OC. [DOI] [PubMed] [Google Scholar]
- 48.Siler TM, Donald AC, O'Dell D, Church A, Fahy WA. A randomized, parallel-group study to evaluate the efficacy of umeclidinium/vilanterol 62.5/25 mug on health-related quality of life in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:971–979. doi: 10.2147/COPD.S102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kerwin EM, Kalberg CJ, Galkin DV, et al. Umeclidinium/vilanterol as step-up therapy from tiotropium in patients with moderate COPD: a randomized, parallel-group, 12-week study. Int J Chron Obstruct Pulmon Dis. 2017;12:745–755. doi: 10.2147/COPD.S119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donohue JF, Soong W, Wu X, Shrestha P, Lei A. Long-term safety of aclidinium bromide/formoterol fumarate fixed-dose combination: results of a randomized 1-year trial in patients with COPD. Respir Med. 2016;116:41–48. doi: 10.1016/j.rmed.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest. 2017;151:340–357. doi: 10.1016/j.chest.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 52.Buhl R, Gessner C, Schuermann W, et al. Efficacy and safety of once-daily QVA149 compared with the free combination of once-daily tiotropium plus twice-daily formoterol in patients with moderate-to-severe COPD (QUANTIFY): a randomised, non-inferiority study. Thorax. 2015;70:311–319. doi: 10.1136/thoraxjnl-2014-206345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tashkin DP, Pearle J, Iezzoni D, Varghese ST. Formoterol and tiotropium compared with tiotropium alone for treatment of COPD. COPD. 2009;6:17–25. doi: 10.1080/15412550902724073. [DOI] [PubMed] [Google Scholar]
- 54.Frith PA, Ashmawi S, Krishnamurthy S, et al. Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: the FLASH randomized controlled trial. Respirology. 2018;23:1152–1159. doi: 10.1111/resp.13374. [DOI] [PubMed] [Google Scholar]
- 55.Celli B, Crater G, Kilbride S, et al. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145:981–991. doi: 10.1378/chest.13-1579. [DOI] [PubMed] [Google Scholar]
- 56.Singh D, Worsley S, Zhu CQ, Hardaker L, Church A. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;15:91. doi: 10.1186/s12890-015-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109:870–881. doi: 10.1016/j.rmed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 58.Vogelmeier CF, Bateman ED, Pallante J, et al. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 59.Zhong N, Wang C, Zhou X, et al. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–1026. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoshino M, Ohtawa J, Akitsu K. Comparison of airway dimensions with once daily tiotropium plus indacaterol versus twice daily Advair((R)) in chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2015;30:128–133. doi: 10.1016/j.pupt.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. doi: 10.1186/1471-2466-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.ZuWallack R, Allen L, Hernandez G, Ting N, Abrahams R. Efficacy and safety of combining olodaterol Respimat(®) and tiotropium HandiHaler(®) in patients with COPD: results of two randomized, double-blind, active-controlled studies. Int J Chron Obstruct Pulmon Dis. 2014;9:1133–1144. doi: 10.2147/COPD.S72482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dahl R, Chapman KR, Rudolf M, et al. Safety and efficacy of dual bronchodilation with QVA149 in COPD patients: the ENLIGHTEN study. Respir Med. 2013;107:1558–1567. doi: 10.1016/j.rmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com. Extracted data summaries are available upon request to the authors.