Abstract
Introduction
Chronic hypoparathyroidism is associated with higher risk of developing chronic kidney disease compared with the general population. This study evaluated changes in estimated glomerular filtration rate (eGFR) over a 5-year period in adult patients with chronic hypoparathyroidism treated with recombinant parathyroid hormone (1-84), rhPTH(1-84), compared with a historical control cohort of patients who did not receive rhPTH(1-84).
Methods
This retrospective cohort study included patients with chronic hypoparathyroidism treated with rhPTH(1-84) in the REPLACE (NCT00732615), RELAY (NCT01268098), RACE (NCT01297309), and HEXT (NCT01199614 and continuation study NCT02910466) clinical trials. A historical control cohort who did not receive parathyroid hormone but who had enrollment criteria similar to those for the clinical trials was selected from the IBM® Explorys electronic medical record database (January 2007–August 2019). Outcomes of interest were the annual rate of change in eGFR from baseline (i.e., eGFR slope) and the predicted eGFR change from baseline at years 1 through 5.
Results
The study comprised 72 adult patients with chronic hypoparathyroidism treated with rhPTH(1-84) and 176 control patients who did not receive rhPTH(1-84). Over 5 years, eGFR remained stable in the rhPTH(1-84) cohort, whereas eGFR declined at a rate of 1.67 mL/min/1.73 m2 per year in the control cohort (P < 0.001 for eGFR slope in the control cohort). At 5 years, predicted eGFR in the rhPTH(1-84) cohort increased from baseline by 1.21 mL/min/1.73 m2, whereas eGFR in the control cohort declined by 10.36 mL/min/1.73 m2, after adjusting for baseline variables. The difference in eGFR slopes between the cohorts over 5 years was 1.37 mL/min/1.73 m2 per year (95% CI 0.62–2.13; P < 0.001).
Conclusion
Long-term treatment with rhPTH(1-84) was associated with stable eGFR compared with eGFR decline in the controls not treated with rhPTH(1-84). Preservation of renal function conferred by rhPTH(1-84) may benefit patients with chronic hypoparathyroidism by reducing risk of long-term renal complications.
Keywords: Electronic health records, Glomerular filtration rate, Hypoparathyroidism, Parathyroid hormone, Retrospective cohort study, rhPTH(1-84)
Key Summary Points
| Why carry out this study? |
| Patients with chronic hypoparathyroidism treated with conventional therapy consisting of calcium and active vitamin D have increased risk of having a decline in estimated glomerular filtration rate (eGFR). |
| The improved biochemical parameters and stabilization of eGFR observed in patients treated with recombinant human parathyroid hormone (1-84), rhPTH(1-84), in long-term, single-arm clinical trials led us to hypothesize that this treatment may reduce the annual rate of eGFR decline compared with patients not treated with rhPTH(1-84). |
| Given the limited number of clinical studies reporting comparative data for eGFR outcomes in patients treated with rhPTH(1-84) versus controls, the hypothesis was tested by comparing data from an rhPTH(1-84)-treated cohort with a historical control cohort derived from an electronic medical record database. |
| This retrospective cohort study assessed change in eGFR over a 5-year period in 72 adult patients with chronic hypoparathyroidism treated with rhPTH(1-84) in clinical trials compared with 176 patients who did not receive rhPTH(1-84) in a real-world setting. |
| What was learned from the study? |
| After adjusting for clinical and demographic differences between cohorts, eGFR remained stable in patients with chronic hypoparathyroidism treated with rhPTH(1-84) compared with eGFR decline over 5 years in patients who were not treated with rhPTH(1-84). |
Introduction
Numerous retrospective studies have reported that patients with chronic hypoparathyroidism treated with conventional therapy had increased risk of developing kidney complications including chronic kidney disease (CKD) and declining estimated glomerular filtration rate (eGFR) [1–6]. Parathyroid hormone (PTH) is the main regulator of serum calcium and phosphate concentrations. PTH deficiency reduces the ability of the renal tubules to reabsorb calcium in the distal nephron and leads to reduced phosphate excretion, resulting in the characteristic features of hypocalcemia and relatively high serum phosphate concentrations [7, 8].
The conventional therapy for chronic hypoparathyroidism consists of oral calcium supplements and active vitamin D [9, 10], but this treatment can lead to hypercalciuria [7, 8]. Hypercalciuria is one of the principal risk factors for development of nephrolithiasis [11] and nephrocalcinosis [12], and may predispose individuals to deteriorating eGFR and renal insufficiency. In a study from Denmark, patients with hypoparathyroidism (n = 688) had an almost fivefold increased risk of renal insufficiency compared with age- and gender-matched controls (n = 2064) [5]. A retrospective cohort study from the USA showed that patients with chronic hypoparathyroidism (n = 8097) had increased risk of developing incident CKD stage 3 or higher, and had increased risk of decline in eGFR ≥ 30% compared with patients without hypoparathyroidism (n = 40,485) [2]. Moreover, a study conducted in Scotland identified a higher risk of renal failure, defined as eGFR < 30 mL/min, in patients with chronic hypoparathyroidism (n = 280) compared with age- and gender-matched controls (n = 1301) and showed that mean serum calcium concentration was associated with renal failure [6].
Recombinant human parathyroid hormone (1-84), rhPTH(1-84), is approved as adjunctive treatment to calcium supplements and active vitamin D for adults with hypoparathyroidism. In the USA, rhPTH(1-84) is indicated to control hypocalcemia, and in Europe it is indicated to control hypoparathyroidism that cannot be adequately controlled with conventional therapy [13, 14]. In long-term, single-arm clinical trials in patients with chronic hypoparathyroidism, treatment with rhPTH(1-84) was associated with maintenance or improvement in mean serum calcium and phosphate levels despite substantial reduction in the amount of oral calcium and active vitamin D supplementation [15, 16]. In those trials, eGFR levels were stable, and mean urinary calcium excretion was decreased to within normal ranges for women and men [15, 16]. In addition, a 5-year retrospective study in patients with chronic hypoparathyroidism reported that treatment with rhPTH(1-84) was associated with stabilization of eGFR, whereas in patients not treated with rhPTH(1-84), eGFR declined [1].
A sustained eGFR decline of greater than 30% for 2 years or more has been proposed as a meaningful clinical endpoint for CKD outcomes [17]. A meta-analysis of 37 randomized controlled studies showed a strong association between eGFR decline of 30% or 40% and development of established endpoints of kidney disease progression [18]. A meta-analysis of patient-level data from 14 observational cohorts (more than three million individuals) from the CKD Prognosis Consortium showed that a steeper slope of eGFR decline was associated with higher risk of development of end-stage kidney disease (ESKD) and that associations were stronger over longer observation periods [19]. According to the Grams et al. [19] study, if a treatment reduced eGFR decline by 0.75 mL/min/1.73 m2 per year (in a population with a 5-year ESKD risk of 8.3%), the risk of developing ESKD would decrease by 1.6%.
We postulated that maintenance of or improvement in biochemical parameters and stabilization of kidney function associated with treatment with rhPTH(1-84) may mitigate the risk of developing kidney complications and CKD. However, further evidence is needed to establish whether treatment with rhPTH(1-84) reduces risk of eGFR decline in patients with chronic hypoparathyroidism compared with patients not treated with rhPTH(1-84). This study therefore evaluated changes in eGFR over a 5-year period in two cohorts of adult patients with chronic hypoparathyroidism: patients treated with rhPTH(1-84) in clinical trials and a historical control cohort of patients who did not receive rhPTH(1-84).
Methods
Selection of the Patient Population and Study Design
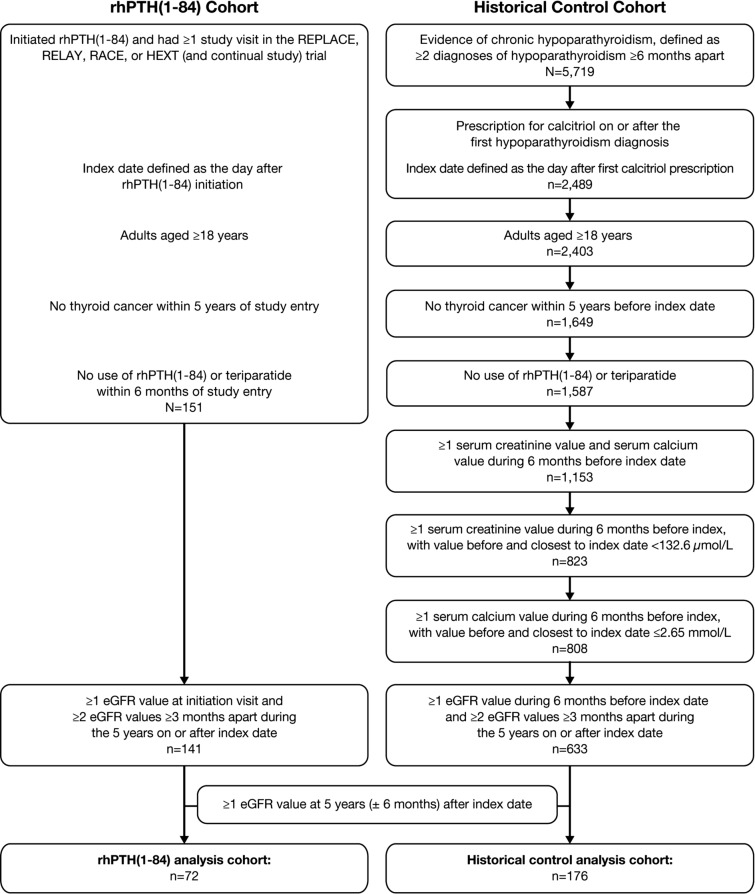
The change in eGFR was evaluated in two cohorts of adult patients with chronic hypoparathyroidism (Fig. 1). The patients treated with rhPTH(1-84) were derived from the REPLACE (NCT00732615), RELAY (NCT01268098), RACE (NCT01297309), and HEXT (NCT01199614 and continuation study NCT02910466) clinical trials. The index date for patients in the rhPTH(1-84) cohort was the day after treatment initiation. The patients in the historical control cohort were not treated with rhPTH(1-84) and were identified from the IBM® Explorys electronic medical record (EMR) database. The EMR database is nationally representative of approximately 15% of the US population. The Explorys EMRs are sourced from ambulatory, inpatient, and post-acute settings and include laboratory measurements, diagnoses, procedures, medications, and demographics. This study used database records from approximately 360 different hospitals and 330,000 different healthcare providers from January 2007 to August 2019.
Fig. 1.
Selection of the study analyses cohorts. eGFR, estimated glomerular filtration rate; rhPTH(1-84), recombinant human parathyroid hormone (1-84)
Patients selected for the control cohort were required to meet criteria that aligned with enrollment criteria in the clinical trials. Patients in the control cohort were therefore required to have at least two diagnoses of hypoparathyroidism occurring at least 6 months apart and at least one prescription for calcitriol after the first diagnosis of hypoparathyroidism, to be at least 18 years old at the index date (for this cohort, the index date was the day after the first calcitriol prescription received on or after the first hypoparathyroidism diagnosis), have no diagnosis of thyroid cancer within 5 years before index date, have no history of rhPTH(1-84) or teriparatide treatments, and have at least one serum creatinine value below 132.6 µmol/L (< 1.5 mg/dL) and at least one serum calcium value of at most 2.65 mmol/L (≤ 10.6 mg/dL) during the 6 months before index date. Hypoparathyroidism was identified in the medical claims using International Classification of Diseases Clinical Modification (ICD-CM) codes ICD-9-CM 252.1 or ICD-10-CM E20.0, E20.8, E20.9, and E89.2.
The analysis cohorts were required to have at least one eGFR measurement at initiation of rhPTH(1-84) treatment or during the 6 months before index date for the control cohort (baseline value), at least two eGFR measurements at least 3 months apart during the 5 years on or after index date, and at least one eGFR measurement at 5 years (± 6 months) after index date. The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [20].
Permission to access and use the trial data was granted by the data owner, Takeda. All patients enrolled in the clinical trials provided written informed consent. Permission to access and use the IBM® Explorys EMR database was granted by IBM. The Explorys database is a nationally representative EMR resource maintained by IBM. Institutional review board approval was not required because the Explorys database consists of pre-existing data that are de-identified in accordance with the Health Insurance Portability and Accountability Act.
Study Outcomes and Statistical Analyses
Outcomes of interest were the annual rate of change in eGFR from baseline (i.e., eGFR slope) and the predicted eGFR change from baseline at years 1 through 5. Continuous variables were reported as means and standard deviations, and categorical variables as frequencies and percentages. Patient demographics and clinical characteristics were compared between cohorts using the Wilcoxon rank-sum tests for continuous variables and chi-square tests for categorical variables.
Difference in annual rate of change in eGFR between patients in the rhPTH(1-84) cohort and the historical control cohort was assessed via a linear mixed model. For both cohorts, the predicted eGFR change from baseline at each year from year 1 to year 5 after the index date was estimated using the adjusted linear mixed model. The multivariable model was adjusted for demographics (age, sex, race) and the following baseline parameters: hypercalciuria, hypertension, type 2 diabetes, acute manifestations of hypoparathyroidism, cardiovascular conditions, and baseline eGFR. Acute hypoparathyroidism manifestations were defined as at least one ICD-9-CM or ICD-10-CM diagnosis code for cardiac dysrhythmia, hypercalcemia, hypocalcemia, laryngeal spasm, muscle spasm, other convulsions, palpitations, tachycardia, tetanic cataract, or tetany. Cardiovascular conditions were defined as at least one ICD-9-CM or ICD-10-CM diagnosis codes, ICD-9-procedure coding system (PCS) or ICD-10-PCS procedure codes, Healthcare Common Procedure Coding System codes or Current Procedural Terminology codes for cerebrovascular disease, coronary artery disease, heart failure, or peripheral vascular disease.
A sensitivity analysis was conducted among the control cohort that included only patients with a record of first calcitriol prescription in the database (i.e., index date) that was 18 months or more after diagnosis of hypoparathyroidism to more stringently match the rhPTH(1–84) cohort in terms of known duration of hypoparathyroidism.
Results
Study Population and Baseline Characteristics
The analysis cohorts consisted of 72 patients treated with rhPTH(1-84) and 176 control patients. Baseline demographics, clinical characteristics, and biochemical parameter levels are summarized in Table 1. At baseline, a lower proportion of patients in the rhPTH(1-84) cohort had hypertension, type 2 diabetes, any acute manifestations of hypoparathyroidism, or cardiovascular conditions of coronary artery disease or heart failure compared with patients in the control cohort (all P < 0.05). At baseline, there was no significant difference in eGFR between cohorts; serum calcium was significantly higher in the rhPTH(1-84) cohort compared with the control cohort (P < 0.001).
Table 1.
Demographics, clinical characteristics, and biochemical parameter level at baseline in patients with chronic hypoparathyroidism
| rhPTH(1-84) cohort (n = 72) |
Historical control cohort (n = 176) |
P value | |
|---|---|---|---|
| Age at index date,a (years), mean ± SD | 47.5 ± 11.0 | 54.1 ± 15.5 | < 0.001** |
| Female, n (%) | 55 (76.4) | 139 (79.0) | 0.78 |
| Race,a n (%) | |||
| White | 70 (97.2) | 145 (82.4) | < 0.001** |
| Black | 0 | 26 (14.8) | < 0.001** |
| Asian, Multi, Other, Unknown | 2 (2.8) | 5 (2.8) | 0.98 |
| Clinical characteristics, n (%) | |||
| Hypertensiona | 18 (25.0) | 86 (48.9) | < 0.001** |
| Type 2 diabetesa | 2 (2.8) | 32 (18.2) | < 0.001** |
| Osteoporosis | 1 (1.4) | 14 (8.0) | 0.07 |
| Hypercalciuriaa | 2 (2.8) | 7 (4.0) | 1.00 |
| Cerebrovascular disease | 1 (1.4) | 12 (6.8) | 0.12 |
| Coronary artery disease | 0 | 16 (9.1) | < 0.01** |
| Heart failure | 0 | 12 (6.8) | < 0.05** |
| Peripheral vascular disease | 1 (1.4) | 14 (8.0) | 0.07 |
| Any acute manifestations of hypoparathyroidism,a n (%) | 16 (22.2) | 121 (68.8) | < 0.001** |
| Hypocalcemia | 5 (6.9) | 104 (59.1) | < 0.001** |
| Hypercalcemia | 1 (1.4) | 19 (10.8) | < 0.05** |
| Cardiac dysrhythmia | 7 (9.7) | 33 (18.8) | 0.12 |
| Palpitations | 0 | 10 (5.7) | 0.07 |
| Muscle spasm | 7 (9.7) | 8 (4.5) | 0.21 |
| Convulsions, not otherwise specified | 0 | 6 (3.4) | 0.19 |
| Tetany | 2 (2.8) | 3 (1.7) | 0.63 |
| Tachycardia | 1 (1.4) | 1 (0.6) | 0.50 |
| Laryngeal spasm | 1 (1.4) | 0 | 0.29 |
| Tetanic cataractb | 0 | 0 | – |
| CKD stage,c n (%) | |||
| Stage 1 | 19 (26.4) | 60 (34.1) | 0.30 |
| Stage 2 | 42 (58.3) | 82 (46.6) | 0.12 |
| Stage 3d | 11 (15.3) | 34 (19.3) | 0.57 |
| eGFR,a mL/min/1.73 m2, mean ± SD | 78.4 ± 17.5 | 81.5 ± 23.0 | 0.39 |
| CKD stage 1 | 101.5 ± 8.1 | 107.6 ± 12.6 | 0.08 |
| CKD stage 2 | 74.8 ± 7.3 | 75.4 ± 7.9 | 0.57 |
| CKD stage 3 | 52.3 ± 5.8 | 50.2 ± 5.9 | 0.21 |
| Serum calcium,c mmol/L, mean ± SD | 2.2 ± 0.2 | 2.0 ± 0.3 | < 0.001** |
Note: Baseline was the period before index date
CKD chronic kidney disease, eGFR estimated glomerular filtration rate, ICD International Classification of Diseases, rhPTH(1-84) recombinant human parathyroid hormone (1-84), SD standard deviation
aLinear mixed models were adjusted for these parameters and sex parameter
bTetanic cataracts were identified using ICD-9 code 366.42 (tetanic cataract), ICD-9 code 366.44 (cataract associated with other syndromes), and ICD-10 code H28 (cataract and other disorders of lens in diseases classified elsewhere)
cClosest measurement before index date (within 6 months pre-index); stage 1 = eGFR ≥ 90 mL/min/1.73 m2, stage 2 = 60 to < 90 mL/min/1.73 m2, stage 3 = 30 to < 60 mL/min/1.73 m2
dNo patients in the rhPTH(1-84) or control cohort had baseline CKD stage 4 or stage 5
**P < 0.05
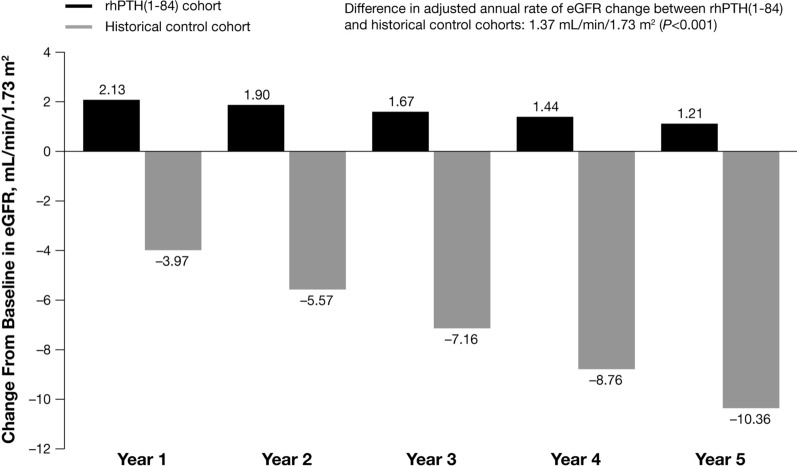
In the unadjusted analyses, eGFR remained stable over 5 years in the rhPTH(1-84) cohort, whereas eGFR declined at a rate of 1.67 mL/min/1.73 m2 per year in the control cohort (P < 0.001 for eGFR slope in the control cohort). In the adjusted analyses, 5 years after the index date, the predicted eGFR in patients in the rhPTH(1-84) cohort increased from baseline by 1.21 mL/min/1.73 m2, whereas the predicted eGFR in patients in the control cohort declined by 10.36 mL/min/1.73 m2 (Fig. 2). The difference in the eGFR slope between the rhPTH(1-84) cohort and control cohort over 5 years was 1.37 mL/min/1.73 m2 per year (95% CI 0.62–2.13; P < 0.001).
Fig. 2.
Predicted yearly eGFR change from baseline derived from adjusted regression model. eGFR, estimated glomerular filtration rate; rhPTH(1-84), recombinant human parathyroid hormone (1-84)
In a sensitivity analysis of patients in the control cohort with an index date at least 18 months after diagnosis of hypoparathyroidism, the size of the cohort was reduced from 176 to 84 patients. In the unadjusted analyses, over 5 years, the eGFR slope in the historical cohort was −1.09 mL/min/1.73 m2 per year (P < 0.001 for the eGFR slope). Similar to the main analysis, in the adjusted analyses, patients in the rhPTH(1-84) cohort had a predicted increase in eGFR (3.04 mL/min/1.73 m2), whereas the predicted eGFR declined among patients in the control cohort (−6.08 mL/min/1.73 m2) at year 5. The difference in the eGFR slope between the rhPTH(1-84) cohort and control cohort over 5 years was 0.75 mL/min/1.73 m2 (95% CI 0.04–1.46; P < 0.05).
Discussion
The results of this study demonstrated that individuals with chronic hypoparathyroidism treated with rhPTH(1-84) had stable eGFR over 5 years compared with the eGFR decline observed in patients not treated with PTH replacement therapy. In this larger study, the unadjusted annual rate of eGFR decline (−1.67 mL/min/1.73 m2 per year) in patients with chronic hypoparathyroidism who did not receive rhPTH(1-84) was similar to that reported in a smaller study by Chen et al. [1] of a regional US electronic health records database (−1.8 mL/min/1.73 m2 per year). In contrast to eGFR decline in the control group, eGFR remained stable in patients treated with rhPTH(1-84); these data are consistent with findings from a prospective US study of 24 patients who received rhPTH(1-84) for 8 years [16]. The consistency of the results using different real-world databases for the control cohorts strengthens the findings from this study and the overall body of evidence supporting a renoprotective effect of rhPTH(1-84) in patients with chronic hypoparathyroidism.
Although the underlying mechanisms by which rhPTH(1-84) appears to confer renoprotection are not fully understood, it is plausible that the restoration of more physiologic calcium and phosphate balance provides collective benefits. In comparison, conventional therapy may induce imbalances in calcium and phosphate homeostasis that can result in long-term kidney complications. In the 24-week REPLACE study, patients with chronic hypoparathyroidism treated with rhPTH(1-84) had significant reductions in oral calcium supplements and active vitamin D compared with patients who received placebo [21]. In the long-term RACE study, reductions in calcium and active vitamin D supplementation were maintained over 5 years [15]. In another long-term study, rhPTH(1-84) treatment over 8 years also led to reduction in oral calcium and vitamin D requirements in patients with chronic hypoparathyroidism that was accompanied by a reduction in mean urinary calcium excretion [16]. In both these long-term rhPTH(1-84) studies, reductions in calcium and active vitamin D supplement requirements were achieved despite the maintenance of normal serum calcium levels [15, 16]. In addition, long-term treatment with rhPTH(1-84) stabilized eGFR and reduced mean urinary calcium excretion [15, 16]. Furthermore, in long-term studies, treatment with rhPTH(1-84) maintained serum phosphate within the recommended range.
The apparent renoprotective effects of rhPTH(1-84) treatment may also result from hemodynamic changes and vasodilatory effects on the kidney through replacement of PTH. Patients treated with rhPTH(1-84) in the current analysis showed a small increase in eGFR. The absence of expected age-related eGFR decline in the rhPTH(1-84) cohort may be due to hemodynamic changes or vasodilatory effects. PTH replacement could exert direct effects via PTH receptors on the kidney tubules leading to a vasodilatory effect at the microcirculatory level in the glomeruli [22]. In addition, PTH replacement could induce indirect effects on the kidney via restoration of normal renal calcium handling and reduction of oral calcium influx, which in turn reduces kidney exposure to hypercalciuria, as well as restoration of phosphate homeostasis. Overall, results from long-term trials and the current study suggest that rhPTH(1-84) is a potential option as a physiologic replacement for the missing hormone that may preserve kidney function.
Complications arising in patients with chronic hypoparathyroidism treated with long-term conventional therapy may contribute toward high healthcare utilization in this patient population and suggest an unmet clinical need for better therapies, particularly among patients with disease that is not adequately controlled [23, 24]. An expert consensus statement from two European Society of Endocrinology-supported 2021 workshops recognizes that adult patients with chronic hypoparathyroidism are at increased risk of developing nephrolithiasis, nephrocalcinosis, and CKD, and recommends frequent monitoring of kidney function [25]. In a retrospective cohort study in England using hospital episode statistics, renal complications were the main driver of a high economic burden on the healthcare system among patients with chronic hypoparathyroidism [25].
This study has important strengths. The Explorys database provided a large sample size of patients from a real-world data source with this rare disorder, as well as detailed biochemical measurements. Many patients in the rhPTH(1-84) cohort in the current study were also included in the Chen study [1]; however, the current study expanded the rhPTH(1-84) analysis set by utilizing additional clinical trial data sets. Furthermore, because the Explorys database is larger and more representative of the entire US population, the control cohort was expanded from 53 to 176 patients. The criteria used to select patients for the control cohort were similar to the enrollment criteria of the clinical trials, including inclusion and exclusion criteria and availability of baseline laboratory measurements. Multivariable regression models accounted for potential differences between patients treated with rhPTH(1-84) and controls by adjusting for potential confounding variables. The inclusion criterion applied to the control cohort for the sensitivity analysis (i.e., first calcitriol prescription in the database for the index date that was at least 18 months after diagnosis of hypoparathyroidism) was stringent, and yet the results of the sensitivity analysis were similar to those of the main analysis.
This study also has its limitations. Management of patients in clinical trials is likely to be different from that of patients treated in a real-world setting with respect to frequency of evaluations and reporting of biochemical parameters. The EMR system contains only information about visits that occurred within the network of providers; hence, any medical visits outside the system were not captured. In common with most observational studies, there may be an effect of unrecorded and/or unmeasured confounders between cohorts that were not accounted for in the analyses that persist after adjusting for baseline differences. Additionally, stratified analyses could not be performed because the etiology of hypoparathyroidism (i.e., surgical or nonsurgical) was not recorded. Oral calcium supplementation, and active vitamin D and non-steroidal anti-inflammatory drug (NSAID) use were not assessed because any over-the-counter use of these medications was not captured in the database. The retrospective design of this study has advantages for studying long-term outcomes in rare diseases, but does not allow for conclusions about causation. However, given accumulating evidence that PTH replacement is associated with lower rate of eGFR decline and relative rarity of chronic hypoparathyroidism in the general population, it may be difficult to recruit patients into randomized trials because they may be reluctant to forego PTH replacement therapy.
Conclusions
After adjustment for differences between cohorts, eGFR remained stable over a 5-year period in patients with chronic hypoparathyroidism treated with rhPTH(1-84) in clinical trials compared with eGFR decline in those in a historical control cohort not treated with rhPTH(1-84). Further research is warranted to better understand the impact of long-term rhPTH(1-84) treatment on the preservation of kidney function in patients with chronic hypoparathyroidism treated in a real-world setting.
Acknowledgements
Funding
This research and the journal’s publication fees were funded by Takeda Pharmaceuticals USA, Inc., Lexington, MA, USA.
Medical Writing and Editorial Assistance
Under the direction of the authors, editorial support was provided by Alan Storey, PhD (ICON plc, Reading, Berkshire, UK), and was funded by Takeda Development Center Americas Inc., Lexington, MA, USA.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Olulade Ayodele, Richard Berman, Angela Lax, Fan Mu, and Elyse Swallow contributed to the study design and data collection. All authors contributed to data analyses and interpretation, and prepared and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Prior Presentations
The Endocrine Society Annual Meeting 2021, March 20–23, 2021, and the European Society of Endocrinology Congress, May 22–26, 2021.
Disclosures
Olulade Ayodele is an employee of Takeda Pharmaceuticals USA, Inc., Lexington, MA, USA. Lars Rejnmark has served as a consultant and speaker for Shire, a Takeda company, and as a speaker for Ascendis Pharma. Fan Mu, Angela Lax, Richard Berman, and Elyse Swallow are employees of Analysis Group, Inc., which received consulting fees from Takeda and other commercial interests. Elvira O. Gosmanova has served as a consultant for Shire, a Takeda company. Elvira O. Gosmanova is an employee of the US Department of Veterans Affairs. Opinions expressed in this article are those of the authors and do not necessarily represent the opinion of the Department of Veterans Affairs.
Compliance with Ethics Guidelines
Permission to access and use the trial data was granted by the data owner, Takeda. All patients enrolled in the clinical trials provided written informed consent. Permission to access and use the IBM® Explorys EMR database was granted by IBM. The Explorys database is a nationally representative EMR resource maintained by IBM. Institutional review board approval was not required as the Explorys database consists of pre-existing data that are de-identified in accordance with the Health Insurance Portability and Accountability Act.
Data Availability
The data sets generated during and/or analyzed during the current study are not publicly available because the Explorys data were used under license. However, the corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1.Chen KS, Gosmanova EO, Curhan GC, et al. Five-year estimated glomerular filtration rate in patients with hypoparathyroidism treated with and without rhPTH(1–84) J Clin Endocrinol Metab. 2020;105:e3557–e3565. doi: 10.1210/clinem/dgaa490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosmanova EO, Chen K, Rejnmark L, et al. Risk of chronic kidney disease and estimated glomerular filtration rate decline in patients with chronic hypoparathyroidism: a retrospective cohort study. Adv Ther. 2021;38:1876–1888. doi: 10.1007/s12325-021-01658-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim SH, Rhee Y, Kim YM, et al. Prevalence and complications of nonsurgical hypoparathyroidism in Korea: a nationwide cohort study. PLoS ONE. 2020;15:e0232842. doi: 10.1371/journal.pone.0232842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell DM, Regan S, Cooley MR, et al. Long-term follow-up of patients with hypoparathyroidism. J Clin Endocrinol Metab. 2012;97:4507–4514. doi: 10.1210/jc.2012-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Underbjerg L, Sikjaer T, Mosekilde L, Rejnmark L. Cardiovascular and renal complications to postsurgical hypoparathyroidism: a Danish nationwide controlled historic follow-up study. J Bone Miner Res. 2013;28:2277–2285. doi: 10.1002/jbmr.1979. [DOI] [PubMed] [Google Scholar]
- 6.Vadiveloo T, Donnan PT, Leese CJ, Abraham KJ, Leese GP. Increased mortality and morbidity in patients with chronic hypoparathyroidism: a population-based study. Clin Endocrinol (Oxf) 2019;90:285–292. doi: 10.1111/cen.13895. [DOI] [PubMed] [Google Scholar]
- 7.Abate EG, Clarke BL. Review of hypoparathyroidism. Front Endocrinol (Lausanne) 2017;7:172. doi: 10.3389/fendo.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilezikian JP. Hypoparathyroidism. J Clin Endocrinol Metab. 2020;105:1722–1736. doi: 10.1210/clinem/dgaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandi ML, Bilezikian JP, Shoback D, et al. Management of hypoparathyroidism: summary statement and guidelines. J Clin Endocrinol Metab. 2016;101:2273–2283. doi: 10.1210/jc.2015-3907. [DOI] [PubMed] [Google Scholar]
- 10.Khan AA, Koch C, Van Uum SHM, et al. Standards of care for hypoparathyroidism in adults: a Canadian and international consensus. Eur J Endocrinol. 2019;180:P1–P22. doi: 10.1530/EJE-18-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letavernier E, Daudon M. Vitamin D, hypercalciuria and kidney stones. Nutrients. 2018;2018:10. doi: 10.3390/nu10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliveira B, Kleta R, Bockenhauer D, Walsh SB. Genetic, pathophysiological, and clinical aspects of nephrocalcinosis. Am J Physiol Renal Physiol. 2016;311:F1243–F1252. doi: 10.1152/ajprenal.00211.2016. [DOI] [PubMed] [Google Scholar]
- 13.Natpar® parathyroid hormone. Dublin, Ireland: Shire Pharmaceuticals Ireland Limited; 2020.
- 14.Natpara® parathyroid hormone. Lexington, MA, USA: Shire-NPS Pharmaceuticals, Inc.; 2020.
- 15.Mannstadt M, Clarke BL, Bilezikian JP, et al. Safety and efficacy of 5 years of treatment with recombinant human parathyroid hormone in adults with hypoparathyroidism. J Clin Endocrinol Metab. 2019;104:5136–5147. doi: 10.1210/jc.2019-01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tay YD, Tabacco G, Cusano NE, et al. Therapy of hypoparathyroidism with rhPTH(1–84): a prospective, 8-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2019;104:5601–5610. doi: 10.1210/jc.2019-00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levey AS, Inker LA, Matsushita K, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2014;64:821–835. doi: 10.1053/j.ajkd.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Lambers Heerspink HJ, Tighiouart H, Sang Y, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. Am J Kidney Dis. 2014;64:860–866. doi: 10.1053/j.ajkd.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Sang Y, Ballew SH, et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30:1746–1755. doi: 10.1681/ASN.2019010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1–84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomized, phase 3 study. Lancet Diabetes Endocrinol. 2013;1:275–83 [erratum: Lancet Diabetes Endocrinol. 2014;2(1):e3]. [DOI] [PubMed]
- 22.Evenepoel P, Bover J, Urena-Torres P. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016;90:1184–1190. doi: 10.1016/j.kint.2016.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Krasner A, Li N, et al. Clinical burden and healthcare resource utilization among patients with chronic hypoparathyroidism, overall and by adequately vs not adequately controlled disease: a multi-country chart review. J Med Econ. 2019;22:1141–1152. doi: 10.1080/13696998.2019.1624081. [DOI] [PubMed] [Google Scholar]
- 24.Fanget F, Demarchi MS, Maillard L, et al. Hypoparathyroidism: consequences, economic impact, and perspectives. A case series and systematic review. Ann Endocrinol (Paris) 2021;82:572–581. doi: 10.1016/j.ando.2021.07.085. [DOI] [PubMed] [Google Scholar]
- 25.Bollerslev J, Rejnmark L, Zahn A, et al. European expert consensus on practical management of specific aspects of parathyroid disorders in adults and in pregnancy: recommendations of the ESE educational program of parathyroid disorders. Eur J Endocrinol. 2021;186:R33–R63. doi: 10.1530/EJE-21-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are not publicly available because the Explorys data were used under license. However, the corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.