Abstract
Background
Radioligand therapy (RLT) with 177Lu-labeled prostate-specific membrane antigen (PSMA) ligands is associated with prolonged overall survival (OS) in patients with advanced, metastatic castration-resistant prostate cancer (mCRPC). A substantial number of patients, however, are prone to treatment failure. We aimed to determine clinical baseline characteristics to predict OS in patients receiving [177Lu]Lu-PSMA I&T RLT in a long-term follow-up.
Materials and methods
Ninety-two mCRPC patients treated with [177Lu]Lu-PSMA I&T with a follow-up of at least 18 months were retrospectively identified. Multivariable Cox regression analyses were performed for various baseline characteristics, including laboratory values, Gleason score, age, prior therapies, and time interval between initial diagnosis and first treatment cycle (intervalDiagnosis-RLT, per 12 months). Cutoff values for significant predictors were determined using receiver operating characteristic (ROC) analysis. ROC-derived thresholds were then applied to Kaplan–Meier analyses.
Results
Baseline C-reactive protein (CRP; hazard ratio [HR], 1.10, 95% CI 1.02–1.18; P = 0.01), lactate dehydrogenase (LDH; HR, 1.07, 95% CI 1.01–1.11; P = 0.01), aspartate aminotransferase (AST; HR, 1.16, 95% CI 1.06–1.26; P = 0.001), and intervalDiagnosis-RLT (HR, 0.95, 95% CI 0.91–0.99; P = 0.02) were identified as independent prognostic factors for OS. The following respective ROC-based thresholds were determined: CRP, 0.98 mg/dl (area under the curve [AUC], 0.80); LDH, 276.5 U/l (AUC, 0.83); AST, 26.95 U/l (AUC, 0.73); and intervalDiagnosis-RLT, 43.5 months (AUC, 0.68; P < 0.01, respectively). Respective Kaplan–Meier analyses demonstrated a significantly longer median OS of patients with lower CRP, lower LDH, and lower AST, as well as prolonged intervalDiagnosis-RLT (P ≤ 0.01, respectively).
Conclusion
In mCRPC patients treated with [177Lu]Lu-PSMA I&T, baseline CRP, LDH, AST, and time interval until RLT initiation (thereby reflecting a possible indicator for tumor aggressiveness) are independently associated with survival. Our findings are in line with previous findings on [177Lu]Lu-PSMA-617, and we believe that these clinical baseline characteristics may support the nuclear medicine specialist to identify long-term survivors.
Keywords: PSMA, Prostate cancer, [177Lu]Lu-PSMA I&T, Radioligand therapy, Overall survival, Prediction
Introduction
Accounting for more than 375,000 deaths annually worldwide, metastatic castration-resistant prostate cancer (mCRPC) requires new treatment modalities [1]. Prostate-specific membrane antigen (PSMA)–targeted radioligand therapies (RLTs) using the β-emitting [177Lu]Lu-PSMA-617 led to increased overall survival (OS) when compared to standard of care [2] and superior biochemical response relative to chemotherapy with cabazitaxel [3]. Despite those promising results, a substantial fraction of patients do not respond to RLT in such a last-line setting, thereby emphasizing the need for reliable outcome predictors prior to treatment initiation. In this regard, multiple studies have suggested relevant prognostic factors for mCRPC patients receiving [177Lu]Lu-PSMA-617, including the presence of visceral metastases [4–7], elevated alkaline phosphatase (AP) [8, 9], or C-reactive protein (CRP) levels [10]. Further studies reported on the impact of prior chemotherapy [7, 11, 12], lactate dehydrogenase (LDH) [8–10, 13], aspartate aminotransferase (AST) [5, 14], and hemoglobin [5, 10] on survival for patients treated with [177Lu]Lu-PSMA-617.
The second most commonly administered radiotracer for PSMA RLT is [177Lu]Lu-PSMA I&T [15], and results of a currently recruiting prospective trial (NCT04647526) will be reported in due course [16]. Despite sharing the same PSMA-binding site, both radiotherapeutics exhibit substantial differences, in particular in regard to the utilized chelator linked to the peptide [17, 18]. Thus, outcome and prognostic parameters identified for [177Lu]Lu-PSMA-617 may not be necessarily applicable to patients treated with [177Lu]Lu-PSMA I&T. Using the latter compound, Heck et al. identified the presence of visceral metastasis and a rising LDH as independent predictors of OS [4], while Gafita et al. reported on the time since diagnosis of prostate cancer (PC) as a prognosticator for OS [19]. Supporting the notion that safety and efficacy profiles of both radioligands are less likely to be interchangeable, the current literature comprises only a limited number of approximately 100 subjects treated with [177Lu]Lu-PSMA I&T, which is in contrast to more than 900 reported patients with PC treated with [177Lu]Lu-PSMA-617 [15].
In this study that included 92 patients, we aimed to expand the current literature on [177Lu]Lu-PSMA I&T and to identify predictive baseline characteristics, focusing on long-term follow-up.
Material and methods
Patient cohort
In this single-center study, we identified 118 men with mCRPC treated with [177Lu]Lu-PSMA I&T. Only patients with a follow-up period of at least 18 months after commencing the first treatment cycle were included. Exclusion criteria was a follow-up of less than 18 months (Fig. 1). All patients signed written informed consent. The local Ethics Committee waived the need for further approval due to the retrospective character of this investigation (waiver no. 20210422 04). Parts of this cohort have been reported in [20]. However, that previous analysis did not focus on identifying prognostic parameters for long-term survival.
Fig. 1.
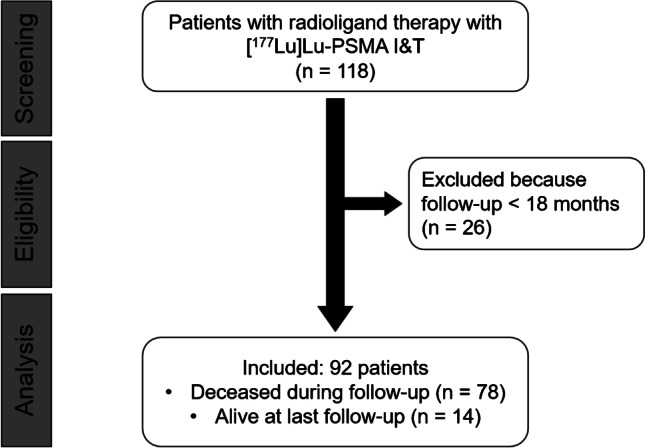
Flowchart of patients of excluded and included subjects
Pretherapy work-up
At the day of admission, pretherapeutic blood samples comprised serum chemistry (prostate-specific antigen (PSA) level, creatinine, LDH, AST, AP, CRP, and bilirubin) and routine hematology (leukocytes, hemoglobin, platelets). For blood draws, we utilized dipotassium ethylenediaminetetraacetic acid (EDTA) tubes (Sarstedt, Nuembrecht, Germany). Probes were then processed with an automated analyzer (Sysmex XN-9000, Kobe, Japan). For serum chemistry, serum-gel tubes were utilized (Sarstedt, Nuembrecht, Germany), followed by an analysis with a fully automated modular analyzer (Roche Cobas, Basel, Switzerland). Patient history was also retrieved from medical records.
Treatment protocol
We conducted routine protocols for RLT. In this regard, [177Lu]Lu-PSMA I&T was synthesized as described elsewhere [20]. A total of 6.0 GBq of [177Lu]Lu-PSMA I&T was then administered every 42–56 days (maximum, 8 cycles per patient). If an individual exhibited markedly decreased renal or hematological function under RLT, the activity was reduced by approximately 20% 12/92 (13%) patients.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 9.3.0 running on Windows (GraphPad Software, San Diego, CA, USA). We presented median and range in parentheses. OS was defined as the time interval of the day of the first cycle until day of death (presented as median). Since nearly all parameters were not normally distributed, comparisons between cohorts were performed using the Mann–Whitney U test. For comparison of dichotomous variables, we applied Fisher’s exact test. Uni- and multivariable Cox regressions were used to identify prognostic baseline parameters (including outlier correction). Cutoffs for survival prediction were determined by receiver operating characteristic (ROC) analysis and the Youden index for maximization of specificity and sensitivity. Kaplan–Meier curves and log-rank comparison were calculated to illustrate separation between responders and non-responders. We considered P < 0.05 statistically significant.
Results
Patients’ characteristics
A total of 92/118 (78.0%) patients were included in the final analysis (age, 71 years (52–90 years)). The median time interval between initial diagnosis and the 1st cycle of RLT (intervalDiagnosis-RLT) was 75.5 (range, 9–364) months. The median initial Gleason score of all patients was 8 (5–10). Patients were treated with a median of three cycles (cumulative activity, 14.9 GBq).
Baseline CRP, LDH, AST, and intervalDiagnosis-RLT are independent predictors of survival
Subdividing patients into those who were dead (n = 63) and those who were alive (n = 29) 18 months after initiation of RLT, no significant differences in previous treatment regimens were observed (P ≥ 0.1). Surviving subjects had a longer intervalDiagnosis-RLT (97 (25–364) vs. 64 (9–238) months, P = 0.006), and a higher number of RLT cycles (6 (2–9) vs. 2 (1–6), P < 0.001), with concomitant increased cumulative activity (33.4 (11.9–89.3) vs. 12.0 (4.9–36.7) GBq, P < 0.001). At baseline, survivors exhibited lower CRP (0.24 (0.04–3.30) vs. 1.47 (0.02–26.6) mg/dl, P < 0.001), lower LDH (209 (118–491) vs. 324 (173–1800) U/l, P < 0.001), lower AST (24.0 (15.5–62.0) vs. 31.8 (15.0–546.7) U/l, P < 0.001), and lower AP levels (89 (38–536) vs. 169 (31–5818) U/l, P < 0.001). Baseline hemoglobin, however, was substantially higher in surviving patients (11.9 (9.3–14.9) vs. 10.7 (6.0–16.1) g/dl, P = 0.002; Table 1).
Table 1.
Baseline patient characteristics
| Entire cohort | Dead (n = 63) | Alive (n = 29) | P | |
|---|---|---|---|---|
| Clinical variables | ||||
| Age at first cycle of PSMA RLT (years) | 71 (52–90) | 69 (52–90) | 73 (55–89) | 0.08 |
| intervalDiagnosis-RLT (months) | 75.5 (9–364) | 64 (9–238) | 97 (25–364) | 0.006 |
| Treatment cycles per patient | 3 (1–9) | 2 (1–6) | 6 (2–9) | < 0.001 |
| Cumulative activity (GBq) | 14.9 (4.9–89.3) | 12.0 (4.9–36.7) | 33.4 (11.9–89.3) | < 0.001 |
| Gleason score | 8 (5–10) | 8 (5–10) | 8 (7–10) | 0.91 |
| Baseline laboratory values | ||||
| PSA (ng/ml) | 192 (0.07–5000) | 208 (0.07–5000) | 93.8 (0.10–1640) | 0.08 |
| CRP (mg/dl) | 0.82 (0.02–26.6) | 1.47 (0.02–26.6) | 0.24 (0.04–3.30) | < 0.001 |
| LDH (37 °C U/l) | 287.5 (118–1800) | 324 (173–1800) | 209 (118–491) | < 0.001 |
| Hemoglobin (g/dl) | 11.3 (6.0–16.1) | 10.7 (6.0–16.1) | 11.9 (9.3–14.9) | 0.002 |
| Creatinine (mg/dl) | 0.92 (0.51–2.41) | 0.89 (0.51–2.41) | 1.02 (0.60–1.81) | 0.24 |
| Bilirubin (mg/dl) | 0.4 (0.1–6.5) | 0.4 (0.2–6.5) | 0.4 (0.1–1.4) | 0.74 |
| AST (37 °C U/l) | 28.1 (15.0–546.7) | 31.8 (15.0–546.7) | 24.0 (15.5–62.0) | < 0.001 |
| AP (37 °C U/l) | 131.5 (31.0–5818) | 169 (31–5818) | 89 (38–536) | < 0.001 |
| Prior treatments (%) | ||||
| Radical prostatectomy | 45.7 | 42.6 | 51.6 | 0.51 |
| Primary radiation therapy to the prostate | 15.2 | 11.5 | 22.6 | 0.22 |
| Antihormonal treatment | 100 | 100 | 100 | 1.0 |
| Enzalutamide | 69.6 | 72.1 | 64.5 | 0.48 |
| Abiraterone | 72.8 | 72.1 | 74.2 | 1.0 |
| Chemotherapy | 69.6 | 75.4 | 58.1 | 0.1 |
intervalDiagnosis-RLT time interval between initial diagnosis and 1st radioligand therapy, PSA prostate-specific antigen, CRP C-reactive protein, LDH lactate dehydrogenase, AST aspartate aminotransferase, AP alkaline phosphatase
On univariate analysis, CRP (HR 1.19, 95% CI 1.12–1.25; P < 0.001), LDH (1.10, 95% CI 1.06–1.14; P < 0.001), hemoglobin (0.72, 95% CI 0.62–0.83; P < 0.001), AST (1.20, 95% CI 1.12–1.30; P < 0.001), AP (1.07, 95% CI 1.04–1.11; P < 0.001), and bilirubin (1.55, 95% CI 1.01–2.16; P = 0.01) reached significance for OS. In addition, intervalDiagnosis-RLT (per 12 months, 0.94, 95% CI 0.90–0.98; P = 0.01) and previous radiation therapy (0.50, 95% CI 0.23–0.95; P < 0.05) were also significant predictors for OS (Table 2).
Table 2.
Univariate and multivariable Cox regressions
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| CRP (per mg/dl) | 1.19 | 1.12–1.25 | < 0.0001 | 1.10 | 1.02–1.18 | 0.01 |
| LDH (per 50 U/l) | 1.10 | 1.06–1.14 | < 0.0001 | 1.07 | 1.01–1.11 | 0.01 |
| Hemoglobin (per g/dl) | 0.72 | 0.62–0.83 | < 0.0001 | 0.91 | 0.77–1.08 | 0.30 |
| AST (per 10 U/l) | 1.20 | 1.12–1.30 | < 0.0001 | 1.16 | 1.06–1.26 | 0.001 |
| AP 37 °C (per 50 U/l) | 1.07 | 1.04–1.11 | < 0.0001 | 1.03 | 0.98–1.07 | 0.30 |
| intervalDiagnosis-RLT* | 0.94 | 0.90–0.98 | 0.01 | 0.95 | 0.91–0.99 | 0.02 |
| Bilirubin (per mg/dl) | 1.55 | 1.01–2.16 | 0.01 | 0.83 | 0.51–1.29 | 0.42 |
| Primary RTx (yes) | 0.50 | 0.23–0.95 | 0.05 | |||
| Chemotherapy (yes) | 1.49 | 0.92–2.50 | 0.11 | 1.43 | 0.86–2.47 | 0.18 |
| PSA (per µg/l) | 1.00 | 0.99–1.00 | 0.13 | |||
| Age at 1st cycle (per year) | 0.98 | 0.96–1.01 | 0.13 | |||
| Enzalutamide (yes) | 1.39 | 0.85–2.37 | 0.21 | |||
| Reduced activity (yes) | 1.28 | 0.64–2.32 | 0.45 | |||
| Gleason score | 1.09 | 0.85–1.39 | 0.51 | |||
| RPE (yes) | 0.88 | 0.55–1.38 | 0.57 | |||
| Abiraterone (yes) | 1.09 | 0.66–1.86 | 0.75 | |||
| Creatinine (per mg/dl) | 0.89 | 0.41–1.79 | 0.75 | |||
HR hazard ratio, CI confidence interval, CRP C-reactive protein, LDH lactate dehydrogenase, AST aspartate aminotransferase, AP alkaline phosphatase, intervalDiagnosis-RLT time interval between initial diagnosis and 1st radioligand therapy, RTx radiation therapy, PSA prostate-specific antigen, RPE radical prostatectomy
*Per 12 months
Parameters reaching significance on univariate analysis were then included for multivariable Cox regression. Previous literature reported on the impact of previous chemotherapies on survival in subjects treated with [177Lu]Lu-PSMA-617 [7], and thus, this clinical variable was also included in our analysis. The following parameters remained significant prognosticators for OS: CRP (1.10, 95% CI 1.02–1.18; P = 0.01), LDH (1.07, 95% CI 1.01–1.11; P = 0.01), AST (1.16, 95% CI 1.06–1.26; P = 0.001), and intervalDiagnosis-RLT (0.95, 95% CI 0.91–0.99; P = 0.02), while all other variables did not reach significance (P > 0.1) (Table 2).
Baseline CRP, LDH, AST, and intervalDiagnosis-RLT differentiate between responders vs. non-responders
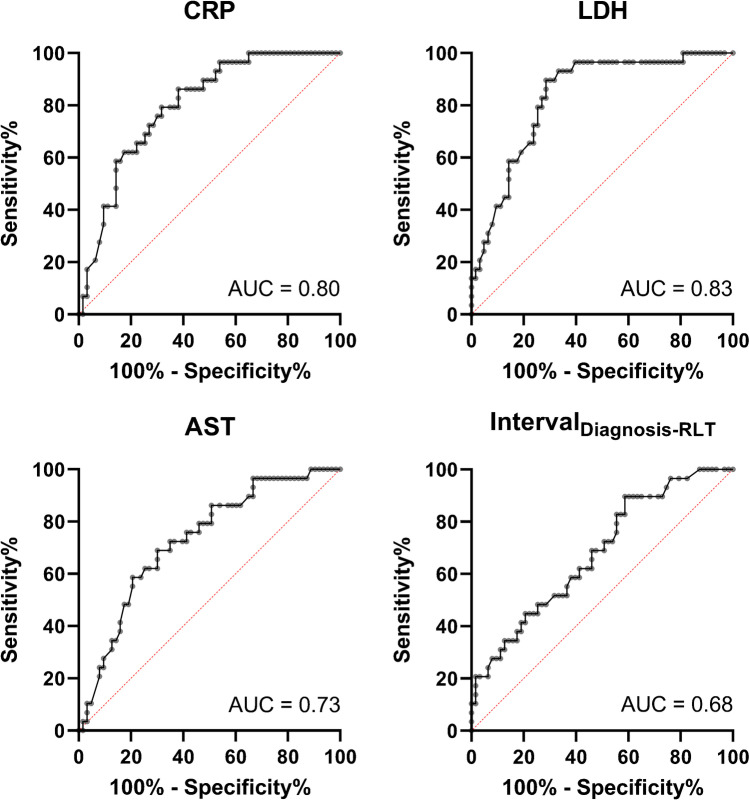
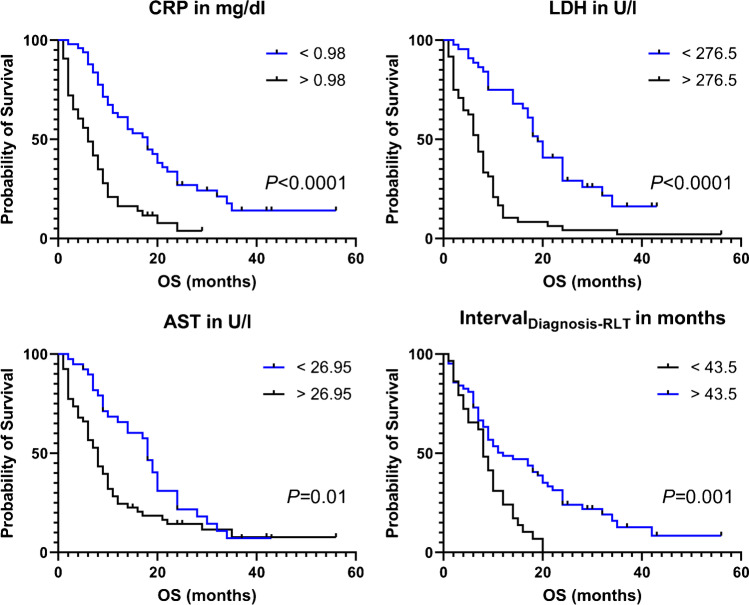
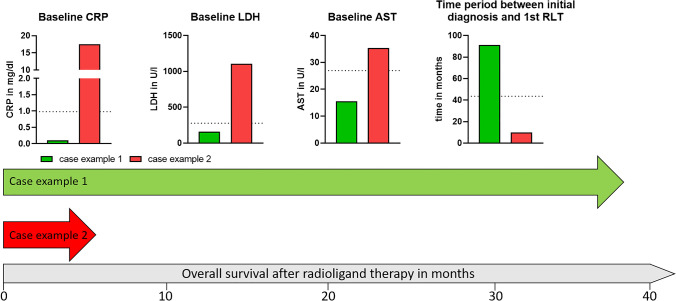
On ROC analysis, the following AUCs were determined: LDH, 0.83 (best cutoff, 276.5 U/l); CRP, 0.80 (best cutoff, 0.98 mg/dl); AST, 0.73 (best cutoff, 26.95 U/l; P < 0.001, respectively); and intervalDiagnosis-RLT, 0.68 (best cutoff, 43.5 months, P = 0.006) (Fig. 2). Applying the derived cutoffs to Kaplan–Meier analyses, baseline CRP (18 vs. 6 months), LDH (19 vs. 7 months), AST (18 vs. 8 months), and intervalDiagnosis-RLT (12 vs. 8 months) separated between responders and non-responders (P ≤ 0.01, respectively) (Fig. 3). Figure 4 illustrates two cases of patients with different outcomes after RLT with [177Lu]Lu-PSMA I&T.
Fig. 2.
Receiver operating characteristics for baseline values of C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and time interval between initial diagnosis and 1st radioligand therapy (intervalDiagnosis-RLT). AUC area under the curve
Fig. 3.
Kaplan–Meier curves for patients treated with radioligand therapy stratified into high- and low-risk patients using predefined cutoff values derived by receiver operating characteristics for C-reactive protein (CRP), lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and time interval between initial diagnosis and 1st radioligand therapy (intervalDiagnosis-RLT). Lower baseline CRP, LDH, and AST predicted a significantly longer median OS. In addition, the median OS of patients with a longer intervalDiagnosis-RLT was also prolonged
Fig. 4.
Examples of two patients with different outcomes after radioligand therapy with [.177Lu]Lu-PSMA I&T. The baseline values of the different parameters are presented in a bar chart. The dotted horizontal lines represent the (ROC-derived) cutoff values, and the large arrows visualize the overall survival
Discussion
In this study that included 92 mCRPC patients treated with [177Lu]Lu-PSMA I&T, lower baseline CRP, LDH, and AST and longer intervalDiagnosis-RLT independently predicted longer OS. Using predefined cutoffs provided by ROC analysis, Kaplan–Meier analyses differentiated between low vs. high risks, with the most prominent segregation for LDH and CRP. Of note, the herein observed follow-up period of 18 months is almost twice as reported previously for patients being treated with [177Lu]Lu-PSMA I&T RLT [4]. Thus, the nuclear medicine practitioner can have certainty that those easily obtainable clinical characteristics can identify long-term survivors prior to therapy.
In recent years, a growing body of literature has reported on the favorable outcome of mCRPC patients treated with [177Lu]Lu-PSMA-617 in retrospective and prospective settings [2, 3, 15]. In this regard, various clinical baseline characteristics have been reported to identify patients that will most likely fail under RLT with this agent, e.g., CRP, LDH, liver enzymes, or AP [5, 9, 10, 14, 21]. However, as stated by Herrmann and coworkers, findings from previous studies using [177Lu]Lu-PSMA-617 have to be interpreted with caution in the context of RLT using [177Lu]Lu-PSMA I&T [22], mainly due to relevant differences in the chemical structure, i.e., using the different chelators DOTA and DOTAGA [17, 18]. Comparative studies on biodistribution and dosimetry showed longer half-lives in the tumor lesions for [177Lu]Lu-PSMA-617, but higher initial tumor uptake for [177Lu]Lu-PSMA I&T [23]. In addition, there is a significant imbalance in the current literature on the number of treated subjects with the latter agent (approximately n = 100) when compared to 617 (> 900) [15]. As such, further investigations focusing on outcome prediction with [177Lu]Lu-PSMA I&T are needed.
We therefore evaluated 92 subjects with the longest follow-up period reported to date and were able to identify comparable baseline characteristics having predictive potential relative to [177Lu]Lu-PSMA-617. In this regard, higher baseline CRP has already been described as a negative predictor of OS after RLT with [177Lu]Lu-PSMA-617 [5, 10, 14]. Although CRP is readily available in the clinic, this parameter is rather non-specific and can be substantially elevated due to inflammatory disease [24]. The influence of LDH on OS after RLT, however, is discussed controversially. For [177Lu]Lu-PSMA-617, elevated LDH has already been described as a negative predictor of OS [5], which was further corroborated by Ferdinandus et al. in an univariate analysis, identifying a comparable cutoff of 240.5 U/l when compared to our threshold (276.5 U/l) [9]. Of note, other univariate analyses failed to confirm LDH as a prognostic factor for OS [8, 10]. Also utilizing [177Lu]Lu-PSMA I&T, Heck et al. demonstrated that elevated LDH was a significant predictor of worse outcome [4], whereas the second most relevant study on this compound yielded no predictive potential of this parameter [19]. Similar to LDH, the predictive performance of AST is also a matter of debate. Ahmadzadehfar et al. reported that elevated AST (≥ 24 U/l) is linked to less favorable outcome for RLT with [177Lu]Lu-PSMA-617 [5], which is comparable to our ROC-derived cutoff of 26.95 U/l. Previous studies utilizing [177Lu]Lu-PSMA I&T did not investigate AST in the context of outcome prediction. Nonetheless, elevated baseline AST seems to be tightly linked to shorter survival regardless of which agent was applied.
Although reported for [177Lu]Lu-PSMA-617 in several studies [5, 8, 9, 11, 21], we could not confirm a prognostic ability of elevated baseline AP for OS after RLT with [177Lu]Lu-PSMA I&T, which is consistent with previous reports with this compound [4, 19]. As such, for this radiotracer, AP may play a less important role for outcome prediction when compared to [177Lu]Lu-PSMA-617.
Beyond laboratory values, longer intervalDiagnosis-RLT also demonstrated independent predictive potential for prolonged OS in our cohort. As a possible explanation, this baseline characteristic may represent tumor aggressiveness, as one may speculate that a longer interval between initial diagnosis and RLT is also linked to a more treatment-sensitive tumor biology. For instance, Barber et al. found an association between intervalDiagnosis-RLT and OS for taxane-naïve patients undergoing RLT with [177Lu]Lu-PSMA-617 in an univariate analysis [25], while Gafita et al. also reported that a longer intervalDiagnosis-RLT is associated with longer OS in men scheduled for [177Lu]Lu-PSMA I&T [19]. As such, this time frame may support a decision-making process to consider RLT for both radiotherapeutics.
The present study may provide in-depth results of patients that have been referred to our center in a real-world scenario with a focus on long-term follow-up. However, given the exploratory design of the study we cannot rule out a possible lack of power for testing the described associations. Therefore, we want to emphasize that the correlations found in our analysis have to be interpreted with caution as they not necessarily mean causation. Moreover, 12/92 patients received reduced activity in at least one cycle, but failed to reach significance in a univariate analysis (Table 2). Future studies may also control for this variable.
Further studies reported on the predictive value of image-based semi-quantification in the context of RLT [26, 27]. In the present study, however, we focused on clinical parameters, which are easy to obtain relative to the time-consuming nature of manual segmentation procedures of PSMA PET/CTs. Given the rather low number of reported subjects treated with [177Lu]Lu-PSMA I&T, we believe that the herein presented results are relevant for the practitioner [22]. Nonetheless, confirmatory clinical trials enrolling a substantially higher number of subjects are warranted, preferably also using PSMA-directed PET findings. In this regard, nomograms for patients treated with [177Lu]Lu-PSMA (including both 617 and I&T) have been established and also consider such PSMA-avid disease sites on molecular imaging. Of note, those risk charts have been provided to the community as online tools and, thus, can be easily tested for patients which had exclusively received [177Lu]Lu-PSMA I&T [6].
Conclusion
In this study that included 92 patients with mCRPC treated with [177Lu]Lu-PSMA I&T, baseline laboratory values (CRP, LDH, and AST) and intervalDiagnosis-RLT (reflecting a possible indicator for tumor aggressiveness) were independent predictors of survival during long-term follow-up. Our findings corroborate with previous findings on [177Lu]Lu-PSMA-617 and we believe that these routinely available parameters may support the nuclear medicine physician to identify individuals that should be scheduled for RLT with [177Lu]Lu-PSMA I&T.
Author contribution
Conceptualization, P.E.H., R.A.W., S.E.S.; methodology, F.X.W.; software, F.X.W., P.E.H.; validation, P.E.H., R.A.W.; formal analysis, P.E.H., F.X.W.; investigation, P.E.H., F.X.W.; resources, A.K.B.; data curation, P.E.H., F.X.W.; writing—original draft preparation, P.E.H., R.A.W.; writing—review and editing, S.E.S., A.S., A.K.B., A.K.S., H.K., S.P.R.; visualization, F.X.W., P.E.H.; supervision, R.A.W., A.K.S.; project administration, P.E.H., R.A.W., A.K.B.; funding acquisition, P.E.H.. All authors have read and agreed to the published version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the IZKF Wuerzburg (grant Z-02/85 to P.H.). This publication was supported by the Open Access Publication Fund of the University of Wuerzburg.
Data availability
The main data presented in this study are available in the article. Detailed information about the image analysis or the overall survivals of the subjects presented in this study is available on request from the corresponding author.
Declarations
Institutional review board
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived for this study by the local Ethics Committee due to the retrospective character of the study (waiver no. 20210422 04).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
This article is part of the Topical Collection on Oncology - Genitourinary.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Philipp E. Hartrampf, Anna Katharina Seitz, Andreas K. Buck and Rudolf A. Werner equally contributed to this study.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Sartor O, de Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofman MS, Emmett L, Sandhu S, Iravani A, Joshua AM, Goh JC, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet (London, England) 2021;397:797–804. doi: 10.1016/s0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 4.Heck MM, Tauber R, Schwaiger S, Retz M, D'Alessandria C, Maurer T, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with (177)Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. doi: 10.1016/j.eururo.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 5.Ahmadzadehfar H, Schlolaut S, Fimmers R, Yordanova A, Hirzebruch S, Schlenkhoff C, et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving [(177)Lu]Lu-PSMA-617 radioligand therapy. Oncotarget. 2017;8:103108–16. doi: 10.18632/oncotarget.21600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gafita A, Calais J, Grogan TR, Hadaschik B, Wang H, Weber M, et al. Nomograms to predict outcomes after (177)Lu-PSMA therapy in men with metastatic castration-resistant prostate cancer: an international, multicentre, retrospective study. Lancet Oncol. 2021;22:1115–1125. doi: 10.1016/s1470-2045(21)00274-6. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadzadehfar H, Rahbar K, Baum RP, Seifert R, Kessel K, Bögemann M, et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [(177)Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 trial) Eur J Nucl Med Mol Imaging. 2021;48:113–22. doi: 10.1007/s00259-020-04797-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahbar K, Boegemann M, Yordanova A, Eveslage M, Schäfers M, Essler M, et al. PSMA targeted radioligandtherapy in metastatic castration resistant prostate cancer after chemotherapy, abiraterone and/or enzalutamide. A retrospective analysis of overall survival. Eur J Nucl Med Mol Imaging. 2018;45:12–9. doi: 10.1007/s00259-017-3848-4. [DOI] [PubMed] [Google Scholar]
- 9.Ferdinandus J, Violet J, Sandhu S, Hicks RJ, Ravi Kumar AS, Iravani A, et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47:2322–2327. doi: 10.1007/s00259-020-04723-z. [DOI] [PubMed] [Google Scholar]
- 10.Grubmüller B, Senn D, Kramer G, Baltzer P, D'Andrea D, Grubmüller KH, et al. Response assessment using (68)Ga-PSMA ligand PET in patients undergoing (177)Lu-PSMA radioligand therapy for metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1063–1072. doi: 10.1007/s00259-018-4236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bräuer A, Grubert LS, Roll W, Schrader AJ, Schäfers M, Bögemann M, et al. (177)Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–1670. doi: 10.1007/s00259-017-3751-z. [DOI] [PubMed] [Google Scholar]
- 12.Kulkarni H, Schuchardt C, Singh A, Langbein T, Baum R. Early initiation of Lu-177 PSMA radioligand therapy prolongs overall survival in metastatic prostate cancer. J Nucl Med. 2018;59:529. [Google Scholar]
- 13.Rathke H, Holland-Letz T, Mier W, Flechsig P, Mavriopoulou E, Röhrich M, et al. Response prediction of (177)Lu-PSMA-617 radioligand therapy using prostate-specific antigen, chromogranin A, and lactate dehydrogenase. J Nucl Med. 2020;61:689–695. doi: 10.2967/jnumed.119.231431. [DOI] [PubMed] [Google Scholar]
- 14.Ferdinandus J, Eppard E, Gaertner FC, Kürpig S, Fimmers R, Yordanova A, et al. Predictors of response to radioligand therapy of metastatic castrate-resistant prostate cancer with 177Lu-PSMA-617. J Nucl Med. 2017;58:312–319. doi: 10.2967/jnumed.116.178228. [DOI] [PubMed] [Google Scholar]
- 15.Sadaghiani MS, Sheikhbahaei S, Werner RA, Pienta KJ, Pomper MG, Solnes LB, et al. A systematic review and meta-analysis of the effectiveness and toxicities of lutetium-177-labeled prostate-specific membrane antigen-targeted radioligand therapy in metastatic castration-resistant prostate cancer. Eur Urol. 2021;80:82–94. doi: 10.1016/j.eururo.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi KN, Metser U, Czernin J, Calais J, Prasad V, Eiber M, et al. Abstract PO-077: Study evaluating metastatic castrate resistant prostate cancer (mCRPC) treatment using 177Lu-PNT2002 PSMA therapy after second-line hormonal treatment (SPLASH) - trial in progress. Clin Cancer Res. 2021;27:PO-077-PO-. 10.1158/1557-3265.Radsci21-po-077.
- 17.Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–920. doi: 10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 18.Weineisen M, Schottelius M, Simecek J, Baum RP, Yildiz A, Beykan S, et al. 68Ga- and 177Lu-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–1176. doi: 10.2967/jnumed.115.158550. [DOI] [PubMed] [Google Scholar]
- 19.Gafita A, Heck MM, Rauscher I, Tauber R, Cala L, Franz C, et al. Early prostate-specific antigen changes and clinical outcome after (177)Lu-PSMA radionuclide treatment in patients with metastatic castration-resistant prostate cancer. J Nucl Med. 2020;61:1476–1483. doi: 10.2967/jnumed.119.240242. [DOI] [PubMed] [Google Scholar]
- 20.Hartrampf PE, Weinzierl F-X, Serfling SE, Pomper MG, Rowe SP, Higuchi T, et al. Hematotoxicity and nephrotoxicity in prostate cancer patients undergoing radioligand therapy with [177Lu]Lu-PSMA I&T. Cancers. 2022;14:647. doi: 10.3390/cancers14030647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khreish F, Ghazal Z, Marlowe RJ, Rosar F, Sabet A, Maus S, et al. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: initial 254-patient results from a prospective registry (REALITY Study) Eur J Nucl Med Mol Imaging. 2021 doi: 10.1007/s00259-021-05525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrmann K, Kratochwil C, Fendler WP, Eiber M, Hustinx R. 2021: the year [(177)Lu]Lu-PSMA-617 RLT PSMA is ready for incorporation into clinical guidelines?: reply to “A perspective on the EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands” by Dr. Germo Gericke. Eur J Nucl Med Mol Imaging. 2021;48:2668–9. doi: 10.1007/s00259-021-05409-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuchardt C, Zhang J, Kulkarni HR, Chen X, Mueller D, Baum RP. Prostate-specific membrane antigen radioligand therapy using (177)Lu-PSMA I&T and (177)Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution and dosimetry. J Nucl Med. 2021 doi: 10.2967/jnumed.121.262713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 25.Barber TW, Singh A, Kulkarni HR, Niepsch K, Billah B, Baum RP. Clinical outcomes of (177)Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J Nucl Med. 2019;60:955–962. doi: 10.2967/jnumed.118.216820. [DOI] [PubMed] [Google Scholar]
- 26.Seifert R, Seitzer K, Herrmann K, Kessel K, Schäfers M, Kleesiek J, et al. Analysis of PSMA expression and outcome in patients with advanced prostate cancer receiving (177)Lu-PSMA-617 radioligand therapy. Theranostics. 2020;10:7812–7820. doi: 10.7150/thno.47251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seifert R, Kessel K, Schlack K, Weckesser M, Kersting D, Seitzer KE, et al. Total tumor volume reduction and low PSMA expression in patients receiving Lu-PSMA therapy. Theranostics. 2021;11:8143–8151. doi: 10.7150/thno.60222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The main data presented in this study are available in the article. Detailed information about the image analysis or the overall survivals of the subjects presented in this study is available on request from the corresponding author.