Abstract
Allergy by cow’s milk proteins is among the major food allergies and could be reduced by the partial hydrolysis of these proteins by proteases, without significantly affecting its physicochemical properties. In addition, the peptides generated through enzymatic hydrolysis of the cow’s milk can present prebiotic and bioactive properties. In this work, the cow’s milk proteins were submitted to a controlled hydrolysis by Novo-Pro D® and the influence of the degree of hydrolysis (DH) on peptide size distribution was evaluated, as well as the prebiotic and antimicrobial properties of milk hydrolysates. It was shown that for DH-10%, all the peptides have sizes lower than 12 kDa which is the size of the most allergenic proteins, without apparent changes in the milk, as long as heating of the hydrolysate is avoided. The protein hydrolysis promoted a great improvement in the milk functional properties. In addition, the obtained milk peptides presented great prebiotic activities, as indicated by the significant improvement of the growth of prebiotic L. acidophilus and L. reuteri and by the production of bacteriocins indicated by the inhibition halos in the growth of a pathogenic microorganism.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05533-x.
Keywords: Milk peptides, Multifunctional properties, Antimicrobial activity, Prebiotics, Enzymatic proteolysis
Introduction
The cow’s milk is one of the most important nutrition sources for humans in the first years of life, being typically composed by water and approximately 12.7% of total solids, with the following distribution: 3.2% proteins; 3.7% fat; 4.8% lactose; and 0.7% ash (Ali et al. 2021). The high nutritional value of cow’s milk is due to its high protein content, mainly composed by caseins (28 g/L, around 80% of total protein), and whey proteins (around 20%): β-lactoglobulin (3.2 g/L), α-lactalbumin (1.2 g/L) and serum albumin (0.5 g/L) (Micinski et al. 2013).
Generally, allergy by the cow’s milk proteins occurs in the first year of life for around 3% of the infants (Feng et al. 2020). This is a serious problem since cow’s milk is largely used to replace breast milk due to its availability, low cost and high nutritional value (Hochwallner et al. 2014). For the allergenic infants, the immune system will recognize all or some of the cow’s milk proteins as harmful substances, which will trigger allergic response, leading to several reactions as infant diarrhea, respiratory asthma and skin allergies (Rangel et al. 2016). The literatures reported that β-lactoglobulin and α-lactalbumin have the highest allergenic potential (Hochwallner et al. 2014). β-Lactoglobulin, in particular, is not found in human milk, which makes its absorption more difficult and may increase its allergenic potential (Feng et al. 2020).
Several methods have been studied to reduce the allergenic potential of the milk proteins, such as heat treatment, glycation reaction, high pressure, lactic acid fermentation, and enzymatic hydrolysis (Abd El-Salam and El-Shibiny 2019; Bu et al. 2009). The enzymatic hydrolysis allows a controlled break down of the milk proteins into small peptide molecules and amino acids, which may not only unfold the conformational epitopes but also reduce the molecular mass, eliminating specific amino acids sequences and so preventing that the Ig-E can identify the milk proteins as foreign compounds in the human body (Li et al. 2016).
In this way, the complete hydrolysis to amino acids assures that the potential allergenic of the cow’s milk proteins is eliminated, reason why they are used in the formulas to replace the breast milk for infants. However, these formulas are expensive and have organoleptic and physical chemical properties very different from milk. Yet, it may be not necessary to proceed a complete hydrolysis of the proteins to reach the desired effect. In fact, the partial enzymatic hydrolysis has been reported to be well succeed in reducing the milk proteins allergy (Sauser et al. 2018). The allergenic potential and greatest reactivity of the immune system is reported to occur for proteins greater than 14 kDa (Hochwallner et al. 2014), The hydrolysis reaction catalyzed by an endoprotease, carried to some specific extent, may eliminate or at least reduce the allergenic potential of the original proteins by transforming them in smaller peptides. Furthermore, the literatures also reported other possible advantages of the protein hydrolysis: the produced peptides of the cow’s milk proteins may act as bioactive peptides (Akan 2021) and as prebiotics (Mohanty et al. 2016; Um et al. 2021), acting as growth factor for probiotic microorganisms. Then, a new product could be generated, using a similar approach that is used for the dairy industry to produce milk without lactose. If two enzymes, lactase and protease, were used, the same product could have several benefic properties, like elimination of lactose, reduced allergenic potential, prebiotic and anti-microbial functions, among others. However, it has to be pointed that if the lactose hydrolysis already modifies the milk properties, like the increase in the sweet taste, the protein hydrolysis may introduce other stronger changes in the milk properties, resulting in undesired bitter taste due to the presence of bitter peptides (Cui et al. 2021), requiring a careful and controlled process design to minimize them.
In this sense, the Novo-Pro D® is a Bacillus licheniformis endoprotease that has subtilisin activity and is produced by submerged cultivation of a genetically modified microorganism, but the enzyme itself is not genetically modified (Lopes et al. 2020). This enzyme has shown promising results in the hydrolysis of proteins from soybean husk (Rojas et al. 2014), casein (Lopes et al. 2020) and whey proteins (Rosa et al. 2018). Rojas et al. (2014) evaluated the proteolysis of soybean husks aiming oligopeptides production and obtained a recovery of around 60% of the protein content as small peptides (86% with molar mass less than 6.5 kDa). Lopes et al. (2020) studied the hydrolysis of casein (26 g/L) at 50 ºC and pH 6.5 and achieved 34% of hydrolysis degree after 2 h of reaction. Rosa et al. (2018) investigated the effect of four different commercial proteases (Novo ProD®, Alcalase®, Pancreas Trypsin® and Flavourzyme®) in the generation of hydrolysates with emulsifying and antioxidant activities from whey protein. After 5 h the Novo ProD® achieved the highest degree of hydrolysis and an increase of 40% in the antioxidant activity. However, there are no reports in the scientific literatures on the study of the reduction of allergenicity and increase in nutritional properties of milk by protein hydrolysis using Novo-Pro D®. In this work, the raw cow’s milk, ultra-high temperature-sterilized (UHT) whole and UHT low fat milks were submitted to a controlled hydrolysis through the action of Novo-Pro D®, in order to investigate the influence of the degree of hydrolysis (DH) on the peptides profile (size distribution). In addition, the milk hydrolysates action as prebiotic and antimicrobial molecules were assessed and an improvement on milk’s properties was achieved.
Materials and methods
Materials and microorganisms
The endoprotease Novo-Pro D® (2537 UCAS/mL and 53.4 UCAS/mgprotein) was kindly donated by Novozymes Latin America Ltda (Araucária, Brazil). Whole (3,3% fat, 30.8 g/L protein) and low fat (1,28% fat, 32.5 g/L protein) UHT milk were Ninho (Nestlé, Araraquara, Brazil), purchased from local markets and raw milk (3.8–4% fat, 28 g/L protein) was purchased from a local farm. The proteins α-lactalbumin, casein, β-lactoglobulin, bovine serum albumin (BSA), LB-agar and Bradford reactant, were from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of analytical grade. The microorganisms used were Lactobacillus acidophilus LA14 (CVS Health/USA), Lactobacillus reuteri LE16 (Acquativa, São Carlos/Brazil) and Escherichia coli K12 MG1655.
Methods
Enzymatic activity
Novo-Pro D® activity was determined by the initial velocity of the hydrolysis of 25 mL of a casein solution (2%, 0.1 mol/L phosphate buffer pH 6.5) at 50 °C. The hydrolysis reaction was carried out in a pHstat model Titrino 907 (Metrohm Ltd., Herisau/Swiss). The number of broken peptide bonds with the time (B x Nb × 1/α) was calculated by measuring the KOH consumed to keep the pH constant during the reaction. The slope of the obtained curve corresponded to the Eq. 1.
| 1 |
where B is the volume of consumed KOH during the reaction time (mL); Nb is the KOH concentration (mol/L); α is the average grade of α-NH2 groups dissociation (tabulated values for several pHs and temperatures, obtained from Adler-Nissen 1986); t is reaction time (minutes); VH is the hydrolysis initial velocity expressed as mmol of peptide bonds per minute (mmol/min). One casein unit (UCas) is the amount of enzyme needed to break one µmol of peptide bonds in one minute, at the described reaction conditions.
Milk hydrolysis
The milk proteins hydrolysis reaction, catalyzed by Novo-Pro D® (1% enzyme protein/milk protein), was proceeded at 50 ºC and pH 6.5. A control experiment, without enzyme, was performed in order to check the milk stability under hydrolysis conditions. The degree of hydrolysis (DH) was calculated by measuring the KOH solution volume needed to keep constant the pH, using the Eq. 2 (Adler-Nissen 1986):
| 2 |
where MP is the protein mass (g) and htot is the total number of peptide bonds potentially breakable in the milk proteins (for cow’s milk htot is 8.1 meq/g according to Adler-Nissen 1986).
Determination of peptide profile of milk hydrolysates
The percentage of proteins/peptides present in each size range, before and after milk hydrolysis, were determined through a size-exclusion chromatography in a HPLC Waters e2695 equipped with a BioSep—SEC s2000 column (Phenomenex), elution with 50 mM phosphate buffer and 0.15 mol/L NaCl, pH 6.8, flow of 1 mL/min, detection UV–visible at 214 nm, injecting 20 µL of sample, and running each sample for 30 min. The peptide profile was obtained by dividing the chromatogram in regions delimited by the retention times of the standards size markers (12 kDa, 29 kDa and 66 kDa) and calculating the area under each detected protein/peptide peak, as described by Gupta (1983). The total chromatogram area is considered as 100% of the proteins/peptides present in the sample and the fraction of the total area in each region, delimited by two size markers, is directly proportional to the concentration of peptides/protein present in this region.
Analysis of milk peptides prebiotic activity
Prebiotic activity was evaluated by measuring the milk hydrolysates influence on the growth of probiotic microrganisms L. acidophilus and L. reuteri. The pre-inoculation was carried out in 10 mL of MRS medium at 37 ºC for 18 h. The fermentation experiments were run in 10 mL of MRS medium pH 6.0 supplemented with milk peptides (1:10 ratio) and maintained at 37 ºC for 24 h with initial OD 0.05 (Milessi et al. 2021). MRS medium without the addition of milk peptides was used as control. Cell growth was evaluated (OD at 600 nm) at the end of the cultivation and production of bacteriocins quantified.
Antimicrobial activities assays
The antimicrobial activities of the milk hydrolysate peptides and the production of bacteriocin by the probiotic microorganisms were determined by the critical dilution technique, observing the formation of inhibition halos using the gram-negative bacteria E. coli K12 MG1655 as control microorganism. The pure milk peptides (without probiotic cultivation) or the supernatant of the lactobacilli fermentation medium (after cells removal, to evaluate the action of the metabolites of the probiotics) were serially diluted (2 and 5x) and 50 μL of each dilution was inoculated into wells of 1.5% LB-agar plates containing the indicator strain and incubated at 37 ºC. The antimicrobial activity as the production of bacteriocins was classified according to the formation and size of the inhibition halos formed and was expressed in Arbitrary Units per milliliter (AU/mL), which was defined as the reciprocal of the highest dilution that presented an inhibition halo (Milessi et al. 2021).
Sugars consumption
The sugar quantification before and after prebiotic activity assays was carried out by High Performance Liquid Chromatography (HPLC) using a Waters e2695 chromatograph with refractive index detector, equipped with a Sugar Pak I column (10 μm, 6.5 × 300 mm; Waters, USA), 0.6 mL/min of ultra-pure water as the mobile phase, at 80 ºC. The injected volume was 20 μL and the refractive index detector was maintained at 40 ºC. The sugar consumption over the cultures was determined according to Eq. 3, where CS0 is the initial sugar concentration and CS is the final sugar concentration (g/L).
| 3 |
Results and discussion
Influence of the milk characteristics on the enzymatic hydrolysis profiles
The milk protein structure, the milk thermal processing and the presence of fat in the milk are factors that can interfere on the enzyme action during milk hydrolysis. Results of Hochwallner et al. (2014) indicated that α-lactalbumin, β-lactoglobulin and casein, separately or together, are the main responsible for the allergenic potential of the milk proteins. These proteins have different structures, which brings the necessity to investigate the individual hydrolysis profile of each of them under similar reaction conditions.
Once only the hydrolysis of casein by Novo-Pro D® is reported in literature (Lopes et al. 2020), the individual hydrolysis of these three main milk proteins by Novo-Pro D® were performed and using protein concentrations similar to the ones present in cow’s milk: 1.5 g/L, 3 g/L and 24 g/L, for α-lactalbumin, β-lactoglobulin and casein, respectively. The observed increase of the DH with the time (Figure S1, Online Resource 1) for the three proteins hydrolysis indicated that all of them can be hydrolyzed by this protease.
The next step was to compare the hydrolysis patterns of the proteins present in ultra high temperature sterilized (UHT) whole milk, UHT low fat milk and raw milk. The hydrolysis conversions were followed for 6 h (Fig. 1). The raw milk presented higher DH than both UHT milks which could be attributed to the conformational change in the structure of the UHT milk proteins due to the high temperature of the sterilization process, making them more resistant to hydrolysis. This sterilization process, which is extensively used in the dairy industry, employs a very high temperature (130–150 °C) for a short time (3–5 s), in order to achieve the dead of all the vegetative forms and most of the spores in the milk. Milk proteins have many Cys groups, which allow the formation of intermolecular disulfide bonds during high temperature processing of milk. Above 70 °C, β-lactoglobulin undergoes irreversible denaturation and polymerization (formation of intermolecular disulfide bonds). α-lactalbumin, on the other hand, is more stable to heat due to the fact that it can almost completely renature by cooling after exposure to temperatures above 62 °C (Morr and Ha 1993). In addition, the presence of contaminant microorganisms with proteolytic action in the raw milk could contribute to achieve higher DHs.
Fig. 1.
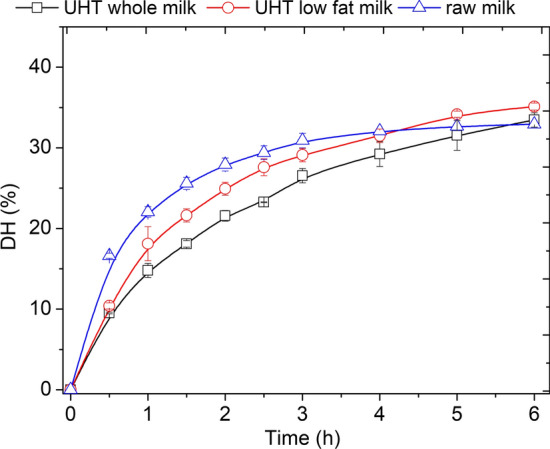
Hydrolysis degree (DH) with the time, in the hydrolysis of proteins present in: UHT whole milk, UHT low fat milk and raw milk, catalyzed by Novo-Pro D.® (1% mgenzymeprotein/mgmilkprotein, pH 6.5, 50 °C, 6 h)
It is important to highlight that in the control experiment it was not observed neither any change in the milk pH (6.5), milk DH (0%) nor in its visual characteristics. In addition, no signs of spoilage were observed, such as sour odor, off-flavor and curdled consistency. Besides, the hydrolysis of milk by proteases for hours at temperatures above 50 °C, such as 70–80 °C, is highly reported in literatures (Liang et al. 2021; Kaur et al. 2021).
In order to get more details, HPLC analysis was carried out to obtain the molecular mass distribution of the proteins before and after the hydrolysis for the three types of milk tested. The results before hydrolysis (Table 1) indicate that the milk protein composition is already affected by both the sterilization and skimming processing. Raw milk does not present peptides between 29 and 66 kDa. This fraction only is present in the UHT milk, being higher for the low fat one. Observing the composition obtained after hydrolysis it is worth to notice that α-lactalbumin (~ 14 kDa) and β-lactoglobulin (~ 18 kDa), reported as the main allergenic factors in the milk (Hochwallner et al. 2014), were degraded by the protease in all tested types of milk, confirming that the used protease is able to reduce the molecular mass of these proteins. It was also observed that, despite of the initial hydrolysis rate of UHT low fat milk being slightly faster than the UHT whole milk, after one hour of hydrolysis all the peptides from raw and UHT whole milks were lower than 12 kDa (below allergenic range), while the fraction between 29 and 66 kDa still remains for the low fat milk even after 6 h of hydrolysis. Therefore, the presence of fat seems to facilitate the enzymatic hydrolysis of milk proteins to the desired range (smaller than 12 kDa). According to Zhang et al. (2020), the milk fat from UHT whole milk can influence the protease morphology, the fat–water emulsion may also contribute with hydrophobic interaction between the enzyme and the substrate, which makes the enzyme active sites more exposed, improving the accessibility between the active sites and the peptide bonds and, consequently, the proteolytic activity.
Table 1.
Molecular mass distribution of proteins in the UHT whole milk, UHT low fat milk and raw milk, before and after hydrolysis catalyzed by Novo-Pro D.® (pH 6.5, 50 ºC, 1% mgenzymeprotein/mgmilkprotein, 6 h)
| Molecular mass | Mass distribution (% w/w) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| UHT whole milk | UHT low fat milk | Raw milk | |||||||
| 0 h | 1 h | 6 h | 0 h | 1 h | 6 h | 0 h | 1 h | 6 h | |
| > 66 kDa | 23.1 | 0.0 | 0.0 | 17.2 | 0.0 | 0.0 | 37.5 | 1.3 | 0.0 |
| 66 kDa > 29 kDa | 23.6 | 0.0 | 0.0 | 44.5 | 31.0 | 16.2 | 1.7 | 0.0 | 0.0 |
| 29 kDa > 12.4 kDa | 12.3 | 0.0 | 0.0 | 9.5 | 6.0 | 0.0 | 42.8 | 0.3 | 0.1 |
| < 12.4 kDa | 40.9 | 100.0 | 100.0 | 28.8 | 63.0 | 83.8 | 18.0 | 98.4 | 99.0 |
In general, the endoprotease action depends on the size of the protein and has preference for specific groups in the substrate which direct the correct fit of the enzyme, facilitating its action. During the heat treatment, β-lactoglobulin associates with milk fat globule membrane through a mechanism which is not dependent on disulfide bounds formation but mainly dependent on hydrophobic interactions and hydrogen bounds (Hansen et al. 2020), which leads to different conformational changes in the proteins depending on the fat content. With this in mind, it can be seen that the fat presence favors Novo-Pro D® action on proteins between 29 and 66 kDa, resulting in 100% of peptides with mass distribution below 12 kDa after only 1 h of hydrolysis using UHT whole milk (Table 1).
Figure S2 in the Online Resource 1 shows the comparative mass molecular distribution for UHT whole milk proteins before and after 6 h of hydrolysis, compared with the position of a 12 kDa marker, at the same elution conditions. The detailed chromatograms for this milk, before and after 6 h of hydrolysis are shown in the Figure S3, in the Online Resource 1. Taking into account these results, it is clear that the presence of fat makes easier the hydrolysis of milk proteins into peptides smaller than 12 kDa, which have less allergenic properties. In addition, besides the probable presence of contaminant proteolytic microorganism in raw milk, the use of this milk has also the drawback of the short time it can be stored before it spoils for experiments runs and the variance between different batches. Then we decided to continue this work using UHT whole milk to take advantage of both the presence of fat and the longer shelf life.
Influence of DH on the molecular mass distribution of the milk proteins
To evaluate the influence of the DH on the molecular mass distribution of the hydrolyzed milk, the UHT whole milk was hydrolyzed by the action of the protease Novo-Pro D® at 50 °C in a pHstat, keeping the pH constant. Samples were withdrawn at different reaction times and heated to 90 °C to deactivate the enzyme and stop the reaction. The DH of each sample was then calculated and the proteins molecular mass distribution evaluated through HPLC analysis. After only few minutes of reaction under the studied conditions, hydrolysate already achieved 5% of DH and contained mostly peptides with less than 12 kDa in its composition (approximately 90%) (Table 2). After 30 min of reaction, all the peptides presented molecular mass lower than 18 kDa and after 60 min DH of 10.5% is reached with 100% of the peptides smaller than 12 kDa, which is below the molecular mass of the most allergenic proteins from milk.
Table 2.
Molecular mass (MM) profile of UHT whole milk proteins without hydrolysis and for different hydrolysis degrees (DH) (%). Reaction conditions: endoprotease Novo-Pro D.® (1% mgenzymeprotein/mgmilkprotein), at pH 6.5, 50 ºC. Peptide profiles determined after thermal inactivation of the samples (90 ºC, 10 min)
| Time (min) | DH (%) | Molecular Mass (% w/w) | |||
|---|---|---|---|---|---|
| MM < 12 kDa | 12 kDa < MM < 18 kDa | 18 kDa < MM < 66 kDa | MM > 66 kDa | ||
| 0 | 0 | 40.9 | 12.3 | 23.6 | 23.1 |
| 4 | 3.5 | 77.4 | 22.1 | 0.03 | 0.44 |
| 6 | 4.4 | 88.5 | 11.4 | 0.06 | 0.10 |
| 8 | 5.1 | 89.1 | 10.8 | 0.00 | 0.14 |
| 10 | 5.7 | 90.1 | 9.8 | 0.00 | 0.12 |
| 15 | 6.3 | 90.2 | 9.7 | 0.00 | 0.09 |
| 30 | 7.1 | 91.3 | 8.5 | 0.00 | 0.00 |
| 60 | 10.5 | 100.0 | 0.0 | 0.00 | 0.00 |
Liang et al. (2021) studied the hydrolysis of cow’s milk by the enzyme Protamex from Novozymes and found that for a DH of 9%, the obtained hydrolysate was mainly composed by peptides with molecular mass lower than 10 kDa. Although different patterns of action are expected from different enzymes, similar results were obtained in the present work, where DH 10% was enough to achieved 100% of peptides molecular mass lower than 12 kDa.
Kaur et al. (2021) studied the hydrolysis of milk protein concentrate by the action of a plant protease actinidin, obtaining a hydrolysis degree of 9.14% at 60 °C after 5 h. Higher DHs can be achieved with longer hydrolysis process as showed in this work, however extensively hydrolyzed milk proteins have poor flavor, bitter taste and high cost (Abd El-Salam and El-Shibiny 2019). Considering that the allergenic potential and greatest reactivity of the immune system occurs for milk proteins greater than 14 kDa (Hochwallner et al. 2014), a product with a DH that satisfies this premise, as the one obtained in the present work, should already considerably reduce allergenic factors and allergic reactions in people sensitive to milk proteins.
At this point, it is important to compare the images of the produced milk hydrolysates at different DH before and after the thermal inactivation of Novo-Pro D® (Fig. 2). The images indicate that at the end of the hydrolysis reaction and prior to heating (Fig. 2b–d) there is a visible change in the visual appearance of milk hydrolysates only for DH-20% (Fig. 2d), while after heating it can be seen an increasing loss of the homogeneity and, the higher the DH before the heating, the bigger the homogeneity loss. The main reason for this phenomenon is probably the heating up to 90 °C, for the enzyme inactivation. In addition, the milk hydrolysis assays were run using a pHstat with controlled pH 6.5 by adding KOH solution, while the thermal inactivation procedure was run without pH control. According to Huppertz (2016), under milk’s natural pH it can be heated at 140 °C for more than 10 min without coagulation, however when the pH is changed or milk is concentrated, the heat stability of milk is reduced. Based on this report and in the fact that no visible coagulation was observed in control experiment without enzyme, it seems very probable that a decrease of the pH due to the continuity of the hydrolysis during the heating for the enzyme inactivation step without pH control have caused the coagulation. Therefore, as long as some enzyme molecules remain active until complete deactivation, the hydrolysis reaction may continue during the thermal inactivation procedure, and with a decrease in the pH the heat coagulation time of milk protein decrease significantly (Dumpler et al. 2020).
Fig. 2.
Photographs of UHT whole milk (a) and milk hydrolysates after the action of Novo-Pro D.® and before heating for enzyme inactivation with DH-5% (b), DH-10% (c) and DH-20% (d) and milk hydrolysates after heating for enzyme inactivation DH-5% (e), DH-10% (f) and DH-20% (g)
These milk hydrolysate changes, observed to occur during the thermal inactivation of the enzyme (Fig. 2e–g), are reported to also occur in the dairy industry during the sterilization of milk at high temperature by a short time (UHT), possibly to a lesser extent. The dairy industry uses additives to avoid protein precipitation (Deeth and Lewis 2016). Since UHT sterilization could also lead to inactivation of the enzyme, this procedure would possibly allow better control of the process and to decrease the deleterious effect of the heating.
A physical–chemical analysis performed according to Brasil (1981) showed that there is no change in milk density with the hydrolysis, remaining around 1.037 ± 0.001 g/mL. Nevertheless, to ensure that no changes in the milk occurs after the protein hydrolysis, it is necessary to perform a complete and specific study to evaluate the influence of the hydrolysis degree of the milk proteins in the sensory, viscosity and other physical–chemical properties of the milk, in order to select the best hydrolysis degree based on the acceptance of the milk and the reduction of the allergenic potential, which will be accessed in the continuity of this work. Most importantly, the results obtained here showed that it is possible to obtain a milk hydrolysate with peptides smaller than 12 kDa without apparent visual changes in the milk aspect as long as enzyme inactivation by heating is avoided. The best alternative should be the use of immobilized enzyme, once this technique would provide an easy separation of the enzyme by microfiltration. This operation would immediately avoid the continuity of hydrolysis and also contribute to eliminate or at least greatly reduces bacterial contamination.
Evaluation of prebiotic properties of milk peptides obtained after hydrolysis with Novo-Pro D®
The hydrolysis of milk proteins can lead to significant changes on its functional properties due to changes on peptides size, structure and hydrophobicity (Abd El-Salam and El-Shibiny 2019). Considering that peptides obtained by the hydrolysis of milk protein may have prebiotic capacities and that the peptide composition varies with DH, not only by increasing the concentration of the smaller ones, but also possibly generating peptides with equals size but different residues sequences, the influence of the addition of hydrolysates obtained with different DH (1.5%; 5.7%; 7.1% and 10.5%) was evaluated on the growth of two lactobacilli that are known to be probiotics, L. acidophilus and L. reuteri.
The probiotic bacteria cultivation in MRS medium supplemented with milk peptides is shown in Fig. 3. UHT whole milk without hydrolysis (DH-zero) containing 30.8 mg/mL of protein was used as positive control and pure MRS medium was used as negative control. It is clear that milk peptides are important growth factors for L. acidophilus and L. reuteri with an increase in the OD of these probiotic microorganisms with the increasing of DH (Fig. 3a). The best performances for both lactobacilli were observed in the tests using DH-7.1%, in which there was total consumption of sugars (glucose and lactose) in 24 h of process (Fig. 3c), as well as higher biomass production (Fig. 3b). Thus, the increase in the DH up to 7.1% and consequently reduction in the size of the peptides (Table 2) positively influences the prebiotic potential of the milk hydrolysates.
Fig. 3.
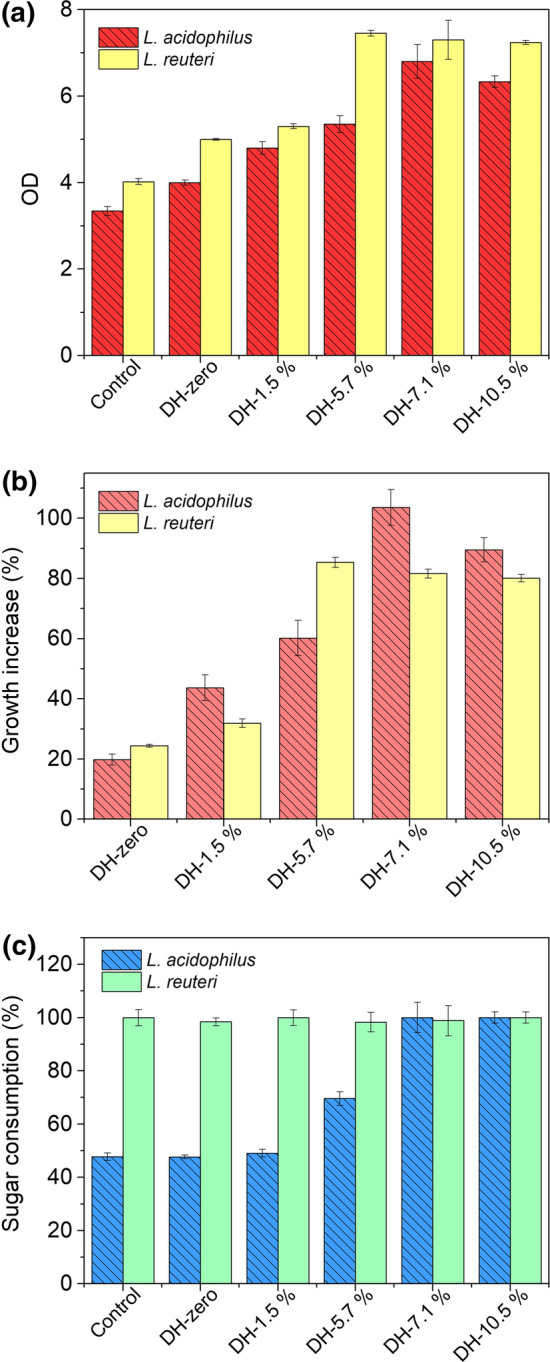
Optical density (OD) (a), growth increase comparing with MRS control assay (b) and sugar consumption (c) after 24 h of cultivation of L. acidophilus and L. reuteri in MRS medium supplemented with milk peptides at different degrees of hydrolysis (DH). Tests were performed at 37 °C and pH 6.0. Results are means of triplicates
Above DH-10.5%, the reduction in the size of peptides should continue, as indicated by the increase in DH, although the methodology used does not allow measuring the distribution in the range below 12 kDa. This greater reduction in peptides’ size, however, does not seem to favor the growth of probiotic microorganisms once no significant increase was observed in the growth of both lactobacilli for DH-10.5%. In fact, a reduction in the growth increase compared to the control experiment was observed from DH-7.1% to DH-10.5% (Fig. 3b), indicating that the growth is influenced not only by the concentration, but also by a specific composition of peptides.
The total sugar consumption in 24 h was also influenced by the DH for L. acidophilus, increasing from 47 to 100% between control experiment and DH-7.1% (Fig. 3c). The presence of peptides clearly increased the sugar consumption rate in these experiments. The same behavior was not observed for L. reuteri that presented 100% of sugar consumption in 24 h in all experiments. This is probably because it is an heterofermentative bacteria (Klantschitsch et al. 1996), with higher versatility in different sugars use. However, the growth increase was evident for both microorganisms. These results clearly show that the peptides obtained by hydrolysis of milk proteins act as prebiotics for these two Lactobacillus, reaching maximum response for DH 7.1%.
Soukolis et al. (2014) also observed the positive effect in the supplementation with milk protein hydrolysates, with significant improvement in the cell viability during storage of probiotic strain L. acidophilus NCIMB 701,748. McComas and Gillilan (2003) studied the influence of milk supplementation with whey protein hydrolysate in the growth of probiotic microorganism. They also observed prebiotic activity by the improvement in the growth of L. acidophilus and Bifidobacterium longum S9, however the observed growth improvement was of 19 and 12.5% for each bacterium, respectively. In the present work, the obtained milk hydrolysates led to substantial higher growth improvement, reaching the double of cell growth for L acidophilus (Fig. 3a). In addition, the produced milk peptides might also represent new biologically active substances to contribute as a supplementation in a more efficient application of L. acidophilus and L. reuteri in the industrial production of pharmaceuticals or functional foods.
Evaluation of bacteriocins production and antimicrobial properties of milk peptides
The functional properties of milk hydrolysates are determined by their protein composition and chain length (Ali et al. 2021). Aiming to evaluate the multifunctional properties of the produced UHT whole milk peptides, the supernatants recovered from the culture broths after the growth of the two probiotic Lactobacillus in the milk hydrolysates with different DHs were inoculated at different dilutions in petri dishes with LB-agar and E. coli K12 MG1655. The results indicated the formation of an inhibition halo and, probably, the presence of bacteriocins as probiotic metabolites for all tested milk protein hydrolysates (Table 3). The maximum bacteriocin production (100 AU/mL) was observed for both for L. acidophilus and L. reuteri when protein hydrolysates with DH-10.5% were added to the culture broths.
Table 3.
Antimicrobial activity (AU/mL), quantified in terms of the size of the inhibition halo formed during cultivation in plates of the indicator bacteria E. coli K12 MG1655 (37 ºC, 24 h), in the presence of supernatants, in different dilutions, of lactobacilli culture broths where milk protein hydrolysates have been added at different degrees of hydrolysis and of pure milk peptides without lactobacilli action, where (−) no antimicrobial activity; ( +) inhibition halo between 2-5 mm; (+ +) inhibition halo between 5-7 mm; (+ + +) inhibition halo between 7 and 10 mm
| D = 1 | D = 2x | D = 5x | AU/mL | |
|---|---|---|---|---|
| L. acidophilus | ||||
| DH-zero | − | − | − | 0 |
| DH-1.5% | + | − | − | 20 |
| DH-5.7% | + | − | − | 20 |
| DH-7.1% | + | + | − | 40 |
| DH-10.5% | + + | + | − | 40 |
| L. reuteri | ||||
| DH-zero | + | − | − | 20 |
| DH-1.5% | + | + | − | 40 |
| DH-5.7% | + | − | − | 20 |
| DH-7.1% | + | + | − | 40 |
| DH-10.5% | + + | + | + | 100 |
| Pure milk peptides | ||||
| DH-zero | + | − | − | 20 |
| DH-1.5% | + | + | − | 40 |
| DH-5.7% | + | − | − | 20 |
| DH-7.1% | − | − | − | 0 |
| DH-10.5% | − | − | − | 0 |
Hayes et al. (2006) investigated the antimicrobial activity of casein-derived peptides present in L. acidophilus DPC6026 culture broth and observed inhibition halos between 1.5 and 2 cm against E. coli strain, with antimicrobial activity in the order of 100 AU/mL. Esmaeilpour et al. (2016) studied the antimicrobial activity of goat milk peptides against E. coli and Bacillus cereus obtained after enzymatic hydrolysis with ficin or trypsin enzymes. The greatest bacterial inhibition (69%) was obtained with the peptides produced using ficin. However, when authors fractionated the produced peptides and investigated the relation between the antimicrobial activity and milk peptide size, they observed that the main factor influencing the antimicrobial activity was the enzyme used for milk hydrolysis, once the range of peptides with higher antimicrobial activity was different when ficin or trypsin were used. In this sense, the enzyme used in milk hydrolysis will influence not only in peptide composition with different residues sequences but also on its antimicrobial capacity. The milk peptides obtained by the action of Novo-Pro D® showed excellent prebiotic activities, demonstrated both by the greater growth of the two probiotic microorganisms in the presence of these peptides and by the bacteriocin production, as indicated by the inhibition of the growth of a pathogenic microorganism.
The literatures also indicated that pure milk peptides can act directly as antibiotics (Wang et al. 2016). Antimicrobial peptides from milk have gained importance due to their positive impact on physiological and metabolic functions of human health (Mohanty et al. 2016). The influence of peptides in the metabolism will vary according to the studied microorganism, once the membrane permeability is the mostly recognized and well-accepted mechanism of action of antimicrobial peptides (Lei et al. 2019). In this sense, considering that the probiotic Lactobacillus tested in the present work were Gram ( +), while the control agent studied is Gram (-), besides milk hydrolysates having great prebiotics activity it is worth to evaluate its direct antimicrobial activity. In order to test this possible action of the peptides obtained by hydrolysis with Novo-Pro D®, it was inoculated directly at different dilutions in petri dishes with LB-agar and E. coli K12 MG1655. The results shown in Table 3 indicate that only the larger oligopeptides seem to have some antimicrobial action against the bacteria tested, obtaining the highest result (40 AU/mL) for the lowest DH tested (DH-1.5%). The non-hydrolyzed whole milk (DH-zero) also showed positive response (20 AU/mL) which indicates that it already contains a small concentration of peptides with antimicrobial action. Indeed, the presence in milk of bioactive peptides that have antimicrobial activities against some pathogenic bacteria such as E. coli is reported. However, the increase in antimicrobial activity only for a small DH and the lack of response for highest DHs indicate that there must be a relation between the benefits obtained with the increase in DH and the properties to be obtained in the final product. In fact, it can be seen in Fig. 3b that lower DH hydrolysates had lower prebiotic activity, leading to lower Lactobacillus growth and lower bacteriocins production (Table 3), which is in accordance with the higher antimicrobial activity observed for the hydrolysates with DH-zero and 1.5%, which leads to infer that, in this case, the smallest peptides (< 18 kDa) have higher prebiotic activity, while the biggest ones (> 18 kDa) present more antimicrobial activity. The results also indicate the importance of continuing studies for both the antimicrobial action of protein hydrolysates as prebiotics and as antimicrobials, investigating the behavior of other lactobacilli and pathogenic microorganisms in the presence of protein hydrolysates.
Conclusions
The protease Novo-Pro D® showed to be efficient for the hydrolysis of all the main cow milk proteins. Comparing UHT whole milk, UHT low fat milk and raw milk, UHT whole milk was selected as substrate, in order to have more control of the possible contamination during assays. Analysis of the size profile of peptides showed that, for a DH-10%, all obtained peptides have size lower than 12 kDa, a condition that is reported to reduce the allergenic potential of the milk proteins. This condition is obtained without visible changes in the milk appearance, as long as heating is avoided. In addition, the obtained peptides presented interesting prebiotic and antimicrobial activities. Then, an important improvement in the milk properties can be obtained with a controlled enzymatic protein hydrolysis using Novo-Pro D®. The deleterious effects of the heating in the product appearance could be overcome by the use of an immobilized protease, which would allow the use of membrane filtration for the reduction of the bacterial contamination and enzyme separation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Prof. Isabel Rocha (University of Minho) for kindly donate Escherichia coli K12 MG1655 strain. This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES, Finance Code 001), Brazilian National Council for Scientific and Technological Development (CNPq) (Grants Number #422131-2016-4 and #439474/2016-7) and São Paulo Research Foundation (FAPESP) (Grant Number #2015/19680-7).
Abbreviations
- AU
Arbitrary units
- BSA
Bovine serum albumin
- DH
Degree of hydrolysis
- HPLC
High performance liquid chromatography
- Ig-E
Immunoglobulin E
- LB-agar
Luria Bertani agar
- MRS
Man, Rogosa and Sharpe
- OD
Optical density
- UHT
Ultra-high temperature
Authors’ contributions
TSM conceived, carried out the experiments and wrote the MS; LAL carried out experiments and edited the MS; PKN conceived and carried out the experiments; PWT conceived, supervised the work and edited the manuscript; RLCG conceived, supervised the work, wrote the MS and administered the project. All authors have given approval to the final version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance Code 001. The authors acknowledge Grants #2015/19680–7, São Paulo Research Foundation (FAPESP), #422131–2016-4 and #439474/2016–7, Brazilian National Council for Scientific and Technological Development (CNPq).
Data availability
All data presented in this article, including Online Resource 1, are also available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Salam MH, El-Shibiny S. Reduction of milk protein antigenicity by enzymatic hydrolysis and fermentation: a review. Food Rev Int. 2019;37(3):276–295. doi: 10.1080/87559129.2019.1701010. [DOI] [Google Scholar]
- Adler-Nissen J. Enzymatic hydrolysis of food proteins. Barking, Essex: Elsevier Applied Science Publishers; 1986. [Google Scholar]
- Akan E. An evaluation of the in vitro antioxidant and antidiabetic potentials of camel and donkey milk peptides released from casein and whey proteins. J Food Sci Technol. 2021;58(10):3743–3751. doi: 10.1007/s13197-020-04832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A, Kamal M, Rahman H, Siddiqui N, Haque A, Saha KK, Rahman A. Functional dairy products as a source of bioactive peptides and probiotics: current trends and future prospectives. J Food Sci Technol. 2021 doi: 10.1007/s13197-021-05091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil (1981) Ministério da Agricultura. Secretaria Nacional de Defesa Agropecuária. Laboratório Nacional de Referência Animal. Métodos analíticos oficiais para controle de produtos de origem animal e seus ingredientes: II–Métodos físicos e químicos. Brasília. (In Portuguese: Minister of Agriculture. National Secretariat for Agricultural Defense. National Animal Reference Laboratory. Official analytical methods for the control of products of animal origin and their ingredients: II–Physical and chemical methods.)
- Bu G, Luo Y, Zheng Z, Zheng H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric Immunol. 2009;20(3):195–206. doi: 10.1080/09540100903026116. [DOI] [Google Scholar]
- Cui Q, Sun Y, Zhou Z, Cheng J, Guo M. Effects of enzymatic hydrolysis on physicochemical properties and solubility and bitterness of milk protein hydrolysates. Foods. 2021;10:2462. doi: 10.3390/foods10102462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeth H, Lewis M. Protein stability in sterilised milk and milk products. Adv Dairy Chem. 2016 doi: 10.1007/978-1-4939-2800-2_10. [DOI] [Google Scholar]
- Dumpler J, Huppertz T, Kulozik U. Heat stability of milk and concentrated milk: past, present, and future research objectives. J Dairy Sci. 2020;103:10986–11007. doi: 10.3168/jds.2020-18605. [DOI] [PubMed] [Google Scholar]
- Esmaeilpour M, Ehsani MR, Aminlari M, Shekarforoush S, Hoseini E. Antimicrobial activity of peptides derived from enzymatic hydrolysis of goat milk caseins. Comp Clin Pathol. 2016;25:599–605. doi: 10.1007/s00580-016-2237-x. [DOI] [Google Scholar]
- Feng N, Zhang H, Li Y, Liu Y, Xu L, Wang Y, Fei X, Tian J. A novel catalytic material for hydrolyzing cow’s milk allergenic proteins: papain-Cu3(PO4)2·3H2O-magnetic nanoflowers. Food Chem. 2020;311:125911. doi: 10.1016/j.foodchem.2019.125911. [DOI] [PubMed] [Google Scholar]
- Gupta BB. Determination of native and denatured milk proteins by high-performance size exclusion chromatography. J Chromatogr A. 1983;282:463–475. doi: 10.1016/s0021-9673(00)91623-6. [DOI] [PubMed] [Google Scholar]
- Hansen SF, Nielsen SD, Rasmusen JT, Larsen LB, Wiking L. Disulfide bond formation is not crucial for the heat-induced interaction between β-lactoglobulin and milk fat globule membrane proteins. J Dairy Sci. 2020;103:5874–5881. doi: 10.3168/jds.2019-18066. [DOI] [PubMed] [Google Scholar]
- Hayes M, Ross RP, Fitzgerald GF, Hill C, Stanton C. Casein-derived antimicrobial peptides generated by Lactobacillus acidophilus DPC6026. Appl Environ Microbiol. 2006 doi: 10.1128/AEM.72.3.2260-2264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochwallner H, Schulmeister U, Swoboda I, Spitzauer S, Valenta R. Cow’s milk allergy: from allergens to new forms of diagnosis, therapy and prevention. Methods. 2014;66:22–33. doi: 10.1016/j.ymeth.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppertz T. Heat stability of milk. In: McSweeney P, O'Mahony J, editors. Advanced dairy chemistry. New York: Springer; 2016. [Google Scholar]
- Kaur S, Huppertz T, Vailjevic T. Milk protein hydrolysis by actinidin: influence of protein source and hydrolysis conditions. Int Dairy J. 2021;118:105029. doi: 10.1016/j.idairyj.2021.105029. [DOI] [Google Scholar]
- Klantschitsch T, Spillman H, Puhan Z. Lactobacillus reuteri: a newcomer in dairy technology. Mljekarsivo. 1996;46:183–196. [Google Scholar]
- Lei J, Sun L, Huang S, Zhu C, Li P, He J, Mackey V, Coy DH, He Q. The antimicrobial peptides and their potential clinical applications. Am J Transl Res. 2019;11(7):3919–3931. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Yu J, Goktepe I, Ahmedna M. The potential of papain and alcalase enzymes and process optimizations to reduce allergenic gliadins in wheat flour. Food Chem. 2016;196:1338–1345. doi: 10.1016/j.foodchem.2015.10.089. [DOI] [PubMed] [Google Scholar]
- Liang X, Yang H, Sun J, Cheng J, Luo X, Wang Z, Yang M, Tao DB, Yue X, Zheng Y. Effects of enzymatic treatments on the hydrolysis and antigenicity reduction of natural cow milk. Food Sci Nutr. 2021;9:985–993. doi: 10.1002/fsn3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LA, Novelli PK, Fernandez-Lafuente R, Tardioli PW, Giordano RLC. Glyoxyl-activated agarose as support for covalently link novo-pro D: biocatalysts performance in the hydrolysis of casein. Catalysts. 2020;10:466. doi: 10.3390/catal10050466. [DOI] [Google Scholar]
- McComas KA, Gillilan SE. Growth of probiotic and traditional yogurt cultures in milk supplemented with whey protein hydrolysate. Food Microbiol Safety. 2003;68(6):2090–2095. doi: 10.1111/j.1365-2621.2003.tb07024.x. [DOI] [Google Scholar]
- Micinski J, Kowalski IM, Zwierzchowski G, Szarek J, Pierozynski B, Zablocka E. Characteristics of cow´s milk proteins including allergenic properties and methods for its reduction. Pol Ann Med. 2013;20:69–76. doi: 10.1016/j.poamed.2013.07.006. [DOI] [Google Scholar]
- Milessi TS, Corradini FAS, Marçal JM, Baldez TO, Kopp W, Giordano RC, Giordano RLC. Xylooligosaccharides production chain in sugarcane biorefineries: from the selection of pretreatment conditions to the evaluation of nutritional properties. Ind Crops Prod. 2021;172:114056. doi: 10.1016/j.indcrop.2021.114056. [DOI] [Google Scholar]
- Mohanty D, Jena R, Choudhury PK, Pattnaik R, Mohapatra S, Saini MR. Milk derived antimicrobial bioactive peptides: a review. Int J Food Prop. 2016;19:837–846. doi: 10.1080/10942912.2015.1048356. [DOI] [Google Scholar]
- Morr CV, Ha YW. Whey protein concentrates and isolates: processing and functional properties. Crit Rev Food Sci Nutr. 1993;33:341–476. doi: 10.1080/10408399309527643. [DOI] [PubMed] [Google Scholar]
- Rangel AHN, Sales DC, Urbano SA, Galvão Júnior JGB, Andrade Neto JC, Macedo CS. Lactose intolerance and cow's milk protein allergy. Food Sci Technol. 2016 doi: 10.1590/1678-457X.0019. [DOI] [Google Scholar]
- Rojas MJ, Siqueira PF, Miranda LC, Tardioli PW, Giordano RLC. Sequential proteolysis and cellulolytic hydrolysis of soybean hulls for oligopeptides and ethanol production. Ind Crops Prod. 2014;61:202–210. doi: 10.1016/j.indcrop.2014.07.002. [DOI] [Google Scholar]
- Rosa LOL, Santana MC, Azevedo TL, Brígida AIS, Godoy R, Pacheco S, Mellinger-Silva C, Cabral LMC. A comparison of dual-functional whey hydrolysates by the use of commercial proteases. Food Sci Technol. 2018;38:31–36. doi: 10.1590/fst.08417. [DOI] [Google Scholar]
- Sauser J, Nutten S, Groot N, Pecquet S, Simon D, Simon HU, Spergel JM, Koletzko S, Blanchard C. Partially hydrolyzed whey infant formula: literature review on effects on growth and the risk of developing atopic dermatitis in infants from the general population. Int Arch Allergy Immunol. 2018;177(2):123–134. doi: 10.1159/000489861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukolis C, Behboudi-Jobbehdar S, Yonejura L, Parmenter C, Fisk I. Impact of milk protein type on the viability and storage stability of microencapsulated Lactobacillus acidophilus NCIMB 701748 using spray drying. Food Bioprocess Technol. 2014;7:1255–1268. doi: 10.1007/s11947-013-1120-x. [DOI] [Google Scholar]
- Um J, Manguy J, Anes J, Jacquier JC, Hurley D, Dillon ET, Wynne K, Fannng S, O´Sullivan M, Shields DC, Enriching antimicrobial peptides from milk hydrolysates using pectin/alginate food-gels. Food Chem. 2021;352:129220. doi: 10.1016/j.foodchem.2021.129220. [DOI] [PubMed] [Google Scholar]
- Wang S, Zeng X, Yang Q, Qiao S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. 2016;17:603. doi: 10.3390/ijms17050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Palmer J, The KH, Calinisan MMA, Flint S. Milk fat influences proteolytic enzyme activity of dairy Pseudomonas species. Int J Food Microbiol. 2020;230:108543. doi: 10.1016/j.ijfoodmicro.2020.108543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data presented in this article, including Online Resource 1, are also available from the corresponding author on reasonable request.



