Abstract
Fruits and vegetable processing industries contribute to the largest portion of food waste. With changing diet habits, the demand for the production and processing of fruits and vegetables has increased greatly to fulfil the rising demand amongst the masses. Waste generation begins from the harvesting of raw material until it gets processed. Pineapple processing industries produce processing waste (peel, core, pomace, and crown) which are rich in various bioactive compounds. In most cases, the by-products contain larger amounts of valuable compounds which have higher nutritional and therapeutic importance than its final produce. Researchers have studied the potential of pineapple wastes primarily for the extraction of enzymes (bromelain, pectinase, xylanase and cellulase) and secondarily as a low-cost substrate to produce dietary fibre, organic acids, and phenolic antioxidants. This review describes the bioactive compounds in pineapple wastes, their extraction techniques, and their potential applications as a polymer material, bio-sorbents, bioethanol and vanillin production, etc. It focuses primarily on bioactive compounds that have functional and medicinal value and can be used independently or incorporated with other ingredients to form the valorised product.
Graphic abstract
Keywords: Pineapple wastes, Low-cost substrate, Bioactive compounds, Extraction, Utilisation
Introduction
Fruits and vegetables play an important role to balance human nutrition needs. Recently, their demand has increased as people prefer to consume food from vegetarian sources. India, with its wide-ranging distributed climatic condition, supports the growth of various horticulture produces. Pineapple is the second most ingested and produced fruit after banana and contributes to around 20% of tropical fruits total production (Pyar et al. 2014). In 2019, the total production of pineapple in India was reported to be 1.71 million tonnes which is nearly 6.07% of total world production (28.17 million tonnes) (FAO 2019). Pineapple production (tonnes/ha) in India accounts to be 16.45, whereas, world production reaches to 25.04. To calculate the average pineapple waste per ha that would have produced in 2019, peel (30%) 4.93 tonnes/ha, core (7%) 1.15 tonnes/ha, crown (13%) 2.14 tonnes/ha and Pulp (50%) 8.22 tonnes/ha (pomace 2.47 tonnes/ha and juice 5.76 tonnes/ha) (Banerjee et al. 2018). Processing leads to the development of food products with balanced nutrients and enhanced shelf life, increasing acceptability and accessibility with ease of consumption. According to FAO reports, one- third of the food produced in the world turns into waste due to improper handling during transportation, spoilage due to microbes and lack of cold storage facilities. Inadequate processing facilities and outdated practices cause huge losses of by-products and their residues during processing.
Considering ease of consumption and health safety as a major concern, humans are shifting preference to consume processed and packaged foods rather than raw form. Also, degrading quality on storage and comparatively lesser shelf life of raw fruits and vegetables made their market shrink. Products like fruit juices, jam, jelly, marmalade, ketchup, sausages, purees are the most common consumables. Moreover, the processing of pineapple creates bio-wastes. Pineapple wastes could be divided into two categories: pineapple on-farm waste (POFW), which includes leaves, roots, stems and other on-field waste which are generated during harvesting and storage and pineapple processing waste (PPW) which are wastes produced during industrial processing of the pineapple as in during pulping, juicing etc., (Banerjee et al. 2018).
Phytochemicals present in food that attunes metabolic processes in the body and helps to promote better health are considered as bioactive food components. They have several beneficial effects on human health, like decreasing enzyme activity, inhibition of receptor activities, induction and inhibition of gene expression (Correia et al. 2012). Pineapple wastes produced during processing are enriched raw material and is mainly composed of DF, pectin, protein, phenolic compounds, vitamins, and minerals (Diaz-Vela et al. 2013). Pineapple is also a rich source of 'bromelain', a proteolytic group of enzymes (Banerjee et al. 2018). As a result, these components can be extracted from the pineapple residues and can be further used for value addition or product innovation.
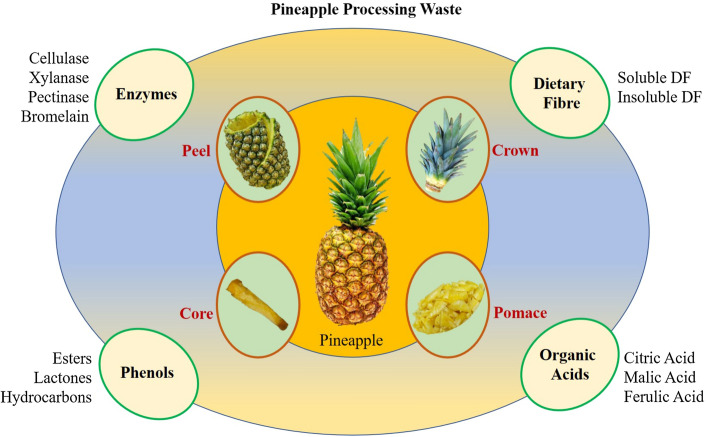
Production of pineapple juice yields pomace, which are potential source of phytochemicals, vitamins, enzymes, carbohydrates, and other components. Such wastes can be better utilised by extracting the bioactive compounds present in different parts of pineapple. Complete utilisation of pineapple fruit and by-products in food, cosmetic and pharmaceutical industries will provide a wide variety of natural compounds with a lower processing cost and higher economic importance (Fig. 1). To create a difference in the present practises of waste handling and tweaking the interest of people for effective utilisation of biomasses generated during fruits and vegetable processing. This article makes an effort to deliver a detailed review on pineapple processing wastes generated during industrial process and create an approach for its valorisation. It discusses about the available nutritional potential, bioactive compounds, their extraction and utilisation in food and pharmaceutical industries. This review may help the researchers and processing experts further utilise the by-products that are presently considered waste.
Fig. 1.
Biorefinery approach for pineapple waste utilization
Dietary fibre (DF)
They comprise a mixture of polymers of plant carbohydrates which includes oligosaccharides and polysaccharides. These include celluloses, hemicelluloses, pectin substances, gums, resistant starch, and inulin associated with other non-carbohydrate components (Elleuch et al. 2011). DF is found to have a positive relationship with human health especially considering they reduce type 2 diabetes and heart diseases. Foods containing DF are generally low GI (glycemic index) foods and, as a result, cause a slower and lesser increase in blood glucose levels and, therefore insulin as well.
Pineapple peels were found to have higher total dietary fibre (TDF) content which was estimated to be 70.6% for dried peel powder. The reported results show pineapple peel are rich in TDFs in comparison to apple and citrus fruits which have 55–65% and 50–70%, respectively. Particularly, insoluble dietary fibre (IDF) made up the highest of the TDFs, which accounts to have 77% of neutral sugars such as glucose, xylose, mannose, arabinose, and galactose (Larrauri et al. 1997). However, Huang et al. (2011) reported different results according to which TDFs constituted 42.2% of the pineapple peel out of which 86.02% belonged to the IDF and the rest belonged to soluble dietary fibre (SDF). The differences may be attributed to various other factors such as differences in geographical conditions, growing conditions, species used for testing, etc. (Selani et al. 2014).
Pomace is the waste generated after juice extraction at the industrial scale and includes left-out portion like seed, skin and pulp. Selani et al. (2014) reported that the pomace contain 45.22% of TDFs, to which 98.18% belongs to the insoluble fraction and results were in agreement with the results reported by Huang et al. (2011). Pineapple core which is another important waste product produced while processing, consists of about 53.59% TDFs, out of which 51.14% are IDF and the rest 2.45% are SDF, by dried weight (Shiau et al. 2015). It can be concluded that pineapples are rich in IDFs, which could be helpful in maintaining good bowel health and insulin sensitivity to result in lowering the risk of diabetes.
The method used for extraction of DF plays an important role in determining the effects that the resulting isolated or extracted DF will have in the food which they are incorporated into or their general effect on the human body. The extraction method varies with the target DF, and also may vary according to their chemical nature, their degree of polymerization and structural properties. Numerous studies have been made in which different parts of pineapple have been treated to various techniques in order to isolate the fibre fraction. DF extraction and estimation were done on different portions of pineapple waste, and the results have been discussed in Table 1.
Table 1.
Extraction of dietary fibre from various parts of pineapple
| Method | Portion of pineapple | SDF (%) | IDF (%) | TDF (%) | Reference |
|---|---|---|---|---|---|
| Alcohol hydrolysis | Pineapple Pomace and Peel | – | – | 44.90–58.48 | Sah et al. (2015) |
| Chemical treatments | Pineapple Pulp and residues | 29.16 | 43.53 | 74.69 | Elena et al. (2014) |
| Acidic Extraction | Pineapple Core | 2.45 | 51.14 | 53.59 | Shiau et al. (2015) |
| Enzyme Digestion | Pineapple Peel | 5.90 | 36.3 | 42.2 | Huang et al. (2011) |
| Steam Explosion | Pineapple Leaves | – | – | 99.25 | Cherian et al. (2010) |
Previous studies illustrate the application of mechanical and chemical methods to carry out the DF extraction from different parts of pineapple waste. The materials were washed with water or digested either in acids, alkalis, alcohols or with the help of enzymes, followed by mechanical techniques like milling and grinding to convert them into nano or micro-sized particles. The TDF present in the different parts were mainly IDF with comparatively smaller amounts of SDF.
Due to the high DF content in pineapple wastes, it has a huge scope of utilisation. The fibres, whether directly or indirectly, have been used in the fortification of various food items to improve the structural and textural properties of food. Prakongpan et al. (2002) used pineapple core fibres and incorporated them into various food items such as doughnuts, beef burgers and golden layered cakes. The result illustrates that large-sized core fibres had the potential to increase the volume of the product and oil reduction, improving emulsifying properties, while the smaller particles had the potential to reduce shrinkage and improve the overall texture of the product. Kengkhetkit and Amornsakchai (2012) used pineapple leaf fibre as a polypropylene reinforcement to sustain high tensile strength and modulus. Due to the structural and functional stability provided by the fibres present in the various parts of pineapple viz. peel, leaf, pomace, etc., these parts or the fibre itself have been used in various ways. Pineapple peel powder has the potential to fortify various integral properties of a probiotic yoghurt (Sah et al. 2016). Pineapple DF has also been used along with water in a species of beef sausage as it not only reduces the fat content of product but also has propensity to improve the overall consistency of the sausage (Henning et al. 2016). The fat reducing property of pineapple fibres was also used to fortify extruded products that are notoriously fibre deficient (Selani et al. 2014). Considering all the previous studies, the major reported functions of pineapple fibre include its fat reducing nature, its ability to provide structural stability and integrity to the product, and its nutritional properties. As a result, these fibre-rich wastes from pineapples can be used to fortify fibre deficient products such as dairy products, extruded products, etc., as well as can be used to reduce fat from various animal and plant based products. These fibres also have the potential to be good packaging materials or as reinforcement materials.
Enzyme availability
Plant polysaccharides are tightly packed in the cellular structure and surrounded by lignin, making them highly resistant to direct enzymatic attacks. Pineapple wastes are mainly composed of cellulose, hemicellulose and pectin substances converted to simpler sugars by treatment. Microbial enzymes are known to be the higher-level enzymes obtained from microorganisms for application in industry. Microbial enzymes produced from pineapple wastes are cellulase, xylanase, pectinase (Table 2).
Table 2.
Overview of enzymes and organic acids produced from different microorganisms
| Microorganisms | Enzyme activity/ Production parameters | Reference | ||
|---|---|---|---|---|
| Enzymes | Cellulase | Aspergillus niger Saccharomyces cerevisiae | ↑ with substrate conc. (up to 3%) | Amaeze et al. (2015) |
| Xylanase | Bacillus pumilus SV-85S | Xylanase activity was optimum at 50 ºC, pH 6 and metal ions KCl, MnCl2 and CaCl2 enhanced the activity | Nagar et al. (2012) | |
| Pectinase | Penicillium chrysogenum | Highest at incubation period of 48 h (220.30 mg−1 protein) and further decreased with increasing incubation period | Okafor et al. (2010) | |
| Organic acids | Citric acid | Aspergillus foetidus ACM 3996 | 16.1 g/100 g (62.4%) yield under conditions, 70% initial moisture, 30 ºC, incubation 4 days and 3% methanol | Tran and Mitchell (1995) |
| Citric acid | Yarrowia lipolytica NCIM 3589 | 202.35 g/kg under conditions, 70.71% initial moisture content, KH2PO4 0.64% (w/w), Na2HPO4 0.69% (w/w) and 0.34% (w/w) yeast extract | Imandi et al. (2008) | |
| L ( +) Lactic acid | Rhizopus oryzae NRRL 395 | 103.69 mg/g yield under optimized conditions (67.53% w/w moisture content, 32.2 ºC, pH 5.6, inoculation size 1 × 107 spores/g and 3 days incubation period) | Zain et al. (2021) | |
| Lactic acid | Lactobacillus delbrueckii | 54.97 g/L (79%) yield under optimized conditions, 40 ºC, pH 6, inoculation size 5%, initial sugar concentration 70 g/L and 5% yeast extract (nitrogen source) | Abdullah and Winaningsih (2020) | |
| Succinic acid | Escherichia coli AFP 184 | 6.26 g/L yield under conditions, 37 ºC, pH 6.6–6.7, inoculation size 5 mL, incubation for 24 h at 200 rpm | Jusoh et al. (2014) | |
Cellulase
Pineapple peel contains lignocellulosic materials such as cellulose, hemicellulose, and lignin, which can be broken down into simple sugars by chemicals or enzymes. Such simple sugars synthesised from lignocellulosic materials can be used for producing bioethanol and other value-added products. Cellulase is the group of hydrolytic enzymes that hydrolyse the β-glycosidic bond of cellulose and produce simple sugars. It is a notable enzyme for industrial saccharification of cellulosic material (Saravanan et al. 2013). These are produced by numerous microorganisms (fungi and bacteria) during their growth on cellulosic materials. Some of the common cellulase producers are Clostridium, Cellulomonas, Thermomonospora, Trichoderma, and Aspergillus species; however, among them fungus, Trichoderma reesei is an efficient producer of cellulase enzymes (Amaeze et al. 2015).
For the saccharification process, the synergistic action of three principal enzymes, namely endo-1,4-β-glucanase, Exo-β-glucanase and β-D-glucosidase, are needed. Because of these principal enzymes synergistic effect, the crystalline and amorphous structure of cellulase is created and hydrolyses cellulose to cellobiose, glucose, and oligosaccharides. Firstly, the enzyme endoglucanase acts on amorphous cellulose fibres, that randomly attacks the glucose polymer chain and releases small fibres consisting of free reducing and non-reducing ends. Secondly, the enzyme exo-β-glucanase acts on the free ends of chain and produces cellobiose. The third enzyme, β-glucosidase, hydrolyses cellobiose and thereby produces glucose which is the resultant compound of saccharification (Amaeze et al. 2015).
An important factor to be considered in cellulase production is thermostability i.e., at higher temperatures, the saccharification process takes place at a faster rate. But at the same time, the stability of an enzyme has to be maintained for the complete saccharification process. Though the enzymes are prepared using thermophilic microorganisms, these enzymes need not be heat stable (Nigam 2013). Thermal activation and stability of cellulases can be achieved by prior heat treatment. Such pre-treatment may increase the efficiency of cellulolytic enzymes (Nigam 2013).
Xylanase
The major component of plant cell wall polysaccharide is hemicellulose, and xylan is the major fraction of it. Though there are many physical, chemical and biochemical methods to produce xylooligosaccharides, the utilisation of microorganisms or their enzymes act as a significant method since they produce lesser undesirable by-products, fewer monosaccharides, and they do not require any special equipment. Xylanase hydrolyses xylan to simpler sugar residues like xylooligosaccharides, xylose, and xylobiose, which are widely used in industries for quality improvement such as food and beverage industries, feedstock improvement, quality improvement of lignocellulosic residues, and texture improvement of bakery products (Goulart et al. 2005). Xylanase can be produced by bacteria like Bacillus pumilus SV-85S (Nagar et al. 2012) and fungus-like Trichoderma viride (Fortkamp and Knob 2014).
Xylan is the complex heteropolysaccharide composed of the main chain of 1,4-β-D-xylose monomers that are partially acetylated and substituted in different degrees by various side chains, mainly single α-D-glucoronosyl, and α-L-arabinosyl units. Since xylan has a complex structure, several hydrolases are required for the complete degradation of xylan. The main enzyme involved in the degradation of xylan is endo-β-(1,4) xylanases.
Pectinase
Pectinases are the group of enzymes that degrade pectic substances which are responsible for maintaining the structural integrity of plant tissues. Pectin is the jelly-like matrix found in the primary cell wall and middle lamella of fruits and vegetables (Sengar et al. 2020). Pectinase converts polygalacturonic acid to monogalacturonic acid by acting on the glycosidic bond of the long carbon chain (polygalacturonase, pectin lyase, and pectate lyase). Moniliella SB9, Penicillium spp., and Aspergillus spp. are among the microbial strains that produce pectinases. Okafor et al. (2010) conducted a study on fruits and vegetable wastes using Aspergillus niger and Penicillium chrysogenum as the potential microbial strains for pectinase production. Results indicated higher pectinase yield (220.3 IU/mg) from pineapple peels using strain P. chrysogenum. These are widely used in the fermentation of coffee and tea, fruit juice industries, extraction of oil and treatment of pectic wastes water produced during fruit juice processing.
Bromelain
Bromelain is an enzyme belonging to the proteolytic group of enzymes that can be extracted from pineapples. As a result of its biological origin and the fact that it has no known side effects on the human body, it has gained popularity as a therapeutic and pharmacological product (Maurer 2001). Bromelain is present in different portions of pineapple (stem, leaves, fruit, and crown) however, the major quantities are found in stem and fruit portion of the plant. Due to the large amount of waste produced by pineapples, which is almost 45–55% of the fruit, it has the potential to be further exploited as a bromelain source. The peels and core of the pineapples, which constitute almost 40–50% of these wastes, have been specifically targeted as potential bromelain sources (Banerjee et al. 2018). Table 3, shows the pineapple part from which the enzyme was extracted and the technique used in doing so.
Table 3.
Extraction of bromelain from different parts of pineapple and the techniques used
| Part of Pineapple | Target compound | Extraction Technique Used | Activity recovery and purification | References |
|---|---|---|---|---|
| Core | Bromelain | Reverse Micellar Extraction → Ultrafiltration | Activity recovery (98.5%), ultrafiltration increased the purification up to 8.9-fold | Hebbar et al. (2012) |
| Core | Bromelain | Reverse Micellar Extraction | Activity recovery of 78.90% and 3.96-fold purification | Chaurasiya and Hebbar (2013) |
| Slat precipitation (ammonium sulfate) | Activity recovery of 86.26%and 3.07-fold purification (50% saturation level) | |||
| Acetone precipitation | Activity recovery (45.11%) and 5.56-fold purification (60% saturation level) | |||
| Peel | Bromelain | Two-phase partitioning system | Activity recovery (113.54%) and purification 2.23-fold | Ketnawa et al. (2010) |
| Peel | Bromelain | C12-8-C12∙2Br (Octame thylene-a,x-bis (dimethyldodecylammonium bromide)) Reverse Micellar Extraction | Activity recovery (163%) and purification 3.3-fold | Wan et al. (2016) |
| DTAB (dodecyl trimethyl ammonium bromide) Reverse Micellar Extraction | Activity recovery (95%) and purification 1.7-fold | |||
| Crown | Bromelain | Size reduction and filtration → purified using High Performance Liquid Chromatography (HPLC) cation exchange resin column → de-saltation (diafiltrator) → freeze drying | Activity recovery:powdered bromelain (529.77 CDU/mg), purified bromelain (501.08 CDU/mg), desalted bromelain (485.78 CDU/mg) and pineapple crown extract (426.49 CDU/mg) | Nadzirah et al. (2012) |
| Core, Peel, Crown and stem | Bromelain | Reverse micellar system of cetyltrimethylammonium bromide (CTAB) | Core: activity recovery (106%) and purification 5.2-fold at pH 4.2. Peel: activity recovery (78%) and purification 2.1-fold at pH 10.5. Crown: activity recovery (54%) and purification 1.7-fold at pH 11.0: activity recovery (80%) and purification 3.5-fold at pH 11.0 | Hebbar et al. (2008) |
| By-products | Bromelain and bioactive peptides | Ultrasound assisted extraction (UAE) and microwave assisted extraction (MAE) | Proteolytic activity: UAE (196.46 ± 3.29 U/mL) and MAE (154.08 ± 1.49 U/mL) | Mala et al. (2021) |
Due to the fact that bromelain has the highest significance among all the enzymes found in pineapple wastes, this review illustrates the bromelain extraction process. Bromelain is an intracellular enzyme or protein, and cell disruption is necessary to release the enzymes in a soluble form. The reverse micellar technique is popular and has been used for extraction of bromelain in various studies over the years. It is a liquid–liquid extraction technique which is used to convert dilute solutions into useful biomolecules. Aqueous pineapple wastes were used for bromelain extraction through reverse micellar extraction (RME) technique (Hebbar et al. 2008). In the other study, ultrafiltration coupled RME was used to extract bromelain from pineapple cores (Hebbar et al. 2012). The results illustrates that the extraction becomes more efficient with the use of ultrafiltration along with RME. Extracts of pineapple waste have also been used to ease the extraction of bromelain from that very part.
The utilisation of these extracted enzymes could create a significant impact in the processing of food industries. Bromelain is believed to be the most useful and the most studied enzyme with the capability of acting as a meat tenderizer. This could be mainly due to its ability to break down collagen in meat. Apart from this, bromelain has been reported to have a number of medical benefits (anti-rombotic and fibrinolytic effects) including other common therapeutic effects on the human body which are, reverse inhibition of platelet aggregation, bronchitis, surgical traumas as well as enhancing drug absorption (Maurer 2001). Besides all these benefits mentioned above, its application in the medicinal field as an anti-cancer agent is conspicuous.
Moreover, the simple partitioning extraction techniques used can cause it to lose its purity and subsequent activity reduction. Hence, purification techniques like RME and the two-phase extraction systems are becoming popular, as they help in extracting the enzymes without much of a loss in purity and as a result, they offer similar effect as its pure form.
On the other hand, pectinases obtained from pineapple processing residues using P. chrysogenum were used to ferment coffee. It helps to remove the mucilaginous coat from coffee beans. The mucilage is a layer which surrounds the coffee beans, and it constitutes about 17% weight of whole cherries (Garg et al. 2016). During fermentation, this mucilaginous layer gets degraded and converted to sugars, contributing to the quality of the coffee bean. Such pectin layer can be removed using pectinase with reduced demucilisation time, which was evident with a reduction in pH value and increased sugar release (Murthy and Naidu 2011).
Organic acids
Though citric acid is found in various acidic fruit juices, pineapple contains 182 mg/100 g of citric acid. About 70% of citric acid is used in the food industry, and 30% have other industrial application. Currently, extraction of citric acid by biological techniques based on the use of micro-fungi is widely used. Citric acid is generally produced by submerged fermentation (SMF). Recent research has been made the solid-state fermentation (SSF) process an alternative with highlighted advantages such as higher production yield, low water requirement and lower operating costs. Microorganisms that produce citric acid are Aspergillus niger, Mucor pirifromis, Penicillium janthinellum, Penicillium restrictum, Trichoderma viride, Ustulina vulgaris and various species of the genera Botrytis, Ascochyta, Absidia, Talaromyces, Acremonium and Eupenicillium (Mengal and Mompon 2011) (Table 2). In pineapple wastes, the concentration of citric acid produced by A. niger was 54.2% in the presence of 4% methanol (Kumar et al. 2003). The other researchers used dried pineapple waste as the sole substrate for citric acid production and reported that SSF using Yarrowia lipolytica produced 202.35 g/kg citric acid (Tran and Mitchell 1995). The other work was done on wet pineapple waste using A. foetidus ACM3996, which produced a higher amount (161 g/kg) of citric acid than other potential wastes such as apple pomace, rice and wheat bran (Tran and Mitchell 1995).
Malic acid is a C4-dicarboxylic acid and can be used as a food additive and synthesise fine chemicals. It could be produced by various techniques such as extraction from plants, enzymatic conversion, and chemical synthesis (Yun et al. 2008). Extraction from fruit or plant is a traditional production method, but this method showed lesser practical use because of its small production capacity. It can be chemically synthesised by the hydration of either maleic or fumaric acid. By using the enzyme fumarase, fumaric acid can be converted to malic acid. However, these processes require complex reaction or expensive enzyme, which make them more difficult to use. To reduce these constraints, Yun et al. (2008) suggested renewable feedstock with Aspergillus species and Schizophyllum commune for malic acid production.
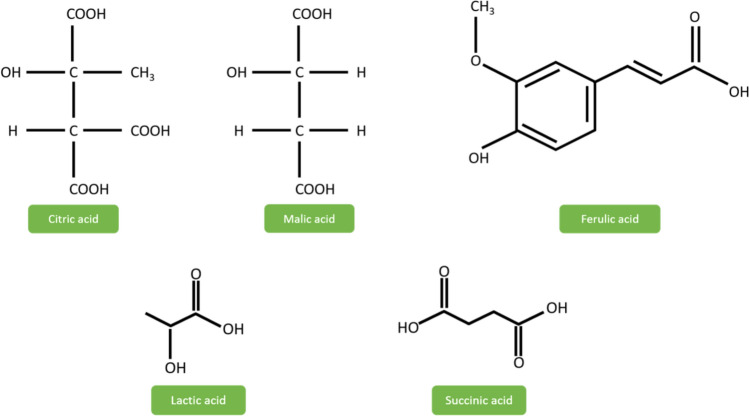
The most abundant hydroxycinnamic acid found in plant cell walls is ferulic acid (Upadhyay et al. 2010) (Fig. 2). It has enormous applications in the medical field, and some of them are arena antioxidant activity, cholesterol-lowering activity, prevention against thrombosis & atherosclerosis, anti-cancer activity, antimicrobial & anti-inflammatory activity. Lun et al. (2014) used autoclaved and non-autoclaved pineapple wastes to produce ferulic acid, which yielded about 3.65 mg/g and 0.64 mg/g, respectively. It concludes the effect of pretreatment conditions, which could increase the yield of ferulic acid.
Fig. 2.
Organic acids present in pineapple processing wastes
Commercially produced succinic acids are conventionally derived from petroleum source and their production involves a chemical process. Pineapple processing waste creates an alternative source for such organic acid production through fermentation processes (Jusoh et al. 2014). Establishing a green technology by reducing the use of toxic chemicals and targeting the bio-based production of organic acids is need of the hour (Nghiem et al. 2017). The popular application of succinic acid in food and beverage industry includes acidity regulation and flavouring agent (sourness). It is also used to produce pharmaceutical intermediates and other non-food application includes use in detergents, cement additives, toners, soldering flux, cosmetics and pigments (Nghiem et al. 2017). Fermentation of pineapple liquid waste using Escherichia coli AFP 184 has reported to yield 6.2 g/L of succinic acid, which was nearly similar to the yield obtained from fermentation of glucose (6.5 g/L) as carbon source (Jusoh et al. 2014). Pineapple liquid waste possess high amount of sugars (100 g/L) and other micro nutrients which plays important role in cell cultivation and fermentation process. The other common microorganisms used for succinic acid production are Anaerobiospirillum succiniciproducens and Actinobacillus succinogenes.
Lactic acid is amongst the important organic acids and has been labelled “generally recognized as safe (GRAS)” by US Food and Drug Administration. They largely find food applications at industrial level for attaining functions such as preservation, pH regulation and flavouring agents (Jantasee et al. 2017). Lactic acids are conventionally synthesized using chemical and fermentation methods; however, microbial fermentation methods are more popular and contributes to 90% of global production. It founds to be more promising method as it uses bio-waste, low energy requirements and no toxic residue production with high product purity (> 95%). Zain et al. (2021) reported production of lactic acid from solid pineapple waste using Rhizopus oryzae NRRL 395. Production yield was found to be 103.69 mg/g after 3 days incubation period under optimized conditions (Table 2). Moreover, pineapple liquid waste has also proved to be a potential substrate for lactic acid production. Abdullah and Winaningsih (2020) studied the effect of various parameters (pH, temperature, inoculation size, nitrogen source and initial sugar concentration) on fermentation yield using Lactobacillus delbrueckii and the maximum attained was 54.97 g/L.
These extracted organic acids have the potential to remove toxic metals like mercury, lead, cadmium, copper, nickel and zinc. Senthilkumaar et al. (2000) reported increase in adsorbent capacity of fruit residues at lower pH with the addition of phosphate groups. On removing heavy metals (Cr, Cu, Pb, Ni, and Zn) from contaminated sewage sludge, citric acid obtained from fermented pineapple wastes with A. niger were used (Del Mundo Dacera and Babel 2008). On the other hand, pineapple plant stem has been used to eliminate Pb(II) ions from aqueous solutions (Ting et al. 2019). To improve the adsorption efficiency of Pb(II), they have modified pineapple plant stem with oxalic acid (OA), and the study showed 30.47 mg/g of Pb(II) ions were adsorbed.
Phenolic compounds and antioxidants
Phenolic compounds are secondary metabolites that are ubiquitous in the plant kingdom. These exist in various forms, each having unique structures and various physiological effects on the human body. They possess numerous phytochemical activities that may boost the human immunity system as well as provide beneficial cardiovascular effects. The reason behind the growing prominence of phenolic compounds is their ability to scavenge free radicals, which may cause numerous diseases in humans. Pineapple waste can be used as a potential source for medicinal benefits through extracting nutraceutical compounds (Teai et al. 2001).
Classification of phenolic compounds
Flavouring agents
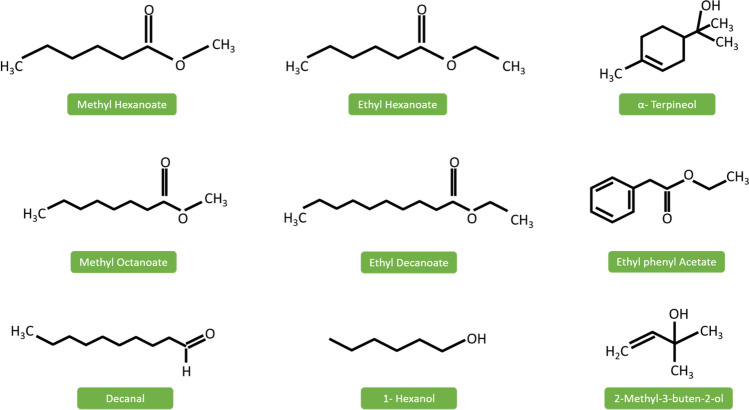
Pineapple consists of both volatile compounds that are perceived by human olfactory systems and non-volatile compounds (sugars and acids) recognised by the tongue (Montero-Calderón et al 2010a, b). Volatile aromatic compounds in pineapple are esters, lactones, acids, hydrocarbons, sulfur-containing compounds and carbonyl compounds (Wei et al. 2011). Its contribution to flavour depends on the cultivar, processing conditions, ethylene control, chemical treatments, maturity and pre-harvest factors such as light, temperature, carbon supply, and water. The major volatile contributor in the pineapple residues are esters (35%), and the characteristic volatile compounds include methyl hexanoate, ethyl hexanoate, ethyl decanoate, methyl octanoate, decanal, nonanal, α-terpineol and methyl 3-(methylthio) propanoate (Barretto et al. 2013). It indicates the potential of pineapple processing wastes to extract and utilise the natural essence compounds.
Esters
Esters are synthesised by reacting carboxylic acids and alcohol in a condensation reaction. Some of the major esters responsible for pineapple flavour include methyl-2-methyl butanoate, ethyl-2-methyl butanoate, ethyl acetate, ethyl hexanoate, ethyl butanoate, ethyl-2-methyl propanoate, methyl hexanoate, methyl butanoate (Zheng et al. 2012) (Fig. 3).
Fig. 3.
Major volatile compounds in pineapple wastes
Other ethyl butyrate names are butyric ether and ethyl butanoate, which is an aromatic ester with the chemical formula (CH3CH2CH2COOCH2CH3). It is produced through a condensation reaction between ethanol and butyric acid and imparts a high resemblance to orange juice or pineapple flavour in alcoholic beverages with an odour threshold of 0.015 ppm. The ester compound in pineapple contributes 44.9% of its aroma (Teai et al. 2001). When the odour activity value (OAV) for a compound is greater than 1, that particular compound will play a critical role in overall fruit flavour. In pineapple pulp, ethyl-2-methyl butanoate has the highest OAV (1693.33), followed by ethyl-3-methylthiopropanoate and ethyl butanoate (Wei et al. 2011).
Lactones
Lactones are cyclic esters formed by successive β-oxidation of saturated and unsaturated hydroxyl acids or their lipid precursors. This β-oxidation process results in a hydroxylated carbon at C4 or C5 position. Whereas γ and δ lactones are formed through internal esterification of C4 (γ) or C5 (δ) hydroxyl group with the carboxylic group (Welsh et al. 1989). It contributes pleasant coconut flavour in some pineapple varieties. Lactones that contributes coconut-like aroma are γ-octalactone, δ-octalactone, and γ-nonalactone (Chen and Martín-belloso 2010).
One of the most important flavour compounds among lactone is 2,5-dimethyl 4-hydroxy 3(2H) furanone. It is commonly called furaneol, DHMF, pineapple furanone (Chen and Martín-belloso 2010). The alternative sources of furaneol are strawberry, raspberry, mango, and other fruits, and its content is directly proportional to the ripeness index and imparts caramel-like sweet and fruity aroma.
Hydrocarbons
Hydrocarbons include simple aliphatic molecules, terpenes, and benzene rings. The two hydrocarbons: 1-(E, Z)-3,5-undecatriene and 1-(E, Z, Z)-3,5,8-undecatetraene are present in fresh-cut pineapple. 1-(E, Z)-3,5-undecatriene gives an oily, waxy and peppery aroma; however, 1-(E, Z, Z)-3,5,8-undecatetraene has a powerful, diffusive and green characteristic odour (Montero-Calderón et al. 2010a). The other common sources are apple, peaches, celery, and parsley.
Other flavour compounds
Carbonyl compounds include aldehydes and ketones, among which nonanal (3.91 µg/kg) and decanal (3.20 µg/kg) are the aldehydes present in pineapple core (Wei et al. 2011). Sulfur-containing compounds are also found in pineapple, however, their concentration is lower than their odour threshold (Montero-Calderón et al. 2010a).
Extraction of such volatile compounds from pineapple processing waste could create a better approach towards their utilisation. Pineapple leaves and peel are rich in esters, ketones, acids, alcohol, aldehydes, and other compounds (Barretto et al. 2013). Despite having several methods for extracting essential oils, the extraction techniques for extracting essential oils from pineapple peels are very few. The extraction techniques used here are hydro-distillation (HD), hydro-distillation with enzyme assisted pretreatment (HDEA) and supercritical fluid extraction (SFE). Among these methods, SFE produced essential oil with a yield of 0.17% (w/w) and solid wax of about 0.64% (w/w)) (Mohamad et al. 2019). The GC–MS analysis of essential oil extracted through SFE has 41 compounds representing 97.33% of essential oil. Among these compounds, propanoic acid ethyl ester was present in a higher proportion (40.25%) and is the most common compound found in other essential oils (Mohamad et al. 2019). The analysis showed that extraction through HD and HDEA yielded hydrosol of about 70.65% (w/w) and 80.65% (w/w), respectively.
Moreover, a higher amount of polyphenolic compounds are present in pineapple wastes, and various extraction techniques have been employed. Solvent–water extraction systems are the most common when it comes to the extraction of polyphenolic compounds. Three different types of systems were used, out of which acetone (50%) and ethanol (70%) were found to be the most efficient (Alothman et al. 2009). Pineapple residues (pulp, seeds and peel) were treated with n-hexane followed by methanol, which was another effective system for the extraction of polyphenolic compounds (Cabral et al. 2009). The methanolic extraction yield and total phenolic content of the pineapple residues were found to be 30.2% and 9.1 mg Gallic acid equivalent/g, respectively. Apart from these residues, pineapple shell powder was also a rich source of polyphenols containing high amounts of Myricetin (59% of the polyphenols identified) and salicylic, tannic, trans-cinnamic, and p-coumaric acids. This fibre-rich powder was found to have high antioxidant activity because of the presence of these compounds (Larrauri et al. 1997). Novel processes like mixing pineapple wastes with soy flour using the microorganism Rhizopus oligosporus also showed positive responses regarding the relation of β-glucosidase found in the extracts and the total phenolic content. These results show that β-glucosidase is involved in releasing polyphenols from its glycosides in the residues (Correia et al. 2004).
Phenolic compounds have lowered the risk of cancer and other chronic diseases like obesity, diabetes, and cardiovascular diseases (Aguilera et al. 2016). Pineapple cannery waste (core and peel) has been used to produce vanillin and vanillic acid (Lun et al. 2014). Pineapple peel contains ferulic acid, which is a precursor for vanillic acid. Vanillin (4 hydroxy 3 methoxy benzaldehyde) is one of the most common ferulic acid derivatives and has a market value of USD 6–20 per kg (Banerjee et al. 2018). It is widely used as a flavouring agent in sweets, confectionery, and baked products. Recently, the demand for natural resources has triggered research in natural vanillin production from natural sources. Lun et al. (2014) used pineapple cannery wastes as the potential substrate for the microbial production of vanillin by Aspergillus niger and Pycnoporuscinnabarinus. The study illustrates 5 g of pineapple cannery waste has produced 141 mg/L of vanillin.
Other by-product utilisation techniques
Biopolymers
Pardo et al. (2014) analysed that high cellulose content in pineapple peels (40.5%) and crown leaves (43.5%) makes it suitable for cellulose derivatives which have potential applications in the polymer industry. Cellulose from pineapple peels is converted into carboxymethyl cellulose and used to prepare green packaging material (Chumee and Khemmakama 2014). Research on the reinforcement of pineapple leaf fibre with polymer composites such as soy-based bioplastic has been carried out to improve their mechanical strength (Liu, Misra, Askeland, Drzal, & Mohanty, 2005). Limited application of soy protein-based bioplastic is due to their low strength and high moisture absorption. The mechanical properties of 30% pineapple leaf fibre reinforced bioplastics were improved when compared with soy-based bio-thermoplastic. When 30% pineapple leaf fibre is reinforced with soy-based plastic, its tensile strength increased from 10 to 30 MPa, flexural strength increased from 10 to 42 MPa and impact strength increased from 35 J/m to 45 J/m (Banerjee et al. 2018). The improved mechanical properties of the composites in the presence of the compatibilizer could be due to the interactions between hydroxyl group in the pineapple leaves and epoxy group in polyester amide grafted glycidyl methacrylate (PEA-g-GMA). Pineapple peel bioplastic application includes green packaging of dehydrated materials (Chumee and Khemmakama 2014).
Bioethanol
The utilisation of pineapple wastes for generating bioethanol has emerged as an important valorisation strategy since the last decade. The major advantage in production of alcohol from pineapple cannery wastes are, nominal cost for ethanol production and eliminating the problem of pineapple waste disposal. Additionally, continuous ethanol production from the pressed juice of pineapple cannery waste, respiration deficient strain Saccharomyces cerevisiae has been used (Nigam 1999). At a dilution rate of 0.05 h−1, the theoretical value of ethanol production was about 92.5% (Upadhyay et al. 2010).
The simple sugars thus obtained from the saccharification process are fermented by yeast-like Saccharomyces cerevisiae and Zymomonasmobilis (Ban-Koffi and Han 1990). Yeast produces the enzyme invertase and zymase, which helps in converting reducing sugars to crude ethanol and CO2. However, the yield of ethanol from lignocellulosic material is limited by two factors, which influence the specific rate of yeast growth and ethanol production, saccharification process (lignocellulosic materials are hard to digest, which in turn affects the yield) and the essential parameters (oxygen, temperature, pH, immobilisation of enzymes and organic acids). Additionally, the major limiting factors are the generation of microbial inhibitors during pretreatment and the limited availability of fermentable sugars. The inhibitors are classified as organic acids, furan derivatives, and phenolic compounds that inhibit the growth of fermenting microorganisms and, hence, reduce ethanol yield.
Xylitol
Xylitol is a sugar alcohol, and it is used as a low-calorie sweetener in the food and beverage industry (Dasgupta et al. 2016). Since xylitol is non-fermentable, it can restore alkaline/acid balance in the mouth. Some of the clinical trials have indicated that xylitol products help to prevent tooth decay (Banerjee et al. 2018), and these are effective in reducing dental caries. Xylitol is also advised as a sugar substitute for diabetics. The prevailing health consciousness regarding naturally derived low-calorie sweeteners has contributed to the rising demand of xylitol. Pineapple peels have 25 to 35% (dry basis) of xylan and could be the possible substrate for xylitol production, which has a market value of USD 4–5 per kg (Banerjee et al. 2018). Commercial production of xylitol suffers from several drawbacks in terms of huge energy requirements, specialised equipment, extensive purification steps, recovery of xylitol, deactivation and recycling of the metal catalyst. Production of xylitol from food wastes would be the cost-effective and attractive alternative for industrial chemical methods.
Conclusion
This review highlights the nutritional, biological and economical importance of industrial wastes generated during pineapple processing. The growing demand of naturally derived bioactives, organic acids and inimitable volatile compounds create an opportunity for the processors in domestic and international markets. It also enlightens the health benefits of pineapple derived DF that could be used as food additive to improve the food texture and consistency. Pineapple waste could be an economical substrate for microbial enzyme production (cellulase, xylanase, pectinase and bromelain). Moreover, esters are defined as major volatile compound present in pineapple wastes including lactones, hydrocarbons, aldehydes, ketones, alcohols. This review also describes the extraction techniques to recover these bioactive components from pineapple waste. There is need of extensive research on application of novel techniques to obtain higher recovery rates of bioactives. Research focused on extraction, purification and valorisation of target compounds from pineapple wastes could open huge market of food, pharmaceutical and chemical industries.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah A, Winaningsih I (2020) Effect of some parameter on lactic acid fermentation from pineapple waste by Lactobacillus delbrueckii. In: AIP Conference Proceedings. AIP Publishing LLC, p 60002
- Aguilera Y, Martin-Cabrejas MA, de Mejia EG. Phenolic compounds in fruits and beverages consumed as part of the mediterranean diet: their role in prevention of chronic diseases. Phytochem Rev. 2016;15:405–423. doi: 10.1007/s11101-015-9443-z. [DOI] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009;115:785–788. doi: 10.1016/j.foodchem.2008.12.005. [DOI] [Google Scholar]
- Amaeze NJ, Okoliegbe IN, Francis ME. Cellulase production by Aspergillus niger and Saccharomyces cerevisiae using fruit wastes as substrates. Int J Appl Microbiol Biotechnol Res. 2015;3:36–44. [Google Scholar]
- Banerjee S, Ranganathan V, Patti A, Arora A. Valorisation of pineapple wastes for food and therapeutic applications. Trends Food Sci Technol. 2018;82:60–70. doi: 10.1016/j.tifs.2018.09.024. [DOI] [Google Scholar]
- Ban-Koffi L, Han YW. Alcohol production from pineapple waste. World J Microbiol Biotechnol. 1990;6:281–284. doi: 10.1007/BF01201297. [DOI] [PubMed] [Google Scholar]
- Cabral A, Oliveira D, Barros I, et al. Total phenolic content and free radical scavenging activities of methanolic extract powders of tropical fruit residues. Food Chem. 2009;115:469–475. doi: 10.1016/j.foodchem.2008.12.045. [DOI] [Google Scholar]
- Chaurasiya RS, Umesh Hebbar H (2013) Extraction of bromelain from pineapple core and purification by RME and precipitation methods. Sep Purif Technol 111:90–97. 10.1016/j.seppur.2013.03.029
- Cherian BM, Leão AL, de Souza SF, et al. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr Polym. 2010;81:720–725. doi: 10.1016/j.carbpol.2010.03.046. [DOI] [Google Scholar]
- Chumee J, Khemmakama P. Carboxymethyl Cellulose from Pineapple Peel : Useful Green Bioplastic. 2014;979:366–369. [Google Scholar]
- Correia RTP, McCue P, Magalhães MMA, et al. Production of phenolic antioxidants by the solid-state bioconversion of pineapple waste mixed with soy flour using Rhizopus oligosporus. Process Biochem. 2004;39:2167–2172. doi: 10.1016/j.procbio.2003.11.034. [DOI] [Google Scholar]
- Correia RT, Borges KC, Medeiros MF, Genovese MI. Bioactive compounds and phenolic-linked functionality of powdered tropical fruit residues. Food Sci Technol Int. 2012;18:539–547. doi: 10.1177/1082013211433077. [DOI] [PubMed] [Google Scholar]
- Dasgupta D, Bandhu S, Adhikari DK, Ghosh D. Challenges and prospects of xylitol production with whole cell bio-catalysis : a review. Microbiol Res. 2016 doi: 10.1016/j.micres.2016.12.012. [DOI] [PubMed] [Google Scholar]
- Del Mundo DD, Babel S. Removal of heavy metals from contaminated sewage sludge using Aspergillus niger fermented raw liquid from pineapple wastes. Bioresour Technol. 2008;99:1682–1689. doi: 10.1016/j.biortech.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Diaz-Vela J, Totosaus A, Cruz-Guerrero AE, De Lourdes P-C. In vitro evaluation of the fermentation of added-value agroindustrial by-products: Cactus pear (Opuntia ficus-indica L.) peel and pineapple (Ananas comosus) peel as functional ingredients. Int J Food Sci Technol. 2013;48:1460–1467. doi: 10.1111/ijfs.12113. [DOI] [Google Scholar]
- Elena M, Pardo S, Elena M, et al (2014) Chemical Characterisation of the Industrial Residues of the Pineapple ( Ananas comosus ). 53–56
- Elleuch M, Bedigian D, Roiseux O, et al. Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications: a review. Food Chem. 2011;124:411–421. doi: 10.1016/j.foodchem.2010.06.077. [DOI] [Google Scholar]
- FAO (2019) FAOSTAT. Food and Agriculture Organization of the United Nations, Rome, Italy. http://www.fao.org/faostat/en/#data
- Fortkamp D, Knob A (2014) High xylanase production by Trichoderma viride using pineapple peel as substrate and its apllication in pulp biobleaching. African J Biotechnol 13:
- Garg G, Singh A, Kaur A, et al (2016) Microbial pectinases: an ecofriendly tool of nature for industries. 3 Biotech 6:1–13 [DOI] [PMC free article] [PubMed]
- Goulart AJ, Carmona EC, Monti R. Partial purification and properties of cellulase-free alkaline xylanase produced by Rhizopus stolonifer in solid-state fermentation. Brazilian Arch Biol Technol. 2005;48:327–333. doi: 10.1590/S1516-89132005000300001. [DOI] [Google Scholar]
- Handbook of Fruit and Vegetable Flavors. Edited by Y. H. Hui. Lebensmittelchemie 64:171–172. 10.1002/lemi.201290031
- Hebbar HU, Sumana B, Raghavarao K. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour Technol. 2008;99:4896–4902. doi: 10.1016/j.biortech.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Hebbar UH, Sumana B, Hemavathi AB, Raghavarao K. Separation and purification of bromelain by reverse micellar extraction coupled ultrafiltration and comparative studies with other methods. Food Bioprocess Technol. 2012;5:1010–1018. doi: 10.1007/s11947-010-0395-4. [DOI] [Google Scholar]
- Henning SSC, Tshalibe P, Hoffman LC. Physico-chemical properties of reduced-fat beef species sausage with pork back fat replaced by pineapple dietary fibres and water. LWT - Food Sci Technol. 2016;74:92–98. doi: 10.1016/j.lwt.2016.07.007. [DOI] [Google Scholar]
- Huang Y-L, Chow C-J, Fang Y-J. Preparation and physicochemical properties of fiber-rich fraction from pineapple peels as a potential ingredient. J Food Drug Anal. 2011;19:318–323. [Google Scholar]
- Imandi SB, Bandaru VVR, Somalanka SR, et al. Application of statistical experimental designs for the optimization of medium constituents for the production of citric acid from pineapple waste. Bioresour Technol. 2008;99:4445–4450. doi: 10.1016/j.biortech.2007.08.071. [DOI] [PubMed] [Google Scholar]
- Jantasee S, Kienberger M, Mungma N, Siebenhofer M. Potential and assessment of lactic acid production and isolation–a review. J Chem Technol Biotechnol. 2017;92:2885–2893. doi: 10.1002/jctb.5237. [DOI] [Google Scholar]
- Jusoh N, Othman N, Idris A, Nasruddin A (2014) Characterization of liquid pineapple waste as carbon source for production of succinic acid. J Teknol 69:
- Kengkhetkit N, Amornsakchai T. Utilisation of pineapple leaf waste for plastic reinforcement: 1. a novel extraction method for short pineapple leaf fiber. Ind Crops Prod. 2012;40:55–61. doi: 10.1016/j.indcrop.2012.02.037. [DOI] [Google Scholar]
- Ketnawa S, Rawdkuen S, Chaiwut P. Two phase partitioning and collagen hydrolysis of bromelain from pineapple peel Nang Lae cultivar. Biochem Eng J. 2010;52:205–211. doi: 10.1016/j.bej.2010.08.012. [DOI] [Google Scholar]
- Kumar D, Jain VK, Shanker G, Srivastava A. Utilisation of fruits waste for citric acid production by solid state fermentation. Process Biochem. 2003;38:1725–1729. doi: 10.1016/S0032-9592(02)00253-4. [DOI] [Google Scholar]
- LC Barretto de, O, Moreira J de J da S, Santos JAB dos, et al. 2013. Characterization and extraction of volatile compounds from pineapple (Ananascomosus L. Merril) processing residues. Food Sci Technol. 33 638 645. 10.1590/s0101-20612013000400007
- Larrauri JA, Rupérez P, SauraCalixto F. Pineapple shell as a source of dietary fiber with associated polyphenols. J Agric Food Chem. 1997;45:4028–4031. doi: 10.1021/jf970450j. [DOI] [Google Scholar]
- Liu W, Misra M, Askeland P, et al (2005) ‘ Green ’ composites from soy based plastic and pineapple leaf fiber : fabrication and properties evaluation. 46:2710–2721. 10.1016/j.polymer.2005.01.027
- Lun OK, Wai TB, Ling LS. Pineapple Cannery Waste as a Potential Substrate for Microbial Biotranformation to Produce Vanillic Acid and Vanillin. 2014;21:953–958. [Google Scholar]
- Mala T, Sadiq MB, Anal AK (2021) Comparative extraction of bromelain and bioactive peptides from pineapple byproducts by ultrasonic‐and microwave‐assisted extractions. J Food Process Eng 44:e13709
- Maurer HR. Bromelain: biochemistry, pharmacology and medical use. Cell Mol Life Sci. 2001;58:1234–1245. doi: 10.1007/PL00000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengal P, Mompon B (2011) Procédé et installation d’extraction sans solvant de produits naturels par micro-ondes. Brevet international, WO 94/26853, 199. 394–409
- Mohamad N, Ramli N, Abd S, et al (2019) Comparison of hydro - distillation , hydro - distillation with enzyme - assisted and supercritical fluid for the extraction of essential oil from pineapple peels. 3 Biotech 9:1–9. 10.1007/s13205-019-1767-8 [DOI] [PMC free article] [PubMed]
- Montero-Calderón M, Rojas-Graü MA, Martín-Belloso O (2010a) Pineapple (Ananas comosus [L.] Merril) flavor. Handb fruit Veg flavors 391–414
- Montero-Calderón M, Rojas-Graü MA, Martín-Belloso O. Aroma profile and volatiles odor activity along gold cultivar pineapple flesh. J Food Sci. 2010;75:506–512. doi: 10.1111/j.1750-3841.2010.01831.x. [DOI] [PubMed] [Google Scholar]
- Murthy PS, Naidu MM. Improvement of robusta coffee fermentation with microbial enzymes. Eur J Appl Sci. 2011;3:130–139. [Google Scholar]
- Nadzirah KZ, Zainal S, Noriham A, et al. Physico-chemical properties of pineapple crown extract variety n36 and bromelain activity in different forms. APCBEE Proc. 2012;4:130–134. doi: 10.1016/j.apcbee.2012.11.022. [DOI] [Google Scholar]
- Nagar S, Mittal A, Gupta VK (2012) Enzymatic Clarification of Fruit Juices (Apple , Pineapple , and Tomato) Using Purified Bacillus pumilus SV-85S Xylanase. 1175:1165–1166. 10.1007/s12257-012-0375-9
- Nghiem N, Kleff S, Schwegmann S. Succinic acid: technology development and commercialization. Fermentation. 2017;3:26. doi: 10.3390/fermentation3020026. [DOI] [Google Scholar]
- Nigam JN. Continuous cultivation of the yeast Candida utilis at different dilution rates on pineapple cannery effluent. World J Microbiol Biotechnol. 1999;15:127–129. doi: 10.1023/A:1008843201019. [DOI] [Google Scholar]
- Nigam PS. Microbial enzymes with special characteristics for biotechnological applications. Biomolecules. 2013;3:597–611. doi: 10.3390/biom3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor UA, Okochi VI, NwodoChinedu S, et al. Pectinolytic activity of wild-type filamentous fungi fermented on agro-wastes. African J Microbiol Res. 2010;4:2729–2734. [Google Scholar]
- Pardo MES, Cassellis MER, Escobedo RM, García EJ. Chemical characterisation of the industrial residues of the pineapple (Ananas comosus) J Agric Chem Environ. 2014;03:53–56. doi: 10.4236/jacen.2014.32b009. [DOI] [Google Scholar]
- Prakongpan T, Nitithamyong A, Luangpituksa P. Extraction and application of dietary fiber and cellulose from pineapple cores. J Food Sci. 2002;67:1308–1313. doi: 10.1111/j.1365-2621.2002.tb10279.x. [DOI] [Google Scholar]
- Pyar H, Liong MT, Peh KK. Potentials of pineapple waste as growth medium for Lactobacillus species. Int J Pharm Pharm Sci. 2014;6:142–145. [Google Scholar]
- Sah BNP, Vasiljevic T, McKechnie S, Donkor ON. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT - Food Sci Technol. 2016;65:978–986. doi: 10.1016/j.lwt.2015.09.027. [DOI] [Google Scholar]
- Saravanan P, Muthuvelayudham R, Viruthagiri T. Enhanced Production of Cellulase from Pineapple Waste by Response Surface Methodology. J Eng (united States) 2013 doi: 10.1155/2013/979547. [DOI] [Google Scholar]
- Selani MM, Brazaca SGC, Dos Santos Dias CT, et al. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014;163:23–30. doi: 10.1016/j.foodchem.2014.04.076. [DOI] [PubMed] [Google Scholar]
- Sengar AS, Rawson A, Muthiah M, Kalakandan SK. Comparison of different ultrasound assisted extraction techniques for pectin from tomato processing waste. Ultrason Sonochem. 2020 doi: 10.1016/j.ultsonch.2019.104812. [DOI] [PubMed] [Google Scholar]
- Senthilkumaar S, Bharathi S, Nithyanandhi D, Subburam V. Biosorption of Toxic Heavy Metals from Aqueous Solutions. 2000;75:163–165. [Google Scholar]
- Shiau S, Wu M, Liu Y. The effect of pineapple core fiber on dough rheology and the quality of mantou. J Food Drug Anal. 2015 doi: 10.1016/j.jfda.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teai T, Claude-Lafontaine A, Schippa C, Cozzolino F. Volatile compounds in fresh pulp of pineapple (Ananas comosus [L.] Merr.) from French polynesia. J Essent Oil Res. 2001;13:314–318. doi: 10.1080/10412905.2001.9712222. [DOI] [Google Scholar]
- Ting VLZ, Ping TY, Abdullah AH. Removal of Pb (II) from aqueous solution by pineapple plant stem. Malaysian J Anal Sci. 2019;23:219–228. [Google Scholar]
- Tran CT, Mitchell DA. Pineapple waste - a novel substrate for citric acid production by solid-state fermentation. Biotechnol Lett. 1995;17:1107–1110. doi: 10.1007/BF00143111. [DOI] [Google Scholar]
- Upadhyay A, Lama JP, Tawata S. Utilization of pineapple waste: a review. J Food Sci Technol Nepal. 2010;6:10–18. doi: 10.3126/jfstn.v6i0.8255. [DOI] [Google Scholar]
- Wan J, Guo J, Miao Z, Guo X. Reverse micellar extraction of bromelain from pineapple peel - effect of surfactant structure. Food Chem. 2016;197:450–456. doi: 10.1016/j.foodchem.2015.10.145. [DOI] [PubMed] [Google Scholar]
- Wei CB, Liu SH, Liu YG, et al. Characteristic aroma compounds from different pineapple parts. Molecules. 2011;16:5104–5112. doi: 10.3390/molecules16065104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh FW, Murray WD, Williams RE, Katz I. Microbiological and enzymatic production of flavor and fragrance chemicals. Crit Rev Biotechnol. 1989;9:105–169. doi: 10.3109/07388558909040617. [DOI] [Google Scholar]
- Yun S, Ho S, Yong T, Yup S. Metabolic Engineering of Escherichia Coli for the Production of Malic Acid. 2008;40:312–320. doi: 10.1016/j.bej.2008.01.001. [DOI] [Google Scholar]
- Zain NAM, Aziman SN, Suhaimi MS, Idris A. Optimization of L (+) lactic acid production from solid pineapple waste (spw) by rhizopus oryzae NRRL 395. J Polym Environ. 2021;29:230–249. doi: 10.1007/s10924-020-01862-0. [DOI] [Google Scholar]
- Zheng LY, Sun GM, Liu YG, et al. Aroma volatile compounds from two fresh pineapple varieties in China. Int J Mol Sci. 2012;13:7383–7392. doi: 10.3390/ijms13067383. [DOI] [PMC free article] [PubMed] [Google Scholar]